Abstract
Several recent studies have shown that chromatin, the DNA-protein complex that packages genomic DNA, has an important function in learning and memory. Dynamic chromatin modification via histone deacetylase (HDAC) inhibitors and histone acetyltransferases (HATs) may enhance hippocampal synaptic plasticity and hippocampus-dependent memory. Little is known about the effects of HDAC inhibitors on extinction, a learning process through which the ability of a previously conditioned stimulus, such as a conditioning context, to evoke a conditioned response is diminished. We demonstrate that administration of the HDAC inhibitors sodium butyrate (NaB) systemically or trichostatin A (TSA) intrahippocampally prior to a brief (3-min) contextual extinction session causes context-evoked fear to decrease to levels observed with a long (24-min) extinction session. These results suggest that HDAC inhibitors may enhance learning during extinction and are consistent with other studies demonstrating a role for the hippocampus in contextual extinction. Molecular and behavioral mechanisms through which this enhanced extinction effect may occur are discussed.
Keywords: Extinction, fear, memory, histone acetylation, hippocampus
Studies of Pavlovian conditioning have long addressed the conditions that strengthen and weaken conditioned behavior. For example, in contextual fear conditioning, as the organism learns that the context is a reliable signal for footstock, the conditioned freezing response evoked by that context increases. If the organism is then exposed to the context in the absence of the expected footshock, the fear response weakens through a process known as experimental extinction. Although there are some important differences in the learning mechanisms that operate during acquisition and extinction, a number of studies have demonstrated many common mechanisms at the behavioral and molecular levels (Bouton et al., 2006; Lattal et al., 2006). Recent molecular studies of learning have addressed the ways in which the regulation of gene transcription contributes to memory formation. Transcription appears to be regulated by the concerted action of multiple transcription factors and cofactors that modify and remodel chromatin, the DNA-protein complex that packages genomic DNA (reviewed in Felsenfeld and Groudine, 2003). There are now several demonstrations that chromatin modifying mechanisms may underlie initial memory formation and consolidation (reviewed in Levenson and Sweatt, 2005; Wood et al., 2006a), but little is known about how these mechanisms contribute to extinction.
Chromatin modifying complexes, which contain histone modifying enzymes like histone acetyltransferases, regulate access to the underlying genomic DNA by relaxing chromatin structure and providing docking sites for additional regulatory factors (reviewed in Berger, 2002; Peterson and Laniel, 2004). Chromatin remodeling complexes, which contain ATP-dependent nucleosome remodeling enzymes, alter histone-DNA contacts to facilitate transcription (reviewed in Saha et al., 2006). Although there is a clear functional interplay between chromatin modifying complexes and chromatin remodeling complexes (Neely and Workman, 2002), significantly more is known about the former with regards to memory processes. Recently, several studies have demonstrated a pivotal role for the histone acetyltransferase (HAT) CREB-binding protein (CBP) and histone deacetyltransferases (HDACs) in memory and synaptic plasticity (Oike et al., 1999; Guan et al., 2002; Bourtchouladze et al., 2003; Alarcon et al., 2004; Korzus et al., 2004; Levenson et al., 2004; Yeh et al., 2004; Wood et al., 2005; Wood et al., 2006b; Vecsey et al., 2007).
CBP is a transcriptional coactivator that mediates transcriptional activation by recruiting basal transcription machinery and by covalent modification of histones via its HAT activity (reviewed in Goodman and Smolik, 2000). Genetically modified Cbp mutant mice exhibit impairments in long-term memory in a variety of behavioral paradigms as well as deficits in long-term potentiation, a form of synaptic plasticity. In three different studies, each representing a different genetically modified Cbp mutant mouse (heterozygous knockout: Alarcon et al., 2004); dominant negative: Wood et al., 2005); and homozygous knockin carrying a mutation in the CREB-binding domain of CBP: Wood et al., 2006b), significant impairments in long-term memory for contextual fear conditioning were observed. In the heterozygous knockout Cbp mutant mouse, the fear memory deficit was ameliorated by treating the mice with an HDAC inhibitor (Alarcon et al., 2004; see also Korzus et al., 2004). HDAC inhibitors block the activity of histone deacetylases and thus increase histone acetylation (Kelly and Marks, 2005; Marks and Dokmanovic, 2005). Recently, we have demonstrated that HDAC inhibition significantly enhances contextual fear conditioning and hippocampal synaptic plasticity (Vecsey et al., 2007). Taken together, these findings suggest that memory can be modulated by manipulating HAT and HDAC activity during initial memory formation and consolidation.
