Abstract
Cypermethrin (CYP) is one of the most common active ingredients in most insecticides, mosquito coils and powder used in Nigeria. dichlorvos (DDVP) is the most indiscriminately used fumigant in most rural and sub-urban areas in Nigeria. These fumigants can easily be accessed without proper method of usage thus exposing the population to their toxic effects. As a result, this study was initiated to determine the effects of sub-acute exposure of CYP and DDVP on some biochemical and histopathological parameters of albino rats. In this study, forty (40) albino rats of 10 groups of 4 rats per group, with one group serving as control, were exposed to these fumigants in a poorly ventilated area for 4hours per day over 2, 4 and 6 weeks. The results showed observable changes in liver enzyme activities (p<0.05) in groups exposed to DDVP for 2, 4 and 6 weeks. The groups exposed to CYP showed mild changes in liver enzyme activities when compared with the DDVP groups. Increase in activity of the liver enzymes was also observed in the groups exposed to a mixture of DDVP+CYP for 2, 4 and 6 weeks. The urea, creatinine and electrolytes levels in all the groups exposed to DDVP, CYP and DDVP+CYP for 2, 4 and 6weeks were significantly (p<0.05) increased. Also WBC and platelets in all the groups exposed to DDVP and CYP recorded significant changes. The histology report of the lungs and liver showed moderate lymphocytic infiltration and hepatocytic steatosis which progressed with duration of exposure to the fumigants, while the kidneys showed no remarkable changes. The results of this study suggest that DDVP and CYP have relative toxic effects in the exposed animals and should be used with caution to avoid human exposure to their visible toxicities.
Keywords: dichlorovos, cypermethrin, fumigant, liver enzymes, sub-acute toxicity
Introduction
Fumigant is any volatile substance used to kill insects, nematodes, and other animals or plants that damage stored foods or seeds. Inadvertent exposure of humans and animals to fumigants is of great danger but the effects are not well quantified as many are not aware of toxic effects of these compounds. Many marketers and farmers through poor handling and ignorance are exposed to these substances. For example dichlorvos or 2,2-dichlorovinyl dimethyl phosphate (DDVP) is widely used worldwide in agriculture for controlling agricultural deleterious insects (Okamura et al., 2005) and thus humans and animals are exposed to its toxicity. Organophosphate compounds such as DDVP are known to cause severe toxicity and death from acute poisoning in many parts of the world (Eddleston, 2000). The widespread use of pesticides produces a number of serious health hazards affecting both humans and animals. Poisoning by these compounds represents a serious public health problem especially in developing countries (Karki et al., 2004). DDVP has been shown to endanger human health (Alavanja et al., 2004; Forget, 1993). Thus ways to reduce human exposure is paramount.
DDVP also known as Ota-piapia in south-east Nigeria is indiscriminately used because of its accessibility and affordability (Nesheim et al., 2002). In Nigeria, DDVP is commonly produced and used as an effective and potent insecticide (Owoeye et al., 2012). The inactivation of acetylcholinesterase by dichlorvos seems to be the major mechanism of toxicity of this compound (Antonijevic et al., 2016; Mostafalou et al., 2017; Mileson et al., 1998). Though, reactive oxygen species (ROS) production by dichlorvos has also been described as one of the mechanisms of its toxicity induction (Eraslan et al., 2010; Eroglu et al., 2013), causing irreversible damage to DNA and membrane lipid peroxidation (Ajiboye, 2010). Studies have also shown that chronic dichlorvos intoxication may result in increased brain intracellular Ca2+ levels and decline in Ca2+-MgATPase activity (Raheja & Gill, 2002), which is deleterious to normal functioning of cells.
cypermethrin is extensively used not only as an ectoparasiticide in animals but also to control many agricultural pests. cypermethrin is classified by the World Health Organisation (WHO) as ‘moderately hazardous’ (WHO, 2009) which interacts with the sodium channels in nerve cells and interferes with other receptors in the nervous system.
Since most people use these fumigants in their houses against insects and pests, this study was initiated to determine the effects of sub-acute exposure of CYP and DDVP on aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), bilirubin (total and direct bilirubin), urea, creatinine and serum electrolytes in albino rats.
