Abstract
Background
This study aimed to investigate the safety and effectiveness of laparoscopic pyloromyotomy for infants with congenital hypertrophic pyloric stenosis.
Material/Methods
The clinical data of 233 infants with congenital hypertrophic pyloric stenosis who were treated at our hospital from January 2013 to January 2018 were analyzed retrospectively. The patients were divided into 2 groups: the laparoscopic group (group A, n=126) and the conventional operation group (group B, n=107).
Results
Laparoscopic surgery was successfully performed in all patients in the laparoscopic group, and none of the surgeries were converted to open surgery. Compared with traditional surgery, laparoscopic surgery has obvious advantages in operation time (29.8±12.9 minutes versus 37.2±17.5 minutes, P=0.012), postoperative feeding time (10.3±2.2 hours versus 15.2±4.1 hours, P=0.035), postoperative hospitalization time (2.8±0.7 days versus 3.5±1.9 days, P=0.013), incision length (0.9±0.2 cm versus 3.3±0.8 cm, P=0.002) and poor wound healing (0 versus 6, P=0.007). No complications, such as bleeding, gastric perforation, duodenal injury, abdominal infection or recurrent vomiting, were observed in the 2 groups. The growth and development (weight and height) of the infants in both groups were normal.
Conclusions
Laparoscopic pyloromyotomy has the same safety and effectiveness as the traditional operation and has the advantages of less trauma, faster recovery and cosmetically pleasing incisions.
MeSH Keywords: General Surgery; Laparoscopy; Surgery Department, Hospital
Background
Congenital hypertrophic pyloric stenosis (CHPS) is the most common malformation of the digestive tract in infants, it has the highest incidence among congenital digestive tract malformations and is the third most common birth defects [1–3]. CHPS is mainly due to the thickening and proliferation of pyloric muscle, which leads to mechanical pyloric obstruction caused by stenosis of the pylorus lumen [4]. The clinical manifestations are projectile vomiting with progressive aggravation 2 to 3 weeks after birth, which often leads to severe dehydration, electrolyte acid-base balance disorder, growth delay, malnutrition, pneumonia, etc. [5,6], but there are several reports in the literature of late presentations of HPS [7].
The traditional pyloromyotomy technique is well-developed with good therapeutic effects, but postoperative recovery is slow, with long incisions and large scars [8,9]. In recent years, with the development of laparoscopic techniques in children, laparoscopic pyloromyotomy has been widely used in the clinic because of its advantages of less trauma, faster postoperative recovery and good cosmetic effects [10,11]. Sathya et al. [12] performed a meta-analysis, which showed that laparoscopic surgery had the same safety and efficacy as open surgery, and the cosmetic effect was better. Oomen et al. [13] retrospectively analyzed the data of 106 open pyloromyotomy and 57 laparoscopic pyloromyotomy that were performed from September 2008 to June 2012, and they obtained the same results. In this study, we compared the safety and effectiveness of laparoscopic and open operations for congenital hypertrophic pyloric stenosis in infants.
Material and Methods
Patients
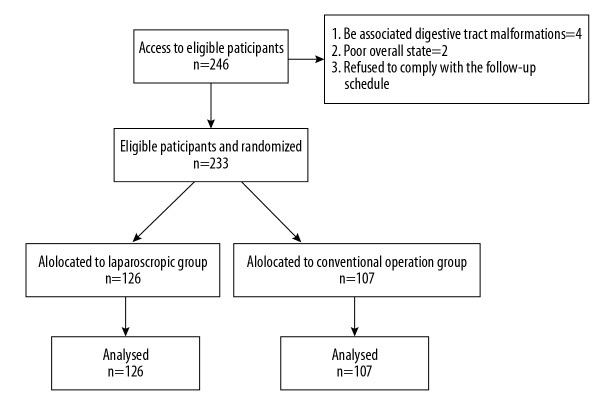
We exceeded the learning curve for laparoscopic pyloromyotomy in January 2013, and this study summarizes the clinical experience since then. We retrospectively analyzed the data of 233 infants with CHPS who were treated at our hospital from January 2013 to January 2018. The patients were divided into 2 groups: the laparoscopic group (group A, n=126) and the conventional operation group (group B, n=107) (Figure 1). All patient preoperative clinical data are shown in Table 1. There were no statistically significant differences in gender, age (group A: 41.4±10.8 days, group B: 38.8±12.1 days), body weight (group A: 4.3±1.3 kg, group B: 4.1±1.5 kg), or preoperative complications, indicating that the 2 groups were homogeneous and comparable. According to the clinical manifestation, physical examination, the results of abdominal color Doppler ultrasound and upper digestive tract radiography, all patients were positively diagnosed with CHPS. All patients were followed for 1 year after discharge, and the symptoms and growth and development were followed. The primary outcomes of the study were the perioperative and postoperative clinical data, such as operation time, postoperative feeding time, postoperative hospitalization time and incision length, and the secondary outcomes of the study were postoperative complications.
