Abstract
Purpose
The aim of this study was to evaluate the clinical outcomes of management of recurrent pterygium with severe symblepharon using mitomycin C, double amniotic membrane transplantation, cryopreserved limbal allograft, and a conjunctival flap.
Patients and Methods
This retrospective case series included 10 eyes of 10 patients with recurrent pterygium with severe symblepharon. Eight patients have diplopia in primary gaze. All patients underwent pterygium excision, application of mitomycin C (MMC), double amniotic membrane transplantation (AMT), cryopreserved limbal allograft (CLA) transplantation, and placement of a conjunctival flap. Outcome measures were visual acuity, astigmatism, and recurrence. Recurrence was defined as the presence of fibrovascular proliferative tissue crossing the limbus.
Results
The patients’ mean age was 73.8 years. The mean follow-up period was 3.0 years. The mean preoperative and postoperative best-corrected visual acuities (logMAR conversion) were 0.43 and 0.30, respectively. The mean preoperative and postoperative astigmatism were –3.89 diopters and –1.54 diopters, respectively, and there was a significant difference. No recurrence occurred in any of the eyes. Symblepharon was released in all eyes. Diplopia in primary gaze was resolved in all eyes.
Conclusion
Management of recurrent pterygium with severe symblepharon using MMC, double AMT, CLA, and a conjunctival flap was an effective treatment.
Keywords: pterygium, symblepharon, limbal allograft, amnion, mitomycin C
Introduction
Recurrent pterygia can cause symblepharon, and in some cases symblepharon may become severe. There have been several reports on recurrent pterygium with symblepharon,1–7 but there have been few reports on recurrent pterygium with severe symblepharon.1,5 Severe symblepharon causes conjunctival fornix shortening,1 fornix obliteration,8 and ocular motility restriction.5 Patients with severe symblepharon suffer from diplopia due to ocular motility restriction. Our previous report demonstrated that pterygium surgery using mitomycin C (MMC), double amniotic membrane transplantation (AMT), and a large conjunctival flap was effective in treating recurrent pterygium with mild symblepharon.7 However, the surgical procedure did not achieve satisfactory results for recurrent pterygium with severe symblepharon. We considered cryopreserved limbal allograft (CLA) can act as a strong barrier to a subconjunctival fibrovascular proliferative tissue, and modified the procedure with CLA for recurrent pterygium with severe symblepharon.
We report the clinical outcomes of management of recurrent pterygium with severe symblepharon using MMC, double AMT, CLA, and a conjunctival flap.
Patients and Methods
The Kurume University institutional review board approved this study. The board waived the requirement for patient consent to review their medical records because identifiable private information was not included and confidentiality of patient data was ensured in this study. A written informed consent was obtained from the patients in the case report to have the case details and accompanying images published.
This study consisted of 10 eyes of 10 patients (6 men and 4 women) with recurrent pterygia with severe symblepharon. Eight of 10 pterygia were large, crossing a point midway between the limbus and the pupillary margin, and 6 of those pterygia encroached on the pupillary axis. Fornix obliteration and ocular motility restriction were seen in all patients. Eight patients had diplopia in primary gaze and the presence of preoperative diplopia was unknown in the other two patients due to suppression of vision. The pterygium surgery was performed at Kurume University Hospital from July 2012 to July 2018.
Amniotic Membrane Preparation
The details of amniotic membrane preparation were described previously.7 In brief, placenta was obtained from healthy mothers and amniotic membrane was peeled off from the chorion, thoroughly washed, and cut into pieces. Each piece of amniotic membrane was placed in a plastic container and preserved at −80°C.