Materials and methods
Fumigants
Fumigants (dichlorvos and cypermethrin, 1000 mg/L each) were purchased from Molon Agrochemical company, GRA Enugu, Nigeria and then prepared in a dilution of 1:1 as recommended by the manufacturer (Hubei Samonda Co. Ltd, China), i.e. 50 ml of fumigant was mixed with 50 ml of distilled water.
Experimental animals
Forty (40) albino rats (Rattus norvegicus) of both sexes aged 6 to 8 weeks with an average weight range of 125.9 g to 135.6 g and mean body weight of 130.0±3.5 g were obtained from the animal house of the animal research unit of the College of Medicine, University of Nigeria, Enugu Campus, Enugu State, Nigeria. They were fed rat chow and water ad libitum. The animals were allowed to acclimatize for 2 weeks before being randomized into 10 groups of 4 rats per group as follows:
Group treatment
Group 0 served as control group and was not exposed to any fumigant; Group 1A was exposed to cypermethrin (50 ml CYP/50 ml distilled water – v/v) for 2 weeks of 4 hours per day at room temperature in a poorly ventilated compartment; Group 1B was exposed to dichlorvos (50 ml DDVP/50 ml distilled water – v/v) for 2 weeks of 4 hours per day at room temperature in a poorly ventilated compartment; Group 1C was exposed to a mixture of dichlorvos and cypermethrin (25 ml DDVP + 25 ml CYP/50 ml distilled water – v/v) for 2 weeks of 4 hours per day at room temperature in a poorly ventilated compartment; Group 2A was exposed to cypermethrin (50 ml CYP/50 ml distilled water – v/v) for 4weeks of 4hours per day at room temperature in a poorly ventilated compartment; Group 2B was exposed to dichlorvos (50 ml DDVP/50 ml distilled water – v/v) for 4 weeks of 4 hours per day at room temperature in a poorly ventilated compartment; Group 2C was exposed to mixture of dichlorvos and cypermethrin (25 ml DDVP + 25 ml CYP/50 ml distilled water – v/v) for 4 weeks of 4 hours per day at room temperature in a poorly ventilated compartment; Group 3A was exposed to cypermethrin (50 ml CYP/50 ml distilled water – v/v) for 6 weeks of 4 hours per day at room temperature in a poorly ventilated compartment; Group 3B was exposed to dichlorvos (50 ml DDVP/50 ml distilled water – v/v) for 6 weeks of 4 hours per day at room temperature in a poorly ventilated compartment; Group 3C was exposed to a mixture of dichlorvos and cypermethrin (25 ml DDVP + 25 ml CYP/50 ml distilled water – v/v) for 6 weeks of 4 hours per day at room temperature in a poorly ventilated compartment.
The doses were obtained using the simplified method of evaluating dose-effect experiments as described by Litchfield et al. (1949).
Exposure of animals to fumigants
Fumigants as described above were poured into an open container for easy diffusion of its odor in and out of the container and inhalation by the animals kept in a metal cage. The metal cage was placed inside a carton. The container was fixed in a corner inside the carton in such a way that the animals were unable to pour away the content of the container in order to prevent body contact.
Collection of blood samples and organs
At the end of 2 weeks, 4 weeks and 6 weeks, blood samples were collected from the rats through ocular puncture without the use of anesthetic agent. Blood samples of the animals were collected into plain tubes and into ethylenediaminetetraacetic acid (EDTA) tubes. The blood samples collected in plain tubes were allowed to clot and were centrifuged to get the serum and stored at 4 °C until used. The animals were sacrificed before the organs (livers, lungs and kidneys) were harvested and used for histopathology analysis.
Biochemical analysis
Aspartate transaminase (AST) and alanine transaminase (ALT) were determined according to the method of Reitman and Frankel (1957). Alkaline phosphatase (ALP) level was assessed using the method described by King (1965). The bilirubin (total and direct bilirubin) levels in serum was determined as described by Dangerfield and Finlayson (1953). Enzymatic method as described by Machado and Horizonte (1958) was used in urea determination. Creatinine was determined as described by Mitchell (1973). Electrolytes were determined using Ion-Selective Electrodes method as described by International Federation of Clinical Chemistry and Laboratory Medicine (IFCC), (2000).
