Abstract
The current problem of increasing antibiotic resistance and the resurgence of numerous infections indicate the need for novel vaccination strategies more than ever. In vaccine development, the search for and the selection of adequate vaccine antigens is the first important step. In recent years, bacterial outer membrane proteins have become of major interest, as they are the main proteins interacting with the extracellular environment. Trimeric autotransporter adhesins (TAAs) are important virulence factors in many Gram-negative bacteria, are localised on the bacterial surface, and mediate the first adherence to host cells in the course of infection. One example is the Neisseria adhesin A (NadA), which is currently used as a subunit in a licensed vaccine against Neisseria meningitidis. Other TAAs that seem promising vaccine candidates are the Acinetobacter trimeric autotransporter (Ata), the Haemophilus influenzae adhesin (Hia), and TAAs of the genus Bartonella. Here, we review the suitability of various TAAs as vaccine candidates.
Keywords: Trimeric autotransporter adhesins, Immunogenicity, Vaccination, Pathogenicity, Virulence
Introduction
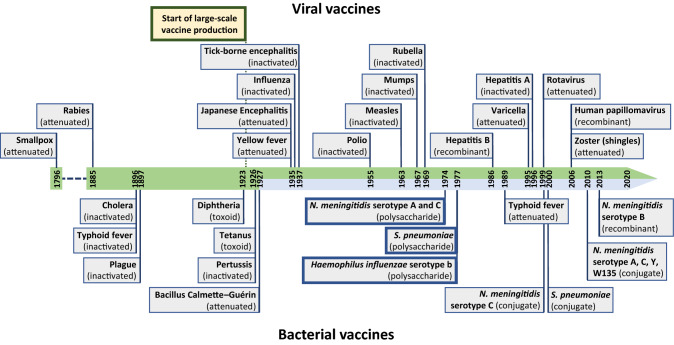
Vaccination against human pathogens was first introduced in medicine in 1796 by Edward Jenner (Fig. 1). He realised that milkmaids who had suffered earlier from cowpox were not infected by smallpox, demonstrating that the inoculated vaccinia virus leads to immunological protection against the variola virus [1]. Nowadays, vaccination represents a life-saving, scientifically accepted, and low-cost procedure to efficiently avoid human infections [2, 3]. Very recently, the national German government announced a program to increase the rate of measles vaccination in the population [4]. Although prophylaxis of infections by vaccination is very effective, there is, unfortunately, only a limited number of licensed vaccines available, most of which target viruses (Fig. 1). Current vaccines do, therefore, not cover most of the infectious diseases and, on top of that, many diseases for which vaccination strategies would be desirable, are on a resurgence (e.g., whooping cough) [5–10]. Novel vaccine formulations or alternative approaches must be investigated and a promising way forward is the use of recombinant vaccine components, developed from, e.g., reverse vaccinology approaches [3, 11]. However, the development of vaccines against emerging infectious diseases including Gram-negative bacteria decelerated in the last decades. Noteworthy is that new vaccines against only three bacterial agents were developed since 1927 (Fig. 1). In this review, we focus on the immunogenicity and vaccine candidacy of trimeric autotransporter adhesins (TAA) as one particular group of outer membrane proteins (OMPs) of Gram-negative bacteria [12–17]
Fig. 1.
Timeline of the development of human vaccines showing the scarcity of newly developed bacterial vaccines since 1927. Viral vaccines are shown above, while bacterial vaccines are shown below the timeline. Only the first developed vaccine against each viral or bacterial species is depicted (except for typhoid fever, N. meningitidis spp. and S. pneumoniae because of the different vaccine compositions). Not all invented, produced or updated vaccine formulation are included, only the major developments. Noteworthy is that vaccines against only three bacterial agents (N. meningitidis spp., S. pneumoniae, and H. influenzae) were developed since 1927 (light blue part in timeline) [1, 135, 251–253]
Principally, the most important conditions necessary to be an effective vaccine component are (i) the in vivo expression of surface epitopes, (ii) a high strain coverage, and (iii) immunogenicity and induction of a protective immune response in the host [18, 19]. In general, bacterial membrane proteins such as TAAs perform numerous important functions in pathogenesis, of which the first interaction with the extracellular environment in the mammalian host is of crucial importance. The extent of virulence of pathogenic organisms depends on various characteristics of both the organism itself (i.e., capacity of entering, infiltrating, and spreading through the host) and the host defence (i.e., immune status and metabolic conditions) [20–22]. It has become evident that TAAs play a prominent role in bacterial pathogenicity, where quick adaptation to changing conditions is crucial. As such, the modular composition of TAAs and their highly repetitive nature makes it possible for rapid adaptation to the host to occur [16, 23]. Moreover, attachment of bacteria via TAAs to the host is the first and absolutely required step in the infection process. Therefore, TAAs are highly suitable as vaccine candidates [12, 23–25].
Trimeric autotransporter adhesins
TAAs are a family of obligate homotrimeric, non-fimbrial, non-pilus bacterial adhesins that have numerous biological functions such as bacterial autoagglutination, binding to extracellular matrix (ECM) proteins and host cells, and the induction of distinct host cell responses. They are widespread in α-, β-, and γ-proteobacteria and primarily ensure the initial adhesion to specific molecular components of both abiotic and biotic surfaces (Fig. 2b) [16, 23, 26]. Former and alternative designations for TAAs are non-fimbrial adhesins (NFAs) and oligomeric coiled-coil adhesins (Ocas) [27–29] of which the latter refers to the presence of coiled coils in the structure of prototypical members of this class [30].
Fig. 2.
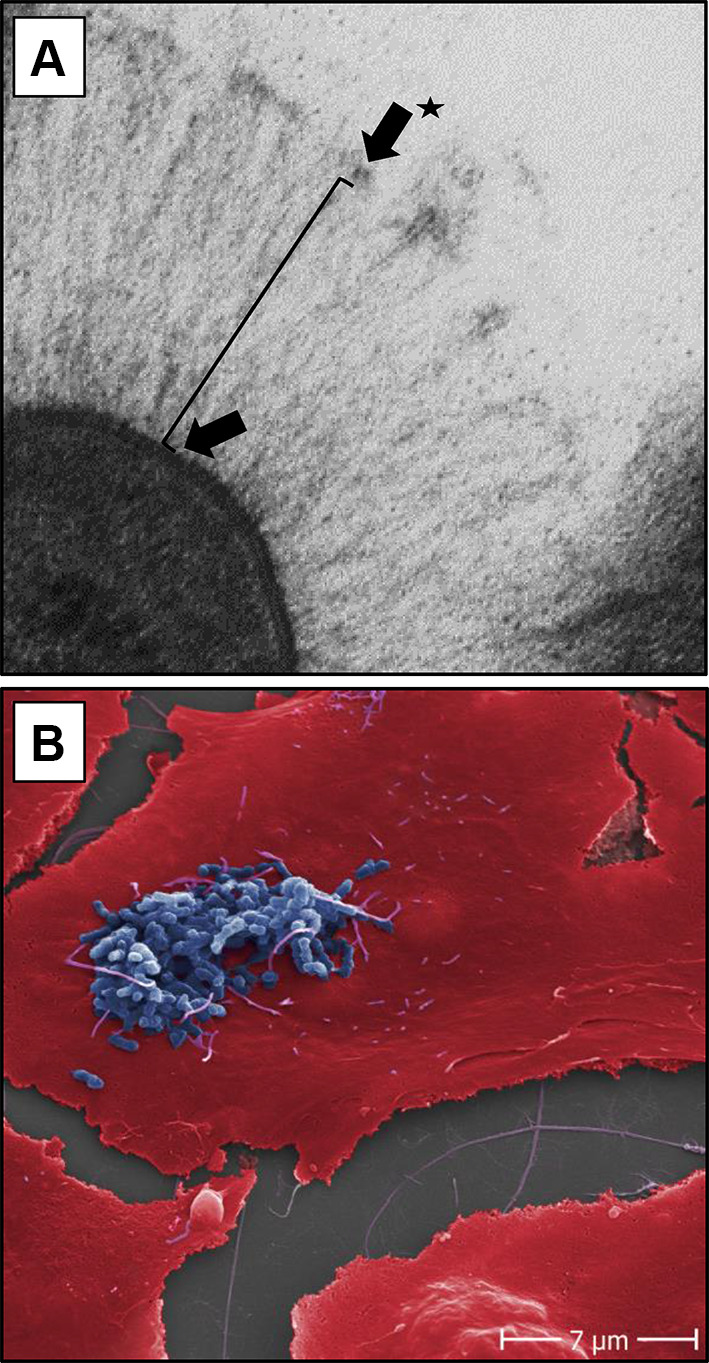
Electron microscopy of B. henselae adhesin A and adherence of B. henselae Marseille to human endothelial cells. a ‘Lollipop-like’ surface structure of the long filamentous BadA with the globular N-terminal head domain (arrow with star), followed by the passenger domain consisting of a neck/stalk domain (black line) and the membrane anchor (not visible) spanning the outer membrane (arrow). bB. henselae Marseille (blue coloured) adhering to the surface of human umbilical vein endothelial cells (red coloured) 30 min upon infection. Scale bare: 7 µm
In general, all TAAs share a common lollipop-like surface structure (Fig. 2a). The C-terminal anchor domain (translocation unit) forms a 12 stranded ß-barrel transmembrane domain followed by a passenger domain consisting of a neck/stalk domain and an N-terminal head domain. The head domain often has a globular structure and is responsible for the majority of the TAA’s biological functions [24, 29, 31]. The anchor domain, which defines the family, is conserved in all TAAs and ensures the autotransporter activity [16, 24, 30].
