Abstract
Metabolic syndrome represents one of the major health, social and economic issues nowadays, and affects more than 25% people worldwide. Being a multifactorial health problem, metabolic syndrome clusters various features, such as obesity, dyslipidemia, hyperglycemia and hypertension. Each of these disturbances represents a risk factor for developing cardiovascular disease. Moreover, patients with metabolic syndrome are more likely to suffer from depression, thus treatment with antidepressants (e.g. venlafaxine) is often neccessary. However, many of the antidepressants themselves may contribute to worsening or even development of the metabolic syndrome, thus creating a “vicious circle”. The aim of this work was to investigate on the animal model of metabolic syndrome, i.e. on hypertriacylglycerolemic rats fed high-fat-fructose diet (HFFD): 1) the effect of a change in diet from HFFD to a standard diet (SD) and the effect of venlafaxine treatment, 2) during HFFD, 3) as well as during a changed diet to SD. We focused on biometric parameters, blood pressure and selected ECG parameters. We observed the reversibility of the present metabolic and cardiovascular changes by switching the HFFD to SD in the last 3 weeks of the experiment. Switch to the standard diet led to decrease of body weight, even in the presence of venlafaxine. Administration of venlafaxine caused the decrease of heart weight/body weight index in rats fed with HFFD compared to the untreated group fed with HFFD for 8 weeks. Blood pressure, which was increased in the HFFD group showed a tendency to decrease to control values after switching to the standard diet . Administration of venlafaxine led to significant increase in all parameters of blood pressure when rats were fed with HFFD throughout the whole experiment. In untreated rats fed with HFFD for 8 weeks, we observed a shorter PQ interval and prolonged QRS complex as well as QTc interval compared to untreated rats with diet switched to SD. This effect was potentiated by venlafaxine administered not only during HFFD but even after switch to SD. Our results point to the fact that metabolic syndrome is clearly affecting the function of the cardiovascular system by modifying blood pressure and electrical activity of the heart. Moreover, administration of venlafaxine may lead to worsening of the observed changes, especially in the presence of high-fat-fructose diet.
Keywords: metabolic syndrome, high-fat-fructose diet, antidepressants, venlafaxine, blood pressure, ECG
Introduction
Metabolic syndrome (MetS) belongs to the most widespread disorders around the world. Its prevalence is rising from year to year, currently affecting more than 25% of the human population and reaching the dimension of an epidemy. MetS is comprised of several independent features – central obesity, hypertension, dyslipidemia, and hyperglycemia, as the result of insulin resistance and impaired glucose tolerance (Gancheva et al., 2017). The pathophysiology of MetS is most likely an interplay between various factors – genetic, metabolic, and environmental (Watanabe et al., 2008; Knezl et al., 2017). Patients suffering from MetS are at higher risk of developing cardiovascular diseases (CVD), such as coronary heart disease, left ventricular dysfunction, myocardial electrical disturbances or sudden cardiac death, as each individual sign and symptom of MetS represents a risk factor for CVD development (Yilmaz et al., 2015; Kurl et al., 2016; Knezl et al., 2017).
A further global health and socio-economic problem with rising prevalence are psychiatric disorders, especially depression. Patients with serious mental illnesses have 2–3 times higher mortality rates and an approximately 30 years shorter life expectancy than the general population. More than 60% of psychiatric patients die mostly due to a non-psychiatric disorder, such as CVD (Alosaimi et al., 2017), which could be, in turn, caused by MetS.
Epidemiological studies have shown an association between MetS and depression (Simon et al., 2006), the prevalence of depression among patients with MetS being as high as 31% (Vancampfort et al., 2014). Obesity is considered to increase the risk of depression, and depression is believed to be a predictive factor for the development of obesity (Gariepy et al., 2010). Various mechanisms have been proposed to mediate the association between depression and MetS, including inflammation, leptin resistance, hypothalamic-pituitary-adrenal (HPA) axis dysregulation, sympatho-adrenal activation, sleep disturbances and poor diet that can result in a decrease in physical activity (Pan et al., 2012). Treatment with antidepressants is therefore often necessary in these patients. However, there is evidence that antidepressants, including venlafaxine, are independently associated with the worsening or even development of MetS, by an increase in systolic and diastolic blood pressure, as well as in LDL cholesterol levels and body weight (McIntyre et al., 2010). Moreover, venlafaxine itself may cause cardiac electrical disturbances, such as QT interval and QRS complex prolongation or lethal forms of dysrhythmias (Khalifa et al., 1999; Bavle, 2015; Vicen et al., 2016; Sasváriová et al., 2018).
Since MetS is a disorder characterized by the dysbalance in energy storage and utilization (Gancheva et al., 2017), one of the most important factors playing a role in the development of MetS is diet. The rising prevalence of MetS may be caused by the general change of lifestyle and eating habits in the modern society, with the trend of high caloric, carbohydrate and fat intake, together with avoidance of physical activity (Finucane et al., 2011), often connected with increased risk of CVD and mortality (Flegal et al., 2007). For experimental observations of the impact of MetS on the cardiovascular system, it is necessary to develop a suitable animal model that would be reliable and would combine as much risk factors of MetS as possible. In the present work, we used as an animal model of MetS hereditary hypertriacylglycerolemic (hHTG) rats fed high-fat-fructose diet (HFFD). Our aim was to investigate 1) the effect of a change in the diet from HFFD to a healthy/standard diet (SD), 2) further the effect of venlafaxine treatment during HFFD, or during achanged diet to SD. We focused on biometric parameters, blood pressure and selected ECG parameters. According to available scientific literature, we expect that we are the first who investigated the effect of venlafaxine treatment in relation to the intake of different dietary composition. We focused on the possibility to reverse the changes by switching HFFD to the standard diet, thus simulating the change of regimen in patients diagnosed with MetS.
