Abstract
Objective
CPP affects approximately 15% of women worldwide and has significant psychological, physical and financial impact on the lives of sufferers. Psychological interventions are often recommended as adjuncts to medical treatment for women with chronic pelvic pain (CPP). This is as women with CPP experience higher rates of mental health concerns and difficulties coping with their pain.. However, recent systematic reviews have highlighted that the efficacy of psychological interventions is not conclusive in this population. This review aimed to identify predictors of mental health outcomes and effective psychological techniques and interventions in women with CPP to inform the development of future psychological therapies.
Methods
Scoping review using the method outlined by Arskey & O’Malley (2005). Relevant databases, reference lists and grey literature were searched to identify effective mental health interventions and predictors of psychological outcomes for women with CPP.
Results
Methodological concerns made identifying predictors of mental health outcomes and effective psychological interventions difficult. However, cognitive behavioural therapy and Mensendieck therapy emerged as therapeutic interventions with the best evidence for women with CPP. A number of useful predictors of mental health outcomes and techniques included in effective interventions were identified.
Conclusion
The evidence provided in this review has the potential to inform future research directions and the development of targeted psychological interventions for women with CPP.
Keywords: chronic, pelvic, pain, psychology, predictors
Plain English Summary
It is not clear whether psychological therapies help women with chronic pelvic pain conditions.
Prior reviews have reported poor study quality in this area of research.
What is known about psychological therapies for women with chronic pelvic pain has not been outlined clearly.
There have not been any studies which have reviewed what predicts mental health outcomes in this group.
This study used a scoping review design to look at what is known about the content of psychological therapies for women with chronic pelvic pain.
This study looked at information on predictors of psychological outcomes.
These pieces of information could be used to form and test future psychological interventions.
Gaps in the research literature and future research directions are also discussed.
Introduction
Chronic pelvic pain (CPP) is defined as pain existing between the hips and below the umbilicus that has been present on most days for more than three months.1,2 It is estimated that around 25% of Australian women and 15% of women worldwide have CPP.3 CPP may be associated with a number of known medical conditions, such as endometriosis or may be unexplained. The exact economic cost of CPP is unknown, but it is believed to be high. For example, a recent assessment of the cost of one pelvic pain condition, endometriosis, reported that endometriosis costs the Australian economy 7.4 billion dollars per year.4 Estimates from the United States have suggested that it costs the US economy 22 billion dollars per annum.5–7 Despite the prevalence of pelvic pain worldwide, economic and personal costs attributed to CPP, there appears to be a limited understanding and awareness of this condition in non-specialist health settings and the general public.2
Best practice guidelines by Meselink, Browenowski and Hughs,8 that were backed by The International Association for the Study of Pain (IASP),1 have recommended multidisciplinary treatment for CPP conditions. However, exactly which professions and interventions are suitable as adjuncts to medical treatments remain unclear. Psychological intervention is a commonly recommended for people with CPP to assist with mental health and pain management. However, as yet, there is limited research available to support this recommendation. The lack of identification of factors that contribute to negative mental health outcomes in women with CPP has hampered the development of effective psychological interventions in this group. There is also no consensus as to which pre-existing psychological interventions are most effective for women with CPP. The limited and poor quality randomized-control trials (RCT) that do exist have failed to provide the evidence-base clinicians require to guide treatment in this area.9
This scoping review considers the current literature relating to psychological interventions and predictors of mental health outcomes for women with CPP. This review has three purposes. Firstly, to identify predictors of mental health outcomes for women with CPP, so that they can be considered as the basis for the development of new psychological therapies to improve mental health for this group. Secondly, to establish which psychological interventions are effective for women with CPP. Thirdly, to establish gaps in the literature and future research directions. This review builds on previous systematic reviews on CPP and commonly associated conditions such as endometriosis9–11 and includes the latest RCTs and qualitative studies. The literature has presented ample evidence for the medical and surgical treatment of CPP, when required, as effective in improving mental health outcomes.12–16 Therefore, studies that focused on predictors of psychological outcomes due to surgical and medical interventions were excluded, as this review aimed to identify predictors that could be targeted by mental health interventions.
Methods
This study used the scoping review methodology outlined by Arskey and O’Malley17 and Peters et al.18,19 and followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) check list.20 The steps that this study followed according to Askey and O’Malley17 included: developing a search strategy based on the concept, context, inclusion and exclusion criteria, study types and outcomes of interest. This search strategy was then developed in Medline, and was then extended to search other databases, key journals, grey literature and reference lists that had different search terms. The results of the scoping exercise were then extracted and mapped in table form to establish the results. The details of this procedure are listed below.
Study Inclusion and Exclusion Criteria
Eligible studies were those that included participants over the age of 18 who met the criteria for CPP, whether associated with a known medical condition or unexplained. Participants had to meet the definition of CPP outlined by the IASP,1 which is pain existing between the hips and below the umbilicus that has been present on most days for more than three months. While CPP may also affect males and girls below the age of 18, this review considers only adult females, as experiences related to chronic pain can differ in younger in comparison to adult age groups, and men compared to women.21
CPP may be present across a wide range of medical conditions. CPP conditions have been separated into gynaecological, urological, gastrointestinal, musculoskeletal, unexplained or other in a paper by Howard et al in 2003.22 The full list as to the conditions across each category can be accessed within Howard et al.22 Disorders were allocated into these categories based on the area of research literature and health professional relevant to them.22 Though CPP conditions across these areas can occur co-morbidly, they must be researched separately so that findings are clinically applicable to each attributed cause and relevant treating professional. Therefore, this review limited the scoping exercise to conditions and studies relevant to gynaecology. This meant that only conditions listed in the gynaecological CPP or unexplained gynaecological pain categories listed in Howard et al.22 were included.
Studies investigating CPP related to gastrointestinal, musculoskeletal and other medical conditions in the Howard22 paper were excluded, as they are regarded as separate areas of investigation in the literature. Participants who reported pelvic pain solely associated with intercourse, their menstrual cycle or cancer, have also been excluded. This is because these conditions are also considered as separate to CPP in the research literature.
Eligible studies must have considered either predictors of psychological outcomes or the effectiveness of a psychological intervention. Treatments other than psychology, no intervention and medical intervention alone were excluded for the psychological intervention component of this review. Studies in the predictor component of the review were excluded if their results could not contribute to the formulation of psychological interventions in future. For instance, studies looking at whether medical intervention or surgery improved mental health outcomes. Studies that considered non-psychological outcome measures have also been excluded for the portion of this review that relates to predictors. Previously conducted systematic and scoping reviews results were excluded.
Concept
There were three key concepts considered as the basis for this review. Firstly, the need to find out what psychological therapies and techniques had previously been considered to be effective for women with CPP. Secondly, the need to establish what predicted mental health outcomes for women with CPP conditions. As if the evidence for the overall therapies was weak, then this information could be used to identify which factors might be worth targeting in the development of future psychological therapies for this group. Thirdly, in summarising the literature on psychological treatments and predictors of mental health outcomes for women with CPP, the gaps in the research literature that need to be addressed most crucially would emerge.
Context
Studies included extended across many different care settings (acute, primary, community), locations (countries, cultures, geographical) and disciplines working within mental health (psychologists, counsellors, social workers, mental health nurses, psychiatrists and medical professionals).
Types of Studies
The review considered quantitative experimental and quasi-experimental designs, including randomized controlled trials, non-randomized controlled trials, before and after studies and interrupted time-series studies. Analytical observational study designs were also considered, including prospective and retrospective cohort studies, case control studies and analytic cross sectional studies. In addition, descriptive observational studies were included, such as case series, individual case reports, analytical observation and descriptive cross-sectional designs.
Qualitative study designs were considered, including designs drawn from perspectives such as phenomenology, grounded theory, ethnography, qualitative description, action and feminist research. Text and opinion papers were also be included in this review.
Published and unpublished studies were sought out using database, google and reference list searches. Papers included were published in English and were from the date range starting with the earliest relevant publication up until March, 2018.
Outcomes
The outcomes of interest for this study were: predictors of mental health outcomes in women with CPP, psychological interventions which have demonstrated effectiveness in improving mental health outcomes for women with CPP, research gaps and consequent future research directions.
Search Strategy
An outline of the Population, Intervention, Comparison, Outcome (PICO) search strategy used in this review can be seen in Table 1. This scoping review followed the search strategy for scoping reviews outlined by Arskey and O’Malley17 with assistance from information drawn from Peters et al.18,19 Relevant studies for including were identified using a four-step search strategy.
Table 1.
Basic Overview of Population, Intervention, Comparison, Outcome (PICO) Search Strategy
| Review Section | Population | Intervention | Comparison | Outcomes |
|---|---|---|---|---|
| Part A: Psychological interventions | Women/woman/females AND Chronic Pelvic Pain | Psychological intervention | Control group OR standard care OR placebo | Any outcomes |
| Part B: Predictors of mental health | Women/woman/females AND Chronic Pelvic Pain | Identification of predictors of mental health outcomes | N/A | Mental health outcomes |
First, a title, abstract and index term search of electronic databases relevant to this topic area was conducted. The databases included Medline, Scopus, Cochrane database, the JBI systematic review library, Web of Science, Embase, Ovid, PubMed, PsychArticles and PsychInfo. An academic librarian from the University of South Australia was consulted in reference to the adequacy of the search. An outline of the search strategy used for this review can be seen in Table 2. This was initially developed in Medline then adjusted according to the Medical Subject Headings (MeSH) terms appropriate for each database.
Table 2.
Sample Search Strategy Used Within Medline Database
| Search Term | Search Strategy |
|---|---|
| Women | 1. Exp women/ |
| 2. (women or woman).mp [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] | |
| 3. 1 or 2 | |
| Pelvic pain | 4. Endometriosis/ |
| 5. Exp Pelvic Pain/ | |
| 6. (persistent pain or Endometrioma* or endometrios*).mp [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] | |
| 7. Chronic Pelvic Pain | |
| 8. 4 or 5 or 6 or 7 | |
| Psychology | 9. Exp Psychotherapy/ |
| 10. mental health/ | |
| Intervention | 11. (narrative therap* or cognitive behavioural therapy psychodynamic or mindfulness or acceptance).mp [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] |
| 12. (Psychologic* and (Therap* or interven*)).mp [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] | |
| 13. or/9–12 | |
| 14. and/3, 8, 13 |
Abbreviations: Exp, exposure; mp, search field; therap, therapy; intervene, intervention.
Second, a hand search of key journals in this field was conducted, to identify articles that may have been missed in the database and reference list searches. This step included searching the journals PAIN, European Journal of Pain, Pain Medicine, Obstetrics and Gynecology, and Journal of Pain.
Third, a search of grey literature from sources including relevant existing networks, organizations and conference proceedings was conducted. This included searches of Proquest dissertation and theses, COS conference papers index, Academic Search Complete, WOS conference proceedings citation index and google scholar. Trial registries including Australia and New Zealand Clinical Trial Registry (ANZCTR), National Institute of Health via Clinicaltrials.gov, and the World Health Organization (WHO) trial registry were searched as part of this step.