Type V secretion systems are autotransporters containing a ß-barrel transmembrane domain [32]. Five different type V secretion systems have so far been identified (type Va, Vb, Vc, Vd, and Ve), all of which are used to transport proteins across the outer membrane in Gram-negative bacteria [26, 33, 34]. The type Vc secretion system is also termed TAA. Several models for the autotransporter mechanism exist, but the details remain unknown [32, 34, 35]. After translocation, the passenger domain remains covalently attached to the anchor domain (Fig. 2a). Previously, it was thought that the translocation of the passenger domain across the outer membrane occurred without any external source of free energy (ion gradients, chaperone proteins, or adenosine triphosphate) [27]. However, recent experimental research on TAAs has demonstrated that the ß-barrel assembly (Bam) complex is likely to catalyse the translocation of the passenger domain across the outer membrane [36], on top of its known function to integrate the ß-barrel anchor domain into the outer membrane. This theory challenges the current ‘autotransporter’ hypothesis, however, does not change the fact that translocation is driven by the free energy of protein folding. The Bam complex consists of five proteins and catalyses the insertion of almost every ß-barrel in the outer membrane of Gram-negative bacteria [33, 34, 37–40].
The use of type V(c) secretion in vaccinology
Even though the exact secretion mechanism of TAAs is still unclear, the Vc secretion system is a potentially valuable feature in the development of multivalent recombinant bacterial vector vaccines [41–44]. For instance, it was suggested for HIV-1 envelope glycoprotein subunits (e.g., gp120) that soluble stabilised trimers generate a stronger immunogenic response in mice compared to monomeric exterior immunogenic glycoproteins [45, 46]. This may be due to the higher stability of trimers in vivo, the presence of multiple, cross-linked epitopes and, in this case, the more faithful representation of the functional envelope glycoprotein complex [45]. In contrast to the type Va secretion system, the type Vc secretion system manages to expose stable trimeric polymers on the outer membrane of Gram-negative bacteria, showing its potential in future vaccine development [23].
In case of the type Va secretion system, autotransport of recombinant heterologous expressed proteins has already been demonstrated to optimise antigen delivery in oral live-attenuated vaccine strains, increasing the immunogenicity and improving the specific immune response [47–49]. Furthermore, Jong et al. emphasized the potential of autotransporter adhesins as a valuable platform to display antigens for the development of multivalent recombinant vector vaccines by successfully expressing various heterologous antigens via the Escherichia coli autotransporter Hbp (type Va secretion system) both in E. coli and in an attenuated Salmonella enterica serovar Typhimurium vaccine strain [50].
Reverse vaccinology and outer membrane vesicles
A more recent vaccine delivery platform is the use of outer membrane vesicles (OMV) because of their high immunogenicity and virulence during infection [42, 51–53]. Recombinant vaccine antigens, such as TAAs, that can be added on OMVs, are primarily selected via reverse vaccinology, which includes in silico genome screening for open reading frames that likely encode for antigenic OMPs [53–55]. OMVs do not replicate, which makes them safer and thus more attractive candidates as vaccine components [56, 57]. However, they do not guarantee broad strain coverage and often mediate protection only against closely related strains [53, 58, 59]. In addition, lipopolysaccharides (LPS) are abundantly present in OMVs causing numerous inflammatory side effects in OMV-based vaccines [60].
TAAs as vaccine (sub)units
The most extensively investigated TAA is the Yersinia adhesin A (YadA) from Yersinia enterocolitica, the prototypical example of this class of adhesins [16, 26, 30]. Furthermore, Neisseria adhesin A (NadA) from Neisseria meningitidis is already one of the main vaccine antigens in the respective multicomponent vaccine, 4CMenB [61]. Other interesting TAAs and potential vaccine antigens are, inter alia, Haemophilus influenzae adhesin (Hia) (H. influenzae) [62], Acinetobacter trimeric autotransporter (Ata) (A. baumannii) [63], Salmonella adhesin A (SadA) (S. enterica) [64], and the ubiquitous surface proteins (UspA1 and UspA2) of Moraxella catarrhalis [65]. The proven immunogenicity of several TAAs makes them a potential target for vaccine development and their use in clinical diagnosis [23, 66]. Below, we discuss the vaccinology prospects of most of the well-studied TAAs (Table 1).
Table 1.
