Abstract
Purpose
Chronic obstructive pulmonary disease (COPD) is one of the leading causes of death worldwide. Impaired lung function is associated with heightened risk for death, cardiovascular events, and COPD exacerbations. However, it is unclear if forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) differ in predictive value.
Patients and Methods
Data from 16,485 participants in the Study to Understand Mortality and Morbidity (SUMMIT) in COPD were analyzed. Patients were grouped into quintiles for each lung function parameter (FEV1 %predicted, FVC %predicted, FEV1/FVC). The four highest quintiles (Q2–Q5) were compared to the lowest (Q1) to assess their relationship with all-cause mortality, cardiovascular events, and moderate-to-severe and severe exacerbations. Cox-regression was used, adjusted for age, sex, ethnicity, body-mass index, smoking status, previous exacerbations, cardiovascular disease, treatment, and modified Medical Research Council dyspnea score.
Results
Compared to Q1 (<53.5% FEV1 predicted), increasing FEV1 quintiles (Q2 53.5–457.5% predicted, Q3 57.5–461.6% predicted, Q4 61.6–465.8% predicted, and Q5 ≥65.8%) were all associated with significantly decreased all-cause mortality (20% (4–34%), 28% (13–40%), 23% (7–36%), and 30% (15–42%) risk reduction, respectively). In contrast, a significant risk reduction (21% (4–35%)) was seen only between Q1 and Q5 quintiles of FVC. Neither FEV1 nor FVC was associated with cardiovascular risk. Increased FEV1 and FEV1/FVC quintiles were also associated with the reduction of moderate-to-severe and severe exacerbations while, surprisingly, the highest FVC quintile was related to the heightened exacerbation risk (28% (8–52%) risk increase).
Conclusion
Our results suggest that FEV1 is a stronger predictor for all-cause mortality than FVC in moderate COPD patients with heightened cardiovascular risk and that subjects with moderate COPD have very different risks.
Keywords: airflow limitation, cardiovascular risk, exacerbation, lung function, lung volumes, death rate
Introduction
Chronic obstructive pulmonary disease (COPD) is a common, progressive disorder of the airways and lung parenchyma and is the fourth leading cause of death.1 Clinical variables that predict mortality are important for identifying patients at highest risk and include lung function, exacerbation burden and comorbidities.1–4 Interestingly, when comparing mortality risk based purely on lung function and the symptoms-exacerbation risk-based Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2013 COPD classification, lung function served as a better predictor.5
It has long been debated whether forced expiratory volume in one second (FEV1) or forced vital capacity (FVC) is the best physiological prognostic measure and if the relationship between FEV1 and mortality is due to airflow limitation or low lung volumes. Analyzing the 7489 participants in the general population in the Atherosclerosis Risk in Communities study, Burney & Hooper concluded that the overall mortality was more strongly related to FVC than to FEV1;6 this was supported by a post hoc analysis of the Burden of Obstructive Lung Disease study reporting that the national COPD related mortality was more strongly associated with the prevalence of spirometric restriction than obstruction.7 In contrast, other analyses, such as the Normative Aging Study concluded that FEV1 is more strongly related to mortality than FVC in a general population.8
Cardiovascular disease (CVD) frequently accompanies COPD.9,10 The close relationship is due to common etiologies (i.e., aging, smoking), increased systemic inflammation, hypoxemia, and increased pulmonary vascular resistance.10 The interplay between CVD and COPD increases the morbidity and mortality of each disorder. For instance, it has been shown that coronary artery calcification, a non-invasive marker for coronary artery disease is associated with increased mortality in COPD.11 However, it is debated if established CVD is an independent risk factor for COPD exacerbations.12,13 Regarding cardiovascular mortality, a strong association has been found with FVC,14 FEV1,15–17 and the rate of lung function decline18 in population-based studies, suggesting that cardiovascular morbidity may be a relevant factor when investigating the relationship between lung function and mortality. Finally, COPD exacerbations pose an increased risk for cardiovascular events.19,20
Given the contrasting findings illustrated above, and the need to risk stratify COPD patients with comorbid CVD, we aimed to examine the prognostic value of the spirometric indices by analyzing the data of the Study to Understand Mortality and Morbidity in COPD (SUMMIT) trial.