Fourth, a search of the reference lists of the relevant articles resulting from the initial database search was conducted once the search results were transported into Covidence (Veritas Health Innovation, 2018), in order to identify any further studies that were not included in the scoping exercise.
Data Extraction
Studies and sources extracted from the search strategy steps were stored in EndNote X8.2 Library23 before being transferred to the Covidence24 program for analysis. To add extra rigor to the review, data extraction quality assessment for the included papers was conducted using the data extraction tools for quantitative and qualitative methodologies by the Johanna Briggs Institute.25 The data extracted included specific details about population, concept, context, and study methodology of significance to the scoping review and its objectives. Extraction and analysis of the data was conducted by two independent reviewers. Conflicts that were not resolved via discussion were resolved by a third independent reviewer.
Data Mapping
The extracted data has been presented using diagrams and tabulation in a manner suited to the objectives of this scoping review. The results have been reported based on the distribution of studies according to date of publication, country of origin, area of practice and research methodology. The tabulated/charted results have been accompanied by a narrative summary that describes how their results relate to the objective and questions of this review.
Results
Selection of Studies
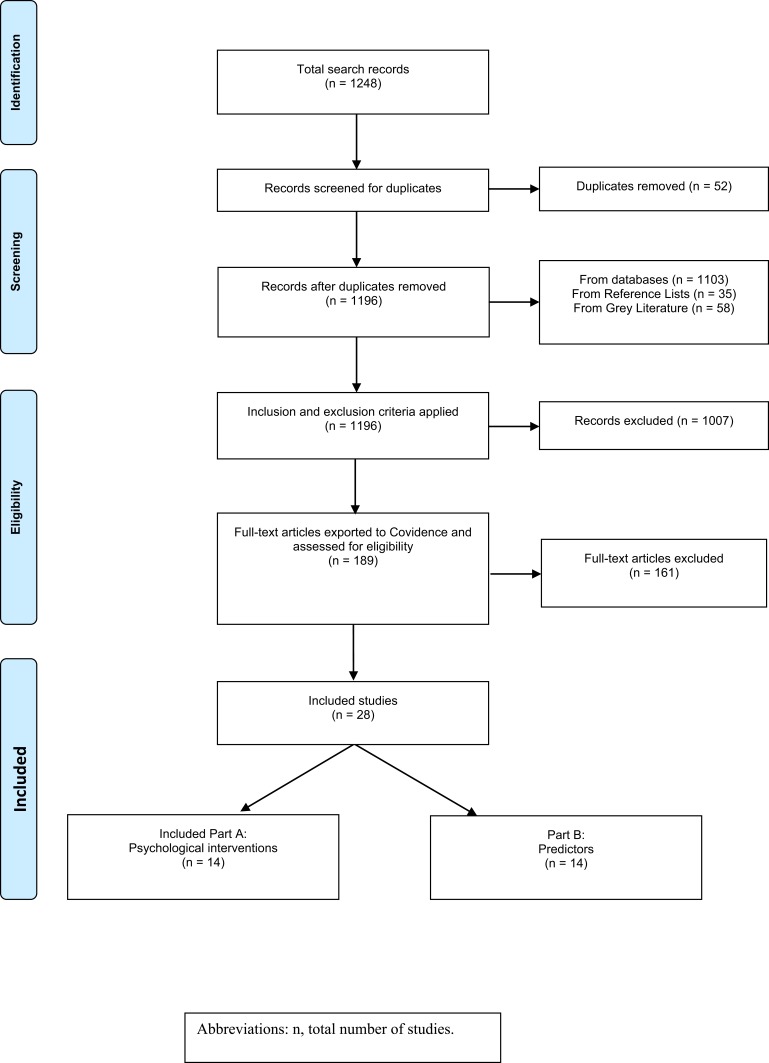
The article selection process is outlined in the Figure 1 PRISMA diagram. From the scoping exercise, 1248 studies were identified for potential inclusion in this review. After duplicate removal and screening, 28 studies met the inclusion criteria, with 14 meeting criteria for the psychological intervention component and 14 meeting criteria for the predictors component. A summary of each study, including characteristics and critique, is presented in Tables 3 and 4.
Figure 1.
PRISMA20 diagram.
Abbreviation: n, total number of studies.
Notes: PRISMA. The PRISMA statement; 2015. Available from: http://www.prisma-statement.org/.20
Table 3.
Overview of Results Extracted for Psychological Interventions for the Treatment of Women with CPP
| Author/Year/Publication Type | Setting, Methodology, Data Collection, Analysis | Participants, Size (n), Diagnoses, Age Range | Intervention | Results | Comments |
|---|---|---|---|---|---|
| Albert32 Journal article | Gynaecology outpatient clinic in Denmark. Pre-post design with 12 months follow up 1988–1997. Qualitative grounded theory, 4 pain drawings, McNemar t-test analyses. | Women with chronic pelvic pain. 18–48 years (M=30.5). Pain duration M=5.25 years. VAS pain score M=2.9. n=53 completed group therapy, n=17 drop outs. | 10 x weekly 2.5 hour group, 6 participants per group. First hour physical therapy combined with relaxation, grounding exercises, visualisation. Last 1.5 hours group psychology therapy (trauma conversations, body and mind connection, pain mechanisms, pelvic anatomy, sexuality, behaviour patterns, communication). | 39% pain free at 12 months follow up. VAS pain score decreased to M=0.9 (p<0.01). Analgesic unit decrease from M=8.5 to M=0.9 units per week. Benzodiazepines use decreased from 16 persons to 0. n=4 returned to work. Decreased sick leave and National Health Service use. Grounded theory identified four themes of improvement related to self-efficacy across therapy: self-knowledge, self-responsibility, self-active and self-control. | Used 2 different therapists, 6 different physiotherapists. Did not separate psychology versus physiotherapy treatment results. Potential confounding, such as other health conditions and prior treatment, were not considered. |
| Chao et al.39 (2015).Journal article | San Francisco hospital and California women’s health centre, USA.1 arm pilot cohort study, pre-post comparison. Measures PHQ-9, SF-36, EHP-5, SHOW-Q, pain catastrophizing scale, MYOP,/30 days health not good. | n=16 women with diagnosed CPP. Mean age 40 (range 23–63). | Monthly group 2-hour sessions based on three components of centering approach: understanding CPP, managing CPP, living with CPP Experiential exercises in pelvic floor therapy, mindfulness, guided imagery, and to provide information about anti-inflammatory diet and herbs provided. Centering Notebook that included educational material for at home. | Statistically significant improvements in EHP-5 score (p=0.01) and SF-36 sub-scales for role limitations, energy/fatigue, and social functioning (p<0.05). Number of unhealthy days reported in the previous month decreased from 24 to 18 (p=0.02). Sexual health outcomes showed statistically significant improvements (p=0.02). Women who attended 4 or more sessions had decreased severity in depressive symptoms (p< 0.05). Patients had improvements in symptom severity (p=0.01) and fewer limitations in activity (p<0.01). | Convenience sample. 61% completed study at follow up, but sample demographically comparable to larger CPP sample from geographic area. Pilot study only, hence no justification for sample size. 16 participants attended 4 or more sessions. |
| Farquhar35 Journal article | 4 arm RCT Measures were VAS pain ratings, pain improvement rating scale, side effects noted. | CEG_A_243337n=84 women with pelvic pain for over 6 months without explanation and venogram score 5+. Age M=29.8. Groups were placebo control (n=7), psychotherapy with placebo (n=26), psychotherapy with MPA (n=26) and MPA only (n=25). | Psychotherapy with MPA and MPA only groups took 50mg of the drug daily for four months. Psychotherapy included 6 x 45 minute sessions held fortnightly. Graded exercise, behavioural analysis of pain responses, positive coping strategies, increasing activity levels. Details of cognitive components and coping skills not published. Baseline measures, then measures at follow up after treatment concluded and at 9 months post treatment. | No significant difference found between psychotherapy group in comparison to placebo group with reference to pain ratings after treatment or at nine months post intervention. | n=12 withdrew, n=7 lost at follow up. Risk of attrition bias (18%). Not blinded to psychotherapy condition Wide confidence intervals with comparison to controls indicates high potential for error. |
| Friggi Sebe Petreluzzi et al.33 | Women’s Health Centre Brazil Within group pre-post design. Measures VAS, PSQ, SF-36, salivary cortisol. | n=26 women with Endometriosis and 7+ years of pain, mean age 32.2 (SD=1.3). | 10 x weekly 2.5 hour sessions. First hour physical therapy. Psychological 1.5 hours (CBT, education (pain, stress, family, endometriosis). Coping skills, breathing exercises, techniques to manage thoughts, symptoms and feelings. | No significant improvements in SF-36 scores after MANOVA conducted. Significant difference in pre versus post treatment salivary cortisol at 8am collection only (p<0.05). Pain intensity (p<0.05) negatively correlated with physical functioning and mental health. Social functioning positively (p<0.05) mental health, role emotional, physical functioning. | Unclear if psychologists worked together or separately. Confounding with potential to influence cortisol not considered Not possible to separate impact of physical versus psychological components of therapy due to study design. |
| Ghaly41 Journal article | Gynaecology clinic, Scotland. Random allocation trial. Measures HADS, MPS, pain duration in months. | n=100 (n=50 scan intervention, n=50 controls) women with CPP, pain duration 6+ months, laparoscopy negative for pathology. Age M=32.2 (SD=1.6), for intervention, M=32.5 (SD=1.5) controls. | Intervention ultrasound scan, education and counselling. Controls ‘wait and see’ with treatment as usual. Re-assessment was 4–9 months post ultrasound. | Difference in results for the 2 groups was significant (p<0.01) on MPS and HADS. | No inferential statistics. n=4 scan, n=6 controls lost to follow up. No power calculation, consistency of intervention not measured. Concealed random allocation but method not specified. Confounding not considered. Performance bias potential due to study design. |
| Hansen et al.28 Journal Article | Follow up from Kold et al.29 longitudinal study. Follow up measures of EHP-30, SF-36 | n=10 women with endometriosis who participated in a study by Kold et al.29 (listed later in this table) none lost to follow up. Age M=48.0 (SD=10.0). | Follow up and measures 6 years post Kold et al.29 study conclusion. | EHP-30 four out of five scales (pain, control and powerlessness, emotional wellbeing, social support) remained significantly improved. All 8 SF-36 scales that showed significant improvement remained improved 9/10 participants still used intervention techniques particularly body scan and breathing meditation. | This study found that results from the original Kold et al.29 study were maintained at 6 year follow up, but there were no significant changes between study conclusion and 6 year results. Hypothesized changes due to changes in psychological and emotional coping rather than pain intensity changes. |
| Haugstad et al.36 Journal article | Female deep pelvic pain clients from a gynaecology outpatient clinic at a tertiary hospital in Norway. RCT, Mesendieck therapy with gynaecology as usual compared to gynaecology as usual controls. Movement quality assessed with the SMT test (gait, movement and posture assessment). VAS pain scale. | n=40 women with deep pelvic pain (n=20 gynaecology treatment with Mensendieck therapy, n=20 standard gynaecology treatment control group). Age M=34.3 (SD=1.97) for therapy group, M=32.30 (SD=1.43). | Mensendieck assessment test with standardized video, then randomized to groups. 10 x 1 hour Mensendieck somatocognitive therapy across 90 days, with gynaecology treatment for intervention group. Gynaecology treatment as usual for control group. Post-treatment Mensendieck test and video. | Mensendieck somatocognitive sessions significantly higher in all aspects of the Mensendieck test (p<0.05). Significant reduction in VAS scores for Mensendieck group (decreased by 48.4%). Gynaecology treatment as usual group no significant difference in SMT or VAS scores. Hypothesis diaphragm breathing assisted with lymphatic drainage. | Inferred long term effects but follow up was after a short time period. May be difficult to incorporate Mensendieck therapy into primary care settings. Confounding considered and potential confounding excluded but not allowed for in final analyses. Researchers not blinded due to study design, unclear if participants blinded. No attrition figures stated. |