Surgical Procedure
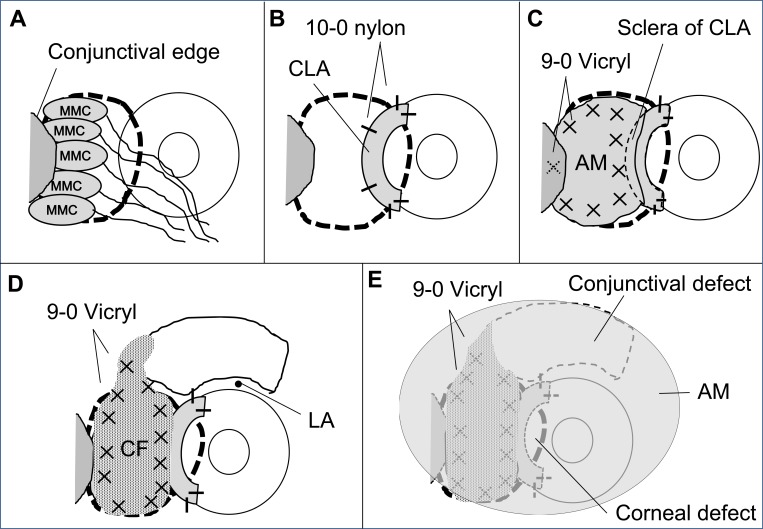
One surgeon (Y.M.) performed all of the surgical procedures after written informed consent was obtained from each patient before the surgical procedure. All patients received topical anesthesia using 4% lidocaine eye drops (Xylocaine, AstraZeneca K.K., Osaka, Japan). Sub-Tenon’s anesthesia with 2% lidocaine was used when the patient experienced pain. Blunt dissection was used to remove the pterygium head from the cornea, the epithelium of the pterygium body from the subconjunctival fibrous tissue, and then the subconjunctival fibrous tissue from the sclera. Removal of the subconjunctival fibrous tissue was extensive, leading to symblepharon lysis, and many pieces of sponge (BEMSHEETS® No.1, KAWAMOTO CORPORATION, Osaka, Japan) soaked in 0.04% MMC were then applied to the adjacent subconjunctival fibrous tissue for 3 minutes. After removing the sponge material, the areas were thoroughly irrigated with 200 to 800 mL of balanced salt solution. The CLA was trimmed and placed on the bare limbus and sclera with 10–0 nylon sutures. Patients were asked to look in the opposite direction to the pterygium excision site to fully expose the bare sclera. Amniotic membrane was placed over the bare sclera to reach the fornix, and the sclera of CLA with the epithelial side up. The amniotic membrane was sutured to the sclera with interrupted 9–0 Vicryl sutures. Patients were again asked to look in the opposite direction to the pterygium excision site to calculate the size of the defect covered by amniotic membrane and to determine the size of conjunctival flap. A conjunctival epithelium (pedicle) flap from the superior area, excluding the limbal area (1–2 mm), was rotated, positioned, and secured on the amniotic membrane with 9–0 Vicryl sutures. The conjunctival flap was large enough to cover the entire amniotic membrane as far as possible. Another amniotic membrane was placed on the ocular surface with 10–0 nylon sutures with the epithelial side down. This second application of amniotic membrane was to cover the conjunctival defect and corneal defect. When the recessed conjunctival edge was not reached to the fornix, one anchoring suture per quadrant was used to secure the recessed conjunctival edge to the skin with a bolster with 4–0 nylon.8 A subconjunctival injection of dexamethasone (Rinderon, Shionogi & Co., Ltd., Osaka, Japan) and gentamicin (Gentacin, MSD K.K., Tokyo, Japan) was given at the inferior temporal fornix, and a therapeutic contact lens was placed over the amniotic membrane. Figures 1 and 2 show the surgical procedure.
Figure 1.
Surgical procedure (schema). (A) Removal of the pterygium head and subconjunctival tissue, and application of pieces of sponge soaked in 0.04% mitomycin C. (B) The CLA was trimmed and placed on the bare limbus and sclera. (C) Amniotic membrane was placed over the bare sclera to reach the fornix, and the sclera of CLA. (D) Large pedicle conjunctival epithelium flap excluding the limbal area (1–2 mm). (E) Amniotic membrane transplantation on the ocular surface.
Abbreviations: MMC, mitomycin C; CLA, cryopreserved limbal allograft, AM, amniotic membrane; CF, conjunctival flap; LA, limbal area.
