Abstract
Studies of contextual fear conditioning have found that ethanol administered prior to a conditioning session impairs the conditioned freezing response during a test session the next day. The present experiments examined the effects of ethanol on extinction, the loss of conditioned responding that occurs as the animal learns that a previously conditioned context no longer signals shock. Ethanol (1.5 g/kg) administered prior to a single (Experiment 1) or multiple (Experiment 2) extinction sessions impaired extinction. Ethanol administered prior to a test session disrupted the expression of freezing after extinction (Experiments 3–5). There was some evidence that ethanol served as an internal stimulus signaling the operation of conditioning or extinction contingencies (Experiments 4–5). In Experiment 6, post-session injections of 1.5 g/kg of ethanol had no effect on extinction with brief (3 min) or long (24 min) exposures to the context. A 3 g/kg dose of ethanol administered after a long extinction session caused higher levels of freezing during further extinction sessions, suggesting that ethanol may have impaired consolidation of the extinction memory or that the aversive properties of ethanol may have been conditioned to the context. Together, these results indicate that ethanol affects extinction by acting on multiple learning and performance processes, including attention, memory encoding, and memory expression.
Keywords: Ethanol, extinction, memory, fear, hippocampus, consolidation, reconsolidation
Studies of the role of ethanol in learning and memory have examined the effects of acute ethanol on memory formation in a number of behavioral preparations. A common finding from many of these studies is that ethanol appears to have deleterious effects on learning and memory, especially in hippocampus-dependent tasks, such as contextual fear conditioning, in which rodents learn to associate a context (such as a conditioning chamber) with a mild footshock (e.g., Gould, 2003). This learning results in a conditioned freezing response when the organism is returned to the context following conditioning. If no shock is presented during these re-exposures to the context, the conditioned freezing response decreases as the organism learns that the context no longer signals the shock. This extinction process suppresses the original context-shock association without severing that association and numerous studies have demonstrated that the behavioral and neurobiological learning processes that underlie extinction have characteristics that are both common to and distinct from those that underlie initial learning (reviewed in Delamater, 2004; Lattal, Radulovic, & Lukowiak, 2006).
Several findings suggest that some of the systems, cellular, and molecular processes that are involved in extinction are affected by ethanol. For example, extinction of context-evoked freezing appears to depend, at least in part, on the hippocampus (e.g., Corcoran & Maren, 2001; Lattal, Barrett, & Wood, 2007; Power, Berlau, McGaugh, & Steward, 2006) and NMDA and GABA receptors may be critical for certain aspects of extinction (e.g., Akirav, Raizel, & Maroun, 2006; Falls, Miserendino, & Davis, 1992; Harris & Westbrook, 1998; Ledgerwood, Richardson, & Cranney, 2005). Ethanol has been hypothesized to affect memory by impairing hippocampal function (e.g., Hoffman & Matthews, 2001; Matthews & Silvers, 2004; Ryabinin, 1998; Weitemier & Ryabinin, 2003; White, Matthews, & Best, 2000) and studies have shown that ethanol directly or indirectly alters NMDA and GABA receptors (e.g., Castellano & Pavone, 1988; Dildy-Mayfield & Leslie, 1989; Lovinger, et al., 1990). Because of the involvement of these systems affected by ethanol in extinction, one might expect that administration of ethanol during extinction should impair the development of extinction.
Several studies have demonstrated that ethanol affects the development of extinction (e.g., Cunningham, 1978, 1979), but the mechanisms underlying this effect remain unclear. Ethanol may impair learning during extinction by altering memory formation or consolidation, or by altering performance or motivation (e.g., Devenport, 1984; Ryabinin, Millar, & Durrant, 2002). It also is possible that instead of affecting the development of the extinction memory, the stimulus properties of ethanol may become part of that extinction memory. In a conditioned suppression task, Cunningham (1979) showed that presenting ethanol during extinction may allow ethanol to create an internal state that becomes specifically associated with extinction. Thus, subsequent testing in the presence of ethanol decreased fear because, during extinction, ethanol acquired the ability to signal the operation of the “no shock” contingencies. Theorizing about the importance of contextual variables in extinction suggests that such internal states may be particularly important in the development and expression of extinction (e.g., Bouton, Kenney, & Rosengard, 1990; Bouton, Westbrook, Corocoran, & Maren, 2006).
Further complicating the potential effects of ethanol on extinction is the possibility that ethanol may affect memory consolidation processes that occur after extinction. Many studies have demonstrated that memories may be sensitive to disruption by pharmacological agents during the period soon after a learning experience. Further, some theories suggest that when a previously formed memory is retrieved, it becomes labile and must be re-consolidated into a more stable state. Thus, ethanol may impair the consolidation of the extinction memory, resulting in sustained high levels of conditioned responding, or it may impair the reconsolidation of the initial memory, resulting in low levels of conditioned responding.
Much of the work examining the effects of ethanol on extinction has focused on the extinction of a discrete Pavlovian cue or instrumental response in rats. Relatively little is known about the effects of ethanol during extinction in hippocampus-dependent tasks, such as contextual fear conditioning. Extinction in this preparation may be particularly sensitive to the memory impairing effects of ethanol because of the effects of ethanol on hippocampal function. Further, extinction of contextual fear may also be sensitive to the state-creating effects of ethanol due to the role of the hippocampus in retrieval of contextual memories (e.g., Corcoran & Maren, 2001, 2004).
The following experiments examine the effects of ethanol on the development, expression, and consolidation of extinction following contextual fear conditioning in C57BL/6 mice. This strain is commonly used in behavioral and neurobiological studies of extinction (e.g., Cain, Blouin, & Barad, 2004; Lattal, et al., 2007; Lattal & Abel, 2001) and also shows an unusually high preference for ethanol, while being much less sensitive to the locomotion-stimulating effects of ethanol compared to other strains (e.g., Phillips, Dickinson, & Burkhart-Kasch, 1994). Experiment 1 examined the effects of different doses of ethanol administered during a single contextual extinction session. Experiment 2 examined effects of ethanol during multiple sessions of extinction in either the conditioned or a novel context. Experiments 3 and 4 examined whether ethanol creates an internal state that may become associated with extinction. Experiment 5 examined whether ethanol may acquire inhibitory properties during extinction. Finally, Experiment 6 examined consolidation and reconsolidation by administering ethanol immediately after short or long contextual extinction sessions. Together, the results of these experiments suggest that ethanol may have multiple effects on the development and expression of extinction.
Experiment 1: Dose effects of ethanol on extinction
This experiment examined the effects of three doses of ethanol on extinction. These doses of ethanol have been examined in several studies of acquisition of contextual fear conditioning (e.g., Gould, 2003; Gould & Lommock, 2003; Weitemier & Ryabinin, 2003). On Day 1, mice received contextual fear conditioning, which consisted of eight unsignaled footshocks delivered over a 24-min session. On Day 2, mice were injected with 0.0, 0.5, 1.0, or 1.5 g/kg of ethanol (20% v/v) prior to a 12-min extinction session in which no shocks were presented in the context. On Day 3, mice received another 12-min extinction session preceded by injections of saline. This served as a common test to evaluate the persistence of extinction from Day 2. If ethanol impairs the development of extinction, then more test freezing should be evident in mice that received higher doses of ethanol during extinction compared to mice that received saline during extinction.
Method
Subjects
Male C57BL/6 mice, aged 2–4 months, served as subjects in this and all subsequent experiments. Mice were housed in groups of four and had free access to food and water in their homecages. All experimental protocols were approved by the Oregon Health & Science University Institutional Animal Care and Use Committee.
Apparatus
Four Coulbourn Instruments mouse conditioning chambers (H10–11M-TC) were used. These chambers measured 18 cm × 18 cm. The front and back walls were Plexiglas and the two side walls were metal. The floor consisted of stainless steel grid rods spaced 6.4 mm apart. The chambers were housed in sound- and light-attenuating shells and a fan provided background noise at 70 dB. A houselight (Coulbourn H11–01M) provided continuous illumination during the sessions. Scrambled shock (2 s, 0.35 mA) was delivered to the grid floor by a computer-controlled shock generator (Coulbourn H13–15). Mounted 18 cm above the floor of each chamber was an automated infrared activity monitor (Coulbourn H24–61). Experimental events were controlled by Graphic State 3.01 software.
Procedure
This and subsequent experiments consisted of conditioning, extinction, and test phases. Prior to conditioning, mice were weighed and handled for several minutes per day for four days. On Day 1, mice received intraperitoneal (IP) injections of saline (volume equivalent to a 1.5 g/kg dose of ethanol) 5–10 prior to the conditioning session, which consisted of a 24-min exposure to the conditioning chamber with eight footshocks delivered at a rate of once every 180 s (variable, range 60–300 s). Mice were split into four groups that were matched for mean percent freezing during the final 12 min of the conditioning session (ns=10 per group). One subject failed to acquire a sufficient level of conditioning to observe effects on extinction (z score = −3.4) and was dropped from the experiment, which reduced the sample size in the 1.5 g/kg EtOH group to nine.
On Day 2, mice were injected IP with saline or 0.5, 1.0, or 1.5 g/kg EtOH (20% v/v) 5–10 min prior to a 12-min extinction session, in which the mice were placed into the context with no shocks delivered. On Day 3, all mice were injected with saline 5–10 min prior to a 12-min test session, which was another nonreinforced exposure to the context.