Materials and methods
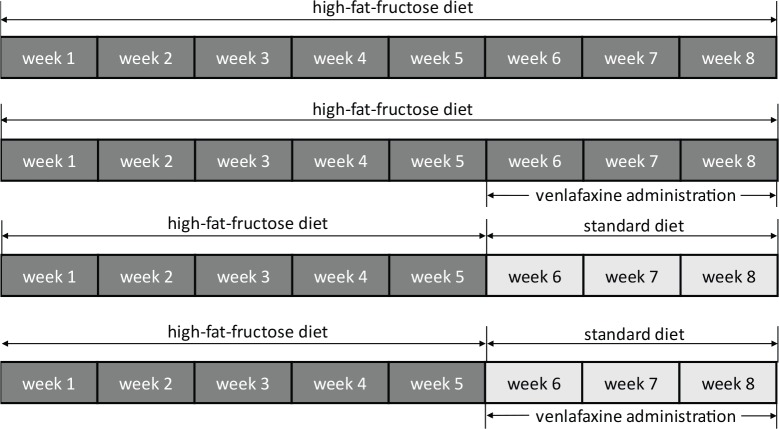
In our 8-week lasting experiment male hereditary hypertriacylglycerolemic rats (hHTG; age: 12 weeks, n=38) were divided into: group HFFD 8 – rats fed 8 weeks with high-fat-fructose diet (HFFD); group HFFD 5+3 – rats fed 5 weeks with HFFD followed by 3 weeks of standard diet (SD); group HFFD 8+VE – rats fed 8 weeks with HFFD and within the last 3 weeks of experiment under the HFFD treated with venlafaxine; group HFFD 5+3+VE – rats fed 5 weeks HFFD, then 3 weeks SD and treated with venlafaxine during the switched diet (Figure 1). HFFD was enriched with 10% of fructose, 7.5% of lard and 1% of cholesterol. The experimental model of metabolic syndrome was designed and prepared at the Institute of Experimental Pharmacology and Toxicology, Centre of Experimental Medicine of the Slovak Academy of Sciences (Kaprinay et al., 2016; 2017).
Figure 1.
Scheme of the experimental protocol.
All of the procedures with animals were performed according to the Principles of Laboratory Animal Care executed by the Ethical Committee of the Institute of Experimental Pharmacology and Toxicology, CEM SAS and by the State Veterinary and Food Administration of Slovak Republic (No. 3635/14-221).
Blood pressure was measured by non-invasive tail-cuff method on the dorsolateral tail artery of a rat, with the MLT125/R cuff (Pulse transducer pressure cuff for rats), using NIBP Controller and Powerlab 8/30 (equipments used were from ADInstruments, Spechbach, Germany) using a rat-friendly procedure (Lipták et al. 2017a). Blood pressure, as well as standard ECG were monitored in conscious rats, using Seiva EKG Praktik Veterinary (Seiva s.r.o., Prague, Czech Republic) (Kraľova et al., 2008). ECG re cordings were analyzed offline (Seiva Database Veterinary, Prague, Czech Republic) and we focused on the duration of PQ interval, QTc interval and of the QRS complex. Corrected QT (QTc) interval was used for elimination of the variability in heart rate and calculated according to the following formula:
QTc=(QT)/(√(RR/200))
where QT – duration of QT interval, RR – the time (ms) between two consecutive R peaks, 200 – physiological heart rate in isolated spontaneously beating rat heart. The susceptibility of the heart to the development of sustained ventricular tachycardia and ventricular fibrillation was evaluated under conditions of isolated spontaneously beating heart perfused according to Langendorff.
After the isolation, hearts were perfused retrogradely through the aorta at constant pressure (80 mmHg). For the assessment of basal diastolic pressure, water-filled latex balloon (size 5; 0.1 ml volume) was inserted into the left ventricle and adjusted to diastolic pressure 8–10 mmHg. The threshold for the development of sustained fibrillation or tachycardia was detected by stimulation (Electrostimulator ST-3, Medicor, Budapest, Hungary) with the pair of electrodes attached to the right ventricle and ECG-like recording was monitored, by pair of eletrodes positioned on the wall of left ventricle. The current intensity was increasing by 5 mA/30 s from 10 mA to 50 mA until the induction of 2 minutes lasting ventricular tachycardia (VT) or fibrillation (VF). Consequently, the flow of the perfusion medium was stopped and time needed for the restoration of the sinus rhythm was recorded (Tribulova et al., 2002; Knezl et al., 2017; Liptak et al., 2017b). The basic parameters of stimulation used were: 10 mA, train duration: 2 s, stimulation rate: 100 pps, delay: 0.1 ms, duration: 0.2 ms. The system BioLab F ver.1 (Institute of Measurement Science, Slovak Academy of Sciences, Bratislava, Slovakia) was used for data collection and offline analysis.
Solutions and chemicals
Composition of the Krebs-Henseleit solution used for isolated heart perfusion was (in mmol/1): NaCl, 118; KCl, 4.75; CaCl2 × 2H2O, 2.5; MgSO4 × 7H2O, 1.2; KH2PO4, 1.18; NaHCO3, 20.0; glucose, 11.1; saturated by the mixture of 95% O2 + 5% CO2, pH=7.4, temperature 37 °C. The chemicals used were from Centralchem (Bratislava, Slovakia) and mikroCHEM (Pezinok, Slovakia). Venlafaxine administered p.o. in dose 10mg/kg/day was from Chemos (Regenstauf, Germany).
Data analysis
All of the obtained data were statistically analyzed in Excel (Microsoft Excel 2016) with standard statistical functions (mean ± SEM) and one-way analysis of variance (ANOVA) was used to evaluate the difference among all experimental groups (SPSS 16.0), using the LSD, Bonferroni and Tukey post-hoc tests. The level of p≤0.05 was considered as a statistically significant difference.
