Abstract
A novel coronavirus (CoV), Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged in late 2019 in Wuhan, China and has since spread as a global pandemic. Safe and effective vaccines are thus urgently needed to reduce the significant morbidity and mortality of Coronavirus Disease 2019 (COVID-19) disease and ease the major economic impact. There has been an unprecedented rapid response by vaccine developers with now over one hundred vaccine candidates in development and at least six having reached clinical trials. However, a major challenge during rapid development is to avoid safety issues both by thoughtful vaccine design and by thorough evaluation in a timely manner. A syndrome of “disease enhancement” has been reported in the past for a few viral vaccines where those immunized suffered increased severity or death when they later encountered the virus or were found to have an increased frequency of infection. Animal models allowed scientists to determine the underlying mechanism for the former in the case of Respiratory syncytial virus (RSV) vaccine and have been utilized to design and screen new RSV vaccine candidates. Because some Middle East respiratory syndrome (MERS) and SARS-CoV-1 vaccines have shown evidence of disease enhancement in some animal models, this is a particular concern for SARS-CoV-2 vaccines. To address this challenge, the Coalition for Epidemic Preparedness Innovations (CEPI) and the Brighton Collaboration (BC) Safety Platform for Emergency vACcines (SPEAC) convened a scientific working meeting on March 12 and 13, 2020 of experts in the field of vaccine immunology and coronaviruses to consider what vaccine designs could reduce safety concerns and how animal models and immunological assessments in early clinical trials can help to assess the risk. This report summarizes the evidence presented and provides considerations for safety assessment of COVID-19 vaccine candidates in accelerated vaccine development.
Keywords: SARS-CoV-2, COVID-19, Vaccine safety, MERS-CoV vaccine, SARS-CoV-1 vaccine, SARS-CoV-2 vaccine, Animal models, Enhanced disease, Vaccine adjuvants
Abbreviations: ACE2, Angiotensin-converting enzyme 2; ADE, Antibody disease enhancement; ARDS, Acute respiratory distress syndrome; B/HPIV3, Bovine/human parainfluenza virus type 3; BC, Brighton Collaboration; BPL, β-Propiolactone; BtCoV, Bat coronavirus; CEPI, Coalition for Epidemic Preparedness Innovations; CNS, Central nervous system; COVID-19, Coronavirus Disease 2019; CRISPR, Clustered regularly interspaced short palindromic repeats; DNA, Deoxyribonucleic acid; DPP4, Dipeptidyl peptidase-4; hACE2, Human ACE2 receptor; HBs, Hepatitis B surface antigen; hDPP4, Human DPP4; IHC, Immunohistochemistry; MERS CoV, Middle East respiratory syndrome coronavirus; mRNA, Messenger RNA; MVA, Modified Vaccinia Virus Ankara; NHP, Non-human primate; Non-SPF, Non-specific pathogen free; NTD, N terminal domain; RAG1, Recombination activating gene 1; RBD, Receptor binding domain; rMVA, Recombinant modified vaccinia virus Ankara; RNA, Ribonucleic acid; RSV, Respiratory syncytial virus; SARS-CoV-1, Severe acute respiratory syndrome coronavirus 1; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; SPEAC, Safety Platform for Emergency vACcines; TCR, T-cell receptor; Tg, Transgenic; Th1, T-helper cell type 1; Th2, T-helper cell type 2; VSV, Vesicular stomatitis virus; WHO, World Health Organization
1. Introduction
Since the identification of a novel coronavirus, SARS-CoV-2, as the cause of pneumonia in patients from Wuhan China, a pandemic has erupted, resulting in enormous health care, social and economic disruption to our global society [1]. As of May 17, 2020 there have been 4,708,415 cases and 314,950 deaths worldwide [2]. In rapid response to the pandemic, academic and industry scientists from around the world have initiated efforts to develop vaccines and therapeutics for disease prevention and patient management. The Coalition for Epidemic Preparedness Innovations (CEPI), a global partnership between public, private, philanthropic, and civil organizations, is funding work to develop SARS-CoV-2 vaccines using a variety of technology platforms. Several vaccine candidates are already in Phase 1 studies with others likely to enter the clinic in the next few months [3].
One of the challenges facing rapid vaccine development for SARS-CoV-2 is the need to adequately assure the safety of these vaccines. One such safety concern is disease enhancement syndrome that occurred in the 1960s with inactivated RSV and measles vaccines. Vaccine-mediated disease enhancement is characterized by a vaccine that results in increased disease severity if the subject is later infected by the natural virus. During early trials with inactivated RSV vaccine, the vaccine did not prevent infection, 80% of those infected required hospitalization and two children died [4]. Lung pathology in patients showed an unexpected inflammatory response with both neutrophils and eosinophils, evidence of immune complex formation and complement activation in small airways [5]. Scientists later learned that the vaccine caused a similar disease enhancement in animals characterized by immunopathology and a T helper cell type 2 (Th2) biased response and antibody responses with poor neutralizing activity [6], [7], [8]. Since that time, the animal models have been relied upon to predict safety for new RSV vaccines that are developed. Of note, the pathogenesis of RSV disease enhancement is distinct from antibody disease enhancement (ADE) which occurs for macrophage tropic viruses, demonstrated most notably for Dengue in humans and the coronavirus feline infectious peritonitis virus in cats, and is directly caused by non-neutralizing or sub-neutralizing antibodies leading to more efficient viral uptake via Fcγ receptor binding [9].
Since pathology consistent with the RSV vaccine enhanced disease (and perhaps ADE) has been demonstrated for some SARS-CoV-1 vaccine candidates in animal models, there is also a concern that a similar syndrome could occur in humans immunized with SARS-CoV-2 candidate vaccines. Therefore, CEPI and the Brighton Collaboration Safety Platform for Emergency vACcines (SPEAC) convened a scientific working meeting https://brightoncollaboration.us/brighton-collaboration-cepi-covid-19-web-conference/) on March 12 and 13, 2020 of experts in the field of vaccine immunology and coronaviruses to discuss current knowledge that could form the basis for the assessment of the risk of enhanced disease during SARS-CoV-2 vaccine development. This consensus report presents considerations for vaccine developers and can serve as a guide for the development and testing of vaccine candidates to avoid these safety concerns. Ultimately, the door to clinical trials is controlled by regulators in the context of the risk/benefit for the entire dataset provided by developers and within the local trial context.
