Abstract
Brown-black pigment network is the hallmark dermoscopic feature of melanocytic lesions. Several non-melanocytic disorders also exhibit a pigment network as one of their main or useful dermoscopic diagnostic features. This article presents a compilation of such disorders to the readers describing their essential dermoscopic features with an emphasis on the characteristics of the pigment network exhibited by them.
Keywords: Dermoscopy, non-melanocytic lesions, pigment network
Introduction
Brown-black pigment network is the quintessential dermoscopic feature of melanocytic lesions (benign, dysplastic and malignant), which is attributable to the increased melanocytes and melanin production. The network pattern corresponds to the melanin in the basal layer of the epidermis. The “pigmented lines” forming the grid correspond to the tips of the rete pegs, and the intervening “holes” to the relatively less pigmented tips of the dermal papillae together with the overlying epidermal suprapapillary plates [Figure 1]. The size of these “holes” depend on the width of the dermal papillae. The network pattern can be regular or typical indicative of benign nature as seen in benign melanocytic lesions and in the normal skin of darker complexion. Irregular or atypical pattern characterize dysplastic nevi and melanoma. The rete ridges on the face are flatter, and hence, the typical pigment network on the face is usually absent. However, a pattern resembling the typical pigment network is evident on the facial skin, wherein the “holes” correspond to the adnexal openings rather than the tips of the dermal papillae, and hence, this pattern is described as pigment “pseudonetwork” [Figure 2].[1]
Figure 1.
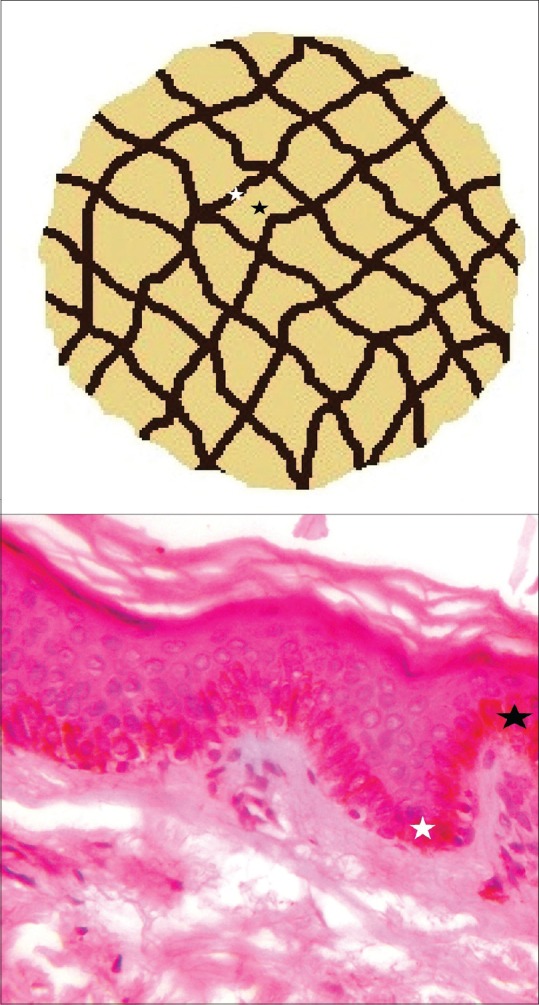
Schematic illustration of a typical pigment network formed by uniform and regular mesh of brown lines. The lines correspond to the tips of the rete pegs (white stars) while the intervening non-pigmented areas to the tips of dermal papillae (black stars)
Figure 2.
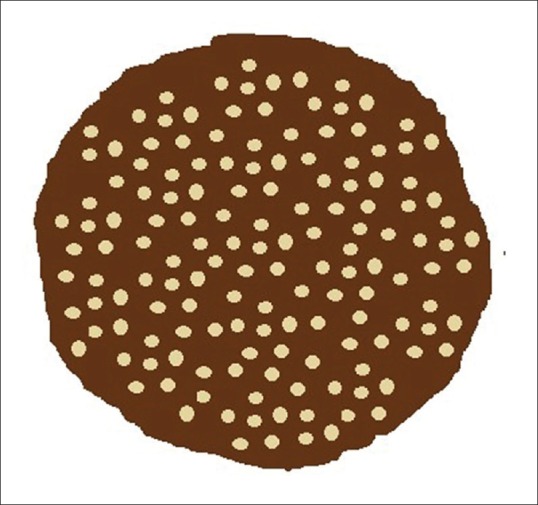
Schematic illustration of pigment pseudonetwork. A uniform homogenous pigmented background is interrupted by the adnexal openings giving a network-like appearance
Non-melanocytic lesions with dermoscopic pigment network
Several non-melanocytic lesions demonstrate a pigment network akin to that seen in melanocytic lesions as one of their main dermoscopic diagnostic criteria, or as an important supportive dermoscopic feature. In these disorders, the pigment network corresponds to increased melanization of the basal layer by the normal number of melanocytes rather than an increase in number of melanocytes. This compilation describes the essential dermoscopic features of such disorders with an emphasis on the pigment network observed in them [Table 1].
Table 1.