Results
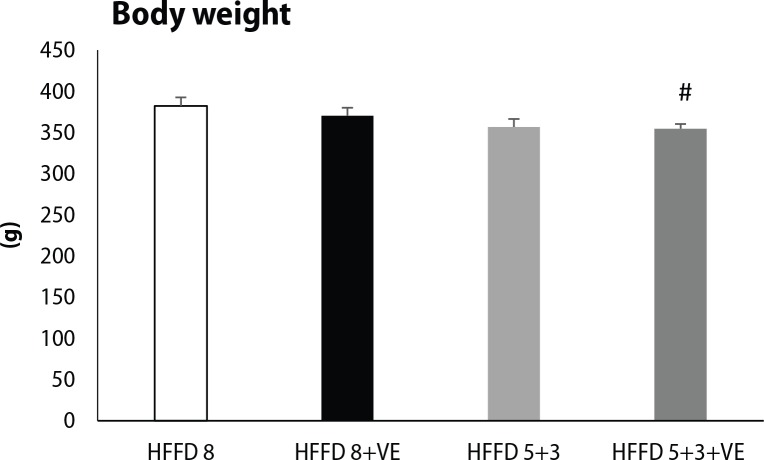
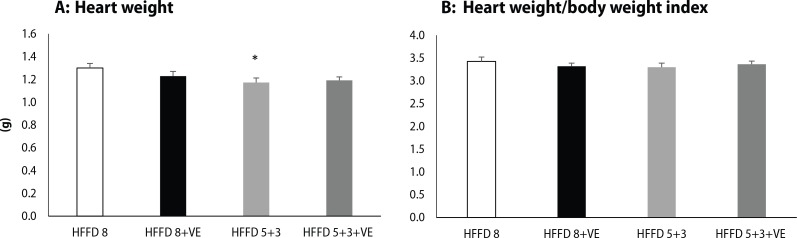
hHTG rats fed with HFFD for 8 weeks had the highest body weight among all of the tested groups. Switch of the HFFD to standard diet caused a decrease in body weight, and moreover, administration of venlafaxine together with standard diet caused a significant lowering of body weight compared to HFFD 8 group (Figure 2). We observed the same trend also in heart weight, when switch to standard diet led to a significant decrease in absolute heart weight (Figure 3A) and heart weight/body weight index (Figure 3B). Venlafaxine showed a tendency to lower heart weight and heart weight/body weight index in the presence of both HFFD and standard diet, however these changes were not statistically significant.
Figure 2.
Final body weight of rats fed high-fat-fructose diet and treated by venlafaxine. HFFD 8 (n=10), HFFD 8+VE (n=9), HFFD 5+3 (n=10), HFFD 5+3+VE (n=9). Values are expressed as mean ± SEM, #p≤0.05 for HFFD 5+3+VE vs. HFFD 8+VE.
Figure 3.
(A) Absolute heart weight isolated from rats fed high-fat-fructose diet and treated by venlafaxine. (B) Heart weight/body weight index of rats fed high-fat-fructose diet and treated by venlafaxine. Legend as in Figure 2. *p<0.05 for HFFD 5+3 vs. HFFD 8.
Analysis of blood pressure (Table 1) showed a significant increase in systolic, diastolic and mean arterial pressure values after 5 weeks of HFFD. Interestingly, values of blood pressure decreased after additional 3 weeks of HFFD close to the control values and also after the switch to standard diet. Administration of venlafaxine potentiated the effect of HFFD on systolic, diastolic, mean arterial and pulse pressure values and further increased these values significantly, yet only in the presence of HFFD.
Table 1.
Effect of venlafaxine administered during HFFD or standard diet in tested rats on blood pressure parameters.
| Group | sBP |
dBP |
|||||
|---|---|---|---|---|---|---|---|
| baseline | 5th week | 8th week | baseline | 5th week | 8th week | ||
| HFFD 8 | 119.66±6.24 | 133.17±3.16† | 125.88±3.52 | 75.14±2.06 | 81.98±4.22 | 77.54±4.18 | |
| HFFD 8+VE | 116.27±4.29 | 125.27±4.3 £ | 132.34±3.32 * | 77.36±2.2 | 82.38±2.03 £ | 84.46±1.35 * | |
| HFFD 5+3 | 124.3±10.36 | 143.6±3.29¤ | 129.46±4.19 € | 73.18±6.09 | 86.24±1.91 | 78.48±8.05 | |
| HFFD 5+3+VE | 122.22±5.96 | 139.73±6.44 | 126.18±5.47 # | 79.94±2.79 | 88.7±3.02§ | 80.92 ±2.72# | |
| Pulse pressure | Mean arterial pressure | ||||||
| HFFD 8 | 44.52±4.56 | 51.19±2.86 | 48.32±3 | 89.98±3.34 | 99.04±3.65† | 93.67±3.71 | |
| HFFD 8+VE | 39.52±2.36 | 42.88±2.62 | 47.88±2.1 | 90.53±2.85 | 96.67±2.72 £ | 100.42±1.98 * | |
| HFFD 5+3 | 51.12±4.73 | 57.36±2.95 | 50.98±3.17 | 90.22±7.45 | 105.36±2.03¤ | 95.47±2.94 € | |
| HFFD 5+3+VE | 42.28±3.26 | 51.04±3.57§ | 45.26±2.98 | 94.04±3.83 | 105.71±4.13§ | 96±3.59# | |
Values of systolic (sBP), diastolic (dBP) blood pressure, pulse pressure and mean arterial pressure at the beginning of the experiment (“baseline”), after 5 weeks of HFFD (5th week) and after 3 weeks of venlafaxine administration (8th week). HFFD 8 (n=10), HFFD 8+VE (n=9), HFFD 5+3 (n=10), HFFD 5+3+VE (n=9). Values are expressed as mean ± SEM
p≤0.05 for HFFD 8; 5th week vs. 8th week
p≤0.05 for HFFD 8+VE, 5th week vs. 8th week
p≤0.05 for HFFD 8+VE, 8th week vs. baseline
p≤0.05 for HFFD 5+3, 8th week vs. 5th week
p≤0.05 for HFFD 5+3, 5th week vs. baseline
p≤0.05 for HFFD 5+3+VE, 5th week vs. baseline
p≤0.05 for HFFD 5+3+VE, 5th week vs. 8th week.