Data Analysis
The dependent variable in all experiments was freezing, which was scored automatically by infrared activity monitors mounted on the ceiling of each chamber. Freezing was defined as a lack movement detected by the activity monitors for > 3 s. Percent time freezing was calculated by summing the total seconds in each bout that satisfied the > 3 s of inactivity criterion and dividing by the total seconds in the time period of interest (e.g., 180 s in many cases). This criterion for automated scoring of freezing correlated well with human observer-scored freezing using a traditional time sampling technique in a pilot experiment in our laboratory (computer-observer, r = .91; observer-observer, r = .93) and has been used successfully in studies from our laboratory and others using Coulbourn activity monitors (e.g., Lattal, et al., 2007; Frick, Kim, & Baxter, 2004). Comparisons were made between groups during the course of extinction to determine whether manipulations affect performance during extinction sessions. To assess the impact of the extinction manipulations on the retention of extinction, comparisons were made during test sessions, in which animals were assessed under common conditions for performance. Data were analyzed using analysis of variance (ANOVA) and Student’s t tests for planned comparisons during the beginning of the test sessions. Post-hoc comparisons were made using the Bonferroni alpha-splitting correction.
Results & Discussion
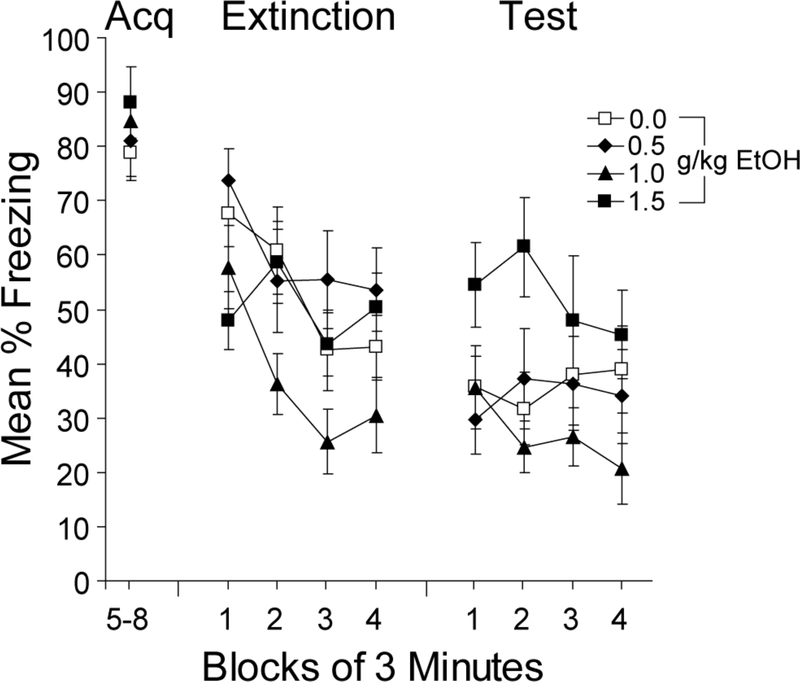
All groups acquired high levels of freezing during conditioning. Freezing during the final 12 min of the conditioning session, which followed saline injections in all groups, is shown in Figure 1 (Acq). During the first 3 minutes of extinction, mice injected with 1.5 g/kg of ethanol appeared to show somewhat less freezing compared to saline and 0.5 g/kg of ethanol (Extinction in Figure 1). Mice injected with 1.0 g/kg appeared to show the most rapid within-session extinction, but by the end of the session, none of the ethanol groups appeared to differ from the saline group. An ANOVA with dose and 3-min time block as factors revealed reliable main effects of dose, F(3,35)=2.9, p<0.05, and time block, F(3, 105)=12.8, p<0.001, as well as a reliable interaction, F(9, 105)=2.1, p<0.05. Analysis of the interaction revealed a reliable main effect of group during the first and third blocks of the extinction session, Fs(3, 35) > 3.1, ps<0.05, suggesting that there were effects of ethanol on the expression of freezing at the beginning of the session and on the rate of extinction. By the final 3 min of the extinction session, there were no reliable group differences in freezing.
Figure 1.
Dose effects of ethanol on extinction of contextual fear. Mean percent freezing is shown for the final 12 min of the 24-min acquisition (Acq) session, which was preceded by saline injections in all mice. Extinction and test session freezing is shown in 3-min time blocks. Mice received injections of saline (0.0), 0.5, 1.0, or 1.5 g/kg (20% v/v) of ethanol prior to extinction and injections of saline prior to acquisition and testing. Bars represent SEMs.
During testing (which followed saline injections in all mice), mice injected with 1.5 g/kg of ethanol prior to extinction froze more during the first half of the test session than did the other groups, which showed similar levels of freezing (Test in Figure 1). An ANOVA with dose and 3-min time block as factors revealed no reliable main effects or interactions over the duration of the 12-min test. However, a one-way ANOVA revealed a reliable main effect of group during the first 6 min of the test session, F(3, 35)=3.6, p<0.05. Mice injected with 1.5 g/kg of EtOH froze more than did mice injected with saline, t(17)=2.7, p<0.05. Mice injected with 0.5 or 1.0 g/kg of EtOH did not differ in freezing from the saline-treated group.
This experiment demonstrates that 1.5 g/kg of ethanol impaired extinction, as revealed in high levels of freezing during a test in the absence of ethanol the day after extinction. These findings extend previous findings showing that ethanol impairs initial learning in contextual fear conditioning (e.g., Gould, 2003) and suggest that similar impairments occur during extinction. In the next experiment, the effects of ethanol injections over the course of three extinction sessions were examined to determine if multiple sessions of extinction can overcome the deleterious effects of ethanol.
Experiment 2: Associative and nonassociative effects of ethanol on extinction
Experiment 2 was designed to examine several issues. First, extinction was conducted over three sessions so that the effects of ethanol on the course of extinction could be tracked more closely. In Experiment 1, groups treated with 1.5 g/kg of ethanol during extinction showed levels of freezing early in testing that were similar to those shown early in extinction, suggesting that little to no extinction occurred. Examining freezing over the course of three extinction sessions should provide a better assessment of whether extinction can occur with this dose of ethanol. A second purpose of this experiment was to examine performance effects of ethanol. Control groups received ethanol injections (1.5 g/kg) but were exposed to a novel context during extinction. Examining freezing in groups exposed to the novel context provided an assessment of the effects of ethanol on performance independent of its effects on extinction. If, during extinction, ethanol causes general increases in activity, which correspond to decreases in freezing, then these differences should be evident in groups that receive exposure to a novel context during extinction. Further, groups exposed to a novel context also constitute No Extinction control groups that do not receive extinction of the conditioning context but are otherwise treated the same as the extinction groups, in terms of injections, handling, and exposure to a context outside of the homecage during extinction. Following extinction, all groups were tested with saline in the original conditioning context. Comparisons during the test of groups that received extinction in the conditioning context with those that received extinction in the novel context provided a measure of the extent to which extinction developed in ethanol-treated mice.
Method
Subjects
Male C57BL/6 mice, aged 2–4 months, served as subjects. They were housed as in Experiment 1.
Apparatus
The four Coulbourn Instruments mouse conditioning chambers described in Experiment 1 were used for the shocked context (Context S). Four additional Coulbourn Instruments mouse conditioning chambers housed in a different room were used as novel contexts (Context N). An opaque white cylinder with a flat acrylic floor (18 cm high, 15 cm diameter) sat on the top of the grid floor. Context N was cleaned with 0.1% acetic acid and houselights and background fans were not activated.
Procedure
Mice were handled and conditioned in Context S as in Experiment 1 and were split into four groups that were matched for mean percent freezing during the final 12 min of the conditioning session. On Days 2–4, mice were injected with saline or 1.5 g/kg EtOH (20% v/v) 5–10 min prior to a 12-min extinction session, in which the mice were placed into the conditioning context (Context S) with no shocks (Ext groups) or into the novel Context N (No Ext groups) with no shocks. On Day 5, all mice were injected with saline 5–10 min prior to a 12-min nonreinforced test session in Context S.
Results & Discussion
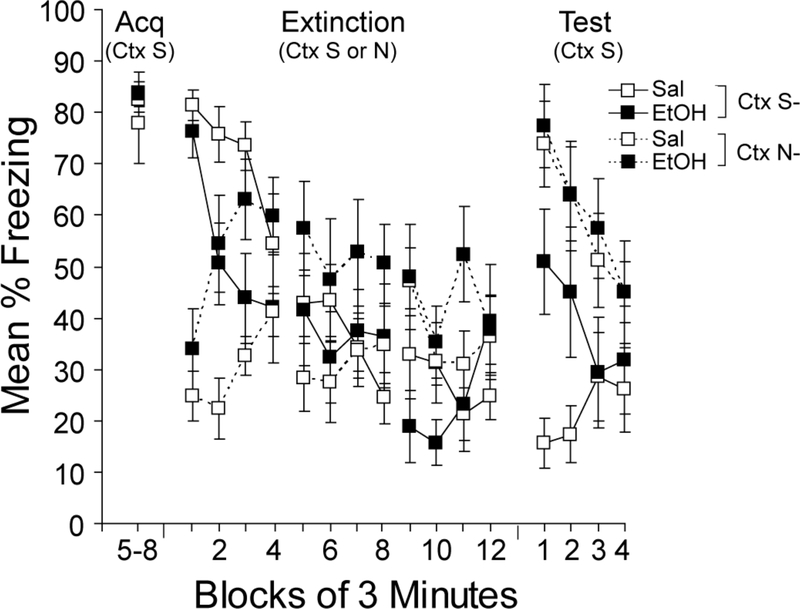
Figure 2 shows the results of acquisition, extinction, and testing in Experiment 2. All groups showed high levels of asymptotic freezing during the last half of the conditioning session in Context S (Acq in Figure 2). During the first 3 min of the first extinction session, all mice froze more in the previously shocked context (Context S) compared to the novel context (Context N), demonstrating that saline- and ethanol-treated mice were able to discriminate the shocked from the unshocked context. However, this context discrimination was lost rapidly within the first extinction session in ethanol-treated mice, as freezing increased in Context S and decreased in Context N. All groups were similar during the next two extinction sessions.