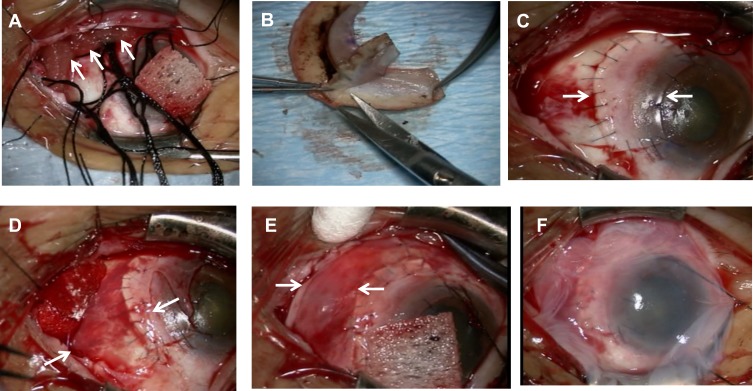
Figure 2.
Surgical procedure (photographs). (A) Application of pieces of sponge (arrows) soaked in 0.04% mitomycin C. (B) The cryopreserved limbal allograft was trimmed. (C) The cryopreserved limbal allograft (between arrows) was placed on the bare limbus and sclera with 10–0 nylon sutures. (D) Amniotic membrane (between arrows) transplantation on the bare sclera and the sclera of the cryopreserved limbal allograft. (E) A conjunctival epithelium flap (between arrows) from the superior area was rotated, positioned, and secured on the amniotic membrane with 9–0 Vicryl sutures. (F) Amniotic membrane was placed on the ocular surface.
The amniotic membrane on the ocular surface was left in place at least 7 days after the surgery. The therapeutic contact lens was left on the cornea for about 1 month. Postoperatively, all patients were treated with topical dexamethasone 0.1% four to eight times daily depending on the degree of hyperemia, nonsteroidal anti-inflammatory agent, and antibiotics for more than a year, after which their use was tapered slowly until they were medication-free. For eyes with severe hyperemia, topical cyclosporine A 0.05% four to ten times daily was added depending on the severity.
Outcomes
Outcome measures were visual acuity before surgery and at the final visit, corneal astigmatism before and at 1, 3, 6, and 12 months after the surgery, and pterygium recurrence. Decimal visual acuities were converted to logMAR units for the analyses. The degree of corneal astigmatism was measured using an automated keratometer (KR8100PA; Topcon, Tokyo, Japan). Recurrence was defined as the presence of fibrovascular proliferative tissue crossing the limbus.3,7
Results
The mean (± SD) age of the patients was 73.8 ± 6.1 years (range, 63–82 years). The mean number of prior pterygium excisions was 1.8 ± 1.0 (range, 1–4). The mean postoperative follow-up duration was 3.0 ± 1.9 years (range, 1.0–6.7 years). The mean surgery duration was 3.8 ± 0.4 hours (range, 3.0–4.4 hours). Three patients had the anchoring suture at the upper lid and the lower lid, one patient had the anchoring suture at the lower lid, and one patient had the anchoring suture at the upper lid and the two anchoring sutures at the lower lid.
There was no significant difference between preoperative (0.43 ± 0.48) and postoperative (0.30 ± 0.40) best-corrected visual acuity (logMAR conversion). Postoperative visual acuity was improved by 0.3 logMAR in 4 eyes, unchanged in 4 eyes, and worsened by 0.3 logMAR in 2 eyes due to cataract. Preoperative astigmatism was measured in 7 eyes. Two eyes had astigmatism that was so high that it could not be measured by the automated keratometer. Astigmatism measurement was not conducted in one eye. There was no significant difference between preoperative [–3.89 ± 1.60 diopters (D)] and postoperative astigmatism 1 month after surgery (–2.35 ± 1.30 D after 1 month), but there was a significant difference between preoperative and postoperative (–1.61 ± 1.17 D after 3 months, –1.29 ± 0.78 D after 6 months, –1.54 ± 1.37 D after 12 months) astigmatism (p < 0.05, Wilcoxon signed-rank test).