Histological preparation and analyses
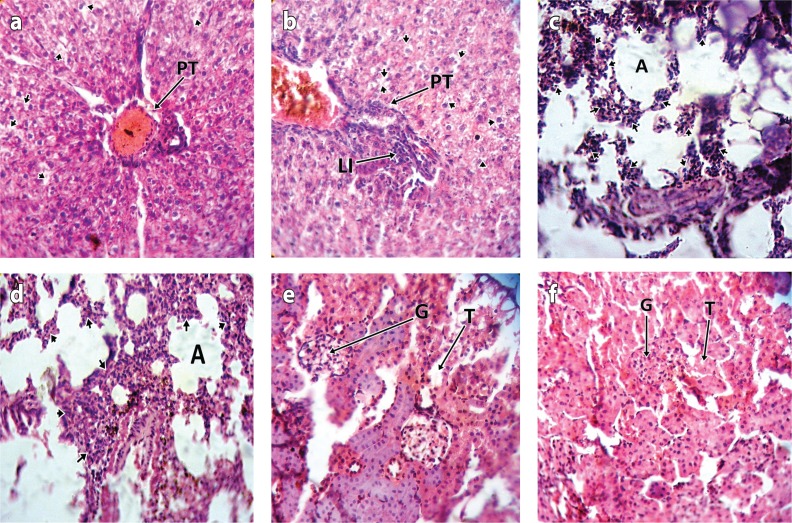
Kidney, liver and lungs were cut in sections at 5 μm and stained using hematoxylin and eosin method for demonstration of general tissue structure. Stained sections were examined using light microscopy and microscopically photographed using Nikon photomicroscope (Figures 1a–f).
Figure 1.
a: Photomicrograph of the liver of test animal treated with cypermethrin for 2 weeks showing mild steatosis of hepatocytes (short arrows) around portal tract (PT) (H & E stain ×400). b: Photomicrograph of the liver of test animal treated with dichlorvos for 2 weeks showing moderate steatosis of hepatocytes (short arrows) and mild lymphocytic infiltration (LI) of portal tract (PT) (H & E stain ×400). c: Photomicrograph of the lung of test animal treated with cypermethrin for 2 weeks showing moderate lymphocytic infiltration (short arrows) of the interstitium (H & E stain ×400). d: Photomicrograph of the lung of test animal treated with dichlorvos for 2 weeks showing moderate lymphocytic infiltration (short arrows) of the interstitium (H & E stain ×400). e: Photomicrograph of the kidney of test animal treated with cypermethrin for 2 weeks showing unremarkable glomeruli (G) and renal tubules (T) (H & E stain ×400). f: Photomicrograph of the kidney of test animal treated with dichlorvos for 2 weeks showing unremarkable glomeruli (G) and renal tubules (T) (H & E stain ×400).
Data analysis
The Statistical Package for Social Science (SPSS) computer software version 17 was used for data analysis. The results of the tests were analyzed using analysis of variance (ANOVA) and student’s t-test at 95% confidence interval with p-value of ≤0.05 considered as significant.
Ethical permit
Ethical permit was sought and obtained from the research and ethics committee of the College of Medicine, University of Nigeria, Enugu Campus. The procedures followed in this study were in accordance with the guide for the care and use of laboratory animals by the National Research Council (National Research Council, 2011)
Results
The results of toxicity study of DDVP inhalation on liver enzymes of rats showed a significant increase in the activities of AST and ALT of rats exposed to DDVP for 2 weeks and 4 weeks and a significant decrease in the activity of AST and ALT of rats exposed to DDVP for 6 weeks when compared with the control. For ALP, there was a significant decrease in the activity of ALP across the three groups and there was a significant decrease in the activity of total bilirubin of rats exposed to DDVP for 4 weeks, while there was a significant decrease in the activity of direct bilirubin in all groups compared with the control (Table 1).
Table 1.