Figure 1.
Details of the study population.
Table 1.
Comparison of preoperative clinical data in laparoscopic group (n=126) and conventional group (n=107).
| Item | Laparoscopic group | Conventional group | P |
|---|---|---|---|
| Age (day) | 41.4±10.8 | 38.8±12.1 | 0.513 |
| Boys/Girls; n (%) | 95(75.4%)/31(24.6%) | 81(75.7%)/26(24.3%) | 0.915 |
| Weight (kg) | 4.3±1.3 | 4.1±1.5 | 0.449 |
| Severe malnutrition; n (%) | 26 (20.6%) | 21 (19.6%) | 0.818 |
| Electrolyte disturbances; n (%) | 105 (83.3%) | 91 (85.0%) | 0.598 |
| Congenital heart disease; n (%) | 13 (10.3%) | 11 (10.3%) | 0.993 |
Inclusion criteria were as follows: the infants were positively diagnosed with congenital hypertrophic pyloric stenosis. Exclusion criteria were as follows: 1) infants with other digestive tract malformations; 2) infants with a poor overall state, such as severe hepatic and renal insufficiency; and 3) refused to sign the consent form for surgery or refused to comply with the follow-up schedule.
Laparoscopic pyloromyotomy
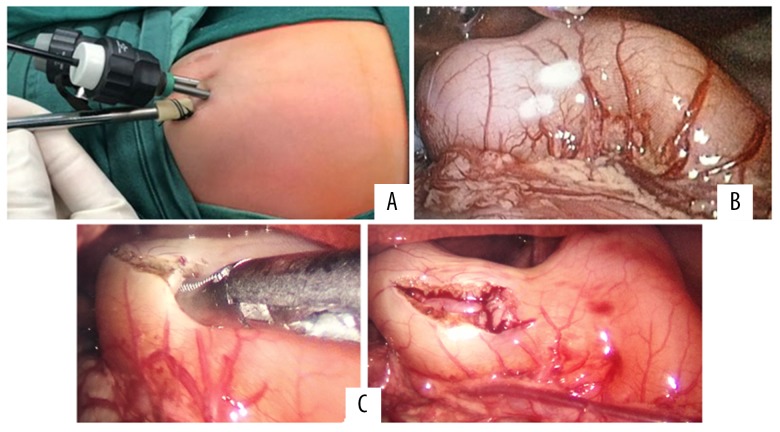
An approximately 0.3-cm incision was made layer-by-layer in the umbilical skin, and a 3-mm trocar was placed directly into the abdomen to establish pneumoperitoneum (5 mmHg). Under laparoscopy, a 3-mm trocar was placed on the right upper edge of the umbilical and the right costal edge. The hypertrophic pyloric stenosis was fully exposed. From the trocar on the right upper edge of the umbilical area, undamaged forceps were placed at the ear of the pyloric tube to catch the duodenum. From the trocar on the right costal edge, the pyloric cutting knife was inserted. From the end of the duodenum to the stomach, the non-vascular area on the anterior wall of the pyloric tube was cut longitudinally (approximately 1.0 cm). Then, we used forceps to open the cut pyloric muscle fully to make the mucous membrane completely bulge. Air (30–40 mL) was slowly injected from the gastric tube to make the mucous membrane bulge; then, we checked whether there was damage to the bulging mucous membrane. If not, we removed the air. After confirming that there was no bleeding, we removed the instrument, eliminated the pneumoperitoneum and closed the incision layer-by-layer (Figure 2).
Figure 2.