Here, we examined the effects of HDAC inhibition during extinction of contextual fear conditioning. We find that systemic treatment with the HDAC inhibitor sodium butyrate as well as intrahippocampal treatment with trichostatin A enhances extinction. This enhancement mirrors that caused by a commonly used behavioral manipulation (duration of the context reexposure period during extinction) and is consistent with other studies demonstrating a role for the hippocampus in the extinction of contextual fear.
Method
Subjects
Male and female C57BL/6 mice obtained from Jackson Laboratories were used. Mice were 8–10 weeks old and had free access to food and water in their home cages. Lights were maintained on a 12:12 hour light/dark cycle, with all behavioral testing carried out during the light portion of the cycle. All experiments were conducted according to National Institutes of Health guidelines for animal care and use and were approved by the Institutional Animal Care and Use Committees of the University of California-Irvine and Oregon Health & Science University.
Cannulations
Mice were anesthetized using isoflurane while immobilized on a Just For Mice stereotax (Stoelting, Wood Dale, Il). Bilateral 22 gauge guide cannulae were used to guide an injection cannula (28 gauge) into the dorsal hippocampus (Plastics One Inc., Roanoke, VA). The guide cannula placement was: AP: −1.7 mm; ML: ± 1.2 mm; DV: 1.5 mm below pedestal. Injection cannulae extended an additional 0.5 mm below the guide cannluae (total depth 2.0 mm). Cannulae placement was verified by sectioning brains on a cryostat and visualizing track position.
Injections
In the systemic injections experiment (Figure 2a), mice received subcutaneous injections of 1.2 g/kg of Sodium Butyrate (NaB; Upstate) dissolved in distilled water or an equivalent volume of distilled water alone (vehicle). This dose has been shown previously to enhance performance after initial conditioning (Levenson et al., 2004). These injections occurred either immediately prior to extinction or 4 hr after extinction. In the intrahippocampal experiment (Figure 2b), mice received bilateral intrahippocampal injections of trichostatin A (TSA; 0.5 μL of 22 mM TSA in 50% ethanol; A.G. Scientific, Inc., San Diego CA) or vehicle (0.5 μL of 50% ethanol) per side from a 5.0 μL Hamilton syringe operated by a Harvard Apparatus Pump II Dual Syringe micropump. Injections occurred over 1 min and injection cannulae were left in place an additional 30 s to allow the fluid to diffuse. Each side was injected individually, one immediately after the other. The entire injection process took approximately 4 min. We have previously shown that TSA used with these injection procedures affects histone acetylation specifically in the hippocampus. Histone acetylation is increased approximately one half-hour post-injection, reaches maximum levels by four hours post-injection and returns to background levels by twenty-four hours post-injection (Vecsey et al., 2007).
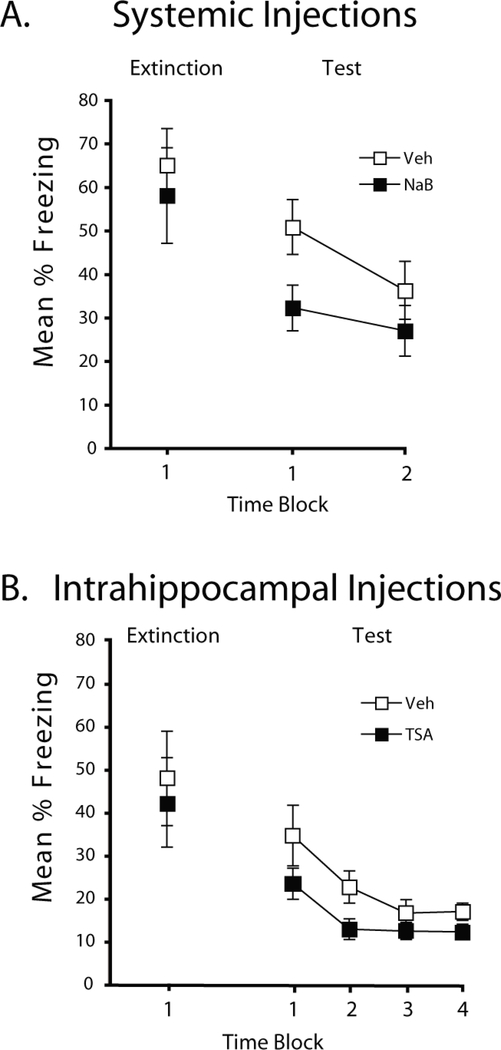
Figure 2.