| Haugstad et al.27 Journal article | 1 year follow up of Haugstad et al.36 study. VAS and GHQ 30 item scale scores at end of treatment in comparison to 1 year post-intervention. | n=16 intervention participants and n=18 control participants, who previously participated in the Haugstad et al.36 study. n=6 lost to follow up. | VAS, SMT and GHQ-30 scales end of treatment in comparison to follow up after 1 year. | At 1 year follow up there were no significant improvements in SMT scales in comparison to follow up post treatment. | Initial power calculation from Haugstad et al.36 was for 20 participants, meaning this study was underpowered. |
| Kames et al.26 | Women with CPP referred to pain management clinic. Case control trial. Measures MMPI, CIPI, BDI, STAI, MPQ, VAS, 3 point scale rating of social and sexual functioning (worse, same, better). | n=22 women with CPP, n=8 waitlist controls with CPP. Pain duration M=4.5 years (SD=3.9), n=2 drop out during intervention, n=6 lost to follow up. | 3 phase trial; evaluation, treatment (6–8 weeks structured protocol), follow up at 6 months. Treatment group completed 2 x weekly acupuncture 1 x weekly psychotherapy sessions. Measures taken pre and post intervention and at 6 month follow up. | No significant change for waitlist controls. For treatment group, STAI, BDI, MPQ affective section, VAS, CIPI total score and activity interference significantly decreased post intervention (p<0.05). MPQ sensory no change in treatment group. At 6 month follow up changes maintained except STAI and MPQ scores, but these still remained significantly lower than pre-treatment measures. | 50% also took tricyclic antidepressant medication prior to commencing the study. 1/3 treatment group prescribed opioids for pain. Psychology therapy structured but not uniform. Short term psychology intervention. Participation rate not stated, no random allocation, no allocation concealment, groups not matched. No sample size calculation reported. High program satisfaction reported. |
| Kold et al.29 | Sample of women with endometriosis only that received treatment at Aarhus hospital Denmark. Within group pre-post comparison design. SF-36, EHP-30 measures. | n=13 women with endometriosis. Ranging from 14–37 years old (Md = 23). | 5 x 1.5 hour group sessions included psycho-education, mindfulness techniques, establishing relationship, group counselling. 5 x 1.5 hour individual sessions mindfulness techniques, supportive counselling, one session with partner. Measures taken pre intervention, post intervention, 6 months and 12 months post intervention. | Significant post intervention improvements in SF-36 bodily pain (p<0.001), physical functioning (p=0.018), role-physical (p=0.019), role-emotional (p=0.005), social functioning (p=0.014), general health (p=0.023). EHP-30 pain scale (p<0.001), control and powerlessness (p<0.001), emotional well-being (p<0.001), social support (p=0.008) significant improvements. No significant different in sexual intercourse scale. | No drop outs. Principal researcher excluded from data collection and analysis. Participant forms self-report and completed at home. No control group for comparison, limits causal conclusions. |
| Meissner et al.30 | German sample. March 2010–2012, RCT design. Measures fMRI of brain connectivity, 11 point numeric pain scale, physical and mental quality of life (SF-12, FW7, HADS anxiety and depression, STAI). | n=67 women with a verified history of endometriosis and pelvic pain (n=35 intervention group, n=32 waitlist controls). Age M=35.6. | Psychotherapy with somatosensory stimulation through acupuncture points versus waitlist control. 30–60 minute psychology sessions which included mindfulness, hypnotherapy, problem solving and CBT. On average 8 (IQR =8–10) sessions of 20–60 minute acupuncture and Chinese medicine sessions attended. | Psychotherapy with somatosensory stimulation significant (p<0.05) decrease in global pain, pelvic pain, dyschezia, depression, anxiety, functional wellbeing and significant increase in quality of life (mental and physical) in comparison to controls. Moderate to large effect sizes. Held at 6 and 12 month follow up. Brain areas and scan no significant findings. | Underpowered for control group at 6 and 24 month follow up. Unusual pain scale and some pain ratings were retrospective. Participants and researchers were not blinded. Confounding not considered. Can not draw conclusions linking brain mechanisms and psychological processes from results. Session content not set. Unsure whether Chinese medicine or psychology or both lead to results. |
| Norman et al.37 | Sample of women from USA, recruited from various pain clinics, media advertisements and through the Endometriosis Association. Prospective RCT. Measures MPQ, SIP, PANAS negative affect, AEQ, CSQ measures. Outcomes were ambivalence, catastrophizing, negative affect, mood. | n=48 with CPP (n=28 disclosure, n=20 positive control groups). Age M= 32.2 (SD=11.5). Endometriosis diagnosed in 50%. n=12 drop out. | Assessment, demographics and medical history taken. Given measures and wrote initial essays. Measures and essay at two month follow up Emotional exposure group wrote about their negative experiences with CPP, controls wrote about positive life experiences unrelated to CPP. | Initially after writing, emotional writing significantly decreased positive mood and increased negative mood, sadness, anger, guilt, fear and decreased happiness. But the disclosure group significant reduction in MPQ evaluative pain (p=0.01). Whereas control group not changed. Writing about stressful aspects of pain improved evaluative dimension of pain. Women with ambivalence to expressing emotions had the most benefit. See Norman et al.37 in Table 3 for moderators of group effects. | M=14.9 years education (11–18), 83% European. No power calculation. Participants were not blinded. 75% of participants had a history of diagnosed depression.8/12women gave reasons for drop out, feedback was it was too upsetting or too hard to think positively of their CPP. Follow up measures completed at home. |
| Poleshuck et al.31 | Women with CPP and MDD from 2 urban obstetrics and gynaecology and family medicine clinics in the USA. RCT design. Measures SCID-IV, BDI, HDRS, MPI, IPP, CSQ, PHQ2, SF36, pain intensity and interference rating. | n=61, women with CPP and MDD diagnoses. IPT treatment group (n=34) enhanced treatment as usual (n=28). IPT age M=36.3 (SD=8.2), treatment as usual M=37.1 (SD=9.8). | IPT versus treatment as usual. IPT group 8 sessionsacross 36 weeks, Assessments completed at 12, 24, 36 weeks at home or medical clinic. | IPT participants had significantly lower adjusted depression scores, significantly lower MDD occurrence, lower interpersonal sensitivity, lower interpersonal ambivalence and lower aggression in comparison to treatment as usual group (p<0.05). No significant differences between groups for pain. | n=24 declined to participate, n=85 agreed to participate but then lost to follow up. IPT group had higher pain ratings that treatment as usual group. No power calculation reported. Medications differed across participants. Treatment as usual group treatment was not standardized. Depression could influence engagement. |
| Zhao et al.38 | Han Chinese women with Endometriosis. Open label RCT. Measures STAI, HADS depression subscale, SF36, Health related quality of life pre and post intervention. Repeat measures | n=100 women with endometriosis. n=50 progressive muscle relaxation and gonadotrophin-releasing hormone agonist therapy group, n=50 controls who only underwent gonadotrophin-releasing hormone agonist therapy. Age range 18–40, split into 3 age groups (<25, 25–30, >30) | Progressive Muscle relaxation and gonadotrophin group did 2 x per week 40 minute group progressive muscle relaxation plus 2 at home CD recording sessions for 2 x day for 12 weeks plus one dose of depot leuprolide 11.25mg IM GnRH therapy. Controls had one dose of depot leuprolide 11.25mg IM GnRH. | Both progressive muscle relaxation and controls reported improved health related quality of life (p<0.01). Significant improvement in state anxiety (p<0.01), trait anxiety (p<0.01) and depression (p=0.01) in progressive muscle relaxation group in comparison to controls due to intervention over time. | n=67 declined to participate. n=13 drop outs. Confounding not considered. Blinding not stated. At home practice sessions self-reported. Stage III and IV endometriosis patients only. Current mental illness was excluded. |
Abbreviations: RCT, Randomized control trial; HPA, hypothalamic-pituitary-Adrenal; n, number of participants; SD, Standard deviation; M, Mean; HADS, Hospital Anxiety and Depression Scale; STAI, State-Trait Anxiety Index; SFHS-12, Short Form Health Survey; CNS, Central Nervous System; CBT, Cognitive Behavioural Therapy; MRI, Magnetic Resonance Imaging; p, significance statistic; PHQ-9, Patient Health Questionnaire-9 item; SF-36, Short Form Health Survey-36 item; EHP-5, Endometriosis Health Profile- 5 item; EHP-30, Endometriosis Health Profile-30 item, SHOW-Q, Sexual Health Outcomes in Women Questionnaire; MYOP, Measure Yourself Medical Outcome Profile; MPA, Medroxyprogesterone Acetate; PSQ, Perceived Stress Questionnaire; VAS, Visual Analogue Scale, MPQ, Magill Pain Questionnaire; HADS, Hospital Anxiety and Depression scale; SMT, Standardized Mensendieck Test; GHQ-30, General Health Questionnaire-30 item; MMPI, Minnesota Multiphasic Personality Inventory; CIPI, Chronic Illness Problem Inventory; BD, Beck Depression Inventory; Md; Mean Difference; fMRI, Functional Magnetic Resonance Imaging; FW-7, Functional Wellbeing 7-item; PANAS, Positive and Negative Affect Scale; AEQ, Ambivalence Over Emotional Expression Questionnaire; SIP, Sickness Impact Profile; IPT, Interpersonal Therapy; SCID-IV, Structured Clinical Interview for the Diagnostic and Statistic Manual fourth-edition; HDRS, Hamilton Rating Scale for Depression; MPI, Multidimensional Pain Inventory; IPP, Inventory of Interpersonal Problems; CSQ, Client Satisfaction Questionnaire; PHQ2, Patient Health Questionnaire 2; MDD, Major Depressive Disorder; PSI, Perceived Stress Index; MPA, medroxyprogesterone.
Table 4.