Patients and Methods
Study Design and Participants
The SUMMIT was a multicenter, randomized, double-blind, parallel-group, placebo-controlled trial assessing the effect of once-daily treatment with fluticasone furoate/vilanterol (100/25 μg), fluticasone furoate (100 μg), vilanterol (25 μg), or matched placebo on mortality in patients with moderate COPD; i.e., FEV1 between 50% and 70% of predicted value, and an increased cardiovascular risk.21 For patients ≥40 years of age, this was defined as any one of the following: established coronary artery disease, established peripheral vascular disease, previous stroke, previous myocardial infarction, or diabetes mellitus with target organ disease. In addition, for patients ≥60 years of age, any one of the criteria applicable for patients ≥40 years of age or two of the following: being treated for hypercholesterolemia, being treated for hypertension, being treated for diabetes mellitus, or being treated for peripheral vascular disease.21 The event-driven study included 16,485 participants and lasted until at least 1000 deaths were recorded. There was no difference between the four treatment arms for the primary outcome of all-cause mortality.22
In this post hoc analysis of the SUMMIT study population, we hypothesized that FVC may be a better predictor of overall mortality and major cardiovascular events than FEV1 and FEV1/FVC, whereas FEV1 and FEV1/FVC would be stronger predictors of COPD exacerbations than FVC.
Major cardiovascular events included cardiovascular death, myocardial infarction, stroke, unstable angina, and transient ischemic attack. We analyzed moderate to severe and severe exacerbations separately. A moderate COPD exacerbation was defined as an exacerbation treated with antibiotics and/or systemic corticosteroids whereas a severe COPD exacerbation required hospitalization.
All participants in the current analysis provided written, informed consent for trial participation. The study was conducted at 1373 sites in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines and was approved by local ethics committees (Supplement 1). Trial registration number: NCT01313676.
Lung Function Measurements
Post-bronchodilator spirometry has been performed according to the European Respiratory Society/American Thoracic Society guidelines.23 Prior to the spirometry long-acting β-agonists and inhaled corticosteroids were withheld for 48 hours, long-acting muscarinic antagonists were withheld for 1 week. Patients with systemic steroid use within 30 days were not included. Lung function measurements at screening were repeated after a 4–10 days run-in period at the baseline visit.
Statistical Analyses
For each measure (percent predicted FEV1, percent predicted FVC and FEV1/FVC ratio), participants were allocated into lung function quintiles (Table 1). The number of volunteers allocated in each quintile was compared between the screening and baseline visit.
Table 1.
Association Between Lung Function Indices and Time to Mortality, Cardiovascular Events, Moderate and Severe Exacerbations, and Severe Exacerbations
| Time to Death Risk Reduction vs. Q1 | Time to First Major Cardiovascular Event Risk Reduction vs. Q1 |
Time to First Moderate/Severe Exacerbation Risk Reduction vs. Q1 | Time to First Severe Exacerbation Risk Reduction vs. Q1 | ||
|---|---|---|---|---|---|
| FEV1% Predicted | Q1 <53.5% | ||||
| Q2 53.5 to 57.5% | 20% (4 to 34%) | 5% (−20 to 25%) | 11% (3 to 19%) | 10% (−5 to 23%) | |
| Q3 57.5 to 61.6% | 28% (13 to 40%) | 15% (−8 to 33%) | 15% (7 to 22%) | 25% (12 to 37%) | |
| Q4 61.6 to 65.8 | 23% (7 to 36%) | 9% (−15 to 28%) | 23% (16 to 30%) | 37% (25 to 47%) | |
| Q5 ≥65.8% | 30% (15 to 42%) | 7% (−18 to 26%) | 27% (20 to 33%) | 40% (28 to 49%) | |
| FVC % Predicted | Q1 <67.4% | ||||
| Q2 67.4 to 73.6% | 14% (−4 to 29%) | 16% (−7 to 34%) | 2% (−8 to 10%) | 4% (−15 to 20%) | |
| Q3 73.6 to 79.5% | 11% (−8 to 27%) | −4% (−30 to 17%) | −4% (−14 to 5%) | 0% (−20 to 17%) | |
| Q4 79.5 to 87.6% | 14% (−4 to 29%) | 11% (−13 to 29%) | −6% (−17 to 3%) | −13% (−34 to 6%) | |
| Q5 ≥87.6% | 21% (4 to 35%) | 21% (−1 to 38%) | −22% (−34 to −11%) | −28% (−52 to −8%) | |
| FEV1/FVC | Q1 <0.51 | ||||
| Q2 0.51 to 0.57 | 0% (−21 to 16%) | −7% (−36 to 16%) | 18% (11 to 25%) | 22% (9 to 33%) | |
| Q3 0.57 to 0.62 | 7% (−12 to 24%) | −8% (−38 to 15%) | 28% (22 to 35%) | 39% (28 to 49%) | |
| Q4 0.62 to 0.66 | 10% (−10 to 26%) | −12% (−43 to 12%) | 29% (22 to 35%) | 41% (29 to 50%) | |
| Q5 ≥0.66 | −5% (−28 to 14%) | −18% (−50 to 8%) | 36% (30 to 42%) | 48% (37 to 57%) | |
Notes: Results are from Cox Proportional Hazard models and are presented as risk reduction compared with Q1 quintile groups (with 95% confidence intervals). These are calculated as (1–hazard ratio) × 100. Negative % reductions indicate increase in risk, i.e., hazard ratio >1. Nominally significant differences are presented in bold (p<0.05, no adjustment for multiplicity).
