Abstract
Background
Among the population without cardiovascular diseases (CVD), it is unclear whether pre-diabetes and/or prehypertension elevated the risk of all-cause and cardiovascular mortality.
Methods
All participants without CVD at baseline were recruited from the 1999–2014 National Health and Nutrition Examination Survey (NHANES), with survival status being updated until 31 December 2015. Cox proportional hazards models and subgroup analyses were performed to estimate hazard ratios (HRs) and 95% confidence interval (CI).
Results
There were 23,622 participants (11,233 [47.6%] male) with mean age of 37.2 years. Compared to participants without prehypertension or pre-diabetes, the HRs for all-cause mortality among participants with prehypertension alone, pre-diabetes alone, and combined pre-diabetes and prehypertension were 1.04 (95% CI: 0.88, 1.24), 0.96 (95% CI:0.76, 1.21), and 1.19 (95% CI:0.98, 1.46), respectively. The corresponding HRs for cardiovascular mortality were 1.51 (95% CI: 0.83, 2.77), 1.40 (95% CI: 0.64, 3.06), and 1.70 (95% CI: 0.88, 3.27), respectively. A subgroup analysis showed that participants with combined pre-diabetes and prehypertension had a higher risk of all-cause mortality among younger participants, higher BMI, white population, and people with elevated non-HDLC. Moreover, the association between combined pre-diabetes and prehypertension and cardiovascular death was only significant among people with elevated non-HDLC.
Conclusion
Pre-diabetes combined with prehypertension might elevate the risk of all-cause mortality among subjects, particularly for those with elevated body weight, high non-HDLC, younger participants or white population.
Keywords: pre-diabetes, prehypertension, cardiovascular disease, CVD, all-cause mortality, cardiovascular mortality
Introduction
Prehypertension was an intermediate status between normal blood pressure and hypertension,1 while pre-diabetes is defined as an metabolic status between normal blood glucose and diabetes mellitus (DM).2 The population with prehypertension/diabetes was at a high risk of developing hypertension and DM,3,4 which were the major risk factors for cardiovascular disease (CVD) or all-cause mortality.5,6 CVD was the common chronic disease worldwide, with high morbidity and mortality.7 In 2016, European Guidelines on CVD prevention in clinical practice suggested to screen for people at relatively low risk of CVD was not effective in reducing the risk of cardiovascular events.8 In 2019, European Society of Cardiology (ESC) Guidelines on diabetes, pre-diabetes, and CVD collaborated with the European Association for the Study of Diabetes and recommended to screen CVD patients for potential type 2 DM.9 At the moment, the common tools for cardiovascular risk assessment included the high-risk Systemic Coronary Risk Estimation (SCORE) and Framingham, which were available to estimate risk of the fatal and non-fatal CVD risks among apparently healthy individuals. In these assessment tools, levels of blood glucose and blood pressure were the important components.10,11 However, it is unclear whether the screening of prehypertension/diabetes among a wider population. Studies on the association between pre-diabetes and/or prehypertension with CVD-related and all-cause mortality have not yielded consistent findings.12–17 To answer this research question, we analyzed data from participants in National Health and Nutrition Examination Survey (NHANES) without CVD at baseline, so as to investigate the impacts of prehypertension and/or pre-diabetes on subsequent occurrence of cardiovascular and all-cause mortality.
Methods
Study Population
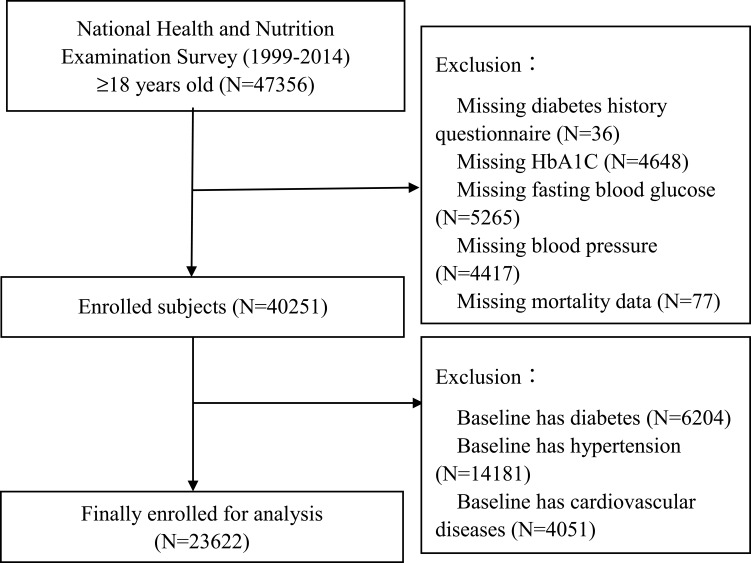
All participants were enrolled from the 1999–2014 NHANES. NHANES was an ongoing cross-sectional study with a series of stratified, multistage probability surveys designed to be representative of the US civilian, non-institutionalized population, which was conducted by the Centers for Disease Control and Prevention.18,19 We included participants ≥ 18-years-old without a history of CVD at baseline. However, participants aged < 18 years, with missing data on diabetes history, hemoglobin A1C (HbA1C), fasting blood glucose (FBG), blood pressure, survival status, and subject with CVD, DM and/or hypertension at baseline were excluded. After applying these exclusion criteria, 23,622 participants were included for data analysis (Figure 1). The survey protocol was approved by the Institutional Review Board of the Centers for Disease Control and Prevention. All participants have provided written informed consent.
