Abstract
Background
Tuberculosis is a communicable disease that is a major cause of ill health, one of the top 10 causes of death worldwide, and the leading cause of death from a single infectious agent, even ranking above human immuno-deficiency virus (HIV/AIDS).
Objective
To assess the prevalence and associated risk factor of Mycobacterium tuberculosis among pulmonary tuberculosis (PTB) suspects attending at Ataye District Hospital from October 1, 2018, to February 30, 2019.
Methodology
A facility-based cross-sectional study was conducted among 423 presumptive tuberculosis patients at Ataye District Hospital. Sputum was processed by MTB/RIF Xpert assay. Data were entered into EpiData 3.1 software and exported to SPSS version 20.0 (SPSS, Chicago, IL, USA) for analysis. Univariate and multivariate analyses were used to examine the relationship between the dependent and independent variables. Variables that show significance at P-value of 0.3 during univariate analysis were selected for multivariable analysis. A P-value of less than or equal to 0.05 was used to indicate statistical significance.
Results
Out of the total study participants, about 60% were male, and 39% were aged between 18 and 24 years. Of the total 423 PTB suspected patients, 38 (8.98%) of them were identified as having PTB by GeneXpert and 2/38 (5.3%) were resistant to rifampicin and 3/38 (7.89%) patients were co-infected with HIV. Participant age between 18 and 24 years and between 25 and 34 years, weight loss, chest pain, having contact history with confirmed PTB cases, utilization of congested transportation, and a history of imprisonment were significantly associated with the prevalence of PTB.
Conclusion
A considerable prevalence of PTB in the area was observed and the magnitude of MDR-TB was low. PTB is still a public health problem in Ethiopia and there is a need for collaborative prevention and control activities in the study area.
Keywords: PTB, Ataye, MTB/RIF Xpert assay, Ethiopia
Background
Tuberculosis (TB) is a communicable disease that is a major cause of ill health, one of the top 10 causes of death worldwide, and the leading cause of death from a single infectious agent, even ranking above human immuno-deficiency virus (HIV/AIDS).1 Tuberculosis is a serious bacterial disease caused by Mycobacterium tuberculosis (M.tb), which mostly affects the lungs to cause pulmonary TB (PTB); the bacteria can affect other organs as well.2 According to a World Health Organization (WHO) report, 4.1% and 19% of newly diagnosed and previously treated TB patients, respectively, were multi-drug resistant tuberculosis (MDR-TB).3 Among retreated patients, the overall prevalence of MDR-TB may be increased up to 66.3%.4 In 2016, there were an estimated 1.3 million TB deaths among HIV negative people and an additional 374,000 deaths among HIV-positive people. Globally, the proportion of people who develop TB and die of the disease was 16% in 2016.3 In 2018, the WHO’s list of 30 high TB burden countries accounted for 87% of the world’s cases; Africa accounts for 24% of TB cases, and based on total TB incidence Ethiopia ranked 12th.1
According to the First Ethiopian National Population-Based Tuberculosis Prevalence Survey TB was recognized as a major public health problem in Ethiopia more than half a century ago.5 Although the effort to control tuberculosis began in the early 1960s with the establishment of TB centers and sanatoria in the country, the prevalence of TB is still increasing. Besides the increment of its prevalence, it can impose a serious public health problem particularly through causing deficiency of some micro-nutrients, like vitamin A and zinc.6 A varied proportion of TB ranges from 10% to 33.8% among presumptive TB cases was indicated in the hospital set up.7–11 In recent years the prevalence of the disease in a prison in the northwest part of the country was as high as 5.3%12 and as low as 3.4%.13
Contact with active tuberculosis cases, smoking cigarettes regularly, and HIV serostatus are important factors for and have an association with PTB.14 In the era of HIV the prevalence of PTB is increasing and it has a strong association with CD4 count, previous history of TB, and smoking.15 A significantly higher number of Rifampicin resistant (RR)-TB and MDR-TB were reported among previously treated patients compared to treatment-naive patients.8
In Ethiopia, a report indicated a relatively higher level of RR-TB among previously untreated cases, which suggests the existence of active person-to-person transmission or the existence of undiagnosed new RR-TB cases, or it is indicative of the performance of TB control programs in the past.8
There was a high prevalence of TB, RR, and TB/HIV co-infection among HIV-positive patients.16,17 People living with HIV have a more than 20-fold increased risk of TB compared to HIV-uninfected people18 and CD4+ lymphocyte count was found to be independently associated with TB/HIV co-infection.17 Since mothers are the primary care givers of children in Ethiopia and being female is a risk factor for RR-TB,8 this may increase the risk of RR-TB transmission among children.