Abbreviations: FEV1, forced expiratory volume in one second; FVC, forced vital capacity.
For primary analyses, lung function data at screening were analyzed. Cox proportional hazards models were applied, adjusted for age, sex, ethnicity, body mass index (BMI), smoking status, previous COPD exacerbations, cardiovascular entry criteria, ischemic and vascular disease indicators, treatment, and modified Medical Research Council (mMRC) dyspnea score. For each outcome, the lowest quintile (Q1) for each lung function measure was used as the reference. Data are expressed as median (95% confidence interval).
Results
Distribution of Lung Function Data
In total, data of 16,485 participants of the intent-to-treat analysis were investigated. Lung function data both at screening and baseline were grouped into quintiles. When comparing the number of patients allocated to each quintile at screening and baseline (at randomization), respectively, only 44%, 54%, and 54% of participants were grouped in the same FEV1, FVC, and FEV1/FVC quintiles, illustrating the variability in these spirometric indices (Table 2).
Table 2.
Distribution of Participants in Lung Function Quintiles at Screening and Baseline
| FEV1 | Screening | |||||
|---|---|---|---|---|---|---|
| Q1: <53.5% (N=3296) | Q2: ≥53.5 to <57.5% (N=3297) | Q3: ≥57.5 to <61.6% (N=3297) | Q4: ≥61.6 to <65.6% (N=3297) | Q5: ≥65.6% (N=3296) | ||
| BASELINE | Q1: <52.3% (N=3296) | 1844 | 858 | 350 | 178 | 65 |
| Q2: ≥52.3 to <56.7% (N=3297) | 941 | 1167 | 741 | 311 | 137 | |
| Q3: ≥56.7 to <61.1% (N=3297) | 319 | 808 | 1109 | 732 | 329 | |
| Q4: ≥61.1 to <66.0% (N=3297) | 119 | 308 | 743 | 1235 | 892 | |
| Q5: ≥66.0% (N=3297) | 73 | 156 | 354 | 841 | 1873 | |
| FVC | Q1: <67.4% (N=3296) | Q2: ≥67.4 to <73.6% (N=3297) | Q3: ≥73.6 to <79.5% (N=3297) | Q4: ≥79.5 to <87.6% (N=3297) | Q5: ≥87.6% (N=3297) | |
| Q1: <52.3% (N=3296) | 2173 | 702 | 264 | 113 | 44 | |
| Q2: ≥65.9 to <72.6% (N=3297) | 798 | 1412 | 739 | 266 | 82 | |
| Q3: ≥72.6 to <78.8% (N=3297) | 224 | 856 | 1357 | 690 | 170 | |
| Q4: ≥78.8 to <87.2% (N=3297) | 68 | 272 | 773 | 1548 | 635 | |
| Q5: ≥87.2% (N=3297) | 33 | 55 | 164 | 680 | 2365 | |
| FEV1/FVC | Q1: <0.51 (N=3296) | Q2: ≥0.51 to <0.57 (N=3297) | Q3: ≥0.57 to <0.62 (N=3297) | Q4: ≥0.62 to <0.66 (N=3296) | Q5: ≥0.66 (N=3297) | |
| Q1: <0.51 (N=3296) | 2480 | 610 | 122 | 37 | 33 | |
| Q2: ≥0.51 to <0.57 (N=3297) | 609 | 1668 | 720 | 196 | 90 | |
| Q3: ≥0.57 to <0.62 (N=3297) | 112 | 718 | 1428 | 757 | 269 | |
| Q4: ≥0.62 to <0.66 (N=3296) | 52 | 181 | 716 | 1407 | 955 | |
| Q5: ≥0.66 (N=3297) | 33 | 103 | 296 | 888 | 1935 | |
Abbreviations: FEV1, forced expiratory volume in one second; FVC, forced vital capacity.