Figure 1.
Research flowchart.
Baseline Assessment
The questionnaires and examinations in NHANES were performed on a standardized manner. Covariates being assessed were socio-demographic, smoking status, education level, dietary energy, medical history (such as hypertension, diabetes, CVD and cancer), medication history ( the use of antihypertensive drugs, hypoglycemic agents, antiplatelet drugs and lipid-lowering drugs), systolic blood pressure (SBP), diastolic blood pressure (DBP), FBG, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C) and HbA1C. Body mass index (BMI) was defined as mass (kg) divided by the square of height (m2). Estimated glomerular filtration rate (eGFR) was calculated using Modification of Diet in Renal Disease formula.20 Non-HDLC was defined as TC minus HDL-C, low HDL-C was defined as HDL-C < 40 mg/dl (men) or < 50 mg/dl (women).21
Blood Pressure Measurement
Blood pressure measurement was measured by a trained physician using a mercury sphygmomanometer (W. A. Baum Co. Inc (1050), Copiague, New York, USA) and an appropriately sized cuff. Three consecutive blood pressure readings were obtained from the same arm. SBP and DBP were defined as the average value of three blood pressure measurements. Hypertension was defined as having a history of hypertension, a SBP ≥140 mmHg, a DPB ≥ 90 mmHg, and/or using antihypertensive medications.22
Definitions of Pre-Diabetes and Prehypertension
According to American Diabetes Association, DM was defined as a self-reported diagnosis that was determined previously by a specialist, being treated with hypoglycemic medications currently, FBG level ≥7.0 mmol/l (126 mg/dl), or HbA1c level ≥ 6.5%.2 Participants were defined as pre-diabetes when their HbA1c level ranged between 5.7% and 6.4% or FBG level ranged between 100 mg/dl and 125 mg/dl2. Participants were classified as having prehypertension if their SBP/DBP ranged from 120 to 139/80 to 89 mm Hg.1
Outcomes
All-cause and cardiovascular mortality were the outcomes of this study. Mortality data were obtained from the NHANES public-use linked mortality files, which captured the vital status and cause of death of survey participants from survey participation to 31 December 2015. Cardiovascular mortality was defined by the International Classification of Diseases, 10th Edition, Clinical Modification System codes (I00-I09, I11, I13, I20-I51) derived from death-certificate data.
Statistical Analysis
All participants were classified into the following four groups: 1) without pre-diabetes nor prehypertension; 2) pre-diabetes alone; 3) prehypertension alone; and 4) combined pre-diabetes and prehypertension. Continuous variables are presented as mean ± standard deviation and categorical variables as percentage where appropriate. Subgroup differences were tested by one-way ANOVA, Student’s t-test, chi-square or fisher exact tests. Survival analysis was performed using standardized Kaplan-Meier curves and Log rank test. Cox proportional hazards regression models were used to estimate hazard ratios (HRs) and 95% confidence interval (CI) for all-cause and cardiovascular mortality. The Model 1 only included the status of prehypertension or pre-diabetes, Model 2 was additionally adjusted for age and gender. The Model 3 was additionally adjusted for smoking status, education, race, BMI, and cancer status at baseline, as well as TC, HDL-C, eGFR, dietary energy, using of statin and antiplatelet drugs. We also performed subgroup analysis according to age (≤ 54, 55–64 or ≥ 65 years), gender (male or female), race (white or non-white), BMI (< 25 or ≥ 25 kg/m2), smoking status (yes or no), non-HDLC (≥ 130 or < 130 mg/dl) and low HDL-C (yes or no), and analyzed their interactions between pre-diabetes and prehypertension status with all-cause and cardiovascular mortality. All statistical analyses were performed using R version 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria), and P<0.05 was considered as statistically significant.
Results
Patient Characteristics
Baseline characteristics were summarized in Table 1. The current study included 23,622 (11,233 [47.6%] male) participants with an average age of 37.2 ± 15.5years. The number of participants without prehypertension nor pre-diabetes, prehypertension alone, pre-diabetes alone, combined prehypertension and pre-diabetes were 12,272 (51.95%), 5,841 (24.73%), 2,757 (11.67%) and 2,752 (11.65%), respectively. There were significant subgroup differences in age, gender, race, smoking status, education level, BMI, FBG, TC, HDL-C, SBP, DBP, eGFR, dietary energy, baseline cancer history, the use of statin and antiplatelet drugs (all P < 0.05).
