Abstract
Objective
The severity of breakthrough cancer pain (BTcP) impacts patients’ quality of life, increases the risk of anxiety and depression, lowers functional capacities, and may lead to poor compliance with cancer treatments. The aim of the current study was to assess, in a real-life setting, patient satisfaction with a fentanyl-pectin-nasal-spray (FPNS) for BTcP management in head and neck (H&N) cancer patients treated by radiotherapy.
Materials and Methods
This non-interventional, prospective study was conducted in 92 adult H&N-cancer patients undergoing radiotherapy and who started FPNS treatment for BTcP. Throughout the radiotherapy period, the patients completed self-diaries to assess their BTcP episodes, FPNS use, satisfaction on FPNS efficiency (primary outcome), tolerability and ease of use.
Results
Prior to FPNS treatment, 86% of the patients were experiencing ≤4 BTcP episodes/day. During the radiotherapy period, the BTcP episodes were treated with a median dose of 100µg of FPNS. Patients were “satisfied/very-satisfied” with the efficiency (73% of assessments), ease of use (87% of assessments) and tolerability (87% of assessments) of FPNS. In total, 27% of patients reported at least one adverse event related to FPNS and 4% of patients discontinued treatment due to adverse events. None of the adverse events were serious. Patient quality of life was maintained throughout the radiotherapy period.
Conclusion
This study showed, in a real-life setting, that a clear majority of H&N cancer patients treated with FPNS for BTcP throughout radiotherapy expressed satisfaction with this analgesic treatment.
Keywords: patient satisfaction, fentanyl nasal spray, breakthrough pain, radiotherapy, head and neck cancer
Introduction
Pain is a common symptom in cancer patients, contributing to poor physical and emotional well-being. Its prevalence depends on disease stage and cancer type.1 The use of opioids is the mainstay of analgesic therapy.2 However, despite a relatively well-controlled baseline pain, the patients can experience pain flares with a fast time to peak intensity, called breakthrough cancer pain (BTcP).
BTcP is defined as "a transient exacerbation of pain that occurs either spontaneously, or in relation to a specific predictable or unpredictable trigger, despite relatively stable and adequately controlled background pain".
The BTcP is characterized by pain episodes of moderate to severe intensity, with rapid onset (minutes) and of relatively short duration (median ≈30 minutes) in a context of background pain controlled by strong opioids.3–5 The previous studies have highlighted that the BTcP is experienced by more than one in two patients with cancer pain.6
The episodes of BTcP severely impacts patients’ quality of life, increases the risk of anxiety and depression, lowers functional capacities,7,8 and may lead to poor compliance with cancer treatments.
BTcP episodes are managed with a specific opioid treatment, self-administered on an “as required” basis. Due to the rapid onset and short duration of BTcP, the reference treatments in numerous countries are fast-acting fentanyl citrates (commonly referred to as rapid-onset opioids, ROOs),9–11 with rapid onset of pain relief (5 to 10 minutes) and lasting for 1 to 2 hours.12–14 However, transmucosal fentanyl was not mentioned in the most recent recommendations of the World Health Organization,15 which fall within a worldwide vision of access and cost of such treatments.
Among the available formulations of fentanyl citrates, the nasal route of the fentanyl pectin nasal spray (FPNS) could be advantageous to patients experiencing oral mucositis and xerostomia, nausea, vomiting, and impaired gastrointestinal function. The clinical efficacy and favorable nasal and overall safety profile of FPNS have been previously demonstrated in cancer patients with BTcP, resulting in patients’ satisfaction in an experimental setting.13,16,17 Nevertheless, overall patient satisfaction in real-life conditions of use has remained little known, whereas patient opinions of treatment efficiency, tolerability and convenience could impact treatment adherence and thus help patients maintain their quality of life, as well as continue their cancer treatment.7,8 As a result, patients may better comply with usage rules for this self-administered opioid.
In patients with head and neck (H&N) cancer, pain is a common condition, present in around 70% of cases, and up to 50% of patients, experience BTcP.18,19 This cancer location and the consequences of pain on patients’ nutritional status make pain management crucial. This is particularly true for those undergoing radiotherapy due to the risk of oral mucositis, with severe BTcP associated with swallowing disorders.20 In this context, the PecDICO study was designed mainly to describe in a real-life setting overall satisfaction with FPNS (efficiency, tolerability, and convenience) for patients with H&N cancer, undergoing radiotherapy and treated with FPNS for BTcP.
Materials and Methods
Study Design
This non-interventional, prospective French study was conducted in patients undergoing radiotherapy for H&N cancer and receiving FPNS for BTcP as usually prescribed in the participating centers. Eligible patients were adults patients (age ≥18 years), with confirmed H&N squamous cell carcinoma treated either with radiotherapy alone or concurrent chemotherapy or biotherapy according to common practices of the centers, with BTcP episodes despite a stable opioid dose regimen (≥ 60mg/day of oral morphine or an equianalgesic dose of any other opioid for ≥1 week) for whom a treatment decision to initiate FPNS had been made at the sole initiative of physicians. The recruited patients were informed on the study and had no objections regarding collection of their personal data. Inclusion and exclusion criteria of the study were consistent with the European Summary of Product Characteristics for FPNS (Kyowa Kirin Pharma, PecFent®).21
The patients’ observation period covered radiotherapy from FPNS initiation and a six-month follow-up after completion of the radiotherapy. FPNS prescription, patient visits, follow-up and data collection were conducted according to the centers’ local standards and at the radiation oncologists’ discretion. The study was conducted in compliance with the ethical standards of the 1964 Helsinki declaration and its later amendments, the deontology guidelines and Good Epidemiology Practices,22 and the French regulation on non-interventional studies. The study protocol was approved by an independent Ethics Committee.
