Abstract
Background:
Tuberculosis (TB) is a major global health problem and leading cause of death. Anti-tubercular therapy (ATT) can lead to various adverse effects including cutaneous reactions. Re-challenge remains the only option to restart the safe therapy with limited number of most efficient primary ATT drugs.
Objectives:
To study the demographic profile, identify the spectrum of cutaneous eruptions, offending drug and the reinstitution of safe ATT.
Materials and Methods:
This was a retrospective study with inclusion of the indoor patients with cutaneous adverse drug reaction secondary to ATT. Hospital records were analyzed regarding demographic characteristics, type of TB, ATT regimen, pattern of drug rash, offending drugs, laboratory parameters, and reinstitution of ATT after re-challenge.
Results:
All the cases (40 patients) were reported in adults with male to female ratio of 1:1.2 and mean age of 50 years. Pulmonary TB was the most common type of TB observed in 24 (60%) patients followed by extra-pulmonary in 16 (40%) patients. Maculopapular rash was the most common (42.5%) type of cutaneous eruptions and ethambutol, the most common (45%) offending drug followed by other first line anti-tubercular drugs. Ten (25%) patients developed multiple drug hypersensitivity on re-challenging. Multiple drug hypersensitivity was seen in 10 (25%) patients.
Conclusion:
Drug reaction to ATT is like a double-edged sword as stopping ATT and starting treatment of reaction with systemic steroids can further aggravate the condition with increased risk of disseminated and multidrug resistant tuberculosis. Re-challenge with ATT not only find out the culprit drug but also helps to restart a safer alternate ATT regimen.
Limitations:
Small sample size, lack of proper hospital records due to which some patients were missed and the fact that re-challenge was not performred in mild lichenoid type rash.
Keywords: Anti-tubercular therapy, cutaneous adverse drug reaction, re-challenge
Introduction
Tuberculosis (TB) is rightly compared to a nine headed dragon especially in developing countries like India. It has existed for millennia and remains a major global health problem. TB is the ninth leading cause of death worldwide and causes ill health for approximately 10 million people each year.[1] First line anti-tubercular (Anti-TB) therapy (ATT) with rifampicin (R), isoniazid (INH), pyrazinamide (Z), ethambutol (E), and streptomycin (S) remains the cornerstone of the treatment. Owing to the increased prevalence of TB worldwide and increased availability of the facilities for diagnosis and treatment, the rate of adverse drug reactions (ADRs) to the first line therapy has also increased. Cutaneous adverse drug reactions (CADRs) are well known side effects of these drugs and can range from a mild pruritus to life threatening toxic epidermal necrolysis (TEN) which require discontinuation of the treatment and may complicate the tuberculosis management. Re-challenge- desensitization remains the only option for re-introducing ATT. There are no clear guidelines regarding re-challenge and only limited studies in the literature. In this study, we report 40 patients presenting with CADR secondary to ATT and successful restart of therapy after re-challenge in 36 patients while continuing ATT in rest of the four patients who had mild reaction.
Materials and Methods
This was a retrospective study conducted in the Department of Dermatology, Venereology and Leprosy of our institution. The study included all indoor patients admitted with the diagnosis of CADRs to ATT from January 2016 to December 2018. Patient's data were analyzed from hospital records regarding demographic characteristics, type of TB, category of directly observed treatment, short course (DOTS), period of latency, previous drug allergies, medical history for risk factors, co-morbidities, and pattern of drug rash. Laboratory investigations recorded were complete blood counts with eosinophil counts, biochemistry including LFTs, RFTs, chest radiography, and HIV serology. Skin biopsy was done in few patients to rule out the differentials. Patients were managed symptomatically for CADR and were re-challenged with ATT as per the institution's protocol, [Figure 1] a modification of European Academy of Allergology and Clinical Immunology guidelines.[2] After finding out the offending drug/drugs and excluding it, patients were restarted on the safer ATT regimen.
Figure 1.
