Abstract
Context:
Facial melanoses decreases the quality of life (QoL). Melasma is the commonest cause but there are various other etiologies for facial pigmentation.
Aims:
To study the clinical profile of patients with facial melanoses and the psychological burden in these patients.
Settings and Design:
All patients having patchy or diffuse facial pigmentation attending the OPD in a tertiary care hospital for a period of 1 year were included in this hospital based cross-sectional study.
Subjects and Methods:
The type, extent, and distribution of the pigmentation was noted and tabulated in all patients. All patients were explained about Skindex-16 questionnaire and asked to complete it.
Statistical Analysis Used:
Student t-test (two tailed, independent) was used to find the significance of study parameters on continuous scale between two groups. Chi-square/Fisher Exact test was used to find the significance of study parameters on categorical scale between two or more groups. Correlation was performed using Spearman corrélation.
Results:
The total number of cases studied was 238 of which 186 (78.2%) were females and 52 (21.8%) were males. The most common diagnosis was melasma seen in 73% of cases. Other conditions noted were phototanning (5.8%), post-inflammatory hyperpigmentation (5.8%), Lichen planus pigmentosus (4.2%), freckles (3.7%), and Nevus of Ota (1.6%). Skindex-16 score against different grades of pigmentation showed that the mean Skindex-16 score was higher in severe cases but there was no statistically significant difference between the groups.
Conclusions:
The extent and severity of facial pigmentation and the decrease in the QoL are not proportional.
Keywords: Clinical profile, facial melanoses, quality of life
Introduction
Appearance of face plays important role in self-perception.[1] Also, skin plays a major role in social and sexual communication.[2] Facial melanoses includes many overlapping clinical conditions and patients may experience severe consequences on self-concept.[1,3,4]
Quality of life (QOL) means a feeling of joy and satisfaction with life and it has gained lot of importance.[5] Skin diseases not only impact QOL of patients and needs to be measured but is helpful for management.[6,7]
Many studies report the impact on the QOL in patients with melasma but not with other causes of facial pigmentation.[8,9] Here we looked into clinical profile of patients with facial melanoses and psychological burden in them.
Subjects and Methods
All patients having patchy or diffuse facial pigmentation attending the OPD in the Department of Dermatology, at a tertiary care hospital for a period of 1 year from April 2015 to March 2016 were included in the study. A complete history and physical examination was conducted in these patients wherein the type, extent, and distribution of the pigmentation was noted and tabulated. Skin biopsy, dermoscopy, and biochemical investigations were done when diagnosis was not clinically confirmatory.
To access the degree and extent of the pigmentation the face was divided into 4 equal quadrants Q1, Q2, Q3, and Q4. The severity of pigmentation in each of these four quadrants was assessed based on 3 variables: percentage of total area involved (A), severity of pigmentation (S), and uniformity of pigmentation (U). A value was assigned for the percentage of area involved: 0 = no involvement; 1 = <10% involvement; 2 = 10–29% involvement; 3 = 30–49% involvement; 4 = 50–69% involvement; 5 = 70–89% involvement; and 6 = 90–100% involvement. The severity of the pigmentation (S) was compared to the normal skin and was graded on a scale of 0 to 3 as follows: 0 = normal skin color; 1 = mild hyperpigmentation; 2 = moderate hyperpigmentation; 3 = severe hyperpigmentation. Score for uniformity of pigmentation (U) was given as 1 = speckled pigmentation and 2 = diffuse pigmentation. Total scoring of pigmentation was calculated using the formula Q1(S+U) A+ Q2(S+U) A+ Q3(S+U) A+ Q4(S+U) A. Pigmentation score was divided as mild, moderate, and severe with scores of 0–15, 16–30 and >31, respectively.
The Skindex is an instrument that measures the effects of a skin disease on a patient's QOL, and has been refined and studied extensively. All patients were explained about Skindex-16 questionnaire and asked to complete it in English or in their native language. The questionnaire in the local language was validated. The native version translation to Kannada was done by 2 language experts and a final version was created. Back translation wasdone to English by 2 independent translators. This was later tested on 20 responders and validated. Scoring ranging from 0 to 96 was done. Permission to use Skindex-16 questionnaire for the study was obtained from Mapi research trust.