Immunogenicity of trimeric autotransporter adhesins and their potential as vaccine (sub)units
| Genus | Species | Licensed vaccine available? | TAA | Est. MW (kDa)a | Strain prevalence | UniProt accession no. | Immunogenicity | Protective properties | Considered as vaccine antigen | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Yersinia spp. | Y. enterocolitica | No | YadA | 47 | High prevalence in both strains with few genomic and phenotypic variants | P31489 | Proven: serum poly- and monoclonal antibodies in rabbit and mice, but no mucosal antibodies in mice | Partly proven in mice | No (not by itself) | [38, 71, 84, 92, 95] |
| Y. pseudo-tuberculosis | No | |||||||||
| Neisseria spp. | N. meningitidis | Yes | NadA | 43 | High prevalence (50–75%) in disease-associated isolates; six NadA variants and 89 distinct nadA allele sequences exist | Q8KH85 | Proven: strong antibody response in mice and bactericidal serum and mucosal antibodies in humans | Proven: in an infant rat infection model and in humans | Yes [licensed vaccine containing NadA available (4CMenB)] | [25, 98, 110, 114, 116, 118, 124] |
| NhhA | 62 | Highly conserved in all meningococcal strains; some isolated MenB strains only (partially) express monomeric NhhA | Q7DDJ2 | Proven: serum antibodies in humans and serum bactericidal antibodies in mice (in conjugation with other antigens) | NA | Yes | [52, 97, 123, 125] | |||
| Haemophilus spp. | H. influenzae |
Yes: targeting Hib No: NTHi and remaining encapsulated H. influenzae |
Hia | 114 | Only present in 25% of NTHi clinical isolates | Q48152 | Proven: opsonophagocytic serum antibodies in guinea pigs and mice | Not proven (but strongly suggested) | Yes (in combination with HMW1, HMW2 and NTHi OMVs) | [62, 133, 136, 139, 145] |
| Hsf | 243 | Present in all encapsulated serotypes and a subset of NTHi | P71401 | Proven: serum polyclonal antibodies in rabbit | NA | No | [129, 132] | |||
| H. ducreyi | No | DsrA | 30 | High prevalence in both H. ducreyi clonal populations with varieties in the DsrA passenger domain | Q9K2H6 | proven: serum antibodies in swine and in mice | Proven: in swine and mice | Yes | [66, 147, 149, 150] | |
| Acinetobacter spp. | A. baumannii | No | Ata | 250 | High prevalence (78%) in monophyletic A. nosocomialis, A. seifertii and A. baumannii | Proven: serum, bactericidal and opsonophagocytic antibodies in mice and rabbit antiserum | Proven: reduction in lung bacterial burdens in mice | Yes | [55, 63, 155, 158, 159] | |
| Moraxella spp. | M. catarrhalis | No | UspA1 | 83,5 | High prevalence of uspA1 (97%) and uspA2 (83%); strain-specific gene differences and variable phenotypes |
A0A3Q9GAK7 |
Proven: serum and mucosal antibodies in children and adults; bactericidal antibodies detected in mouse and guinea pig anti-sera | Proven: pulmonary clearance of bacteria in immunised mice | Yes (in the past) | [18, 163, 168, 172, 173, 175–177, 179] |
| UspA2 | 59,5 |
B5L5X1 |
||||||||
| Escherichia spp. | E. coli (EHEC, STEC, EAEC, ExPEC and VTEC) | No | EibA | 42 | NA | Q9LA60 | NA | NA | No | [186–189, 197–199] |
| EibC | 53 | NA | Q9LA56 | |||||||
| EibD | 54 | NA | Q9MCI8 | |||||||
| EibE | 52 | NA | Q9LA53 | |||||||
| EibF | 49 | NA | Q8VW24 | |||||||
| EibG | 54 | Low prevalence in STEC (15%) | Q0EAF1 | |||||||
| E. coli (STEC) | Saa | 56 | High prevalence in specific LEE-negative STEC strainsb | Q93F81 | Proven: polyclonal serum antibodies in mice | NA | Yes (for LEE-negative STEC strains) | [182] | ||
| E. coli (UPEC and ExPEC) | UpaG | 178 | Low prevalence in UPEC (21%), mostly related to E. coli B2 and D phylogenetic groups and frequently associated with ExPEC | A0A0H2VCA1 | Proven: serum antibodies in mice and rabbit | Proven: in mice after active and passive immunisation targeting ExPEC | Yes | [183, 184, 195] | ||
| E. coli (EHEC) | EhaG | 160 | NA | Q7DJ60 | Proven: serum antibodies in rabbit | No protective properties | No | [204] | ||
| Salmonella spp. | S. enterica (serovar Typhimurium) | Yes | SadA | 147 | High prevalence in S. enterica strains and highly conserved sequence | Q8ZL64 | Proven: IgG response in mice | Modest protection in mice | No (not by itself) | [64, 204, 209, 210] |
| Bartonella spp. | B. bacilliformis | No | BbadA (BrpA) | 130 | NA |
A0A3G2T987 A1UT92 |
NA | NA | Yes | [211, 225, 226] |
| BbadB (BrpB) | 132 | A1URN1 | ||||||||
| BbadC (BrpC) | 57 | A1URM8 | ||||||||
| B. henselae | No | BadA | 328 | highly conserved in B. henselae; variable length in passenger domain | Q5MWV9 | Proven: serum antibodies in rabbit and IgG antibodies in patient sera | NA | No | [24, 232, 238, 240, 241] | |
| B. quintana | No | Vomp A | 101 | Heterogeneity in vomp gene locus from B. quintana human isolates; highly conserved genes; variably expressed | Q64HS9 | Proven: immunoreactive in human sera infected with B. quintana | NA | Yes | [212, 213, 220] | |
| Vomp B | 109 | Q64HS8 | ||||||||
| Vomp C | 104 | Q64HS7 | NA | No | ||||||
| Vomp D | 99 | Q64HT0 |
NA not assessed
aMonomeric
bLocus of enterocyte effacement (LEE) pathogenicity island
Yersinia spp. TAA
YadA is a TAA present on the bacterial surface of Y. enterocolitica and Yersinia pseudotuberculosis. Yersinia pestis harbours the yadA gene, but the TAA is not expressed due to a frameshift mutation in the yadA gene [67].
Infections of Y. enterocolitica and Y. pseudotuberculosis are caused by the ingestion of contaminated food or water and can cause acute enteritis and lymphadenitis (pseudoappendicitis) in the gastrointestinal tract [68, 69], sometimes followed by sequelae such as arthritis and septicaemia [70]. Subsets of Y. pseudotuberculosis are the causative agent of, e.g., Far East scarlet-like fever [69].
Currently, there are no licensed vaccines targeting Y. pestis and Y. pseudotuberculosis [71]. Earlier human vaccines comprising live-attenuated Yersinia strains or killed whole-cell bacteria [72] often caused severe side reactions or proved to be too reactogenic, respectively [72–75]. Some vaccines are in clinical trials (e.g., rF1-V and RYpVax) and seem the ideal approach to overcome more outbreaks of Y. pestis by providing pre-exposure prophylaxis to combat infection for individuals with a high risk of exposure [71]. Important to note is, however, the fact that Y. pestis does not express YadA precluding its use as a potential plague vaccine candidate.
Successful first attempts to develop effective vaccines against Y. enterocolitica were established using different Yersinia proteins. In 1996, Noll and Autenrieth used heat shock proteins (Yersinia HSP60) with IL-12 as adjuvant in their vaccine development [76]. They suggested that microbial heat shock proteins would be promising vaccine candidates. Palmer et al. demonstrated the ability of Y. enterocolitica to modulate the immune response via OMPs [77]. More recently, the effective use of a bivalent fusion protein consisting of immunologically active regions of Y. pestis LcrV (i.e., a 35 kDa secreted protein that mediates the transport effector proteins into the host cell [71, 75]) and YopE proteins gave mice immunogenic protection upon delivery of lethal Y. enterocolitica [78]. New screening approaches for the development of vaccine candidates are still necessary, for instance, in vivo signature-tagged mutagenesis to target genes for novel virulence factors [79] or the use of reverse vaccinology to screen for antigenic OMPs.
The immunodominant YadA of Y. enterocolitica has a monomeric molecular weight of approximately 47 kDa [31, 38] and the yadA gene is located on the 64-75 kb Yersinia virulence plasmid (pYV) [80, 81]. Although discovered in 1981 as ‘protein 1’ [82, 83], YadA is still investigated to unravel its complex structure, to clarify the autotransporter mechanism and to identify its biological functions [16, 36].
Between the different Yersinia strains, highly homologous YadA proteins exist [84]. Different pathogenic and virulence functions are attributed to YadA in Y. enterocolitica and Y. pseudotuberculosis [80, 85]. For example, a short amino acid sequence was identified within the N-terminal head domain of YadA from Y. pseudotuberculosis that mediates uptake in human cells and promotes binding to the ECM protein fibronectin [84]. Later, it was shown that a similar stretch also exists in distinct strains of Y. enterocolitica, but only in those of serotype O:9. There, the stretch was crucial for efficient binding of the serum protein vitronectin [86]. Furthermore, the YadA-passenger domain confers serum resistance and is important for the pathogenicity of Y. enterocolitica [30, 87]. In addition, Schütz et al. demonstrated that the trimeric stability of YadA is crucial for full pathogenicity of Y. enterocolitica [88]. YadA itself induces the production of proinflammatory cytokines, including interleukin-8 (IL-8) and this process is triggered via the adhesion to β1-integrins [89, 90].