Predictors of Mental Health Outcomes in Women with CPP
| Author/Year | Setting, Methodology, Data Source | Participants, Size (n), Diagnoses, Age. | Results | Comments |
|---|---|---|---|---|
| Allaire et. al.45 | Women with CPP from an interdisciplinary pain clinic including counselling intervention in British Columbia. 1 year prospective cohort study design. EHP-30, service access reports, VAS pain ratings. | n=296 women with CPP. Age M=34.3 (SD=7.6). | Multi-disciplinary treatment in a team including psychology improved median pain severity and decreased emergency and physician visits after 1 year. Quality of life on the EHP-30 pain subscale improved. Higher chronic pain severity at 1 year associated with higher pain catastrophizing score at baseline. Counselling intervention included mindfulness skills (body scan, breathing, progressive muscle relaxation) and CBT to challenge thinking and beliefs. | 57% response rate at 1 year follow up. May not be generalizable to other cohorts. Did not consider potential moderators of catastrophizing at baseline. Participants were reported to have 2 visits to counselling. |
| Donatti et al.48 | Brazilian sample of women with Endometriosis. Prospective explorative within group design. COPE, BDI, LISS measures. | n=171 women with endometriosis. Age M= 39.5 (SD=5.6) years of age. | Higher depression scores predicted higher maladaptive strategies in solving daily life problems. Increased CPP severity predicted increased stress and increased depression scores. Participants who used positive coping strategies had lower depression scores and adapted better to stress. | Statistics appeared to be correlational than inferential and were not well described. Not reflective of population as a whole. No inferential statistics. Confounding not considered. Withdrawal participants not described. |
| Facchin et al.72 | Women with endometriosis from an Italian obstetrics and gynaecology department sample recruited 2012–2014. Cross-sectional study, between groups comparisons. Investigated whether endometriosis predicted personality factors on the TCI-R 240 item scale. | n=133 split into 3 groups: endometriosis with pain (n=58), endometriosis no pain (n=24) and controls no endometriosis or pain (n=51). | Women with pain and endometriosis had lower novelty seeking, lower exploratory excitability, higher harm avoidance, lower responsibility and higher fatigability in comparison to pain free endometriosis and control groups. Higher CPP severity predicted higher harm avoidance and lower self-directedness. | Small control sample, groups not matched. May not be generalizable to other cohorts. Included pain with sex and periods associated with endometriosis diagnoses. Model of personality related to Cloningers’ model (1998). |
| Facchin et al.46 | Italian women with endometriosis from a gynaecology department, recruited 2015–2017. Cross sectional study, within group design. Measures HADS, RRS for mental health outcomes. Rosenburg self-esteem scale, emotional self-efficacy scale. | n=210 endometriosis patients. Age M=36.7 (SD=7). | Being in a stable relationship was associated with decreased rumination on the RRS. Pelvic pain severity predicted anxiety, depression and rumination. Greater self-esteem, body esteem and emotional self-efficacy were associated with less anxiety, depression and rumination. | Potential confounding (eg Fertility, pregnancy, sexual concerns, beliefs about gender, cultural differences) was not considered. |
| Facchin et al.47 | Italian women with Endometriosis recruited from an endometriosis service 2014–2015. Grounded theory based interviews looking at how endometriosis affects mental health. Textual analysis of transcripts. Qualitative study. | n=74 women with Endometriosis. Age M= 36.44 (SD=6.9). | Women with high distress reported regular life disruption as a theme, where those without distress did not. Life disruption and continuity of care were affected by: pathway to diagnoses, quality of doctor-patient relationship, support (intimate and financial), female identity (body impact, fertility, sexuality), meaning of life with endometriosis. | One institution limits generalizability of results. Cultural and gender factors not considered. |
| Kaya et al.54 | Women with PP from Inonu University Medical Facility in Turkey, collected January – April 2000. Cross-sectional survey, quasi-experimental case control design. Measures BDI, BAI, STAI, GRIS. | n=19 women with CPP. Age M= 34.1 (SD=9.3), n=25 healthy controls, age M=30.6 (SD=7.3). | Avoidance, dissatisfaction and non-sensuality subscales of the GRIS positive correlation with depression and anxiety scores. Linked anxiety, depression and sexual dysfunction in women with CPP. | Underpowered. Non-parametric statistics. Statistical investigation stopped at correlations for some associations. |
| Low et al.52 | Women with CPP who presented to a UK gynaecology clinic with concerns related to CPP, infertility or both. Between groups comparison. EPQ, BDI, GHQ-30, STAI, GRIMS, MPQ-SF short form measures. | 3 groups of women separated by referral reason. n=61 CPP referral group, n=12 infertility referral group, n=15 CPP and infertility referral group. Measures completed 2 weeks pre-treatment and 13 months post-treatment. | Concluded anxiety associated with pain not fertility. Less psychopathy associated with less pain. | Small group sizes and no power calculation noted. Confounding not considered. n=58 had prior treatment, n=50 had prior surgical treatment. Pre and post assessments in different settings. CPP higher anxiety pre and post treatment in comparison to infertility group. |
| Norman et al.37 | US obstetrics and gynaecology sample of women with CPP. Prospective RCT. Demographics, medical history taken at assessment. Measures essays, MPQ, SIP, PANAS negative affect, AEQ, CSQ at assessment and two month follow up. | n=48 (n=28 disclosure, n=20 control groups). 18–64 (M=38.2, SD= 11.5) years old. n=12 drop out. For written emotional exposure, CPP group wrote about their negative experiences with CPP, controls wrote about positive life experiences unrelated to CPP | Baseline catastrophizing, negative affect and ambivalence were found to predict daily disability. However, baseline catastrophizing was no longer a significant moderator of daily disability over and above negative affect and baseline ambivalence. | No power calculation and high drop-out rate. Participants predominantly Caucasian. 75% of women reported previous depression diagnoses which may have influenced negative affect and ambivalence. |
| Oniszczenko et al.58 | Sample of women hospitalized for gynaecological reasons in Polish hospital. Cross-sectional study. PTSD-FVIT, FCB-TI, MSI, GHQ-28. | n=136 women with CPP, 18–60 (M=34.60, SD=9.92) years old. n=10 drop outs. | Emotional reactivity, anxiety and lovability explained 8%, 34% and 6% of the variance in PTSD FVIT scores respectively, 48% of total variance. Suggested esteem has a protective role in trauma for women with CPP. | No power calculation. Participants were not blinded. 75% of participants had a history of diagnosed depression. 8/12 women gave reasons for drop out, feedback was it was too upsetting or too hard to think positively of their CPP. Multiple diagnoses included. Follow up measures were completed at home. |
| Petreluzzi et al.51 | Women with CPP recruited from Brazilian Women’s Health Centre for endometriosis group, Between groups comparison. PSQ, HRQOL- SF-36, HPA axis activity from salivary cortisol, VAS for pain scores measures. | n=93 women with endometriosis and CPP non responsive to surgical or pharmacological treatment, n=82 controls made up of university staff and student volunteers. Endometriosis diagnosed by laparoscopy or laparotomy. Age endometriosis group M=33.85 (SD=1.04) controls M=30.9 (SD=0.92). | PSQ stress score higher for women with constant versus intermittent pain, pain intensity did not differ between groups. | Mental health conditions such as anxiety and depression can influence salivary cortisol. Antidepressants can be used to manage CPP in women and may influence cortisol concentrations. Participants had different levels of endometriosis. Did not consider many confounding variables. |
| Spinhoven et al.57 | Women with CPP recruited from gynaecology departments of two academic hospitals in the Netherlands. Cohort study, correlational design. CPP sample was one of four samples in this study. SDQ-20 and self-report measures. | n=52 women who had CPP for over six months. Age M= 37.8 (SD=9.7) years. | Positive association between self-reported physical abuse and SDQ-20 scores for somatoform dissociation remained after general psychopathy removed as a mediating factor. Suggested considering dissociation in physical abuse history. | Physical abuse was self-reported. |
| Toomey et al.55 | Women with CPP from a hospital based CPP clinic in North Carolina. Between groups design. Abuse history questionnaire, MPQ, MPI, FIS, SLC-90 measures. | n=36 women with CPP, n=19 reported abuse history, n=17 reported no abuse history. VAS pain M=4.91 (SD=5.95), age M=30.31 (SD=9.56) years. | 19/36 participants reported prior abuse. Abuse group reported less perceived control, greater punishing responses to pain, high somatization, higher global distress in comparison to non-abuse group. | t-test only for statistical analyses and no consideration of potential confounding. |
| Vannuccini et al.49 | Italian sample of women with endometriosis. Observational cross-sectional design. PHQ measure. | Women with endometriosis n=134. Age M= 34.8 (SD=6.3) years, BMI M=22.0 (SD=3.7). | Having a mental health condition was highly correlated with pain symptoms. Pain positively correlated with incidence of multiple psychiatric disorders, pain and somatoform disorders were positively correlated. | Chronic pain often diagnosed as somatoform disorder hence high association pain and somatoform disorder diagnoses. Response rate 89.3%. |
| Weijenborg et al.50 | Women with CPP who attended gynaecology outpatient clinic at Leiden University in the Netherlands, between July 2001-January 2006. Retrospective cohort study. Intervention was hospital team treatment. Rand-36, HADS. PCCL, McGill VAS on MPQ Dutch version. | n=84 women with CPP completed pre and post measures. Age M= 40.2 (SD=11.3) years. | Reduction in pain intensity and catastrophizing, as well as improvements to depressive symptoms and SF-36 physical scores at follow up in comparison to baseline. Baseline PCCL internal pain control subscale score was associated in changes in depression scores and baseline pain intensity. PCCL catastrophizing and internal pain control subscales were negatively correlated. | 64% response rate that completed baseline and follow up measures. Correlation analyses. |
Abbreviations: BAI, Beck Anxiety Inventory; BDI, Beck Depression Inventory; CPP, chronic pelvic pain; COPE, Brief Coping Orientation to Problems Experienced; EHP-30, Endometriosis Health Profile 30- item; GRIS, Golombok-Rust Inventory of Sexual Satisfaction; HADS, Hospital Anxiety and Depression Scale; n, sample size; LISS, Lipps Stress Symptom Inventory for Adults; M Mean; SD, standard deviation, RCT, randomized control trial; RRS, Ruminative Response Scale; STAI, Speilberger State Trait Anxiety Index; VAS, Visual Analogue Scale; EPQ, Eysenck Personality Questionnaire; GHQ-30, General Health Questionnaire 30-item; GRIMS, Golombok-Rust Inventory of Marital State; MPQ-SF, Mcgill Pain Questionnaire- short form; MPQ, Mcgill Pain Questionnaire; PTSD, Post-Traumatic Stress Disorder; PTSD-FVIT, Post-Traumatic Stress Disorder Factorial Version Inventory; FCB-TI, Formal Characteristics of Behaviour Temperament Inventory; MSI, Multi-dimensional Self-esteem Inventory; PSQ, Perceived Stress Index; HRQOL, Health Related Quality of Life; SF-36, Short Form Survey 36-item; SDQ-20, Somatoform Dissociation Questionnaire; PHQ, Patient Health Questionnaire; PCCL, Pain Appraisal and Pain Coping Scale; GHQ-28, General Health Questionnaire 28-item; FIS; Functional Interference Scale; SCL-90, Symptom Checklist 90 item; HPA, Hypothalamic-Pituitary-Adrenal; TCI-R, Temperament and Character Inventory –Revised; SIP, Sickness Impact Profile; PANAS, Positive and Negative Affect Schedule; AEQ, Achievement Emotions Questionnaire; CSQ, Client Satisfaction Questionnaire; PCOS, Polycystic Ovary Syndrome.