Non-melanocytic lesions exhibiting pigment network on dermoscopy
| Conditions | Essential dermoscopic features | Dermoscopic pigment network |
|---|---|---|
| Dermatofibroma | Central white scar-like bright white areas with or without shiny white lines, and a peripheral delicate pigment network | Delicate network of thin brown lines, predominantly at the periphery and occasionally throughout the lesion |
| Pigmented purpuric dermatosis | Coppery-brown background, brown globules, red dots and globules, gray dots, linear or curved vessels, and brown pigment network | Interconnecting brown lines commonly at the center of the lesions |
| Diabetic dermopathy | Central white scar-like patch with peripheral brown pigment network | Light brown to dark brown lines in a fishnet pattern Darker at the periphery and faint toward the center |
| Cutaneous mastocytosis | Yellow-orange blot, reticular brown pigment network, reticulate vascular pattern and light brown blot | Light to dark brown network throughout the lesion |
| Syringoma | Brown background/pseudonetwork, larger eccrine openings, brown reticulate pigmentation, and multifocal hypopigmentations | Faint network of thin brown lines at the periphery Dark brown prominent pigment network throughout the lesional surface (authors’ personal observation) |
| Accessory nipple | Central white scar-like area and a peripheral pigment network | Brownish thin interconnecting lines over a background of diffuse tan background in a peripheral (commonly) or central location |
| Cutaneous lichen sclerosus | Bright white areas with yellowish follicular plugs | Brown pigment network-like areas occasionally seen in atrophic lesions |
| Morphea | White cloudy areas described as “fibrotic beams” | Brown pigment network-like areas occasionally seen in inflammatory-sclerotic, sclerotic, and atrophic lesions |
| Neurofibroma | Peripheral pigment network, peripheral brown halo, pink-red structureless areas, fingerprint-like structures, scar-like areas, fissures, and blood vessels | Peripheral pigment network is predominantly seen in neurofibromas associated with underlying café au lait macules |
| Solar lentigo | Homogenous brown pattern, brown lines in “finger print” pattern, or a pigment network | Faint brown pigment network throughout the lesion |
| Café au lait macules | Brown homogenous or reticulate pattern of pigmentation | Reticulate pattern was seen on the non-facial lesions. Facial lesions showed homogenous pigmentation with perifollicular halo (see text) |
| Becker’s melanosis | Pigment network, focal hypopigmentation, skin furrow hypopigmentation, hair follicles, perifollicular hypoigmentation, and blood vessels | Pigment network seems to be regular and uniform (see text) |
| Pigmented seborrheic keratosis | Milia-like cysts, comedo-like openings, furrows, vessels, diffuse pigmentation or pigment network | Pigment network formed by dark wide grids and the intervening holes corresponding also to keratin filled structures apart from tips of the dermal papillae |
Dermatofibroma
Dermatofibroma is a common fibrohistiocytic tumor clinically characterized by a smooth, well-defined dome-shaped circumscribed lesion predominantly affecting the lower extremities of adults.[2] The classic dermoscopic features of dermatofibroma include central white scar-like area, which on polarized dermoscopy appears as bright white areas with or without shiny white lines (chrysalis or crystalline structures), and a peripheral delicate pigment network [Figure 3]. The former corresponds to fibrosis in the papillary dermis and the pigment network to increased melanization of the basal layer attributable to the preceding inflammation.[3] Zaballos et al. observed such pigment network in 296 of the 412 dermatofibromas assessed by them, and it was found to be predominantly peripheral in location and characterized by delicate thin lines of light brown color forming a regular (typical) mesh. In other instances, the network was found to be throughout the lesion, and also as a prominent and irregular network.[4]
Figure 3.
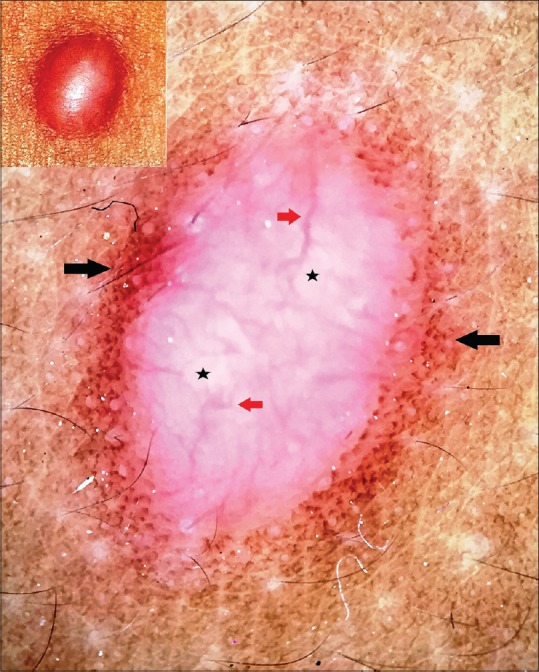
Polarized dermoscopy of dermatofibroma showing a central homogenous bright white background (black stars) with linear and branching vessels (red arrows) and a peripheral discrete tan-brown pigment network (black arrows) merging imperceptibly with the surrounding skin. [DermLite™ DL3 (3Gen Inc., San Juan Capistrano, CA, USA), Original magnification × 10]
Pigmented purpuric dermatoses
Pigmented purpuric dermatoses (PPDs) are a group of chronic conditions of undefined etiology, characterized by bilaterally symmetrical purpuric and pigmented macules, and patches involving the lower legs. Five clinical forms have been described namely progressive PPD (Schamberg disease), purpura annularis telangiectodes (Majocchi's disease), lichen aureus, pigmented purpuric lichenoid dermatosis, and eczematid-like purpura (of Doucas and Kapetanakis). The prototype of the PPDs is the Schamberg disease, which is characterized by asymptomatic irregular red to brown papules and plaques with typical “cayenne pepper” spots appearing within and at the edge of older lesions. All the clinical forms histologically exhibit “endocapillaritis” characterized by endothelial swelling of superficial vessels, perivascular T-cell infiltration, extravasation of red blood cells, and hemosiderin-laden macrophages in the interstitium.[5] In addition, an increased melanization of basal layer as well as upper dermal melanophages in the vicinity of the dermo-epidermal junction are seen. The predominant dermoscopic features of PPD include a coppery-brown background (owing to cellular infiltrate and hemosiderin-laden macrophages), brown globules (hemosiderin), red dots and globules (red blood cell extravasation), gray dots (hemosiderin-laden macrophages in the upper dermis), linear or curved vessels (vasodilation), and brown interconnecting lines (pigment network due to increased melanization of the basal layer).[6,7] This pigment network is usually in the center of the lesion [Figure 4].[8,9]
Figure 4.