HDAC inhibition enhances extinction of contextual fear. A) During a 3-min extinction session, mice injected systemically with NaB or Vehicle prior to extinction did not differ, but during testing, mice injected with NaB showed less freezing compared to vehicle-treated mice (p<0.05). B) Mice administered TSA or vehicle via intrahippocampal cannulae prior to the extinction session exhibited enhanced extinction during testing. Mice showed comparable freezing during acquisition and extinction, but TSA treated mice showed significantly lower freezing during testing (p<0.05). Percent freezing is shown during the 3-min extinction session and in 6-min time blocks during testing.
Contextual Fear Conditioning
Fear conditioning experiments were performed using a set of four Coulbourn Instruments mouse conditioning chambers at OHSU (Figures 1a and 2a) or a set of four PhenoTyper conditioning chambers at UC Irvine (Figures 1b and 2b; PhenoTyper 3000 with shock floor, Noldus Information Technology, Leesburg, VA). Mice were handled for five consecutive days for two minutes each day prior to conditioning.
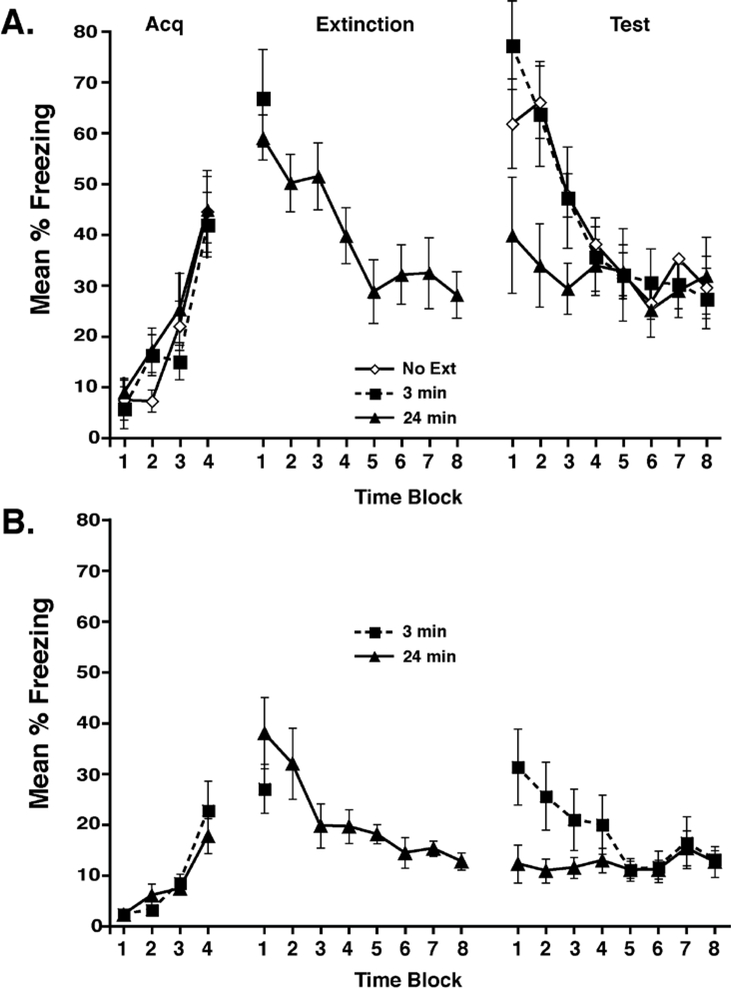
Figure 1.
Mice re-exposed to the conditioned context for 24 min exhibited less freezing during testing than those re-exposed for 3 min. A) Mice were conditioned on Day 1, re-exposed to the conditioned context on Day 2 for 3 or 24 min, and tested on Day 3. Both groups showed comparable freezing during the acquisition and extinction sessions, but mice that were re-exposed for 3 min had significantly higher freezing during testing than those exposed for 24 min (p<0.05). B) The protocol from A was used with different conditioning chambers and a different tracking system (see Methods for details). Again, mice re-exposed to the conditioned context for 24 min showed less freezing than the mice re-exposed for 3 min (p<0.05). Percent freezing is shown during the acquisition, extinction, and testing periods in 3-min time blocks.
Experiment 1 (Figures 1a and 1b). On Day 1, mice were placed into the conditioning chamber and received 2-s 0.35 mA scrambled footshocks, 2.5, 5, 9, and 11.5 min after placement into the chamber. Mice were removed from the chamber after a total of 12 min. Performance during this session was used to match levels of conditioning in mice assigned to different extinction groups. On Day 2, 24 hr after conditioning, mice were returned to the conditioning chambers for a 3- or 24-min extinction session, during which no shocks were administered. A third group of mice did not receive extinction, but were handled with the other groups on Day 2. On Day 3, all mice were returned to the conditioning chambers for a 24-min extinction test, in which no shocks were presented. For Figure 1a, sample sizes were as follows: 3-min group (n=9); 24-min group (n=10); no extinction group (n=9). For Figure 1B, sample sizes were as follows: 3-min group (n=10); 24-min group (n=10).