Improving case-detection and diagnostic capacity for TB and MDR-TB is a priority in the care and control of TB. Reductions of the time to provide results and increasing the diagnostic sensitivity by directly detecting mutations in the genes that determine resistance to different anti-TB drugs are becoming possible via the introduction of molecular diagnostic facilities for anti-TB resistance.19 The introduction and rapid scale-up of the Xpert MTB/RIF assay in Ethiopia are therefore essential to address the issues. The assay has good sensitivity than and comparable specificity and positive predictive value with light-emitting diode fluorescent microscopy.20,21 It also has an increased positivity rate in comparison with ZN microscopy.22 The performance of GeneXpert in the detection of RR is similar to other molecular techniques like Genotype MTBDRplus.23 The rpoB gene, which is a target gene for RIF, covers all the mutations found in more than 99.5% of all RR strains. There is no cross-reactivity with non-tuberculosis mycobacterium and TB and RR were correctly detected in the presence of non-tuberculosis DNA or a mix of susceptible strains and resistant strains.24
There are limited data on the prevalence of PTB and associated risk factors among presumptive PTB patients in Ethiopia and there is no report specifically in Ataye. Therefore, the aim of this study was to determine the prevalence of TB and associated risk factors among presumptive PTB patients at Ataye Hospital, North Shoa, Ethiopia.
Methods
Study Design, Area, and Period
A facilitybased cross-sectional study was conducted at Atay District hospital from October 2018 to February 2019 GC. The hospital is found in the town of Ataye, in the regional state of Amhara, Ethiopia; the town is located 270 km from Addis Ababa which is the capital city of the country. Ataye is surrounded by Antsokiya Woreda in the north, Kewoit Woreda in the South, Jile Timuga Woreda in the East, and Menth Woreda in the west.
Study Population
The source population was all PTB suspected patients attending Ataye District Hospital; the study population included all TB suspected patients who fulfill the inclusion criteria and who were enrolled in the hospital during the study period.
Sample Size Determination
A single population proportion formula was used to estimate the sample size and the following assumption was considered: 95% confidence interval (Zα/2 = 1.96), 10% proportion from previous work,11 and 3% margin of error.
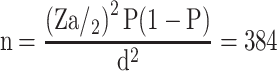 |
The calculated sample size was 384, but in order to compensate for inadequate sample specimens and minimize errors due to sample collection, it was decided to consider an additional 10% of the minimum sample size which made the final sample size 423.
Sample Collection and Processing
Before the actual specimen collection, the patients were instructed about the procedure for how to collect the sputum specimen. The sputum sample was processed by following the SOPs of MTB/RIF Xpert assay processing techniques. First, the label of the falcon tube was checked with the patient identification number. The sample was collected, prepared, and incubated by adding a reagent buffer with a 1:2 proportion sample and reagent, respectively. The incubated sample was examined to detect Mycobacterium tuberculosis by using a GeneXpert machine, model number 900–0513Gx-IvR2/Rev.C.2 (Cepheid, Inc.) with the molecular technique (MTB/RIF Xpert Assay).
Data Processing and Analysis
The collected data were entered into “EpiData 3.1” software and exported to SPSS version 20.0 (SPSS, Chicago, IL, USA) for analysis. Descriptive statistics including frequencies and proportions were used to summarize the data. Univariate and multivariate analyses were used to examine the relationship between the dependent variables and independent variables [socio-demographic characteristics, clinical characteristics, and predisposing factors]. Variables that show significance at P-values of 0.3 during univariate analysis were selected for multivariable analysis. Adjusted odds ratios (AOR) and their 95% confidence intervals (CIs) were used as indicators of the strength of association. P-value <0.05 was used to indicate statistical significance.
Ethical Consideration
Ethical clearance was obtained from the Department of Medical Laboratory Science, college of Medicine and Health Sciences, Wollo University. Permission to conduct the study was also obtained from Ataye District Hospital. Additionally, after explaining the importance, purpose, and procedure of the study briefly a written consent was obtained from study participants. A parent or legal guardian provided written informed consent for any participant under the age of 18 years. Any patient who was not willing to take part in the study had the full right not to do so and the confidentiality of the study participants was also strictly maintained. Any study participant who found to be infected with the bacterium was referred to a physician for treatment. This study was fully conducted in accordance with the ethical consideration declaration of Helsinki.
Results
Socio-Demographic-Related Data
In this research work, a total of 423 PTB suspected individuals participated. Out of the total study participants, about 60% were male, 39% were aged 18–24 years, 66% were single, 51% were Illiterate and 58% were rural area residents (Table 1).
Table 1.