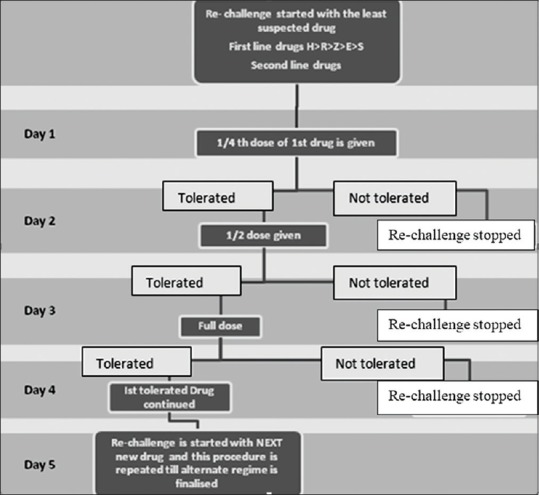
Flow chart showing Re-challenge Protocol
Results
A total of 40 patients with CADR to ATT required hospitalization during the study period of 3 years. The study comprised of 18 males and 22 females with male to female ratio of 1:1.2. All the cases reported were adults with age range of 19 to 80 years and mean age of 50 years [Figure 2]. Pulmonary TB was the most common type of TB seen in 24 (60%) patients while extra-pulmonary disease constituted the remaining 16 (40%) patients. Tubercular lymphadenopathy was most common type among extra pulmonary TB in 7 (17.5%) patients followed by pleural effusion 3 (7.5%), abdominal tuberculosis 2 (5%), tuberculoma, TB osteomyelitis, disseminated TB, and tubercular meningitis in 1 (2.5%) patient each. Majority of the patients, 31 (77.5%) were on category I, DOTS. The high-risk factor identified in our patients were elderly age 19 (47%), polypharmacy 10 (25%), pre-existing renal disease 3 (7.5%), diabetes mellitus 5 (12.5%), smoking 12 (30%) and alcohol intake 11 (27%). But none of the patient was found to be HIV positive or having auto-immune disease. Latent period between drug intake and onset of rash varied between 3 days to 150 days however the mean duration was 33 days. Among clinical patterns of drug rash, maculopapular was the most common type in 17 (42.5%) patients, followed by urticarial in 7 (17.5%), lichenoid drug rash in 5 (12.5%) patients. Ten (25%) patients had severe rash in the form of Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), 5 (12.5%) patients had exfoliative dermatitis and Acute Generalised Exanthematous Pustulosis (AGEP) was seen in 3 (7.5%) patients [Figure 3].
Figure 2.
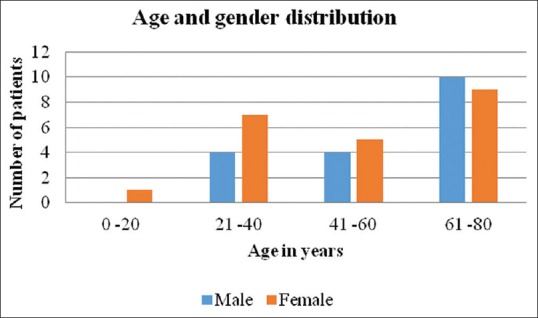
Demographic profile of patients with age and sex distribution
Figure 3.
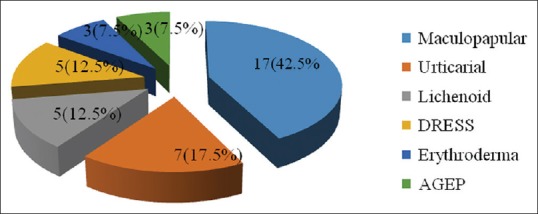
Pattern of cutaneous adverse drug reaction seen with anti-tubercular drugs
Systemic involvement was seen in 32 (80%) patients. Eosinophilia (eosinophil count more than 500/cu.mm) was seen in 32 (80%) patients. Fifteen (37.5%) patients had evidence of transient hepatitis and 3 (7.5%) patients had deranged renal functions. In 5 (12.5%) patients, deranged LFTs, RFTs, and eosinophilia were present along with significant lymphadenopathy thus meeting the criteria for DRESS.