Table 1.
Baseline Characteristics of the Participants
| Overall | No Pre-Diabetes | Pre-Diabetes | P-value | |||
|---|---|---|---|---|---|---|
| No PreHTN | PreHTN | No PreHTN | PreHTN | |||
| (n=23,622) | (n=12,272) | (n=5841) | (n=2757) | (n=2752) | ||
| Age, y | 37.2 ± 15.5 | 32.2 ± 12.6 | 39.7 ± 16.2 | 42.2 ± 15.5 | 49.4 ± 16.2 | <0.001 |
| Gender, % | <0.001 | |||||
| Male | 11,233 (47.6%) | 4562 (37.2%) | 3618 (61.9%) | 1327 (48.1%) | 1726 (62.7%) | |
| Female | 12,389 (52.4%) | 7710 (62.8%) | 2223 (38.1%) | 1430 (51.9%) | 1026 (37.3%) | |
| Ethnicity, % | <0.001 | |||||
| Non-white | 13,235 (56.0%) | 6866 (55.9%) | 3005 (51.4%) | 1745 (63.3%) | 1619 (58.8%) | |
| White | 10,387 (44.0%) | 5406 (44.1%) | 2836 (48.6%) | 1012 (36.7%) | 1133 (41.2%) | |
| Smoking, % | 8998 (38.1%) | 4104 (33.4%) | 2436 (41.7%) | 1167 (42.3%) | 1291 (46.9%) | <0.001 |
| Less than high school education, % | 4950 (21.0%) | 2205 (18.0%) | 1210 (20.7%) | 724 (26.3%) | 811 (29.5%) | <0.001 |
| BMI, kg/m2 | 27.3 ± 6.04 | 26.1 ± 5.49 | 28.0 ± 6.16 | 28.7 ± 6.26 | 29.8 ± 6.58 | <0.001 |
| SBP, mmHg | 115 ± 11.0 | 108 ± 7.34 | 125 ± 6.56 | 110 ± 6.90 | 127 ± 6.46 | <0.001 |
| DBP, mmHg | 67.9 ± 10.8 | 64.5 ± 9.36 | 73.4 ± 11.3 | 66.0 ± 8.91 | 73.4 ± 10.8 | <0.001 |
| TC, mg/dl | 193 ± 41.4 | 187 ± 40.5 | 197 ± 41.6 | 198 ± 41.0 | 206 ± 40.0 | <0.001 |
| HDL-C, mg/dl | 53.4 ± 15.5 | 55.1 ± 15.3 | 52.9 ± 16.0 | 51.0 ± 15.0 | 49.7 ± 14.7 | <0.001 |
| Non-HDL-C, mg/dl | 140 ± 41.6 | 132 ± 39.7 | 144 ± 42.2 | 147 ± 41.6 | 157 ± 40.9 | <0.001 |
| Energy intake, kcal | 2290 ± 1080 | 2260 ± 1060 | 2380 ± 1160 | 2200 ± 1050 | 2250 ± 999 | <0.001 |
| eGFR, mL/min/1.73m2 | 98.6 ± 29.7 | 104 ± 32.2 | 95.1 ± 27.0 | 93.8 ± 25.1 | 88.2 ± 21.9 | <0.001 |
| Baseline cancer, % | 980 (4.1%) | 355 (2.9%) | 274 (4.7%) | 163 (5.9%) | 188 (6.8%) | <0.001 |
| Statin use, % | 608 (2.6%) | 139 (1.1%) | 167 (2.9%) | 134 (4.9%) | 168 (6.1%) | <0.001 |
| Antiplatelet drugs, % | 16 (0.1%) | 2 (0.0%) | 6 (0.1%) | 3 (0.1%) | 5 (0.2%) | 0.008 |
| Low HDLC, % | 6840 (29.0%) | 3319 (27.0%) | 1560 (26.7%) | 995 (36.1%) | 966 (35.1%) | <0.001 |
| All-cause mortality, % | 1039 (4.4%) | 345 (2.8%) | 336 (5.8%) | 118 (4.3%) | 240 (8.7%) | <0.001 |
| Cardiovascular mortality, % | 98 (0.4%) | 19 (0.2%) | 39 (0.7%) | 12 (0.4%) | 28 (1.0%) | <0.001 |
Note: Data are mean ± SD or percentage.
Abbreviations: BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; HTN, hypertension.