No recurrence occurred in any of the eyes. Symblepharon was released in all eyes, but the conjunctival sac was shortened in 2 eyes. Diplopia in primary gaze has been resolved in all eyes. Corneal and conjunctival epithelialization was observed in 5 eyes when the amniotic membrane on the ocular surface was removed 9.7 ± 3.1 days (range, 7–17 days) after the surgery. Other five patients were treated with autologous serum eye drops 20% until the conjunctiva had epithelialized. The conjunctiva in all patients had epithelialized within 8.5 ± 15.6 days (range, 0–48 days) after the amniotic membrane removal. A small recurrent conjunctival epithelial defect (2 mm or less in diameter) was seen in the area close to the CLA in three eyes 32.7 ± 25.0 days (range, 7–57 days) after the conjunctiva had epithelialized. The conjunctiva in the eyes had epithelialized by autologous serum eyedrops 20%, but scleral stromalysis was observed in one eye after the epithelialization. The patient underwent scleral transplantation and there was no recurrence. A non-infectious corneal ulcer (about 3 mm in diameter) was seen in the center of CLA in two eyes 4.4 and 5.5 years after the surgery. The ulcer in the eyes resolved with therapeutic contact lens and 2% rebamipide ophthalmic suspension. Topical cyclosporine A 0.05% was used in 6 eyes because of severe hyperemia for more than a year, after which its use was tapered. Intraocular pressure (IOP) increased to more than 21 mmHg in 7 eyes, and the IOP was controlled with topical antiglaucoma medications.
Case Report
Case 1
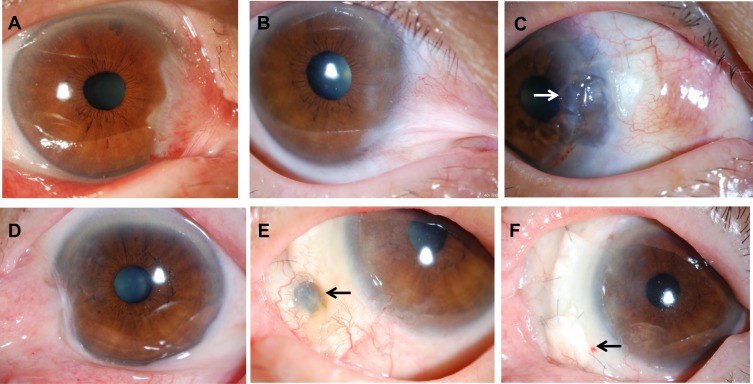
A 68-year-old male who had diplopia in primary gaze had undergone pterygium excision 2 times in the past. He had undergone excision of a recurrent pterygium combined with application of MMC, double AMT, and a large conjunctival flap, but recurrence was documented 12 months after the surgery. He had diplopia in primary gaze again, and he underwent pterygium surgery using MMC, double AMT, CLA, and a conjunctival flap. Symblepharon was released and diplopia in primary gaze resolved. A non-infectious corneal ulcer was seen in the center of CLA 5.5 years after the surgery. The center of CLA became thinner, but there has been no recurrence for 5.6 years. Figure 3a–c show preoperative and postoperative appearances of Case 1.
Figure 3.
Preoperative and postoperative appearances. Upper column, Case 1 and lower column, Case 2. (A) Case 1. Before excision of a recurrent pterygium combined with application of mitomycin C, double amniotic membrane transplantation, and a large conjunctival flap. (B) Before the surgery. (C) At 5.6 years after the surgery. Symblepharon was released and the center of CLA became thinner (arrow), but there has been no recurrence. (D) Case 2. Before the surgery. (E) Scleral stromalysis was observed 6 months after the surgery (arrow). (F) Scleral transplantation was performed for the scleral stromalysis 2 years after the surgery (arrow).
Case 2
An 80-year-old female who had diplopia in primary gaze had undergone pterygium excision in the past. She underwent pterygium surgery using MMC, double AMT, CLA, and a conjunctival flap. Scleral stromalysis was observed 6 months after the surgery, and she underwent scleral transplantation. Symblepharon was released and diplopia in primary gaze resolved. There has been no recurrence for 3.2 years. Figure 3–f show preoperative and postoperative appearances of Case 2.
Figures 4 and 5 show the pre- and postoperative photographs of other cases.