FEV1, FVC, and FEV1/FVC as Predictors for All-Cause Mortality, Cardiovascular Events, and COPD Exacerbations
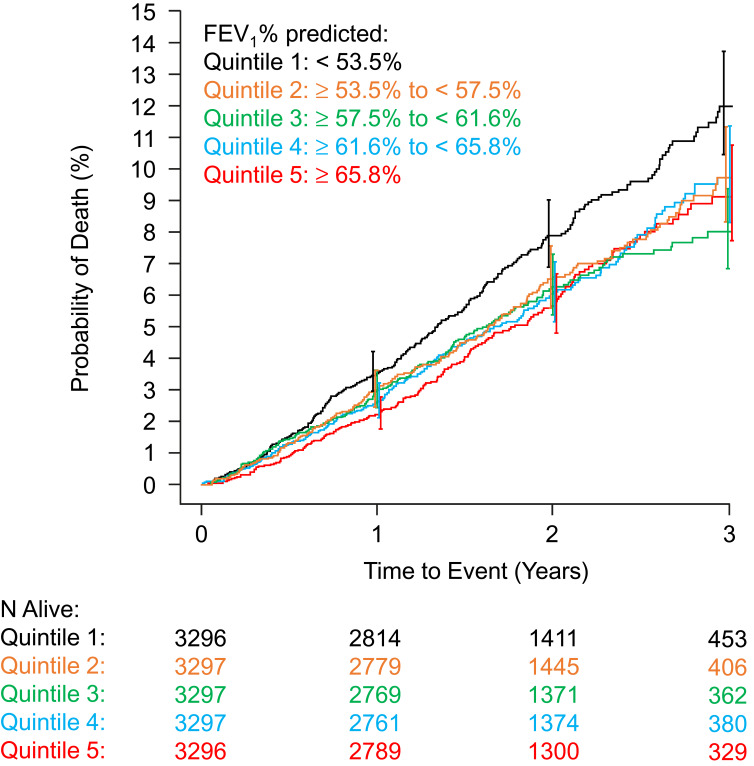
Compared with patients with the lowest FEV1 (Q1, <53.5% predicted), each of the higher quintiles was associated with better survival, ranging from a 20% lower risk in Q2 to a 30% lower risk in Q5 (Table 1, Figure 1). Although there was a trend for decreasing mortality along the increasing quintiles of FEV1/FVC (7.8%, 6.7%, 5.9%, 5.4%, and 5.9%, from Q1 to Q5), this did not reach the level of significance. In contrast, for FVC only mortality in quintile 5 (FVC≥87.6% predicted) differed significantly from that of quintile 1 (FVC<67.4% predicted). No lung function indices were predictive of a major cardiovascular event.
Figure 1.
Kaplan–Meier plot showing unadjusted relationship between FEV1% predicted at screening and all-cause mortality.
Abbreviations: FEV1, forced expiratory volume in one second; N, number of patients.
Compared with quintile 1, higher FEV1 and FEV1/FVC quintiles were all associated with a reduction of risk of moderate-to-severe and severe exacerbations (Table 1). Unexpectedly, quintile 5 of FVC showed an increased risk of a moderate-to-severe and severe exacerbation compared to quintile 1. Despite analyzing FEV1 within a narrow range (50–70%), there was a strong association between FEV1 and exacerbation risk, with the lowest FEV1 quintile having a 27% higher risk of moderate/severe exacerbations compared with the highest FEV1 quintile (≥65.8%), and a 40% increase for severe exacerbations.
Discussion
Analyzing the results of the SUMMIT trial, we found that FEV1 was a better predictor for mortality and exacerbation risk than FVC, while neither of them was associated with the risk for major cardiovascular events. The clearer relationship between mortality and FEV1 than with FVC suggests that airflow limitation, rather than lung volume, predicts mortality in patients with COPD and heightened cardiovascular risk.