Incidence of Cardiovascular and All-Cause Mortality
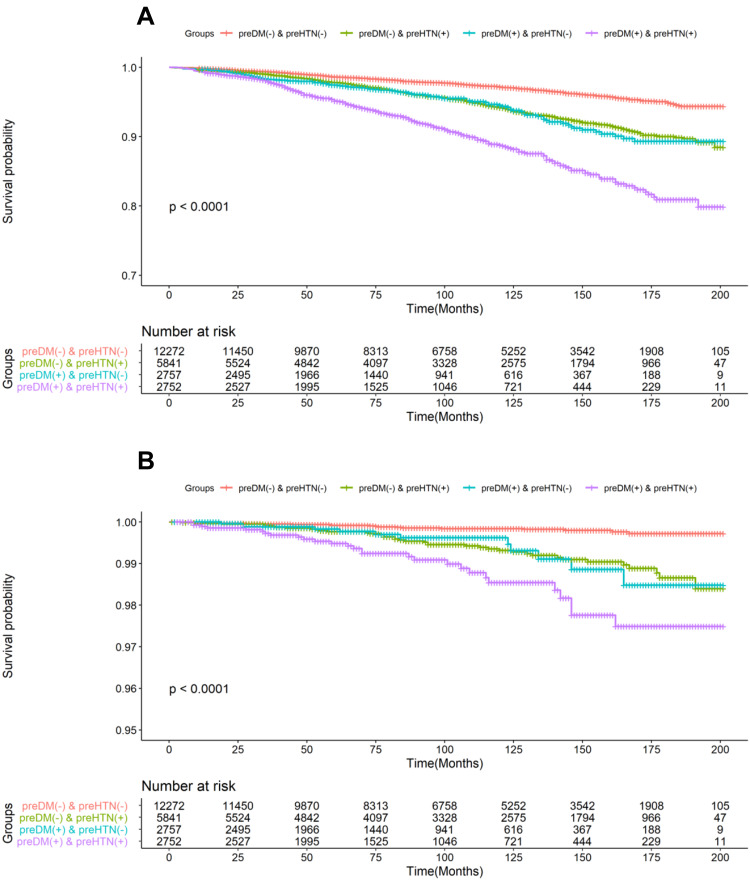
The incidence rate of all-cause and cardiovascular among prehypertension and/or pre-diabetes was shown in Table 1. There were 98 cases of (0.41%) cardiovascular and 1,039 cases of (4.40%) all-cause mortality occurred during the mean follow-up time of 8.41 years. The cumulative survival probability of all-cause and cardiovascular mortality among participants with prehypertension and/or pre-diabetes was demonstrated in Figure 2.
Figure 2.
Kaplan-Meier analysis for all-cause and cardiovascular mortality in different groups. (A), all-cause mortality; (B), cardiovascular mortality.
Abbreviations: DM, diabetes mellitus; HTN, hypertension.
Risk for Cardiovascular and All-Cause Mortality
As shown in Table 2, comparing to participants with no pre-diabetes nor prehypertension group, the Model 1 HRs for all-cause mortality for those with prehypertension alone, with pre-diabetes alone, and with combined pre-diabetes and prehypertension were 1.98 (95% CI:1.71, 2.30), 2.09 (95% CI:1.69, 2.57) and 3.97 (95% CI:3.36, 4.68). The Model 1 HRs for cardiovascular mortality were 4.17 (95% CI:2.41, 7.21), 3.95 (95% CI:1.91, 8.15) and 8.54 (95% CI:4.76, 15.32), respectively. In the fully adjusted model (Model 3), the HRs for all-cause mortality for those with prehypertension alone, with pre-diabetes alone, and combined pre-diabetes and prehypertension were 1.04 (95% CI: 0.88, 1.24), 0.96 (95% CI:0.76, 1.21), and 1.19 (95% CI:0.98, 1.46). Meanwhile, the Model 3 HRs for cardiovascular mortality were 1.51 (95% CI: 0.83, 2.77), 1.40 (95% CI:0.64, 3.06), and 1.70 (95% CI:0.88, 3.27), respectively for those with prehypertension alone, with pre-diabetes alone, and combined pre-diabetes and prehypertension.
Table 2.
Multivariate Cox Regression Analysis for Mortality in Different Models
| All-Cause Mortality | ||||
|---|---|---|---|---|
| HRs (95% CI) | ||||
| Case/total | Model1 | Model2 | Model3 | |
| N=23,622 | N=23,622 | N=19,674 | ||
| preDM (-) & preHTN (-) | 345/12,272 | 1.00(ref) | 1.00(ref) | 1.00(ref) |
| preDM (-) & preHTN (+) | 336/5841 | 1.98(1.71,2.3) | 0.96(0.82,1.13) | 1.04(0.88,1.24) |
| preDM(+) & preHTN (-) | 118/2757 | 2.09(1.69,2.57) | 0.92(0.74,1.14) | 0.96(0.76,1.21) |
| preDM(+) & preHTN(+) | 240/2752 | 3.97(3.36,4.68) | 1.06(0.89,1.27) | 1.19(0.98,1.46) |
| Cardiovascular mortality | ||||
| HRs (95% CI) | ||||
| Case/total | Model1 | Model2 | Model3 | |
| N=23,622 | N=23,622 | N=19,674 | ||
| preDM (-) & preHTN (-) | 19/12,272 | 1.00(ref) | 1.00(ref) | 1.00(ref) |
| preDM (-) & preHTN (+) | 39/5841 | 4.17(2.41,7.21) | 1.46(0.83,2.56) | 1.51(0.83,2.77) |
| preDM(+) & preHTN (-) | 12/2757 | 3.95(1.91,8.15) | 1.21(0.58,2.54) | 1.40(0.64,3.06) |
| preDM(+) & preHTN(+) | 28/2752 | 8.54(4.76,15.32) | 1.36(0.73,2.51) | 1.70(0.88,3.27) |
Notes: Data are HRs and 95% CI; model1, unadjusted; model2, adjusted for age and gender; model3, adjusted for age, gender, smoking, education, race, body mass index, cancer at baseline, total cholesterol, high-density-lipoprotein cholesterol, dietary energy intake, estimated glomerular filtration rate, the use of statin or antiplatelet drugs.