Socio-Demographic Characteristics of PTB Suspected Cases at Ataye District Hospital from October 1, 2018, to February 30, 2019 (N=423)
| Parameter | MTB Detected No. (%) | MTB Not Detected No. (%) | Frequency No. (%) |
|---|---|---|---|
| Sex | |||
| Male | 24 (63.16) | 229 (59.48) | 253 (59.8) |
| Female | 14 (36.84) | 156 (40.52) | 170 (40.2) |
| Age | |||
| <18 yrs | 4 (10.53) | 47(12.21) | 51(12.1) |
| 18–24 yrs | 6 (15.79) | 159 (41.3) | 165 (39) |
| 25–34 yrs | 12 (31.58) | 67 (17.4) | 79 (18.7) |
| 35–44 yrs | 5 (13.16) | 75 (19.48) | 80(18.9) |
| ≥45 yrs | 11 (28.95) | 37 (9.61) | 48 (11.3) |
| Marital Status | |||
| Single | 20 (52.63) | 259 (67.27) | 279 (66) |
| Married | 17(44.74) | 93(24.16) | 110(26) |
| Divorced | 0 | 9 (2.34) | 9 (2.1) |
| Widowed | 1 (2.63) | 24 (6.23) | 25 (5.9) |
| Residency | |||
| Rural | 23 (60.53) | 224 (58.18) | 247 (58.4) |
| Urban | 15 (39.47) | 161 (41.82) | 176 (41.6) |
| Educational Level | |||
| Illiterate | 11 (28.95) | 205 (53.25) | 216 (51) |
| Read and write | 9 (23.68) | 62 (16.1) | 71 (16.8) |
| Primary/Secondary | 14 (36.84) | 92 (23.9) | 106 (25.1) |
| Higher Education | 4 (10.3) | 26 (6.75) | 30 (7.1) |
| Monthly Income | |||
| <500 | 3 (7.9) | 60 (15.58) | 63 (14.9) |
| 500–1000 | 9 (23.68) | 76 (19.74) | 85 (20.1) |
| 1001–1500 | 5 (13.16) | 97 (25.19) | 102 (24.1) |
| >1500 | 21 (55.26) | 152 (39.48) | 173 (40.9) |
Clinical Presentation-Related Data
All study participants showed clinical signs and symptoms of PTB. The main symptoms of the participants were cough 412 (97.4%), productive sputum 411 (97.2%), night-sweating 395 (93.4%), chest pain 302 (71.4%), fever 267 (63.1%), loss of appetite 300 (70.9%), fatigue 413 (97.6%), and excessive weight loss 279 (66%). About half of the study participants were coming to the health facility between 2 and 5 weeks after the onset of clinical signs and symptoms (Table 2).
Table 2.
Clinical Signs and Symptoms of PTB Suspected Cases at Ataye District Hospital from October 2018 to February 2019 (N=423)
| Parameter | MTB Detected No. (%) | MTB Not Detected No. (%) | Total No. (%) |
|---|---|---|---|
| Chronic Cough | |||
| Yes | 36 (94.74) | 376 (97.66) | 412 (97.4) |
| No | 2 (5.26) | 9 (2.34) | 11 (2.6) |
| Sputum Production | |||
| Yes | 38 (100) | 373 (96.88) | 411 (97.2) |
| No | 0 | 12 (3.12) | 12 (2.8) |
| Night Sweat | |||
| Yes | 37 (97.37) | 358 (92.99) | 395 (93.4) |
| No | 1 (2.63) | 27 (7.01) | 28 (6.6) |
| Fatigue | |||
| Yes | 38 (100) | 375 (97.4) | 413 (97.6) |
| No | 0 | 10 (2.6) | 10 (2.4) |
| Shortness of Breath | |||
| Yes | 38 (100) | 346 (89.87) | 384 (90.8) |
| No | 0 | 39 (10.13) | 39 (9.2) |
| Loss of Appetites | |||
| Yes | 35 (92.11) | 265 (68.83) | 300 (70.9) |
| No | 3 (7.89) | 120 (31.17) | 123 (29.1) |
| Weight Loss | |||
| Yes | 35 (92.11) | 244 (63.38) | 279 (66) |
| No | 3 (7.89) | 141 (36.62) | 144 (34) |
| Chest Pain | |||
| Yes | 35 (92.11) | 267 (69.35) | 302 (71.4) |
| No | 3 (7.89) | 118 (30.65) | 121 (28.6) |
| Fever | |||
| Yes | 29 (76.32) | 238 (61.82) | 267 (63.1) |
| No | 9 (23.68) | 147 (38.18) | 156 (36.9) |
| Duration of Coming at H/F | |||
| After Developing S/S? | |||
| Before 2 wks | 4 (10.53) | 64 (16.62) | 68 (16.1) |
| 2–5 wks | 31 (81.58) | 181 (47.01) | 212 (50.1) |
| >5 wks | 3 (7.89) |
|
143 (33.8) |
Associated Factor-Related Characteristics
Out of the total study participants, 352 (83.2%) had frequent utilization of congested transportation, 80 (18.9%) had contact history with known TB infected patients, 29 (6.9%) had a previous history of PTB and 23 (5.4%) had a history of cigarette smoking (Table 3).
Table 3.