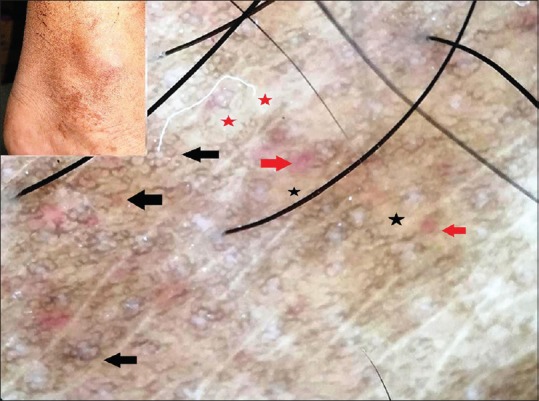
Polarized dermoscopy of Schamberg disease showing a coppery-brown background (black stars), red globules (red arrows), grayish-white areas (red stars), and a brown pigment network (black arrows). [DermLite™ DL3 (3Gen Inc., San Juan Capistrano, CA, USA), Original magnification × 10]
Diabetic dermopathy
Diabetic dermopathy is the most common cutaneous manifestation of long-standing diabetes usually seen in the elderly and characterized by asymptomatic erythematous or brown, round to oval atrophic lesions predominantly involving the shins in a bilateral and symmetrical manner (diabetic shin spots). In established lesions, the intensity of pigmentation reflects the extent of atrophy, with the most pigmented lesion being the most atrophic. In some instances, the brown color gradually fades and is replaced by hypopigmentation, especially in the center [Figure 5a]. Histologically, the epidermis is atrophic with attenuation of the rete ridges, thinning of the Malpighian layer, hyperkeratosis of stratum corneum, and variable melanization of the basal layer. Dermal changes include thickening and fragmentation of collagen bundles, increased capillaries with evidence of microangiopathy (endothelial proliferation, hyalinization of the vessel walls, and luminal occlusion), and dermal melanophages and hemosiderin deposits.[10] Central white scar-like patch with peripheral brown pigmentation in a fishnet pattern has been described as a frequent dermoscopic feature in diabetic dermopathy. The former is attributable to dermal fibroplasia and overlying atrophy of the epidermis (thinning and effacement of rete ridges), and the latter to the melanization of the basal layer in the persistent rete ridges.[11] Authors have observed similar features except for the pigment network being throughout the lesion albeit faint and lesser toward the central hypopigmented area [Figure 5b].
Figure 5.
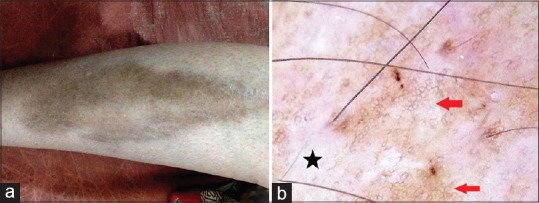
Diabetic dermopathy [a]. Polarized dermoscopy showing central scar-like bright white area (black star) and a peripheral discrete brown pigment network (red arrows) [b]. [DermLite™ DL3 (3Gen Inc., San Juan Capistrano, CA, USA), Original magnification × 10]
Cutaneous mastocytosis
Cutaneous mastocytosis is a mast cell proliferative disorder affecting the skin. The disease may be primarily cutaneous or the skin lesions reflect systemic mastocytosis. The latter requires systemic screening to identify and establish the diagnosis. Hence, “mastocytosis in the skin” is an appropriate description. Urticaria pigmentosa (most common), diffuse cutaneous mastocytosis, telangiectasia macularis eruptiva perstans, and solitary cutaneous mastocytoma are the frequent forms of cutaneous mastocytoses. Histology of cutaneous mastocytosis is characterized by diffuse proliferation of mast cells in the dermis together with increased melanization of the basal layer (especially the rete ridges) and dilated blood vessels. Vano-Galvan et al. observed four distinctive dermoscopic features – yellow-orange blot (corresponding to diffuse dermal mast cell infiltrate), reticular vascular pattern (owing to vasodilatation), light brown blot, and reticular brown pigment network. The latter is seen throughout the lesion [Figure 6] and is attributable to basal layer melanization secondary to inflammatory process in the dermis due to increased mast cells.[12,13] Authors have observed a similar pigment network in a case of solitary cutaneous mastocytoma.[14]
Figure 6.