Statistical analysis
Results on continuous measurements were presented as Mean ± SD (Min–Max) and results on categorical measurements were presented in number (%). Significance was assessed at 5% level of significance. Analysis of variance (ANOVA) was used to find the significance of study parameters between three or more groups of patients. Student t-test (two tailed, independent) was used to find the significance of study parameters on continuous scale between two groups (inter-group analysis) on metric parameters. Chi-square/Fisher Exact test was used to find the significance of study parameters on categorical scale between two or more groups. Corrélation was performed using Spearman corrélation. The P value <0.05 was considered to be significant and P value <0.001 as highly significant.
Results
The total number of cases studied was 238 of which 186 (78.2%) were females and 52 (21.8%) were males. The mean duration of the disease was 3.78 months. Family history was seen in 9.2% of cases. The most common diagnosis was melasma seen in 73% of cases. Other conditions noted were phototanning (5.8%), post-inflammatory hyperpigmentation (PIH, 5.8%), Lichen planus pigmentosus (4.2%), freckles (3.7%), and Nevus of Ota (1.6%). Acanthosis nigricans, Becker's nevus, pigmentary demarcation lines, Nevus spilus, sebborhoeic melanosis, post-chikungunya pigmentation were noted in 0.42% of cases.
Mean pigmentation score noted was 20.39 ± 14.27. Mild pigmentation was seen in 41.17% of patients, moderate in 42% of cases, and severe pigmentation was seen in 16.8% of cases. Mean Skindex-16 score was 23.25 ± 19.10 [Table 1]. The mean Skindex scores in different conditions was tabulated and it was highest in post-chikungunya pigmentation followed by pigmentary demarcation lines (PDL) with seborrhoeic melanosis [Table 2]. It was least in patients having melasma with PIH. This was when we considered patients having one single diagnosis or when they had overlapping conditions. When there was a single diagnosis, post-chikangunya pigmentation patients had the maximum Skindex scores followed by Nevus of Ota and it was least in Nevus Spilus.
Table 1.
Skindex scoring among the patients
| Skindex-16 | Mean score |
|---|---|
| Symptoms | 1.12±3.01 |
| Emotions | 17.32±12.66 |
| Social | 4.78±7.51 |
| Total | 23.25±19.10 |
Table 2.
Skindex-16 score in different diseases
| Diagnosis | Number of cases | Mean Skindex-16 score |
|---|---|---|
| Acanthosis nigricans | 2 | 57 |
| Beckers nevus + LPP | 2 | 28 |
| Freckles | 6 | 9.5 |
| Freckles + PIH | 2 | 9 |
| LPP | 10 | 19.6 |
| Melasma | 176 | 23.3 |
| Melasma + Freckles | 2 | 16 |
| Melasma + PDL | 2 | 1 |
| Melasma + PIH | 2 | 0 |
| Nevus of Ota | 4 | 38.5 |
| Nevus Spilus | 2 | 2 |
| PDL + Seborrhoeic melanosis | 2 | 60 |
| Phototanning | 14 | 14.9 |
| PIH | 10 | 29 |
| Post-chikungunya pigmentation | 2 | 81 |
In the Skindex score, the mean symptom score was 1.12 ± 3.01, emotion score was 17.32 ± 12.66. The mean Skindex-16 score of female cases was more than male cases but was weakly significant (P = 0.08; P < 0.10). But when the mean emotion Skindex-16 score was compared it was noted that the score of female cases was more than male cases and it was statistically significant (P = 0.045; P < 0.05).
Scatter plot graph was used to correlate between pigmentation score and Skindex-16 score, r value was 0.0409 suggesting a weak positive correlation between pigmentation score and Skindex-16 score [Figure 1]. Skindex-16 score against different grades of pigmentation showed that the mean Skindex-16 score was higher in severe cases but there was no statistically significant difference between the groups [Table 3].
Figure 1.
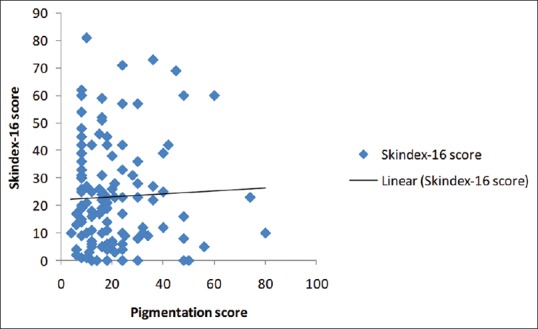
Correlation between pigmentation score and skindex-16 score (scatter plot graph)
Table 3.
