COVID-19 pandemics has shocked our world in a few months, not only by attacking individual health, but also public health and economic systems, the way people relate to each other, but has also changed scientific and editorial practices.
By now, more than 4.7 million persons have been infected by SARS COV 2 virus, and more than 315,000 have died worldwide. As there is no vaccine to prevent the disease, or a specific therapeutic drug to treat patients, health care systems treat the sick with supportive measures, hoping that each persons’ immunologic system can confront the disease. But the rush of scientists to quickly understand the virus and its behavior, and to design proper prevention and therapeutical interventions must not sacrifice rigorous science, as vital decisions must be taken daily not only by health care workers but also by national policymakers.
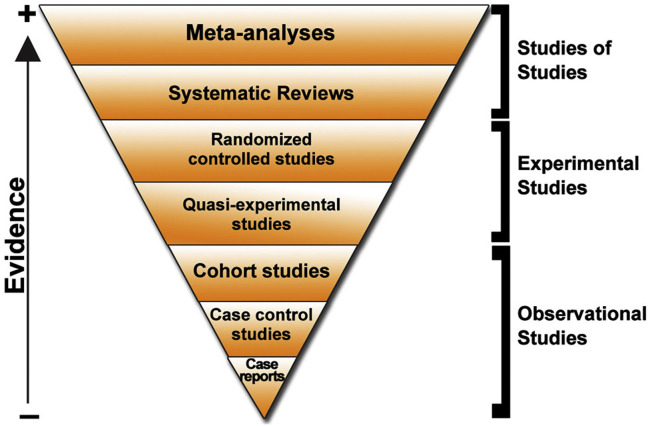
Clinical research and Evidence based medicine have been the tools by which physicians and public health policymakers take informed decisions. Both strategies follow strict rules in order to make strong scientific observations and recommendations. Retrospective analysis of uncontrolled clinical experience often leads to erroneous conclusions about the efficacy of a treatment. Thus, solid scientific conclusions must be derived from randomized controlled studies. Furthermore, systematic reviews and meta-analysis confirm valuable findings. Validity then, in therapeutic trials, depends on the power of the methods and the degree in which they can be generalized in clinical settings (Figure 1 ).
Figure 1.
Type of studies and force of evidence.
Evidence based medicine refers to the process of systematically reviewing, appraising and using clinical research findings to deliver optimal clinical care to patients. The combination of principles and methods ensure that medical decisions, guidelines and policies are based on the current best evidence.
COVID-19 has quickly spread globally, causing countries health systems to collapse due to the great number of simultaneous patients with moderate and severe disease. Daily, doctors and administrators must urgently decide on the best treatment or recommendation in the field of public health, with very scarce information, as it is a new disease (1). The possibility of making mistakesincreases. For example, treatments based on what is known about the pathogenesis of the disease led very early to point out that the use of steroids should not be recommended due to the possibility of disease spreading. However, the role of cytokine storm as a complication was later identified and the use of steroids is now known to improve patients' conditions and prevent the use of ventilators (2).
From the ethical point of view, it is considered that, given the imminent possibility of a patient's death and the lack of proof that a treatment is useful, but having the possibility that it will produce some benefit, treatment should be offered. The problem is that it may become routine to treat in that way without having clear evidence of its benefit or even exposing patients to unnecessary risk. Furthermore, many of these studies are either not properly reviewed in a research ethics committee or are poorly designed (3).