Assessments
At enrollment (baseline visit), after receiving patients’ consent, radiation oncologists collected the patients’ demographic and clinical characteristics, including cancer history, nutritional status, comorbidities and history, and pain management. During the radiotherapy period, clinicians collected weekly data on patient health performance status (Eastern Cooperative Oncology Group, ECOG), nutritional status, background pain and BTcP management including FPNS treatment, as well as a full assessment of the radiotherapy regimen at the end of the period. Patients used a BTcP diary to complete information about pain intensity (using a 0–10 rating scale), the number of BTcP episodes, and FPNS use. Around 3 and 6 months after radiotherapy completion, the patients attended follow-up visits and the response to the cancer treatment was evaluated by the clinician.
During the radiotherapy period, as well as during the follow-up visits at 3 and 6 months, patients assessed weekly their satisfaction on the efficiency, tolerability, and ease of use of FPNS in the previous 24 hours, using a 4-point Likert scale (not satisfied, slightly satisfied, satisfied, very satisfied). At every study visit, patients estimated their quality of life using the Brief Pain Inventory (BPI) short-form,23 and the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC-QLQ-C30).24
Safety data for adverse events (AEs) potentially related to FPNS were collected throughout the entire study period.
Statistical Methods
Based on findings from a previous interventional study,25 it was hypothesized that at least 80% of all the weekly patient satisfaction assessments on treatment efficiency would be positive during radiotherapy (ie satisfied or very satisfied). With an absolute precision of 4% and an associated confidence interval (CI) of 95%, 384 weekly patient assessments were expected during radiotherapy,26 corresponding to around 100 enrolled patients.
Patient and disease characteristics, background and breakthrough cancer pain, treatments for cancer and pain (including FPNS), and patients’ quality of life were described using standard descriptive statistics. The proportion of all the patients’ assessments considered as positive for FPNS efficiency (primary criterion), tolerability, and ease of use during the radiotherapy period was described with its associated 95% CI. After univariate analysis performed on baseline patient, disease, and BTcP characteristics, as well as on pain management including FPNS use, a multivariable, stepwise logistic regression analysis was performed to search for predictive independent factors associated with patient satisfaction regarding FPNS efficacy. Safety data were described over the study period for AEs potentially related to FPNS treatment and specific events (mainly misuse or abuse, and overdose). Missing data were not replaced. Statistical analyses were performed using SAS® software, version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Patient Disposition
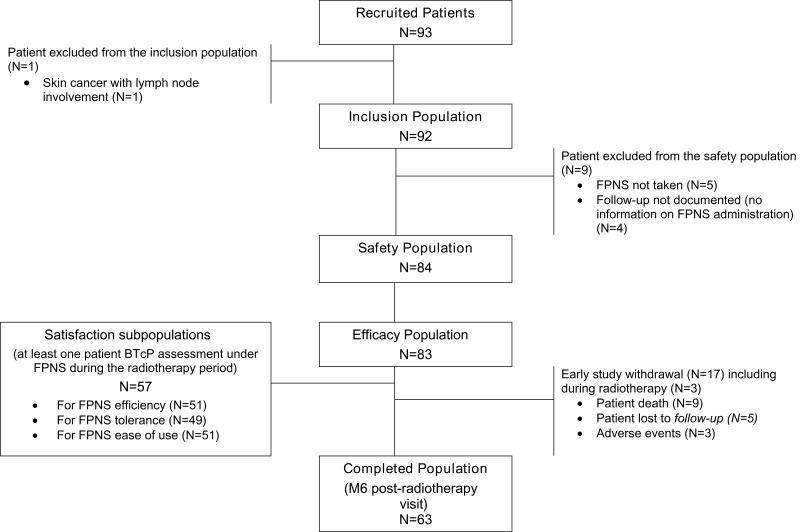
Between November 2014 and June 2016, 93 patients were included by eight French radiotherapy centers. Of the enrolled patients, 92 (99%) were retained in the inclusion population (description of patients, disease, and treatments at baseline), 84 patients (90%) in the safety population (description of FPNS tolerability), and 83 patients (89%) in the efficacy population since they had at least one follow-up visit (cancer and pain management during follow-up, patient satisfaction and quality of life) (Figure 1).
Figure 1.
Study population flowchart.
Abbreviations: M6, month 6; FPNS, fentanyl pectin nasal spray.
Patient and Disease Baseline Characteristics
The demographic and clinical baseline characteristics of the population are summarized in Table 1. At inclusion, 97% of patients had dysphagia. Nutritional management was provided in 82% of patients (oral supplements: 65%, nasogastric intubation: 15%, and/or gastrostomy: 15%). In addition, weight loss over 10% was experienced by 22% of patients within the previous month. The radiotherapy started within a median time of 4 weeks (range: 0–8) prior to enrollment (ie before starting the FPNS). Of the 61 patients (66%) who received chemotherapy for H&N cancer (Table 1), radiotherapy was concomitant in 80% of them (n=49).
Table 1.