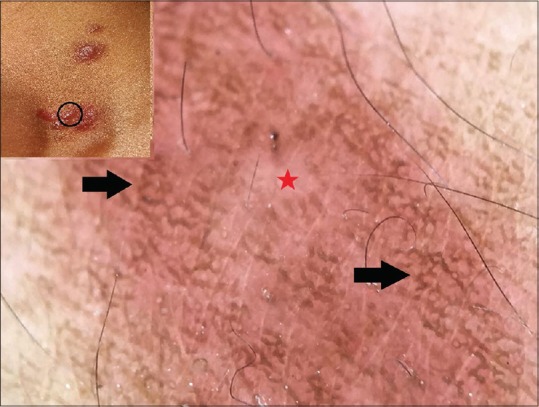
Polarized dermoscopy of urticaria pigmentosa showing an erythematous to faint brown background (red star) and overlying brown pigment network (black arrows). [DermLite™ DL3 (3Gen Inc., San Juan Capistrano, CA, USA), Original magnification × 10]
Syringoma
Syringoma is a benign tumor arising from the eccrine ducts. It occurs predominantly in women and principally involves the periorbital area (most commonly below the lower eyelids). The lesions are characterized by skin-colored or light brown small discrete papules. Rarely, involvement of other sites (chest and neck) and different clinical forms (linear or eruptive) are seen as well. Histology of syringoma is typical and is characterized by dermal aggregates of convoluted and cystic eccrine ducts in a fibrous stroma with a pathognomonic tail-like strands of cells extending from a side of the duct into the stroma giving a “tadpole appearance.”[15] The dermoscopic features of syringoma described in the literature include a brown pigment pseudonetwork with larger and prominent eccrine openings,[16] and the presence of faint delicate brown pigment network surrounding a central homogenous brownish area interspersed with multifocal hypopigmentations.[17] The pigment network has been speculated to be due to the fibrotic dermal stroma inducing thickening of the overlying epidermis and basal layer melanosis.[17] In authors' personal observation, similar features were noted although the pigment network was conspicuously thick and distributed throughout the lesion [Figure 7].
Figure 7.
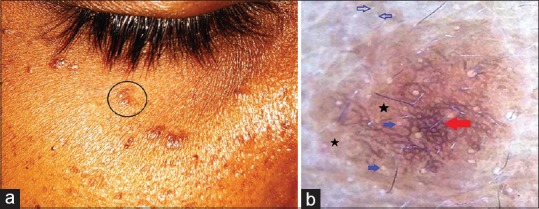
Periorbital syringoma [a]. Polarized dermoscopy showing a brown background (black stars) with overlying dark brown lines in a reticulate fashion (red arrow). In addition, note the larger and prominent eccrine openings on the lesional surface (blue solid arrows) than on surrounding normal skin (blue hollow arrows) [b]. [DermLite™ DL3 (3Gen Inc., San Juan Capistrano, CA, USA), Original magnification × 10]
Accessory nipple
Accessory or supernumerary nipple (polythelia) is a rare developmental anomaly usually characterized by a solitary, asymptomatic, pigmented papule or nodule with a central umbilication resembling a fibroma. They can occur anywhere along the embryonic milk line but are frequently seen on the anterior torso. Histologically, they are characterized by variable amounts of smooth muscles and ductal structures that either open into the pilosebaceous units or enter the overlying epidermis, which is thickened, papillomatous, and exhibits basal layer hyperpigmentation.[18] Kamińska-Winciorek et al. observed a central white scar-like area and a peripheral network-like structure made up of brownish thin interconnecting lines over a diffuse tan background as the most common dermoscopic feature in 19 accessory nipples. Such a network was also noted to be centrally located in some of the cases.[19] A similar feature was noted in five cases by Oztas et al.[20] as well, and Cabo et al.[21] has also previously highlighted the peripheral pigment network in accessory nipples. This dermoscopic pattern is akin to that seen in dermatofibroma (see above), which also is a clinical differential diagnosis for accessory nipple.[19,22] From the histological features, this pigment network should be attributable to basal layer hyperpigmentation.
Morphea and cutaneous (non-genital) lichen sclerosus
Although statistically insignificant, Errichetti et al. observed a brownish reticulate pigmentation in a few cases of morphea and cutaneous lichen sclerosus as features additional to the principal findings of “fibrotic beams” or “white clouds” (attributable to dermal fibrosis) in morphea, and bright white areas (attributable to hyalinization of the upper dermis together with epidermal atrophy) and yellowish follicular plugs in cutaneous lichen sclerosus. The pigment network is due to epidermal hyperpigmentation and was observed in the inflammatory-sclerotic, sclerotic and atrophic lesions of morphea, and sclerotic lesions of cutaneous lichen sclerosus.[23] Figure 8 exemplifies the pigment network in atrophic stage of morphea.