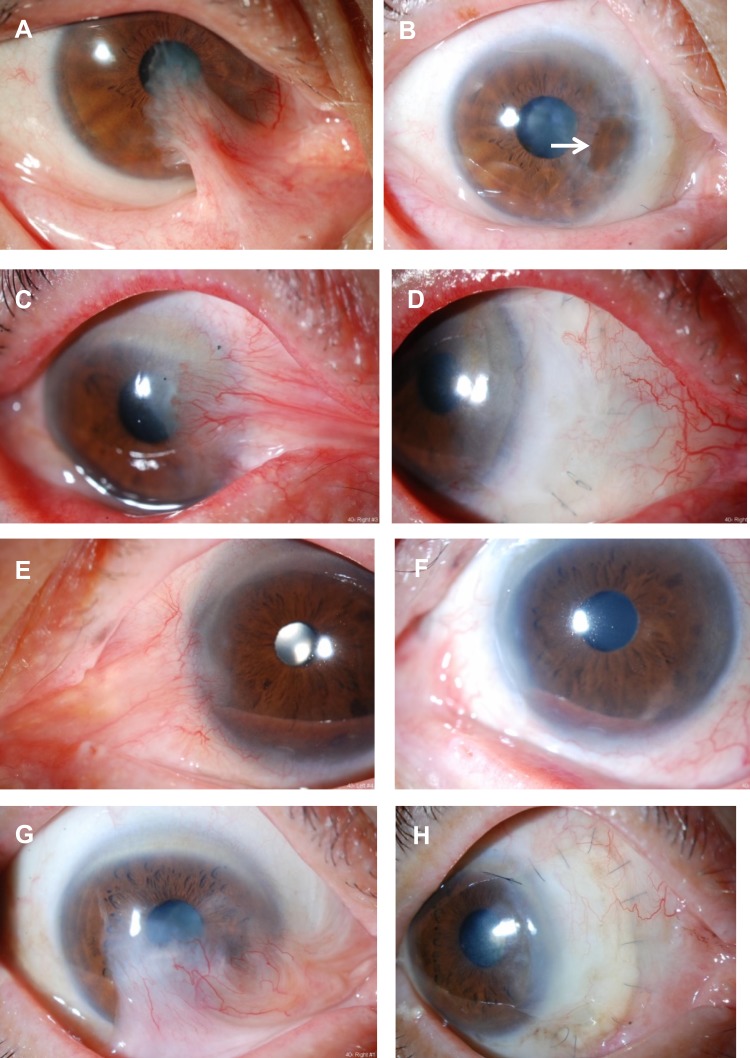
Figure 4.
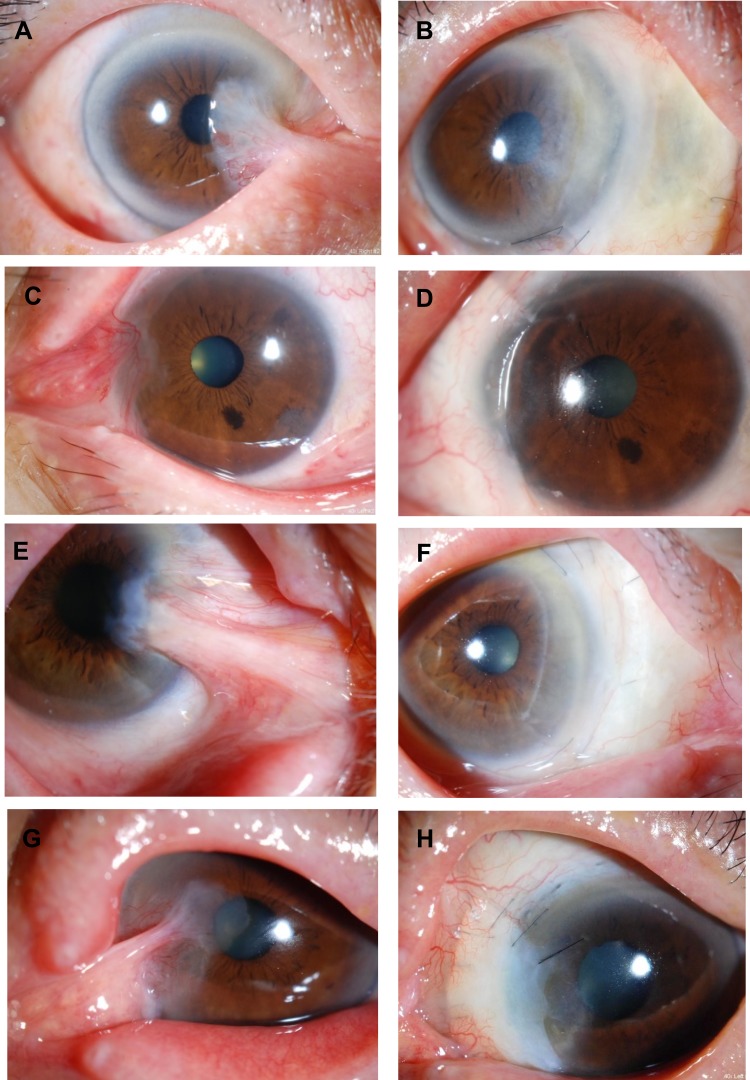
Preoperative and postoperative appearances in other cases. Left column, eyes before surgery and right column, after surgery. (A) A 63-year-old female had undergone pterygium surgery two times in the past. Before the surgery. (B) At 5.5 years after the surgery. Symblepharon was released and the center of the CLA became thinner (arrow), but there has been no recurrence. (C) A 72-year-old male had undergone pterygium surgery four times in the past. Before the surgery. (D) At 3 years after the surgery. (E) An 82-year-old male had undergone pterygium surgery three times in the past. Before the surgery. (F) At 2.5 years after the surgery. The conjunctival sac was shortened. (G) A 73-year-old male had undergone pterygium surgery once in the past. Before the surgery. (H) At 1 year after the surgery.
Figure 5.
Preoperative and postoperative appearances in other cases. Left column, eyes before surgery and right column, after surgery. (A) A 73-year-old male had undergone pterygium surgery two times in the past. Before the surgery. (B) At 2 years after the surgery. (C) A 70-year-old female had undergone pterygium surgery once in the past. Before the surgery. (D) At 2 years after the surgery. (E) An 81-year-old female had undergone pterygium surgery once in the past. Before the surgery. (F) At 1 year after the surgery. (G) A 76-year-old male had undergone pterygium surgery once in the past. Before the surgery. (H) At 11 months after the surgery. The conjunctival sac was shortened.
Discussion