Patient and Disease Baseline Characteristics – Inclusion Population (N= 92)
| Total (n=92) | |
|---|---|
| Malea, n (%) | 73 (80.2) |
| Age, years | |
| Median (range) | 59 (34–81) |
| Body mass index ≥ 25 kg/m2, n (%) | 33 (35.9) |
| ECOG Performance status ≤ 2a, n (%) | 90 (100) |
| Dysphagia, n (%) | |
| Grade 0 | 3 (3.3) |
| Grade 1 | 19 (20.7) |
| Grade 2 | 56 (60.9) |
| Grade 3 | 6 (6.5) |
| Grade 4 | 8 (8.7) |
| Time from cancer diagnosisa, months | |
| Median (range) | 3.73 (1.8–14.6) |
| Disease stage (TNM classification)a, n (%) | |
| Stage I | 7 (7.8) |
| Stage II | 8 (8.9) |
| Stage III | 18 (20.0) |
| Stage IV A | 43 (47.8) |
| Stage IV B | 6 (6.7) |
| Stage IV C | 2 (2.2) |
| Other (unknown T, N or M) | 6 (6.7) |
| H&N cancer location, n (%) | |
| Oral cavity | 22 (23.9) |
| Oropharynx | 36 (39.1) |
| Hypopharynx | 15 (16.3) |
| Larynx | 13 (14.1) |
| Nasopharynx | 1 (1.1) |
| Oral cavity and oropharynx | 1 (1.1) |
| Hypopharynx and oropharynx | 3 (3.3) |
| Nasopharynx and oropharynx | 1 (1.1) |
| H&N cancer treatment, n (%) | |
| Radiotherapy | 92 (100.0) |
| Chemotherapy | 61 (66.3) |
| Surgery | 27 (29.3) |
| Targeted therapy | 15 (16.3) |
Notes: aMissing data: sex (1); ECOG performance status (2); Time to diagnosis at enrollment (2); Disease stage (2).
Abbreviations: ECOG, Eastern Cooperative Oncology Group; H&N, head and neck; TNM, tumor, node, metastasis.
Breakthrough Cancer Pain
Prior to enrollment, the first BTcP episode occurred in a median of 4 days (range: 0–78) after the start of the radiotherapy treatment and most patients (86%) experienced ≤4 episodes/day with variable intensity (Table 2). Most patients (90%) reported “predictable” pain.
Table 2.
Breakthrough Cancer Pain Characteristics at Enrollment – Inclusion Population (N= 92)
| Total (n=92) | |
|---|---|
| Time between the first BTcP episode and enrollment, days | N=92 |
| Median (range) | 4.0 (0.0–78.0) |
| Type of pain, n (%) | N=92 |
| Nociceptive | 42 (45.6) |
| Mixed | 50 (54.3) |
| Neuropathic | 0 (0.0) |
| Cause of BTcP, n (%) | N=92 |
| Procedural pain | 7 (7.6) |
| Predictable pain | 83 (90.2) |
| Spontaneous pain | 28 (30.4) |
| Patient perception of BTcP intensity in the 24 h before enrollment (0–10 scale) | N=43 |
| Median (range) | 5.0 (0.0–10.0) |
| BTcP intensity | |
| ≤4 | 20 (39.2) |
| [4–6] | 11 (21.6) |
| [6–10] | 20 (39.2) |
| Patient count of BTcP in the 24 h before enrollment | N=43 |
| Median (range) | 3.0 (0.0–15) |
| ≤4 BTcP episodes | 37 (86.0) |
Abbreviation: BTcP, breakthrough cancer pain.
At enrollment, the pattern of background cancer pain treatment was similar in both efficacy and inclusion populations. During the radiotherapy period, 54% of patients experienced changes in background opioid treatment. Nevertheless, the total daily dose of opioids taken by the 75 patients treated both at enrollment and at the end of radiotherapy showed overall stability between both time-points (median difference: 0.0 mg, range: −720–990).
The Table 3 details the BTcP characteristics during the radiotherapy period, as well as the use of FPNS.
Table 3.
Breakthrough Cancer Pain Characteristics and Use of FPNS During Radiotherapy
| Initial Assessmenta | W1 | W2 | W3 | W4 | W5 | W6 | End of Radiotherapy | |
|---|---|---|---|---|---|---|---|---|
| Investigator Assessments of BTcP (N=83) | N=83 | N=74 | N=55 | N=38 | N=22 | N=10 | N=1 | N=79 |
| Type of pain, n (%) | N=83 | N=74 | N=54 | N=38 | N=22 | N=10 | N=1 | N=78 |
| No pain | 1 (1.4) | 1 (1.9) | 1 (2.6) | 0 | 0 | 0 | 7 (9.0) | |
| Nociceptive | 36 (43.4) | 29 (39.2) | 18 (33.3) | 9 (23.7) | 2 (9.1) | 1 (10.0) | 0 | 25 (32.1) |
| Neuropathic | 0 | 0 | 0 | 1 (2.6) | 1 (4.5) | 0 | 0 | 1 (1.3) |
| Mixed | 47 (56.6) | 44 (59.5) | 35 (64.8) | 27 (71.1) | 19 (86.4) | 9 (90.0) | 1 (100.0) | 45 (57.7) |
| Causes of BTcP, n (%)b | N=83 | N=73 | N=53 | N=37 | N=22 | N=10 | N=1 | N=71 |
| Procedural pain | 6 (7.2) | 3 (4.1) | 1 (1.9) | 2 (5.4) | 2 (9.1) | 0 | 0 | 2 (2.8) |