ATT was withheld in all except four patients who had lichenoid drug rash and had already completed intensive phase of treatment. These were managed with oral anti-histaminic, short course oral steroids along with continuation of their regular ATT. Other patients were initiated on oral steroids; clinical response appeared in 7–14 days with total duration of steroids therapy ranging from 10 to 110 days. Thirty-six (90%) patients were then re-challenged with ATT as per protocol. The commonest implicated drug was found to be ethambutol (E) in 18 (45%) patients followed by pyrazinamide (Z) in 8 (20%), isoniazid (H) in 7 (17.5%), rifampicin (R) in 6 (15%), streptomycin (S) in 3 (7.5%), and levofloxacin in 2 (5%) patients [Figure 4]. Ten (25%) patients developed drug rash to more than one ATT drug on re-challenging. Eight (20%) patients developed rash to 2 ATT drugs out of which 4 patients developed rash to HE and 1 patient each developed rash to RE, ZH, SZ, ZE. One patient developed rash to 3 ATT drugs (HZE) and one patient developed rash to four drugs including three 1st line ATT drugs (except R) and levofloxacin.
Figure 4.
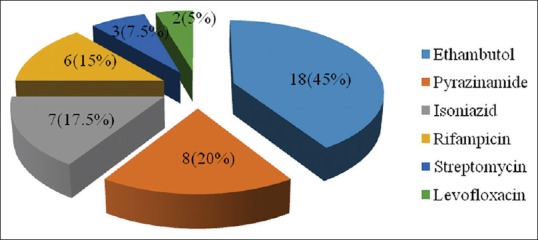
Commonly implicated drugs after re-challenge
Discussion
Adverse drug reaction as defined by WHO is “a response to a drug that is noxious and unintended and occurs at doses normally used in human for the prophylaxis, diagnosis or therapy of disease, or for modification of physiological function.”[3] The majority (75–80%) of adverse drug reactions are caused by predictable, nonimmunologic effects of drugs. The remaining 20–25% events are caused by unpredictable effects that may or may not be immune-mediated. Immune-mediated reactions account for 5–10% of all drug reactions.[4] The incidence of cutaneous adverse drug reactions (CADRs) reported in patients on antitubercular therapy is 5.7%.[5] Various type of rash observed are urticarial drug rash, maculopapular rash, lichenoid drug rash, acute generalized exanthematous pustulosis (AGEP), exfoliative dermatitis, drug reaction with eosinophilia and systemic symptoms (DRESS), Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN).
Many patients are at increased risk of developing adverse drug reactions. Various risk factors associated are genetic susceptibility,[6] elderly age group, female gender, diabetes, organ failure, poly-pharmacy, infections such as HIV,[7] EBV, autoimmune diseases (rheumatoid arthritis, Sjogren's disease, SLE), malignancy especially hematological[7] and probably fixed dose combinations of ATT.
Elderly age group is more prone to adverse reactions due to polypharmacy, reduced ability of kidney to excrete the drugs, variable drug absorption, and metabolism by liver. During old age the amount of water decreases in the body and comparative amount of fat increases. So, water soluble drugs reach higher concentration and fat soluble drugs accumulate more due to increased fat to store them.[8] In this study majority of the patients i.e. 19 (47.5%) patients were in the age group of 61–80 years. The mean age in our study was 50 years which was comparable to the study by Tan et al.[5] and Sood A et al.[9]
CADRs are less common in males, as androgens are considered to be microsomal inducers and females as compared to males have lower body weight, organ size, more body fat, different gastric motility, and reduced glomerular filtration rate altering the pharmacokinetic and pharmacodynamics of drugs.[8] In the present study, females outnumbered males with male to female ratio of 1: 1.2. Female preponderance has been observed by David et al.[10] However a male dominance was observed by others including Sinha K et al.,[11] Sharma et al.,[12] Suthar et al.[13] and Patel et al.[14]
Diabetes mellitus was present in 12.5% patients and most of them presented with multiple drug hypersensitivity. Patients with diabetes are more prone to ADRs due to oxidative stress and polypharmacy.[15] Chronic renal failure affects both renally excreted drugs and also drugs metabolized by the liver through the effect of uremia caused by renal failure.[8] We had 7.5% patients with deranged renal functions. History of alcohol consumption was present in 27% patients. Chronic alcoholism activates enzymes which convert some drugs into toxic metabolites which damage liver and affect metabolism of drugs.[6] Smoking affects the metabolic process by acting as liver enzyme inducer of hepatic cytochrome P-450.[8] Thirty percent of our patients were smokers. None of the patients in our study was immunocompromised or had any autoimmune disease.