Figure 2.
Effects of ethanol on multiple sessions of extinction in the shocked (Ctx S) or novel (Ctx N) context. Mean percent freezing is shown for the final 12 min of the 24-min acquisition (Acq) session, which was preceded by saline injections in all mice. Freezing is shown in blocks of 3 min for each of the three 12-min extinction sessions in Context S (the shocked context) or N (the novel context) and for the single test session in Context S. Bars represent SEMs.
A context (N or S) x injection (saline or ethanol) x 3-min extinction block ANOVA revealed a reliable main effect of extinction block, F(11, 308)=8.5, p<0.001 and reliable interactions between context and injection, F(1, 28)=5.3, p<0.05, and context and extinction block, F(11, 308)=11.3, p<0.001, as well as a reliable 3-way interaction, F(11, 308)=3.9, p<0.001. Because of the three-way interaction, the effects of context and injection were examined in each individual extinction session. During the first session of extinction, the main effect of context and the context x injection interaction were reliable, Fs(1,28)>15.7, ps<0.001, as were the interaction between block and context, F(3, 84)=15.6, p<0.001, and the 3-way interaction, F(3, 84)=3.8, p<0.05. Further exploration of the 3-way interaction revealed that during the first three minutes of Ext 1, mice froze more in Context S compared to Context N, regardless of injection (reliable main effect of context in the first 3 min, F(1, 28)=83.1, p<0.001, but no reliable main effect of injection or interaction, Fs(1,28)<1.8, ps>0.20). This context discrimination was lost more rapidly in ethanol-treated mice and those mice actually froze more in Context N compared to Context S during the last half of the session, t(14)=3.7, p<0.005. There were no reliable effects during the second extinction session and only a reliable main effect of context in the third extinction session, F(1, 28)=5.4, p<0.05.
When all mice were tested in the conditioning context (Context S) in the presence of saline (Test in Figure 2), those groups that received exposure to Context N during extinction showed equally high levels of freezing regardless of whether they were injected with saline or ethanol during extinction. This finding suggests that exposure to ethanol in the absence of extinction in the conditioned context did not disrupt freezing to that context. The lowest levels of freezing were observed in mice injected with saline prior to the extinction sessions in Context S. Mice injected with ethanol prior to extinction in Context S showed slightly less freezing compared to the groups that did not receive extinction, but much higher freezing compared to the group injected with saline prior to extinction in Context S.
A context x injection x 3-min test block ANOVA conducted on the test data revealed a reliable main effect of context, F(1, 28)=13.1, p<0.001, and test block, F(3,84)=9.3, p<0.001, as well as all 2- and 3-way interactions with test block, Fs(3, 84)>3.2, ps<0.05. Further exploration of the 3-way interaction revealed that during the first 3 min, mice that received saline or ethanol in Context N during extinction did not differ. Mice that received saline in Context S froze less compared to the other groups, ps<0.05, but mice that received ethanol in Context S were not statistically different from the groups that received extinction in Context N.
The results of Experiment 2 replicate and extend those of Experiment 1, which found that a 1.5 g/kg dose of ethanol impaired extinction after a single session. In Experiment 2, this effect occurred even after three sessions of extinction. Further, the results of Experiment 2 show that although some extinction may occur when ethanol is injected prior to extinction sessions relative to no-extinction controls, the retention of extinction is greatly impaired, as evident in the large difference in test performance between mice that received saline or ethanol prior to extinction in the conditioning context.
There are two general ways to think about the impairment in extinction caused by ethanol in Experiments 1 and 2. One is that 1.5 g/kg of ethanol administered prior to extinction impaired the formation of the extinction memory, meaning that the animal did not fully learn that the context no longer signals the shock. Because this learning is impaired, the extinction memory is weaker than the conditioning memory, resulting in higher levels of freezing. Another interpretation is that the stimulus properties of ethanol created as an internal context during extinction in the 1.5 g/kg group and that testing in the absence of ethanol resulted in higher levels of freezing because a critical stimulus cue necessary for the retrieval of the extinction memory was absent. The purpose of Experiments 3 and 4 was to determine whether ethanol could serve as an internal context that selectively retrieves memories from extinction.
Experiment 3: Ethanol as a cue for extinction
Many studies have shown that extinction is particularly sensitive to manipulations of context – when the context between extinction and testing changes, the extinguished behavior is renewed (e.g., Bouton & Bolles, 1979). Bouton and colleagues have built a theory around such findings that suggests that memories formed during extinction are particularly dependent on contextual cues for retrieval. When those extinction cues are present, the extinction memory is retrieved and conditioned responding is low, but when those cues are absent, the extinction memory is poorly retrieved and conditioned responding is high. Such an account would suggest that the impairment in extinction caused by ethanol in Experiments 1 and 2 was not due to a memory storage deficit, but was instead due to a critical retrieval cue for extinction (ethanol) being absent when testing occurred in the presence of saline.
In Experiment 3, mice received conditioning following saline injections. Mice then received a single 12-min extinction session preceded by injections of saline or 1.5 g/kg EtOH. The next day, half of the mice from each group was tested following saline injections; the other half was tested following ethanol injections. Thus, the conditioning-extinction-testing injections were S-S-S, S-S-E, S-E-S, and S-E-E, where S was saline and E was ethanol. If ethanol serves as a context for extinction, changing the context before testing should renew conditioned freezing. That is, more test freezing should be observed in the presence of ethanol in mice that received conditioning and extinction with saline (S-S-E) compared to mice that received extinction with ethanol (S-E-E). Similarly, in mice that are tested with saline, there should be more freezing in mice extinguished with ethanol (S-E-S) compared to those extinguished with saline (S-S-S).
Method
Subjects & Apparatus
Male C57BL/6 mice, aged 2–4 months, served as subjects. They were housed as in the previous experiments. The apparatus consisted of the four square chambers used in the previous experiments.
Procedure
Mice were handled as before. On Day 1, all of the mice received injections of saline prior to a contextual conditioning session. The conditioning session consisted of 8 shocks in 24 min as in Experiments 1 and 2. Mice were split into two groups that were matched for mean percent freezing during the final 12 min of the conditioning session. On Day 2, one group of mice (n=24) received injections of saline and the other group (n=24) received injections of 1.5 g/kg ethanol prior to a 12-min extinction session. On Day 3, groups again were divided, resulting in half of the animals from each group receiving saline and the other half receiving ethanol prior to a 12-min test session. Thus, there were four possible conditioning-extinction-testing groups: saline-saline-saline (SSS), saline-saline-ethanol (SSE), saline-ethanol-saline (SES), saline-ethanol-ethanol (SEE; n=12 per group).
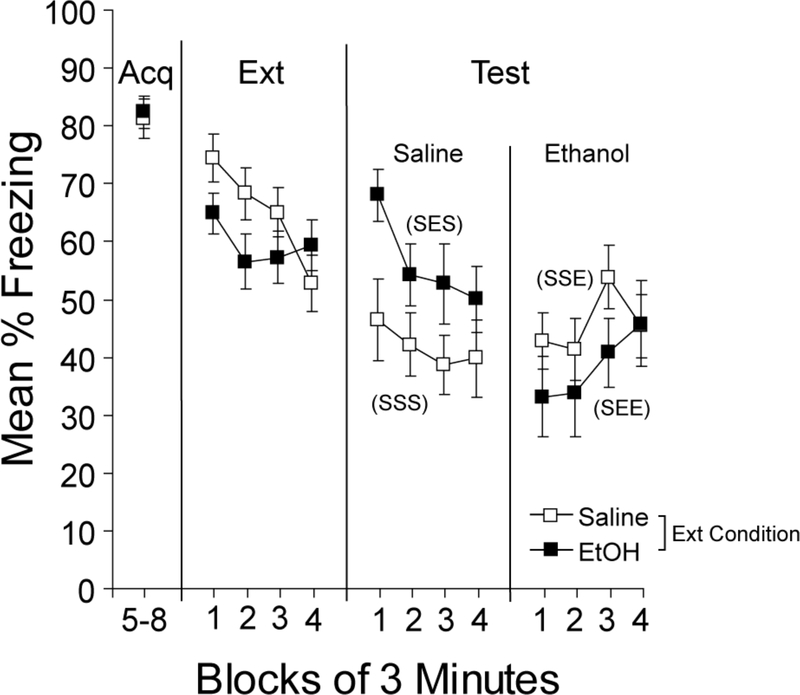
Results & Discussion
Figure 3 shows the results of acquisition, extinction, and testing in Experiment 3. All groups showed high levels of freezing during the final 12-min of the acquisition session (Acq in Figure 3). Ethanol injections prior to the extinction session caused a reduction in freezing relative to saline injections (Extinction in Figure 3). An extinction injection (saline or ethanol) x 3-min extinction block ANOVA revealed a reliable main effect of extinction injection, F(1, 46)=7.1, p<0.01, a reliable main effect of extinction block, F(3, 138)=14.5, p<0.001, and a reliable interaction, F(3, 138)=2.8, p<0.05. Exploration of the interaction revealed differences in freezing as a function of injection during the first 3 min, F(1, 46)=9.7, p<0.01, but not during the final 3 min, F(1,46)<1.0, of the extinction session. Thus, ethanol caused a reduction in freezing early in extinction, but there was no difference between ethanol- and saline-treated mice by the end of the session.