| Predictable pain | 75 (90.4) | 63 (86.3) | 49 (92.5) | 33 (89.2 | 19 (86.4) | 10 (100.0) | 1 (100.0) | 64 (90.1) |
| Spontaneous pain | 23 (27.7) | 23 (31.5) | 10 (18.9) | 8 (21.6) | 6 (27.3) | 1 (10.0) | 0 | 19 (26.8) |
| Patient assessments of BTcP (at least one completed, N=58) |
N=50 | N=52 | N=41 | N=29 | N=15 | N=9 | N=3 | N=15 |
| Intensity of BTcP episodesd | N=48 | N=52 | N=40 | N=29 | N=15 | N=8 | N=3 | N=15 |
| Mean (SD) | 5.1 (2.3) | 4.9 (2.1) | 5.1 (1.8) | 5.2 (1.9) | 5.0 (1.7) | 3.4 (2.2) | 4.1 (3.4) | 4.4 (2.0) |
| Median (range) | 5.0 (0.0–10.0) |
5.1 (1.0–9.1) |
5.0 (0.0–8.1) |
5.4 (1.0–8.3) |
5.0 (1.6–8.5) |
3.6 (0.6–6.5) |
5.3 (0.3–6.8) |
4.0 (0.0–8.0) |
| BTcP intensity by classd, n (%) | ||||||||
| ≤4 | 19 39.6%) | 17 (32.7%) | 9 (22.5%) | 11 (37.9%) | 4 (26.7%) | 6 (75.0%) | 1 (33.3%) | 9 (60.0%) |
| [4–6] | 11 (22.9%) | 18 (34.6%) | 19 (47.5%) | 10 (34.5%) | 8 (53.3%) | 0 | 1 (33.3%) | 3 (20.0%) |
| >7 | 18 (37.5%) | 17 (32.7%) | 12 (30.0%) | 8 (27.6%) | 3 (20.0%) | 2 (25.0%) | 1 (33.3%) | 3 (20.0%) |
| Number of BTcP episodesd | N=48 | N=52 | N=40 | N=29 | N=15 | N=8 | N=3 | N=15 |
| Mean (SD) | 3.3 (2.9) | 3.3 (2.3) | 3.2 (1.9) | 3.4 (2.1) | 3.6 (1.9) | 2.2 (1.8) | 2.5 (0.9) | 2.2 (1.1) |
| Median (range) | 3.0 (0.0–15.0) |
3.0 (0.0–11.1) |
3.1 (0.0–7.5) |
3.3 (0.0–8.3) |
3.3 (0.3–6.0) |
2.0 (0.1–5.7) |
3.0 (1.5–3) |
2.0 (0.0–4.0) |
| Number of BTcP episodes by classd, n (%) | ||||||||
| ≤4 | 34 (85.0) | 33 (73.3) | 24 (66.7) | 17 (70.8) | 8 (61.5) | 6 (85.7) | 3 (100.0) | 14 (100.0) |
| >4 | 6 (15.0) | 12 (26.7) | 12 (33.3) | 7 (29.2) | 5 (38.5) | 1 (14.3) | 0 | 0 |
| Use of FPNS, n (%) | N=74 | N=55 | N=38 | N=22 | N=10 | N=1 | N=79 | |
| Yes | 70 (94.6) | 50 (90.9) | 31 (81.6) | 20 (90.9) | 9 (90.0) | 1 (100.0) | 60 (75.9) | |
| Not applicablec | 0 | 1 (1.8) | 3 (7.9) | 2 (9.1) | 1 (10.0) | 0 | 9 (11.4) | |
| Dose of FPNS/BTcP episode, µg | N=70 | N=50 | N=31 | N=20 | N=9 | N=1 | N=59 | |
| Median (range) | 100 (100–400) |
100 (100–400) |
100 (100–400) |
100 (100–400) |
100 (100–400) |
100 (100–800) |
Notes: Missing data: type of pain (W2, n=1; end of radiotherapy, n=1), causes of BTcP (W2, n=1), average duration of BTcP episodes (W2, n=1), intensity of BTcP episodes (Day 1, n=2; W2, n=1; W5, n=1); number of BTcP episodes (Day 1, n=10; W1, n=7; W2, n=5; W3, n=5; W5, n=2; W5, n=2; end of radiotherapy, n=1), dose of FPNS per BTcP episode (end of radiotherapy, n=1).aInvestigator initial assessment: enrollment visit; patient initial assessment: at first FPNS intake; bMultiple answers possible per patient; cTreatment discontinued since last visit; dBTcP episodes (treated or not with FPNS) within the last 24 hours.
Abbreviations: BTcP, breakthrough cancer pain; FPNS, fentanyl pectin nasal spray; SD, standard deviation; W, week.
Therapeutic Management of Cancer
The mean duration of radiotherapy was 7.1±0.8 weeks and its median total dose was 70.0 Gy (range: 11.5–70.0) in fractions of 2.0 Gy per day. Two thirds of the patients (63%) received combined chemotherapy during follow-up (platinum-based regimen in 83% of the cases). Radiotherapy was prematurely discontinued in only one patient (1%). Of the 69 patients (83%) with at least one assessment of response to treatment after radiotherapy completion (at the follow-up visits at 3 and 6 months), an overall response (either complete and/or partial) was observed in 84% of the cases.
Patient Satisfaction with Fentanyl Pectin Nasal Spray
Patient satisfaction with FPNS was analyzed in those who completed at least one weekly evaluation after the first FPNS intake (assessment of efficiency over the previous 24 hours: N=51; on tolerability: N=49; on ease of use: N=51). On these bases, respectively 80%, 90% and 88% of patients were “satisfied” or “very satisfied” with the treatment at least once. High rates of satisfaction were also observed when proportions were calculated on all the patient assessments: 73% (95% CI 65–81%) for FPNS efficiency, and 87% (95% CI 81–93%) for both tolerability and ease of use (Table 4).
Table 4.