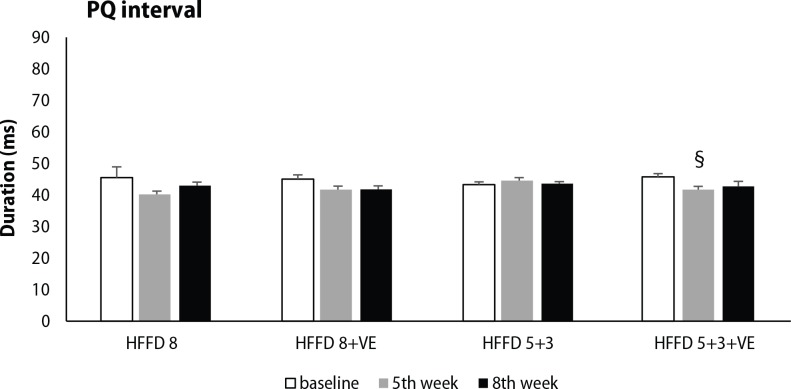
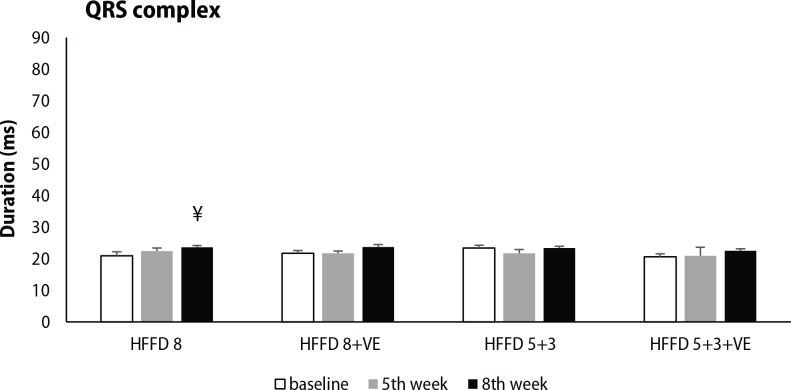
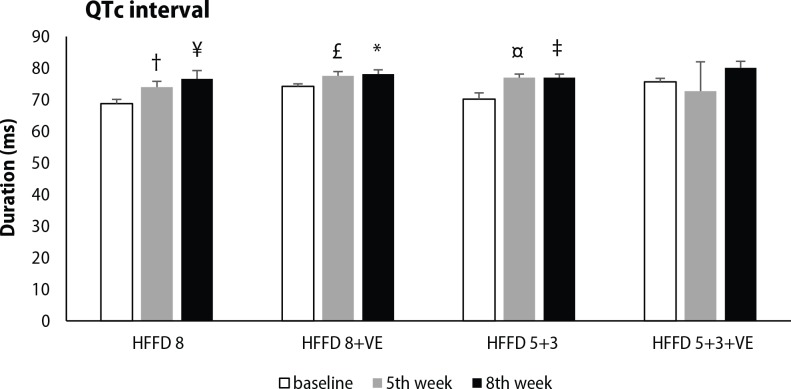
ECG analysis was focused on selected parameters of cardiac electrical activity, including PQ interval, QRS complex and QTc interval duration. High-fat-fructose diet without treatment showed a tendency to shorten PQ interval during 8 weeks, while this change was significant in groups HFFD 8+VE and HFFD 5+3+VE after 5 weeks of HFFD. Administration of venlafaxine displayed only a minor effect on PQ interval duration (Figure 4). Significant prolongation of QRS complex was seen after 8 weeks of HFFD compared to baseline values. Venlafaxine non-significantly potentiated the effect of 5-week lasting HFFD on QRS complex duration, independently from the diet applied during its administration in the following 3 weeks (Figure 5). Significant prolongation of QTc interval was induced by five weeks lasting HFFD in almost all of the groups tested (except HFFD 5+3+VE group). This change was not modified by the switch to standard diet. Moreover, longer duration of HFFD either without or with venlafaxine administration led to further QTc interval prolongation (Figure 6).
Figure 4.
Changes in PQ interval duration, measured at the beginning of the experiment, after 5 weeks of HFFD and after venlafaxine administration during the next 3 weeks in rats fed HFFD or SD. Legend as in Figure 2. §p<0.05 for HFFD 5+3+VE, 5th week vs. baseline. Legend as in Figure 2.
Figure 5.
Changes in QRS complex duration, measured at the beginning of the experiment, after 5 weeks of HFFD and after venlafaxine administration during the next 3 weeks in rats fed with HFFD or SD. Legend as in Figure 2. ¥p<0.05 for HFFD 8 8th week vs. baseline.
Figure 6.
Changes in QTc interval duration, measured at the beginning of the experiment, after 5 weeks of HFFD and after venlafaxine administration during the following 3 weeks in rats fed HFFD or SD. Legend as in Figure1. † p<0.05 for HFFD 8, 5th week vs. 8th week; ¥ p<0.05 for HFFD 8. 8th week vs. baseline; £ p<0.05 for HFFD 8+VE, 5th week vs. 8th week; *p<0.05 for HFFD 8+VE, 8th week vs. baseline; ¤ p<0.05 for HFFD 5+3, 5th week vs. baseline; ‡ p<0.05 for HFFD 5+3, 8th week vs. baseline. ¥ p<0.05 for HFFD 8 8th week vs. baseline. Legend as in Figure 2.