Abbreviations: DM, diabetes mellitus; HTN, hypertension; HR, hazard ratios; CI, confidence interval.
Subgroups Analysis
We performed subgroup according to age, gender, ethnicity, smoking status, BMI status, status of non-HDL-C status and low HDL-C status (Table 3). BMI and race interacted significantly with the association between pre-diabetes/prehypertension status and all-cause mortality (both P for interaction < 0.05), while only race interacted significantly pre-diabetes/prehypertension status to influence the association with cardiovascular mortality (P-interaction=0.029). Participants with combined pre-diabetes and prehypertension had a higher risk of all-cause mortality among those aged ≤ 54 (HR: 1.49 [95% CI: 1.09, 2.05]) compared to people aged 55–64 0.99 [95% CI: 0.59, 1.65]) or ≥ 65 1.16 [95% CI: 0.84, 1.60]) years. Similarly, the association was stronger among white population (HR: [1.47 (95% CI: 1.12, 1.94)]) than non-white population (0.98 [95% CI: 0.74, 1.32]), for people with elevated non-HDLC (HR: [1.30 (95% CI: 1.03, 1.64)]) than those with normal non-HDLC (0.90 [95% CI: 0.61, 1.31]). When using cardiovascular mortality as outcome, participants who combined pre-diabetes and prehypertension have higher risk of cardiovascular death among those with high non-HDLC (HR: [2.92 (95% CI: 1.26,6.77)]) comparing with people with normal non-HDLC (0.36 [95% CI: 0.09,1.50]).
Table 3.
Multivariate Cox Regression Analysis for Mortality in Different Models by Subgroups
| All-Cause Mortality | ||||||
|---|---|---|---|---|---|---|
| HRs (95% CI) | ||||||
| preDM (-)& preHTN (-) |
preDM (-)& preHTN (+) |
preDM(+)& preHTN (-) |
preDM(+)& preHTN(+) |
|||
| Subgroup | Cases/total | P-interaction | ||||
| Age, y | 0.128 | |||||
| ≥65 | 413/1543 | 1.00(ref) | 1.09(0.80,1.49) | 0.96(0.64,1.44) | 1.16(0.84,1.60) | |
| 55–64 | 142/1941 | 1.00(ref) | 1.11(0.7,1.75) | 1.09(0.61,1.94) | 0.99(0.59,1.65) | |
| ≤54 | 484/20,138 | 1.00(ref) | 1.02(0.8,1.31) | 1.01(0.71,1.43) | 1.49(1.09,2.05) | |
| Gender | 0.227 | |||||
| Male | 648/11,233 | 1.00(ref) | 1.02(0.82,1.27) | 0.97(0.72,1.31) | 1.13(0.89,1.45) | |
| Female | 391/12,389 | 1.00(ref) | 1.04(0.78,1.37) | 0.88(0.59,1.31) | 1.28(0.92,1.80) | |
| Race | <0.001 | |||||
| Non-white | 534/13,235 | 1.00(ref) | 1.11(0.87,1.40) | 0.79(0.56,1.12) | 0.98(0.74,1.32) | |
| White | 505/10,387 | 1.00(ref) | 1.05(0.82,1.35) | 1.16(0.83,1.61) | 1.47(1.12,1.94) | |
| BMI, kg/m2 | 0.034 | |||||
| ≥25 | 574/14,074 | 1.00(ref) | 1.14(0.90,1.44) | 1.08(0.80,1.47) | 1.38(1.07,1.78) | |
| <25 | 430/9337 | 1.00(ref) | 0.92(0.71,1.20) | 0.77(0.52,1.14) | 0.91(0.66,1.26) | |
| Smoking | 0.760 | |||||
| Yes | 593/8998 | 1.00(ref) | 1.12(0.90,1.39) | 1.13(0.84,1.51) | 1.22(0.95,1.57) | |
| No | 388/11,971 | 1.00(ref) | 0.95(0.72,1.25) | 0.72(0.48,1.08) | 1.21(0.88,1.67) | |
| Low HDL-C | 0.246 | |||||
| Yes | 298/6840 | 1.00(ref) | 1.02(0.74,1.41) | 0.90(0.59,1.37) | 1.12(0.79,1.59) | |
| No | 738/16,773 | 1.00(ref) | 1.06(0.86,1.29) | 0.97(0.73,1.29) | 1.22(0.96,1.55) | |