Patient Satisfaction with Fentanyl Pectin Nasal Spray During Radiotherapy – Total Weekly Assessments of Patients for Treated BTcP Episodes in the Previous 24 Hours
| Total | |
|---|---|
| Positive assessmenta of FPNS efficacy, n (%) | N=120 |
| No | 32 (26.7) |
| Yes | 88 (73.3) |
| 95% CI | 65.4–81.2 |
| Positive assessmenta of FPNS tolerability, n (%) | N=115 |
| No | 15 (13.0) |
| Yes | 100 (87.0) |
| 95% CI | 80.8–93.1 |
| Positive assessmenta of FPNS ease of use, n (%) | N=119 |
| No | 15 (12.6) |
| Yes | 104 (87.4) |
| 95% CI | 81.4–93.4 |
Notes: aPositive assessment: patient “satisfied” or “very satisfied” on the weekly evaluation.
Abbreviations: BTcP, breakthrough cancer pain; CI, Confidence Interval; FPNS, fentanyl pectin nasal spray.
Univariate analysis highlighted that patient satisfaction with FPNS efficiency was associated (p ≤0.1) with the following parameters: pain caused by radiotherapy procedures, location of H&N cancer in the larynx, and T1/T2 disease stage at baseline. Based on these three parameters, multivariate analysis showed only one baseline parameter was an independent predictive factor of patient satisfaction: T1/T2 disease stage (odd ratio: 3.46; 95% CI: 1.14–10.52; p=0.029).
Safety of FPNS
At least one adverse event (AE) potentially related to FPNS was reported in 21 patients (27%) in the safety population, and 47 related AEs were reported, all considered not serious by radiation oncologists (Table 5). The most common related AE was dizziness (11 events reported in 9 patients, 11%). Five AEs led to treatment discontinuation in 3 patients (4%): hypotension and dizziness (n=1), nasal intolerance (n=1), and nausea and dizziness (n=1). Specific events related to FPNS use were experienced by 4 patients (5%): 2 cases of lack of efficacy, 2 cases of misuse or abuse, and 1 overdose. During follow-up, 9 patients (11%) had died due to non-related events: disease progression (n=7), refractory hypoxemia (n=1), and cachexia with septic shock (n=1).
Table 5.
Adverse Events Related to FPNS - Safety Population (n=84)
| Adverse Events Related to FPNS | ||
|---|---|---|
| Number of AEs | Patients - N (%) | |
| Any FPNS-related AEs | 47 | 23 (27.4) |
| Any FPNS-related SAEs | 0 | 0 (0.0) |
| FPNS-related AEs | 47 | 23 (27.4) |
| Dizziness | 11 | 9 (11) |
| Constipation | 7 | 6 (7) |
| Somnolence | 6 | 6 (7) |
| Nausea | 6 | 5 (6) |
| Nasal intolerance | 8 | 4 (5) |
| Hypotension | 3 | 2 (2) |
| Vomiting | 2 | 2 (2) |
| Tremor | 1 | 1 (1) |
| Headache | 1 | 1 (1) |
| Insomnia | 1 | 1 (1) |
| Fatigue | 1 | 1 (1) |
Abbreviations: AE, adverse event; FPNS, fentanyl pectin nasal spray; SAE, serious adverse event.
Patient Health Condition and Quality of Life Over the Study Period
During radiotherapy, the proportion of patients with an ECOG score ≤2 varied between 81% and 100% depending on study visits. The proportion of patients with improvement increased between enrollment and the follow-up visits at 3 months (39%), and 6 months (50%).
During the radiotherapy visits, approximatively 90% of the patients reported dysphagia and this proportion decreased to 50% and 43% at the 3-month and 6-month visits, respectively. At these times (radiotherapy, 3-month and 6-month visits), 20%, 54% and 68% of patients had no more specific nutritional management.
Brief Pain Inventory (BPI)
In 47 patients (57%) with assessments at both enrollment and end of radiotherapy, overall pain intensity slightly decreased [median score at enrollment according to the 0–10 scale: 4 (range: 1–8); median change: −0.6 (range: −5.8–4.0)]. In addition, the percentage of improvement in pain under ongoing treatments increased from 52% to 66%, using a 0–100% scale. However, the impact of pain on patient quality of life remained broadly stable (baseline median score: 3, range: 0–10; median change: −0.3; range: −5.3; 3.9).
EORTC-QLQ C30
Depending on the domain studied in the questionnaire, 46 or 47 patients (55% or 57%) gave an assessment at both enrollment and end of radiotherapy. A clinically significant improvement in functional scores was more frequent than a worsening for “mood” (40% vs 26%), whereas it was the opposite for “physical” (40% vs 49%), “activity” (24% vs 33%), and “cognitive” (26% vs 40%). Regarding the symptom scores, a clinically-significant improvement was more frequent than a worsening for “pain” (41% vs 26%), “insomnia” (40% vs 17%), and “loss of appetite” (32% vs 23%) while worsening was more frequently observed for “nausea and vomiting” (23% vs 34%). At enrollment, the median score for patient quality of life was 58.3 (range: 16.7; 91.7), using a 0–100 scale; and 61% of them reported a clinically-significant improvement or stability at the end of the radiotherapy (46% and 15%, respectively).
Discussion
The severity of BTcP impacts patients’ quality of life, increases the risk of depression and anxiety, and lowers functional capacities.7,8 FPNS has a rapid onset consistent with the timeline of BTcP, and incorporates a proprietary pectin-based gelling agent that reduces drip and run-off.27 This real-world study, conducted in patients undergoing radiotherapy for H&N cancer, showed high rates of patient satisfaction regarding the efficiency, tolerability and ease of use of FPNS during radiotherapy.