Figure 3.
Effects of ethanol during extinction and testing. Mean percent freezing is shown for the final 12 min of the 24-min acquisition (Acq) session, which was preceded by saline injections in all mice. Freezing is shown in blocks of 3 min for extinction sessions preceded by ethanol or saline and for the test session, also preceded by ethanol or saline. Test labels in parentheses represent injections during acquisition, extinction, and testing (S or E; e.g., SES represents saline during acquisition, ethanol during extinction, and saline during testing). Bars represent SEMs.
During testing, mice that received ethanol during extinction appeared to freeze more during the test after saline injections (Group SES in Figure 3) compared to mice that received saline during extinction (Group SSS in Figure 3), consistent with the results of Experiments 1 and 2. During testing with ethanol, there appeared to be slightly less freezing in the group that received ethanol during extinction (Group SEE in Figure 3) relative to mice that received saline during extinction (Group SSE in Figure 3), though this difference was small. A 3-way ANOVA with extinction injection (saline or ethanol), test injection (saline or ethanol), and 3-min test block as factors conducted on freezing during the 12-min test session revealed a reliable main effect of test block, F(3, 132)=12.1, p<0.001, as well as reliable interactions between extinction and test injection, F(1, 44)=4.4, p<0.05, and between test injection and test block, F(3, 132)=9.9, p<0.001. Exploration of the test injection x test block interaction revealed that ethanol reduced freezing during the first 3 min of the test, F(1, 44)=12.3, p<0.001, but by the end of the session, there was no effect of test injection, F(1, 44)<1.0, suggesting that ethanol caused a general reduction in freezing early in the test session. Exploration of the extinction x test injection interaction found that Group SES froze more during the test session compared to Group SSS, F(1,22)=7.0, p<0.05, but there were no differences in freezing between Group SEE and SSE, F(1,22)<1.0.
The findings from Experiment 3 suggest that ABA renewal may occur when ethanol is the extinction context and saline is the testing context (as in Experiments 1 and 2), relative to the AAA group that received saline during extinction and testing. When testing occurred with ethanol, mice that received ethanol during extinction froze less compared to mice that received saline during ethanol, consistent with AAB renewal in Group SSE relative to Group SEE, but this difference was not reliable. It is possible that the single extinction session was insufficient for the mouse to associate the extinction context with ethanol. Thus, with additional extinction sessions, the association between the context and ethanol may be more readily established, allowing ethanol to selectively modulate test performance.
Experiment 4: Internal context effects of ethanol on acquisition and extinction
The goal of Experiment 4 was to further investigate the contextual properties of ethanol during extinction. By conducting three extinction sessions, this experiment attempted to establish a stronger association between the extinction context and ethanol, which may result in ethanol’s becoming a better retrieval cue for extinction. Studies of conditioned place preferences induced by ethanol have demonstrated that the context-ethanol association is strengthened with increased numbers of context-ethanol pairings (e.g., Cunningham, Tull, Rindall, & Meyer, 2002). Further, in addition to receiving ethanol prior to extinction or test sessions, some mice in Experiment 4 also received injections of ethanol prior to the initial conditioning session. Thus, the internal context (or state)-creating effects of ethanol could be compared during acquisition and extinction, which allowed for a more complete analysis of ethanol’s effects on contextual renewal.
In Experiment 4, mice were conditioned in the presence of ethanol (1.5 g/kg) or saline. Half of the mice from each group then received saline or ethanol prior to each of three consecutive extinction sessions. Each of those groups was then split again and received ethanol or saline prior to a test session. This resulted in a total of eight groups. If ethanol creates an internal state that serves as a retrieval cue for memory, then mice that are conditioned in the presence of ethanol should show more freezing during extinction trials in the presence of ethanol compared to saline. Additionally, mice that receive conditioning with ethanol followed by extinction with saline should show renewal of freezing when testing occurs with ethanol compared to saline if ethanol acquires the properties of a context during conditioning and extinction.
Method
Subjects & Apparatus
Male C57BL/6 mice, aged 2–4 months, served as subjects. They were housed as in the previous experiments. The apparatus consisted of the four square chambers used in the previous experiments.
Procedure
Mice were handled as in Experiments 1 and 2. On Day 1, half of the mice received injections of saline (n=32) and the other half received injections of 1.5 g/kg ethanol (n=32) prior to a contextual fear conditioning session. The conditioning session consisted of 8 shocks in 24 min as in the previous experiments. On Days 2–4, half of the mice from each group (n=16) received injections of saline and half received injections of ethanol prior to a 12-min extinction session. Thus, there were four possible conditioning-extinction treatments: salinesaline, saline-ethanol, ethanol-saline, or ethanol-ethanol. After three sessions of extinction, groups again were divided, resulting in half of the animals from each group receiving saline and the other half receiving ethanol prior to a 12-min test session. Thus, there were eight possible conditioning-extinction-testing groups: saline-saline-saline, saline-saline-ethanol, saline-ethanol-saline, saline-ethanol-ethanol, ethanol-saline-saline, ethanol-saline-ethanol, ethanol-ethanol-saline, or ethanol-ethanol-ethanol (n=8 per group). If one thinks of the conditioning treatment as A and the extinction treatment as B, all possible conditioning-extinction-testing combinations were examined: A-A-A, A-A-B, A-B-A, and A-B-B, with ethanol and saline being counterbalanced between A and B.
Results & Discussion
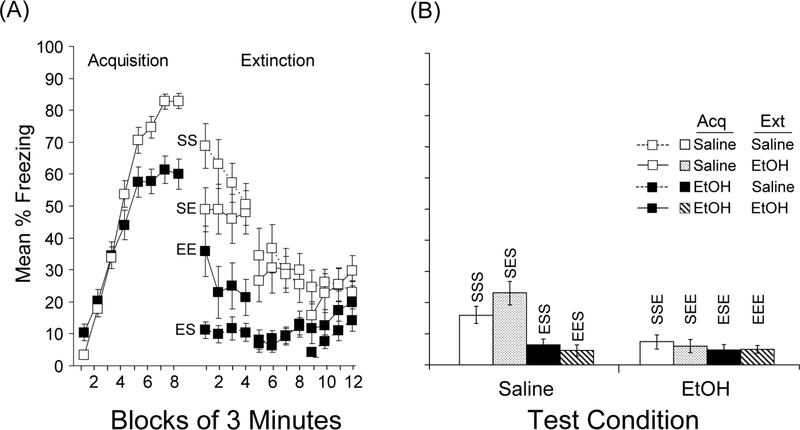
Figure 4 shows the results of conditioning, extinction, and testing in Experiment 4. An examination of the conditioning data over the course of the 24-min conditioning session reveals that mice injected with ethanol prior to acquisition reached a lower level of asymptotic freezing compared to mice injected with saline. An injection x 3-min conditioning block ANOVA confirmed this difference with a reliable injection x block interaction, F(7,434)=10.2, p<0.001, as well as reliable main effects of injection, F(1,62)=5.4, p<0.05, and block, F(7,434)=199.6, p<0.001.
Figure 4.
Effects of ethanol during acquisition, extinction, and testing. (a) Mean percent freezing is shown in blocks of 3 min for the 24-min acquisition session and the three 12-min extinction sessions. Mice received saline (open squares) or ethanol (closed squares) prior to acquisition. Groups were subdivided and received saline (Groups SS and ES) or ethanol (Groups SE and EE) prior to extinction. (b) Mean percent freezing is shown for the first 6 min of the test session, as a function of test injection (saline or ethanol). Labels above the bar denote the injection prior to acquisition, extinction, and testing (S or E). Bars represent SEMs.
During the initial 3 minutes of the first extinction session (Ext 1 in Figure 4a), mice froze more when injected with the agent that was injected prior to acquisition; mice injected with ethanol prior to acquisition froze more when injected with ethanol prior to extinction (Group EE in Figure 4a) compared to saline (Group ES in Figure 4a) and mice injected with saline prior to acquisition froze more when injected with saline prior to extinction (Group SS in Figure 4a) compared to ethanol (Group SE in Figure 4a). This suggests that ethanol increased the expression of freezing during extinction if it was also injected prior to conditioning, but decreased freezing if it was not injected prior to conditioning, consistent with a state-dependent learning and retrieval process. Overall, mice injected with saline during conditioning froze more than did mice injected with ethanol prior to conditioning regardless of what was injected during extinction. An acquisition injection x extinction injection x 3-min extinction block ANOVA during the first extinction session revealed main effects of acquisition injection, F(1, 60)=43.3, and extinction block, F(3, 180)=4.5, ps<0.005. It also revealed a reliable interactions between acquisition and extinction injection, F(1,44)=6.5, p<0.05, as well as a reliable 3-way interaction, F(3, 180)=3.7, p<0.05. Further analysis of the interaction revealed that in the groups that received ethanol during conditioning, more extinction freezing initially (first 3 min) occurred in the group that also received ethanol during extinction, p<0.05. Conversely, in the groups that received saline during conditioning, more extinction freezing occurred in the group that received saline during extinction, p<0.05. By the final extinction session, there was still a reliable main effect of acquisition injection, F(1, 60)=10.3, and extinction block, F(3, 180)=5.1, ps<0.005, but there were no other reliable main effects or interactions.