Fixed dose combination ATT was started in India in 2016 when intermittent therapy was changed to daily regimen as per body weight of the patient and we noticed a slightly increased rate of drug reaction after this, however this may be due to increased rate of TB detection due to better National Programme implementation, adherence to treatment due to better tracking system, early detection of CADR or probably due to increased dosage of drugs given in daily than thrice weekly regimen.
Latent period between intake of drug and onset of rash varied between 3 days to 150 days however the mean duration was 33 days. The onset of urticarial rash was seen within days to weeks while lichenoid rash was seen after months of taking ATT. But majority of the patients developed rash within 2 months of treatment that is before the completion of intensive phase of ATT. Our results are in agreement with other studies who have documented this aspect.[16,17]
The most common type of rash seen with ATT was maculopapular rash (42.5%) followed in frequency by urticarial, lichenoid, DRESS, AGEP, and exfoliative dermatitis. Our results were comparable to other studies by Thong et al.[18] and Tan WC et al.,[5] however Lehloenya[19] et al. reported SJS/TEN as the most common type rash, because they studied only the severe forms of drug reactions in their study.
The patients in our study were managed with oral steroids till the rash and systemic symptoms subsided. Stopping ATT and treatment with steroids increases the risk of disseminated disease and multidrug resistant tuberculosis. Therefore, we believe that re-challenge should be initiated as early as possible considering the relative safety of re-challenging. There are no specific re-challenge guidelines, we re-challenged with each ATT drug as per our institution's protocol till culprit drug was found and final regimen established.
Drug causality can be found out with the help of various provocation tests including prick test, patch test, lymphocyte transformation test (LTT), and oral provocation test (OPT) or re-challenge. OPT is the gold standard as other tests have low sensitivity and are not easily available in resource poor setting where TB is most prevalent.[20]
Re-challenge is defined as a controlled administration of a drug in order to diagnose drug hypersensitivity reactions.[2] Tuberculosis outcomes are better if re-challenge is undertaken and only the offending drug is removed from the treatment regimen.[20] Re-challenge is of utmost importance because of increased burden of TB in India/World, limited number of first line ATT drugs, increased toxicity of second line drugs and keeping second line drugs reserve for resistant cases. It helps in avoiding treatment interruption due to ADR thereby decreasing morbidity, mortality and transmission rate. Interruption of therapy during the intensive phase is associated with a three times higher risk of death.[21] The sequence of re-challenge is still a matter of debate whether most effective drugs, rifampicin, and isoniazid should be re-challenged first or the drugs least likely to cause a reaction. However, all first line drugs cause CADR and no good studies quantify the contribution of each drug. So, it is suggested to re-challenge with rifampicin and isoniazid to decrease the chances of resistance as use of isoniazid and rifampicin in the treatment regimen of tuberculosis is associated with superior outcome. More than 90% of re-challenge reactions occur within 72 hours. So, re-challenging with a new first line drug every 96 hours is recommended, while monitoring closely for features of a re-challenge reaction.[20]
Among the ATT drugs, Tan et al.[5] reported pyrazinamide as the commonest drug causing CADR (38%); however, most common drug implicated in our study was ethambutol (45%) as depicted in Table 1.
Table 1.
Comparison of our study with various other studies
| Thong et al. (2014) | Tan WC et al. (2007) | Lehloenya et al. (2011) | Our Study | |
|---|---|---|---|---|
| Maculopapular | 8 (72%) | 34 (72.3%) | 2 | 17 (42.5%) |
| Urticarial | 1 | 4 (8.5%) | - | 7 (17.5%) |
| AGEP | - | - | - | 3 (7.5%) |
| Erythema multiforme | - | 2 (4.2%) | - | - |
| SJS/TEN | - | - | 13/17 (20/26%) | - |
| DRESS | 2 | - | 25 (38%) | 5 (12.5%) |
| Erythroderma | - | 1 | - | 3 (7.5%) |
| Lichenoid Rash | - | 1 | 3 | 5 (12.5%) |
| Other | - | 1 (Generalized pruritus) | 5 (SJS-TEN) | - |
| Total | 11 | 47 | 65 | 40 |
Ten (25%) patients in our study showed multiple drug sensitivity, 1 of them to 3 drugs and one to 4 ATT drugs. Lehloenya et al.[22] reported multiple drug hypersensitivity (MDH) in 12.5% cases co-infected with HIV.