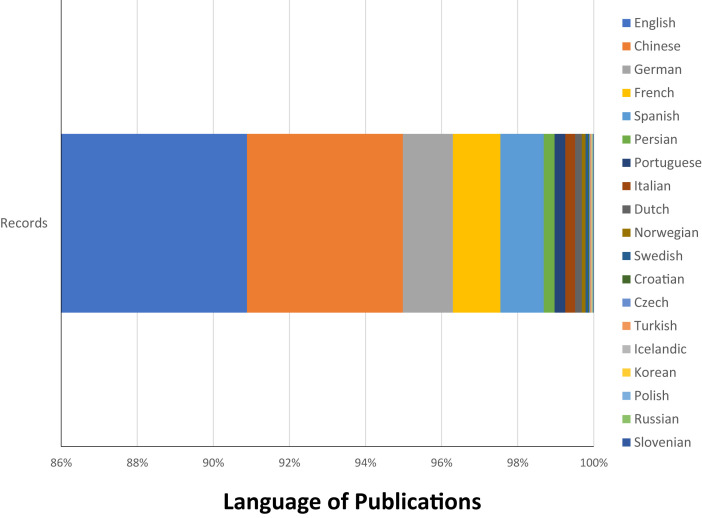
Scientists have relentlessly rushed to analyze information, but the strongest evidence flows very slowly. In the past few weeks, we have seen an exponential growth of publications related to COVID-19. Torres-Salinas D, (4) recently reported 9,435 documents retrieved by Dimensions by April 8th, 2020, with an exponential growth (R2) of 0.92, with more than 500 new documents published daily. An analysis by our team revealed 9,381 documents in Scopus and 3,697 by Web of Science by April 30th, 2020 (Table 1 ). More than 90% of the published information is in English language, followed by Chinese, German, French and Spanish (Figure 2 ). The journals with the highest publication of COVID-19 articles are: British Medical Journal, The Lancet, Journal of Medical Virology, and Nature.
Table 1.
Published documents in Web of Science (WoS)/Scopus by April 30th, 2020
| WoS |
Scopus |
||||
|---|---|---|---|---|---|
| No. | No. | No. | |||
| General Internal Medicine | 717 | Orthopedics | 28 | Arts and Humanities | 23 |
| Public Environmental Occupational Health | 167 | ObstetricsGynecology | 27 | Biochemistry, Genetics and Molecular Biology | 662 |
| InfectiousDiseases | 164 | Dentistry Oral Surgery Medicine | 26 | Business, Management and Accounting | 41 |
| Virology | 163 | Business Economics | 25 | ChemicalEngineering | 30 |
| Science, Technology, OtherTopics | 138 | Psychology | 25 | Chemistry | 38 |
| Microbiology | 109 | Social Sciences Other Topics | 23 | Dentistry | 80 |
| Research Experimental Medicine | 107 | Biomedical Social Sciences | 21 | Earth and PlanetarySciences | 9 |
| Biochemistry Molecular Biology | 101 | Nursing | 21 | Energy | 6 |
| Immunology | 96 | UrologyNephrology | 21 | Engineering | 60 |
| Radiology Nuclear Medicine Medical Imaging | 91 | Tropicla Medicine | 20 | EnvironmentalScience | 159 |
| Surgery | 91 | NutritionDietetics | 17 | HealthProfessions | 152 |
| PharmacologyPharmacy | 84 | Transplantation | 17 | Immunology and Microbiology | 778 |
| Oncology | 77 | Rheumatology | 14 | MaterialsScience | 32 |
| Cardiovascular systemCardiology | 75 | GovernmentLaw | 13 | Mathematics | 25 |
| CellBiology | 74 | Medical Informatics | 13 | Medicine | 6044 |
| VeterinaySciences | 66 | Parasitology | 13 | Multidisciplinary | 176 |
| Engineering | 64 | Biophysics | 12 | Neuroscience | 216 |
| Pediatrics | 64 | IntegrativeComplementary Medicine | 12 | Nursing | 223 |
| Chemistry | 59 | Material Science | 11 | Pharmacology, Toxicology and Pharmaceutics | 271 |
| HealthCareSciencesServices | 55 | Mathematical Computational Biology | 11 | Physics and Astronomy | 32 |
| NeurosciencesNeurology | 51 | Anthropology | 10 | Social Sciences | 207 |
| RespiratorySystem | 49 | ComputerScience | 10 | Veterinary | 92 |
| Anesthesiology | 48 | Education Educational Research | 10 | Undefined | 25 |
| Dermatology | 41 | GeneticsHeredity | 10 | ||
| Life Sciences Biomedicine Other Topics | 39 | Allergy | 9 | ||
| Hematology | 38 | Mathematics | 9 | ||
| Emergency Medicine | 37 | Medical Laboratory Technology | 9 | ||
| GastroenterologyHepatology | 35 | Sport Sciences | 9 | ||
| Ophthalmology | 35 | Pathology | 8 | ||
| Otorhinolaryngology | 35 | Rehabilitation | 8 | ||
| EnvironmentalSciencesEcology | 32 | Medical Ethics | 7 | ||
| Psychiatry | 32 | Substance Abuse | 7 | ||
| Biotechnology Applied Microbiology | 30 | Agriculture | 6 | ||
| GeriatricsGerontology | 29 | Food Science Technology | 6 | ||
| EndocrinologyMetabolism | 28 | Other | 88 | ||
Retrieval of information strategy: (coronavirus) OR (SARS-CoV-2) OR (2019-nCoV) OR (novel coronavirus) OR (Coronavirus disease-19). Subcategories “Psychology”, “Agricultural and Biological Science”, “Computer Science and Decision Sciences” and “Economics”, Econometrics and finance” were excluded.