Risk Factor-Related Characteristics Among PTB Suspected Cases at Ataye District Hospital from October 2018, to February 2019 (N=423)
| Parameter | MTB Detected No. (%) | MTB Not Detected No. (%) | Total No. (%) |
|---|---|---|---|
| No of Family Members | |||
| 1 up to 3 | 8 (21.05) | 82 (21.3) | 90 (21.3) |
| 4 up to 6 | 28 (73.68) | 234 (60.78) | 262 (61.9) |
| More than 6 | 2 (5.26) | 69 (17.92) | 71 (16.8) |
| Contact History with Known TB-iInfected pt | |||
| Yes | 19 (50) | 61 (15.84) | 80(18.9) |
| No | 19 (50) | 324 (84.16) | 343(81.1) |
| Contact with Confirmed TB | |||
| Family member | 3 (15.79) | 38 (62.3) | 41 (51.25) |
| Friends | 11 (57.89) | 11 (18.03) | 22 (27.5) |
| Neighbor | 4 (21.05) | 12 (19.67) | 16(20) |
| Others | 1 (5.26) | 0 | 1 (1.25) |
| Frequent Utilization of Congested Transportation | |||
| Yes | 37 (97.37) | 315 (81.82) | 352 (83.2) |
| No | 1 (2.63) | 70 (18.18) | 71 (16.8) |
| Imprisonment History | |||
| Yes | 15 (39.47) | 81 (21.04) | 96 (22.7) |
| No | 23 (60.53) | 304 (78.96) | 327 (77.3) |
| Previous PTB History | |||
| Yes | 6 (15.79) | 23 (5.97) | 29 (6.9) |
| No | 32 (84.21) | 362 (94.03) | 394 (93.1) |
| HIV Sero Status | |||
| Sero-reactive | 3 (7.89) | 11 (2.86) | 14 (3.3) |
| Sero-non-reactive | 31 (81.58) | 331 (85.97) | 362 (85.6) |
| Unknown | 4 (10.53) | 43 (11.17) | 47 (11.11) |
| Cigarette Smoking History? | |||
| Yes | 3 (7.89) | 20 (5.19) | 23 (5.4) |
| No | 35 (92.11) | 365 (94.81) | 400 (94.6) |
| History of Drinking Alcohol? | |||
| Yes | 11 (28.95) | 90 (23.38) | 101 (23.9) |
| No | 27 (71.05) | 295 (76.62) | 322 (76.1) |
Prevalence and Association of PTB
Of the total 423 PTB suspected patients, 38 (9%) of them were identified as having PTB, and two of these 38 (5.3%) were resistant to rifampicin. One of the rifampicin-resistant patients was a previously treated case and about three 3/38 (7.89%) patients were co-infected with HIV. Each variable from socio-demographic characteristics, clinical characteristics, and predisposing factors that had a P-value of 0.3 were subjected to multivariate analysis. Among the socio-demographic characteristics, age had a significant association with PTB. Participant ages between 18 and 24 years [AOR = 6.7; CI 1.5 −30.7] and between 25 and 34 years [AOR= 4.5; CI 1.2–16.4] were significantly associated with the prevalence of PTB (Table 4).
Table 4.
Univariate & Multivariate Analysis of Socio-Demographic Characteristics of PTB Suspected Study Participants at Ataye District Hospital from October 1, 2018, to February 30, 2019 (N=423)
| Variables | COR [95% CI] | P-value | AOR [95% CI] | P-value |
|---|---|---|---|---|
| Age | 0.001 | *0.044 | ||
| <18 | 2.26 [0.6–8.3] | 0.22 | 1.7 [0.24–12.05] | 0.598 |
| 18–24 | 7.9 [2.74–22.7] | <0.001 | 6.7 [1.5 −30.7] | *0.014 |
| 25–34 | 4.75 [1.7–13.2] | 0.003 | 4.5 [1.2–16.4] | *0.023 |
| 35–44 | 1.77 [0.52–6] | 0.36 | 1.44 [0.38 −5.4] | 0.59 |
| ≥45 | 1 | 1 | ||
| Educational level | 0.054 | 0.623 | ||
| Illiterate | 0.35 [0.1–1.18] | 0.089 | 0.87 [0.18–4.28] | 0.87 |
| Read and write | 0.94 [0.27–3.34] | 0.928 | 1.34 [0.3–5.97] | 0.7 |
| Primary/Secondary | 0.99 [00.3–3.26] | 0.986 | 0.68 [0.17–2.69] | 0.58 |
| Higher education | 1 | 1 | ||
| Marital status | 0.074 | 0.91 | ||
| Single | 1.85 [0.24–14.4] | 0.56 | 0.59 [0.06–6.44] | 0.67 |
| Married | 4.4 [0.56–34.6] | 0.16 | 0.83 [0.07–10.5] | 0.89 |
| Divorced | 0.99 [0.00—] | 0.99 | 0.000 [0.000—] | 0.99 |
| Widowed | 1 | 1 | ||
| Family size | 0.158 | 0.102 | ||
| 1 up to 3 | 3.37 [0.7–16.4] | 0.13 | 1.54 [0.27–8.87] | 0.63 |
| 4 up to 6 | 4.13 [0.96–17.77] | 0.057 | 3.5 [0.76–16.17] | 0.11 |
| More than 6 | 1 | 1 | ||
| Monthly income | 0.140 | 0.42 | ||
| <500 | 0.362 [0.104–1.258] | 0.110 | 0.38 [0.07–2.06] | 0.26 |
| 500–1000 | 0.857 [0.375–1.962] | 0.715 | 0.9 [0.32–2.54] | 0.83 |
| 1001–1500 | 0.373 [0.14–1.02] | 0.055 | 0.5 [0.17–1.46] | 0.2 |
| >1500 | 1 | 1 |
Note: Only significant values (P-value) are indicated by an asterisk indicator (*).
Loss of appetite, weight loss, chest pain, duration of coming to the health facility after developing signs and symptoms had an association in univariate analysis. But in multivariate analysis, only weight loss [AOR = 6.3; CI 1.06–37.3] and chest pain [AOR = 5.58 CI 1.58–19.7] were significantly associated with PTB (Table 5).
Table 5.