Mean± SD values of some liver enzymes of rats exposed to DDVP through inhalation.
| Group | AST (U/L) | ALP (U/L) | ALT (U/L) | T.BILL (mg/l) | D.BILL (mg/l) |
|---|---|---|---|---|---|
| Control | 80.50±7.00ae | 362.25±180.80a | 81.00±10.03ac | 6.05±0.42ac | 2.67±0.34a |
| 1B | 93.75±9.28b | 881.25±189.21bf | 105.50±11.90ba | 6.37±0.70ac | 1.49±0.41bc |
| 2B | 89.00±1.24be | 1166.00±59.93c | 101.25±20.17be | 4.62±1.80b | 1.55±0.12bc |
| 3B | 75.75±6.65a | 710.50±187.96f | 73.75±15.94cf | 5.90±0.25ac | 1.97±0.42bc |
Data in the same column bearing different superscripts differ significantly. (p<0.05) DDVP: Dichlorovos. 1B = exposed to DDVP for 2 weeks, 2B = exposed to DDVP for 4 weeks and 3B = exposed to DDVP for 6 weeks.
There was a significant decrease in the activity of AST in the group exposed to CYP for 2 weeks, which significantly increased in week 4 and week 6. There was also a significant increase in the activity of ALT in week 2, which decreased with no significant difference in week 4 and week 6. The ALP activity increased significantly in weeks 2, 4 and 6 of exposure while the direct bilirubin activity decreased with duration of exposure of CYP (Table 2).
Table 2.
Mean± SD values of some liver enzymes of rats exposed to CYP DDVP through inhalation.
| Group | AST (U/L) | ALP (U/L) | ALT (U/L) | T.BILL (mg/l) | D.BILL (mg/l) |
|---|---|---|---|---|---|
| Control | 80.50±7.00ae | 362.25±180.80a | 81.00±10.03ac | 6.05±0.42ac | 2.67±0.34a |
| 1A | 76.00±7.87a | 1032.25±9.53c | 94.25±4.03ab | 6.32±0.99ac | 2.27±0.30a |
| 2A | 83.25±7.13ba | 902.50±47.74bcf | 80.00±5.29ac | 6.12±0.87a | 1.92±0.29bc |
| 3A | 85.00±3.65ba | 1020.75±5.37bc | 82.75±1.50ac | 4.77±0.05b | 1.80±0.66bc |
Data in the same column bearing different superscripts differ significantly. (p<0.05) CYP: Cypermethrin, 1A = exposed to CYP for 2 weeks, 2A = exposed to CYP for 4 weeks and 3A = exposed to CYP for 6 weeks.
The AST, ALT and ALP activities significantly increased after 2-week exposure and decreased with duration of exposure of the mixture of DDVP+CYP and the bilirubin activity decreased with duration of exposure (Table 3).
Table 3.
Mean± SD values of some liver enzymes of rats exposed to a mixture of DDVP +CYP DDVP through inhalation.
| Group | AST (U/L) | ALP (U/L) | ALT (U/L) | T.BILL (mg/l) | D.BILL (mg/l) |
|---|---|---|---|---|---|
| Control | 80.50±7.00ae | 362.25±180.80a | 81.00±10.03ac | 6.05±0.42ac | 2.67±0.34a |
| 1C | 93.00±7.25b | 1169.50±65.22c | 110.00±28.08b | 5.85±1.31ac | 1.27±0.25bc |
| 2C | 79.75±5.43ae | 1009.50±37.11bc | 82.25±5.31ac | 4.00±0.93b | 1.00±0.74bd |
| 3C | 60.25±8.13d | 849.75±148.43bf | 72.50±3.87cf | 4.20±0.08b | 1.32±0.43bd |
Data in the same column bearing different superscripts differ significantly. (p<0.05) DDVP: Dichlorovos, CYP: Cypermethrin. 1C = exposed to the mixture of DDVP +CYP for 2 weeks, 2C = exposed to the mixture of DDVP +CYP for 4 weeks and 3C = exposed to the mixture of DDVP + CYP for 6 weeks.