Psychological Interventions and Techniques Which Lead to Improved Outcomes
The use of CBT, Interpersonal Therapy and Mensendieck therapies were associated with improved mental health and pain outcomes.26–31 Techniques to manage thinking, identification and regulate emotions and coping skills were common features included in the CBT interventions. Studies such as Kames et al.26 utilized cognitive therapy in conjunction with education about pain, emotions, thinking and behavioural patterns and saw decreased pain affect, anxiety, depression, VAS pain scores, activity interference and problems related to chronic illness. They reported that at six months these changes were maintained and participants reported improved social activity, sexual activity and 65% improvement in pain scores. Decreased medication use and decreased morning cortisol levelswere seen following CBT interventions that included physical components. Changes to the Hypothalamic-Pituitary-Adrenal (HPA) axis, the interactions between the hypothalamus in the brain, pituitary gland and adrenals as part of the bodies’ stress response system, were also seen following CBT interventions with physical components.32–34
The findings with reference to interpersonal therapy varied, though there was consensus that it improved mental health outcomes. Interpersonal therapy significantly lowered depression scores, interpersonal sensitivity, interpersonal ambivalence and aggression in women with CPP in comparison to controls in the study by Poleshuck et al.31 However, there was no impact on pain outcomes reported in this study. Three studies found that improvements in mental health and pain outcomes were sustained or improved at long term follow up, with follow up being conducted after 6 months, one year or six years.26–28 One earlier study found no significant differences between psychotherapy and placebo groups on pain outcomes.35
Only one study examined the effect of Mensendieck somatocognitive therapy on CPP. It was found that women with CPP who completed Mensendieck somatocognitive therapy and gynaecology intervention reported significantly improved motor functions, coping on the GHQ-30 scale, anxiety, insomnia and distress as well as a 50% decrease in VAS pain score in comparison to those who only engaged with gynaecology at one year post intervention.27,36
A number of psychological techniques were used across interventions. These were either used as part of a wider psychological therapy as a whole or on their own. Mindfulness and relaxation exercises were commonly utilized with therapeutic interventions, with progressive muscle relaxation, sensory training, body scan and breathing exercises specifically mentioned. Hypnotherapy, problem solving, pacing, and written expression of emotions related to CPP were other techniques listed as part of interventions.28,29,37,38 Sleep and pain management were also mentioned, though the details of what was covered in these interventions was not published. At 12 month follow up the study by Kold et al.29 showed that psycho-education and mindfulness intervention lead to significant improvements in body pain, physical functioning, role physical and role emotional scores on the Short Form Health Survey 36-item (SF-36). The same study also reported improvements on pain scale, control and powerlessness, emotional wellbeing, social support measures on the Endometriosis Health Profile 30-item (EHP-30). Hansen et al.28 reported that these outcomes held at six year follow up and that ninety percent of participants still used the skills that they had learned in the study. Emotional writing was found to assist women with CPP in expressing and processing the stressful emotions associated with their pain and consequently was attributed to improved MPQ pain scores after two months despite the technique initially elevating guilt, anger, sadness, fear and unhappiness.37
Incorporating Pain Education in Psychological Interventions for CPP
Pain education emerged as one of the key components across psychological interventions that improved mental health outcomes for women with CPP conditions. Chao et al.39 found that pain education alone lead to improvements in physical health, energy, perceived limitations, social functioning and sexual health outcomes. However, this approach did not impact on physical functioning, emotional wellbeing or general health. Using grounded theory as a qualitative approach, Beissner et al.40 found that pain drawings increased in size and detail following psycho-education for pain and that women had a better visual and verbal understanding of pain with improved coping and education. Topics of pain education and management reported by the included studies were: types of pain, causes of pain, chronic pain treatment and diagnosis, mind-body interactions, tolerance, triggers and CPP management. Education on self-care, nutrition, communication, anxiety, sexual intercourse and intimacy were also seen in some interventions.26,29,39 An older study found that ultrasound in combination with education and counselling improved reported pain and anxiety.41 Interestingly, improvements in sex and intimacy related concerns only occurred in interventions specifically directed towards these areas, not with general mindfulness or psycho-education about pain.26,28,29,39
Overlap Between Mental and Physical Health
The studies which reported improvements in mental health outcomes were most commonly those that incorporated both mental and physical health components. Therapies utilizing CBT in combination with physical interventions reported improvements in pain severity, anxiety, perceived stress, physical and mental quality of life, global pain, pelvic pain, depression, functional wellbeing, physical functioning post-intervention.27,30,33,36,40 The physical therapy interventions and techniques that were reported as effective when in combination with psychological therapy included the following: acupuncture, Mensendieck therapy (lymphatic drainage, breathing, postural considerations), breathing exercises, stretching, medical treatment, medications, stretching, pelvic exercises, joint movement, body awareness training, massage, moving areas inactive due to pain, light daily exercise.27,30,33,36,38,40 Zhao et al.38 found that progressive muscle relaxation twice daily in conjunction with Leuprolide significantly improved depression, state and trait anxiety in women with CPP in comparison to controls. Leuprolide is a gonadotrophin releasing hormone that works by signalling the pituitary gland to stop stimulating the ovaries to produce estrogen. Thus, it induces amenorrhea, reducing pain, which has a cyclical worsening. Therefore, it is unclear whether the results of this study were obtained due to the progressive muscle relaxation component or the effect that Leuprolide had on the pituitary or estrogen for the women in this study. Interestingly, Meissner et al.30 found that CBT and acupuncture applied in combination did not influence brain connectivity observed using fMRI scan, despite the therapy leading to improvements in mental health outcomes.
Measures Used Across Studies
The most common measures of mental health in these studies used one or more of the Hospital Anxiety and Depression Scale (HADS), State-Trait Anxiety Inventory (STAI), Patient Health Questionnaire 9-item (PHQ-9), (SF-36), Beck Depression Inventory (BDI) or a pain catastrophizing scale to quantify the degree of anxiety, depression, and quality of life reported by subjects. These measures all have demonstrated reliability and validity in the research literature.42–44 Table 3 presents the results of the mental health intervention component of this scoping review.
Predictors of Mental Health Outcomes for Women with CPP
Pain as a Predictor of Mental Health Outcomes for Women with CPP
Pain severity and frequency predicted a number of mental health outcomes. Higher pain severity predicted higher anxiety, pain catastrophizing, depression, stress, harm avoidance, lower self-directedness and rumination.45–49 Baseline pain intensity was found to be positively correlated with depression scores.50 Constant pain led to higher reported stress for women with CPP in comparison to those with intermittent pain. Pain intensity was negatively correlated with physical functioning and general health for women who had endometriosis and CPP.51 Trait anxiety was also found to be associated with pain but not fertility.52 One study found that pain was correlated with the presence of reported somatoform disorder diagnoses.49
Anxiety and Depression Predicted Mental Health Outcomes for Women with CPP
Depression and anxiety, which includes the concepts of catastrophizing, pain catastrophizing and stress, predicted mental health outcomes across many studies. Catastrophizing was found to be negatively correlated with internal pain control and positively correlated with depression scores.50 Pain catastrophizing specifically was found to moderate physical and mental health outcomes, a finding supported by the wider literature on other chronic pain conditions.53 However, Norman et al.37 found that catastrophizing no longer acted as a significant moderator of daily disability once considering baseline negative affect and ambivalence over emotional expression as mediators.
Overall mental health scores on the SF-36 were negatively correlated with perceived stress for women with CPP and endometriosis. The same study also found that salivary cortisol was decreased in women with CPP and endometriosis in comparison to controls without. Therefore, poorer quality of life for women with CPP and endometriosis could be due to decreased cortisol levels and higher perceived stress.51 However, it was unclear whether this was a feature of endometriosis, pain or an adaptive response.
Anxiety and depression were found to be correlated for women with CPP. Physical quality of life, depressive symptoms and catastrophizing were improved when women with CPP engaged with a hospital team for treatment, emphasizing the importance of multi-disciplinary care.50
The Importance of Coping Skills
Maladaptive coping skills and avoidance emerged as key predictors of sexual dysfunction, esteem, depression and anxiety symptoms. Women with CPP were found to have higher avoidance, dissatisfaction and non-sensuality in sexual dysfunction in comparison to controls and that these were linked to their anxiety and depression symptoms.54 Higher depression scores also lead to more difficulties with problem solving for women with CPP.48 Positive coping skills, stable relationships, good self and body esteem were all associated with less depression symptoms and decreased thinking styles associated with depression, such as rumination.46,48
Supportive Relationships Improved Outcomes
Access to supports emerged as a key predictor of mental health outcomes. Grounded theory investigation showed that life disruption and continuity of care predicted pathways to diagnoses, doctor-patient relationship quality, intimate and financial support, female identity, body impact, fertility, sexuality and life meaning for women with endometriosis.47 Women with CPP who reported a shorter time since diagnosis reported higher HADS anxiety scores.46 These results suggest that access to medical and personal supports has the potential to improve mental health outcomes for women with CPP. However, it was interesting that no studies examined whether access to psychology for support was viewed as beneficial by women with CPP conditions.
When Abuse and Esteem Issues Were Present, Outcomes Were Worse
Abuse and esteem related beliefs predicted outcomes such as PTSD symptomology, depression and anxiety. Women with CPP who reported abuse reported less perceived control, greater punishing responses to pain, higher somatization and greater global distress than those who did not.55 Self-reported physical abuse in women with CPP was found to be positively correlated with somatoform dissociation, which can include loss of sensations, movement difficulties and analgesia.56 This was shown to occur after controlling for general psychopathy as a mediator. There were no recorded differences in dissociation between women with CPP who reported physical in comparison to sexual abuse.57 Emotional reactivity, anxiety and lovability on the FCB-TI scale were all found to correlate positively with PTSD symptom presence for women with CPP who had a history of trauma, and together predicted 48% of the variance in PTSD symptoms.58 Table 4 provides an outline of the results obtained for the predictors component of this scoping review.
Discussion
This scoping review aimed to identify predictors of mental health outcomes for women with CPP conditions and determine effective psychological interventions for this group. Following the scoping exercise, 14 studies met the inclusion criteria for each component of this review.
The Effectiveness of Psychological Interventions for Women with CPP Showed Varied Results
For the psychological interventions component, CBT, interpersonal therapy and Mensendieck therapies emerged as the interventions with the best evidence for use with women who have CPP at present.26,28-31,35,36 There were also a range of psychological techniques included in effective interventions without being attached to a wider psychological intervention. Techniques such as thought challenging, emotional regulation, relaxation and mindfulness skills were common across studies. These findings are a useful basis for the development of future psychological interventions for women with CPP. However, design flaws across noted across this study and problems with quality reported by all three previous systematic reviews in this area, mean that this evidence is regarded as weak at this stage.9–11
There is a large amount of research in the chronic pain literature giving evidence of overlap between the brain, body, emotions and cognition and the importance of explaining these dynamics to chronic pain sufferers as part of treatment.59 Recent research has highlighted the roles of the innate, central and peripheral nervous system and aspects in which they overlap with reference to maintaining chronic pain. This highlights why people with chronic pain can experience widespread and changing physical sensations which are genuinely experienced. Innate immune system activation has particularly been linked to chronic pain in females.59–62 There is also evidence that certain psychiatric concerns often co-morbidly occurring with CPP, such as Major Depressive Disorder, could result from changes in communication between immune and nervous system pathways.63 There is also suggestion that female and male pain as well as visceral in comparison to peripheral neuropathic pain may have different mechanisms.60 This evidence highlights the importance of considering both physical and mental health components for women with CPP, multi-disciplinary care and the importance of pain education.