Figure 4b shows the results of testing after extinction. Freezing was generally low during the test, regardless of what was injected prior to the test. The highest levels of freezing appeared to occur in mice that were injected with saline during conditioning and testing, though the difference between Groups SES and SSS did not appear as large as in previous experiments. Freezing appeared to be lowest in mice that received ethanol during conditioning or during the test, regardless of extinction history. In no case did ethanol appear to increase freezing in the direction predicted by a state-dependent learning process. For example, mice that received conditioning after ethanol injections and extinction after saline injections showed no renewal of freezing when ethanol was administered prior to the test session (Group ESE compared to ESS and EEE in Figure 4b).
An ANOVA conducted on the first 6 min of the test session with acquisition injection, extinction injection, and test injection as factors revealed reliable main effects of acquisition injection, F(1, 56)=8.4, p<0.005, and test injection, F(1, 56)=7.5, p<0.01, as well as a reliable acquisition injection x test injection interaction, F(1, 56)=6.4, p<0.05. There was no reliable main effect of extinction injection, nor were there reliable interactions involving extinction injection, suggesting that extinction treatment had little effect on test performance. Post-hoc comparisons found that Groups SES did not differ from Group SSS, but that Group SES differed from Group SEE, Bonferroni-adjusted p<0.01. Thus, extinction in Group SES appears to be more complete in this experiment than in Experiment 2, which delivered the same number of extinction sessions.
These data demonstrate that some of the deficit in initial conditioning and extinction caused by ethanol can be attributed to state-dependent learning effects. During the first extinction session, freezing was higher when extinction was preceded by the same injection (saline or ethanol) that occurred prior to conditioning, consistent with the idea that ethanol increased freezing if conditioning occurred in the presence of ethanol but decreased freezing if conditioning occurred in the absence of ethanol. Thus, ethanol may have served as a retrieval cue for the initial context-shock memory in mice conditioned with ethanol.
It is important to note that although there was some evidence for state-dependent expression of freezing at the outset of extinction, overall there was less freezing in mice injected with ethanol prior to conditioning compared to mice injected with saline prior to conditioning. This demonstrates that not all of the difference in freezing at the outset of extinction can be attributed to state-dependent learning during conditioning. Indeed, there likely were differences in learning as reflected in the lower asymptote of freezing reached by ethanol-treated mice during conditioning (Acquisition in Figure 4a). This deficit in initial acquisition may have also impacted ethanol’s ability to renew freezing during a test in Group ESE because poor retention of conditioning (~10% freezing during Ext 1 in Figure 4a) coupled with a large amount of extinction may severely attenuate any renewal effect (e.g., Denniston, Chang, & Miller, 2003).
There was some evidence that ethanol also may have acted as a context during extinction. The difference during testing between Group SES and SEE is consistent with the idea that ethanol served as an extinction context. However, this interpretation is complicated by the general finding that freezing was low when ethanol was injected prior to the test. Ethanol did not increase freezing in a way that might be expected if it served as an internal context (e.g., there were no differences in test freezing between Groups ESE and EEE or between Groups SSE and SEE). Thus, there was some evidence for renewal in this experiment, but these findings are complicated by the generally low levels of freezing evident in all groups tested with ethanol.
The most simple explanation for the low levels of test freezing in the presence of ethanol in Experiments 3 and 4 is that ethanol had unconditioned stimulatory effects on behavior, which may have impaired the expression of freezing. This seems unlikely because ethanol caused an increase in freezing to a novel context in Experiment 2 (Ext 1 in Figure 2), and ethanol increased freezing during extinction in mice that received conditioning in the presence of ethanol in Experiment 3 (Ext 1 in Figure 3). Further, some studies suggest that the sensitizing effects of ethanol (2.0 g/kg) on locomotion are small in the C57BL/6 strain (e.g., Phillips, Dickinson, & Burkhart-Kasch, 1994) and may be restricted to conditions with higher doses of ethanol (2.5 g/kg) coupled with previous ethanol intake (Lessov, Palmer, Quick, & Phillips, 2001). Nonetheless, it still is possible that motor activation contributed to the low levels of test freezing caused by ethanol. It also is possible that although ethanol may create a powerful internal state, this state may not be strong enough to cause contextual renewal. In Experiment 5, we attempt to explicitly endow ethanol with inhibitory properties during extinction, which may result in a more selective modulation of test performance by ethanol after extinction.
Experiment 5: Inhibitory properties of ethanol
In Experiments 3 and 4, there was some evidence that ethanol served as an internal context. Groups that received ethanol during extinction froze less during a test with ethanol compared to a test with saline, suggesting that ethanol may have served as a context for extinction. However, this interpretation is complicated because ethanol administered prior to a test generally decreased freezing, even in groups that received conditioning and extinction in the presence of saline (i.e., there was no reliable AAB renewal when ethanol was B). This was true after one session of extinction resulting in moderate to high levels of freezing (Experiment 3) or three sessions of extinction resulting in low levels of freezing (Experiment 4). It is possible that the inability to observe an increase in test freezing caused by ethanol was due to different reasons in the two experiments. A single extinction session in Experiment 3 may have been insufficient to establish an ethanol-context association, meaning that ethanol during the test could not retrieve the extinction memory, and a floor effect on freezing following extensive extinction may have masked some of the contextual renewal effects of ethanol in Experiment 4.
The purpose of Experiment 5 was to attempt to circumvent both of these issues by examining the ability of ethanol to modulate performance to a conditioned, but not extinguished context based on ethanol’s associative history with another context during extinction. Thus, the goal of this experiment was to explicitly make ethanol a conditioned inhibitor, signaling the absence of an expected shock, and assess that inhibition by testing the ability of ethanol to modulate performance to a different context after extinction. Mice were conditioned in two counterbalanced contexts (A and B) and received injections of either saline or ethanol (1.5 g/kg) prior to three consecutive extinction session in Context B. Saline-treated mice received ethanol injections 4 hr after the extinction session to control the potential sensitizing effects of repeated exposure to ethanol. Each group was divided in half and injected with saline or ethanol prior to a nonreinforced test session in Context A. If ethanol acquires the ability to signal the absence of shock during extinction, this ability may be expected to transfer to other conditioned contexts, resulting in a reduction of freezing in that context. Thus, this training should make ethanol a conditioned inhibitor, resulting in less freezing in Context A in mice that received ethanol prior to extinction in Context B compared to mice that received saline prior to extinction in Context B.
Method
Subjects & Apparatus
Male C57BL/6 mice, aged 2–4 months, served as subjects. They were housed as in previous experiments. The apparatus consisted of the four square chambers and four round chambers used in Experiment 2. A grid floor with 7.9 mm spacing between bars was used in the round chambers.
Procedure
Mice were handled as in Experiments 1 and 2. On Days 1 and 2, mice were pre-exposed to Contexts A and B (square and circular chambers, counterbalanced) for 12 min. The interval between each exposure was 1–2 hr and the order of exposures was counterbalanced within and between days. On Day 3, mice received a single conditioning session in both Contexts A and B (order counterbalanced). These sessions consisted of 8 shocks in 24 min, as in previous experiments. Conditioning of the two contexts was separated by 1–2 hr. On Days 4–6, mice received injections of 1.5 g/kg EtOH (n=24) or saline (n=23; matched for performance during the final half of each conditioning session) 5–10 prior to 12-min nonreinforced exposures to Context B. Four hours after the extinction session, mice were injected with saline or ethanol (whichever was not injected prior to extinction), thus matching the overall exposure to ethanol in the two groups. On Day 7, half of the mice from each group were injected with saline prior to a 12-min nonreinforced exposure to Context A; the other half of the mice were injected with ethanol prior to this exposure. On Day 8, mice received a second 12-min test session preceded by injection of saline or ethanol (whichever was not injected prior to the first test).
Results & Discussion
During the final 12 min of conditioning in Contexts A and B, mean percent freezing was 83.4 and 82.3, respectively. Figure 5 shows the results of extinction and testing. During extinction in Context B, ethanol administered prior to the session greatly attenuated the expression of freezing during the beginning of each extinction session. Separate ANOVAs with extinction injection (ethanol or saline) and 3-min time block revealed reliable two-way interactions in each session, Fs(3, 135)>3.8, ps<0.05, confirming that the groups differed in freezing at the onset of each session, but reached common levels by the end of the session.
Figure 5.
Effects of ethanol on extinction and on freezing in a separately conditioned context. Freezing is shown in blocks of 3 min for the three extinction sessions in Context B preceded by ethanol or saline and for the test session in Context A, also preceded by ethanol or saline. Bars represent SEMs.