2. Animal models of SARS-CoV-1 and MERS CoV
Dr. Kanta Subbarao, director of the WHO Collaborating Centre for Reference and Research on Influenza and Professor in the Department of Microbiology and Immunology at the University of Melbourne, and Dr. Stanley Perlman, Professor in the Departments of Microbiology and Immunology and Pediatrics at the University of Iowa, both reviewed their work and that of others in animal models developed for SARS-CoV-1 and MERS-CoV. The lessons from these models can inform the development priorities for useful SARS-CoV-2 animal models to address both efficacy and safety.
In inbred mouse strains, SARS-CoV-1 replicates efficiently in the respiratory tract and can cause pneumonitis, but clinical signs and pneumonia were only observed in old BALB/c mice [10]. Subsequent passage of SARS-CoV-1 through mouse lungs resulted in the isolation of virus that caused severe disease in both young and old mice [11], [12]. This virus was used in many subsequent studies. Ferret models of SARS-CoV-1 also demonstrate virus replication in respiratory tracts with induction of a neutralizing antibody response but also demonstrated little evidence of clinical disease [13]. Hamsters, in contrast to mice and ferrets, demonstrate high levels of viral replication, develop pneumonitis, and can be shown to have clinical signs of disease [14]. Following the identification of human ACE2 as the receptor for SARS-CoV-1, transgenic murine models expressing human ACE2 receptor (hACE2) were developed and shown to develop mild pulmonary disease. Of note, these mice also developed lethal viral encephalitis, attributed to viral spread through the olfactory nerve, despite the relative scarcity of hACE2 expression in the brain which may have relevance to SARS-CoV-2 disease [15].
Efficacy of several SARS-CoV-1 vaccines was evaluated in these models with spike (S) protein based vaccines demonstrating neutralizing antibody and protection against pulmonary replication of the challenge virus in mice and hamsters [16]. For DNA vaccine studies, it was shown that candidate vaccines encoding the S protein conferred antibody mediated protection from challenge in mice and that vaccines encoding the N protein induced humoral and cellular immunity [17], [18]. For vectored vaccines expressing SARS-CoV-1 proteins, it was shown that viral proteins were expressed in mice, ferrets, and hamsters. In these studies, neutralizing antibodies were elicited by B/HPIV3, VSV, rabies, MVA and adeno viruses expressing S protein, that protected against SARS-CoV-1 replication in lungs of challenged animals. However, one MVA vaccine expressing the S-protein did not protect against infection [16].
In contrast to SARS-CoV-1, inbred mice were found to be resistant to MERS-CoV, thus infection was studied by creating models that expressed the MERS receptor, human DPP4 (hDPP4). Ad5-hDPP4 transduced mice could be infected with MERS virus but infection was associated with minimal clinical disease except in immunocompromised mice that developed weight loss after infection. Of note, hDPP4-transgenic mice developed lethal viral encephalitis with concurrent inflammatory changes on histopathological examination of the lung, similar to hACE2-Tg mice with SARS-CoV-1. Subsequently, investigators developed mice “knocked-in” for expression of hDPP4 and after virus passage in these mice, identified mouse-adapted MERS strains that caused more severe disease and increased histopathology with more pulmonary edema than those infected with the original MERS strain [19]. Importantly, mice without functional T cells, such as RAG1-/- and TCR alpha-/-, had delayed viral clearance whereas mice that could not produce antibodies, muMT mice, did not show delay in clearance. Similar models were developed by CRISPR/Cas9 mutagenesis of two residues in the mouse ACE2 molecule, followed by mouse adaptation with serial passage, leading to an ARDS model of lethal infection [20], [21]. Taken together this evidence supports the notion that T cells are important in viral clearance for MERS [22].
Non-human primate (NHP) models have also been established for both SARS-CoV-1 and MERS-CoV. There was evidence of upper respiratory and lower respiratory tract SARS-CoV-1 replication in African green monkeys to a greater extent than in cynomolgus macaques, and least in rhesus macaques, with little evidence of clinical disease in all three species [23]. Of note, consistent with findings in older humans and mice, increased pathology has been documented in aged cynomolgus macaques with SARS-CoV-1 wild type infection [24]. There is some controversy on the disease severity in the MERS models with different groups seeing different levels of pathology. This has not been resolved [25], [26].
3. Enhanced disease following SARS-CoV-1 vaccines
Both vaccine efficacy and safety have been studied in animal models with many SARS-CoV-1 candidate vaccines. The group of experts discussed how the vaccine models were utilized to characterize the response of specific vaccines and to examine both disease enhancement and antibody dependent enhancement (ADE) signals.
There is evidence for disease enhancement in vaccinated animals after challenge with live virus in multiple studies with SARS–CoV-1 vaccine candidates as summarized in Table 1 . We are limiting our comments in this report to data in animal models and not discussing in vitro data except to mention that there is some evidence of ADE in human primary monocytes [27], [28]. Different animal models exhibit different pulmonary pathology but generally are characterized by cellular infiltrates including eosinophils. In this summary, we provide an overview of the consensus opinion on vaccine related outcomes in animal models that were of concern for risk of disease enhancement and could guide assessments of SARS-CoV-2 vaccine candidates.
Table 1.
Evidence of enhanced disease in SARS-CoV-1 vaccine candidates.
| Animal Model | Vaccine | Adjuvant | Immunopathology | Reference |
|---|---|---|---|---|
| Murine1 | VEE Replicon Particles expressing N protein | – | YES | Deming 2006 |
| Murine2 | Recombinant Vaccinia virus expressing N protein | – | YES | Yasui 2008 |
| Murine3 | Inactivated Whole Virus | Alum | YES | Bolles 2011 |
| – | YES | |||
| Murine4 | Replicon Particles expressing S protein | – | YES | Sheahan 2011 |
| Murine5 | Inactivated Whole Virus and S protein vaccines | Alum | YES | Tseng 2012 |
| – | YES | |||
| Ferret6 | Recombinant Modified Vaccinia Virus Ankara (rMVA) expressing S protein | – | YES† | Weingartl 2004 |
| NHP7 | Modified Vaccinia Ankara (MVA) virus encoding full-length S protein | – | YES | Liu 2019 |
| Passive anti-S sera | N/A | YES | ||
| NHP7 | Inactivated Whole Virus | – | YES | Wang 2016/2020 |
| Passive Human SARS Antiserum | N/A | YES |
Young and senescent female BALB/c mice.