Figure 2.
Language of published documents.
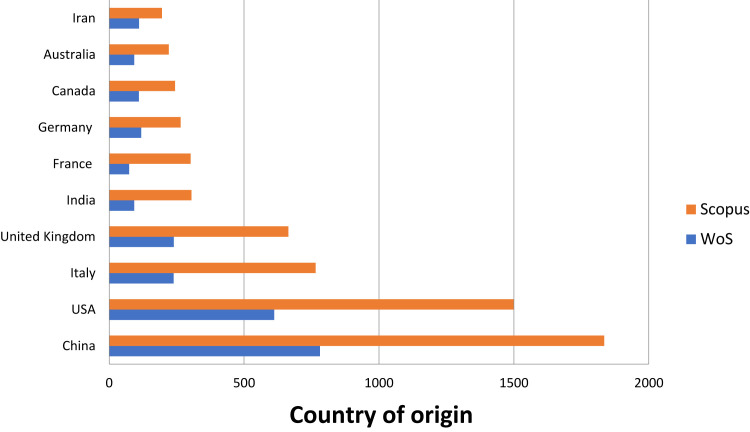
The majority of the publications have been originated in China and the United States of America, followed by Italy, United Kingdom and India (Figure 3 ).
Figure 3.
Country of origin of publication.
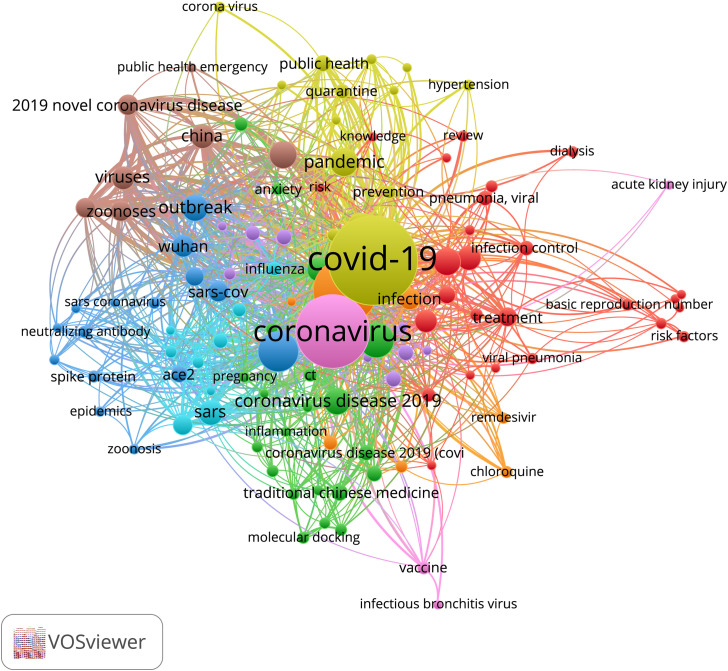
Co-occurrence of keywords for published articles shows that “COVID-19”, “Coronavirus”, “Pandemic”, “Outbreak”, “Wuhan”, “Coronavirus disease 2019”, “Viruses”, “China” are the most frequent. Co-occurrence is an indicator of semantic proximity in which it is observed that the keywords have coincidences between the analyzed documents (larger clusters). (Figure 4 ).