Figure 8.
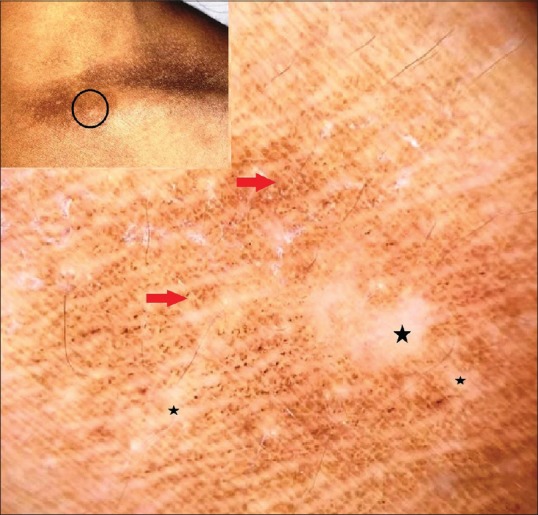
Polarized dermoscopy of morphea (atrophic stage) showing a homogenous white globules and structureless areas (black stars) with discrete pigment network (red arrows). [DermLite™ DL3 (3Gen Inc., San Juan Capistrano, CA, USA), Original magnification × 10]
Neurofibroma
Duman et al. characterized dermoscopic features of cutaneous neurofibromas in neurofibromatosis type 1 after analyzing 26 lesions in five patients. Seven predominant features were observed – a peripheral pigment network, a peripheral halo of brown pigmentation, pink-red structureless areas, fingerprint-like structures, scar-like areas, fissures, and blood vessels. The peripheral pigment network was predominantly observed in the lesions associated with underlying café au lait macules (CALMs). Other pigmentary features (halo of brown pigmentation and finger-print structures) were also more frequently observed in neurofibromas associated with CALMs compared to those without.[24] Considering the histological features of CALMs (see below), the pigment network is attributable to increased melanization of basal layer by the normal numbers of melanocytes. Similar features were observed by the authors [Figure 9].
Figure 9.
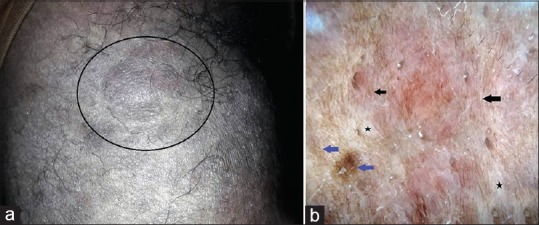
A case of neurofibromatosis type 1 [a]. Polarized dermoscopy of a neurofibroma in this patient showing brown fingerprint patterns (black arrows), pigment network (blue arrows), and white globules (scar-like areas, black stars). [DermLite™ DL3 (3Gen Inc., San Juan Capistrano, CA, USA), Original magnification × 10] [b]
Solar lentigo
As opposed to lentigo simplex, wherein the increased basal layer melanization is due to excessive melanin produced by the increased number of melanocytes, solar lentigo is characterized by increased basal layer melanization due to excessive melanin production by the normal number of melanocytes. This histological change is reflected dermoscopically as uniform diffuse pigmentation, as fingerprint structures (pigmented lines configured in a pattern resembling dermatoglyphics), or as a faint pigment network. Solar lentigo may evolve into pigmented seborrheic keratosis.[25]
Pigmented seborrheic keratosis
A brown pigment network akin to melanocytic lesions (grid and holes as described above) can be seen in seborrheic keratosis, especially in the pigmented variants [Figure 10] owing to basal layer hypermelanization. Braun et al. described this feature in pigmented seborrheic keratosis as “network-like structures” and observed that the lines forming the grid were often hyperpigmented and wider and ended abruptly at the periphery. The intervening holes may also represent fissures and comedo-like openings apart from the dermal papillary tips.[26] Squillace et al. characterized ten dermoscopic patterns in “difficult-to-diagnose” seborrheic keratosis, which included a “reticular” pattern characterized by a regular grid of thin brown lines over a diffuse light brown background.[27] In demoscopic analysis of 72 “atypical” seborrheic keratoses, Mazzeo et al. characterized eight dermoscopic patterns, which also included a “reticular” pattern seen in 30 cases that was characterized by straight lines intersecting each other at right angles and at regular intervals to form a pigment network.[28]
Figure 10.