Some research has been carried out towards the immunogenicity of YadA. For example, poly- and monoclonal antibodies against YadA were obtained and antigens were identified upon immunisation with live bacteria [91–93]. According to Tahir et al., it is of interest to use purified YadA or killed Y. enterocolitica instead of live bacteria in vaccines [94]. They indicated that live Y. enterocolitica can prevent the host from recognising other than N-terminal epitopes of YadA. Finally, in 2017, Tsugo et al. immunised mice subcutaneously either with recombinantly expressed YadA (group 1), with inactivated Y. pseudotuberculosis strongly expressing YadA (group 2), or just with phosphate-buffered saline (group 3—control). Survival rates after exposure to pathogenic Y. pseudotuberculosis were 100% (group 1), 60% (group 2), and 0% (group 3), respectively [95]. However, the recombinantly expressed YadA proteins did not induce mucosal immunity as measured by IgG secretion. The authors concluded that YadA shows promising results as a vaccine component, but more research towards its safety, immunogenicity, and protective properties is necessary [95].
Neisseria meningitidis TAAs
The Neisseria adhesin A (NadA) and the Neisseria Hia/Hsf homologue A (NhhA) are both OMPs belonging to the class of TAAs. Both adhesins are present on certain genetic lineages of the Gram-negative bacterium Neisseria meningitidis [16, 96, 97].
Neisseria meningitidis is a human-specific Gram-negative pathogen and is the causative agent of meningococcal meningitis and sepsis [98, 99] with over 500,000 meningococcal cases each year worldwide [61, 100–102]. Twelve meningococcal serogroups have been classified based on their capsular polysaccharides. The serogroups A, B, C, W-135, X, and Y are most associated with invasive diseases [102–104]. Currently, serogroup B meningococci (MenB) causes most of the epidemic and endemic meningococcal diseases and is responsible for one-third of the meningococcal infections [52, 105]. Despite antibiotic treatment and partially effective vaccines, the progression of the disease is quick and has high mortality rates (5–15%) [98, 100].
In general, three types of meningococcal vaccines are available: polysaccharide vaccines, polysaccharide–protein conjugated vaccines and vaccines based on OMPs (developed via reverse vaccinology) [13, 103]. In the case of polysaccharide vaccines, bi-, tri-, or tetravalent vaccines exist, of which only the tetravalent vaccine is still available in Europe [103]. Effective tetravalent conjugate polysaccharide vaccines, combination vaccines, or monovalent vaccines against N. meningitidis serogroups A, C, W-135 and Y have been available since the early 1990s, are licensed, and are in clinical use [103]. The MenB capsular polysaccharide, however, shows high similarities with N-acetyl neuraminic acid on the surface of human fetal neural tissues and is, therefore, poorly immunogenic [25, 106, 107]. A protective capsular polysaccharide-based vaccine against serogroup B is thus not being pursued [52, 98]. Nevertheless, recently, two protein-based MenB vaccines were approved and licensed in several countries [105]. In 2013, the four component MenB vaccine 4CMenB (Bexsero®), using an OMV and three recombinant proteins [two protein–protein fusions and a single antigen (NadA)] was approved by the European Union (EU) [13, 103, 108]. Later, the recombinant protein vaccine MenB-FHbp was licensed in the USA (2014) and the EU (2018). It contains two variants of the meningococcal surface protein factor H-binding protein (FHbp) [58, 109].
Neisseria meningitidis adhesin A
NadA is a phase-variable ca. 43 kDa OMP of which the expression is mainly regulated by the transcriptional regulator NadR [96, 110, 111]. NadA plays a crucial role in the attachment of N. meningitidis to epithelial cells via ß1-integrins and in its subsequent invasion during the infection process [112, 113]. NadA is immunogenic, induces a protective bactericidal response, and has self-adjuvanting activity [114–116]. Furthermore, two genetically distinct groups of NadA exist that share only 46–50% identity and that do not show immunological cross reactivity [117, 118]. Group I (sharing ca. 95% sequence identity) consists of the variants NadA1, NadA2, and NadA3, while Group II (sharing ca. 90% sequence identity) consists of the variants NadA4, NadA5, and NadA6 [98, 118, 119]. Variants are classified based on their main variant group and small mutations [118, 119]. For example, NadA4 is mainly associated with carriage strains [98, 119, 120]. The crystal structures of NadA5 and NadA3 are available and provide valuable information for further investigations on their biological functions and on the effectiveness and structure of NadA as a vaccine antigen [61, 98, 121].
The nadA gene is present in approximately 30% of N. meningitidis isolated strains and in 75% of hypervirulent N. meningitidis serogroup B lineages [112, 117, 122]. Comanducci et al. demonstrated via dot-blot hybridization and PCR that 47% of 150 representatives of disease-associated isolates harbour the nadA gene [25]. In case of commensal strains derived from healthy carriers, nadA is present in 16.2% of 154 isolates [117].
Currently, NadA is the only TAA that is used as a component in a licensed vaccine, as NadA3 is a major antigen in the multicomponent vaccine 4CMenB [61, 96, 98]. In 2002, Comanducci et al. were the first to propose NadA as a vaccine candidate against MenB by demonstrating the strong inducement of antibodies upon immunisation of mice with NadA and showing protective features in an infant rat model [25]. Two years later, NadA was proven to be the only antigen out of 23 selected meningococcal proteins that elicits a strong antibody response in convalescent infant patients suffering from a meningococcal infection [123]. In 2006, Giuliani et al. described a universal vaccine against MenB that makes use of 5 antigens discovered by reverse vaccinology and aluminium hydroxide as an adjuvant [124]. In 2013, the 4CMenB vaccine was approved by the EU, promptly followed by a vaccination campaign in infants in the UK [13, 96]. Summarised, NadA and its discovery via reverse vaccinology, its analysis as an essential pathogenicity factor of N. meningitidis, and the further development as a vaccine component serve as a role model to expedite the development of TAA-based vaccines.
Neisseria meningitidis Hia/Hsf homologue A
NhhA was the first vaccine candidate against MenB and was described using whole genome sequencing to identify possible vaccination targets [54]. NhhA shows high similarities with the TAAs Hia and Haemophilus surface fibril (Hsf) of Haemophilus influenzae, is immunogenic in humans in conjugation with other antigens (e.g., TbpA, Omp85, or NspA), and facilitates bacterial attachment to host epithelial cells during infection by binding heparan sulphate and laminin [97, 99, 125, 126]. Furthermore, NhhA mediates serum resistance, induces macrophage apoptosis, reduces phagocytosis, and protects the bacteria against complement-mediated killing [99, 127]. Moreover, the nhha gene is highly conserved in all meningococcal strains [19, 97].
All these features suggest that NhhA is a promising vaccine candidate [23, 111]. Peak et al. immunised mice with OMVs containing various NhhA constructs, demonstrating protective properties of truncated NhhA against heterologous NhhA-expressing N. meningitidis strains [97]. A later study showed an enhanced immunogenicity against NhhA when its membrane anchor domain was coupled to the Moraxella IgD-binding protein providing a more effective vaccine [52].
However, it was found that a subset of clinical isolated MenB strains only (partially) express the monomeric form of NhhA, caused by a single natural mutation (glycine to aspartic acid) in the C-terminal passenger domain. Accordingly, loss in trimerization, surface exposure and adhesive features was observed. These findings question the vaccine candidacy of NhhA because of the need for broad strain coverage [128].
Haemophilus spp. TAAs
Two different TAAs are expressed on the outer membrane of H. influenzae, H. influenzae adhesin (Hia), and H. surface fibril (Hsf) [129, 130].