Association of PTB with Clinical Characteristics Among Study Participants at Ataye District Hospital from October 1, 2018, to February 30, 2019 (N=423)
| Variables | COR [95% CI] | P-value | AOR [95% CI] | P-value |
|---|---|---|---|---|
| Chronic Cough | ||||
| Yes | 0.431 [0.090–2.071] | 0.293 | 0.58 [0.1–3.3] | 0.54 |
| No | 1 | 1 | ||
| Loss of Appetites | ||||
| Yes | 5.28 [1.59–17.52] | 0.006 | 1.15 [0.19–7.05] | 0.88 |
| No | 1 | 1 | ||
| Weight Loss | ||||
| Yes | 6.74 [2.04–22.32] | 0.002 | 6.3 [1.06–37.3] | *0.043 |
| No | 1 | 1 | ||
| Chest Pain | ||||
| Yes | 5.16 [1.56–17.10] | 0.007 | 5.58 [1.58–19.7] | *0.008 |
| No | 1 | 1 | ||
| Fever | ||||
| Yes | 1.990 [0.92–4.32] | 0.082 | 1.35 [0.58–3.17] | 0.49 |
| No | 1 | 1 | ||
| Duration of coming at H/F | 0.001 | 0.006 | ||
| After Developing S/S? | ||||
| Before 2 wks | 1 | 1 | ||
| 2–5 wks | 2.740 [0.9–8.1] | 0.067 | 1.74 [0.56–5.4] | 0.34 |
| >5 wks | 0.34 [0.08–1.58] | 0.169 | 0.25 [0.05–1.19] | 0.08 |
Note: Only significant values (P-value) are indicated by an asterisk indicator (*).
In the univariate analysis of predisposing factors, there are four variables that had an association with PTB but in multivariate analysis three were significantly associated. The patients that had a contact history with confirmed PTB cases, who utilize congested transportation, and who had a history of imprisonment were 5.5 times [AOR = 5.57; CI 2.69–11.56], 7.7 times [AOR = 7.73; CI 1.004–59.6] and 2.26 times [AOR = 2.26; CI 1.07–4.8], respectively, more at risk of acquiring the disease in comparison with those without (Table 6).
Table 6.
Association of Predisposing Factors with PTB Among Study Participants at Ataye District Hospital from October 1, 2018, to February 30, 2019 (N=423)
| Variables | COR [95% CI] | P-value | AOR [95% CI] | P-value |
|---|---|---|---|---|
| Contact history with TB patient | <0.001 | *<0.001 | ||
| Yes | 5.31 [2.60–10.61] | 5.57 [2.69–11.56] | ||
| No | 1 | 1 | ||
| Congested Transportation | ||||
| Yes | 8.222 [1.11–60.94] | 0.039 | 7.73 [1.004–59.6] | *0.05 |
| No | 1 | 1 | ||
| Imprisonment History | ||||
| Yes | 2.45 [1.22–4.91] | 0.012 | 2.26 [1.07–4.8] | *0.034 |
| No | 1 | 1 | ||
| Previous PTB History | ||||
| Yes | 2.95 [1.120–7.773] | 0.029 | 2.3 [0.81–6.62] | 0.12 |
| No | 1 | 1 | ||
| HIV sero status | 0.285 | 0.2 | ||
| Sero-reactive | 1 | 1 | ||
| Sero-non-reactive | 0.343 [0.091–1.297] | 0.115 | 0.28 [0.07–1.15] | 0.076 |
| Unknown | 0.341 [0.07–1.753] | 0.198 | 0.35 [0.06–2.08] | 0.25 |
Note: Only significant values (P-value) are indicated by an asterisk indicator (*).
Discussion
This research work was conducted to address the prevalence of PTB at Ataye hospital by using the geneXpert diagnostic instrument and it is also conducted to provide good information concerning the associated risk factors of the problem. After completing the study we have obtained a result that indicates PTB is still public health concern and we have outlined some important clinical and associated factors of PTB for the community.
The result indicated the overall prevalence of PTB among suspected cases was 8.98%, which was in agreement with studies conducted in Ethiopia.11,14 In comparison with the present study, a higher rate of prevalence of TB was reported in a study conducted in Nigeria (22.9%),25 in Addis Ababa (15.11%),8 Gambella regional state, southwest Ethiopia (20%),16 northwest Ethiopia (24.6%)10 and Debre Markos (23.2%).7 The discrepancy might be due to variation in the nature of study populations, study setting, and study design. In contrast, there were other studies that had lower reports of the prevalence of PTB among suspected cases: 2.6%,26 4.6%,27 5.3%,12 6.5%,28 and 7.2%.15 This lower prevalence in comparison with the current study might be due to variation in study setting and study population.
In the current study, the rate of TB/HIV co-infection was 3/38 (7.89%), which is almost in line with studies conducted in Ethiopia.11,15 It was also comparable with a WHO report that indicates 8% of new HIV patients in 2018 were diagnosed with TB.1 In contrast, a higher TB/HIV co-infection result was reported from several studies conducted in high HIV setting areas in Ethiopia (35.5%,16 23.8%,10 and 16.6%7), in a systematic review report (22%),29 and in a prison (27%).12 A still higher rate of TB/HIV co-infection was reported from MDR-TB suspected cases in the country (56.6%).30 With the high rates of TB/HIV co-infection revealed by these studies there was also a higher prevalence of PTB cases; this, in turn, showed a significant role of HIV in the transmission of PTB.