The overlap between brain, body and cognition may explain why many studies that effectively improved mental health outcomes for women with CPP utilized both physical and mental health components. The overlap between mental and physical health components is widely known in the literature on both mental health and chronic pain. For instance, it is known that cognitive symptoms of anxiety can lead to physical symptoms such as muscle tension.69 There is a wide and emerging research base on chronic pain and the role of the brain.59
Psychological interventions using mental health intervention in combination with physical health interventions included in this review also saw significant reductions in pain severity and anxiety outcomes. However, this also made it impossible to decipher which psychological interventions and techniques specifically improved mental health outcomes for women with CPP. For instance, Mensendieck somatocognitive therapy, aims to train muscle groups in defined movement patterns, improved posture, movement, sitting and breathing. In studies utilizing this therapy in conjunction with cognitive techniques, such as those by Haugstad et al.,27,36 it was not possible to isolate whether findings were due to the cognitive components, the physical aspects or the combination of both. For studies using pain education and cognitive components, such as Kames et al.,26 it was not possible to attribute findings to the education component, cognitive aspects or both. This makes it difficult to decipher which techniques and combinations were effective and therefore worthwhile considerations for the development of future interventions for women with CPP. Some studies did not report which techniques or therapeutic approached that they used.26,30 Future studies would benefit from investigating whether set psychological interventions and techniques are effective for women with CPP conditions before considering combining them with therapies from other disciplines.
Pain education is an important component of psychological interventions for women with CPP conditions. Many interventions included in this review had included components of pain education, as it has been shown to help people with chronic pain understand and manage their pain and related psychological concerns.59 However, pain education was not consistent across studies. No studies reported what women with CPP thought of their interventions or pain education components of them, so it was not possible to see what they may have found most useful. However, the results suggested that when aiming for improvements in sexual intimacy concerns, specific psycho-education in this area is required as general pain education did not improve outcomes in this area.
Research into other chronic pain conditions tends to widely support the use of CBT to improve mental health outcomes, and somatocognitive therapy has evidence for use across gynaecological conditions.27,64,65 However, in the chronic pain literature, interpersonal therapy has been shown to be less beneficial to sufferers than CBT.65 Pain catastrophizing and perceived injustice, which have been shown to moderate physical and mental functioning in other chronic pain samples, were only considered in a few studies.53 Interventions and techniques that have widely been accepted as having good evidence in the wider chronic pain literature, such ACT hypnosis, have yet to be independantly tested with reference to CPP.66–68 For instance, techniques associated with ACT or hypnosis were mentioned across studies in this review, but they were not utilized as a whole therapy and the details of what exactly was used were not reported. Therefore, it would be beneficial to investigate whether these and other therapies and techniques with established evidence in the chronic pain literature are also effective for women with CPP. It would only be feasible to label the most effective psychological interventions and techniques for women with CPP once enough quality RCT studies have been conducted including therapies known to be beneficial from the wider chronic pain literature.
Predictors of Mental Health in Women with CPP
Our aim of identifying factors that predicted mental health outcomes yielded several results. Pain severity and frequency, anxiety, maladaptive coping styles, support access, esteem issues and abuse all emerged as predictors worth considering in the development of future psychological interventions for women with CPP. Future studies investigating the effectiveness of psychological therapies and techniques specifically targeting these aspects would be worthwhile. For instance, pain management, including techniques such as pacing and challenging beliefs around pain, could offer ways of managing frequent and severe pain experiences.73 CBT and ACT have a wide evidence base for addressing anxiety and thinking styles such as catastrophizing.66,67 Dialectical Behaviour Therapy and techniques drawn from it, such as radical acceptance and distress tolerance skills, have been shown to address long term maladaptive coping modes and behaviours in other populations.72,74
Interestingly, there was also no consideration of long term personality traits with the potential to influence results and participant engagement.72 Findings from Vannuccini et al.49 with reference to somatization being more present for women with CPP should be regarded with care, as it is common practice for all chronic pain conditions to be diagnosed as Somatic Symptom Disorder (chronic pain), previously labelled as Somatoform Disorder.
Anxiety and associated concepts such as catastrophizing emerged as key predictors of mental health outcomes. Anxiety and depression were found to be correlated for women with CPP, as supported in the wider mental health and chronic pain literature.69 Catastrophizing emerged as a key predictor of daily disability, as is supported in the wider chronic pain literature. However, findings by Norman et al.37 suggested that catastrophizing no longer predicted disability once the influence of negative affect and ambivalence over emotional expression were removed. Ambivalence over emotional expression as measured on the AEQ looks at conflict as to whether to express emotions. A recent study on Irritable Bowel Syndrome showed that depression scores were significantly reduced when participants completed relaxation training, but only if they had a high level of ambivalence over emotional affect at baseline.70 Therefore, psychological therapies including work on coping with and communicating emotions may prove beneficial. These results highlight that understanding and management of anxiety, stress and catastrophizing are key for mental health interventions for women with CPP. In future, it would be interesting to investigate whether findings on emotional ambivalence over emotional affect are replicable and consider interactions with mood in relation to it.
Coping skills and support emerged as key predictors of mental health outcomes. Avoidance of different types is a key feature of many mental health concerns, including anxiety, depression, chronic pain and PTSD. Because of this, many psychological therapies with demonstrated effectiveness for mental health and chronic pain conditions, such as CBT and ACT, include work on avoidance. Therefore it was not surprising that it emerged as a predictor of mental health outcomes for women with CPP. The results also suggested that coping skills, such as problem solving, and good supports in medical and personal relationships improved mental health outcomes for women with CPP.46,48,54
Abuse and esteem related concerns also emerged as predictors of mental health outcomes. This highlights the importance of identifying whether there are problems related to esteem or abuse as part of psychological intervention for women with CPP. Though the literature debates whether abuse history is more prevalent for women with CPP, these results indicate that when problems related to trauma are present it can influence aspects such as pain and the processing of emotions for women with CPP conditions. Elevated autonomic arousal, difficulties processing emotions and esteem concerns are all aspects seen in Posttraumatic Stress Disorder (PTSD). These aspects are specifically targeted by psychological interventions for PTSD, such as Cognitive Processing Therapy and Eye Movement Desensitisation and Reprocessing (EMDR). A study of 230 female public hospital patients with CPP by De Deus et al.75 found that 15.8% of their sample reported previously experiencing physical abuse and 11% sexual abuse. A large proportion (70%) of their sample reported that the onset of their CPP had occurred following a significant life incident, with the most common incidents reported being conflict or trauma. They also found that their sample reported an overall pain reduction of 39.2% following treatment (laparoscopic removal of endometriosis, pharmacological treatment, psychotherapy), a history of sexual abuse or abortion was associated with less pain reduction due to these treatments. Therefore, future research could consider potential overlap between trauma and PTSD symptoms and chronic pain experienced by women with CPP. These results also highlight that assessment for trauma and esteem related difficulties and potential psychological interventions targeting these aspects could be worthwhile future research.55,57,58
The qualitative studies included in the intervention and predictor stages provided some interesting insights into how women with CPP experience psychological interventions for their pain using grounded theory. Albert32 found that improvements to self-efficacy was the main theme reported by women across their intervention. Self-efficacy reported was composed of improved self-knowledge, responsibility for meeting their own needs, self-control over physical and verbal expressions of their pain and actively being able to express their needs and work with their pain. Facchin et al found that life and care disruption due to CPP lead to higher distress for women with CPP. They found that diagnostic pathways, doctor-patient relationships, financial and personal supports, female identity and life meaning with pain were reported to impact on care disruption for these women. The qualitative results stress the importance of incorporating appropriate multi-disciplinary care and clear communication in any treatments for women with CPP. They also suggest that future psychological treatments need to help women in navigate these factors as well as assisting them managing the impact that their pain has on their lives.
Methodological limitations across both components of the review made it difficult to establish whether findings were valid and identify what aspects should be included in future interventions for women with CPP. Methodological limitations for research with reference to CPP and psychology are widely acknowledged by the systematic reviews in this area,9–11 and this was a key consideration as to why the present study chose a scoping design for the review. Key problems included the following: poor consideration of potential confounding variables, methodological errors, invalid measures, low samples sizes, variations in session length, failure to report intervention content, and no reported intention to treat or power calculation analyses reported. Across the intervention studies, reasons for drop outs were only sought and reported in some studies, with women with CPP who dropped out reporting reasons such as the intervention was “too upsetting” or it was “too hard to think positively” about their pain.37 Participation rates in some studies left them underpowered or at risk of selection bias, with drop-out rates reported as high as 45%.32,39,40 As many different scales were used to measure mental health outcomes, result comparison across studies was difficult. The choice of scales in some studies also meant results were limited. For instance, pain severity and pain duration were commonly assessed using Visual Analog Scale (VAS) and “days per month” respectively. However, a VAS scale will only allow linear data analysis, without regard for an individual’s experience of pain. In contrast, measures such as the McGill Pain Questionnaire (MPQ), Endometriosis Health Profile 30-item (EHP-30) and Multidimensional Pain Inventory (MPI) are sensitive to aspects of pain that including word descriptors, additional numerical scales and ratings of pain intensity, though these were not as commonly utilized.71 Stabbing pain was often assessed using a dichotomous scale, which is problematic as this does not give information about factors such as whether stabbing pain was movement related suggesting a musculoskeletal mechanism, or not. Methodological limitations such as these reduce the ability to make meaningful conclusions and comparisons across studies.
Conclusions
Overall, the findings were varied with reference to the effectiveness of psychological interventions and predictors of mental health outcomes for women with CPP. However, the findings were extremely relevant to clinical practice and the development of future research and interventions in this area. The evidence base supported the role of pain education, CBT, Mensendieck therapy and interpersonal therapy, multiple psychological techniques and consideration of the overlap between mental and physical health. Pain severity, negative affect and emotional ambivalence all emerged as predictors of note with reference to mental health outcomes. However, many therapies and techniques with a good evidence base in the wider chronic pain literature, such ACT and hypnosis, were not represented in the literature for CPP. The studies included in this review were also plagued by methodological difficulties weakening the strength of findings. This made it hard to establish conclusive results as to which psychological therapies and techniques were most beneficial. These make distinguishing effective interventions and strong predictors that could form the base of these difficult. Future research needs to formulate psychological interventions with careful reference to predictors of psychological outcomes for women with CPP. Body and mind interactions must also be kept in mind in the development of future treatments for this population, as the most effective interventions included information and treatment on this. This is strongly supported by the wider literature on other chronic pain conditions. Consideration needs to be given to appropriate methodology and potential confounding in doing so, in order to ensure the production of quality research in this area to guide practice and research.