Haemophilus influenzae is a human specific, Gram-negative pathogen categorised into two different groups, the polysaccharide encapsulated (serotypes a–f), and the unencapsulated group often referred to as non-typeable H. influenzae (NTHi) [129, 131, 132]. H. influenzae serotype b (Hib) encapsulated strains are considered most virulent and are a major agent of respiratory tract systemic infections. Infections can lead to acute epiglottitis, sepsis, acute meningitis, and pneumonia. NTHi mainly causes local diseases such as bronchitis, otitis media, and sinusitis [131, 133, 134].
Current vaccines are mainly against the most virulent Hib. The earlier polysaccharide-based vaccines showed only short-term protection for children under 18 months after various trials were undertaken in 1977 [135]. The first conjugate vaccine was introduced in 1992. In total, four different conjugate vaccines have been licensed, each with different immunologic properties [136]. In 2012, it was concluded that the invasive disease caused by Hib had been virtually eliminated since the introduction of the vaccine [136, 137]. However, Hib vaccines do not protect against other serotypes. There are currently no approved vaccines against the remaining capsulated H. influenzae nor against NTHi, and so research is thus needed [138–140]. For instance, recent studies on the prevention of chronic obstructive pulmonary disease (COPD) focused on the immunogenicity of various vaccine formulations consisting mainly of NTHi and Moraxella catarrhalis surface proteins [141].
Two relevant candidate vaccine antigens are the surface proteins Hia and Hsf. Both TAAs contain several repetitive domains, are homologous in their N- and C-termini, and show an overall sequence identity of 81% and 72%, respectively [132].
Haemophilus influenzae surface fibril
Hsf has a monomeric molecular weight of 243 kDa [132], represents a major virulence factor of H. influenzae, and is presented in all encapsulated serotypes and a subset of NTHi [132]. The binding of vitronectin by Hsf inhibits the formation of the membrane attack complex and thus facilitates the invasion of lung epithelial cells [131]. Furthermore, Hsf mediates adherence to host epithelial surface integrins via bridge formation with vitronectin. Hsf is not frequently mentioned as potential vaccine antigen, but Hallström et al. demonstrated reduced survival of a Hsf-deficient mutant when incubated with human serum [129, 142].
Haemophilus influenzae adhesin
In contrary with Hsf, Hia is only present in 25% of NTHi clinical isolates and has a monomeric molecular weight of 114 kDa [130, 131, 133]. Hia is a major adhesin in NTHi strains and performs a crucial role in the infection and colonisation of the upper respiratory tract [143]. In addition, Hia is highly immunogenic in humans and a strong antibody induction was observed during naturally acquired infections [144, 145]. However, to qualify as a vaccine antigen, a broad strain coverage is required. A vaccine that comprises Hia, combined with both surface adhesins HMW1 and HMW2, would be active against 95% of all NTHi [130, 133, 144, 146]. HMW1 and HMW2 are both immunogenic surface adhesins expressed by approximately 75% of NTHi strains [130, 146]. Winter and Barenkamp demonstrated in 2017 the protective ability of OMVs, overexpressing HMW1 and HMW2 or Hia, as vaccine antigens in a rodent otitis media model [62].
Haemophilus ducreyi serum resistance A
The TAA of Haemophilus ducreyi called the ‘ducreyi serum resistance A’ (DsrA) is a proven virulence factor and thus a potential target as vaccine antigen [147]. H. ducreyi is a pathogen that causes the genital ulcer disease chancroid, for which no vaccines are available [148]. Fusco et al. demonstrated the immunogenic and protective properties of a recombinant form of the N-terminal passenger domain of DsrA (rNT–DsrAI), administered bi-weekly in Freund’s adjuvant against infection with experimental H. ducreyi in swine [66]. It was subsequently found that the humoral immune response in mice upon intramuscularl administration of rNT–DsrAI with alum is highly persistent and of superior quality and quantity compared to subcutaneous administration [149]. Furthermore, a Th2-type immune response was observed using Freund’s adjuvant, alum, or imiquimod as adjuvant [149]. Nonetheless, H. ducreyi is divided into two clonal populations with varieties in the passenger domain of DsrA, meaning that antibodies recognising class I DsrA do not recognise class II DsrA [147, 149, 150].
Acinetobacter baumannii TAA
Ata is a TAA present on the bacterial surface of the Gram-negative A. baumannii, one of the major causative agents of hospital-acquired infections worldwide [151, 152]. Characteristically, A. baumannii strains possess the ability to acquire resistance genes rapidly against all commonly used antimicrobial compounds. The dissemination of carbapenem-resistant and in general multidrug-resistant Acinetobacter spp. strains is one of the most urgent health risks of our time and threatens to undo a century of medical progress [153]. Consequently, A. baumannii is the number one pathogen on the ‘WHO priority pathogens list for R&D of new antibiotics’ [154]. Effective antibiotic treatment is thus complicated and alternative therapy strategies are urgently needed [63, 151, 152, 155].
Vaccination can become a valuable alternative for shortcoming antibiotic treatments against multi-resistant pathogenic strains. Currently, no vaccines against A. baumannii are licensed. However, promising vaccine candidates with immunogenic and protective properties have been described, including outer membrane complexes, OmpA and Ata itself [155–157].
Ata was first described in 2012 while searching for novel virulence factors of A. baumannii [158]. The ata gene was detected in 44 out of 75 collected A. baumannii isolates of which 43 showed additional Ata expression on its outer membrane [158]. More recently via phylogenetic profiling, 78% of monophyletic A. nosocomialis, A. seifertii, and A. baumannii showed presence of the ata gene [159]. Ata mediates binding to ECM proteins, under static and dynamic flow conditions [160], plays a crucial role in biofilm formation, mediates virulence in vitro and in vivo, and is hence an important virulence factor [63, 158, 159]
Bentancor et al. demonstrated in a pneumonia infection model in immunocompetent and immunocompromised mice the promising bactericidal, opsonophagocytic, and protective features of Ata-induced antibodies against inter alia two heterologous unrelated multidrug resistant A. baumannii strains [63]. Nevertheless, to increase the efficacy and strain coverage, the combination of Ata proteins from various isolates was suggested. In addition, the use and effectiveness of reverse vaccinology in the search for potential vaccine antigens against A. baumannii were recently re-emphasized [55, 156].
Moraxella catarrhalis TAAs
Moraxella catarrhalis expresses two different TAAs on its outer membrane, the ubiquitous surface protein A1 (UspA1) and the ubiquitous surface protein A2 (UspA2) [18, 161].
Moraxella catarrhalis is a Gram-negative and a human-specific bacterium of the respiratory tract [162, 163]. It was previously classified as Micrococcus catarrhalis, Neisseria catarrhalis, and Branhamella catarrhalis [164]. M. catarrhalis is a commensal coloniser of the nasopharynx and represents a causative agent of otitis media in (young) children. The role of M. catarrhalis as causative agent of COPD has long been underestimated, however, is a frequent pathogen in the acute exacerbation phase of the disease [141, 165]. Other related illnesses are meningitis, sinusitis and pneumonia [18, 162]. Diseases caused by M. catarrhalis are a serious burden for health systems worldwide [166, 167]. Moreover, M. catarrhalis produces ß-lactamases and is thus resistant against various important antibiotics [18]. Alternative therapies, such as M. catarrhalis vaccines, are, therefore, highly desirable [168].
Currently, no licensed vaccines are available to prevent M. catarrhalis-associated diseases, but several candidate vaccines are being developed [165, 168, 169]. Potential M. catarrhalis vaccine antigens are adhesive proteins (e.g., OMP CD, Moraxella IgD-binding protein, UspA1 and UspA2), proteins involved in nutrient acquisition (e.g., oligopeptide permease protein A, transferrin-binding proteins, and OMP E), lipooligosaccharides, or other Moraxella surface proteins [18, 170, 171]. Numerous OMPs including UspA1 and UspA2 are main virulence factors of M. catarrhalis and play an important role in the first adherence to the epithelial host cells, during the infection process, and the subsequent disease development [163].