BALB/c mice.
Aged BALB/c mice.
Young and aged BALB/c mice.
Female BALB/c mice.
Mustela putorius furo, castrated males.
Chinese rhesus macaque.
Acute hepatitis.
In murine models, evidence for vaccine related disease enhancement has been demonstrated for inactivated whole vaccine (with and without alum), vectored vaccine expressing N protein (but not seen with vectored vaccine expressing S protein in same report), a replicon particle platform expressing S protein, and a vectored vaccine expressing S proteins. In general, the pathology described included pulmonary infiltrates often with eosinophils observed. Th2 dominant responses were documented in some reports by expression of Th2 driven cytokines [29], [30], [31], [32], [33]. In a ferret model, hepatitis was demonstrated in animals vaccinated with a recombinant modified vaccinia virus Ankara vaccine expressing S protein and then challenged with virus [34] although questions have been raised about this study [35].
Of note, mouse models have also shown evidence of enhanced disease for inactivated and recombinant adenovirus 5-based MERS-CoV vaccine [36], [37].
Non-human primate models have also produced evidence of enhanced disease after SARS-CoV-1 vaccine immunization. Chinese macaques immunized with a modified vaccinia virus expressing S protein then challenged with SARS-CoV-1 did not develop clinical disease, but histopathology showed lung injury. This injury was characterized by decreased wound healing, and increased pro-inflammatory macrophages expressing IL-6, IL-8, and CCL2 [38]. This report also demonstrated that passively administered anti-S antibody was associated with lung pathology after challenge with the live virus although the mechanism may not be through Fc receptor and thus not classic “ADE”. Of note, a second report similarly demonstrates the effect with certain anti-S antibody preparations and without Fc involvement [39], [40]. The relevance of these reports remains unclear as there are multiple studies with administration of neutralizing monoclonal antibodies to different models that did not induce disease enhancement. Other investigators have reported absence of disease enhancement in both hamsters and monkeys immunized with a whole inactivated vaccine although these models differed in a number of ways, most notably by the use of BPL (β-Propiolactone) instead of formalin for inactivation of the virus [41], [42]. Finally, we note that there has not been an agreed upon positive control applied in these animal studies and thus interpretations are hampered.
4. SARS-CoV-2 murine and NHP models newly developed
Animal models with SARS-CoV-2 are being rapidly developed by multiple research groups. Dr. Qin Chuan, Professor and Director of the Institute of Laboratory Animal Science, Comparative Medicine Center of the Peking Union Medical College presented data on SARS-CoV-2 infection in both transgenic mice and rhesus macaque models.
Human ACE2 transgenic mice (hACE2 Tg) aged 4–6 weeks and 6–11 months of age were studied and hACE2 expression was observed in lung, heart, kidney and intestinal tissues. Following intranasal inoculation with SARS-CoV-2, weight loss was observed, and viral RNA was detected in the lungs as well as in the intestine [43].
Gross pathology demonstrated swollen and enlarged lungs with moderate interstitial pneumonia. Histological studies documented an accumulation of inflammatory cells including monocytes and lymphocytes in alveolar interstitium, with thickening of alveolar walls. SARS-CoV-2 S protein was detected by IHC in alveolar macrophages and epithelia [43].
NHP were also infected with SARS-CoV-2 with 3 rhesus macaques aged 3–4 years inoculated intratracheally and although no fever was observed, weight loss and asthenia were seen on multiple days. Viral RNA was detected from nasal and throat swabs and to a lesser degree in anal specimens, peaking on days 3 to 7 and lasting until day 11 post infection. One animal was euthanized on day 7 for necropsy and viral RNA was detected in multiple organs including CNS, skeletal muscle and heart. For the two surviving rhesus macaques, positive neutralization titers were documented by day 11 post infection. There was radiographic evidence of multiple ground glass opacities in the lungs on days 3, 5 and 7 post infection. Microscopically the lung lesions represented an acute interstitial pneumonia characterized by mild to moderate thickening of alveolar septum, increased number of macrophages, degeneration of pneumocytes and an inflammatory cell infiltration. Presence of viral antigen was confirmed, predominately in alveolar monocytes and macrophages [44]. Analysis of blood samples showed a decline in counts of total white blood cells, lymphocytes and monocytes with no observed changes in percentages. A decrease in both CD3 + CD4 + and CD3 + CD8 + T-cell counts was observed. Importantly, these hematological findings are similar to those seen in SARS-CoV-2 infected patients.
This model could serve as a critical tool for detailed studies of pathogenesis and the evaluation of intervention strategies including vaccines. Of note, following the meeting another group has confirmed SARS-CoV-2 infection in rhesus macaques with viral antigen detected in type I and type II pneumocytes and diffuse pulmonary alveolar damage noted [45]. Experts agreed that these models and others under development should be utilized to evaluate vaccine candidates for any evidence of disease enhancement as specified in later sections.
5. COVID-19 vaccine design considerations for efficacy and safety
5.1. Structure and function of S glycoproteins in coronavirus
Design of safe and effective COVID-19 vaccines can be informed by knowledge of previous coronavirus vaccine development activities and shared elements of viral pathogenesis for non-coronaviruses such as RSV. Specific epitope targets for potent neutralizing antibody, platforms for inducing both neutralizing antibody and effective T cell responses, and adjuvants for improving immunogenicity were presented at the conference. We review first the structure and function of the major target of COVID-19 vaccines, spike (S) glycoprotein.
Ralph Baric PhD, Professor in the Department of Epidemiology at the University of North Carolina Chapel Hill School of Medicine presented a review of the structure and function of coronavirus (CoV) S glycoprotein highlighting priorities for the development of vaccine and immune therapeutics. There is a long history of emerging CoVs with acceleration of cross-species movement and emergence of highly pathological strains in the last 16 years, including SARS-CoV-1, MERS-CoV, and SARS-CoV-2, and this trend is likely to increase in the future. Phylogenetic relationships within CoVs have been established, and Group 2B includes SARS-CoV-1 and SARS-like CoVs including SARS-CoV-2, BtCoV WIV1 and BtCoV SHC014. Similarly, Group 2C are MERS-like CoVs which are also poised for human emergence. Within Group 2B, known SARS-like CoVs are divided into high or low pre-epidemic potential. High risk features include use of ACE2 for cell entry, growth in primary human airway cells, causing ARDS, causing age-related disease severity, and escape from existing immune therapeutics. Drivers of CoV evolution include the high mutation rate of the RNA-dependent RNA polymerase paired with the regulated fidelity complex. CoVs also demonstrate high rates of RNA recombination as during mixed infection up to 25% of progeny are recombinant, and modular evolution allows CoVs to swap whole genes or portions of key proteins between strains. The S protein itself, which regulates host range, tissue tropism, and transmissibility, can tolerate a high mutation rate while retaining its function.