Previous general population studies highlighted the predictive role of FVC versus FEV1.6,14 There are possible explanations for the discrepancies. First, the current study included only patients with an obstructive lung function pattern.21 A restrictive pattern may also be common in the general population and associated with poverty7 and morbid obesity, which may both lead to increased mortality.24 Although our analysis was adjusted for BMI, socioeconomic data were unavailable and several other variables, such as very severe heart failure (which may lead to reduced FVC), were not included, which may have influenced our findings. While inclusion into SUMMIT was not restricted by FVC as it was for FEV1, we only included patients with moderate COPD, which imposed some restrictions on FVC indirectly. In this sense, the previously seen association between reduced lung volumes and cardiovascular outcomes may have been driven by subjects with very low FVC.14
We found a steep gradient between FEV1 quintiles and exacerbation risk. As the FEV1 cut-off value generally applied for separating moderate from severe COPD is quite arbitrary, our findings add to the increasing perception that, for usual clinical care, these arbitrary FEV1 cut-off values hold little clinical value.
Our article also highlights the obscurity of lung function values from a single spirometry. Only approximately half of the participants allocated to different lung function quintiles at screening were grouped in the same quintile at baseline. Indeed, approximately 1% of patients changed from the lowest to highest quintiles or vice versa. This can be likely explained by the methodological variability of lung function measurements and physiological variability of the airway caliber.
Our analysis has limitations. Only patients with moderate COPD were included and a wider lung function range would likely have provided more robust data. Lung function aside, hypoxia, hypercapnia, BMI, dyspnea, and exercise capacity are also strong predictors of mortality in COPD.1 Although our analyses were adjusted for BMI and mMRC dyspnea score, blood gas values and exercise capacity test were not available in SUMMIT. However, patients on long-term oxygen were excluded, as were those with very severe heart failure, severe renal failure, or whose life expectancy from diseases other than vascular disease and COPD was under 3 years. We therefore do not believe that our findings are the result of confounding by these other risk factors.
Confirming previous findings, lower FEV1 and FEV1/FVC were associated with higher risk for COPD exacerbations.13,25,26 Increased disease severity is associated with heightened airway and systemic inflammation.27 The ECLIPSE study highlighted the potential role of persistent systemic inflammation leading to frequent exacerbations.28 However, analyzing the SUMMIT data, the levels of systemic inflammatory biomarkers did not relate to the frequency of flare-ups.29 Interestingly we found that the highest FVC quintile was associated with an increased exacerbation risk. This, together with the gradually increased risk for exacerbation with FEV1 decline suggest that more severe emphysema may be related to higher number of exacerbations. This is in line with the ECLIPSE study;25 however, no computed tomography was performed in the SUMMIT trial. Our results are similar to the findings of the TIOSPIR study showing that larger FVC was associated with increased exacerbation risk.12
Conclusion
In conclusion, we found strong relationships between FEV1 and exacerbation rate and all-cause mortality, but not with major cardiovascular events, in SUMMIT. These were stronger than for FVC and stronger than we anticipated for patients with moderate COPD and a limited FEV1 range.
Acknowledgments
AB and JV are supported by the National Institute of Health Research Manchester Biomedical Research Centre (NIHR Manchester BRC). Editorial support (in the form of editorial alignment with journal guidance) was provided by Kirsty Millar, MSc, of Gardiner-Caldwell Communications (Macclesfield, UK), and was funded by GlaxoSmithKline plc.
Funding Statement
This study was funded by GlaxoSmithKline plc (HZC113782/NCT01313676).
Abbreviations
BMI, body mass index; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; mMRC, modified Medical Research Council; SUMMIT, Study to Understand Mortality and Morbidity.
Data Sharing
The authors confirm that they intend to share individual deidentified participant data, including demographics, comorbidities, mortality, lung function, and treatment. In addition, other study-related documents will be made available, including raw dataset, analysis ready dataset, reporting and analysis plan, clinical study report, case report forms, and protocol.
It is GSK policy to provide access to patient-level data within 6 months of publishing the results of the primary endpoints of the study. Researchers can enquire about the availability of data from GSK clinical studies that are not listed on www.clinicalstudydatarequest.com before they submit a research proposal. To access data for other types of GSK sponsored research, for study documents without patient-level data and for clinical studies not listed, please submit an enquiry via www.clinicalstudydatarequest.com.