UspA1 and UspA2 are TAAs with a predicted molecular weight of ca. 83.5 and ca. 59.5 kDa, respectively [172]. They are immunogenic [161, 173] and play an important role in serum resistance [174]. In addition, UspA1 and UspA2 are identified as one of the main targets of antibodies to surface epitopes in patients with COPD [175, 176]. Earlier, UspA1 and UspA2 were considered as promising vaccine candidates [18, 171, 173, 177]. However, a high degree of sequencing diversity in the uspA1 and uspA2 genes was demonstrated [163, 178] resulting in strain-specific differences and variable phenotypes [179]. In addition, to evade acquired immunity from the host while maintaining serum resistance and adhesive features, regions of uspA genes can swap between other uspA genes from the same strains [180]. Consequently, both TAAs lately lost major interest as potential vaccine antigens [18]. A possible solution might be to target conserved motifs of known function that are present in both UspA proteins [e.g., domains responsible for binding with ECM proteins or proteins from the carcinoembryonic antigen related cell adhesion molecule (CEACAM) subfamily] [180].
Escherichia coli TAAs
Four different TAAs have been characterised from pathogenic Escherichia coli, in particular the E. coli immunoglobulin binding (Eib) proteins [181], the Shiga toxin-producing E. coli auto-agglutinating adhesin (Saa) [182], the uropathogenic E. coli adhesin G (UpaG) [183], and, most recently, the enterohemorrhagic E. coli adhesin G (EhaG) [184].
Currently, no broadly protective vaccines against pathogenic E. coli are available [185, 186], but some vaccines have reached clinical trial status [187–189]. Most research concerning vaccine development against pathogenic E. coli is done for the enterotoxigenic E. coli (ETEC) expressing enterotoxins and colonisation factors (i.e., usually fimbriae or fibrillae) upon infection [190], as this bacterium is an important cause of bacterial diarrhoea (travellers’ diarrhoea) in developing and middle-income countries [187]. ETEC vaccine development is currently one of the WHO priorities [191, 192]. Two vaccines against ETEC are in phase II clinical trials. To broaden the vaccine coverage, novel immunogenic, conserved and virulent antigens must be reviewed, e.g., non-fimbrial surface adhesins [193]. Promising research to identify potential protective antigens is ongoing [185, 194–196].
Escherichia coli immunoglobulin binding proteins
Eib proteins are mostly found in intimin-negative, shiga toxin-producing enterohaemorrhagic E. coli (EHEC) strains [197, 198]. Shiga toxin-producing E. coli (STEC) causes severe diseases in humans such as haemorrhagic colitis or haemolytic–uremic syndrome (HUS) [199]. Eib genes occur in various pathogenic and multidrug-resistant E. coli strains, for example enteroaggregative E. coli (EAEC), extraintestinal pathogenic E. coli (ExPEC), and verotoxigenic E. coli (VTEC) [199–201]. No licensed vaccines against STEC-associated diseases are available [196].
Currently, six homologous Eib proteins are described (EibA, EibC, EibD, EibE, EibF, and EibG) [181, 197, 198]. They are all TAAs and mutually share a high similarity in their passenger domain and C-terminus. Eib proteins are major virulence factors, as they (i) mediate serum resistance; (ii) play a major role in adherence to epithelial cells; and (iii) are receptors for IgAs and IgGs, binding non-immunologically to the Fc portion of immunoglobulins (Ig) [197, 198, 202]. To the best of our knowledge, no research has been carried out on their potential as vaccine components.
Shiga toxin-producing E. coli auto-agglutinating adhesin
In 2001, the gene for Saa was isolated from a large, virulence-related plasmid in a STEC strain negative for the locus for enterocyte effacement. Saa mediates autoaggregation and adherence to human epithelial type 2 cells, shows variation in size for different STEC strains, and has just ca. 25% identity with the Eib proteins. Furthermore, Saa was not proven to contribute to serum resistance. Nevertheless, in vitro adherence of saa-positive STEC strains was inhibited upon application of a polyclonal antiserum that was raised against purified Saa, emphasizing its potential as a vaccine antigen [182].
Uropathogenic E. coli adhesin G
Escherichia coli UpaG, was identified by Durant et al. via reverse vaccinology [195]. UpaG, characterised in the uropathogenic E. coli (UPEC) strain CFT073, mediates binding to ECM proteins and bladder epithelial cells, and promotes bacterial cell aggregation and biofilm formation [183]. The upaG gene in UPEC shows extensive sequence variation with the upaG gene in ExPEC strains [184].
Furthermore, UpaG was proven to induce protective antibodies in a mouse model against lethal sepsis due to virulent extraintestinal isolates of E. coli [195]. UpaG shows a wide strain distribution and is present in both commensal and pathogenic strains (e.g., in ExPEC strains) [203], suggesting that it is important in efficient colonisation of the urinary tract [183].
Enterohaemorrhagic E. coli adhesin G
The most recently identified TAA is EhaG which occurs in EHEC strains. EhaG is a positional orthologue of UpaG [184, 204], but has significant sequence differences in the passenger domain and has some divergent functional characteristics. EhaG also mediates bacterial binding to ECM proteins, autoaggregation, and biofilm formation. Other than UpaG, EhaG promotes adherence to intestinal epithelial cells. In addition, EhaG is highly conserved in diarrheagenic E. coli strains [184]. Some of these features indicate that EhaG is suitability as a potential vaccine candidate, but more research on it is certainly necessary.
Salmonella enterica adhesin A
Salmonella adhesin A (SadA) is a TAA expressed in vivo on the bacterial surface of the pathogenic Salmonella enterica (serovar Typhimurium) during infections [64, 205].
Salmonella enterica causes significant morbidity and mortality worldwide in humans and cattle [206]. Moreover, Salmonella is the most frequent bacterial cause of foodborne disease in the US and is responsible for the majority of foodborne outbreaks in the European Union [207]. Infection with S. enterica can result in enteric salmonellosis and sometimes manifests as septicaemia. When not self-limiting, Salmonella-infected patients are treated via antimicrobial therapy. Consequently, multidrug-resistant S. enterica are on the rise [206, 208].
Currently, three types of licensed Salmonella vaccines exist: (i) a whole-cell live-attenuated vaccine (Vivotif®); (ii) a polysaccharide unconjugated vaccine; and (iii) a polysaccharide-conjugated vaccine (the latter commercialised under several names), all against one S. enterica serovar Typhi [209]. Furthermore, vaccination therapy against Salmonella spp. does exist for livestock breeding; for instance, an attenuated S. enterica serovar Typhimurium was designed providing higher cross protection against Salmonella serovars in swine [210]. Despite various studies and existing vaccines, there is still a need for safer and well-defined Salmonella vaccines.
The TAA SadA has an approximate trimeric size of 426 kDa and promotes biofilm formation and autoaggregation, but does not mediate serum resistance and does not bind ECM proteins. In addition, no distinction in virulence was observed between wild-type S. enterica and SadA-deficient S. enterica. SadA, nonetheless, plays an important role in adherence to and invasion of intestinal epithelial cells. Large surface structures such as LPS or fimbria inhibit the function of SadA, suggesting a specific role during certain conditions in colonisation and infection of epithelial cells. Moreover, SadA is highly conserved within S. enterica strains and is considered as a positional orthologue of UpaG and EhaG in E. coli, but with some different functions [64, 204]. An immunological IgG response was observed upon immunisation of mice with purified SadA (and Alum as adjuvant). However, IgG antibodies to SadA give only a limited protection compared to the PBS control, and therefore, the development of an effective vaccine against S. enterica might involve multiple antigens in parallel [64].