The organization of the SARS-CoV-2 genome has been elucidated and SARS-CoV-2, like SARS-CoV-1, has been shown to use hACE2 for cell entry. Group 2B viruses have fourteen contact interfaces between their S protein and ACE2. Variation across the interface sites can facilitate orthologous species ACE2 receptor usage, since as few as seven interface sites are needed for entry. The prefusion structure of the S glycoprotein has three major antigenic domains, receptor binding domain (RBD), N terminal domain (NTD), and S2. Epitopes on SARS-CoV-1 RBD have been identified as targets for neutralizing antibodies. Analyzing the variations and conserved regions in the S protein of Group 2B SARS-like CoVs, shows conserved sites on the S2 region that could be targeted in broad-based therapeutics against multiple CoVs.
Dr. Baric stressed that there is a large reservoir of SARS-like and MERS-like CoVs poised for emergence in humans. Two priorities are immediate vaccine candidates specific for SARS-CoV-2 and development of broad-based vaccines protective against antigenically distinct CoVs destined to emerge in the future. Key priorities for the development of a SARS-CoV-2 vaccine include characterization the SARS-CoV-2 neutralizing epitope map, identification of broadly cross-reactive neutralizing epitopes, identification of putative enhancing epitopes that might potentiate disease in vivo, identification of key T cell epitopes across outbred populations, and determination of correlates of protective immunity.
5.2. Preserving neutralization sensitive epitopes on spike proteins
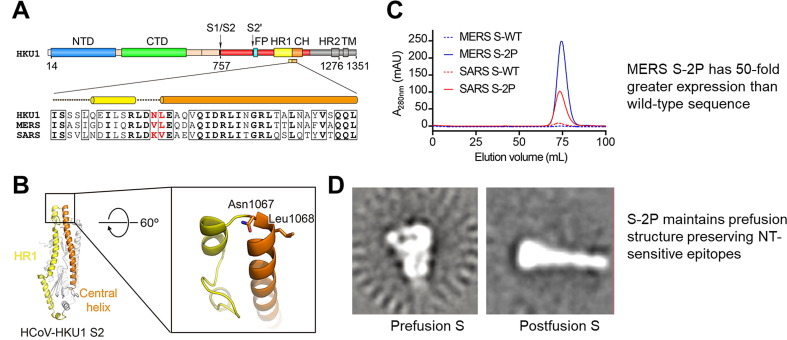
Barney Graham, MD PhD, Deputy Director of the NIH Vaccine Research Center presented data on the immunogenicity and neutralizing efficacy of truncated spike (S) antigens, with a focus on SARS-CoV-2. Class I fusion proteins (such as S protein) are common among enveloped viruses including RSV, parainfluenza viruses, and coronaviruses and have been successfully stabilized in their pre-fusion conformations. This approach has been shown to preserve neutralization-sensitive epitopes, avoid antibodies that are non-neutralizing, and improve expression in transfected cells, thus aiding in manufacturing and immunogenicity of gene-based vectors. The S proteins of SARS-CoV-1 and MERS-CoV have both been successfully stabilized by introducing two proline residues to the top of the central helix, preventing heptad assembly and stabilizing the S2 region and the entire S protein as a result (Fig. 1 ) [46].
Fig. 1.
2P mutation stabilizes MERS and SARS CoV S; improves expression, prefusion structure, and immunogenicity.
The SARS-CoV-2 S protein structure was solved shortly after its emergence and shows similar structure and mobility as the SARS-CoV-1 S [47]. The timing from first knowledge of SARS-CoV-2 to the beginning of the Phase 1 study was a remarkable sixty-five days. The advantages of mRNA vaccines include ability to create a highly precise type of protein to elicit the correct antibodies, to elicit T cell responses that are Th1 predominant, and the rapidity of manufacturing. Of course, disadvantages include the novel nature of both mRNA and DNA vaccines without any licensed vaccine with either technology to date and lack of experience for mass production. Therefore, multiple platforms for SARS-CoV-2 are under development that mitigate against some of the potential disadvantages of nucleic acid vaccines.
6. Effects of adjuvants on immune response and implications for COVID-19 vaccines
Although mRNA and DNA vaccines elicit T cell responses without adjuvants, adjuvants may be important for subunit and whole cell inactivated vaccines to increase their immunogenicity and drive an immune response that could limit the risk of disease enhancement. Multiple SARS-CoV-2 vaccines are in development including vectored vaccines, whole cell inactivated vaccines, and recombinant protein vaccines. The experts discussed how the choice of adjuvants will be important for both efficacy and safety with these platforms.
Dr. Arnaud Didierlaurent from the Centre of Vaccinology at the University of Geneva presented background on the effects of different adjuvants on animal and human immune responses. Several adjuvants are now being used in commercial vaccines or are in clinical development [48]. Oil-in-water emulsions such as MF59 or AS03 have been shown to increase the breadth of the antibody repertoire, binding affinity and affinity maturation when compared to unadjuvanted vaccines [49], [50] In human studies with influenza vaccines, H5N1 vaccine adjuvanted with MF59 (squalene-based emulsion) increased the levels of H5-specific antibody for subclasses IgG1 and IgG3 and the binding to FcγR2 but not to FcγR3 when compared to alum adjuvanted vaccines. This demonstrates that the use of an adjuvant can skew the functionality profile of antigen-specific antibodies, with the potential to activate different innate effectors based on their FcγR expression [51]. Use of squalene-based emulsion vaccines for influenza have also been shown to increase CD4 + T cell response frequencies and cross-reactivity. Even if pre-existing cross-reactive antibodies are present prior to immunization, such adjuvants could activate naïve B cells and promote the adaptability of memory B cells [52], [53], [54], [55].