There have been several approaches for recurrent pterygium with symblepharon (Table 1). 1–7 There have been a few reports on recurrent pterygium with severe symblepharon.1,5 Recurrent pterygium with severe symblepharon causes severe barrier function destruction in the limbal area and severe fibrosis.1 It is considered that limbal autograft, limbal allograft, limbal conjunctival autograft, and conjunctival autograft can act as a barrier to subconjunctival fibrovascular proliferative tissue. Limbal autograft and limbal conjunctival autograft could cause limbal stem cell deficiency at the donor site. One of our patients with severe symblepharon who had diplopia in primary gaze underwent pterygium surgery with MMC, double AMT, and a large conjunctival flap, but recurrence occurred (Case 1). We considered that a conjunctival autograft was not a sufficient barrier in cases with recurrent pterygium with severe symblepharon, and we modified the surgical procedure with CLA. CLA can act as a strong barrier because CLA is thicker than a conjunctival autograft and amniotic membrane. A CLA is stored in a deep freezer and is readily available. A limbal allograft can be also a strong barrier, but requires a fresh donor cornea and rejection is considered to be a concern.
Table 1.
Previous Report of Surgery for Recurrentpterygium with Symblepharon
| Authors | Year | Surgery | No. of Eyes with Symblepharon | Recurrence Rate |
|---|---|---|---|---|
| Shimazaki et al1 | 1998 | AMT+limbal autograft | 4 | 25% |
| Prabhasawat et al2 | 2001 | AMT+MMC of 5-fluorouracil | 13 | 23% |
| Miyai et al3 | 2005 | AMT+limbal allograft+MMC | 10 | 0% |
| Yao et al4 | 2006 | AMT+limbal conjunctival autograft+MMC | 7 | 14% |
| Farid et al5 | 2005 | Limbal allograft | 1 | 0% |
| Ono et al6 | 2016 | AMT+preserved limbal allograft+MMC | 23 | Unknown |
| Monden et al7 | 2018 | Double AMT+largeconjunctival autograft+MMC | 10 | 0% |
Abbreviations: AMT, amniotic membrane transplantation; MMC, mitomycin C.