Of the 120 patient evaluations of FPNS efficiency during the 24-hours prior to weekly radiotherapy visits, 73.3% (95% CI: 65.4–81.2) were positive, which was consistent with findings of a previous clinical trial (70% of BTcP episodes were considered positive).25 In addition, 80% of treated patients had at least one positive assessment of FPNS efficiency. TNM stage T1-2 was the only independent predictive factor of patient satisfaction regarding FPNS efficiency (p=0.029). For tolerability and ease of use, 90% and 88% of patients respectively were “satisfied” or “very satisfied” with FPNS at least once, and 87% (95% CI 81–93%) of all the patient assessments were positive for both parameters. These high rates of satisfaction are consistent with previous findings: in a recent study conducted in German palliative care centers, FPNS received a rating of “very good” or “good” for tolerability from 87% of cancer patients;28 ease of use had a positive satisfaction evaluation in previous studies in up to 77% of patients.13,16 All these results were also corroborated by the small number of FPNS discontinuations due to adverse events or perceived inefficacity (10%) during the radiotherapy period. Altogether, these results show the real-life usefulness of FPNS treatment in a specific cancer patient population in which pain management is crucial: patients older than those included in previous interventional studies on FPNS,13,16 H&N cancer - considered particularly painful and with consequences on patients’ nutritional status (22% of patients had weight loss ≥10% within the month prior to enrollment), disease stage IV (55% of cases), ongoing radiotherapy leading to frequent oral mucositis with severe BTcP associated with swallowing disorders (76% of patients with grade 2–4 dysphagia at enrollment), and combined chemotherapy (53% of patients). In this context, BTcP management with FPNS may help patients to continue their cancer treatment, particularly radiotherapy (only one premature discontinuation was observed in the PecDICO study).
In addition, using the 0–100% scale of the BPI, the percentage of pain improvement with ongoing treatments increased from 52% before starting FPNS to 66% at the end of radiotherapy, confirming the real-life efficacy of the treatment. Nevertheless, the median BPI score for the impact of pain on patient quality of life remained stable over this period. This result was consistent with the overall score of the EORTC QLQ-C30, which only showed a slightly higher improvement than worsening during radiotherapy (46% vs 39%), suggesting that BTcP management is not enough to improve quality of life in such patients. That being said, the overall quality of life of H&N cancer patients was maintained under radiotherapy.
Before enrollment, background pain treatment was based on ER/LA opioids (transdermal fentanyl: 92%) in combination with IR oral opioids in 40% of patients, with a daily dosage of at least 60 mg, in agreement with the definition of controlled background pain as required for the use of FPNS. Most BTcP episodes were predictable, with very variable intensity.
FPNS showed an acceptable safety profile, similar to that observed in previous controlled clinical trials,13,16 and in a recent German non-interventional study:28 events related to FPNS were typical of opioids and none of them were serious. The rate of treatment withdrawal due to adverse events (6%) was in accordance with the 5% already reported in a previous clinical trial.13 In addition, of all the BTcP episodes treated with self-administrated FPNS in the PecDICO study, only 2 cases of misuse or abuse, and 1 overdose were reported, confirming that the treatment may be used safely in cancer outpatients.
The main limitation of this study was related to the limited number of participating radiotherapy centers (n=8), and which were not randomly selected, preventing the study from reflecting the overall practices of French radiation oncologists managing pain related to H&N cancer. These participating clinicians, practicing throughout France, belonged to a group of radiation oncologists regularly prescribing FPNS to treat patients’ BTcP, and agreeing in their daily practice with the French regulatory authorities’ definition of BTcP.11 Consequently, this selection of clinicians made it possible to circumvent the varied definitions of BTcP still present at the time of start of the study. In our study, the stricter BTcP definition applied, relating to the usual practices of selected radiation oncologists, should lead to robust efficiency results even if the downside was a smaller patient population. Another potential limitation of the PecDICO study was the amount of missing data during patient follow-up - notably regarding the completion of BTcP diaries by patients - which may have led to some reporting bias, as often in real-life studies, particularly in cancer patients with an advanced disease stage.
Conclusions
This study, together with previously reported evidence, tend to show that in H&N cancer patients treated with strong opioids (at least 60mg per day of oral morphine equivalent) for background pain, the addition of FPNS for BTcP treatment may help to maintain their quality of life and limit radiotherapy discontinuations. The majority of patients using FNPS throughout radiotherapy were satisfied with the efficacy, tolerability and ease of use of the treatment. FPNS had an acceptable safety profile, with non-serious events related to FPNS common to typical of opioids. Furthermore, the self-administration of FPNS was mainly in agreement with recommendations showing that cancer outpatients can handle this treatment securely and safely even in frail patients undergoing radiotherapy for H&N cancer.
Acknowledgments
The authors would like to thank all the patients included and who participated in this study. Assistance funded by Kyowa Kirin Pharma was provided by STATITEC (Carine Vera, logistic services and monitoring, data management, and statistical analysis), and AUXESIA (Marie-Odile Barbaza, MD, medical writing assistance under the direction of the authors).
Funding Statement
The study was supported by Kyowa Kirin Pharma.
Abbreviations
AE, adverse event; BTcP, breakthrough cancer pain; CI, Confidence Interval; ECOG, Eastern Cooperative Oncology Group; ER/LA, extended-release/long-acting; FPNS, fentanyl pectin nasal spray; H&N, head and neck; IR, immediate release; ROO, rapid-onset opioids.
Data Sharing Statement
Data are not publicly available, but may be provided upon request.