In addition to antibodies, adjuvants can promote cellular responses. Human malaria challenge studies provided early evidence that the choice of adjuvants (combined with the malaria antigen RTS,S) was critical in achieving optimal protection and highlighted the importance of cellular response [56]. More recently, studies with Hepatitis B Surface Antigen (HBs) vaccine adjuvanted with AS01, AS03, AS04 or alum showed that vaccines formulated with AS01 and AS03 induced the highest antibody levels while AS01 promoted best HBs-specific CD4 T cell response [57]. These differences were associated with the magnitude of the initial inflammatory response triggered by the different adjuvanted formulations [57], [58]. Interestingly, although the level of CD4 T cell response was lower in the alum group compared to the AS01 group, both adjuvants led to similar memory subset profiles and cytokine production profiles (polyfunctionality) and neither induced Th2 cytokines nor a CD8 induced response upon peptide restimulation. This indicates that use of alum may not necessarily lead to Th2 skewing in humans. Recently a number of systems biology studies have revealed that specific early signatures (e.g., interferon-dependent pathways) induced by adjuvanted vaccines are often associated with protective responses [59] but the impact of these early signals on functional features of antibodies and the quality of T cell response are not well established yet.
Although adjuvant selection is best performed in early clinical studies, animal models could be useful in determining the immune profile of adjuvanted vaccines. NHP models are well-established to assess immune responses to vaccination and elicit immune responses in closer parallel to humans than mice. For example, in non-human primates, adjuvant choice affects antibody half-life, antibody glycosylation and antibody binding to FcγRs, indicating effects on both antibody quality and function, like what is observed in humans [60]. When adeno-based vectored vaccines are given to humans or NHPs, both groups develop similar antibody function profiles. Additionally, NHPs and humans tend to show similarities in terms of “ranking” of adjuvants and innate immune pathways triggered by adjuvants. Overall, NHPs could be utilized to evaluate COVID-19 vaccine candidates with and without adjuvants and guide in the selection of vaccines that elicit desired attributes that could reduce the risk of vaccine-mediated enhanced disease.
Given the unprecedented demand for an effective vaccine, the use of adjuvants may be critical for subunit vaccines in providing antigen-dose sparing, increased immunogenicity, breadth and duration of response, potentially eliciting cross-protection against new CoV strains and minimizing the risk of enhanced disease.
7. Consensus considerations on the assessment of the risk of disease enhancement with COVID-19 Vaccines:
Following the presentations, attendees participated in discussion of the suggested consensus statements and all attendees were asked to comment on the draft statements available online. These comments were reviewed and discussed again on the second day of the meeting and resulted in the summary consensus statement that follows.
Murine models for assessment of vaccine-related disease enhancement
-
•
SARS-CoV-2 has a low affinity for murine ACE2 receptor and murine models will require the use of hACE2 transgenic mice, preferably with a ‘knock-in’ approach. Preliminary data indicate the possibility of infecting hACE2 transgenic mice with demonstration of viral replication and mild lung lesions. Mouse adaptation of SARS-CoV-2, as done with SARS-CoV-1, will likely be required to obtain a virus that causes more severe disease in mice. Models that develop acute lung injury with some lethality and that mimic the human condition will be important for evaluating vaccine safety.
-
•
Previous studies from SARS-CoV-1 and MERS-CoV indicated that some vaccines, especially those using whole inactivated virus, could enhance the disease induced in mice challenged with SARS-CoV-1 or MERS-CoV. The lung lesions were highly inflammatory, with a dominance of eosinophil infiltration and occurred in animals despite presence of a neutralizing antibody response and reduced challenge virus replication in the lungs. Such studies have not yet been completed for SARS-CoV2.
-
•
In mice, this immunopathology was considered a consequence of a dominant Th2 type response to the vaccine antigens. It was not seen after including adjuvants (e.g. CpG) in the vaccine or other vaccine formulations known to drive immune responses towards Th1. The timing of challenge after vaccination may be critical. It would be of major interest to explore the outcome following challenge at later timepoints when antibodies are significantly decaying.
-
•
One should be aware of the potential confounding effect of cell-culture excipients in the vaccine and challenge strain material. It is known that impurities such as fetal calf serum in the preclinical vaccine preparation may induce eosinophil influx in any mouse model if the challenge strain also contains the same excipients.
-
•
In these models, characterization of the immune response to the candidate vaccine (e.g., IgG isotypes, Th2 markers) may have some predictive value.
-
•
Other small animal models which can be infected by SARS-CoV-2 can be considered (e.g. ferret, hamster). Development of small animal models of severe disease will also inform studies of vaccine-enhanced disease.
Non-human primate models for assessment of vaccine-mediated enhanced disease
-
•
Non-human primates (NHP) are of primary interest in view of their ACE2 homology with hACE2. Preliminary studies indicate the possibility of inducing some COVID-19 lung pathological features after infection, without clinical signs, in Rhesus macaques. African Green monkeys may be more susceptible to COVID-19, but the model suffers from some limitations (e.g. access, genetic polymorphism).
-
•Previous studies with SARS candidate vaccines have suggested a risk of enhanced pathology in NHPs after viral challenge. Eosinophilic infiltrates were not prominent. The mechanism is still incompletely defined but there is evidence for a role of non-neutralizing antibodies. Non- or incompletely neutralizing antibodies may contribute to:
-
othe formation of pathogenic immune complexes and
-
oFc-mediated viral capture by monocytes/macrophages that may favor excessive T-cell activation and inflammation.
-
o
-
•
Enhanced pathology was seen following passive transfer of IgG from immunized NHPs
General considerations on animal models
-
•
Although existing animal models of COVID-19 imperfectly reproduce the human disease, they appear useful for assessing the risk of disease enhancement. Vaccine responses are closer to human responses in NHPs than in mice. Therefore, it is likely that data obtained from NHP studies are more significant. However, there is an urgent need to standardize the NHP model (read-out of disease enhancement, timing of challenge, age) and to include appropriate controls (i.e., a vaccine that induces enhanced pathology and disease) and a sufficient number of animals to be confident of findings in outbred species. It is important to control for potential co-infection, including with other coronaviruses, in all non-SPF models.
-
•
Potential markers of safety in these animal models could include:
-
o
the relative levels of neutralizing vs non-neutralizing antibodies,
-
o
antibody affinity,
-
o
T-cell response profile,
-
o
quantitative virology in the upper and lower respiratory tract
-
o
characterization of lung histopathology with immunohistochemistry for viral antigen and immune cell markers.