Intraoperative findings of hypertrophic pyloric stenosis in 5-week-old infant. (A) The position of the trocars. (B) Exposure of the hypertrophic pylorus. (C) Incision and dissection of pyloric muscle fully to make the mucosa completely bulge.
Traditional pyloromyotomy
After anesthesia, the patient was placed in a supine position, and then we routinely disinfected and draped the surgical area. A 3- to 4-cm longitudinal transverse incision was made in the right upper abdomen, and then we cut the skin, subcutaneous tissue, and muscle layer-by-layer. After entering the abdomen, the second and third fingers of the right hand explored the hypertrophic pyloric tube along the great bend of the stomach to the distal side, and then, the pyloric tube was gently raised out of the incision. The first and second fingers of the left hand fixed the pyloric tube. From the end of the duodenum to the stomach, the nonvascular area on the anterior wall of the pyloric tube was cut longitudinally (approximately 1.0 cm) under direct visualization. Then, we used the mosquito pliers to open the cut pyloric muscle fully to make the mucous membrane completely bulge. Air (30–40 mL) was slowly injected from the gastric tube to make the mucous membrane bulge; then, we checked whether there was damage to the bulging mucous membrane. If not, we removed the air. After confirming that there was no bleeding, we placed the pyloric tube back into the abdominal cavity and closed the incision layer-by-layer.
Statistical analysis
SPSS was used for statistical analysis. Continuous data are presented as the mean±the standard deviation and range. A normal distribution test was carried out on all the data, and a nonparametric test was used for data that did not have a normal distribution. Clinical parameters between the two groups were compared with an independent samples t-test. The χ2 or Fisher test was used to categorize the variables. A P value of <0.05 was defined as statistically significant.
Results
Laparoscopic surgery was successfully performed in all patients in the laparoscopic group, and none of the procedures were converted to open surgery. The operation time (29.8±12.9 minutes versus 37.2±17.5 minutes, P=0.012), postoperative feeding time (10.3±2.2 hours versus 15.2±4.1 hours, P=0.035), postoperative hospitalization time (2.8±0.7 days versus 3.5±1.9 days, P=0.013), incision length (0.9±0.2 cm versus 3.3±0.8 cm, P=0.002) in group A were significantly shorter than those in group B. There was no significant difference in the amount of bleeding (1.3±3.5 mL versus 2.2±2.5 mL, P=0.308) between group A and group B (Table 2).
Table 2.
Comparison of perioperative and postoperative clinical data in both groups.
| Item | Laparoscopic group | Conventional group | P |
|---|---|---|---|
| Operative time (min) | 29.8±12.9 | 37.2±17.5 | 0.012 |
| Volume of bleeding (mL) | 1.3±3.5 | 2.2±2.5 | 0.308 |
| Postoperative feeding time (h) | 10.3±2.1 | 15.2±4.1 | 0.035 |
| Postoperative hospitalization time (d) | 2.8±0.7 | 3.5±1.9 | 0.013 |
| Incision length (cm) | 0.9±0.2 | 3.3±0.8 | 0.002 |
The incidence of poor wound healing was 0% in group A and 5.6% (6 out of 107) in group B, and the difference was statistically significant (P=0.007). No complications, such as bleeding, gastric perforation, duodenal injury, abdominal infection or recurrent vomiting, occurred after surgery in the 2 groups. The healing time of the upper abdominal incision was generally 7–9 days. If the incision was still not healed after 9 days, we consider that to be poor wound healing.
One year after the operation, there was no recurrent vomiting in the 2 groups. The growth and development (height and weight) of the 2 groups were normal.
Discussion
Pyloromyotomy is the most effective surgery for the treatment of CHPS [14]. Presently, the most common surgical methods are open pyloromyotomy and laparoscopic pyloromyotomy. Open pyloromyotomy is a classical method with definite curative effects, but the incision is long, the recovery is slow, and the scar after healing is large and not cosmetically pleasing [8,9]. Since Alain performed laparoscopic pyloromyotomy in 1991 [15], laparoscopic treatment for CHPS has been widely recognized and popularized [10,11,16,17].