Rifampicin resistant PTB isolates found by geneXpert were strong predictors of MDR-TB.28 Even though the numbers of RR-TB (2/38; 5.3%) in the present study were less than in similar studies in Ethiopia8,10 and in Nigeria,25 this can still imply a serious public health problem in the study area. The report of RR-TB in a study conducted in Gambella, Ethiopia16 was comparable with the current study, and no RR-TB was also indicated in a study conducted in northeast Ethiopia.26 This indicates a limited circulation of the MDR-TB into the community.
In the present study, those individuals who had a close contact history with confirmed PTB cases [either MDR or DS-TB], which accounts for 4.49%, were 5.57 times more likely to develop the diseases in comparison with those who did not. In this regard, a study conducted in Addis Ababa reported a higher prevalence (8.45%).28 Similar studies conducted in Ethiopia also indicated that a history of contact with TB patients had a strong association.12,31 Another study in Ethiopia indicated the prevalence of TB among presumptive PTB suspected who had contacts with MDR-TB patients was 45% and among presumptive PTB suspected who had contacts with DS-TB patients was 34%.32 Having a contact history with MDR-TB infected cases was an important predictor (8/9) to develop RR-TB. But out of 14 study subjects who had a history of contact with DS-TB only 1 had RR-TB result.32 Similarly, in the current study, only one study subject developed RR-TB.
Several studies in Ethiopia7,8,15 and elsewhere33,34 indicated that a history of previous TB treatment had strong association with the development of PTB and MDR-TB; whereas in the present study it was not associated with PTB. On the other hand, like the current study, there were reports that showed sex, residence, education,14 previous history of TB, and HIV serostatus10 were insignificant variables. In contrast to the present study, some research conducted in Debre-Markos Referral Hospital indicated sex, residence, and HIV serostatus were significantly associated with PTB.7
In this research work, the participant’s age had a significant association with the occurrence of PTB. Participants aged between 18 and 24 years had about 6.7 times and those aged between 25 and 34 years had 4.5 times the likelihood of developing PTB, in reference to the age category ≥45 years. This indicated the young and adult working population was at risk of being affected by TB and it is suggested that TB is circulating in the community. In considering the proportion of PTB patients from the total study participants in each age category, those participants whose age was ≥45 years were highly affected (11/48; 22.92%) followed by age category between 18 and 24 years (12/79; 15.19%). This indicated the young and the old population group were highly affected by PTB.
Utilization of congested transportation and having an imprisonment history had a significant association with PTB. According to one study conducted in Ethiopia, there were around 4086 prisoners who were affected by TB and the pooled prevalence was 8.33%.35 This population group may be a source of infection, if they use the congested transportation system and live or work in a high density population group.
Production of sputum, cough more than two weeks, loss of appetite, weight loss, and development of chest pain are among the clinical signs and symptoms from patients who are presumptive PTB.28,36 In the present study, loss of appetite, weight loss, and chest pain were significant clinical features of patients in the univariate analysis, but weight loss and chest pain remain as having significant association in the multivariate model. Weight loss among PTB patients has an association with treatment failure.37 In a study conducted in Addis Ababa, weight loss, loss of appetite and shortness of breath were significant clinical features in the univariate analysis, but unlike the present study, none of them were significantly associated in multivariate models.28
Limitations of the Study
This study was conducted with a few limitations. First, it is conducted in a single institution. It would be better if we incorporated a number of health institutions for a better representation of study subjects. Second, the study lacks any other diagnostic tool that used as comparators such as culture (LJ or MGIT), sputum smear, and chest X-ray results. The GeneXpert test result is the sole diagnostic tool for TB and RIF resistance for eligible cases. Even though the current study has these limitations it provides baseline information on the implementation of Xpert for detection of MTB and RIF resistance and assessment of different risk factors among presumptive PTB cases in the study area.
Conclusions
The overall prevalence of PTB among presumptive cases was 38 (9%) and from these PTB confirmed cases two (5.3%) were resistant to rifampicin; three (7.89%) patients were co-infected with HIV. Age, weight loss, chest pain, contact history with confirmed PTB cases, utilization of congested transportation, and having a history of imprisonment were variables that had an association with PTB. Therefore, the results of the study indicate PTB is still a public health problem in Ethiopia and there is a need for collaborative and intensified prevention and control activities of TB in the study area. Clinicians and physicians should give attention to the clinical signs and symptoms while they are detecting PTB and treating their patients.
Acknowledgments
Our appreciation goes to Wollo University and Ataye District Hospital for their technical support during the whole research activity and to all study participants and the staff members of Ataye District Hospital laboratory personnel, where this study was conducted.
Funding Statement
This study was conducted with the researcher’s funding.
Abbreviations
HIV, human immuno-deficiency virus; MDR-TB, multi-drug resistant tuberculosis; PTB, pulmonary tuberculosis; RR, rifampicin resistance; TB, tuberculosis; WHO, World Health Organization.