-
•
Passive transfer in NHPs of human antibodies generated during Phase 1 trials, followed by viral challenge could be considered to assess the risk of disease enhancement.
-
•
Challenge of immunized animals with a closely related heterologous CoV strains may assess the risk of enhancement during future outbreaks.
-
•
In case of disease enhancement, in-depth studies in animal models may give some indications on the mechanism of immunopathology. They can inform human trial designers on the critical immunological risk markers to be monitored in Phase 1 trials.
-
•
Based on previous experience with SARS and other viral diseases, it may be useful to evaluate the risk of disease enhancement for COVID-19 vaccines (particularly those including whole virions or N protein) in an established NHP model before advanced clinical development.
During the Vaccine Design session, the group of Experts suggested that consideration should be given to the following:
-
•
Caution should be observed when developing vaccines to avoid inducing predominant Th2 responses and non-neutralizing antibodies.
-
•
Vaccines inducing strong neutralizing antibodies, predominant Th1 responses and balanced CD4/CD8 and polyfunctional T cell responses are less likely to induce immunopathology.
-
•
Given what will be the unprecedented demand for an effective vaccine, the use of adjuvants may be critical for sub-unit vaccines in providing increased immunogenicity, breadth of response, dose sparing, duration of response, potentially cross-protection against new CoV strains, and possibly minimize the risk of enhanced disease. Preference should be given to Th1-driving adjuvants with an established safety profile in humans.
-
•
Understanding the role cross-reacting antibodies from prior coronavirus infections may have on natural disease caused by SARS-CoV-2 or if they influence the risk of enhanced disease following vaccination may inform vaccine design.
-
•
Data are needed on whether antibody waning could increase the risk of enhanced disease on exposure to virus in the long term.
It was the opinion of the Experts that animal data to support clinical development could address:
-
•
Post-vaccination (neutralizing) antibody responses, and T cell analysis to demonstrate a Th1 response.
-
•
Post-vaccination challenge data from NHPs with careful evaluation for immunopathology and quantitative virology in the animals.
-
•
Small animal data may also provide important supporting evidence of safety, and hamster, ferret and mouse models are likely to be available for developers.
-
•
Where possible, immunopathology experiments with a positive control (e.g., formalin inactivated alum-adjuvanted SARS-CoV-1 or SARS-CoV-2 vaccine) and a mock-immunized negative control will provide best guidance. It was felt that it will be important to establish broadly accepted endpoints and scoring systems to allow comparison of various vaccine candidates. WHO is working on this issue.
-
•
For vaccine constructs likely to induce a predominant Th2 response, the group felt that animal studies should be considered before entering human Phase 1 trials in more than one animal species including NHPs where possible. It was noted that the absence of a Th2 response does not eliminate the risk of enhanced disease.
-
•
For vaccine constructs which are already known to induce neutralizing antibody and Th1 responses, it was the consensus of the group that while Phase 1 studies are cautiously proceeding with careful review of safety data, animal studies run in parallel could provide useful information for the further clinical development
-
•
Suggestive data in animal models should not by default prevent clinical development of vaccine candidates; potential risk should be thoroughly evaluated by developers and regulators on a vaccine product-specific basis.
Regarding Phase 1 clinical trials, it was the opinion of the Experts that:
-
•
Since not all studies that have begun or are about to begin will prescreen to determine preimmunization serostatus of participants, although this shall be determined retrospectively, appropriate baseline blood specimens should be obtained and stored. Because the virus is spreading rapidly, such specimens will allow assessment of the immune response in both seronegative and seropositive persons as both are likely to be vaccinated.
-
•
Level of neutralizing antibodies and determination of the relative ratio of binding to neutralizing antibodies will be important to assess the potential risk of enhanced disease. Also, detection of initial priming that includes CD8 T cells and/or a CD4 Th1 biased response is likely to mitigate the risk of disease enhancement. Determination of memory responses will be useful, particularly if SARS-CoV-2 continues to circulate.
-
•
Consideration should be given to the use of post-vaccination sera from vaccinees which could be used for antibody transfer studies in animals to look for enhanced disease and for evidence of cross-protection against other coronaviruses.
-
•
Monitoring for enhanced disease in immunized participants may require longer follow-up than is usual in Phase 1 trials but need not delay Phase 2 trials.
-
•
Investigators on the call requested frequent updating with both preclinical and evolving clinical data that are being developed by the different academic and industrial developers to help in decision-making about the various vaccine clinical trials. Creation of a central information hub was encouraged for this purpose.
-
•
Participants on the call expressed the need for standardization of protocols, data collection forms, critical assays (including reagents) and biobanking of samples from initial clinical trials to allow future re-assay once standards are agreed to and enable comparison of results across trials
Concluding remarks
-
•
The group of Experts considers that the demonstration of some disease enhancement with any candidate vaccine after viral challenge in animal models should not necessarily represent a no-go signal for deciding whether to progress into early trials in clinical development of a COVID-19 vaccine.
-
•
Continuous monitoring of this risk during clinical trials in an epidemic context will be needed.
-
•
Each observed effect should be discussed by the developers with their regulators who will ultimately define the actual requirements for clinical studies.
The considerations in this document should be interpreted as general scientific remarks based on current knowledge to inform a research agenda that could be beneficial for vaccines in development. These considerations are not of a regulatory nature and cannot in any sense replace the need for proper regulatory consultations on the requirements for vaccines clinical trials. Vaccine developers are therefore encouraged to seek individual scientific advice from regulatory authorities.
Disclaimer
The findings, opinions, conclusions, and assertions contained in this document are those of the individual authors. They do not necessarily represent the official positions of any participant’s organization (e.g., government, university, or corporations) and should not be construed to represent any Agency determination or policy.
Declaration of Competing Interest
RB has collaborations with VaxArt, Takeda, Moderna, Eli Lily, and Pfizer. SB is a consultant for GSK on matters unrelated to the topic of this manuscript. CD is a consultant to Medicago on their vaccine programs; her husband owns stock in Dynavax Technologies Corporation. BSG is a named inventor on patent applications related to coronavirus vaccines and monoclonal antibodies. AJP is Chair of UK Dept. Health and Social Care's (DHSC) Joint Committee on Vaccination & Immunisation (JCVI) and is a member of the WHO's SAGE. AJP is an NIHR Senior Investigator. PL, DA, SRA, RTC, AMD, SDM, DM, SP, PAP, CQ, and KS declare no competing financial interests or personal relationships that could have appeared to influence the work reported in this manuscript.