This study retrospectively analyzed the clinical data of laparoscopic and open pyloromyotomy in our hospital in the last 5 years. The results show that the success rate of the laparoscopic group was the same as that of the traditional surgery group, both of which reached 100% in this study. No recurrence of recurrent vomiting occurred after surgery and follow-up, and the growth and development (height and weight) of the patients in both groups were normal. The operation time, postoperative feeding time, postoperative hospitalization time, incision length and the incidence of poor wound healing in the laparoscopic group were significantly lower than those in the open group. The results were similar to those of Mahida et al. [18] and St Peter et al. [19]. Clinical data of 1143 pyloromyotomy patients, which included 674 patients (59%) who underwent a laparoscopic procedure, were analyzed by Mahida et al. [18], and the results showed that patients with laparoscopic surgery recovered more quickly and had shorter hospital stays. St Peter et al. [19] conducted a prospective randomized controlled study to compare the clinical effects of laparoscopic surgery and open surgery, and the results showed that pain and complications after laparoscopic surgery were lower, and the cosmetic effect was better.
Laparoscopic surgery had the following advantages. First, the magnification and clarity of the visual field is beneficial to successful surgery and reduces the probability of damage to normal tissue. Second, the stomach and the pylorus are not required to be operated on outside of the abdominal cavity, and the interference of the abdominal organs is small, which is beneficial to the recovery of gastrointestinal function after the operation. Third, the operation time is shorter. In the direct view of laparoscopy, the pyloric tube is easier to find. However, in open surgery, the pyloric tube should be found under the abdominal incision, the operation should be carried out outside of the incision, and it takes a long time for the incision to close on the abdomen. Fourth, the operation incision is small, the postoperative scar is not obvious and is cosmetically pleasing, the trauma is minimal, and the recovery speed is fast. Although there are many advantages, it is undeniable that a certain learning curve exists for the laparoscopic operation, in particular for infants, for whom the operation space for the laparoscopic operation is smaller, the surgical instrument is thinner, and the operation is more difficult [20]. CHPS mostly occurs in newborns or infants, and laparoscopic surgery has higher requirements for anesthesiologists and surgeons. Under laparoscopy, it is necessary to adjust the firepower of the electric knife when cutting the pyloric sarcosis layer and master the contact time and force between the knife and the pylorus to avoid the delayed perforation of the gastrointestinal tract caused by the skin effect of the electric knife. When the pyloric muscle is longitudinally cut, the muscle layer should be deep enough to ensure its complete separation. When the pyloric muscle layer is separated passively, it is necessary to ensure that the action is soft and slow to not injure the pyloric mucous membrane and to avoid perforation. If necessary, the 2 sides can be separated alternately from the outside.
There are several limitations in this study. First, the sample size was small. Second, it was a retrospective and single-center study. Third, the follow-up period was short.
Conclusions
Laparoscopic pyloromyotomy has the same early clinical effectiveness and safety as the traditional operation, and it also has the advantages of minimal trauma, quick recovery and a smaller and cosmetically pleasing incision.
Acknowledgements
We highly acknowledge the contribution by the participating doctors: Yi-fan Fang, Bing Zhang, Yu Lin, Jian-xi Bai.
Abbreviations
- CHPS
congenital hypertrophic pylorus stenosis
Footnotes
Conflict of interests
None.