Disclosure
AB reports fees from AstraZeneca, Chiesi, and Sandoz. PL reports grants from AstraZeneca and GlaxoSmithKline and fees from Boehringer Ingelheim, AstraZeneca, Novartis, and GlaxoSmithKline. JAA, CC, and JCY are employees of and hold stock in GlaxoSmithKline plc. PMAC reports fees from GlaxoSmithKline, Boehringer Ingelheim, and AstraZeneca. BRC reports fees from GlaxoSmithKline. NJC and IJD are employed by Veramed Ltd, a Contract Research Organization undertaking contracted statistical analyses of studies funded by GlaxoSmithKline plc. FJM reports grants, personal fees, non-financial support from AstraZeneca, personal fees, non-financial support from Boehringer Ingelheim, non-financial support from Proterrix Bio, personal fees from CME Outfitters, personal fees from Gala, Asthma DSMB from Genentech/Roche, grants, personal fees, non-financial support from GlaxoSmithKline, personal fees from INOVA Fairfox, personal fees from Miller Communications, personal fees from National Association for Continuing Education, personal fees from Novartis, personal fees from Pearl, personal fees from PeerView, personal fees from Physicians Education Resource, personal fees from Prime Education, personal fees from Sunovion, personal fees from Teva, personal fees, non-financial support from UpToDate, personal fees from WebMD/Medscape, during the conduct of the study; personal fees from Gentech, and is involved with COPD CME for UpToDate. DEN reports fees from GlaxoSmithKline. JV reports fees from GSK, AstraZeneca, Boehringer-Ingelheim, Chiesi, and Novartis. The authors report no other conflicts of interest in this work.
References
- 1.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Eur Respir J. 2017;49(3):1700214. doi: 10.1183/13993003.00214-2017 [DOI] [PubMed] [Google Scholar]
- 2.Prudente R, Franco EAT, Mesquita CB, Ferrari R, de Godoy I, Tanni SE. Predictors of mortality in patients with COPD after 9 years. Int J Chron Obstruct Pulmon Dis. 2018;13:3389–3398. doi: 10.2147/COPD.S174665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soriano JB, Lamprecht B, Ramirez AS, et al. Mortality prediction in chronic obstructive pulmonary disease comparing the GOLD 2007 and 2011 staging systems: a pooled analysis of individual patient data. Lancet Respir Med. 2015;3(6):443–450. doi: 10.1016/S2213-2600(15)00157-5 [DOI] [PubMed] [Google Scholar]
- 4.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–1012. doi: 10.1056/NEJMoa021322 [DOI] [PubMed] [Google Scholar]
- 5.Goossens LM, Leimer I, Metzdorf N, Becker K, Rutten-van Molken MP. Does the 2013 GOLD classification improve the ability to predict lung function decline, exacerbations and mortality: a post-hoc analysis of the 4-year UPLIFT trial. BMC Pulm Med. 2014;14:163. doi: 10.1186/1471-2466-14-163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burney PG, Hooper R. Forced vital capacity, airway obstruction and survival in a general population sample from the USA. Thorax. 2011;66(1):49–54. doi: 10.1136/thx.2010.147041 [DOI] [PubMed] [Google Scholar]
- 7.Burney P, Jithoo A, Kato B, et al. Chronic obstructive pulmonary disease mortality and prevalence: the associations with smoking and poverty–a BOLD analysis. Thorax. 2014;69(5):465–473. doi: 10.1136/thoraxjnl-2013-204460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiss ST, Segal MR, Sparrow D, Wager C. Relation of FEV1 and peripheral blood leukocyte count to total mortality. The normative aging study. Am J Epidemiol. 1995;142(5):493–498; discussion 499–503. doi: 10.1093/oxfordjournals.aje.a117665 [DOI] [PubMed] [Google Scholar]
- 9.Sin DD, Man SF. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation. 2003;107(11):1514–1519. doi: 10.1161/01.CIR.0000056767.69054.B3 [DOI] [PubMed] [Google Scholar]
- 10.Rabe KF, Hurst JR, Suissa S. Cardiovascular disease and COPD: dangerous liaisons? Eur Respir Rev. 2018;27(149):180057. doi: 10.1183/16000617.0057-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams MC, Murchison JT, Edwards LD, et al. Coronary artery calcification is increased in patients with COPD and associated with increased morbidity and mortality. Thorax. 2014;69(8):718–723. doi: 10.1136/thoraxjnl-2012-203151 [DOI] [PubMed] [Google Scholar]