Skindex-16 score against different grades of pigmentation
| Pigmentation score | Number of cases | Mean skindex-16 score |
|---|---|---|
| Mild (0-15) | 98 | 22.6735±18.87 |
| Moderate (16-30) | 100 | 22.64±17.81 |
| Severe (>31) | 40 | 26.1±23.20 |
When the Skindex-16 score in different age groups was compared, the mean Skindex-16 score in age group 45–50 years was less compared to younger age groups. But there was no statistically significant difference between the various groups.
Discussion
Facial pigmentation is a common dermatological complaint. In a study done in Brazil, pigmentation disorders were the main cause for dermatological complaint in 23.6% of men and 29.9% of women.[10] Facial melanoses includes many overlapping clinical conditions. Melasma, Riehl's melanosis, lichen planus pigmentosus, Erythromelanosis peribuccale pigmentaire of Brocq (EPP), Poikiloderma of Civatte, Erythromelanosis follicularis of face and neck, Nevus of Ota, etc., are few of the causes of facial pigmentation. Etiology is not known in many conditions and diagnosis is based mostly on clinical features. Treatment of facial melanoses is not only challenging but also requires therapy to maintain remission.[3]
The commonest cause of pigmentation noted in our study was melasma which was seen in 73% of the patients and was similar to other studies.[10,11]
PIH and phototanning was the next common complaint in our study. Both were seen in 5.8% of patients. It is common in skin of color and can be secondary to skin diseases or caused by esthetic or light-based treatments.[12] Pigmentary disorders were the third most commonly cited skin disorder in a study done on 2000 dermatology patients of black origin in Washington DC and in this study PIH was more frequent than melasma.[13]
The skin diseases have an impact on QOL of the patient owing to the high visibility of skin diseases. Patients with skin conditions on the face are at risk of having depression and can suffer from feelings of loneliness and isolation.[1] In the study by Seite et al., it was noted that patients with pigmentary disorders are more affected by depression than patients with other skin conditions of the face like acne, acne scars, and rosacea.[4]
Health-related quality of life (HRQOL) is dependent not only on physical health but also on social and psychological well-being. Several instruments have been developed to measure the HRQOL and SKINDEX 16, dermatology life quality index, dermatology specific quality of life are few of them.[1,5] Skindex-16 is a self-administered questionnaire and has been studied extensively. It is used for measuring the QOL in skin diseases and measures the burden of symptoms, social function, and emotional state.[2] We have used Skindex-16 because it measures “bother rather than frequency of experience,” as described by Mary-Margaret Chren which assesses the QOL better.[14] Mean Skindex-16 score was 23.25 ± 19.10 in our study.
In our study, the mean Skindex-16 score of female cases was more than male cases but it was weakly significant (P = 0.08; P < 0.10). But there was a statistically significant difference when only the emotion Skindex-16 scores were compared. In the study by Abolfotouh et al. in Saudi Arabia wherein the QOL in patients with skin disease in Saudi Arabia was studied, they noted that the disability suffered by female patients was more than that of males.[2] This may be because of the fact that women are more conscious about their appearance in public.
In the study by Balakrishnan et al. where they studied the QOL in patients with facial blemishes, they found that the extent or the type of facial blemish did not influence the HRQOL but the mere presence of the facial blemish reduced the HRQOL.[1] This is similar to what we inferred from our study where in there was no statistically significant difference in the Skindex value when compared to the degree of pigmentation. Few other studies also did not show correlation between severity of melasma and QOL.[15,16] This can be because just the mere presence of pigmentation worries the patient and whether it is little or more, blemish is considered unattractive. Also stoicism may play a role.