Experiment 2 (Figures 2a and 2b). Mice received contextual fear conditioning as in Experiment 1. On Day 2, 24 hr after conditioning, mice were returned to the conditioned context immediately following subcutaneous injections of NaB (1.2 g/kg NaB; n=8) or vehicle (water; n=12; Figure 2a) or bilateral intrahippocampal injections of TSA (0.5 μL of 22 mM TSA; n=15) or vehicle (0.5 μL of 50% EtOH; n=13; Figure 2b). They remained in the context for 3 min without receiving shock, and then were returned to their home cages. On Day 3, mice received a 12-min extinction test in the NaB experiment and a 24-min extinction test in the TSA experiment. In a follow-up experiment, mice received the 12-min conditioning and 3-min extinction sessions, but injections of NaB (1.2 g/kg NaB; n=8) or vehicle (water; n=8) occurred 4 hr after the extinction session. These mice then received a 12-min extinction test session.
Data Analysis
Conditioning was assessed by measuring freezing behavior (Fanselow, 1980). For Figures 1a and 2a, freezing was defined by the absence of detected movement for at least 3 s using Coulbourn infrared activity monitors, as in other studies (e.g., Lee & Kim, 1998). For Figures 1b and 2b, freezing was scored using EthoVision 3.1 (EthoVision 3.1, Noldus Information Technology, Leesburg, VA). The behavior of each mouse was sampled at 1 s intervals and the percentage of those intervals in which the mouse froze was calculated.
Analyses of variance (ANOVAs) with repeated measures were performed in all experiments to examine the changes within the extinction and test sessions. Planned comparisons during the initial periods of the tests were conducted using Student’s t tests with alpha levels held at 0.05.
Results
The overall aim of this study was to examine the ability of histone deacetylase (HDAC) inhibitors to facilitate extinction. To do this, we first needed to identify a period of extinction exposure that would not entirely extinguish conditioned behavior so that any floor effects could be avoided. Thus, in the first experiment we determined the effects of two context exposure durations (3 or 24 min) during extinction. Figure 1 shows the results from two replications of this experiment in different laboratories. There were no differences during conditioning or during the initial 3-min of extinction, ts<1.0. A 1-way repeated measures ANOVA revealed a reliable main effect of extinction time block in the 24-min groups, demonstrating within-session changes in freezing during extinction (ps<0.001). During testing, mice that received the 24-min exposure to the context during extinction froze less compared to mice that received a 3-min exposure or no extinction. A group x test time block ANOVA revealed a reliable main effect of test block, (Figure 1a: F(7,175)=16.4, p<0.001; Figure 1b: F(7, 126)=3.1, p<0.005) and a reliable interaction (Figure 1a: F(14,175)=2.9, p<0.001; Figure 1b: F(7, 126)=3.3, p<0.005). Analysis of the interaction revealed that the 24-min extinction group froze less during the first 6 min of the test compared to the other groups in Figure 1a (p<0.05) and 1b (p<0.05). Further, the 3-min and the No Ext groups in Figure 1a did not differ. In extinction and the tests, freezing was not eliminated, but appeared to reach asymptotic levels after about 15 min in the context.
Importantly, these results were observed in two different laboratories, using different conditioning chambers and different behavioral scoring analyses (see Methods for details), even though overall freezing levels appeared higher in Figure 1a relative to Figure 1b. Together, these results demonstrate that a longer reexposure period to the conditioned context results in greater extinction relative to a shorter exposure, and this is true at different parts of the behavioral scale. These findings also demonstrate that extinction occurs rapidly within the test session, suggesting that enhancements in extinction are best detected early in the test. Further, data in Figure 1a demonstrate that a 3-min reexposure period did not generate much, if any, extinction at the behavioral level relative to a group that received no extinction session. This extinction duration is therefore appropriate for examining the ability of HDAC inhibitors to enhance extinction.