Availability of Data and Material
There are no material outputs from this study, and all data are those presented in the manuscript.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the ethical guidelines of the concerned institutions and after obtaining consent to participate from each study subject. A parent or legal guardian provided written informed consent for any participant under the age of 18 years.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.World Health Organization. Global tuberculosis report: 2019.
- 2.Chheng P, Tamhane A, Natpratan C, et al. Pulmonary tuberculosis among patients visiting a voluntary confidential counseling and testing center, Cambodia. Int J Tuberculosis Lung Dis. 2008;12(3):S54–S62. [PubMed] [Google Scholar]
- 3.World Health Organization. Global World Tuberculosis Report 2017. Geneva, Switzerland: World Health Organization; 2017. [Google Scholar]
- 4.Diarra B, Goita D, Tounkara S, et al. Tuberculosis drug resistance in Bamako, Mali, from 2006 to 2014. BioMed Central Infect Dis. 2016;16(1):714. doi: 10.1186/s12879-016-2060-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alebachew Z, Kebede A, Tsegaye F. First Ethiopian National Population Based Tuberculosis Prevalence Survey; July 2011. [Google Scholar]
- 6.Keflie TS, Samuel A, Woldegiorgis AZ, Mihret A, Abebe M, Biesalski HK. Vitamin A and zinc deficiencies among tuberculosis patients in Ethiopia. J Clin Tuberculosis Other Mycobacterial Dis. 2018;12:27–33. doi: 10.1016/j.jctube.2018.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mulu W, Abera B, Yimer M, Hailu T, Ayele H, Abate D. Rifampicin-resistance pattern of Mycobacterium tuberculosis and associated factors among presumptive tuberculosis patients referred to debre markos referral hospital, Ethiopia: a cross-sectional study. BioMed Central Res Notes. 2017;10(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arega B, Menbere F, Getachew Y. Prevalence of rifampicin resistant Mycobacterium tuberculosis among presumptive tuberculosis patients in selected governmental hospitals in Addis Ababa, Ethiopia. BioMed Central Infect Dis. 2019;19(1):307. doi: 10.1186/s12879-019-3943-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belay M, Bjune G, Abebe F. Prevalence of tuberculosis, HIV, and TB-HIV co-infection among pulmonary tuberculosis suspects in a predominantly pastoralist area, northeast Ethiopia. Glob Health Action. 2015;8(1):27949. doi: 10.3402/gha.v8.27949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaleta KN, Gizachew M, Gelaw B, Tesfa H, Getaneh A, Biadgo B. Rifampicin-resistant Mycobacterium tuberculosis among tuberculosis-presumptive cases at university of gondar hospital, northwest Ethiopia. Infect Drug Resist. 2017;10:185. doi: 10.2147/IDR.S135935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nugussie DA, Mohammed GA, Tefera AT. Prevalence of smear-positive tuberculosis among patients who visited saint paul’s specialized hospital in Addis Ababa, Ethiopia. Biomed Res Int. 2017;2017:1–5. doi: 10.1155/2017/6325484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gebrecherkos T, Gelaw B, Tessema B. Smear positive pulmonary tuberculosis and HIV co-infection in prison settings of North Gondar Zone, Northwest Ethiopia. BioMed Central Public Health. 2016;16(1):1091. doi: 10.1186/s12889-016-3761-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gizachew Beza M, Hunegnaw E, Tiruneh M. Prevalence and associated factors of tuberculosis in prisons settings of East Gojjam Zone, Northwest Ethiopia. Int j Bacteriol. 2017;2017:1–7. doi: 10.1155/2017/3826980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tulu B, Dida N, Kassa Y, Taye B. Smear positive pulmonary tuberculosis and its risk factors among tuberculosis suspect in South East Ethiopia; a hospital based cross-sectional study. BioMed Central Res Notes. 2014;7(1):285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mama M, Manilal A, Tesfa H, Mohammed H, Erbo E. Prevalence of pulmonary tuberculosis and associated factors among HIV positive patients attending antiretroviral therapy clinic at Arba Minch general hospital, southern Ethiopia. Open Microbiol J. 2018;12(1):163. doi: 10.2174/1874285801812010163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ejeta E, Beyene G, Bonsa Z, Abebe G. Xpert MTB/RIF assay for the diagnosis of Mycobacterium tuberculosis and Rifampicin resistance in high human immunodeficiency virus setting in gambella regional state, southwest ethiopia. J Clin Tuberculosis Other Mycobacterial Dis. 2018;12:14–20. doi: 10.1016/j.jctube.2018.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wondimeneh Y, Muluye D, Belyhun Y. Prevalence of pulmonary tuberculosis and immunological profile of HIV co-infected patients in Northwest Ethiopia. BioMed Central Res Notes. 2012;5(1):331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Getahun H, Gunneberg C, Granich R, Nunn P. HIV infection—associated tuberculosis: the epidemiology and the response. Clin Infect Dis. 2010;50(Supplement_3):S201–S7. doi: 10.1086/651492 [DOI] [PubMed] [Google Scholar]