Ethics Approval and Informed Consent
In agreement with the French regulation for non-interventional studies, the study protocol #CP090/13, Version 1.0 dated March 25, 2014) was approved by the Consultative Committee on Information Processing for Research in the Field of Health (Comité Consultatif sur le Traitement de l’Information en Matière de Recherche dans le Domaine de la Santé, CCTIRS) and was authorized by the National Commission on Data Processing and Liberties (Commission Nationale de l’Informatique et des Libertés, CNIL) prior to implementation, on respectively June 18, 2014 and October 14, 2014. Similarly, tacit approval by the French College of Physicians (Conseil National de l’Ordre des Médecins, CNOM) was obtained on April 12, 2015.
In agreement with the French laws on non-interventional studies, the participating clinicians gave an oral information to patients on the study, supported by an information sheet provided to each patient. Patients gave then a verbal consent to participating to the study without any objections for the use of his/her personal data.
Author Contributions
All authors were involved in the study design, data interpretation, revising the manuscript critically for intellectual content, and in the final approval of the version to be published. YP, RJB, GB, CS, AR, GJ, MB, and BP were also involved in data acquisition. VB and XA were also involved in the study conception. All authors agree to be accountable for all aspects of the work.
Disclosure
YP has participated in advisory boards for BMS, Kyowa Kirin Pharma, Merck, MSD, and Pierre Fabre Oncology, and in speakers’ bureau for BMS and MSD. He is also a paid consultant to MSD and BMS. RJB and AR have participated in advisory boards for Kyowa Kirin Pharma. GB has participated in advisory boards for BMS, Janssen and Sanofi, and he is also a paid consultant to Janssen. GJ has participated in advisory boards for Kyowa Kirin Pharma and Pierre Fabre Oncology, and in speakers’ bureau for BMS and Merck. VB and XA are employees at Kyowa Kirin Pharma. BP, GC, MB and CS have no relationships to declare. The authors report no other conflicts of interest in this work.
References
- 1.Burton AW, Fanciullo GJ, Beasley RD, Fisch MJ. Chronic pain in the cancer survivor: a new frontier. Pain Med. 2007;8(2):189–198. doi: 10.1111/j.1526-4637.2006.00220.x [DOI] [PubMed] [Google Scholar]
- 2.Fallon M, Giusti R, Aielli F, et al. Management of cancer pain in adult patients: ESMO clinical practice guidelines†. Ann Oncol. 2018;29(Supplement_4):iv166–iv191. doi: 10.1093/annonc/mdy152 [DOI] [PubMed] [Google Scholar]
- 3.Davies AN, Dickman A, Reid C, Stevens A-M, Zeppetella G. science committee of the association for palliative medicine of Great Britain and Ireland. The management of cancer-related breakthrough pain: recommendations of a task group of the science committee of the association for palliative medicine of Great Britain and Ireland. Eur J Pain. 2009;13(4):331–338. doi: 10.1016/j.ejpain.2008.06.014 [DOI] [PubMed] [Google Scholar]
- 4.Mercadante S, Radbruch L, Caraceni A, et al. Episodic (breakthrough) pain: consensus conference of an expert working group of the European association for palliative care. Cancer. 2002;94(3):832–839. doi: 10.1002/cncr.10249 [DOI] [PubMed] [Google Scholar]
- 5.Davies AN, Dickman A, Farquhar-Smith P, Webber K, Zeppetella J. incorrect use of the english language term “episodic.”. J Pain Symptom Manage. 2016;52(5):e1. doi: 10.1016/j.jpainsymman.2016.06.002 [DOI] [PubMed] [Google Scholar]
- 6.Deandrea S, Corli O, Consonni D, Villani W, Greco MT, Apolone G. Prevalence of breakthrough cancer pain: A systematic review and a pooled analysis of published literature. J Pain Symptom Manage. 2014;47:57–76. doi: 10.1016/j.jpainsymman.2013.02.015 [DOI] [PubMed] [Google Scholar]
- 7.Breivik H, Cherny N, Collett B, et al. Cancer-related pain: a pan-European survey of prevalence, treatment, and patient attitudes. Ann Oncol. 2009;20:1420–1433. doi: 10.1093/annonc/mdp001 [DOI] [PubMed] [Google Scholar]
- 8.Portenoy RK, Bruns D, Shoemaker B, Shoemaker SA. Breakthrough pain in community-dwelling patients with cancer pain and noncancer pain, part 1: prevalence and characteristics. J Opioid Manag. 6(2):97–108. doi: 10.5055/jom.2010.0009 [DOI] [PubMed] [Google Scholar]
- 9.CI RIPAMONTI, BAREGGI C. Pharmacology of opioid analgesia: clinical principles In: Bruera ED, Portenoy RK, editors. Cancer Pain. Cambridge: Cambridge University Press; 2009:195–229. doi: 10.1017/CBO9780511642357.012 [DOI] [Google Scholar]
- 10.Caraceni A, Davies A, Poulain P, Cortés-Funes H, Panchal SJ, Fanelli G. Guidelines for the management of breakthrough pain in patients with cancer. J Natl Compr Canc Netw. 2013;11(Suppl 1):S29–36. doi: 10.6004/jnccn.2013.0211. [DOI] [PubMed] [Google Scholar]
- 11.HAS. Les médicaments des accès douloureux paroxystiques du cancer; 2014. Available from: http://www.has-sante.fr/portail/jcms/c_952643/les-medicaments-des-acces-douloureux-paroxystique. Accessed November12, 2019.