We next examined how systemic or intrahippocampal HDAC inhibition affects extinction learning. As can be seen in Figure 2a, systemic injections of NaB prior to extinction resulted in less freezing during a test compared to vehicle injections. There were no differences between the groups during acquisition (mean percent freezing during the final 6 min of acquisition was 50.4 (SEM=8.6) and 56.4 (SEM=6.5) in mice treated with NaB or Vehicle, respectively, t(17)<1.0, p=.57. One mouse failed to show conditioning (mean percent freezing during acquisition was 2.8%) and was discarded from the experiment. NaB injections did not affect freezing during extinction, t(17)<1.0, p=.72, but appeared to reduce freezing during the test session. A group x test block ANOVA revealed a reliable main effect of 6-min time block (F(1,17)=6.3, p<0.05). A planned comparison revealed that mice treated with NaB froze less during the first 6 min of the test session compared to vehicle-treated mice, t(17)=2.3, p<0.05. A follow-up experiment demonstrated that injections of NaB 4 hr after extinction did not affect test freezing (mean percent freezing during the test was 62.6 and 61.0 in NaB- and vehicle-treated mice, respectively, t(14)<1.0, p=.86). These results demonstrate that a temporally contiguous relation between NaB and extinction was critical for the enhanced extinction effect.
To more precisely determine what brain region may be affected by HDAC inhibition during extinction learning, we next tested the ability of TSA delivered via intrahippocampal cannulae to facilitate extinction. Because TSA begins to affect histone acetylation in the hippocampus approximately 30 min after intrahippocampal delivery (Vecsey et al., 2007), injections were given immediately prior to the 3-min extinction period so that it would be active during memory consolidation. As shown in Figure 2b, the vehicle and TSA groups exhibited similar freezing during the conditioning and extinction periods, t(26)<1.0, but when tested 24 hours after extinction on the third day, the TSA group showed reduced freezing in comparison to the vehicle group. A group x test block ANOVA revealed a reliable main effect of 6-min time block during the test (F(3,78)=1.6, p<0.001) and a planned comparison revealed that mice treated with TSA froze less during the first 6 min of the test session compared to vehicle-treated mice, t(26)=3.2, p<0.05. Together, our systemic NaB and site-specific TSA results demonstrate that two biochemically distinct HDAC inhibitors can facilitate extinction learning.
Discussion
We show that HDAC inhibitors, which increase histone acetylation and modulate gene expression, facilitate extinction after contextual fear conditioning. Intraperitonael delivery of sodium butyrate (NaB) or intrahippocampal delivery of trichostatin A (TSA) prior to a brief extinction session caused a reduction in freezing one day later, relative to vehicle-treated animals. Although NaB and TSA are both HDAC inhibitors, they are from structurally distinct classes, with NaB belonging to the aliphatic acid class and TSA belonging to the hydroxamate class. Both NaB and TSA inhibit class I (nuclear localization) and II (shuttle between nucleus and cytoplasm) HDACs at millimolar concentrations in vivo and nanomolar concentrations in cell culture, which results in histone hyperacetylation and subsequent transcriptional regulation of specific genes (Marks et al., 2004; Marks and Dokmanovic, 2005). Together, our results demonstrate that two biochemically distinct HDAC inhibitors, delivered via different methods (systemic or intrahippocampal), have similar effects on extinction.
These results are consistent with our previous study in which we examined the effect of HDAC inhibition on initial learning of contextual fear conditioning as well as on hippocampal synaptic plasticity. Post-conditioning intrahippocampal injections of TSA enhanced memory for contextual fear conditioning and this enhancement appeared to require the transcription factor cAMP response element binding protein (CREB) (Vecsey et al., 2007). With regards to synaptic plasticity, TSA and NaB enhanced LTP in the Schaeffer collateral pathway of the hippocampus through a CREB-dependent mechanism. Importantly, the enhancement in LTP was also shown to require the transcriptional coactivator and histone acetyltranferase CREB-binding protein (CBP) and the interaction between CREB and CBP (Vecsey et al., 2007). These previous results suggest that HDAC inhibitors may facilitate CREB:CBP-dependent gene expression underlying initial memory consolidation. Additionally, previous studies have found that systemic delivery of HDAC inhibitors enhances initial learning for contextual fear conditioning and fear-potentiated startle and may involve ERK/MAPK and NF-kB signaling, respectively (Levenson et al., 2004; Yeh et al., 2004; Chwang et al., 2006). Similar mechanisms may also be involved in HDAC inhibition-dependent enhancement of extinction, but further work characterizing the time course of these effects is needed to make inferences about memory consolidation processes during extinction.