- 12.Calverley PM, Tetzlaff K, Dusser D, et al. Determinants of exacerbation risk in patients with COPD in the TIOSPIR study. Int J Chron Obstruct Pulmon Dis. 2017;12:3391–3405. doi: 10.2147/COPD.S145814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoogendoorn M, Feenstra TL, Boland M, et al. Prediction models for exacerbations in different COPD patient populations: comparing results of five large data sources. Int J Chron Obstruct Pulmon Dis. 2017;12:3183–3194. doi: 10.2147/COPD.S142378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman GD, Klatsky AL, Siegelaub AB. Lung function and risk of myocardial infarction and sudden cardiac death. N Engl J Med. 1976;294(20):1071–1075. doi: 10.1056/NEJM197605132942001 [DOI] [PubMed] [Google Scholar]
- 15.Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest. 2005;127(6):1952–1959. doi: 10.1378/chest.127.6.1952 [DOI] [PubMed] [Google Scholar]
- 16.Speizer FE, Fay ME, Dockery DW, Ferris BG Jr. Chronic obstructive pulmonary disease mortality in six U.S. cities. Am Rev Respir Dis. 1989;140(3 Pt 2):S49–S55. doi: 10.1164/ajrccm/140.3_Pt_2.S49 [DOI] [PubMed] [Google Scholar]
- 17.Duong M, Islam S, Rangarajan S, et al. Mortality and cardiovascular and respiratory morbidity in individuals with impaired FEV1 (PURE): an international, community-based cohort study. Lancet Glob Health. 2019;7(5):e613–e623. doi: 10.1016/S2214-109X(19)30070-1 [DOI] [PubMed] [Google Scholar]
- 18.Tockman MS, Pearson JD, Fleg JL, et al. Rapid decline in FEV1. A new risk factor for coronary heart disease mortality. Am J Respir Crit Care Med. 1995;151(2 Pt 1):390–398. doi: 10.1164/ajrccm.151.2.7842197 [DOI] [PubMed] [Google Scholar]
- 19.Donaldson GC, Hurst JR, Smith CJ, Hubbard RB, Wedzicha JA. Increased risk of myocardial infarction and stroke following exacerbation of COPD. Chest. 2010;137(5):1091–1097. doi: 10.1378/chest.09-2029 [DOI] [PubMed] [Google Scholar]
- 20.Kunisaki KM, Dransfield MT, Anderson JA, et al. Exacerbations of chronic obstructive pulmonary disease and cardiac events. A post hoc cohort analysis from the SUMMIT randomized clinical trial. Am J Respir Crit Care Med. 2018;198(1):51–57. doi: 10.1164/rccm.201711-2239OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vestbo J, Anderson J, Brook RD, et al. The Study to Understand Mortality and Morbidity in COPD (SUMMIT) study protocol. Eur Respir J. 2013;41(5):1017–1022. doi: 10.1183/09031936.00087312 [DOI] [PubMed] [Google Scholar]
- 22.Vestbo J, Anderson JA, Brook RD, et al. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet. 2016;387(10030):1817–1826. doi: 10.1016/S0140-6736(16)30069-1 [DOI] [PubMed] [Google Scholar]
- 23.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 24.Masters RK, Reither EN, Powers DA, Yang YC, Burger AE, Link BG. The impact of obesity on US mortality levels: the importance of age and cohort factors in population estimates. Am J Public Health. 2013;103(10):1895–1901. doi: 10.2105/AJPH.2013.301379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mullerova H, Maselli DJ, Locantore N, et al. Hospitalized exacerbations of COPD: risk factors and outcomes in the ECLIPSE cohort. Chest. 2015;147(4):999–1007. doi: 10.1378/chest.14-0655 [DOI] [PubMed] [Google Scholar]
- 26.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi: 10.1056/NEJMoa0909883 [DOI] [PubMed] [Google Scholar]
- 27.Barnes PJ, Burney PG, Silverman EK, et al. Chronic obstructive pulmonary disease. Nat Rev Dis Primers. 2015;1:15076. doi: 10.1038/nrdp.2015.76 [DOI] [PubMed] [Google Scholar]
- 28.Agusti A, Edwards LD, Rennard SI, et al. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS One. 2012;7(5):e37483. doi: 10.1371/journal.pone.0037483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Celli BR, Anderson JA, Brook R, et al. Serum biomarkers and outcomes in patients with moderate COPD: a substudy of the randomised SUMMIT trial. BMJ Open Respir Res. 2019;6(1):e000431. doi: 10.1136/bmjresp-2019-000431 [DOI] [PMC free article] [PubMed] [Google Scholar]