During testing in Context A, freezing was reduced by injections of ethanol prior to the test in both groups, but this reduction appeared to be greater in the group that received ethanol during extinction. As in previous experiments, this effect appeared most pronounced early in the test session. An ANOVA with extinction and test injections (ethanol or saline) as factors revealed no reliable main effect of extinction injection, but did reveal a reliable main effect of test injection F(1, 43)=25.2, p<0.001, as well as a reliable interaction, F(1, 43)=4.6, p<0.05, during the first 3 min of testing in each context. Further analysis of the interaction revealed that during the first 3 min of the test with ethanol, mice injected with ethanol during extinction froze less compared to mice injected with saline during extinction, p<0.05. During the first 3 min of the test with saline, mice injected with saline during extinction froze less compared to mice injected with ethanol during extinction, p<0.05. These findings suggest that ethanol may modulate test freezing as a function of its history during extinction.
Unlike Experiments 4 and 5, this experiment demonstrated clear differential modulation of test freezing by ethanol as a function of ethanol’s history during extinction. This may have occurred because ethanol acquired inhibitory, or at least occasion setting, properties during extinction. It also is possible that inhibition did not develop to ethanol, but that a more general state-dependent learning process coupled with a failure to discriminate the two contexts contributed to the test results. In either case, however, this experiment demonstrates that ethanol may become incorporated as part of the content of the learning that occurs about the context during extinction.
Experiment 6: Effects of ethanol on consolidation of extinction
In Experiments 1–5, injections of ethanol occurred prior to the conditioning, extinction, or testing session. The purpose of Experiment 6 was to examine whether injections of ethanol after a brief or long extinction session would affect memory consolidation during extinction. Several studies suggest that manipulations that impair memory consolidation may disrupt the development of extinction or the expression of the original fearful memory. Further, there is some evidence that the effects of a pharmacological manipulation on behavior during extinction may depend on how much extinction occurs during the session. With brief sessions, which may cause little extinction, the reconsolidation of the original memory may be impaired, resulting in a loss of freezing. With long sessions, which should cause more extinction, the consolidation of the extinction memory may be impaired, resulting in sustained high levels of freezing (e.g., Suzuki, et al., 2004).
Some studies suggest that alcohol may enhance memory when administered after a learning experience, either by promoting memory consolidation or blocking retroactive interference (e.g., Knowles, & Duka, 2004; Mueller, Lisman, & Spear, 1983; Parker, et al., 1980). One of the procedural advantages to administering a drug after the extinction session is that the performance effects of the drug are less worrisome because the drug is not active during the extinction session. Consequently, Experiment 6 examined the effects of a higher dose of ethanol (3.0 g/kg) in addition to the 1.5 g/kg used in Experiments 1–5. Contextual extinction sessions were either 3 or 24 min, durations that result in different amounts of extinction (Lattal, et al, 2007).
Method
Subjects & Apparatus
Male C57BL/6 mice, aged 2–4 months, served as subjects. They were housed as in previous experiments. The apparatus consisted of the four square chambers used in Experiment 2.
Procedure
Mice were handled as in Experiments 1 and 2. On Day 1, mice received conditioning, which consisted of 8 shocks in 24 min, as in previous experiments. On Days 2–4, mice received extinction, consisting of either a 3- or 24-min exposure to the context in the absence of shock. Injections of saline, 1.5 g/kg, or 3.0 g/kg of EtOH (matched for performance during the final half of the conditioning session) were administered immediately after each of the first three 3- or 24-min extinction sessions. Each of the 3-min groups had 8 mice. The 24-min groups were run in two replications with total sample sizes of 10, 10, and 17 for the saline, 1.5 g/kg, and 3.0 g/kg groups, respectively. Because of this additional replication within the 24-min groups, separate statistical analyses were conducted on the 3- and 24-min conditions.
Results & Discussion
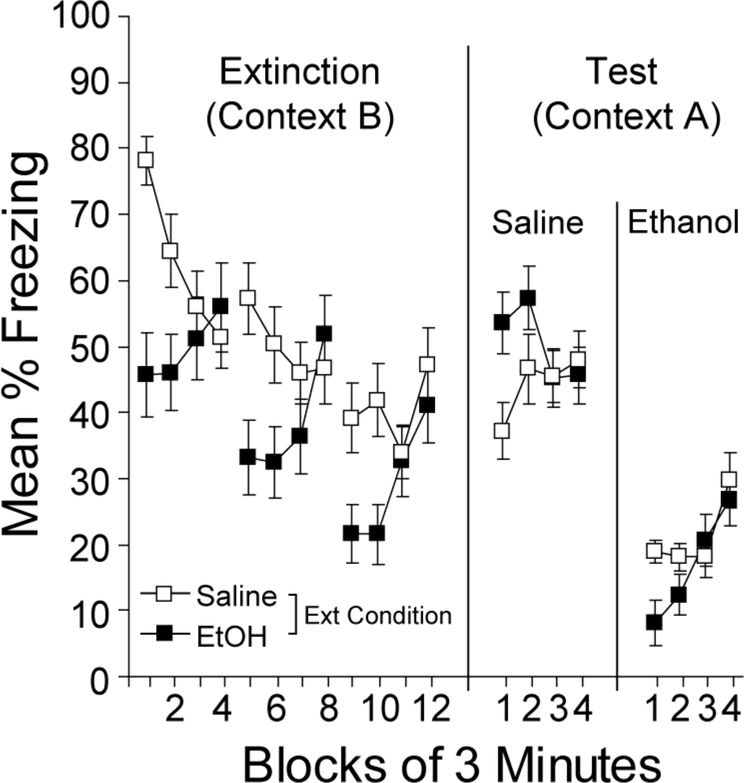
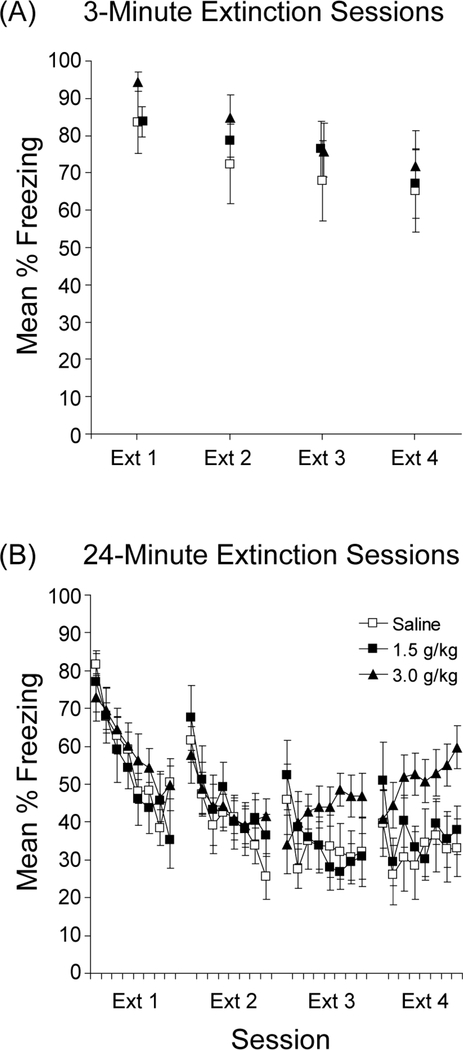
During the final 12-min of conditioning, mice froze an average of 76.2% and 73.9% in the 3- and 24-min conditions, respectively. There were no differences in the groups assigned to different ethanol treatments. Figure 6a shows the results of extinction with 3-min context exposures. Over the course of four 3-min extinction sessions, freezing decreased in all groups and there appeared to be no differences in the rate of decrease as a function of the dose of post-session EtOH (Figure 6a). A dose x session ANOVA revealed a reliable main effect of extinction session, F(3, 63)=8.9, p<0.001, but no reliable main effect of dose, F(2,21)<1.0, or interaction, F(6, 63)=<1.0, confirming that post-session injections of ethanol were without effect on extinction with short sessions.
Figure 6.
Effects of post-session injections of ethanol on short or long extinction sessions. (a) Mean percent freezing is shown for each of four 3-min extinction sessions. (b) Mean percent freezing is shown for each of four 24-min extinction sessions. Each tick mark represents one 3-min block. Bars represent SEMs.
Similarly, ethanol administered following longer extinction sessions (24 min) were generally without effect, as can be seen in Figure 6b. However, as extinction developed, the high dose of ethanol (3 g/kg) appeared to cause an increase in freezing within Sessions 3 and 4 of extinction (Ext 3 and Ext 4 in Figure 6b). Individual 3-min block x dose ANOVAs conducted on each 24-min extinction session revealed only a reliable main effect of block during Ext 1 and Ext 2, Fs(7, 238)>14.7, ps<0.001. However, the block x dose interaction was reliable during Ext 3, F(14, 238)=4.1, p<0.001, and Ext 4, F(14, 238)=2.0, p<0.05, suggesting that the within-session change in freezing differed among the groups. Further analyses of these interactions using one-way ANOVAs revealed the groups differed in freezing during the final half of Ext 3, F(2, 34)=3.6, p<0.05, and Ext 4, F(2, 34)=4.9, p<0.05. During the final half of Ext 4, freezing was higher in the 3.0 g/kg group relative to the other groups, ts(25)>2.5, ps<0.05.