Significant increase in values of K+, Na+, Cl– and urea and a significant decrease in HCO3 – were observed in the groups when compared with the control (Table 4).
Table 4.
Mean± SD values of serum urea, creatinine and some electrolytes of rats exposed to DDVP DDVP through inhalation.
| Group | K+ | Na+ | Cl– | HCO3 – | UREA (mg/l) | CREAT (mg/l) |
|---|---|---|---|---|---|---|
| Control | 3.72±0.23a | 124.50±5.19a | 89.50±5.19a | 25.25±3.50a | 4.42±1.99a | 96.25±11.44a |
| 1B | 6.37±0.82bf | 136.75±4.03bc | 89.00±4.24a | 24.75±3.09a | 6.45±1.42ac | 112.00±2.94b |
| 2B | 5.42±0.50c | 135.00±5.47bc | 97.25±6.80bc | 22.75±1.50ab | 7.22±0.60bd | 57.24±65.24e |
| 3B | 4.92±0.25d | 135.00±3.91bc | 96.00±5.94bc | 19.00±5.16bc | 6.27±0.55ac | 6.00±2.16g |
Data in the same column bearing different superscripts differ significantly. (P<0.05) DDVP: Dichlorovos, 1B = exposed to DDVP for 2 weeks, 2B = exposed to DDVP for 4 weeks and 3B = exposed to DDVP for 6 weeks.
The results of exposure of rats to CYP and DDVP+CYP revealed significantly increased levels of K+, Na+, Cl–, HCO3 – and urea and a significant decrease in creatinine across the groups when compared with the control (Tables 5 and 6).
Table 5.
Mean± SD values of serum urea, creatinine and some electrolytes of rats exposed to CYP DDVP through inhalation.
| Group | K+ | Na+ | Cl– | HCO3 – | UREA (mg/l) | CREAT (mg/l) |
|---|---|---|---|---|---|---|
| Control | 3.72±0.23a | 124.50±5.19a | 89.50±5.19a | 25.25±3.50a | 4.42±1.99a | 96.25±11.44a |
| 1A | 5.22±0.35c | 141.50±1.29c | 95.00±3.65b | 26.00±3.16a | 5.15±0.31ad | 90.25±6.89c |
| 2A | 6.32±0.76bf | 140.75±4.50c | 105.50±7.04c | 28.25±2.21ac | 8.92±2.62b | 90.00±6.83c |
| 3A | 4.52±0.68d | 139.00±2.16c | 99.25±3.36d | 27.50±5.44ac | 8.10±2.15b | 5.50±3.41g |
Data in the same column bearing different superscript differ significantly. (p<0.05) CYP: Cypermethrin. 1A = exposed to CYP for 2 weeks, 2A = exposed to CYP for 4 weeks and 3A = exposed to CYP for 6 weeks.
Table 6.
Mean± SD values of serum urea, creatinine and some electrolytes of rats exposed to a mixture of DDVP +CYP DDVP through inhalation.
| Group | K+ | Na+ | Cl– | HCO3 – | UREA (mg/l) | CREAT (mg/l) |
|---|---|---|---|---|---|---|
| Control | 3.72±0.23a | 124.50±5.19a | 89.50±5.19a | 25.25±3.50a | 4.42±1.99a | 96.25±11.44a |
| 1C | 6.57±0.76bf | 134.75±6.22bc | 89.25±4.78a | 17.75±5.61b | 5.17±1.73ad | 124.00±7.16d |
| 2C | 5.57±0.37c | 138.50±3.87bd | 97.75±6.13bc | 23.75±3.21a | 5.60±1.00ad | 29.00±43.42f |
| 3C | 4.85±0.36d | 136.00±3.55bc | 99.50±1.73d | 27.00±4.69ac | 8.77±2.60b | 5.75±2.21g |
Data in the same column bearing different superscripts differ significantly. (P<0.05) DDVP: Dichlorovos, CYP: Cypermethrin. 1C = exposed to a mixture of DDVP +CYP for 2 weeks, 2C = exposed to a mixture of DDVP +CYP for 4 weeks and 3C = exposed to a mixture of DDVP + CYP for 6 weeks
Histology of the lung and liver showed moderate lymphocytic infiltration and hepatocytic steatosis thus progressing with duration of exposure to fumigants, while the kidney showed no remarkable changes.