Acknowledgments
Acknowledgement
We acknowledge the financial support provided by the Coalition for Epidemic Preparedness Innovations (CEPI) for our work under a service order entitled Safety Platform for Emergency vACcines (SPEAC) Project with the Brighton Collaboration, a program of the Task Force for Global Health, Decatur, GA, USA. We thank the following persons for their assistance: (1) the ~90 attendees of the meeting from many countries globally who provided invaluable discussion to move the field forward; (2) Angel Honrado and Imanol Urkola of WeDO, Jim Mootrey and Chantal Veira, Lisa Chung, Matt Dudley, and Gabriella Corrigan of the SPEAC/TFGH team for their logistical support; (3) other SPEAC Executive Board (Barbara Law, Wan-Ting Huang, Marc Gurwith, Miriam Sturkenboom) for their oversight and planning.
All authors attest they meet the ICMJE criteria for authorship.
References
- 1.Zhu N. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.COVID-19 Data Center. 2020, Johns Hopkins Coronavirus Resource Center.
- 3.Lurie N. Developing Covid-19 Vaccines at Pandemic Speed. N Engl J Med. 2020 doi: 10.1056/NEJMp2005630. [DOI] [PubMed] [Google Scholar]
- 4.Kim H.W. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89(4):422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 5.Polack F.P. A role for immune complexes in enhanced respiratory syncytial virus disease. J Exp Med. 2002;196(6):859–865. doi: 10.1084/jem.20020781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connors M. Enhanced pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of interleukin-4 (IL-4) and IL-10. J Virol. 1994;68(8):5321–5325. doi: 10.1128/jvi.68.8.5321-5325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connors M. Pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of CD4+ T cells. J Virol. 1992;66(12):7444–7451. doi: 10.1128/jvi.66.12.7444-7451.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delgado M.F. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med. 2009;15(1):34–41. doi: 10.1038/nm.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smatti M.K., Al Thani A.A., Yassine H.M. Viral-Induced Enhanced Disease Illness. Front Microbiol. 2018;9:2991. doi: 10.3389/fmicb.2018.02991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts A. Aged BALB/c mice as a model for increased severity of severe acute respiratory syndrome in elderly humans. J Virol. 2005;79(9):5833–5838. doi: 10.1128/JVI.79.9.5833-5838.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frieman M. Molecular determinants of severe acute respiratory syndrome coronavirus pathogenesis and virulence in young and aged mouse models of human disease. J Virol. 2012;86(2):884–897. doi: 10.1128/JVI.05957-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts A. A mouse-adapted SARS-coronavirus causes disease and mortality in BALB/c mice. PLoS Pathog. 2007;3(1) doi: 10.1371/journal.ppat.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu Y.K. The SARS-CoV ferret model in an infection-challenge study. Virology. 2008;374(1):151–163. doi: 10.1016/j.virol.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts A. Severe acute respiratory syndrome coronavirus infection of golden Syrian hamsters. J Virol. 2005;79(1):503–511. doi: 10.1128/JVI.79.1.503-511.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCray P.B., Jr Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J Virol. 2007;81(2):813–821. doi: 10.1128/JVI.02012-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enjuanes L. Vaccines to prevent severe acute respiratory syndrome coronavirus-induced disease. Virus Res. 2008;133(1):45–62. doi: 10.1016/j.virusres.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Z.Y. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature. 2004;428(6982):561–564. doi: 10.1038/nature02463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim T.W. Generation and characterization of DNA vaccines targeting the nucleocapsid protein of severe acute respiratory syndrome coronavirus. J Virol. 2004;78(9):4638–4645. doi: 10.1128/JVI.78.9.4638-4645.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li K. Mouse-adapted MERS coronavirus causes lethal lung disease in human DPP4 knockin mice. Proc Natl Acad Sci U S A. 2017;114(15):E3119–E3128. doi: 10.1073/pnas.1619109114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cockrell A.S. A mouse model for MERS coronavirus-induced acute respiratory distress syndrome. Nat Microbiol. 2016;2:16226. doi: 10.1038/nmicrobiol.2016.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Douglas M.G. Adaptive evolution influences the infectious dose of MERS-CoV necessary to achieve severe respiratory disease. Virology. 2018;517:98–107. doi: 10.1016/j.virol.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao J. Recovery from the Middle East respiratory syndrome is associated with antibody and T-cell responses. Sci Immunol. 2017;2(14) doi: 10.1126/sciimmunol.aan5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McAuliffe J. Replication of SARS coronavirus administered into the respiratory tract of African Green, rhesus and cynomolgus monkeys. Virology. 2004;330(1):8–15. doi: 10.1016/j.virol.2004.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smits S.L. Exacerbated innate host response to SARS-CoV in aged non-human primates. PLoS Pathog. 2010;6(2) doi: 10.1371/journal.ppat.1000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson R.F. 3B11-N, a monoclonal antibody against MERS-CoV, reduces lung pathology in rhesus monkeys following intratracheal inoculation of MERS-CoV Jordan-n3/2012. Virology. 2016;490:49–58. doi: 10.1016/j.virol.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson R.F. Intratracheal exposure of common marmosets to MERS-CoV Jordan-n3/2012 or MERS-CoV EMC/2012 isolates does not result in lethal disease. Virology. 2015;485:422–430. doi: 10.1016/j.virol.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaume M. Anti-severe acute respiratory syndrome coronavirus spike antibodies trigger infection of human immune cells via a pH- and cysteine protease-independent FcgammaR pathway. J Virol. 2011;85(20):10582–10597. doi: 10.1128/JVI.00671-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yip M.S. Antibody-dependent infection of human macrophages by severe acute respiratory syndrome coronavirus. Virol J. 2014;11:82. doi: 10.1186/1743-422X-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bolles M. A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J Virol. 2011;85(23):12201–12215. doi: 10.1128/JVI.06048-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deming D. Vaccine efficacy in senescent mice challenged with recombinant SARS-CoV bearing epidemic and zoonotic spike variants. PLoS Med. 2006;3(12) doi: 10.1371/journal.pmed.0030525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheahan T. Successful vaccination strategies that protect aged mice from lethal challenge from influenza virus and heterologous severe acute respiratory syndrome coronavirus. J Virol. 2011;85(1):217–230. doi: 10.1128/JVI.01805-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tseng C.T. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS ONE. 2012;7(4) doi: 10.1371/journal.pone.0035421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yasui F. Prior immunization with severe acute respiratory syndrome (SARS)-associated coronavirus (SARS-CoV) nucleocapsid protein causes severe pneumonia in mice infected with SARS-CoV. J Immunol. 2008;181(9):6337–6348. doi: 10.4049/jimmunol.181.9.6337. [DOI] [PubMed] [Google Scholar]