Figure 4.
Co-occurrence of keywords.
Funding opportunities for COVID-19 research projects have appeared worldwide, and many free COVID-19 resource centers have been created in order to bring information to scientists and health care workers: For example, Elsevier https://www.elsevier.com/connect/coronavirus-information-center and The Lancet https://www.thelancet.com/coronavirus?dgcid=kr_pop-up_tlcoronavirus20, among many others.
But does all this information add up to our knowledge of the disease? Glasziou PP, et al. published, in a very recent editorial in BMJ (5), their concern about the quality of the research that is being done and published, principally related to low quality of trials (low sample size, non-randomization or patients, poor outcome measures, etc.), repeated trials and poor reporting. Measurement errors are increasingly evident due to the lack of sensitivity and specificity of tests to diagnose SARS-CoV-2, either by molecular biology or by antibody measurements, and possible confusion biases generated by a lack of control of all the potential variables that can influence the results in most studies.
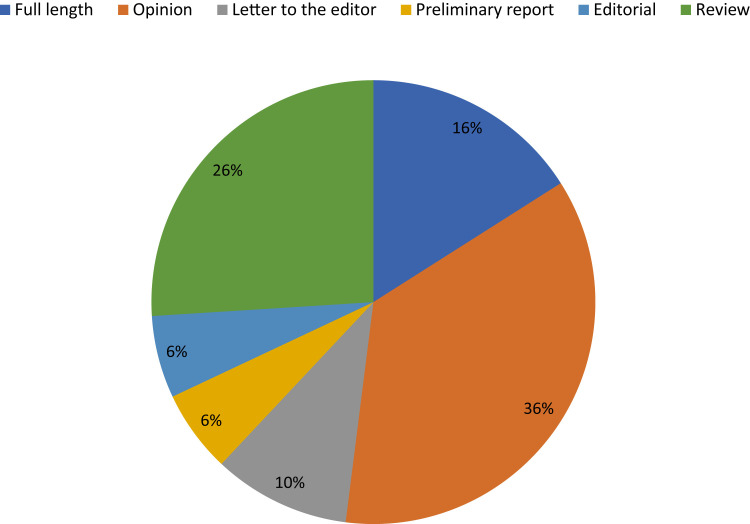
A big problem with what is being published is the lack of original findings, as almost half correspond to editorials, opinions, letter to the editor, commentaries, news, proceedings/conference or data paper, book chapter, short survey or reprint (Figure 5 ).
Figure 5.
Type of publication (Scopus outside circle, WoS inside circle).
Unfortunately, another problem that cannot be neglected is that a lot of information is supported by the pharmaceutical industry. There is a clear intention to help, but it is known that it is an unfair race, where some powerful companies that have a greater potential to disseminate the results of a study, which favor their products, and on the other hand, smaller companies find it more difficult to get their information properly and quickly to users (6).
Publication during the pandemic has also become complicated as Scientific journals have had to adapt to manage regular submissions along with an increasing amount of manuscripts related to COVID-19, in many cases with a shortage in personnel and a shortage of experts available for peer review, as many of them are attending doctors in COVID hospitals.
The flaws of peer review, slow traditional publication times, and the urgent need to share information have led to the rise of Pre prints, (manuscripts submitted to publicly accessible repositories, which may or may not be later submitted to a formal Scientific Journal). COVID-19 has promoted the use of repositories such as BioRxiv and MedRxiv to make communication more agile, open and accessible. Outbreak Science Rapid PREreview, an open-source platform for rapid review of preprints related to COVID-19 (7) has been recently created.