Multiple drug hypersensitivity is a predilection to react to different chemically and structurally unrelated drugs which are metabolized by different pathways with no evidence of cross reactivity.[22] Specific types of drugs, higher concentration, fixed dose combination and long lasting treatment predisposes to MDH. MDH develops as a consequence of massive T cell activation due to long lasting hypersensitivity to different drugs. Reactions may be simultaneous, sequential, or distant (after long interval).[23] Fixed dose combination and higher doses of INH, pyrazinamide, ethambutol, and streptomycin can lead to MDH with ATT. One of our elderly female patients, known case of air born contact dermatitis secondary to parthenium and diabetes, developed hypersensitivity to 3 primary anti-tubercular drugs and 1 secondary ATT (levofloxacin) was desensitized to isoniazid and rifampicin.
Conclusion
Adverse drug reaction to anti-tubercular drugs is like a double-edged sword as stoppage of ATT and simultaneous treatment of CADR with systemic steroid further increases the risk of disseminated and multidrug resistant tuberculosis, a biggest threat to community. Re-challenge is a ray of hope as it decreases the risk of ATT interruption/default. The offending drug can be found out by re-challenge and safer ATT regimen can be restarted. Any type of cutaneous drug reaction may develop from any of the first line drugs. Hence, the patient should be counseled regarding ADRs and physician should have high suspicion of ADR/CADR for early detection and reinstitution of alternate regime. However, multidrug hypersensitivity is another challenge in reinstitution of safe ATT regime.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.World Health Organization. Global Tuberculosis Report 2017. Introduction. [Last accessed on 2019 Mar 10]. pp. 1–5. Available from: https://www.who.int/tb/publications/global_report/gtbr2017 .
- 2.Aberer W, Bircher A, Romano A, Blanca M, Campi P, Fernandez J, et al. Drug provocation testing in the diagnosis of drug hypersensitivity reactions: General considerations. Allergy. 2003;58:854–63. doi: 10.1034/j.1398-9995.2003.00279.x. [DOI] [PubMed] [Google Scholar]
- 3.Edwards IR, Aronson JK. Adverse drug reactions: Definitions, diagnosis and management. Lancet. 2000;356:1255–9. doi: 10.1016/S0140-6736(00)02799-9. [DOI] [PubMed] [Google Scholar]
- 4.Wintroub BU, Stern R. Cutaneous drug reactions: Pathogenesis and clinical classification. J Am Acad Dermatol. 1985;13:833–45. doi: 10.1016/s0190-9622(85)70156-9. [DOI] [PubMed] [Google Scholar]
- 5.Tan WC, Ong CK, Kang SC, Razak MA. Two years review of cutaneous adverse drug reaction from first line antituberculous drugs. Med J Malaysia. 2007;62:143–6. [PubMed] [Google Scholar]
- 6.Resende LS, Santos-Neto ET. Risk factors associated with adverse reactions to antituberculosis drugs. J Bras Pneumol. 2015;41:77–89. doi: 10.1590/S1806-37132015000100010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernández-Salazar A, Rosales SP, Rangel-Frausto S, Criollo E, Archer-Dubon C, Orozco-Topete R, et al. Epidemiology of adverse cutaneous drug reactions. A prospective study in hospitalized patients. Arch Med Res. 2006;37:899–902. doi: 10.1016/j.arcmed.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Alomar MJ. Factors affecting the development of adverse drug reactions. Saudi Pharm J. 2014;22:83–94. doi: 10.1016/j.jsps.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sood A, Bansal R, Sharma A, Himani H, Bhagra S, Kansal D. Profile of adverse drug reactions in patients on anti-tubercular drugs in a sub Himalayan rural tertiary care teaching hospital. Int J Res Med Sci. 2016;4:4465–71. [Google Scholar]