- 34.Weingartl H. Immunization with modified vaccinia virus Ankara-based recombinant vaccine against severe acute respiratory syndrome is associated with enhanced hepatitis in ferrets. J Virol. 2004;78(22):12672–12676. doi: 10.1128/JVI.78.22.12672-12676.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts A. Animal models and antibody assays for evaluating candidate SARS vaccines: summary of a technical meeting 25–26 August 2005, London, UK. Vaccine. 2006;24(49–50):7056–7065. doi: 10.1016/j.vaccine.2006.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agrawal A.S. Immunization with inactivated Middle East Respiratory Syndrome coronavirus vaccine leads to lung immunopathology on challenge with live virus. Hum Vaccin Immunother. 2016;12(9):2351–2356. doi: 10.1080/21645515.2016.1177688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hashem A.M. A Highly Immunogenic, Protective, and Safe Adenovirus-Based Vaccine Expressing Middle East Respiratory Syndrome Coronavirus S1-CD40L Fusion Protein in a Transgenic Human Dipeptidyl Peptidase 4 Mouse Model. J Infect Dis. 2019;220(10):1558–1567. doi: 10.1093/infdis/jiz137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu L. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight. 2019:4(4). doi: 10.1172/jci.insight.123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Q. Immunodominant SARS Coronavirus Epitopes in Humans Elicited both Enhancing and Neutralizing Effects on Infection in Non-human Primates. ACS Infect Dis. 2016;2(5):361–376. doi: 10.1021/acsinfecdis.6b00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Q. Correction: Immunodominant SARS Coronavirus Epitopes in Humans Elicited Both Enhancing and Neutralizing Effects on Infection in Non-human Primates. ACS Infect Dis. 2020 doi: 10.1021/acsinfecdis.0c00148. [DOI] [PubMed] [Google Scholar]
- 41.Qin E. Immunogenicity and protective efficacy in monkeys of purified inactivated Vero-cell SARS vaccine. Vaccine. 2006;24(7):1028–1034. doi: 10.1016/j.vaccine.2005.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberts A. Immunogenicity and protective efficacy in mice and hamsters of a beta-propiolactone inactivated whole virus SARS-CoV vaccine. Viral Immunol. 2010;23(5):509–519. doi: 10.1089/vim.2010.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bao L. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature. 2020 doi: 10.1038/s41586-020-2312-y. [DOI] [PubMed] [Google Scholar]
- 44.Yu P. Age-related rhesus macaque models of COVID-19. Animal Model Exp Med. 2020;3(1):93–97. doi: 10.1002/ame2.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rockx B. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science. 2020 doi: 10.1126/science.abb7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pallesen J. Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc Natl Acad Sci U S A. 2017;114(35):E7348–E7357. doi: 10.1073/pnas.1707304114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wrapp D. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O'Hagan D.T. Towards an evidence based approach for the development of adjuvanted vaccines. Curr Opin Immunol. 2017;47:93–102. doi: 10.1016/j.coi.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 49.Khurana S. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Sci Transl Med. 2011;3(85):p. 85ra48. doi: 10.1126/scitranslmed.3002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khurana S. AS03-adjuvanted H5N1 vaccine promotes antibody diversity and affinity maturation, NAI titers, cross-clade H5N1 neutralization, but not H1N1 cross-subtype neutralization. NPJ Vaccines. 2018;3:40. doi: 10.1038/s41541-018-0076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boudreau C.M. Selective induction of antibody effector functional responses using MF59-adjuvanted vaccination. J Clin Invest. 2020;130(2):662–672. doi: 10.1172/JCI129520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Couch R.B. Superior antigen-specific CD4+ T-cell response with AS03-adjuvantation of a trivalent influenza vaccine in a randomised trial of adults aged 65 and older. BMC Infect Dis. 2014;14:425. doi: 10.1186/1471-2334-14-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Del Giudice G., Rappuoli R., Didierlaurent A.M. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin Immunol. 2018;39:14–21. doi: 10.1016/j.smim.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 54.Galson J.D. Investigating the effect of AS03 adjuvant on the plasma cell repertoire following pH1N1 influenza vaccination. Sci Rep. 2016;6:37229. doi: 10.1038/srep37229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moris P. H5N1 influenza vaccine formulated with AS03 A induces strong cross-reactive and polyfunctional CD4 T-cell responses. J Clin Immunol. 2011;31(3):443–454. doi: 10.1007/s10875-010-9490-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun P. Protective immunity induced with malaria vaccine, RTS, S, is linked to Plasmodium falciparum circumsporozoite protein-specific CD4+ and CD8+ T cells producing IFN-gamma. J Immunol. 2003;171(12):6961–6967. doi: 10.4049/jimmunol.171.12.6961. [DOI] [PubMed] [Google Scholar]
- 57.Leroux-Roels G. Impact of adjuvants on CD4(+) T cell and B cell responses to a protein antigen vaccine: Results from a phase II, randomized, multicenter trial. Clin Immunol. 2016;169:16–27. doi: 10.1016/j.clim.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 58.Burny W. Different Adjuvants Induce Common Innate Pathways That Are Associated with Enhanced Adaptive Responses against a Model Antigen in Humans. Front Immunol. 2017;8:943. doi: 10.3389/fimmu.2017.00943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harandi A.M. Systems analysis of human vaccine adjuvants. Semin Immunol. 2018;39:30–34. doi: 10.1016/j.smim.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 60.Francica J.R. Innate transcriptional effects by adjuvants on the magnitude, quality, and durability of HIV envelope responses in NHPs. Blood Adv. 2017;1(25):2329–2342. doi: 10.1182/bloodadvances.2017011411. [DOI] [PMC free article] [PubMed] [Google Scholar]