Disclosure
Dr Susan Evans is chair of the Pelvic Pain Foundation of Australia. She is also founding directors of Alyra Biotech and Dr Susan Evans Pty Ltd companies. She has a patent pending for Alyra Biotech. She also receives payment for teaching in pelvic pain management, book royalties for her book Endometriosis and Pelvic Pain. She does not offer or develop services for the psychological management of pelvic pain. The authors report no other conflicts of interest in this work.
References
- 1.International Association for the Study of Pain. Classification of chronic pain; 2011. Available from: https://www.iasp-pain.org/PublicationsNews/Content.aspx?ItemNumber=1673.Accessed May02, 2020.
- 2.Pelvic Pain Steering Committee. The $6 billion dollar woman and the $600 million girl: the pelvic pain report; 2011. Available from: http://fpm.anzca.edu.au/documents/pelvic_pain_report_rfs.pdf.Accessed May02, 2020.
- 3.Ahangari A. Prevalence of Chronic Pelvic Pain among women: an updated review. Pain Physician. 2014;17(2):E141–E147. [PubMed] [Google Scholar]
- 4.Ernst & Young. The cost of Endometriosis; 2019. Available from: https://www.pelvicpain.org.au/wp-content/uploads/2019/06/The-Cost-of-Endo.5June2019-Infographic_Cost-of-Endo-.pdf.Accessed May02, 2020.
- 5.Simoens S, Hummelshoj L, D’Hooghe T. Endometriosis cost estimates and methodological perspectives. Human Reprod Update. 2007;13(4):394–404. doi: 10.1093/humupd/dmm010 [DOI] [PubMed] [Google Scholar]
- 6.Peters KM, Wrigley P, Fraser I The cost of endometriosis in Australia. 12th world congress on endometriosis (30 April −3 May); 2014. Available from https://www.endometriosisaustralia.org/research.Accessed May02, 2020.
- 7.Soliman AM, Yang H, Due EX, Kelley C, Winkel C. (2016). The direct and indirect costs associated with endometriosis: a systematic literature review. Human Reprod. 2016;31(4):712–722. doi: 10.1093/humrep/dev335 [DOI] [PubMed] [Google Scholar]
- 8.Meselink B, Baranowski A, Hughs J. Abdominal and Pelvic Pain: From Definition to Best Practice. US, IASP; 2014. [Google Scholar]
- 9.Stones W, Cheong YC, Howard FM, Singh S. Interventions for treating chronic pelvic pain in women. Cochrane Database Syst Rev. 2005;2. doi: 10.1002/14651858.CD000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Niekerk L, Weaver-Pirie B, Matthewson M. psychological interventions for endometriosis related symptoms: a systematic review with narrative data synthesis. Arch Women’s Mental Health. 2019;2019:1–3. [DOI] [PubMed] [Google Scholar]
- 11.Andrews J, Yunker AJ, Reynolds WS, Likis FE, Sathe NA, Jerome RN. Noncyclic chronic pelvic pain therapies for women: comparative effectiveness review no. 41. Agency Healthcare Res Qual. 2012;11(12). Available from: www.effectivehealthcare.ahrq.gov. [PubMed] [Google Scholar]
- 12.Fritzer N, Keckstein J, Thomas A, Hudelist G. Radical laparoscopic resection of bowel endometriosis: long-term implications for psychological well-being. J Gynecol Surg. 2012;28(3):183–187. doi: 10.1089/gyn.2011.0078 [DOI] [Google Scholar]
- 13.Fry RP, Beard RW, Crisp AH, McGuigan S. Sociopsychological factors in women with chronic pelvic pain with and without pelvic venous congestion. J Psychosom Res. 1997;42(1):71–85. doi: 10.1016/S0022-3999(96)00231-0 [DOI] [PubMed] [Google Scholar]
- 14.Lamvu G, Williams R, Zolnoun D, et al. Long-term outcomes after surgical and nonsurgical management of chronic pain: one year after evaluation in a pelvic pain speciality clinic. Am J Obstetrics Gynaecol. 2006;195(2):591–598. doi: 10.1016/j.ajog.2006.03.081 [DOI] [PubMed] [Google Scholar]
- 15.Lazzeri L, Vannuccini S, Orlandini C, et al. Surgical treatment affects perceived stress differently in women with endometriosis: correlation with pain severity. Fertil Steril. 2015;103(2):433–438. doi: 10.1016/j.fertnstert.2014.10.036 [DOI] [PubMed] [Google Scholar]
- 16.Rowlands IJ, Teede H, Lucke J, Dobson AJ, Mishra GD. Young women’s psychological distress after a diagnosis of polycystic ovary syndrome or endometriosis. Human Reprod. 2016;31(9):2072–2081. doi: 10.1093/humrep/dew174 [DOI] [PubMed] [Google Scholar]
- 17.Arskey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. doi: 10.1080/1364557032000119616 [DOI] [Google Scholar]
- 18.Peters MJ, Godfrey C, McInerney P, Baldini Soares C, Khahil H, Parker D. Chapter 11: scoping reviews The Johanna Briggs Institute Reviewers’ Manual. The Johanna Briggs Institute; 2015a. Available from https://wiki.joannabriggs.org/display/MANUAL/Chapter+11%3A+Scoping+reviews. Retrieved 19December, 2019. [Google Scholar]
- 19.Peters MJ, Godfrey C, McInerney P, Baldini Soares C, Khahil H, Parker D. (2015b). Guidance for conducting systematic scoping reviews. Int J Evidence Based Healthcare. 2015b;13(3):141–146. doi: 10.1097/XEB.0000000000000050 [DOI] [PubMed] [Google Scholar]
- 20.PRISMA. The PRISMA statement; 2015. Available from: http://www.prisma-statement.org/.Accessed May02, 2020.
- 21.Rustøen T, Wahl AK, Hanestad BR, Lerdal A, Paul S, Miaskowski C. Age and the experience of chronic pain: differences in health and quality of life among younger, middle-aged, and older adults. Clin J Pain. 2005;21(6):513–523. doi: 10.1097/01.ajp.0000146217.31780.ef [DOI] [PubMed] [Google Scholar]
- 22.Howard FM. (2003). Chronic Pelvic Pain. Obstetrics Gynaecol. 2003;101(3):594–611. [DOI] [PubMed] [Google Scholar]
- 23.Clarivate Analytics. Endnote X8.2 Software; 2017. Available from: http://endnote.com/downloads.Accessed May02, 2020.
- 24.Veritas Health Innovation. Covidence systematic review software; 2018. Available from: www.covidence.org.Accessed May02, 2020.
- 25.Johanna Brigs Institute. Critical appraisal tools; 2019. Available from: https://joannabriggs.org/critical_appraisal_tools.Accessed May02, 2020.
- 26.Kames LD, Rapkin AJ, Naliboff BD, Afifi S, Ferrer-Brechner T. (1990). Effectiveness of an interdisciplinary program for the treatment of chronic pelvic pain. Pain. 1990;41(1):41–46. doi: 10.1016/0304-3959(90)91107-T [DOI] [PubMed] [Google Scholar]
- 27.Haugstad GK, Haugstad TS, Kirste UM, et al. Continuing improvement of chronic pelvic pain in women after a shirt-term Mensendieck somatocognitive therapy: results of a one year follow up study. Am J Obs Gynae. 2008;199(6):615.e1–615.e8. doi: 10.1016/j.ajog.2008.06.019 [DOI] [PubMed] [Google Scholar]
- 28.Hansen KE, Kesmodel U, Kold M, Forman A. Long-term effects of mindfulness-based psychological intervention for coping with pain in endometriosis: a six-year follow up on a pilot study. Nord Psychol. 2017;69(2):100–109. doi: 10.1080/19012276.2016.1181562 [DOI] [Google Scholar]
- 29.Kold M, Hansen T, Vedsted-Hansen FA. Mindfulness-based psychological intervention for coping with pain in endometriosis. Nord Psychol. 2012;64(1):2–16. doi: 10.1080/19012276.2012.693727 [DOI] [Google Scholar]
- 30.Meissner K, Schweizer-Arau A, Limmer AM, et al. Psychotherapy with somatosensory stimulation for endometriosis-associated pain: a randomized control trial. Obs Gynae. 2016;128(5):1134–1142. doi: 10.1097/AOG.0000000000001691 [DOI] [PubMed] [Google Scholar]
- 31.Poleshuck EL, Gamble SA, Bellenger K, et al. Randomized controlled trial of interpersonal psychotherapy versus enhanced treatment as usual for women with co-occurring depression and pelvic pain. J Psychosom Res. 2014;77(4):264–272. doi: 10.1016/j.jpsychores.2014.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Albert H. Psychosomatic group treatment helps women with chronic pelvic pain. J Psychosom Obstet Gynaecol. 1999;20(4):216–225. doi: 10.3109/01674829909075598 [DOI] [PubMed] [Google Scholar]
- 33.Friggi Sebe Petrelluzzi K, Garcia MC, Petta CA, et al. Physical therapy and psychological intervention normalize cortisol levels and improve vitality in women with endometriosis. J Psychosom Obs Gyn. 2012;33(4):191–198. doi: 10.3109/0167482X.2012.729625 [DOI] [PubMed] [Google Scholar]
- 34.Miller WL, Fluck MD. Chapter 13: adrenal cortex and its disorders In: Brook CG, Clayton PE, Brown RS, editors. Brook’s Clinical Pediatric Endocrinology. 4th ed. UK: Wiley Blackwell; 2014:283–326. [Google Scholar]
- 35.Farquhar M, Rogers V, Franks S, Pearce S, Wadsworth J, Beard RW. A randomized control trial of medroxyprogesterone acetate and psychotherapy for the treatment of pelvic congestion. Br J Obstet Gynaecol. 1989;96(10):1153–1162. doi: 10.1111/j.1471-0528.1989.tb03190.x [DOI] [PubMed] [Google Scholar]
- 36.Haugstad GK, Haugstad TS, Kirste UM, et al. Mensendieck somatocognitive therapy as a treatment approach to chronic pelvic pain: results of a randomized controlled intervention study. Am J Obs Gynae. 2006;194(5):1303–1310. doi: 10.1016/j.ajog.2005.10.793 [DOI] [PubMed] [Google Scholar]
- 37.Norman SA, Lumley MA, Dooley JA, Diamond MP. For whom does it work? Moderators of the effects of written emotional exposure in a randomized trial among women with chronic pelvic pain. Psychosom Med. 2004;66(2):174–183. doi: 10.1097/01.psy.0000116979.77753.74 [DOI] [PubMed] [Google Scholar]
- 38.Zhao L, Wu H, Zhou X, Wang Q, Zhu W, Chen J. Effects of progressive muscle relaxation on anxiety, depression and quality of life in endometriosis patients under gonadotrophin releasing hormone therapy. Eur J Obstetrics Gynaecol Reprod Biol. 2012;162(2):211–215. doi: 10.1016/j.ejogrb.2012.02.029 [DOI] [PubMed] [Google Scholar]
- 39.Chao MT, Abercrombie PD, Santana T, Duncan LG. Applying the RE-AIM framework to evaluate integrative medicine group visits among diverse women with chronic pelvic pain. Pain Manage Nurs. 2015;16(6):920–929. doi: 10.1016/j.pmn.2015.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beissner F, Preibisch C, Schweizer-Arau A, Popvici RM, Meissner K. Psychotherapy with somatosensory stimulation for Endometriosis associated pain: the role of the anterior hippocampus. Biol Psychiatry. 2018;84(10):734–742. doi: 10.1016/j.biopsych.2017.01.006 [DOI] [PubMed] [Google Scholar]