Recent work examining the role of CBP, HDACs, and histone acetylation in memory processes has demonstrated that inhibiting CBP activity and decreasing histone acetylation impairs long-term memory and long-term potentiation (Oike et al., 1999; Guan et al., 2002; Bourtchouladze et al., 2003; Alarcon et al., 2004; Korzus et al., 2004; Wood et al., 2005; Wood et al., 2006b). Conversely, increasing histone acetylation (via HDAC inhibitors) appears to enhance memory and synaptic plasticity (Levenson et al., 2004; Yeh et al., 2004; Vecsey et al., 2007). These studies indicate that enzymes involved with modifying chromatin via histone acetylation are pivotal for memory formation. This is particularly interesting because structural changes at the cellular level may lead to lasting changes in memory and behavior. Chromatin modifying and remodeling complexes and epigenetic mechanisms of transcription have been shown to maintain cellular memory (cell fate; Turner, 2002, 2003). They may also potentially underlie the strengthening and maintenance of synaptic connections required for long-term changes in behavior and memory (Weaver et al., 2004). Thus, it is intriguing to speculate that modulating molecular mechanisms involved in modifying chromatin (such as HDAC inhibitors) may have long-lasting effects, which may be relevant to enhancing the persistence of extinction and possibly minimizing relapse in clinical settings. In support of this idea, a very recent study by (Bredy et al., 2006) found that valproic acid (VPA), which like NaB is an HDAC inhibitor in the aliphatic acid class, may enhance long-term memory for extinction. How HDAC inhibitors may be affecting gene expression required for different memory processes remains to be understood. HDAC inhibitors can increase as well as decrease the expression of specific genes (Fass et al., 2003); thus, one should not assume that the effects of HDAC inhibitors on memory are mediated only by increases in gene expression. It is possible that in addition to promoting memory by increasing gene expression, HDAC inhibitors may promote memory by decreasing the expression of memory suppressor genes, which may impair memory storage (Abel & Kandel, 1998; Abel, et al., 1998). Further, under some circumstances, HDAC inhibitors may actually impair memory storage depending on which genes are expressed or suppressed.
In addition to further clarification of the molecular effects of HDAC inhibitors, a critical behavioral issue to be resolved is determining the persistence of the effects of HDAC inhibitors on extinction. In our experiments, extinction was enhanced 24 hr after the extinction trial. A 24-hr interval is often used to measure “long-term” memory, but questions about the persistence of extinction effects are best answered in further experiments examining spontaneous recovery over much longer intervals (see Lattal et al., 2004; Isiegas et al., 2006). Clearly, although our results suggest that extinction may be enhanced by HDAC inhibitors, much more work is needed to determine the persistence of these effects across longer retention intervals. Indeed, some studies of agents that may enhance the development of extinction have found that enhancements in extinction do not prevent spontaneous recovery (e.g., Isiegas et al., 2006), contextual renewal (e.g., Woods and Bouton, 2006), or US reinstatement (e.g., Fischer et al., 2004) of the conditioned behavior, meaning that the therapeutic value of such agents needs to be examined closely.
There are several mechanisms through which extinction may be enhanced. HDAC inhibition may enhance the formation of a new “context-no shock” memory. This may occur through enhancements in consolidation of this memory. Studies of initial learning have demonstrated that post-session injections of TSA into the hippocampus result in enhancements in freezing the next day, consistent with the notion that TSA enhanced consolidation (Vecsey, et al., 2007). Further, TSA begins to affect histone acetylation approximately 30 minutes after injection into the hippocampus, suggesting that the effects on extinction in our experiments were due to enhancements in consolidation. Nonetheless, future work will need to examine the effects of HDAC inhibitors injected at different times before and after an extinction session to characterize the effects on memory encoding and consolidation (e.g., Abel & Lattal, 2001).
It also is possible that behavioral extinction may be enhanced because HDAC inhibition may weaken, perhaps temporarily, some aspect of the original memory. Such depressive actions on the original memory often are described as impairments in reconsolidation of that memory. This is possible, but it is important to note that may theories of extinction have described depressions in aspects of the original memory (such as the CS or US representation) without appealing to reconsolidation processes (e.g., Ledgerwood et al., 2005; Rescorla and Cunningham, 1978; Robbins, 1990; Schmajuk and Larrauri, 2006), so the implications of an account in terms of impairments in reconsolidation need to be explored in the context of other theories of extinction.
There are multiple mechanisms that may result in enhancements in extinction and there clearly are many unanswered questions about how these basic mechanisms may interact to cause persistent extinction. Our results suggest that HDAC inhibitors enhance extinction for contextual fear, perhaps by modulating transcription in the hippocampus, a region that has been implicated in other studies of extinction of conditioned fear (Szapiro et al., 2003; Rossato et al., 2006; Fischer et al., 2007; Power et al., 2006). Further studies evaluating the persistence of these effects will be informative about the potential for HDAC inhibitors, which are being widely tested in clinical trials for neurodegenerative diseases, as part of a treatment intervention for psychiatric disorders, such as post-traumatic stress disorder and substance abuse that involve failures in extinction.