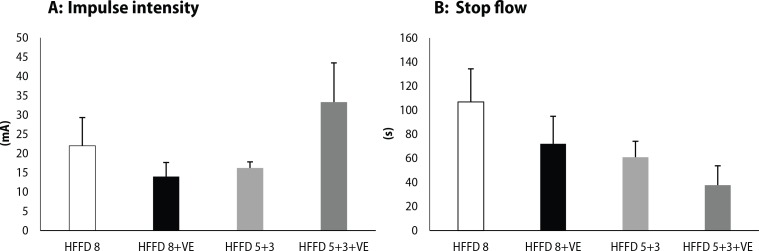
Detection of the threshold for the development of sustained ventricular tachycardia or fibrillation showed that administration of venlafaxine to rats fed HFFD for 8 weeks led to higher susceptibility to life-threatening dysrhythmias (Figure 7A). However, the proarrhythmogenic potential of venlafaxine was not observed in the group where HFFD was changed for the last 3 weeks to standard diet. On the other hand, administration of venlafaxine shortened the time needed for the recovery of the sinus rhythm after VT/VF and consequent „stop-flow“ (discontinuation of the flow of perfusion medium), compared to HFFD 8 group (Figure 7B). Return to the standard diet led to shortening of the „stop-flow“ time, even in the presence of venlafaxine, in comparison with rats fed with the high-fat-fructose diet for 8 weeks.
Figure 7.
(A) Intensity of the impulse (mA) needed for induction of 2 minutes lasting sustained ventricular tachycardia or fi brillation. (B) The time (s) needed for the restoration of the sinus rhythm after „stop fl ow“ (discontinuation of the fl ow of perfusion medium). Legend as in Figure 2.
Discussion
Hereditary hypertriacylglycerolemic rats fed with a high-fat diet were previously defined by our colleagues as a non-obese animal model of metabolic syndrome (MetS). The model was characterized by disturbance in glucose and lipid metabolism, hepatic steatosis, oxidative stress and endothelial damage (Kaprinay et al., 2016). In this study, it has been proposed that addition of fructose to the diet could better simulate the western-type diet and lead to major changes not only in biochemical parameters but also in biometric parameters of the heart, blood pressure, and cardiac electrical activity. Moreover, foras-much as patients with MetS are more likely to suffer from depression and take antidepressants, it is necessary to know if the antidepressants (e.g. venlafaxine) could work as protection or potentiate the changes caused by MetS, or if the changes could be attenuated by turning back to the standard diet.
Obesity is one of the key symptoms of MetS. Even though the experimental model used by us is considered as a non-obese, animals fed with HFFD for 8 weeks had the highest body weight and by switching to the standard diet, we observed a decrease in body weight. According to Alosaimi et al. (2017), administration of venlafaxine and mirtazapine lead to the development of central obesity, and together with high correlation with other independent symptoms of MetS, represents a potential cardiometabolic risk factor for depressive patients. In our study, administration of venlafaxine led to a decrease in body weight, slightly in rats fed with HFFD for 8 weeks (HFFD 8+VE group) and significantly in the group fed for the last 3 weeks with the standard diet (HFFD 5+3+VE).
Lower heart weight/body weight index was observed in group HFFD 5+3, and the administration of venlafaxine caused a decrease in heart weight independently from the length of HFFD. Other studies showed that in animal models of metabolic syndrome, with a high fructose diet, abnormalities in myocardial structure (Cheng et al., 2014) and cardiac hypertrophy were present (Delbosc et al., 2005; Jover et al., 2017). Due to a high intake of fat and fructose, hepatic and intracardial lipid accumulation was previously observed, which could affect not only morphology but also the function of the organs. On the other hand, antidepressant paroxetine was shown to decrease the heart weight by reduction of myocyte size (Toscano et al., 2008).
In the present work, analysis of blood pressure showed a significant increase of blood pressure already after 5 weeks of HFFD compared to baseline values, however, further 3 weeks of modified diet resulted in a decrease in blood pressure in the HFFD 8 group, what may be a sign of some kind of adaptation. The development of prehypertension or even hypertension is consistent with other studies using similar models. Wong et al. (2018) used a special diet consisting of fructose, sweetened condensed milk, Hubble Mendel and Wakeman salt mixture, ghee and powdered rat chow. After 8 weeks, they observed a significant increase in blood pressure and the development of MetS. Combination of high fat and high fructose diet caused an increase in systolic blood pressure after 28 weeks in male Sprague-Dawley rats (Gradel et al., 2018). On the other hand, change of a lifestyle could reverse individual components of MetS, as well as hypertension. Hinderliter et al. (2014) examined overweight hypertensive or prehypertensive patients who underwent intervention on their lifestyle. Only the change of the diet caused a decrease in blood pressure by 11.2 mmHg after 16 weeks, and together with weight management intervention led to weight loss about 8.7 kg. This is in agreement with our results when blood pressure, which was increased in HFFD group, showed a tendency to decrease after switching to the standard diet. We observed a decrease in systolic, diastolic, mean arterial pressure and pulse pressure after the switch to the standard diet.
Venlafaxine has been reported to accelerate hypertension (Kivrak et al., 2014) or even lead to the development of hypertension in previously healthy patients (Mbaya et al., 2007), and in the worst cases it resulted in a hypertensive crisis (Khurana et al., 2003). Hypertensive effect of venlafaxine is connected mainly with higher doses or overdose, hypertensive effect in lower doses is rare. This could be explained by the character of venlafaxine, which is an antidepressant with a dual mechanism of action – it inhibits the reuptake of both serotonin and noradrenaline. However, the affinity of venlafaxine to serotonin receptors is 30-fold higher than to noradrenaline receptors in therapeutic doses, and its adrenergic effect could be observed mainly in higher doses (Harvey et al., 2000). Some studies evaluated the association between various antidepressants and MetS in depressive patients, but the majority of them are focused on selective serotonin reuptake inhibitors (citalopram, paroxetine) or tricyclic antidepressants (imipramine, clomipramine). This study showed that even though venlafaxine did not cause weight gain, it aggravated the effect of the modified diet and had a hypertensive effect only in the presence of HFFD (HFFD 8+VE group).