- 41.Ghaly AF. The psychological and physical benefits of pelvic ultrasonography in patients with chronic pelvic pain and negative laparoscopy. A random allocation trial. J Obstet Gyn. 1994;14(4):69–271. [Google Scholar]
- 42.Osman A, Barrios FX, Kopper BA, Haptmann W, Jones J, O’Neill E. Factor structure, reliably and validity of the Pain Catastrophizing Scale. J Behav Med. 1997;20(6):589–605. doi: 10.1023/A:1025570508954 [DOI] [PubMed] [Google Scholar]
- 43.Julian LJ. Measures of anxiety. Arthritis Care Res. 2011;63(11):1–19. doi: 10.1002/acr.20561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blackwell TL, McDermott AN. Review of the patient health questionnaire-9 (PHQ-9). Rehabil Counselling Bull. 2014;57(4):246–248. [Google Scholar]
- 45.Allaire C, Williams C, Bodmer-Roy S, et al. Chronic pelvic pain in an interdisciplinary setting: 1 year prospective cohort. Am. J Obstet Gynecol. 2018;218(114):1–12. doi: 10.1016/j.ajog.2017.10.002 [DOI] [PubMed] [Google Scholar]
- 46.Facchin F, Barbara G, Dridi D, et al. Mental health in women with endometriosis: searching for predictors of psychological distress. Human Reprod. 2017a;32(9):1855–1861. doi: 10.1093/humrep/dex249 [DOI] [PubMed] [Google Scholar]
- 47.Facchin F, Saita E, Giussy B, Dhouha D, Vercellini P. (2017b) ‘Free butterflies will come out of these deep wounds’ A grounded theory of how endometriosis affects women’s psychological health. J Health Psychol. 2017b;23(4):538–549. doi: 10.1177/1359105316688952 [DOI] [PubMed] [Google Scholar]
- 48.Donatti L, Ramos DG, Andres MP, Passman LJ, Podgaec S. Patients with endometriosis using positive coping strategies have less depression, stress and pelvic pain. Einstein. 2017;15(1):65–70. doi: 10.1590/s1679-45082017ao3911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vannuccini S, Lazzeri L, Orlandini C, et al. Mental health, pain symptoms, and systemic comorbidities in women with endometriosis: a cross-sectional study. J Psychosom Obstet Gynaecol. 2017;39(4):315–320. doi: 10.1080/0167482X.2017.1386171 [DOI] [PubMed] [Google Scholar]
- 50.Weijenborg P, Le Kuile MM, Gopie JP, Spinhoven P. Predictors of outcome in a cohort of women with chronic pelvic pain: a follow up study. Eur J Pain. 2009;13(7):769–775. doi: 10.1016/j.ejpain.2008.09.002 [DOI] [PubMed] [Google Scholar]
- 51.Petrelluzzi KF, Garcia MC, Petta CA, Grassi-Kassisse DM, Spadari-Bratfisch RC. Salivary cortisol concentrations, stress and quality of life in women with endometriosis and chronic pelvic pain. Stress. 2008;11(5):390–397. doi: 10.1080/10253890701840610 [DOI] [PubMed] [Google Scholar]
- 52.Low WY, Low WY, Sutton CJ. Patients with chronic pelvic pain and/or infertility: psychological differences pre and post treatment. J Psychosomatic Obstetrics Gynecol. 1994;15(1):45–52. doi: 10.3109/01674829409025628 [DOI] [PubMed] [Google Scholar]
- 53.Suso-Ribera C, Garcia-Palacios A, Botella C, Ribera-Canudas MV. Pain catastrophizing and its relationship with health outcomes: does pain intensity matter? Pain Res Manag. 2017;2017:1–8. doi: 10.1155/2017/9762864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaya B, Unal S, Ozenli Y, Gursoy N, Tekiner S, Kafkasli A. Anxiety, depression, and sexual dysfunction in women with chronic pelvic pain. Sexual Relat Therapy. 2006;21(2):187–196. doi: 10.1080/14681990500359897 [DOI] [Google Scholar]
- 55.Toomey TC, Hernandez JT, Gittelman DF, Hulka JF. Relationship of sexual and physical abuse to pain and psychological assessment variables in chronic pelvic pain in chronic pelvic pain patients. PAIN. 1993;53(1):105–109. doi: 10.1016/0304-3959(93)90062-T [DOI] [PubMed] [Google Scholar]
- 56.Nijenhuis ERS, Spinhoven P, Vanderlinden J, Van Dyck R, van der Hart O. Van der Hart O. Somatoform dissociative reactions as related to animal defensive reactions, to predatory threat and injury. J Abnorm Psychol. 1998;107(1):63–73. doi: 10.1037/0021-843X.107.1.63 [DOI] [PubMed] [Google Scholar]
- 57.Spinhoven P, Roelofs K, Moene F, et al. Trauma and dissociation in conversion disorder and chronic pelvic pain. Int J Psychiatry Med. 2004;34(4):305–318. doi: 10.2190/YDK2-C66W-CL6L-N5TK [DOI] [PubMed] [Google Scholar]
- 58.Oniszczenko W, Szulc J, Bulsa M, Zebielowicz D. Post-traumatic stress symptoms in women with gynaecologic pathology: the role of temperament, self-esteem and mental health. Curr Issues Personality Psychol. 2016;4(4):196–205. doi: 10.5114/cipp.2016.61680 [DOI] [Google Scholar]
- 59.Mosely L, Butler DS. 15 years of explaining pain: the past, the present and the future. J Pain. 2015;16(9):807–813. doi: 10.1016/j.jpain.2015.05.005 [DOI] [PubMed] [Google Scholar]
- 60.Grace P, Hutchinson M, Maier S, Watkins L. Pathological pain and neuro-immune influences. Nat Rev Immunol. 2014;14(4):217–231. doi: 10.1038/nri3621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ji RR, Nackley A, Huh Y, Terrando N, Maxiner W. Neuro-inflammation and central sensitization in chronic and widespread pain. Anesthesiology. 2018;129(2):343–366. doi: 10.1097/ALN.0000000000002130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bellavance MA, Rivest S. The HPA-Immune axis and the immunomodulatory actions of glucocorticoids in the brain. Front Immunol. 2014;5:136. doi: 10.3389/fimmu.2014.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dantzer R. Neuroimmune interactions: from the brain to the immune system and vice versa. Physiol Rev. 2017;98(1):477. doi: 10.1152/physrev.00039.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eccleston C, Crombez G. Advancing psychological therapies for chronic pain. F1000res. 2017;11(6):461. doi: 10.12688/f1000research.10612.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van Hees ML, Rotter T, Ellerman T, Evers SM. The effectiveness of individual interpersonal psychotherapy as a treatment for major depressive disorder in adult outpatients: a systematic review. BMC Psychiatry. 2013;13(1):22. doi: 10.1186/1471-244X-13-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ehde DM, Dillworth TM, Turner JA. Cognitive behavioral therapy for individuals with chronic pain: efficacy, innovations and directions for research. Am Psychologist. 2014;69(2):153–166. doi: 10.1037/a0035747 [DOI] [PubMed] [Google Scholar]
- 67.Hughes LS, Clark J, Colclough JA, Dale E, McMillan D. Acceptance and Commitment Therapy (ACT) for chronic pain: a systematic review and meta-analysis. Clin J Pain. 2017;33(6):552–568. doi: 10.1097/AJP.0000000000000425 [DOI] [PubMed] [Google Scholar]
- 68.Sturgeon JA. Psychological therapies for the management of chronic pain. Psychol Res Behav Manag. 2014;10(7):115–124. doi: 10.2147/PRBM.S44762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scott KM, Bruffaerts R, Tsang A, et al. Depression-anxiety relationships with chronic physical conditions: results from the world mental health surveys. J Affect Disord. 2007;103(1–3):113–120. doi: 10.1016/j.jad.2007.01.015 [DOI] [PubMed] [Google Scholar]
- 70.Holmes H, Thakur ER, Carty JN, et al. Ambivalence over emotional expression and perceived social constraints as moderators of relaxation training and emotional awareness and expression training for irritable bowel syndrome. Gen Hosp Psychiatry. 2018;53:38–43. doi: 10.1016/j.genhosppsych.2018.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kumar P, Tripathi L. Challenges in pain assessment: pain intensity scales. Indian J Pain. 2014;28(2):61–70. doi: 10.4103/0970-5333.132841 [DOI] [Google Scholar]
- 72.Facchin F, Barbara G, Saita E, Erzegovesi S, Martoni RM, Varcellini P. Personality in women with endometriosis: temperament and character dimensions and pelvic pain. Human Reprod. 2016;31(7):1515–1521. doi: 10.1093/humrep/dew108 [DOI] [PubMed] [Google Scholar]
- 73.Eccleston C. Role of psychology in pain management. Br J Anesthesia. 2001;87(1):144–152. doi: 10.1093/bja/87.1.144 [DOI] [PubMed] [Google Scholar]
- 74.Linton SJ. Applying Dialectical Behavior Therapy to chronic pain: a case study. Scand J Pain. 2010;1(1):50–54. doi: 10.1016/j.sjpain.2009.09.005 [DOI] [PubMed] [Google Scholar]
- 75.De Deus JM, Santos AF, Bosquetti RB, Pofhal L, Neto OA. Analysis of 230 women with chronic pelvic pain assisted at a public hospital. Rev Dor. 2014;15(3):191–197. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- International Association for the Study of Pain. Classification of chronic pain; 2011. Available from: https://www.iasp-pain.org/PublicationsNews/Content.aspx?ItemNumber=1673.Accessed May02, 2020.
- Pelvic Pain Steering Committee. The $6 billion dollar woman and the $600 million girl: the pelvic pain report; 2011. Available from: http://fpm.anzca.edu.au/documents/pelvic_pain_report_rfs.pdf.Accessed May02, 2020.
- Ernst & Young. The cost of Endometriosis; 2019. Available from: https://www.pelvicpain.org.au/wp-content/uploads/2019/06/The-Cost-of-Endo.5June2019-Infographic_Cost-of-Endo-.pdf.Accessed May02, 2020.
- Peters KM, Wrigley P, Fraser I The cost of endometriosis in Australia. 12th world congress on endometriosis (30 April −3 May); 2014. Available from https://www.endometriosisaustralia.org/research.Accessed May02, 2020.
- PRISMA. The PRISMA statement; 2015. Available from: http://www.prisma-statement.org/.Accessed May02, 2020.
- Clarivate Analytics. Endnote X8.2 Software; 2017. Available from: http://endnote.com/downloads.Accessed May02, 2020.
- Veritas Health Innovation. Covidence systematic review software; 2018. Available from: www.covidence.org.Accessed May02, 2020.
- Johanna Brigs Institute. Critical appraisal tools; 2019. Available from: https://joannabriggs.org/critical_appraisal_tools.Accessed May02, 2020.