This experiment demonstrates that when injections followed a brief extinction trial, which some have suggested favors reconsolidation processes, there was no effect on performance, suggesting that ethanol did not disrupt the reconsolidation of the context-shock memory. This finding is consistent with studies of ethanol’s effects on initial consolidation of contextual fear (Gould & Lommock, 2003). However, when a high dose of ethanol (3.0 g/kg) followed a long extinction trial, freezing in later sessions remained high, consistent with the idea that the consolidation of the extinction memory may have been disrupted. This needs to be interpreted cautiously, however, as other studies have demonstrated that post-session injections of ethanol can condition aversions to the physical contexts that precede them (e.g., Cunningham, Okorn, & Howard, 1997). Thus, instead of impairing consolidation, these injections may have resulted in further aversive conditioning of the context.
General Discussion
The experiments reported here demonstrate that ethanol administered prior to an extinction session affects the development and persistence of extinction. A relatively high dose of ethanol (1.5 g/kg) administered during extinction resulted in higher levels of freezing during an ethanol-free test session relative to lower ethanol doses (Experiment 1) or saline (Experiments 1–5), consistent with the idea that ethanol impaired the formation of the extinction memory. However, when testing occurred in the presence of ethanol, there was no evidence for an impairment in memory for extinction (Experiments 3 and 4). In Experiment 5, ethanol caused a greater reduction in freezing in a second conditioned context if extinction in the first context occurred in the presence of ethanol compared to saline, suggesting that ethanol may have acquired inhibitory stimulus properties during extinction. Experiment 6 demonstrated that post-session injections of 1.5 g/kg of ethanol were largely without effect on extinction. However, freezing remained high when repeated extinction sessions were followed by 3.0 g/kg of ethanol, consistent with the idea that post-session injections of ethanol may have served as an aversive US (Cunningham, et al., 1997) or that the consolidation of the extinction memory was impaired. Together, these findings suggest that multiple mechanisms may contribute to the effects of ethanol on the development and expression of extinction.
In several of these experiments, more freezing during a drug-free test occurred in mice that received ethanol during extinction, compared to mice that did not receive ethanol during extinction. One interpretation of this pattern is that ethanol impaired the neuronal processing required for the development of the extinction memory; mice did not learn that the context no longer signaled shock during extinction and froze more during testing because the extinction memory was weakly formed. One way that this may happen is that ethanol may impair attention, causing the extinction experience to be poorly learned because the animal does not fully process its environment. The possible effects of ethanol on attention and memory encoding have been offered to explain other ethanol-induced learning and memory deficits (e.g., Givens, 1997; Gould, 2003; see also Ryabinin, et al., 2002). Such an account fits with much of the conditioning and extinction data here and is consistent with the local effects of ethanol on performance, which generally were to decrease the expression of freezing. If ethanol prevents the mouse from fully processing its environment, those cues in the context that are associated with shock will not be able to evoke as much freezing (e.g., Estes, 1950; Harris, 2006).
Any effects of ethanol on memory in these experiments likely were caused by effects on attention or memory encoding because Experiment 6 found that the 1.5 g/kg dose of ethanol that affected extinction when administered before the extinction session in other experiments had no effect when administered after the session. Some have suggested that when a memory is retrieved, that memory is vulnerable to disruption until it has been reconsolidated into a fixed state (e.g., Nader, et al., 2000). None of our experiments found results consistent with the idea that ethanol affected this putative reconsolidation process. Indeed, performance during brief (3 min) and long (24 min) extinction sessions, which cause different amounts of extinction in this laboratory (e.g., Lattal, et al., 2007), were unaffected by post-session injections of 1.5 g/kg ethanol.
Although a high dose of ethanol had no effect when administered after brief extinction sessions (3 min), it did result in higher levels of freezing following long extinction sessions (24 min). This is consistent with the idea that a high dose of ethanol impaired the consolidation of the extinction memory, but it also is consistent with the idea that post-session injections of ethanol functioned as an aversive unconditioned stimulus which may have entered into associations with the context during extinction (e.g., Cunningham et al., 1997; see also Bevins, Rauhut, McPhee, & Ayres, 2000). Clearly, future research will need to disentangle the effects of post-session injections of ethanol on memory consolidation from effects on aversive contextual conditioning.
Although the findings from several of these experiments are consistent with the interpretation that ethanol impaired the encoding of the extinction memory, the results from Experiments 3–6 also demonstrate that ethanol may become part of the memory that forms during conditioning and extinction. The best evidence for ethanol as part of the original memory formed during conditioning came from Experiment 4. In that experiment, more freezing during the early part of extinction occurred when the extinction injection (saline or ethanol) was identical to the injection prior to conditioning. This finding is consistent with the idea that ethanol had stimulus properties that were encoded as part of the conditioning memory; testing in the presence of ethanol introduced a stimulus that was present during conditioning, allowing that original memory to be more easily retrieved (e.g., Hernandez, Valentine, & Powell, 1986; Lowe, 1983).
The best evidence for ethanol as part of the extinction memory came from the repeated observation that when ethanol was administered during extinction, test freezing was higher after saline than after ethanol injections. This finding is consistent with observations that following extinction of a discrete CS in a context different from conditioning, conditioned responding returns when the CS is tested in the context of conditioning. This renewal phenomenon is one of the driving forces behind Bouton’s (1991) memory retrieval theory of extinction, which suggests that the expression of the extinction memory is particularly dependent on retrieval cues. These retrieval cues can be external, often contextual cues or discrete cues associated with extinction (e.g., Brooks & Bouton, 1993; Brooks, Vaughn, Freeman, & Woods, 2004); temporal, such as the pattern of stimulus presentation during extinction (e.g., Bouton & Garcia-Gutierrez, 2006); or internal, such as a drug state associated with extinction (Cunningham, 1979; Bouton, et al., 1990). Renewal has been demonstrated most often in ABA situations, in which conditioning of a discrete cue (such as a white noise) occurs in Context A, followed by extinction in Context B, followed by testing in Context A. Other experiments have shown AAB and ABC renewal, demonstrating that renewal does not depend simply on a return to the conditioning context (e.g., Bouton & Ricker, 1994).
The difficulty with a renewal account for the present experiments is that ABA renewal occurred only when A was saline and B was ethanol. In Experiment 4, when A was ethanol and B was saline, there was relatively little conditioning and no renewal after extinction. Further, although the direction of test performance in Experiment 3 was consistent with AAB renewal, the difference between the AAB and ABB groups was not reliable. Although a finding of AAB renewal would have been a powerful demonstration of the internal contextual properties of ethanol, it is important to consider that renewal effects with pharmacological manipulations are complicated because one of the contexts (saline or vehicle injections) likely does not create an internal state that differs much from the animal’s basal state. Thus, the two internal contexts (drug versus vehicle) are not counterbalanced in terms of their salience and the animal’s history with them, which may make those two internal contexts differentially effective in triggering contextual renewal. Further, if ethanol impairs attention or hippocampal processing, this may result in even weaker renewal because of the organism’s inability to fully process its environment, which could cause a particular problem in observing AAB renewal when B is ethanol.
A selective effect of ethanol was observed in Experiment 5, in which extinction in one context (Context B) preceded by ethanol administration caused ethanol to subsequently reduce freezing in a second conditioned, but not extinguished context (Context A). Importantly, the magnitude of this reduction depended on the associative history of ethanol with Context B; ethanol caused a greater reduction of test freezing if it had been paired with Context B compared to if it had been unpaired with Context B. This is consistent with the idea that ethanol may acquire inhibitory stimulus properties during extinction that transfer to other conditioned contexts. These findings replicate and extend those of Cunningham (1979) who found evidence that ethanol may acquire inhibitory properties when paired with a discrete cue undergoing extinction. The broader implication of Experiment 5 is that the state-dependent learning effects during extinction may best be characterized by testing the ability of a pharmacological agent paired with extinction to transfer to other conditioned stimuli. It is important to note that these potential effects of ethanol as a conditioned inhibitor differ from those of physical contexts associated with extinction. Extinction contexts often fail a transfer test like that used in Experiment 5, leading to the suggestion that extinction contexts are negative occasion setters (e.g., Bouton & Swartzentruber, 1986; Nelson, 2002). An important line of future work will therefore be to characterize the contextual effects of ethanol by examining the implications of inhibitory and more general modulatory accounts of the effects of ethanol during extinction.
Overall, these results are consistent with multiple effects of ethanol on learning and performance during extinction. Ethanol may create an internal context that becomes associated with extinction and perhaps acquires inhibitory properties when extinction occurs in the presence of ethanol. Additionally, ethanol may affect attention, disrupting the processing of the contextual stimuli. When ethanol follows extinction sessions, the aversive properties of ethanol may further condition the context during extinction and the memory-altering effects of ethanol may impair consolidation of the extinction memory. These findings paint a complicated picture about ethanol and extinction, but they also have larger implications for current thinking about extinction. Many variables determine the rate, persistence, and expression of extinction and it is likely that any pharmacological manipulation will affect any number of these variables. It therefore is critical to examine not just how a manipulation appears to affect the development of the extinction memory, but also to consider how that manipulation affects performance and attention during extinction and testing.
Acknowledgments
This work was supported by a pilot project grant from the Portland Alcohol Research Center (AA10760) and by grant MH077111 to KML. I thank DeeAnna Duffield for assistance with data collection and Ellen Walker for conducting pilot experiments. I also thank Chris Cunningham for helpful comments on an earlier version of the manuscript.