| Non-HDLC | 0.627 | |||||
| ≥130 | 664/13,236 | 1.00(ref) | 1.08(0.87,1.34) | 0.99(0.74,1.32) | 1.30(1.03,1.64) | |
| <130 | 372/10,377 | 1.00(ref) | 0.95(0.71,1.28) | 0.88(0.58,1.31) | 0.90(0.61,1.31) | |
| Cardiovascular mortality | ||||||
|---|---|---|---|---|---|---|
| HRs (95% CI) | ||||||
| preDM (-)& preHTN (-) |
preDM (-)& preHTN (+) |
preDM(+)& preHTN (-) |
preDM(+)& preHTN(+) |
|||
| Subgroup | Cases/total | P-interaction | ||||
| Age, y | 0.359 | |||||
| ≥65 | 58/1543 | 1.00(ref) | 0.95(0.4,2.26) | 1.03(0.35,3.00) | 1.52(0.66,3.51) | |
| 55–64 | 13/1941 | 1.00(ref) | 4.11(0.49,34.54) | 3.41(0.3,38.9) | 1.96(0.19,19.92) | |
| ≤54 | 27/20,138 | 1.00(ref) | 2.09(0.81,5.41) | 1.70(0.43,6.69) | 1.50(0.37,6.17) | |
| Gender | 0.320 | |||||
| Male | 65/11,233 | 1.00(ref) | 1.51(0.73,3.13) | 1.03(0.37,2.83) | 1.57(0.72,3.42) | |
| Female | 33/12,389 | 1.00(ref) | 1.37(0.46,4.14) | 2.30(0.66,8.03) | 2.04(0.61,6.91) | |
| Race | 0.029 | |||||
| Non-white | 42/13,235 | 1.00(ref) | 1.86(0.80,4.34) | 1.09(0.32,3.66) | 1.18(0.42,3.31) | |
| White | 56/10,387 | 1.00(ref) | 1.42(0.60,3.39) | 1.75(0.61,4.99) | 2.27(0.94,5.49) | |
| BMI, kg/m2 | 0.448 | |||||
| ≥25 | 53/14,074 | 1.00(ref) | 1.57(0.67,3.70) | 1.87(0.68,5.12) | 2.03(0.84,4.93) | |
| <25 | 40/9337 | 1.00(ref) | 1.36(0.58,3.23) | 0.78(0.20,2.98) | 1.11(0.40,3.09) | |
| Smoking | 0.780 | |||||
| Yes | 59/8998 | 1.00(ref) | 1.85(0.84,4.09) | 1.34(0.47,3.86) | 1.87(0.80,4.39) | |
| No | 39/11,971 | 1.00(ref) | 1.14(0.43,2.97) | 1.53(0.47,4.96) | 1.54(0.53,4.44) | |
| Low HDL-C | 0.976 | |||||
| Yes | 21/6840 | 1.00(ref) | 1.23(0.38,4.01) | / | 1.00(0.29,3.43) | |
| No | 77/16,773 | 1.00(ref) | 1.71(0.84,3.46) | 2.23(0.95,5.23) | 2.07(0.95,4.49) | |
| Non-HDLC | 0.250 | |||||
| ≥130 | 66/13,236 | 1.00(ref) | 1.79(0.78,4.15) | 2.43(0.92,6.42) | 2.92(1.26,6.77) | |
| <130 | 32/10,377 | 1.00(ref) | 1.18(0.48,2.90) | 0.45(0.09,2.22) | 0.36(0.09,1.50) | |
Notes: Data are HRs and 95% CI; Adjusted for age, gender, smoking, education, race, body mass index, cancer at baseline, total cholesterol, high-density-lipoprotein cholesterol, energy intake, estimated glomerular filtration rate, the use of statin, antiplatelet drugs.
Abbreviations: DM, diabetes mellitus; HTN, hypertension; HR, hazard ratios; CI, confidence interval; BMI, body mass index; HDL-C, high-density lipoprotein cholesterol.
Discussion
In the present study, we found that no obvious association of prehypertension alone or pre-diabetes on the subsequent occurrence of cardiovascular nor all-cause mortality among subjects without CVD at baseline. According to subgroup analysis, BMI and race interacted significantly with the association of combined pre-diabetes and prehypertension status with all-cause mortality, while only race interacted significantly with the association of cardiovascular mortality. The impact of combined pre-diabetes and prehypertension on all-cause mortality appeared to be stronger among participants aged ≤ 54 years, BMI ≥ 25 kg/m2, white population and subject with elevated non-HDLC.