- 12.Zeppetella G, O’Doherty CA, Collins S. Prevalence and characteristics of breakthrough pain in cancer patients admitted to a hospice. J Pain Symptom Manage. 2000;20(2):87–92. doi: 10.1016/S0885-3924(00)00161-5. [DOI] [PubMed] [Google Scholar]
- 13.Portenoy RK, Burton AW, Gabrail N, Taylor D. A multicenter, placebo-controlled, double-blind, multiple-crossover study of fentanyl pectin nasal spray (FPNS) in the treatment of breakthrough cancer pain. Pain. 2010;151:617–624. doi: 10.1016/j.pain.2010.07.028 [DOI] [PubMed] [Google Scholar]
- 14.Coluzzi PH, Schwartzberg L, Conroy JD, et al. Breakthrough cancer pain: A randomized trial comparing oral transmucosal fentanyl citrate (OTFC®) and morphine sulfate immediate release (MSIR®). Pain. 2001;91:123–130. doi: 10.1016/S0304-3959(00)00427-9 [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. WHO guidelines for the pharmacological and radiotherapeutic management of cancer pain in adults and adolescents; January 2019. Available from: https://apps.who.int/iris/bitstream/handle/10665/279700/9789241550390-eng.pdf?ua=1. Accessed May8, 2020. [PubMed]
- 16.Davies A, Sitte T, Elsner F, et al. Consistency of efficacy, patient acceptability, and nasal tolerability of fentanyl pectin nasal spray compared with immediate-release morphine sulfate in breakthrough cancer pain. J Pain Symptom Manage. 2011;41:358–366. doi: 10.1016/j.jpainsymman.2010.11.004 [DOI] [PubMed] [Google Scholar]
- 17.Fisher A, Watling M, Smith A, Knight A. Pharmacokinetic comparisons of three nasal fentanyl formulations; pectin, chitosan and chitosan-poloxamer 188. Int J Clin Pharmacol Ther. 2010;48:138–145. doi: 10.5414/CPP48138 [DOI] [PubMed] [Google Scholar]
- 18.Van Den Beuken-van Everdingen MHJ, De Rijke JM, Kessels AG, Schouten HC, Van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007;18:1437–1449. doi: 10.1093/annonc/mdm056 [DOI] [PubMed] [Google Scholar]
- 19.Bhatnagar S, Upadhyay S, Mishra S. Prevalence and characteristics of breakthrough pain in patients with head and neck cancer: a cross-sectional study. J Palliat Med. 2010;13:291–295. doi: 10.1089/jpm.2009.0266 [DOI] [PubMed] [Google Scholar]
- 20.Prieto I, Pardo J, Perez-Casas A, Olivera J, Luna J, Vara J. Fentanyl pectin nasal citrate (FPNC) to control breakthrough pain (BP) and improve dysphagia in head-and-neck cancer patients receiving radiation therapy (RT). Int J Radiat Oncol. 2012;84:S517. doi: 10.1016/j.ijrobp.2012.07.1378 [DOI] [Google Scholar]
- 21.European Medicines Agency. PecFent. Summary of Product Characteristics. Available from: https://www.ema.europa.eu/en/documents/product-information/pecfent-epar-product-information_en.pdf. Accessed November12, 2019.
- 22.ADELF. Recommendations for professional standards and good epidemiological practices (version France 2007). Available from: http://adelf.isped.u-bordeaux2.fr/Portals/0/DOCUMENTATION/Recommandations_Version_Anglais-Janv.2008.pdf. Accessed November12, 201. [PubMed]
- 23.Cleeland CS, Ryan KM. Pain assessment: global use of the brief pain inventory. Ann Acad Med Singapore. 1994. [PubMed] [Google Scholar]
- 24.Aaronson NK, Ahmedzai S, Bergman B, et al. The European organization for research and treatment of cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365 [DOI] [PubMed] [Google Scholar]
- 25.Taylor D, Galan V, Weinstein SM, Reyes E, Pupo-Araya AR, Rauck R. Fentanyl pectin nasal spray in breakthrough cancer pain. J Support Oncol. 2010. [PubMed] [Google Scholar]
- 26.Czernichow P, Chaperon J, Le Coutour X. Enquêtes descriptives PAR sondages In: Epidémiologie. Connaissances et pratiques [Epidemiology. Knowledge and practices]. Paris: Masson; 2001:84. [Google Scholar]
- 27.Castile J, Cheng YH, Simmons B, Perelman M, Smith A, Watts P. Development of in vitro models to demonstrate the ability of PecSys®, an in situ nasal gelling technology, to reduce nasal run-off and drip. Drug Dev Ind Pharm. 2013;39:816–824. doi: 10.3109/03639045.2012.707210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ueberall MA, Lorenzl S, Lux EA, Voltz R, Perelman M. Efficacy, safety, and tolerability of fentanyl pectin nasal spray in patients with breakthrough cancer pain. J Pain Res. 2016;9:571–585. doi: 10.2147/JPR.S106177 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- HAS. Les médicaments des accès douloureux paroxystiques du cancer; 2014. Available from: http://www.has-sante.fr/portail/jcms/c_952643/les-medicaments-des-acces-douloureux-paroxystique. Accessed November12, 2019.
- World Health Organization. WHO guidelines for the pharmacological and radiotherapeutic management of cancer pain in adults and adolescents; January 2019. Available from: https://apps.who.int/iris/bitstream/handle/10665/279700/9789241550390-eng.pdf?ua=1. Accessed May8, 2020. [PubMed]
- European Medicines Agency. PecFent. Summary of Product Characteristics. Available from: https://www.ema.europa.eu/en/documents/product-information/pecfent-epar-product-information_en.pdf. Accessed November12, 2019.
- ADELF. Recommendations for professional standards and good epidemiological practices (version France 2007). Available from: http://adelf.isped.u-bordeaux2.fr/Portals/0/DOCUMENTATION/Recommandations_Version_Anglais-Janv.2008.pdf. Accessed November12, 201. [PubMed]