CLA can induce astigmatism and worse visual acuity. In the present study, there was a significant difference between the mean preoperative (–3.89 D) and postoperative (–1.54 D) astigmatism, but there was no significant difference between preoperative and postoperative best-corrected visual acuity (logMAR conversion). CLA did not induce astigmatism and affect visual acuity.
MMC has an anti-fibrotic effect and has been commonly used in surgery for recurrent pterygium. A severe complication of MMC use is scleral stromalysis.9 Lindquist TP et al emphasized that the bare sclera surgical technique is not recommended because it lends a higher rate of recurrence and a greater chance of stromalysis. Our procedure is not the bare sclera technique and MMC was applied to the adjacent subconjunctival fibrous tissue not to the sclera, but scleral stromalysis occurred in 1 case in which MMC inadvertently touched with sclera. The patient underwent scleral transplantation 6 months after the pterygium surgery. Scleral stromalysis was not observed in our previous report.7 In the present study MMC was more broadly applied than in cases of our previous report because of the severity of symblepharon and fibrosis, and the complications associated with MMC use were likely to occur. A recurrent epithelial defect of the conjunctiva was seen in the area close to the CLA in 4 eyes after the surgery. This may be a complication of MMC. The conjunctiva in the eyes had epithelialized by autologous serum eyedrops 20%. A non-infectious corneal ulcer was seen in the center of CLA in two eyes 4 to 5 years after the surgery. This may be not a complication of MMC because there was a distance between the ulcer and the place of MMC application. Because the center of CLA was in the lid fissure and the place elevated a bit, the center of CLA was likely to be dry and to cause ulcer. Autologous serum eyedrops have been used for dry eyes and epithelial defects10,11 and they were effective for the treatment of persistent conjunctival epithelial defect. We consider that it is necessary to use MMC for recurrent pterygium because it has severe fibrosis, but MMC should be used with caution.
Cyclosporine A 0.05% is also effective in inhibiting fibroblast proliferation.12 Cyclosporine A was actively involved in the migration of pterygium fibroblasts.13 Six of 10 eyes in this study were treated with topical cyclosporine A 0.05% four to ten times daily depending on the degree of hyperemia.
We used two pieces of amniotic membrane as described in our previous report.7 One piece of amniotic membrane is placed on the bare sclera and the sclera of CLA under the conjunctival flap. Because the conjunctival flap in our procedure involves only epithelium, the amniotic membrane may also substitute for Tenon’s capsule under the flap. The other piece of amniotic membrane is placed on the ocular surface. This second application facilitates epithelialization at the donor conjunctival site and the area of the corneal defect after pterygium excision. It is considered that the double amniotic membrane transplantation promotes epithelialization and prevents fibrosis and inflammation. Different from our previous report, after excising the recurrent pterygium, CLA is placed on the bare limbus and sclera, and then one piece of amniotic membrane is placed on the bare sclera and the sclera of CLA. Because in cases with recurrent pterygium with severe symblepharon the size of the bare sclera become large but the size of harvested conjunctival flap is limited, the amniotic membrane on the bare sclera may not be covered by the conjunctival flap. In those cases, we expect the surrounding conjunctival epithelium to epithelialize on the amniotic membrane. The other piece of amniotic membrane is placed on the ocular surface. This second application facilitates epithelialization at the amniotic membrane, the donor conjunctival site and the area of the corneal defect after pterygium excision.