HFFD caused also changes in cardiac electrical activity. We observed PQ interval shortening after 5 weeks of HFFD in all groups (except HFFD 5+3 group), but there were no further changes either after another 3 weeks of the diet or after venlafaxine administration. Even though PQ interval prolongation is considered a risk factor for the development of atrial fibrillation (Schumacher et al., 2018), short PQ interval may be observed due to conduction abnormalities in the atrioventricular node and/or His-Purkyne network and may predispose the individuals to dysrhythmias (Moro & Cosio, 1980). PQ interval was found to be shortened in obese patients with no cardiovascular disease (Bilora et al., 1999).
Significant prolongation of the QRS complex was present only in the group exposed to HFFD during whole experiment (HFFD 8 rats). This is consistent with the study by Axelsen et al. (2015), who observed prolongation of the QRS complex in rats fed with high fructose diet (FFFR), used as a model of diet-induced pre-diabetes. QRS complex widening has been previously seen also in other studies focused on experimental diabetes, such as ZDF rats (Howarth et al., 2008) or type 2 diabetic Goto-Kazaki rats (Yang et al., 1990). Cardiac electro-physiological abnormalities found in FFFR and ZDF rats may be caused by intramyocardial lipid accumulation found in these two models. Ectopic lipid accumulation could lead to lipotoxicity and cardiac energy alterations together with morphological and functional mitochondrial disturbances, as well as arrhythmogenesis due to abnormal cardiac conduction (van de Weijer et al., 2011; Elezaby et al., 2015). Khalifa et al. (1999) have shown, that venlafaxine blocks the fast inward sodium current (INa) in guinea pig ventricular myocytes, which would explain the prolongation in QRS complex, however, in our experiment the duration of QRS complex was not further significantly increased in other experimental groups.
ECG analysis showed significant prolongation of QTc interval as soon as after 5 weeks of HFFD and the values were even higher after further 3 weeks in the group fed HFFD during the whole experiment (HFFD 8). Moreover, switch to the standard diet and also venlafaxine administration did not reverse the effect of HFFD and the QTc interval duration was still increased. This points to the fact, that administration of HFFD to hHTG rats leads to irreversible prolongation of ventricular repolarization, representing a risk factor for the development of ventricular dysrhythmias and sudden cardiac death. Patients with MetS are more likely to have a prolonged QTc interval, which increases the risk of cardiovascular mortality (Li et al., 2009). Additionally, venlafaxine may increase the duration of QTc interval in therapeutic doses, which could occur due to inhibition of the outward delayed rectifier potassium current (IKr) in ventricular myocytes (Lestas et al., 2005; Bavle, 2015).
A long QTc interval represents a risk factor for the development of dysrhythmias, especially life-threatening ventricular tachycardia (VT) or fibrillation (VF). By electrical stimulation, we evaluated the threshold for the development of sustained VT/VF (i.e. lasting more than 2 minutes). The lower the intensity of the impulse needed, the more susceptible is the heart for VT/VF occurrence. Our results showed that venlafaxine lowered the fibrillation threshold, but also shortened the time needed for the restoration of the sinus rhythm in the presence of high-fat fructose diet, in comparison to the HFFD 8 group. On the other hand, after the switch to the standard diet, the threshold for sustained VT/VF was markedly higher and the restoration of the sinus rhythm was more rapid. Vicen et al. (2016) showed that venlafaxine administered in a single dose to Wistar rats caused a significantly higher incidence of life-threatening dysrhythmias, compared to the control group. In clinical practice, venlafaxine showed a potential to induce tachycardia in a dose-dependent manner (Abozguia et al., 2006). Moreover, administration of high-fat diet even without fructose significantly decreased the fibrillation threshold in hHTG rats (Knezl et al., 2017).
Conclusions
From our present results, we could conclude that the administration of high-fat-fructose diet to hHTG rats led to the development of MetS symptoms and altered the cardiac electrical function. Switch to the standard diet, as a non-pharmacological way of alleviating the manifestations of metabolic syndrome, was partly able to reverse the cardiovascular alterations of animal MetS, however, some of them, such as QTc interval prolongation, persisted. Venlafaxine mostly potentiated changes caused by the modified diet, especially in the presence of 8-week HFFD and seems to be potent in the progression of MetS. Improvement of a life-style, at least by modification of diet, may decrease the cardiometabolic risk of venlafaxine, nevertheless cli nicians should be aware of the potential danger.
Acknowledgement
Supported by grants VEGA 2/0120/19, VEGA 2/0124/19 and UK/315/2019.
Sasváriova M. contributed by measuring of ECG and blood pressure, administration of venlafaxine and by obtaining biometric data of rats. She is corresponding author and the main author of the paper. Micháliková D. contributed by measuring blood pressure, administration of venlafaxine. Tyukos Kaprinay B. contributed by measuring blood pressure and administration of venlafaxine. Salvaras L. contributed by obtaining the biometric data. Knezl V. supervised the isolated and perfused heart experiments by Langendorff. Gáspárová Z. contributed as Head of the project related with metabolic syndrome. She revised the manuscript. Stankovičová T. contributed by the idea to test venlafaxine effect in different diet composition. She supervised the blood pressure and ECG analysis (MS) and biometric data collection (LS). She revised the manuscript.