- 10.David P, Thappa DM. Adverse cutaneous drug reactions: Clinical pattern and causative agents in a tertiary care centre in South India. Indian J Dermatol Venereol Leprol. 2004;70:20–4. [PubMed] [Google Scholar]
- 11.Sinha K, Marak IR, Singh WA. Adverse drug reactions in tuberculosis patients due to directly observed treatment strategy therapy: Experience at an outpatient clinic of a teaching hospital in the city of Imphal, Manipur, India. J Assoc Chest Physicians. 2013;1:50–3. [Google Scholar]
- 12.Sharma VK, Sethuraman G, Kumar B. Cutaneous adverse drug reactions: Clinical pattern and causative agents - A 6-year series from Chandigarh, India. J Postgrad Med. 2001;47:95–9. [PubMed] [Google Scholar]
- 13.Suther JV, Desai SV. A study of adverse cutaneous drug reactions in outdoor patients attending to Skin and VD Department of Shree Krishna Hospital, Karamsad. Int J Res Pharm Biomed Sci. 2011;2:2229–31. [Google Scholar]
- 14.Patel RM, Marfatia YS. Clinical study of cutaneous drug eruptions in 200 patients. Indian J Dermatol Venereol Leprol. 2008;74:430–6. doi: 10.4103/0378-6323.42883. [DOI] [PubMed] [Google Scholar]
- 15.Yew WW, Chan DP, Leung CC, Zhang Y, Wang R, Ng P, et al. Can toxicities induced by antituberculosis drugs be better managed in diabetic patients? Eur Respir J. 2017;50:1700409. doi: 10.1183/13993003.00409-2017. [DOI] [PubMed] [Google Scholar]
- 16.Castro AT, Mendes M, Freitas S, Roxo PC. Incidence and risk factors of major toxicity associated to first-line antituberculosis drugs for latent and active tuberculosis during a period of 10 years. Rev Port Pneumol. 2015;21:144–50. doi: 10.1016/j.rppnen.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Yee D, Valiquette C, Pelletier M, Parisien I, Rocher I, Menzies D. Incidence of serious side effects from 1st line anti-tuberculosis drugs among patients treated for active tuberculosis. Am J Respir Crit Care Med. 2003;167:1472–7. doi: 10.1164/rccm.200206-626OC. [DOI] [PubMed] [Google Scholar]
- 18.Thong BY, Chia FL, Tan SC, Tan TC, Leong KP, Tan JW, et al. A retrospective study on sequential desensitization-rechallenge for antituberculosis drug allergy. Asia Pacific allergy. 2014;4:156–63. doi: 10.5415/apallergy.2014.4.3.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehloenya RJ, Todd G, Badri M, Dheda K. Outcomes of reintroducing antituberculosis drugs following cutaneous adverse drug reactions. Int J Tuberc Lung Dis. 2011;15:1649–55. doi: 10.5588/ijtld.10.0698. [DOI] [PubMed] [Google Scholar]
- 20.Kakande B, Lehlohnya RJ. Drug reactions associated with anti-tubercular drugs. Cur Allergy Clin Immunol. 2015;28:264–7. [Google Scholar]
- 21.Chemotherapy and management of tuberculosis in the United Kingdom: Recommendations 1998. Joint Tuberculosis Committee of the British Thoracic Society. Thora×. 1998;53:536–48. [PMC free article] [PubMed] [Google Scholar]
- 22.Lehloenya RJ, Wallace J, Todd G, Dheda K. Multiple drug hypersensitivity reactions to anti-tuberculosis drugs: Five cases in HIV-infected patients. Int J Tuberc Lung Dis. 2012;16:1260–4. doi: 10.5588/ijtld.11.0187. [DOI] [PubMed] [Google Scholar]
- 23.Pichler WJ, Srinoulprasert Y, Yun J, Hausmann O. Multiple drug hypersensitivity. Int Arch Allergy Immunol. 2017;172:129–38. doi: 10.1159/000458725. [DOI] [PMC free article] [PubMed] [Google Scholar]