- 19.Parrish NM, Carroll KC. Role of the clinical mycobacteriology laboratory in diagnosis and management of tuberculosis in low-prevalence settings. J Clin Microbiol. 2011;49(3):772–776. doi: 10.1128/JCM.02451-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taddese B, Desalegn D, Misganaw A, Kitila K, Balcha H. Comparison of GeneXpert against light-emitting diode fluorescent microscopy for the diagnosis of pulmonary tuberculosis in addis Ababa, Ethiopia. J Microbiol Biochem Technol. 2018;10(1):12–15. doi: 10.4172/1948-5948.1000388 [DOI] [Google Scholar]
- 21.Khan AS, Ali S, Khan MT, et al. Comparison of GeneXpert MTB/RIF assay and LED-FM microscopy for the diagnosis of extra pulmonary tuberculosis in Khyber Pakhtunkhwa, Pakistan. Braz j Microbiol. 2018;49(4):909–913. doi: 10.1016/j.bjm.2018.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Habte D, Melese M, Hiruy N, et al. The additional yield of GeneXpert MTB/RIF test in the diagnosis of pulmonary tuberculosis among household contacts of smear positive TB cases. Int J Infect Dis. 2016;49:179–184. doi: 10.1016/j.ijid.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 23.Mechal Y, Benaissa E, Benlahlou Y, et al. Evaluation of GeneXpert MTB/RIF system performances in the diagnosis of extrapulmonary tuberculosis. BioMed Central Infect Dis. 2019;19(1):1–8. doi: 10.1186/s12879-018-3567-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. Xpert MTB/RIF implementation manual: technical and operational ‘how-to’; practical considerations. World Health Organization; 2014: 9241506709. [PubMed] [Google Scholar]
- 25.Ikuabe PO, Ebuenyi ID. Prevalence of rifampicin resistance by automated Genexpert rifampicin assay in patients with pulmonary tuberculosis in Yenagoa, Nigeria. Pan Afr Med J. 2018;29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Semunigus T, Tessema B, Eshetie S, Moges F. Smear positive pulmonary tuberculosis and associated factors among homeless individuals in dessie and debre birhan towns, Northeast Ethiopia. Ann Clin Microbiol Antimicrob. 2016;15(1):50. doi: 10.1186/s12941-016-0165-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eliso E, Medhin G, Belay M. Prevalence of smear positive pulmonary tuberculosis among outpatients presenting with cough of any duration in Shashogo Woreda, Southern Ethiopia. BioMed Central Public Health. 2015;15(1):112. doi: 10.1186/s12889-015-1411-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinshaw W, Kebede A, Bitew A, et al. Prevalence of tuberculosis, multidrug resistant tuberculosis and associated risk factors among smear negative presumptive pulmonary tuberculosis patients in Addis Ababa, Ethiopia. BioMed Central Infect Dis. 2019;19(1):641. doi: 10.1186/s12879-019-4241-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teweldemedhin M, Asres N, Gebreyesus H, Asgedom SW. Tuberculosis-Human Immunodeficiency Virus (HIV) co-infection in Ethiopia: a systematic review and meta-analysis. BioMed Central Infect Dis. 2018;18(1):676. doi: 10.1186/s12879-018-3604-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mesfin EA, Beyene D, Tesfaye A, et al. Drug-resistance patterns of Mycobacterium tuberculosis strains and associated risk factors among multi drug-resistant tuberculosis suspected patients from Ethiopia. PLoS One. 2018;13(6):e0197737. doi: 10.1371/journal.pone.0197737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berju A, Haile B, Nigatu S, Mengistu A, Birhan G. Smear-positive tuberculosis prevalence and associated factors among pregnant women attending antenatal care in north gondar zone hospitals, Ethiopia. Int J Microbiol. 2019;2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hiruy N, Melese M, Habte D, et al. Comparison of the yield of tuberculosis among contacts of multidrug-resistant and drug-sensitive tuberculosis patients in Ethiopia using GeneXpert as a primary diagnostic test. Int J Infect Dis. 2018;71:4–8. doi: 10.1016/j.ijid.2018.03.011 [DOI] [PubMed] [Google Scholar]
- 33.Elduma AH, Mansournia MA, Foroushani AR, et al. Assessment of the risk factors associated with multidrug-resistant tuberculosis in Sudan: a case-control study. Epidemiol Health. 2019;41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diandé S, Badoum G, Combary A, et al. Multidrug-resistant tuberculosis in Burkina Faso from 2006 to 2017: results of national surveys. Eur J Microbiol Immunol (Bp). 2019;9(1):23–28. doi: 10.1556/1886.2018.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melese A, Demelash H. The prevalence of tuberculosis among prisoners in Ethiopia: a systematic review and meta-analysis of published studies. Arch Public Health. 2017;75(1):37. doi: 10.1186/s13690-017-0204-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hargreaves N, Kadzakumanja O, Phiri S, et al. What causes smear-negative pulmonary tuberculosis in Malawi, an area of high HIV seroprevalence? Int j Tuberculosis Lung Dis. 2001;5(2):113–122. [PubMed] [Google Scholar]
- 37.Diallo A, Dahourou DL, Tassembedo S, Sawadogo R, Meda N, Meda N. Factors associated with tuberculosis treatment failure in the Central East Health region of Burkina Faso. Pan Afr Med J. 2018;30(1). doi: 10.11604/pamj.2018.30.293.15074 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There are no material outputs from this study, and all data are those presented in the manuscript.