Bartonella TAAs
Bartonella quintana, B. bacilliformis, and B. henselae are clinically the three most important Bartonella species each expressing one or more TAAs [211, 212]. B. quintana expresses four variably expressed outer membrane proteins (VompA–D) [213], B. bacilliformis the B. bacilliformis adhesins A, B, and C (Brps, also designated as BbadA–C) and B. henselae the B. henselae adhesin A (BadA). Antimicrobial treatment of Bartonella spp.-associated diseases depends solely on the clinical situation and immunological status of the patient and less on the infective species. Consequently, no general treatment recommendation does exist for all Bartonella spp.-associated diseases [212, 214].
Variably expressed outer membrane proteins
Bartonella quintana is transmitted via the human body louse and is the causative agent of trench fever. Infections with B. quintana can lead to endocarditis, bacillary angiomatosis and peliosis hepatis in immunocompromised patients [211, 215–217]. Until now, no vaccines exist or are being developed against B. quintana infections [212].
Bartonella quintana expresses four TAAs called VompA–D, which are encoded by four genes (vompA, vompB, vompC, and vompD) and are tandemly arranged in a 12.8 kb gene locus [213]. The domain structure of the four ca. 100 kDa VompA–D is highly conserved, except for the major variable region in the N-terminal half of the stalk [213]. This region might be responsible for the variable phenotypes amongst the VompA–D which causes diversity in adhesion specificity, e.g., expression of VompA mediates autoaggregation of B. quintana [218]. Vomps are involved in bacterial cell adhesion to endothelial HUVEC cells [219], but do not seem important for bacterial adherence to epithelial HeLa-229 and phagocytic THP-1 cells [215]. Vomps are, therefore, important virulence factors and are crucial for the course of infection [213, 218].
The immunogenicity of Vomps and their suitability as a vaccine antigen have been described. While analysing protective and diagnostically relevant B. quintana antigens, 24 immunoreactive membrane proteins were identified of which, among others, VompA and VompB were most frequently recognised by sera from B. quintana-infected patients [220]. Further research to classify both TAAs as vaccine antigens is, however, necessary.
Bartonella bacilliformis adhesins A–C
Bartonella bacilliformis is the causative agent of Carrion’s disease, a biphasic illness restricted to the South American Andes [221]. The pathogen can infect human erythrocytes causing a serious acute hemolytic anaemia called ‘Oroya fever’ with high mortality rates in untreated patients. In a second chronic phase, B. bacilliformis infects endothelial cells and stimulates cell proliferation which results in the formation of blood-filled nodular haemangioma-like lesions in the skin known as ‘verruga peruana’ [221].
Currently, no vaccine is available for B. bacilliformis. However, vaccines against B. bacilliformis infections should be effective, as indigenous people in B. bacilliformis endemic regions seem less susceptible to infections and hemolytic diseases compared to non-indigenous people [222]. In addition, antiflagellin antiserum significantly reduced in vitro human erythrocyte invasion by the pathogen as compared to the controls [223].
One of the few attempts to prepare a vaccine against the Carrion’s disease was performed in 1943 by Howe and Hertig. The vaccine contained a formalin suspension of four B. bacilliformis strains. Twenty-two Peruvian guards working in a region notorious for frequent incidents of Carrion’s disease were subcutaneously vaccinated. The vaccine did not prevent infection, but alleviated the severity of the Carrion’s disease [221, 224]. Nonetheless, as the highly deadly Carrion’s disease affects mostly indigenous people with only limited medical care, the most promising and effective strategy to fight this disease is the development of a vaccine evoking both humoral and cellular immune responses [221, 225, 226].
In B. bacilliformis, three genes encoding for putative TAAs were identified [211]. The B. bacilliformis adhesins A–C (BbadA–C), originally called Bartonella repeat proteins (Brps), share common domain features with other TAAs of the genus Bartonella. The 130 kDa monomeric BbadA shows a highly similar head structure compared to BadA of B. henselae. In contrast, BbadB and the much shorter BbadC have more in common with the VompA–C of B. quintana. The role of B. bacilliformis adhesins during the infection process remains unclear.
Among other candidates [226, 227], the TAAs BbadA–C have been described as potential antigen candidates in vaccines [226]. More research towards antigenic candidates is, however, necessary and ongoing.
Bartonella henselae adhesin A
Bartonella henselae is the etiologic agent of cat scratch disease (CSD) and vasculoproliferative disorders. CSD is a self-limiting disease, but can be life-threatening for immunocompromised patients [29, 211]. Cats and dogs are the main reservoir of B. henselae, and the role of ticks in the transmission of B. henselae to humans remains unclear [29, 228, 229].
Unlike research towards clinical serodiagnostic tools including immunogenic proteins of B. henselae [230–233], and towards the development of feline vaccines [234–237], there has been no research towards the development of human vaccines preventing B. henselae infections. One possible obstacle in this research is the variable gene pool of B. henselae strains promoting antigenic variation and defining the specific immune response [238].
An important pathogenicity factor of B. henselae is the TAA BadA. BadA is a large (ca. 240 nm and ca. 328 kDa monomeric) outer membrane protein primarily responsible for the first interaction of the pathogen with endothelial host cells and ECM proteins (e.g., collagen, fibronectin, and laminin) (Fig. 2). Expression of BadA correlates with the secretion of angiogenic cytokines and activation of hypoxia-inducible factor (HIF)-1, the key transcription factor involved in angiogenesis [24, 219, 239, 240]. Despite length variations in the neck-stalk region, BadA seems to be highly conserved within B. henselae strains [241].
BadA is an immunodominant and immunogenic protein and regularly found in sera of patients (75%) infected with B. henselae [231, 240]. A mixture of immunodominant proteins including BadA seems the most plausible approach to develop an effective vaccine [232, 238].
Other TAAs
The Brucella suis trimeric autotransporter F (BtaF) and E (BtaE) are described as a promising immunogenic target for vaccination against mucosal B. suis infections [242]. Other research concerning the vaccine development targeting melioidosis and glanders caused by Burkholderia pseudomallei and Burkholderia mallei, respectively, is ongoing and describes various expressed TAAs with immunogenic properties such as BPSL2063 [243] and BimA [244, 245].
Finally, vaccine development against animal pathogenic bacteria expressing TAAs is of high veterinary importance. For example, AhsA (designated according to gene locus ahsA) of Mannheima haemolytica A1, the principal cause of bovine pneumonic pasteurellosis, promotes colonisation and subsequent infection via its ability to bind collagen and, more importantly, is suggested to be immunogenic in calves [246]. Furthermore, the TAA HMTp210 is the major hemagglutinin antigen of Avibacterium paragallinarum, which can cause infectious coryza in poultry. A variable region within HMTp210 is proposed as a candidate for recombinant vaccine production [247–249]. Lastly, Actinobacillus pleuropneumoniae adhesin 1 (Apa1) and 2 (Apa2) are two TAAs expressed on the bacterial surface of Actinobacillus pleuropneumoniae, the causative agent of porcine pleuropneumonia. The main functional head domain, Apa2H1, activates dendritic cells and provides effective protection in mice against lethal infections with A. pleuropneumoniae by both reducing bacterial colonisation and dissemination [250].
Conclusion
Remarkably, it is still only NadA of all TAAs that is used as a main vaccine antigen in the respective multicomponent vaccine 4CMenB. Nonetheless, TAAs largely fulfil the requirements to be considered as potential vaccine antigens. Immunogenicity was demonstrated for many of the TAAs (Table 1). Moreover, seven out of nine already assessed TAAs (YadA, NadA, DsrA, Ata, UspA1-2, UpaG, and SadA) induced a protective host response upon infection with the respective pathogen.