Discussion
Serum enzyme levels are considered indicators of overall health status of an individual, especially in hepatocyte injury and related stress (Khan et al., 2009). Small amounts of intracellular enzymes are present in the blood as a result of normal cell turnover. When damage to cells occurs, increased amounts of enzymes will be released and their concentrations in blood will rise.
The increase in activity of serum AST observed in DDVP exposure for 2 and 4weeks and the decrease observed in week 6 of exposure (Table 1) suggest toxic and harmful effects of DDVP on the liver. The decreased activity of serum AST observed after 2-week exposure of CYP and then rapid increase in 4 and 6weeks of exposure (Table 2) showed toxicity of CYP on the liver. Similar effect was also observed in the exposure of DDVP+CYP. The increase in AST activity of the groups exposed to DDVP observed in this study agrees with earlier investigations by Atef (2010) and Ajiboso (2012) on effects of a class of organophosphate insecticides in rats.
High levels of ALT usually indicate a damaged liver while most low ALT levels indicate malnutrition as a major cause of low blood ALT levels (Gimson, 1996). The mild decrease in activity of liver ALT in groups exposed to DDVP and DDVP+CYP for 6weeks may be due to malnutrition. According to Lum (1995), malnutrition is one of the conditions that leads to low serum ALT. The decreased serum activity of ALT in this study may be attributed to malnutrition that resulted from withdrawal from food by animals due to continuous and prolonged exposure to DDVP and DDVP+CYP. ALT activity was not altered in the groups exposed to CYP for 4 and 6weeks (Table 2). Impairment in alkaline phosphatase of rats exposed to other classes of insecticides such as DDT, malathion, phosalone and elsan, as reported by Saigal et al. (1982), was also observed in this study with altered activity of serum ALP of groups exposed to DDVP, CYP and DDVP+CYP for 2, 4, 6 weeks.
The increased bilirubin levels observed in rats exposed to DDVP and CYP for 2 weeks may be attributed to the breakdown of hemoglobin and the subsequent mild decrease may be due to the withdrawal from food in the exposed groups. A similar finding was reported by Ajiboso et al. (2012).
Increased creatinine levels of rats exposed for 2 weeks showed the idiosyncratic hepatotoxic properties of DDVP and DDVP+CYP. The increase in creatinine levels of groups exposed to DDVP in this study agrees with the result obtained by Atef (2010). However the decrease in creatinine levels for groups exposed to CYP for 2, 4, and 6weeks may be attributed to malnutrition. It was the same for the rats exposed to DDVP+CYP and DDVP for 4 and 6 weeks, which agrees with the report of Ajiboso et al. (2012). The kidney maintains blood creatinine within a normal range. Creatinine has been found to be a fairly reliable indicator of kidney function and an elevated creatinine levels suggests impaired kidney function or kidney disease (Al-Jassabi et al., 2011). In this study, there was an increase in the levels of urea for all groups exposed to DDVP, CYP and DDVP+CYP for 2 weeks, 4 weeks and 6 weeks, that may be attributed to high body muscle mass breakdown generating more waste nitrogen due to prolonged inhalation of toxic compounds. The balance of electrolytes in the body is essential for normal body function of cells and organs. The observed increase in values of sodium, potassium, chloride and bicarbonate ions was as a result of impaired kidney function due to prolonged exposure of toxic compounds (DDVP and CYP).
The histology report of the lungs and liver showed moderate lymphocytic infiltration and hepatocytic steatosis which progressed with duration of exposure to DDVP and CYP that could be attributed to the toxic effect of the fumigants on the organs. Notably, lungs are the first site of metabolism of gaseous poison, followed by the liver. However the kidneys showed no remarkable changes which did not correspond with the result of biochemical analysis of this study, and the reports by Owoeye et al. (2012) and Blair et al. (1976). The changes observed in the biochemical analysis may be attributed to malnutrition because these fumigants could cause loss of appetite in the experimental animals.