REFERENCES
- Abozguia K, Chudley S, Gammage M. Dose-dependent Venlafaxine-induced sinus tachycardia. Int J Cardiol. 2006;113:E9–10. doi: 10.1016/j.ijcard.2006.01.072. [DOI] [PubMed] [Google Scholar]
- Alosaimi FD, Abalhassan M, Alhaddad B, Alzain N, Fallata E, Alhabbad A, Alas-siry MZ. Prevalence of metabolic syndrome and its components among patients with various psychiatric diagnoses and treatments: A cross-sectional study. Gen Hosp Psychiatry. 2017;45:62–69. doi: 10.1016/j.genhosppsych.2016.12.007. [DOI] [PubMed] [Google Scholar]
- Bavle A. Venlafaxine induced QTc interval prolongation in a therapeutic dose. Asian J Psychiatr. 2015;16:63–64. doi: 10.1016/j.ajp.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Bilora F, Vettore G, Barbata A, Pastorello M, Petrobelli F, San Lorenzo I. Electrocardiographic fi ndings in obese subjects. Minerva Gastroenterol Dietol. 1999;45:193–197. [PubMed] [Google Scholar]
- Burke V, Beilin LJ, Cutt HE, Mansour J, Wilson A, Mori TA. Eff ects of a lifestyle programme on ambulatory blood pressure and drug dosage in treated hypertensive patients: a randomized controlled trial. J Hypertens. 2005;23:1241–9. doi: 10.1097/01.hjh.0000170388.61579.4f. [DOI] [PubMed] [Google Scholar]
- Delbosc S, Paizanis E, Magous R, Araiz C, Dimo T, Cristol JP, Cros G, Azay J. Involvement of oxidative stress and NADPH oxidase activation in the development of cardiovascular complications in a model of insulin resistance, the fructose-fed rat. Atherosclerosis. 2005;179:43–49. doi: 10.1016/j.atherosclerosis.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Dupas J, Feray A, Goanvec C, Guernec A, Samson N, Bougaran P, Guerrero F, Mansourati J. Metabolic Syndrome and Hypertension Resulting from Fructose Enriched Diet in Wistar Rats. Biomed Res Int. 2017;2017:2494067. doi: 10.1155/2017/2494067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elezaby A, Sverdlov AL, Tu VH, Soni K, Luptak I, Qin F, Liesa M, Shirihai OS, Rimer J, Schaff er JE, Colucci WS, Miller EJ. Mitochondrial remodeling in mice with cardiomyocyte-specifi c lipid overload. J Mol Cell Cardiol. 2015;79:275–283. doi: 10.1016/j.yjmcc.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, Farzadfar F, Riley LM, Ezzati M. Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Body Mass Index) National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specifi c excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028–2037. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- Gancheva S, Galunska B, Zhelyazkova-Savova M. Diets rich in saturated fat and fructose induce anxiety and depression-like behaviors in the rat: is there a role for lipid peroxidation? Int J Exp Pathol. 2017;98:296–306. doi: 10.1111/iep.12254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariepy G, Nitka D, Schmitz N. The association between obesity and anxiety disorders in the population: a systematic review and meta-analysis. Int J Obes (Lond) 2010;34:407–419. doi: 10.1038/ijo.2009.252. [DOI] [PubMed] [Google Scholar]
- Gradel AKJ, Salomonsson M, Sørensen CM, Holstein-Rathlou NH, Jensen LJ. Long-term diet-induced hypertension in rats is associated with reduced expression and function of small artery SKCa, IKCa, and Kir2.1 channels. Clin Sci (Lond) 2018;132:461–474. doi: 10.1042/CS20171408. [DOI] [PubMed] [Google Scholar]
- Harvey AT, Rudolph RL, Preskorn SH. Evidence of the dual mechanisms of action of venlafaxine. Arch Gen Psychiatry. 2000;57:503–509. doi: 10.1001/archpsyc.57.5.503. [DOI] [PubMed] [Google Scholar]
- Hinderliter AL, Sherwood A, Craighead LW, Lin PH, Watkins L, Babyak MA, Blumenthal JA. The long-term eff ects of lifestyle change on blood pressure: One-year follow-up of the ENCORE study. Am J Hypertens. 2014;27:734–741. doi: 10.1093/ajh/hpt183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth FC, Jacobson M, Shafi ullah M, Adeghate E. Long-term effects of type 2 diabetes mellitus on heart rhythm in the Goto-Kakizaki rat. Exp Physiol. 2008;93:362–369. doi: 10.1113/expphysiol.2007.040055. [DOI] [PubMed] [Google Scholar]
- Cheng S-M, Cheng Y-J, Wu L-Y, Kuo C-H, Lee Y-S, Wu M-C, Huang C-Y, Ting H, Lee S-D. Activated apoptotic and anti-survival eff ects on rat hearts with fructose induced metabolic syndrome. Cell Biochem Funct. 2014;32:133–141. doi: 10.1002/cbf.2982. [DOI] [PubMed] [Google Scholar]
- Jover B, Reynes C, Rugale C, Reboul C, Jeanson L, Tournier M, Lajoix AD, Desmetz C. Sodium restriction modulates and prevents cardiac remodeling in a rat model of Metabolic syndrome. Biochim Biophys Acta Mol Basis Dis. 2017;1863:1568–1574. doi: 10.1016/j.bbadis.2017.02.026. [DOI] [PubMed] [Google Scholar]
- Kaprinay B, Lipták B, Slovák L, Švík K, Knezl V, Sotníkoví R, Gáspárová Z. Hypertriglyceridemic rats fed high fat diet as a model of metabolic syndrome. Physiol Res. 2016;65:S515–S518. doi: 10.33549/physiolres.933524. [DOI] [PubMed] [Google Scholar]
- Kaprinay B, Gáspárová Z, Lipták B, Frimmel K, Sotníková R. Endothelial dysfunction in experimental models of metabolic syndrome - effect of fructose. Eur Pharm J. 2017;64:4–6. [Google Scholar]
- Khalifa M, Daleau P, Turgeon J. Mechanism of sodium channel block by venlafaxine in guinea pig ventricular myocytes. J Pharmacol Exp Ther. 1999;291:280–284. [PubMed] [Google Scholar]
- Khurana RN, Baudendistel TE. Hypertensive crisis associated with venlafaxine. Am J Med. 2003;115:676–677. doi: 10.1016/s0002-9343(03)00472-8. [DOI] [PubMed] [Google Scholar]