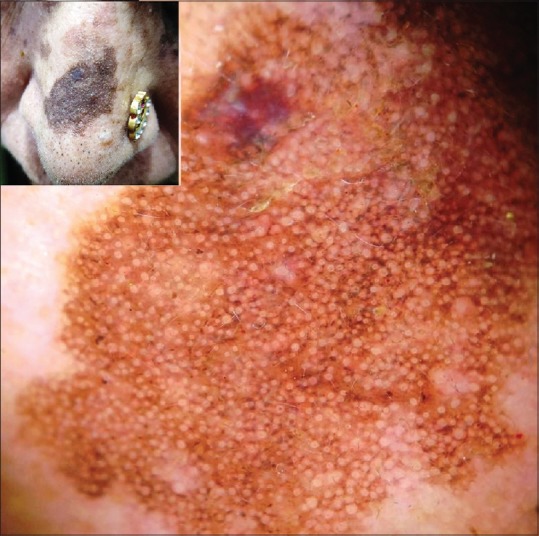
Polarized dermoscopy of a flat pigmented seborrheic keratosis on the nose showing a conspicuous pigment “pseudonetwork.” [DermLite™ DL3 (3Gen Inc., San Juan Capistrano, CA, USA), Original magnification × 10]
Café au lait macule
Café au lait macules are characterized by light brown to tan pigmented macules of varying sizes. Multiple CALMs are one of the earlier markers of neurofibromatosis type 1, which is the most common neurocutaneous syndrome associated with CALMs. The CALMs are also seen in several other genetic disorders such as McCune-Albright syndrome, Watson syndrome, Fanconi anemia, and a few others.[29] Solitary CALMs are usually isolated lesions without any syndromic associations.[30] Histologically, the essential feature is the hypermelanization of basal layer of the epidermis and presence of macromelanosomes in the basal keratinocytes.[31] Dermoscopically, Luk et al. observed a homogenous brown pigmentation with perifollicular halo, and a reticular pattern of brown pigmentation in the CALMs of face and neck in 15 children.[32] These features should be attributable to increased basal layer melanization and melanosomes in the basal keratinocytes. It should be noted however that the CALMs associated with neurofibromatosis type 1 may also show an increase in the melanocyte number.[33,34] [Figure 11] depicts the reticulate brown pigmentation of a CALM in a case of neurofibromatosis type 1 observed by the authors.
Figure 11.
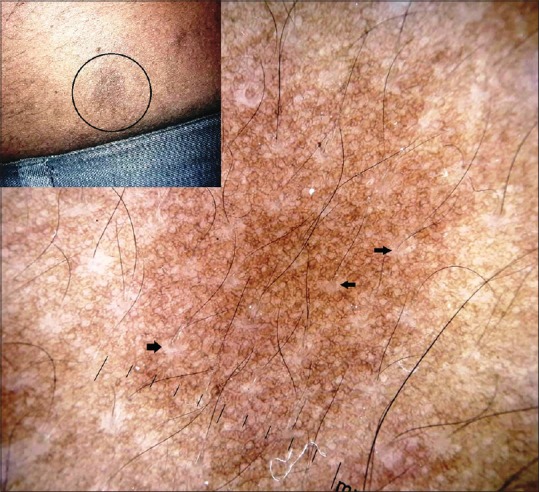
Polarized dermoscopy of a café au lait macule in the patient of neurofibromatosis type 1 [Figure 9a] showing a diffuse reticulate pattern of brown pigmentation with perifollicular hypopigmentation (black arrows). [DermLite™ DL3 (3Gen Inc., San Juan Capistrano, CA, USA), Original magnification × 10]
Becker's melanosis
Becker's melanosis (or nevus) is an acquired hamartoma occurring in late childhood or adolescence. It is more common in males and frequently presents as macular hyperpigmentation with irregular borders. It may be associated with lesional hypertrichosis. Increased frequency in males, onset in adolescence, and increased lesional expression of androgenic receptors and mRNA are all suggestive of androgenic role in its pathogenesis.[35] Ingordo et al. compared nine dermoscopic features (target network, focal thickening of network lines, target globules, skin furrow hypopigmentation, focal hypopigmentation, hair follicles, perifollicular hypopigmentation, vessels, and target vessels) in medium to large congenital melanocytic nevi and Becker nevus in adult males. It was observed that the pigment network, focal hypopigmentation, skin furrow hypopigmentation, hair follicles, perifollicular hypopigmentation, and vessels were the main dermoscopic features in Becker nevus. Pigment network, globules, target globules, homogeneous diffuse pigmentation, hyperpigmented areas, and blotches were more frequent in congenital melanocytic nevi than in Becker nevus. In regard to the pigment network, focal thickening of network lines was more frequent in congenital melanocytic nevi than in Becker nevus.[36] Authors have observed similar features [Figure 12].
Figure 12.
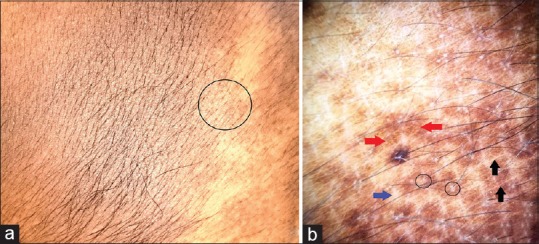
Becker's melanosis [a]. Polarized dermoscopy showing pigment network (red arrows) with perifollicular hypopigmentation (black circles), focal hypopigmentation (blue arrow), and skin furrow hypopigmentation (black arrows) [b]. [DermLite™ DL3 (3Gen Inc., San Juan Capistrano, CA, USA), Original magnification × 10]
Other conditions
A brown pigment network pattern has occasionally been described in Kaposi sarcoma[13] and erythema ab igne.[37] An atypical pigment network akin to that of melanoma has been reported in a case of pigmented Bowen's disease as well.[38]
Conclusion
It is important to be aware of the nature and patterns of dermoscopic pigment network observed in the disorders discussed in this article and to be able to differentiate with those in the melanocytic lesions. This would not only help in making an appropriate diagnosis but also avoid unnecessary and erroneous categorization of such lesions into the melanocytic group.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Pigment network. [Last accessed on 2019 May 16]. Available from: https://dermoscopedia.org/Pigment_network .