In conclusion, exposure to fumigants (DDVP and CPY) caused no mortality but induced biochemical alterations in Wistar rats and these disturbances in biochemical parameters and histology of vital organs, such as the liver and kidneys, could be attributed to toxic effects of DDVP and CPY.
REFERENCES
- Ajiboso SOO, Gbate M, Ajari OI, Adeyemo SO. Subchronic inhalation toxicity studies of 2,2-dichlorovinyl dimethyl phosphate (DDVP) in albino rats. Adv Biol Res. 2012;6(4):133–140. [Google Scholar]
- Ajiboye TO. Redox status of the liver and kidney of 2,2-dichlorovinyl dimethyl phos-phate (DDVP) treated rats. Chem Biol Interact. 2010;185:202–207. doi: 10.1016/j.cbi.2010.03.022. [DOI] [PubMed] [Google Scholar]
- Al-Jassabi S, Saad A, Azirun MS, Al-Omari A. The role of silymarin in prevention of alloxan-induced diabetes mellitus in Balb/C Mice. Am Eurasian J Toxicol Sci. 2011;3(3):172–176. [Google Scholar]
- Alavanja MC, Hoppin JA, Kamel F. Health effects of chronic pesticide exposure: cancerand neurotoxicity. Annu Rev Public Health. 2004;25:155–197. doi: 10.1146/annurev.publhealth.25.101802.123020. [DOI] [PubMed] [Google Scholar]
- Antonijevic E, Musilek K, Kuca K, Djukic-Cosic D, Vucinic S, Antonijevic B. Therapeutic and reactivating efficacy of oximes K027 and K203 against a direct acetylcholinesterase inhibitor. Neurotoxicology. 2016;55:33–39. doi: 10.1016/j.neuro.2016.05.006. [DOI] [PubMed] [Google Scholar]
- Atef MA. Physiological and histopathological investigations on the effects of α-lipoic acid in rats exposed to Malathion. J Biomed Biotech. 2010;203503:1–8. doi: 10.1155/2010/203503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair D, Dix KM, Hunt PF, Thorpe E, Stevenson DE, Walker AI. dichlorvos : A 2-year inhalation carcinogenesis study in rats. Arch. Toxicol. 1976;35:281–294. doi: 10.1007/BF00570270. [DOI] [PubMed] [Google Scholar]
- Burnett RW, Covington AK, Fogh-Andersen N, Külpmann WR, Lewenstam A, Maas AH, Müller-Plathe O, VanKessel AL, Zijlstra WG. Use of ion-selective electrodes for blood-electrolyte analysis. Recommendations for nomenclature, definitions and conventions. International Federation of Clinical Chemistry and Laboratory Medicine (IFCC). Scientific Division Working Group on Selective Electrodes. Clin Chem Lab Med. 2000;38(4):363–370. doi: 10.1515/CCLM.2000.052. [DOI] [PubMed] [Google Scholar]
- Dangerfield WG, Finlayson R. Estimation of bilirubin in serum. J Clin Pathol. 1953;6(3):173–177. doi: 10.1136/jcp.6.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddleston M. Patterns and problems of deliberate self-poisoning in the developing world. QJM. 2000;93:715–31. doi: 10.1093/qjmed/93.11.715. [DOI] [PubMed] [Google Scholar]
- Eraslan G, Kanbur M, Silici S, Karabacak M. Beneficial effect of pine honey on trichlorfon induced biochemical alterations in mice. Ecotoxicol Environ Saf. 2010;73:1084–91. doi: 10.1016/j.ecoenv.2010.02.017. [DOI] [PubMed] [Google Scholar]
- Eroglu S, Pandir D, Uzun FG, Bas H. Protective role of vitamins C and E in dichlorvos induced oxidative stress in human erythrocytes in vitro. Biol Res. 2013;46:33–38. doi: 10.4067/S0716-97602013000100005. [DOI] [PubMed] [Google Scholar]
- Forget G. Balancing the need for pesticides with the risk to human health. In: Forget G, Goodman T, de Villiers A, editors. Impact of Pesticide Use on Health in Developing Countries. Ottawa: IDRC; 1993. p. 2. [Google Scholar]