Source of support: Departmental sources
References
- 1.Wang J, Waller DK, Hwang LY, et al. Prevalence of infantile hypertrophic pyloric stenosis in Texas 1999–2002. Birth Defects Res A Clin Mol Teratol. 2008;82(11):763–67. doi: 10.1002/bdra.20527. [DOI] [PubMed] [Google Scholar]
- 2.MacMahon B. The continuing enigma of pyloric stenosis of infancy: A review. Epidemiology. 2006;17(2):195–201. doi: 10.1097/01.ede.0000192032.83843.c9. [DOI] [PubMed] [Google Scholar]
- 3.Feng Z, Nie Y, Zhang Y, et al. The clinical features of infantile hypertrophic pyloric stenosis in Chinese Han population: Analysis from 1998 to 2010. PLoS One. 2014;9(2):e88925. doi: 10.1371/journal.pone.0088925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ranells JD, Carver JD, Kirby RS. Infantile hypertrophic pyloric stenosis: Epidemiology, genetics, and clinical update. Adv Pediatr. 2011;58(1):195–206. doi: 10.1016/j.yapd.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Everett KV, Capon F, Georgoula C, et al. Linkage of monogenic infantile hypertrophic pyloric stenosis to chromosome 16q24. Eur J Hum Genet. 2008;16(9):115l–54. doi: 10.1038/ejhg.2008.86. [DOI] [PubMed] [Google Scholar]
- 6.Walker K, Halliday R, Holland AJ, et al. Early developmental outcome of infants with infantile hypertrophic pyloric stenosis. J Pediatr Surg. 2010;45(12):2369–72. doi: 10.1016/j.jpedsurg.2010.08.035. [DOI] [PubMed] [Google Scholar]
- 7.Pogorelić Z, Čagalj IČ, Žitko V, et al. Late-onset hypertrophic pyloric stenosis in a 14-weeks-old full-term male infant. Acta Medica (Hradec Kralove) 2019;62(2):82–84. doi: 10.14712/18059694.2019.108. [DOI] [PubMed] [Google Scholar]
- 8.Peters B, Oomen MW, Bakx R, et al. Advances in infantile hypertrophic pyloric stenosis. Expert Rev Gastroenterol Hepatol. 2014;8(5):533–41. doi: 10.1586/17474124.2014.903799. [DOI] [PubMed] [Google Scholar]
- 9.Cai BL, Zhang YX. Progress in the study of surgical methods for congenital hypertrophic pylorus stenosis. International Journal of Pediatrics. 2016;43(3):201–3. [Google Scholar]
- 10.Zhang YX, Nie YQ, Xiao X, et al. Treatment of congenital hypertrophic pyloric stenosis with endoscopic pyloromyotomy. Chin J Pediatr. 2008;46(4):247–51. [PubMed] [Google Scholar]
- 11.Aspelund G, Langer JC. Current management of hypertrophic pyloric stenosis. Semin Pediatr Surg. 2007;16(1):27–33. doi: 10.1053/j.sempedsurg.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Sathya C, Wayne C, Gotsch A, et al. Laparoscopic versus open pyloromyotomy in infants: A systematic review and meta-analysis. Pediatr Surg Int. 2017;33(3):325–33. doi: 10.1007/s00383-016-4030-y. [DOI] [PubMed] [Google Scholar]
- 13.Oomen M, Bakx R, Peeters B, et al. Laparoscopic pyloromyotomy, the tail of the learning curve. Surg Endosc. 2013;27(10):3705–9. doi: 10.1007/s00464-013-2951-2. [DOI] [PubMed] [Google Scholar]
- 14.Fan JF, Wang DF, Pu X, et al. [Treatment strategy of congenital hypertrophic pyloric stenosis]. Chinese Journal of Pediatric Surgery. 2015;36(11):818–23. [in Chinese] [Google Scholar]
- 15.Alain JL, Grousseau D, Terrier G. Extramucosal pyloromyotomy by laparoscopy. Surg Endosc. 1991;5(4):174–75. doi: 10.1007/BF02653256. [DOI] [PubMed] [Google Scholar]
- 16.Miyata S, Cho J, Matsushima K, et al. Operative outcomes of infantile hypertrophic pyloric stenosis in patients with congenital heart disease. J Pediatr Surg. 2016;51(11):1755–58. doi: 10.1016/j.jpedsurg.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Bataineh ZA, Novotny NM. A novel nonelectrosurgical technique for incising the pylorus in laparoscopic pyloromyotomy. J Laparoendosc Adv Surg Tech A. 2018;28(2):235–36. doi: 10.1089/lap.2017.0555. [DOI] [PubMed] [Google Scholar]
- 18.Mahida JB, Asti L, Deans KJ, et al. Laparoscopic pyloromyotomy decreases postoperative length of stay in children with hypertrophic pyloric stenosis. J Pediatr Surg. 2016;51(9):1436–39. doi: 10.1016/j.jpedsurg.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 19.St Peter SD, Holcomb GW, 3rd, Calkins CM, et al. Open versus laparoscopic pyloromyotomy for pyloric stenosis: A prospective randomized trial. Ann Surg. 2006;244(3):363–70. doi: 10.1097/01.sla.0000234647.03466.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang ZQ, Tang HJ. [Clinical effect of laparoscopic surgery in the treatment of congenital hypertrophic pylorus stenosis]. Chinese and Foreign Medical Research. 2014;21:149–50. [in Chinese] [Google Scholar]