When the Skindex scores in individual conditions were considered, Skindex cores of pigmentation due to melasma was less than conditions like acanthosis nigricans, Nevus of Ota, PIH and post-Chikangunya pigmentation. Any sort of pigmentation on the face does impact the QOL of the patients but not many studies have taken into consideration other causes of pigmentation of the face while studying the QOL impairment. Many studies report that there was no relation with age and psychological impairment caused by skin disease as seen in our study.[17,18] But in the study by Abolfotouh et al. there was significantly higher scores in older patients.[2]
In conclusion, facial melanoses decreases the QOL but the extent and severity of facial pigmentation and the decrease in the QOL does not appear to be proportional. Together with melasma other conditions causing hyperpigmentation on the face does impact the QO L of the patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Balakrishnan R, McMichael AJ, Hu JY, Camacho FT, Shew KR, Bouloc A, et al. Correlates of health-related quality of life in women with severe facial blemishes. Int J Dermatol. 2006;45:111–5. doi: 10.1111/j.1365-4632.2004.02371.x. [DOI] [PubMed] [Google Scholar]
- 2.Abolfotouh MA, Al-Khowailed MS, Suliman WE, Al-Turaif DA, Al-Bluwi E, Al-Kahtani HS. Quality of life in patients with skin diseases in central Saudi Arabia. Int J Gen Med. 2012;5:633–42. doi: 10.2147/IJGM.S33276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khanna N, Rasool S. Facial melanoses: Indian perspective. Indian J Dermatol Venereol. 2011;77:552–64. doi: 10.4103/0378-6323.84046. [DOI] [PubMed] [Google Scholar]
- 4.Seite S, Deshayes P, Dréno B, Misery L, Reygagne P, Saiag P, et al. Interest of corrective makeup in the management of patients in dermatology. Clin Cosmet Investig Dermatol. 2012;5:123–8. doi: 10.2147/CCID.S33172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halioua B, Beumont M, Lunel F. Quality of life in dermatology. Int J Dermatol. 2000;39:801–6. doi: 10.1046/j.1365-4362.2000.00793.x. [DOI] [PubMed] [Google Scholar]
- 6.Finlay AY. Quality of life indices. Indian J Dermatol Venereol Leprol. 2004;70:143–48. [PubMed] [Google Scholar]
- 7.Ongenae K, Van Geel N, De Schepper S, Naeyaert JM. Effect of vitiligo on self-reported health-related quality of life. Br J Dermatol. 2005;152:1165–72. doi: 10.1111/j.1365-2133.2005.06456.x. [DOI] [PubMed] [Google Scholar]
- 8.Lieu TJ, Pandya AG. Melasma quality of life measures. Dermatol Clin. 2012;30:269–80. doi: 10.1016/j.det.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Leeyaphan C, Wanitphakdeedecha R, Manuskiatti W, Kulthanan K. Measuring melasma patients quality of life using willingness to pay and time trade-off methods in thai population. BMC Dermatology. 2011;11:16. doi: 10.1186/1471-5945-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Handel AC, Miot LDB, Miot HA. Melasma: A clinical and epidemiologicals review. An Bras Dermatol. 2014;89:771–82. doi: 10.1590/abd1806-4841.20143063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sivayathorn A. Melasma in orientals. Clin Drug Investig. 1995;10:24–40. [Google Scholar]
- 12.Nouveau S, Agrawal D, Kohli M, Bernerd F, Misra N, Nayak CS. Skin hyperpigmentation in Indian population: Insights and best practice. Indian J Dermatol. 2016;61:487–95. doi: 10.4103/0019-5154.190103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halder RM, Grimes PE, McLaurin CI, Kress MA, Kenney JA., Jr Incidence of common dermatoses in a predominantly black dermatologic practice. Cutis. 1983;32:388–90. [PubMed] [Google Scholar]
- 14.Chren MM. The Skindex instruments to measure the effects of skin disease on quality of life. Dermatol Clin. 2012;30:231–6. doi: 10.1016/j.det.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yalamanchili R, Shastry V, Betkerur J. Clinico-epidemiological study and quality of life assessment in Melasma. Indian J Dermatol. 2015;60:519. doi: 10.4103/0019-5154.164415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kothari P, Sharma YK, Patvekar MA, Gupta A. Correlating Impairment of quality of life and severity of Melasma: A Cross-sectional study of 141 patients. Indian J Dermatol. 2018;63:292–6. doi: 10.4103/ijd.IJD_10_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Hoqail I. Impairment of quality of life among adults with skin disease in King Fahad Medical City, Saudi Arabia. J Family Community Med. 2009;16:105–9. [PMC free article] [PubMed] [Google Scholar]
- 18.Linnet J, Jemec GBE. An assessment of anxiety and dermatology life quality in patients with atopic dermatitis. Br J Dermatol. 1999;140:268–72. doi: 10.1046/j.1365-2133.1999.02661.x. [DOI] [PubMed] [Google Scholar]