Acknowledgments
This work was supported by grants from NIH (MH074547 and MH077111) and a pilot grant from the Portland Alcohol Research Center (AA10760) to KML. We thank DeeAnna Duffield for assistance with data collection and Sara Cabrera for critical reading of the manuscript.
References
- Abel T, Kandel E (1998) Positive and negative regulatory mechanisms that mediate long-term memory storage. Brain Res Brain Res Rev 26:360–378. [DOI] [PubMed] [Google Scholar]
- Abel T, Lattal KM (2001) Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr Opin Neurobiol 11:180–187. [DOI] [PubMed] [Google Scholar]
- Abel T, Martin KC, Bartsch D, Kandel ER (1998) Memory suppressor genes: inhibitory constraints on the storage of long- term memory. Science 279:338–341. [DOI] [PubMed] [Google Scholar]
- Alarcon JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER, Barco A (2004) Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron 42:947–959. [DOI] [PubMed] [Google Scholar]
- Berger SL (2002) Histone modifications in transcriptional regulation. Curr Opin Genet Dev 12:142–148. [DOI] [PubMed] [Google Scholar]
- Bourtchouladze R, Lidge R, Catapano R, Stanley J, Gossweiler S, Romashko D, Scott R, Tully T (2003) A mouse model of Rubinstein-Taybi syndrome: defective long-term memory is ameliorated by inhibitors of phosphodiesterase 4. Proc Natl Acad Sci U S A 100:10518–10522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S (2006) Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry 60:352–360. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Wu H, Crego C, Zellhoefer J, Sun YE, Barad MG (2007) Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learn Mem 14:268–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chwang WB, O’Riordan KJ, Levenson JM, Sweatt JD (2006) ERK/MAPK regulates hippocampal histone phosphorylation following contextual fear conditioning. Learn Mem 13:322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS (1980) Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci 15:177–182. [DOI] [PubMed] [Google Scholar]
- Fass DM, Butler JE, and Goodman RH (2003). Deacetylase activity is required for cAMP activation of a subset of CREB target genes. J Biol Chem 278, 43014–43019. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G, Groudine M (2003) Controlling the double helix. Nature 421:448–453. [DOI] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Schrick C, Spiess J, Radulovic J (2004) Distinct roles of hippocampal de novo protein synthesis and actin rearrangement in extinction of contextual fear. J Neurosci 24:1962–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Radulovic M, Schrick C, Sananbenesi F, Godovac-Zimmermann J, Radulovic J (2007) Hippocampal Mek/Erk signaling mediates extinction of contextual freezing behavior. Neurobiol Learn Mem 87:149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman RH, Smolik S (2000) CBP/p300 in cell growth, transformation, and development. Genes Dev 14:1553–1577. [PubMed] [Google Scholar]
- Guan Z, Giustetto M, Lomvardas S, Kim JH, Miniaci MC, Schwartz JH, Thanos D, Kandel ER (2002) Integration of long-term-memory-related synaptic plasticity involves bidirectional regulation of gene expression and chromatin structure. Cell 111:483–493. [DOI] [PubMed] [Google Scholar]
- Isiegas C, Park A, Kandel ER, Abel T, Lattal KM (2006) Transgenic inhibition of neuronal protein kinase A activity facilitates fear extinction. J Neurosci 26:12700–12707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly WK, Marks PA (2005) Drug insight: Histone deacetylase inhibitors--development of the new targeted anticancer agent suberoylanilide hydroxamic acid. Nat Clin Pract Oncol 2:150–157. [DOI] [PubMed] [Google Scholar]
- Korzus E, Rosenfeld MG, Mayford M (2004) CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron 42:961–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattal KM, Rapoport DA, Abel T (2004) Different long-term effects of hippocampal protein synthesis inhibition after acquisition and retrieval of contextual fear conditioning. In: Society for Neuroscience. San Diego, CA. [Google Scholar]
- Lattal KM, Radulovic J, Lukowiak K (2006) Extinction: does it or doesn’t it? The requirement of altered gene activity and new protein synthesis. Biol Psychiatry 60:344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J (2005) D-cycloserine facilitates extinction of learned fear: effects on reacquisition and generalized extinction. Biol Psychiatry 57:841–847. [DOI] [PubMed] [Google Scholar]