- 2.Kamino H, Meehan SA, Pui J. Fibrous and fibrohistiocytic proliferations of the skin and tendons. In: Bolognia JL, Jorizzo JL, Rapini RP, editors. Dermatology. 2nd ed. London: Elsevier; 2008. pp. 1813–29. [Google Scholar]
- 3.Arpaia N, Cassano N, Vena GA. Lessons on dermoscopy: Dermatofibroma (a case report and personal considerations) Dermatol Surg. 2004;30:421. doi: 10.1111/j.1524-4725.2004.30112.x. [DOI] [PubMed] [Google Scholar]
- 4.Zaballos P, Puig S, Llambrich A, Malvehy J. Dermoscopy of dermatofibromas: A prospective morphological study of 412 cases. Arch Dermatol. 2008;144:75–83. doi: 10.1001/archdermatol.2007.8. [DOI] [PubMed] [Google Scholar]
- 5.Cox NH, Piette WW. Purpura and microvascular occlusion. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook's Textbook of Dermatology. 8th ed. Oxford: Wiley-Blackwell; 2010. pp. 491–51. [Google Scholar]
- 6.Ozkaya DB, Emiroglu N, Su O, Cengiz FP, Bahali AG, Yildiz P, et al. Dermatoscopic findings of pigmented purpuric dermatosis. An Bras Dermatol. 2016;91:584–7. doi: 10.1590/abd1806-4841.20165124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Çakmak SK, Kılıç A, Yorulmaz A, Onan D, Yayla D, Artüz F. Dermoscopic findings in patients with pigmented purpuric dermatoses. Acta Dermatovenerol Croat. 2016;24:291–5. [PubMed] [Google Scholar]
- 8.Zaballos P, Puig S, Malvehy J. Dermoscopy of pigmented purpuric dermatoses (lichen aureus): A useful tool for clinical diagnosis. Arch Dermatol. 2004;140:1290–1. doi: 10.1001/archderm.140.10.1290. [DOI] [PubMed] [Google Scholar]
- 9.Portela PS, Melo DF, Ormiga P, Oliveira FJ, Freitas NC, Bastos Júnior CS. Dermoscopy of lichen aureus. An Bras Dermatol. 2013;88:253–5. doi: 10.1590/S0365-05962013000200013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George SMC, Walton S. Diabetic Dermopathy. Br J Diabetes Vasc Dis. 2014;14:95–7. [Google Scholar]
- 11.Nischal KC, Khopkar US. Dermoscopic diagnosis of diabetic dermopathy: A skin marker of diabetic microangiopathy. In: Khopkar US, editor. Dermoscopy and Trichoscopy in the Diseases of Brown Skin Atlas and Short Text. 1st ed. New Delhi: Jaypee Brothers Medical Publishers; 2012. pp. 265–9. [Google Scholar]
- 12.Vano-Galvan S, Alvarez-Twose I, De las Heras E, Morgado JM, Matito A, Sánchez-Muñoz L, et al. Dermoscopic features of skin lesions in patients with mastocytosis. Arch Dermatol. 2011;147:932–40. doi: 10.1001/archdermatol.2011.190. [DOI] [PubMed] [Google Scholar]
- 13.Arpaia N, Cassano N, Vena GA. Lessons on dermoscopy: Pigment network in nonmelanocytic lesions. Dermatol Surg. 2004;30:929–30. doi: 10.1111/j.1524-4725.2004.30275.x. [DOI] [PubMed] [Google Scholar]
- 14.Adya KA, Inamadar AC, Palit A. Dermoscopy of cutaneous mastocytoma. Indian Dermatol Online J. 2018;9:218–9. doi: 10.4103/idoj.IDOJ_193_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calonje E. Tumours of the skin appendages. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook's Textbook of Dermatology. 8th ed. Oxford: Wiley-Blackwell; 2010. pp. 531–44. [Google Scholar]
- 16.Ankad BS, Sakhare PS, Prabhu MH. Dermoscopy of non-melanocytic and pink tumors in brown skin: A descriptive study. Indian J Dermatopathol Diagn Dermatol. 2017;4:41–51. [Google Scholar]
- 17.Hayashi Y, Tanaka M, Nakajima S, Ozeki M, Inoue T, Ishizaki S, et al. Unilateral linear syringoma in a Japanese female: Dermoscopic differentiation from lichen planus linearis. Dermatol Reports. 2011;3:e42. doi: 10.4081/dr.2011.e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weedon D. Miscellaneous conditions. In: Weedon D, editor. Weedon's Skin Pathology. 3rd ed. Edinburgh: Churchil Livingstone Elsevier; 2010. pp. 502–9. [Google Scholar]
- 19.Kamińska-Winciorek G, Szymszal J, Silny W. Dermoscopy of accessory nipples in authors' own study. Postepy Dermatol Alergol. 2014;31:127–33. doi: 10.5114/pdia.2014.43189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oztas MO, Gurer MA. Dermoscopic features of accessory nipples. Int J Dermatol. 2007;46:1067–8. doi: 10.1111/j.1365-4632.2007.03283.x. [DOI] [PubMed] [Google Scholar]