When comparing with previous literatures, our study has demonstrated some inconsistent findings. The Women’s Health Initiative study showed that prehypertension was associated with an increased risk of cardiovascular death in postmenopausal women.23 The Framingham Heart Study also found that prehypertension associated with an increased risk of CVD.24 A recent meta-analysis of 47 cohort studies showed that prehypertension was associated with an increased risk of total CVD, and the effective control of prehypertension could prevent more than 10% CVD cases.25 Moreover, a meta-analysis demonstrated that prehypertension was associated with CVD mortality, but not with all-cause mortality.12 Meanwhile, our findings were similar to a previous study that pre-diabetes did not associate with the risk of cardiovascular and all-cause mortality.26 Zhang et al demonstrated that impaired glucose tolerance or impaired fasting glucose greatly increased the CVD risk among people with prehypertension.27 A meta-analysis of 53 prospective cohort studies revealed that pre-diabetes might lead to an increased risk of cardiovascular and all-cause mortality.15 Moreover, the Strong Heart Study found that comparing with people with normal levels of blood pressure and blood glucose, prehypertension and/or pre-diabetes significantly increased the risk of subsequent cardiovascular events.27 In addition, a meta-analysis revealed that among patients with clinical history of CVD but without hypertension, antihypertensive treatment was associated with a decreased risk of composite CVD events and all-cause mortality.28 The main reason for inconsistent findings might be attributed by the few number of mortality cases in our study population, which reduced the statistical power. In addition, the study population, adjusting confounding factors and the follow-up time may also have an impact on the results.
As revealed by subgroup analysis, the association between combined pre-diabetes and prehypertension status with all-cause mortality appeared to be stronger among participants who were younger, had elevated body weight, white population or people with high non-HDLC. Our results suggested screening and management of blood glucose/pressure may be necessary for the CVD-free population at a younger age, particularly for those with elevated BMI or dyslipidemia. According to American Diabetes Association, pre-diabetes screening should be performed among people with overweight or obese who had at least one cardiometabolic risk factor.2 However, European Society of Cardiology recommends that individuals with pre-diabetes but without CVD were not necessarily at elevated cardiovascular risk, the risk scoring for CVD should be calculated in the same way as the general population.29 Although prehypertension or pre-diabetes alone may not predict the risk for cardiovascular event, combined prehypertension and pre-diabetes may enhance the detrimental effect on cardiovascular health. In addition, without any intervention, many prehypertensive and pre-diabetes participants may develop hypertension and diabetes. Therefore, early screening of blood glucose and blood pressure in specific subjects including young and middle-aged, overweight/obesity, dyslipidemia population, may be beneficial.
Our study had several limitations that should be taken into consideration. First, some baseline variables were self-reported, which may introduce recall errors. Second, this study did not consider the confounding effects of physical activity, triglyceride, low-density lipoprotein cholesterol and some other variables. Third, FBG and HbA1C were measured only once at baseline, and we were not able to examine postprandial blood glucose nor performed oral glucose tolerance test which might underestimate the total number of pre-diabetes. In addition, we only measured blood pressure at baseline, which may also lead to inaccurate diagnosis of prehypertension. Despite these limitations, NHANES have a rigorous and standardized study protocol, and have an extensive quality control procedure in data collection, which are the advantages of this study.
In conclusion, combined prehypertension and pre-diabetes might elevate the risk of all-cause mortality in participants without CVD at baseline. It is worthwhile to explore the most suitable population for prehypertension/diabetes screening. However, for subjects without a history of CVD, more well-designed prospective cohort studies are needed in the future to clarify whether should be early screening or intervention for prehypertension or pre-diabetes.
Funding Statement
This work was supported by the Science and Technology Program of Guangzhou (No.201604020143, No.201604020018, No.201604020186 and No.201803040012), the National Key Research and Development Program of China (No.2017YFC1307603, No.2016YFC1301305) and the Key Area R&D Program of Guangdong Province (No.2019B020227005).
Disclosure
The authors declare that they have no conflicts of interest.