But the quality and scientific robustness of some of these articles has led to further retraction of papers, (like the one posted on BioRxiv in late January claiming that the similarities between SARS COV 2 coronavirus and HIV-1 were “unlikely to be fortuitous in Nature”, which led to a conspiracy theory that the virus was a man-made bioweapon, and was later retracted after thousands of scientists cleared out that “although there were some genetic similarities between the two viruses, these similarities are shared by many other viruses as well”), creating confusion among information consumers. Also, a problem is that dissemination of the information occurs as if they were final results accepted by the scientific community.
Editorial processes have been modified in response to COVID-19 pandemic, for SARSCoV2 papers and for regular submissions. The Journal of Clinical Investigation (8), Cell systems (9) and eLife (10), for example, relaxed some of their policies on regular submissions, as many laboratories have closed or established social distancing policies during the pandemic, and will allow authors more flexible times to respond to reviewers, flexible times for reviewers, or curtail requests for additional experiments. For COVID-19 papers, many journals offer expedite peer review.
Archives of Medical Research has adapted to this crisis by speeding up editorial processes for COVID-19 manuscripts. Editors daily analyze newly arrived documents and make a first editorial decision. Daily follow up of “in process” manuscripts are done and peer reviewers are urged to speed their analysis. “Accepted for publication” reviews and original research (Biomedical, Clinical or Epidemiological) are all peer-reviewed, as we are engaged with maintaining our editorial quality. We know peer review is not perfect, but is still better than the alternatives.
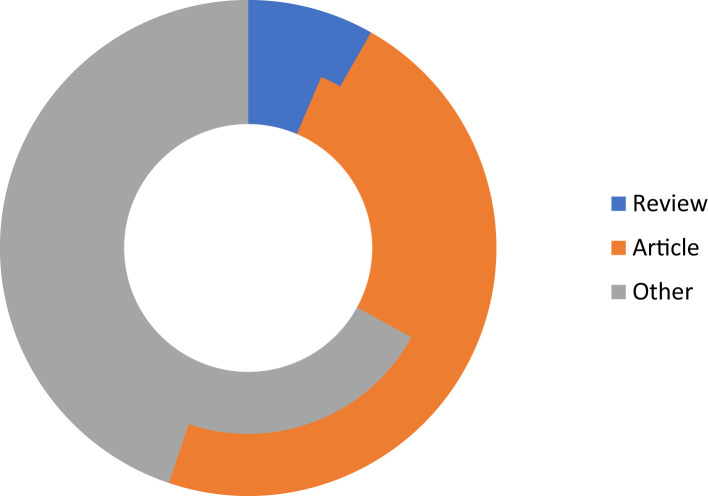
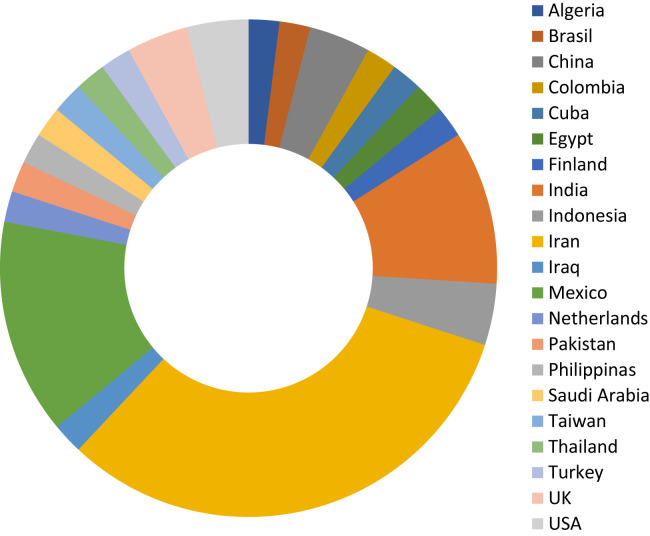
We want to thank the scientific community as an increasing amount of manuscripts have arrived to our Journal in the last two months, from countries worldwide (Figure 6 ), and we countersign our commitment to fulfill our authors and our readers expectations. As to April 30th, 2020, one third of the incoming manuscripts were accepted and sent to on-line publication (average of 4.3 d), and 14% are still under peer review. Figure 7 shows the type of articles that have been received.