- Gimson AE. Fulminant and late onset hepatic failure. British J Anaesth. 1996;77(1):90–98. doi: 10.1093/bja/77.1.90. [DOI] [PubMed] [Google Scholar]
- Karki P, Ansari JA, Bhandary S, Koirala S. Cardiac and electrocardiographical manifestations of acute organophosphate poisoning. Singapore Med J. 2004;45:385–389. [PubMed] [Google Scholar]
- Khan A, Faridi HAM, Ali M, Khan MZ, Siddique IH, Ahmad M. Effects of cypermethrin on some clinico-hematobiochemical and pathological parameters in male dwarf goats (Capra hircus) Exp Toxicol Pathol. 2009;61:151–160. doi: 10.1016/j.etp.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Litchfield JT, Wilcoxon F. A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther. 1949;96(2):99–113. [PubMed] [Google Scholar]
- Lum G. Low activities of aspartate and alanine aminotransferase. Lab Med. 1995;26(4):273–276. [Google Scholar]
- Machado M, Horizonte B. Simple and rapid method for determination of urea by urease. Rev Assoc Med Bras. 1958;4:364–367. [PubMed] [Google Scholar]
- Mileson BE, Chambers JE, Chen WL, Dettbarn W, Ehrich M, Eldefrawi AT, Gaylor DW, Hamernik K, Hodgson E, Karczmar AG, Padilla S, Pope CN, Richardson RJ, Saunders DR, Sheets LP, Sultatos LG, Wallace KB. Common mechanism of toxicity: A case study of organophosphorus pesticides. Toxicol Sci. 1998;41:8–20. doi: 10.1006/toxs.1997.2431. [DOI] [PubMed] [Google Scholar]
- Mitchell RJ. Improved method for specific determination of creatinine in serum and urine. Clin Chem. 1973;19:408–410. [PubMed] [Google Scholar]
- Mostafalou S, Abdollahi M. Pesticides: an update of human exposure and toxicity. Arch Toxicol. 2017;91(2):549–599. doi: 10.1007/s00204-016-1849-x. [DOI] [PubMed] [Google Scholar]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. 8th edition. Washington (DC): National Academies Press (US; 2011. [PubMed] [Google Scholar]
- Nesheim ON, Fishel FM, Mossler MA. Toxicity of pesticides. Gainesville University of Florida Institute of Food and Agricultural Sciences; 2002. http://edis.ufl.edu/P1008. [Google Scholar]
- Okamura A, Kamijima M, Shibata E, Ohtani K, Takagi K, Ueyama J, Watanabe Y, Omura M, Wang H, Ichihara G, Kondo T, Nakajima T. A comprehensive evaluation of the testicular toxicity of dichlorvos in Wistar rats. Toxicology. 2005;213:129–37. doi: 10.1016/j.tox.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Owoeye O, Edem FV, Akinyoola BS, Rahaman S, Akang EE, Arinola GO. Histological changes in liver and lungs of rats exposed to dichlorvos before and after vitamin supplementation. Eur J Anat. 2012;16(3):190–198. [Google Scholar]
- Owoeye O, Edem FV, Akinyoola BS, Rahaman S, Akang EE, Arinola GO. Histological changes in liver and lungs of rats exposed to dichlorvos before and after vitamin supplementation. Eur J Anat. 2012;16(3):190–198. [Google Scholar]
- Raheja G, Gill KD. Calcium homeostasis and dichlorovos induced neurotoxicity in rat brain. Mol Cell Biochem. 2002;232:13–18. doi: 10.1023/a:1014873031013. [DOI] [PubMed] [Google Scholar]
- Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28(1):56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- Saigal S, Bhatnagar VK, Malviya AN. Effect of selected pesticides on alkaline and acid phosphatase in the rat. Toxicol Lett. 1982;12(2–3):177–180. doi: 10.1016/0378-4274(82)90182-5. [DOI] [PubMed] [Google Scholar]
- The WHO recommended classification of pesticides by hazard and guidelines to classification . International Programme on Chemical Safety (IPCS) 2009. [Google Scholar]