References
- 1.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2 [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes–2019. Diabetes Care. 2019;42:S13–S28. doi: 10.2337/dc19-S002 [DOI] [PubMed] [Google Scholar]
- 3.Egan BM, Stevens-Fabry S. Prehypertension–prevalence, health risks, and management strategies. Nat Rev Cardiol. 2015;12:289–300. doi: 10.1038/nrcardio.2015.17 [DOI] [PubMed] [Google Scholar]
- 4.Beulens J, Rutters F, Ryden L, et al. Risk and management of pre-diabetes. Eur J Prev Cardiol. 2019;26:47–54. doi: 10.1177/2047487319880041 [DOI] [PubMed] [Google Scholar]
- 5.Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957–967. doi: 10.1016/S0140-6736(15)01225-8 [DOI] [PubMed] [Google Scholar]
- 6.Wang H, Ba Y, Cai RC, et al. Association between diabetes mellitus and the risk for major cardiovascular outcomes and all-cause mortality in women compared with men: a meta-analysis of prospective cohort studies. BMJ Open. 2019;9:e24935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roth GA, Johnson C, Abajobir A, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70:1–25. doi: 10.1016/j.jacc.2017.04.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37:2315–2381. doi: 10.1093/eurheartj/ehw106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323. [DOI] [PubMed] [Google Scholar]
- 10.D’Agostino RS, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the framingham heart study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579 [DOI] [PubMed] [Google Scholar]
- 11.Conroy RM, Pyorala K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. doi: 10.1016/S0195-668X(03)00114-3 [DOI] [PubMed] [Google Scholar]
- 12.Huang Y, Su L, Cai X, et al. Association of all-cause and cardiovascular mortality with prehypertension: a meta-analysis. Am Heart J. 2014;167:160–168. doi: 10.1016/j.ahj.2013.10.023 [DOI] [PubMed] [Google Scholar]
- 13.Guo X, Zhang X, Zheng L, et al. Prehypertension is not associated with all-cause mortality: a systematic review and meta-analysis of prospective studies. PLoS One. 2013;8:e61796. doi: 10.1371/journal.pone.0061796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang S, Wu H, Zhang Q, et al. Impact of baseline prehypertension on cardiovascular events and all-cause mortality in the general population: a meta-analysis of prospective cohort studies. Int J Cardiol. 2013;168:4857–4860. doi: 10.1016/j.ijcard.2013.07.063 [DOI] [PubMed] [Google Scholar]
- 15.Huang Y, Cai X, Mai W, et al. Association between prediabetes and risk of cardiovascular disease and all-cause mortality: systematic review and meta-analysis. BMJ. 2016;355:i5953. doi: 10.1136/bmj.i5953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang Y, Cai X, Chen P, et al. Associations of prediabetes with all-cause and cardiovascular mortality: a meta-analysis. Ann Med. 2014;46:684–692. doi: 10.3109/07853890.2014.955051 [DOI] [PubMed] [Google Scholar]
- 17.Ford ES, Zhao G, Li C. Pre-diabetes and the risk for cardiovascular disease: a systematic review of the evidence. J Am Coll Cardiol. 2010;55:1310–1317. doi: 10.1016/j.jacc.2009.10.060 [DOI] [PubMed] [Google Scholar]
- 18.JA F. NHANES. Diabetes Educ. 2017;43:151. doi: 10.1177/0145721717698651 [DOI] [PubMed] [Google Scholar]
- 19.Kim C, Bullard KM, Herman WH, et al. Association between iron deficiency and A1C Levels among adults without diabetes in the National Health and Nutrition Examination Survey, 1999–2006. Diabetes Care. 2010;33:780–785. doi: 10.2337/dc09-0836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levey AS, Coresh J, Bolton K, et al. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 21.Jacobson TA, Ito MK, Maki KC, et al. National lipid association recommendations for patient-centered management of dyslipidemia: part 1–full report. J Clin Lipidol. 2015;9:129–169. doi: 10.1016/j.jacl.2015.02.003 [DOI] [PubMed] [Google Scholar]
- 22.Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 23.Hsia J, Margolis KL, Eaton CB, et al. Prehypertension and cardiovascular disease risk in the Women’s Health Initiative. Circulation. 2007;115:855–860. doi: 10.1161/CIRCULATIONAHA.106.656850 [DOI] [PubMed] [Google Scholar]
- 24.Qureshi AI, Suri MF, Kirmani JF, et al. Is prehypertension a risk factor for cardiovascular diseases? Stroke. 2005;36:1859–1863. doi: 10.1161/01.STR.0000177495.45580.f1 [DOI] [PubMed] [Google Scholar]
- 25.Han M, Li Q, Liu L, et al. Prehypertension and risk of cardiovascular diseases: a meta-analysis of 47 cohort studies. J Hypertens. 2019;37:2325–2332. doi: 10.1097/HJH.0000000000002191 [DOI] [PubMed] [Google Scholar]
- 26.Kiviniemi AM, Lepojarvi ES, Tulppo MP, et al. Prediabetes and risk for cardiac death among patients with coronary artery disease: the ARTEMIS study. Diabetes Care. 2019;42:1319–1325. doi: 10.2337/dc18-2549 [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Lee ET, Devereux RB, et al. Prehypertension, diabetes, and cardiovascular disease risk in a population-based sample: the strong heart study. Hypertension. 2006;47:410–414. doi: 10.1161/01.HYP.0000205119.19804.08 [DOI] [PubMed] [Google Scholar]
- 28.Thompson AM, Hu T, Eshelbrenner CL, et al. Antihypertensive treatment and secondary prevention of cardiovascular disease events among persons without hypertension: a meta-analysis. JAMA. 2011;305:913–922. doi: 10.1001/jama.2011.250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cosentino F, Grant PJ. The 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: new features and the ‘Ten Commandments’ of the 2019 Guidelines are discussed by Professor Peter J. Grant and Professor Francesco Cosentino, the Task Force chairmen. Eur Heart J. 2019;40:3215–3217. doi: 10.1093/eurheartj/ehz687 [DOI] [PubMed] [Google Scholar]