Figure 6.
Country of origin of COVID-19 manuscripts received in Archives of Medical Research (up to April 30th, 2020).
Figure 7.
Type of article related to COVID-19 received in Archives of Medical Research (up to April 30th, 2020).
In a scenario like the one that we are experiencing nowadays, the responsibility of all actors to ensure that the published information is useful is very important (11). Researchers must reflect on their responsibility and remember that, although we are experiencing an emergency, there must be robust scientific results. This is a good time to search for the interaction between the need to do (treat patients) and the need to learn (try treatments) (12).
Universities, Institutions, Hospital Centers where the research studies are being carried out must supervise that the projects are being properly evaluated by the research and ethics committees and not be carried away by the pressure of who should publish first, but who is doing better research, and whose evidence will be more useful to patients. Financial institutions that support research, in addition to guaranteeing that the research carried out complies with all the appropriate ethical and methodological requirements, must avoid duplication of information and over investing. Open and accessible databases must be generated in different languages for researcherś consultation. Priority should be given to research that has the greatest application in the shortest term.
Journals must continue to ensure that published articles comply to methodological and ethical quality standards, and have no conflict of interest. Impartiality, transparency, objectivity and confidentiality must always be observed. The “urge to publish” must never prevail over good editorial practices.
(ARCMED_2020_770)
References
- 1.Zhai M.Z., Lye C.T., Kesselheim A.S. Need for transparency and reliable evidence in emergency use authorizations for coronavirus disease 2019 (COVID-19) Therapies. JAMA Intern Med. 2020:E1–E2. doi: 10.1001/jamainternmed.2020.2402. [DOI] [PubMed] [Google Scholar]
- 2.Sanders J.M., Monogue M.L., Jodlowski T.Z. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323:1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 3.Goodman J.L., Borio L. Finding effective treatments for COVID-19: scientific integrity and public confidence in a time of crisis. JAMA. 2020;323:1899–1900. doi: 10.1001/jama.2020.6434. [DOI] [PubMed] [Google Scholar]
- 4.Torres-Salinas D. Vol. 29. 2020. p. e290215. (Ritmo de crecimiento diario de la producción científica sobre Covid-19. Análisis en bases de datos y repositorios en acceso abierto. El profesional de la información). [DOI] [Google Scholar]
- 5.Glasziou P.P., Sanders S., Hoffmann T. Waste in COVID-19 research. BMJ. 2020;369:m1847. doi: 10.1136/bmj.m1847. [DOI] [PubMed] [Google Scholar]
- 6.Bero L.A. Producing independent, systematic review evidence: cochrane's response to COVID-19. Am J Public Health. 2020:e1–e2. doi: 10.2105/AJPH.2020.305734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johansson M., Saderi D. Fast peer review for COVID 19 preprints. Nature. 2020;579:29. doi: 10.1038/d41586-020-00613-4. [DOI] [PubMed] [Google Scholar]
- 8.Ahima R., Jackson S., Casadervall A. Changing the editorial process at JCI and JCI Insight in response to COVID-19 pandemic. J Clin Invest. 2020;130:2147. doi: 10.1172/JCI138305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Justman Q. Keeping the wheels of the scientific endeavor turning during the COVID-19 Pandemic. Cell Syst. 2020;10:307. doi: 10.1016/j.cels.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisen M., Akhmanova A., Behrens T. Publishing in the time of COVID 19. eLife. 2020;9:e57162. doi: 10.7554/eLife.57162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ioannidis J., Greenland S., Hlatky M. Increasing value and reducing waste in research design, conduct, and analysis. Lancet. 2014;383:166–175. doi: 10.1016/S0140-6736(13)62227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angus D.C. Optimizing the trade-off between learning and doing in a pandemic. JAMA. 2020;323:1895–1896. doi: 10.1001/jama.2020.4984. [DOI] [PubMed] [Google Scholar]