- Kıvrak Y, Güvenç TS, Akbulut N, Yağcı I, Çığşar G, Gündüz S, Balcı B. Accelerated Hypertension after Venlafaxine Usage. Case Rep Psychiatry. 2014;2014:659715. doi: 10.1155/2014/659715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knezl V, Sotní ková R, Brnoliaková Z, Stankovič ová T, Bauer V, Bezek Š. Monotherapy of experimental metabolic syndrome: II. study of cardiovascular eff ects. Interdiscip Toxicol. 2017;10:86–92. doi: 10.1515/intox-2017-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurl S, Laaksonen DE, Jae SY, Makikallio TH, Zaccardi F, Kauhanen J, Ronkainen K, Laukkanen JA. Metabolic syndrome and the risk of sudden cardiac death in middle-aged men. Int J Cardiol. 2015;203:792–797. doi: 10.1016/j.ijcard.2015.10.218. [DOI] [PubMed] [Google Scholar]
- Letsas K, Korantzopoulos P, Pappas L, Evangelou D, Efremidis M, Kardaras F. QT interval prolongation associated with venlafaxine administration. Int J Cardiol. 2006;109:116–117. doi: 10.1016/j.ijcard.2005.03.065. [DOI] [PubMed] [Google Scholar]
- Li W, Bai Y, Sun K, Xue H, Wang Y, Song X, Fan X, Song H, Han Y, Hui R. Patients with metabolic syndrome have prolonged corrected QT interval (QTc) Clin Cardiol. 2009;32:E93–99. doi: 10.1002/clc.20416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipták B, Kaprinay B, Gáspárová Z. A rat-friendly modifi cation of the non-invasive tail-cuff to record blood pressure. Lab Animal. 2017a;46:251–253. doi: 10.1038/laban.1272. [DOI] [PubMed] [Google Scholar]
- Mbaya P, Alam F, Ashim S, Bennett D. Cardiovascular eff ects of high dose venlafaxine XL in patients with major depressive disorder. Human Psychopharmacology. 2007;22:129–133. doi: 10.1002/hup.834. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Young Park K, Law CWY, Sultan F, Adams A, Lourenco MT, Lo AKS, Soczynska JK, Woldeyohannes H, Alsuwaidan M, Yoon J, Kenedy SH. The association between conventional antidepressants and the metabolic syndrome. CNS Drugs. 2010;24:741–753. doi: 10.2165/11533280-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Moro C, Cosío FG. Electrophysiologic study of patients with short P-R interval and normal QRS complex. Eur J Cardiol. 1980;11:81–90. [PubMed] [Google Scholar]
- Pan A, Keum N, Okereke OI, Sun Q, Kivimaki M, Rubin RR, Hu FB. Bidirectional association between depression and metabolic syndrome: a systematic review and meta-analysis of epidemiological studies. Diabetes Care. 2012;35:1171–1180. doi: 10.2337/dc11-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasváriová M, Tyukos-Kaprinay B, Salvaras L, Belovičová K, Bögi E, Knezl V, Barteková M, Stankovičová T, Dubovický M. Eff ect of pre-gestational stress and prenatal venlafaxine administration on cardiovascular system of rat off spring. Eur Pharm J. 2018;65:17–22. [Google Scholar]
- Schumacher K, Büttner P, Dagres N, Sommer P, Dinov B, Hindricks G, Boll-mann A, Kornej J. Association between PR interval prolongation and electro-anatomical substrate in patients with atrial fi brillation. PLoS One. 2018;13:e0206933. doi: 10.1371/journal.pone.0206933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon GE, Von Korff M, Saunders K, Miglioretti DL, Crane PK, van Belle G, Kessler RC. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry. 2006;63:824–830. doi: 10.1001/archpsyc.63.7.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano AE, Amorim MA, de Carvalho Filho EV, Aragão Rda S, Cabral-Filho JE, de Moraes SR, Manhaes-de-Castro R. Do malnutrition and fl uoxetine neonatal treatment program alterations in heart morphology? Life Sci. 2008;82:21–22. doi: 10.1016/j.lfs.2008.03.013. [DOI] [PubMed] [Google Scholar]
- van de Weijer T, Schrauwen-Hinderling VB, Schrauwen P. Lipotoxicity in type 2 diabetic cardiomyopathy. Cardiovasc Res. 2011;92:10–18. doi: 10.1093/cvr/cvr212. [DOI] [PubMed] [Google Scholar]
- Vicen M, Gulač P, Stankovičová T. Changes in electrical activity of heart during ischemic-reperfusion injury modifi ed by the administration of antidepressants. Eur Pharm J. 2016;1:5–8. [Google Scholar]
- Vancampfort D, Correll CU, Wampers M, Sienaert P, Mitchaell AJ, De Herdt A, Probst M, Scheewe W, De Hert M. Metabolic syndrome and metabolic abnormalities in patients with major depressive disorder: a meta-analysis of prevalence and moderating variables. Psychol Med. 2014;44:2017–2028. doi: 10.1017/S0033291713002778. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Tanabe N, Watanabe T, Roden D M, Sasaki S. Aizawa Y. Metabolic syndrome and risk of development of atrial fi brillation. Circulation. 2008;117:1255–1260. doi: 10.1161/CIRCULATIONAHA.107.744466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SK, Chin KY, Suhaimi FH, Ahmad F, Ima-Nirwana S. The Eff ects of a Modifi ed High-carbohydrate High-fat Diet on Metabolic Syndrome Parameters in Male Rats. Exp Clin Endocrinol Diabetes. 2018;126:205–212. doi: 10.1055/s-0043-119352. [DOI] [PubMed] [Google Scholar]
- Yang Q, Kiyoshige K, Fujimoto T, Katayama M, Fujino K, Saito K, Nakaya Y, Mori H. Signal-averaging electrocardiogram in patients with diabetes mellitus. Jpn Heart J. 1990;31:25–33. doi: 10.1536/ihj.31.25. [DOI] [PubMed] [Google Scholar]
- Yilmaz H1, Özcan KS, Sayar N, Kemaloglu T, Gungor B, Erer B, Yilmaz M, Gurkan U, Cakmak N, Oz D, Calik AN, Bolca O. Metabolic syndrome is associated with atrial electrical and mechanical dysfunction. Med Princ Pract. 2015;24:147–152. doi: 10.1159/000368754. [DOI] [PMC free article] [PubMed] [Google Scholar]