We report the clinical outcomes of management of recurrent pterygium with severe symblepharon using MMC, double AMT, CLA, and a conjunctival flap. No recurrence occurred in any of the eyes during the follow-up duration. This combined procedure was effective in the treatment of recurrent pterygium with severe symblepharon.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Shimazaki J, Shinozaki N, Tsubota K. Transplantation of amniotic membrane and limbal autograft for patients with recurrent pterygium associated with symblepharon. Br J Ophthalmol. 1998;82(3):235–240. doi: 10.1136/bjo.82.3.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prabhasawat P, Tesavibul N. Preserved amniotic membrane transplantation for conjunctival surface reconstruction. Cell Tissue Bank. 2001;2(1):31–39. doi: 10.1023/A:1011597332277 [DOI] [PubMed] [Google Scholar]
- 3.Miyai T, Hara R, Nejima R, Miyata K, Yonemura T, Amano S. Limbal allograft, amniotic membrane transplantation, and intraoperative mitomycin C for recurrent pterygium. Ophthalmology. 2005;112(7):1263–1267. doi: 10.1016/j.ophtha.2005.01.037 [DOI] [PubMed] [Google Scholar]
- 4.Yao YF, Qiu WY, Zhang YM, Tseng SC, Mitomycin C. amniotic membrane transplantation and limbal conjunctival autograft for treating multirecurrent pterygia with symblepharon and motility restriction. Graefes Arch Clin Exp Ophthalmol. 2006;244(2):232–236. doi: 10.1007/s00417-005-0010-y [DOI] [PubMed] [Google Scholar]
- 5.Farid M, Lee N. Ocular surface reconstruction with keratolimbal allograft for the treatment of severe or recurrent symblepharon. Cornea. 2005;34(6):632–636. doi: 10.1097/ICO.0000000000000423 [DOI] [PubMed] [Google Scholar]
- 6.Ono T, Mori Y, Nejima R, Tokunaga T, Miyata K, Amano S. Long-term follow-up of transplantation of preserved limbal allograft and amniotic membrane for recurrent pterygium. Graefes Arch Clin Exp Ophthalmol. 2016;254(12):2425–2430. doi: 10.1007/s00417-016-3483-y [DOI] [PubMed] [Google Scholar]
- 7.Monden Y, Hotokezaka F, Yamakawa R. Recurrent pterygium treatment using mitomycin C, double amniotic membrane transplantation, and a large conjunctival flap. Int Med Case Rep J. 2018;11:47–52. doi: 10.2147/IMCRJ.S150969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kheirkhah A, Blanco G, Casas V, Hayashida Y, Raju VK, Tseng SCG. Surgical strategies for fornix reconstruction based on symblepharon severity. Am J Ophthalmol. 2008;146(2):266–275. doi: 10.1016/j.ajo.2008.03.028 [DOI] [PubMed] [Google Scholar]
- 9.Lindquist TP, Lee WB. Mitomycin C-associated scleral stromalysis after pterygium: surgery. Cornea. 2015;34(4):398–401. doi: 10.1097/ICO.0000000000000384 [DOI] [PubMed] [Google Scholar]
- 10.Poon AC, Geerling G, Dart JK, Fraenkel GE, Daniels JT. Autologous serum eyedrops for dry eyes and epithelial defects: clinical and in vitro toxicity studies. Br J Ophthalmol. 2001;85(10):1188–1197. doi: 10.1136/bjo.85.10.1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geerling G, Maclennan S, Hartwig D. Autologous serum eye drops for ocular surface disorders. Br J Ophthalmol. 2004;88(11):1467–1474. doi: 10.1136/bjo.2004.044347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viveiros MM, Kakizaki FY, Hércules LA, Padovani CR, Candeias JM, Schellini SA. In vitro study of cyclosporine A 0.05 % on primary and recurrent pterygium fibroblasts. Int Ophthalmol. 2016;36(2):237–242. doi: 10.1007/s10792-015-0106-2 [DOI] [PubMed] [Google Scholar]
- 13.Kim YH, Jung JC, Jung SY, Kim YI, Lee KW, Park YJ. Cyclosporine A downregulates MMP-3 and MMP-13 expression in cultured pterygium fibroblasts. Cornea. 2015;34(9):1137–1143. doi: 10.1097/ICO.0000000000000477 [DOI] [PubMed] [Google Scholar]