- Lee H, Kim JJ (1998) Amygdalar NMDA receptors are critical for new fear learning in previously fear-conditioned rats. J Neurosci 18:8444–8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson JM, Sweatt JD (2005) Epigenetic mechanisms in memory formation. Nat Rev Neurosci 6:108–118. [DOI] [PubMed] [Google Scholar]
- Levenson JM, O’Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD (2004) Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem 279:40545–40559. [DOI] [PubMed] [Google Scholar]
- Marks PA, Dokmanovic M (2005) Histone deacetylase inhibitors: discovery and development as anticancer agents. Expert Opin Investig Drugs 14:1497–1511. [DOI] [PubMed] [Google Scholar]
- Marks PA, Richon VM, Miller T, Kelly WK (2004) Histone deacetylase inhibitors. Adv Cancer Res 91:137–168. [DOI] [PubMed] [Google Scholar]
- Neely KE, Workman JL (2002) Histone acetylation and chromatin remodeling: which comes first? Mol Genet Metab 76:1–5. [DOI] [PubMed] [Google Scholar]
- Oike Y, Hata A, Mamiya T, Kaname T, Noda Y, Suzuki M, Yasue H, Nabeshima T, Araki K, Yamamura K (1999) Truncated CBP protein leads to classical Rubinstein-Taybi syndrome phenotypes in mice: implications for a dominant-negative mechanism. Hum Mol Genet 8:387–396. [DOI] [PubMed] [Google Scholar]
- Peterson CL, Laniel MA (2004) Histones and histone modifications. Curr Biol 14:R546–551. [DOI] [PubMed] [Google Scholar]
- Power AE, Berlau DJ, McGaugh JL, Steward O (2006) Anisomycin infused into the hippocampus fails to block “reconsolidation” but impairs extinction: the role of reexposure duration. Learn Mem 13:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA, Cunningham CL (1978) Recovery of the US representation over time during extinction. Learning & Motivation 9:373–391. [Google Scholar]
- Robbins SJ (1990) Mechanisms underlying spontaneous recovery in autoshaping. Journal of Experimental Psychology: Animal Behavior Processes 16:235–249. [Google Scholar]
- Rossato JI, Bevilaqua LR, Lima RH, Medina JH, Izquierdo I, Cammarota M (2006) On the participation of hippocampal p38 mitogen-activated protein kinase in extinction and reacquisition of inhibitory avoidance memory. Neuroscience 143:15–23. [DOI] [PubMed] [Google Scholar]
- Saha A, Wittmeyer J, Cairns BR (2006) Chromatin remodelling: the industrial revolution of DNA around histones. Nat Rev Mol Cell Biol 7:437–447. [DOI] [PubMed] [Google Scholar]
- Schmajuk NA, Larrauri JA (2006) Experimental challenges to theories of classical conditioning: application of an attentional model of storage and retrieval. J Exp Psychol Anim Behav Process 32:1–20. [DOI] [PubMed] [Google Scholar]
- Szapiro G, Vianna MR, McGaugh JL, Medina JH, Izquirdo I (2003) The role of NMDA glutamate receptors, PKA, MAPK, and CAMKII in the hippocampus in extinction of conditioned fear. Hippocampus 13:53–58. [DOI] [PubMed] [Google Scholar]
- Turner BM (2002) Cellular memory and the histone code. Cell 111:285–291. [DOI] [PubMed] [Google Scholar]
- Turner BM (2003) Memorable transcription. Nat Cell Biol 5:390–393. [DOI] [PubMed] [Google Scholar]
- Vecsey CG, Hawk JD, Lattal KM, Stein JM, Fabian SA, Attner MA, Cabrera SM, McDonough CB, Brindle PK, Abel T, Wood MA (2007) Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. In Press. [DOI] [PMC free article] [PubMed]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ (2004) Epigenetic programming by maternal behavior. Nat Neurosci 7:847–854. [DOI] [PubMed] [Google Scholar]
- Wood MA, Hawk JD, Abel T (2006a) Combinatorial chromatin modifications and memory storage: a code for memory? Learn Mem 13:241–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood MA, Attner MA, Oliveira AMM, Brindle PK, Abel T (2006b) A transcription factor-binding domain of the coactivator CBP is essential for long-term memory and the expression of specific target genes. Learn Mem, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood MA, Kaplan MP, Park A, Blanchard EJ, Oliveira AM, Lombardi TL, Abel T (2005) Transgenic mice expressing a truncated form of CREB-binding protein (CBP) exhibit deficits in hippocampal synaptic plasticity and memory storage. Learn Mem 12:111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods AM, Bouton ME (2006) D-cycloserine facilitates extinction but does not eliminate renewal of the conditioned emotional response. Behav Neurosci 120:1159–1162. [DOI] [PubMed] [Google Scholar]
- Yeh SH, Lin CH, Gean PW (2004) Acetylation of nuclear factor-kappaB in rat amygdala improves long-term but not short-term retention of fear memory. Mol Pharmacol 65:1286–1292. [DOI] [PubMed] [Google Scholar]