- 21.Cabo H, Stolz W. Pigment network: A clue to dermatoscopic diagnosis of melanocytic lesions – Supernumerary nipple: Another exception to the rule. Dermatol Surg. 2004;30:1068–9. doi: 10.1111/j.1524-4725.2004.30319.x. [DOI] [PubMed] [Google Scholar]
- 22.Blum A, Roehm S. Accessory nipple looks like dermatofibroma in dermoscopy. Arch Dermatol. 2003;139:948–9. doi: 10.1001/archderm.139.7.948-b. [DOI] [PubMed] [Google Scholar]
- 23.Errichetti E, Lallas A, Apalla Z, Di Stefani A, Stinco G. Dermoscopy of morphea and cutaneous lichen sclerosus: Clinicopathological correlation study and comparative analysis. Dermatology. 2017;233:462–70. doi: 10.1159/000484947. [DOI] [PubMed] [Google Scholar]
- 24.Duman N, Elmas M. Dermoscopy of cutaneous neurofibromas associated with neurofibromatosis type 1. J Am Acad Dermatol. 2015;73:529–31. doi: 10.1016/j.jaad.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 25.Dermoscopy: Dermoscopy of benign melanocytic lesions. [Last accessed on 2019 May 21]. Available from: https://www.dermnetnz.org/cme/dermoscopycourse/dermoscopy-of-benign-melanocytic-lesions/
- 26.Braun RP, Rabinovitz HS, Krischer J, Kreusch J, Oliviero M, Naldi L, et al. Dermoscopy of pigmented seborrheic keratosis: A morphological study. Arch Dermatol. 2002;138:1556–60. doi: 10.1001/archderm.138.12.1556. [DOI] [PubMed] [Google Scholar]
- 27.Squillace L, Cappello M, Longo C, Moscarella E, Alfano R, Argenziano G. Unusual dermoscopic patterns of seborrheic keratosis. Dermatology. 2016;232:198–202. doi: 10.1159/000442439. [DOI] [PubMed] [Google Scholar]
- 28.Mazzeo M, Manfreda V, Diluvio L, Dattola A, Bianchi L, Campione E. Dermoscopic analysis of 72 “atypical” seborrheic keratoses. Actas Dermosifiliogr. 2019 doi: 10.1016/j.ad.2018.10.014. pii: S0001-7310 (18) 30532-5. [DOI] [PubMed] [Google Scholar]
- 29.James WD, Sheth RD. Café au lait spots. [Last accessed on 2019 May 24]. Available from: https://emedicinemedscapecom/article/911900-overview#a4 .
- 30.James WD, Sheth RD. Café au lait spots clinical presentation. [Last accessed on 2019 May 24]. Available from: https://emedicinemedscapecom/article/911900-clinical#b2 .
- 31.James WD, Sheth RD. Café au lait spots work up. [Last accessed on 2019 May 24]. Available from: https://emedicinemedscapecom/article/911900-workup#c7 .
- 32.Luk DC, Lam SY, Cheung PC, Chan BH. Dermoscopy for common skin problems in Chinese children using a novel Hong Kong-made dermoscope. Hong Kong Med J. 2014;20:495–503. doi: 10.12809/hkmj144245. [DOI] [PubMed] [Google Scholar]
- 33.James WD, Sheth RD. Café au lait spots. [Last accessed on 2019 May 24]. Available from: https://emedicinemedscapecom/article/911900-overview#a5 .
- 34.Crowson AN, Zeller S, Barnhill RL. Disorders of pigmentation. In: Barnhill RL, Crowson AN, Magro CM, Pipekorn MW, editors. Dermatopathology. 3rd ed. New York: McGraw Hill companies, Inc; 2010. pp. 338–61. [Google Scholar]
- 35.Nair PA, Modasia K, Bhavsar N, Navadiya R. Dermatoscopy of Becker's nevus. Egypt J Dermatol Venerol. 2019;39:37–9. [Google Scholar]
- 36.Ingordo V, Iannazzone SS, Cusano F, Naldi L. Dermoscopic features of congenital melanocytic nevus and Becker nevus in an adult male population: An analysis with a 10-fold magnification. Dermatology. 2006;212:354–60. doi: 10.1159/000092286. [DOI] [PubMed] [Google Scholar]
- 37.Senhaji G, El Jouari O, Douhi Z, Mernissi FZ. A historical misleading case of erythema ab igne in a young female patient. Our Dermatol Online. 2018;9:454–5. [Google Scholar]
- 38.Stante M, de Giorgi V, Massi D, Chiarugi A, Carli P. Pigmented Bowen's disease mimicking cutaneous melanoma: Clinical and dermoscopic aspects. Dermatol Surg. 2004;30:541–4. doi: 10.1111/j.1524-4725.2004.30173.x. [DOI] [PubMed] [Google Scholar]