References
- Akirav I, Raizel H and Maroun M (2006). Enhancement of conditioned fear extinction by infusion of the GABA agonist muscimol into the rat prefrontal cortex and amygdala. Eur J Neurosci 23, 758–64. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Rauhut AS, McPhee JE and Ayres JJB (2000). One-trial context fear conditioning with immediate shock: The roles of transport and contextual cues. Journal of Experimental Psychology: Animal Behavior Processes 28, 162–171. [DOI] [PubMed] [Google Scholar]
- Bouton ME (1991). Context and retrieval in extinction and in other examples of interference in simple associative learning Current topics in animal learning: Brain, emotion, and cognition. Dachowski L and Flaherty CF. Hillsdale, NJ, USA, Lawrence Erlbaum Associates, Inc: 25–53. [Google Scholar]
- Bouton ME and Bolles RC (1979). Contextual control of the extinction of conditioned fear. Learning & Motivation 10, 445–466. [Google Scholar]
- Bouton ME and Garcia-Gutierrez A (2006). Intertrial interval as a contextual stimulus. Behav Processes 71, 307–17. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Kenney FA and Rosengard C (1990). State-dependent fear extinction with two benzodiazepine tranquilizers. Behav Neurosci 104, 44–55. [DOI] [PubMed] [Google Scholar]
- Bouton ME and Ricker ST (1994). Renewal of extinguished responding in a second context. Animal Learning & Behavior 22, 317–324. [Google Scholar]
- Bouton ME and Swartzentruber D (1986). Analysis of the associative and occasion-setting properties of contexts participating in a Pavlovian discrimination. Journal of Experimental Psychology: Animal Behavior Processes 12, 333–350. [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA and Maren S (2006). Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry 60, 352–60. [DOI] [PubMed] [Google Scholar]
- Brooks DC and Bouton ME (1993). A retrieval cue for extinction attenuates spontaneous recovery. Journal of Experimental Psychology: Animal Behavior Processes 19, 77–89. [DOI] [PubMed] [Google Scholar]
- Brooks DC, Vaughn JM, Freeman AJ and Woods AM (2004). An extinction cue reduces spontaneous recovery of ataxic ethanol tolerance in rats. Psychopharmacology (Berl) 176, 256–65. [DOI] [PubMed] [Google Scholar]
- Cain CK, Blouin AM and Barad M (2004). Adrenergic transmission facilitates extinction of conditional fear in mice. Learn Mem 11, 179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano C and Pavone F (1988). Effects of ethanol on passive avoidance behavior in the mouse: involvement of GABAergic mechanisms. Pharmacol Biochem Behav 29, 321–4. [DOI] [PubMed] [Google Scholar]
- Corcoran KA and Maren S (2001). Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. J Neurosci 21, 1720–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA and Maren S (2004). Factors regulating the effects of hippocampal inactivation on renewal of conditional fear after extinction. Learn Mem 11, 598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL (1978). Alcohol interacts with flavor during extinction of conditioned taste aversion. Physiological Psychology 6, 510–516. [Google Scholar]
- Cunningham CL (1979). Alcohol as a cue for extinction: State dependency produced by conditioned inhibition. Anim Learn Behav 7, 45–52. [Google Scholar]
- Cunningham CL, Okorn DM and Howard CE (1997). Interstimulus interval determines whether ethanol produces conditioned place preference or aversion in mice. Animal Learning & Behavior 25, 31–42. [Google Scholar]
- Cunningham CL, Tull LE, Rindal KE and Meyer PJ (2002). Distal and proximal pre-exposure to ethanol in the place conditioning task: tolerance to aversive effect, sensitization to activating effect, but no change in rewarding effect. Psychopharmacology (Berl) 160, 414–24. [DOI] [PubMed] [Google Scholar]
- Delamater AR (2004). Experimental extinction in Pavlovian conditioning: behavioural and neuroscience perspectives. Q J Exp Psychol B 57, 97–132. [DOI] [PubMed] [Google Scholar]
- Denniston JC, Chang RC and Miller RR (2003). Massive extinction treatment attenuates the renewal effect. Learning and Motivation 34, 68–86. [Google Scholar]
- Devenport LD (1984). Extinction-induced spatial dispersion in the radial arm maze: arrest by ethanol. Behav Neurosci 98, 979–85. [DOI] [PubMed] [Google Scholar]
- Dildy JE and Leslie SW (1989). Ethanol inhibits NMDA-induced increases in free intracellular Ca2+ in dissociated brain cells. Brain Res 499, 383–7. [DOI] [PubMed] [Google Scholar]
- Estes WK (1950). Toward a statistical theory of learning. Psychological Review 57, 94–107. [Google Scholar]
- Falls WA, Miserendino MJ and Davis M (1992). Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci 12, 854–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Kim JJ and Baxter MG (2004). Effects of complete immunotoxin lesions of the cholinergic basal forebrain on fear conditioning and spatial learning. Hippocampus 14, 244–54. [DOI] [PubMed] [Google Scholar]
- Givens B (1997). Effect of ethanol on sustained attention in rats. Psychopharmacology (Berl) 129, 135–40. [DOI] [PubMed] [Google Scholar]
- Gould TJ (2003). Ethanol disrupts fear conditioning in C57BL/6 mice. J Psychopharmacol 17, 77–81. [DOI] [PubMed] [Google Scholar]
- Gould TJ and Lommock JA (2003). Nicotine enhances contextual fear conditioning and ameliorates ethanol-induced deficits in contextual fear conditioning. Behav Neurosci 117, 1276–82. [DOI] [PubMed] [Google Scholar]
- Harris JA (2006). Elemental representations of stimuli in associative learning. Psychol Rev 113, 584–605. [DOI] [PubMed] [Google Scholar]
- Harris JA and Westbrook RF (1998). Evidence that GABA transmission mediates contextspecific extinction of learned fear. Psychopharmacology (Berl) 140, 105–15. [DOI] [PubMed] [Google Scholar]
- Hernandez LL, Valentine JD and Powell DA (1986). Ethanol enhancement of Pavlovian conditioning. Behav Neurosci 100, 494–503. [DOI] [PubMed] [Google Scholar]
- Knowles SK and Duka T (2004). Does alcohol affect memory for emotional and non-emotional experiences in different ways? Behav Pharmacol 15, 111–21. [DOI] [PubMed] [Google Scholar]
- Lattal KM, Barrett R, & Wood MA (2007). Systemic or intrahippocampal delivery of histone deacetylase inhibitors facilitates fear extinction. Behavioral Neuroscience, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattal KM, Radulovic J and Lukowiak K (2006). Extinction: does it or doesn’t it? The requirement of altered gene activity and new protein synthesis. Biol Psychiatry 60, 344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R and Cranney J (2005). D-cycloserine facilitates extinction of learned fear: effects on reacquisition and generalized extinction. Biol Psychiatry 57, 841–7. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G and Weight FF (1990). NMDA receptor-mediated synaptic excitation selectively inhibited by ethanol in hippocampal slice from adult rat. J Neurosci 10, 1372–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe G (1983). Alcohol and state-dependent learning. Subst Alcohol Actions Misuse 4, 273–82. [PubMed] [Google Scholar]
- Matthews DB and Silvers JR (2004). The use of acute ethanol administration as a tool to investigate multiple memory systems. Neurobiol Learn Mem 82, 299–308. [DOI] [PubMed] [Google Scholar]
- Mueller CW, Lisman SA and Spear NE (1983). Alcohol enhancement of human memory: tests of consolidation and interference hypotheses. Psychopharmacology (Berl) 80, 22630. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE and LeDoux JE (2000). Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406, 722–726. [DOI] [PubMed] [Google Scholar]
- Nelson JB (2002). Context specificity of excitation and inhibition in ambiguous stimuli. Learning and Motivation 33, 284–310. [Google Scholar]
- Parker ES, Birnbaum IM, et al. (1980). Retrograde enhancement of human memory with alcohol. Psychopharmacology (Berl) 69, 219–22. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Dickinson S and Burkhart-Kasch S (1994). Behavioral sensitization to drug stimulant effects in C57BL/6J and DBA/2J inbred mice. Behav Neurosci 108, 789–803. [DOI] [PubMed] [Google Scholar]
- Power AE, Berlau DJ, McGaugh JL and Steward O (2006). Anisomycin infused into the hippocampus fails to block “reconsolidation” but impairs extinction: the role of re-exposure duration. Learn Mem 13, 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryabinin AE (1998). Role of hippocampus in alcohol-induced memory impairment: implications from behavioral and immediate early gene studies. Psychopharmacology (Berl) 139, 34–43. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Miller MN and Durrant S (2002). Effects of acute alcohol administration on object recognition learning in C57BL/6J mice. Pharmacol Biochem Behav 71, 307–12. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, et al. (2004). Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci 24, 4787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitemier AZ and Ryabinin AE (2003). Alcohol-induced memory impairment in trace fear conditioning: a hippocampus-specific effect. Hippocampus 13, 305–15. [DOI] [PubMed] [Google Scholar]
- White AM, Matthews DB and Best PJ (2000). Ethanol, memory, and hippocampal function: a review of recent findings. Hippocampus 10, 88–93. [DOI] [PubMed] [Google Scholar]