The reason for this ‘scarcity’ of TAAs as vaccine (sub)units may be that research on TAAs itself is still fairly new, especially research towards their applicability as potential vaccines. The current trend to use OMVs in vaccines (as in 4CMenB) and to apply reverse vaccinology to identify new vaccine antigens might give a boost for the usage of TAAs as vaccine antigens.
TAAs showing the highest potential as vaccine targets are Hia of H. influenzae, DsrA of H. ducreyi, Ata of A. baumannii, UpaG of uropathogenic E. coli and EhaG of enterohaemorrhagic E. coli. TAAs that are no longer of major interest as vaccine targets are NhhA of N. meningitidis and UspA1 and UspA2 of M. catarrhalis due to their irregular expression patterns and high degree of diversity, respectively. Other TAAs, including YadA of Y. enterocolitica, Saa of Shiga toxin-producing E. coli, BbadA–C of B. bacilliformis, and VompA and VompB of B. quintana show promising results as potential future vaccine candidates. In conclusion, more extensive research bringing more insights in the functionality and effectiveness of TAAs as vaccines is necessary.
Acknowledgements
This research was supported by the Viral and Bacterial Adhesin Network Training (ViBrANT) Program funded by the European Union’s HORIZON 2020 Research and Innovation Program under the Marie Sklodowska-Curie Grant Agreement No 765042, by the Deutsche Forschungsgemeinschaft [DFG FOR 2251], by the Robert Koch-Institute, Berlin, Germany (Bartonella consiliary laboratory, 1369-354) and by the LOEWE Center DRUID (Novel Drug Targets against Poverty-Related and Neglected Tropical Infectious Diseases, project C2). We thank Stephan Göttig (Frankfurt am Main, Germany), Monika Schütz (Tübingen, Germany) and Ulrich Vogel (Würzburg, Germany) for proofreading this review. We also thank Jürgen Berger and Katharina Hipp (all Max Planck-Institute, Tübingen, Germany) for the electron microscopy. This article is published as part of the Special Issue on “Vibrant ITN”.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Arno Thibau, Email: arno.thibau@kgu.de.
Alexander A. Dichter, Email: alexander.dichter@kgu.de
Diana J. Vaca, Email: diana.vaca@kgu.de
Dirk Linke, Email: dirk.linke@ibv.uio.no.
Adrian Goldman, Email: a.goldman@leeds.ac.uk.
Volkhard A. J. Kempf, Email: volkhard.kempf@kgu.de
References
- 1.Riedel S. Edward Jenner and the history of smallpox and vaccination. Baylor Univ Med Cent Proc. 2005;18:21–25. doi: 10.1080/08998280.2005.11928028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giersing BK, Modjarrad K, Kaslow DC, et al. Report from the World Health Organization’s Product Development for Vaccines Advisory Committee (PDVAC) meeting, Geneva, 7–9th Sep 2015. Vaccine. 2016;34:2865–2869. doi: 10.1016/j.vaccine.2016.02.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giersing BK, Modjarrad K, Kaslow DC, et al. The 2016 vaccine development pipeline: a special issue from the World Health Organization Product Development for Vaccine Advisory Committee (PDVAC) Vaccine. 2016;34:2863–2864. doi: 10.1016/j.vaccine.2016.04.041. [DOI] [PubMed] [Google Scholar]
- 4.Spahn J (Bundesgesundheitsminister) (2019) Impfpflicht soll Kinder vor Masern schützen. In: Bundesministerium für Gesundh. https://www.bundesgesundheitsministerium.de/impfpflicht.html?fbclid=IwAR0aG2Asp6v4c9oWjGOG-v6TJq85dqr2f8UdzQcMrioqS0yjhSO_GqQH7Tw. Accessed 25 Jul 2019
- 5.Gasperini G, Biagini M, Arato V, et al. Outer membrane vesicles (OMV)-based and proteomics-driven antigen selection identifies novel factors contributing to Bordetella pertussis adhesion to epithelial cells. Mol Cell Proteomics. 2018;17:205–215. doi: 10.1074/mcp.RA117.000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark TA, Messonnier NE, Hadler SC. Pertussis control: time for something new? Trends Microbiol. 2012;20:211–213. doi: 10.1016/j.tim.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Wantuch PL, Avci FY. Invasive pneumococcal disease in relation to vaccine type serotypes. Hum Vaccine Immunother. 2019;15:874–875. doi: 10.1080/21645515.2018.1564444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones KE, Patel NG, Levy MA, et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drake JM, Brett TS, Chen S, et al. The statistics of epidemic transitions. PLoS Comput Biol. 2019;15:e1006917. doi: 10.1371/journal.pcbi.1006917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morens DM, Folkers GK, Fauci AS. The challenge of emerging and re-emerging infectious diseases. Nature. 2004;430:242–249. doi: 10.1038/nature02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michalik M, Djahanshiri B, Leo JC, Linke D. Reverse vaccinology: the pathway from genomes and epitope predictions to tailored recombinant vaccines. In: Thomas S, editor. Vaccine design: methods and protocols. New York: Springer New York; 2016. pp. 87–106. [DOI] [PubMed] [Google Scholar]
- 12.Hu Y-F, Zhao D, Yu X-L, et al. Identification of bacterial surface antigens by screening peptide phage libraries using whole bacteria cell-purified antisera. Front Microbiol. 2017;8:82. doi: 10.3389/fmicb.2017.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masignani V, Pizza M, Moxon ER. The development of a vaccine against Meningococcus B using reverse vaccinology. Front Immunol. 2019;10:751. doi: 10.3389/fimmu.2019.00751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Post DMB, Slütter B, Schilling B, et al. Characterization of inner and outer membrane proteins from Francisella tularensis strains LVS and Schu S4 and identification of potential subunit vaccine candidates. MBio. 2017;8:e01592-17. doi: 10.1128/mBio.01592-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van der Pol L, Stork M, van der Ley P. Outer membrane vesicles as platform vaccine technology. Biotechnol J. 2015;10:1689–1706. doi: 10.1002/biot.201400395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linke D, Riess T, Autenrieth IB, et al. Trimeric autotransporter adhesins: variable structure, common function. Trends Microbiol. 2006;14:264–270. doi: 10.1016/j.tim.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Qin W, Wang L, Zhai R, et al. Trimeric autotransporter adhesins contribute to Actinobacillus pleuropneumoniae pathogenicity in mice and regulate bacterial gene expression during interactions between bacteria and porcine primary alveolar macrophages. Antonie Van Leeuwenhoek. 2016;109:51–70. doi: 10.1007/s10482-015-0609-x. [DOI] [PubMed] [Google Scholar]
- 18.Ren D, Pichichero ME. Vaccine targets against Moraxella catarrhalis. Expert Opin Ther Targets. 2016;20:19–33. doi: 10.1517/14728222.2015.1081686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peak IRA, Srikhanta Y, Dieckelmann M, et al. Identification and characterisation of a novel conserved outer membrane protein from Neisseria meningitidis. FEMS Immunol Med Microbiol. 2000;28:329–334. doi: 10.1111/j.1574-695X.2000.tb01494.x. [DOI] [PubMed] [Google Scholar]
- 20.Cordwell SJ, Nouwens AS. Proteome analysis of outer membrane and extracellular proteins from Pseudomonas aeruginosa for vaccine discovery. In: Grandi G, editor. Genomics Proteomics and Vaccines. Chichester, England: Wiley; 2004. pp. 285–304. [Google Scholar]
- 21.Finlay BB, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev. 1997;61:136. doi: 10.1128/.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Casadevall A, Pirofski L. Host-pathogen interactions: redefining the basic concepts of virulence and pathogenicity. Infect Immun. 1999;67:3703. doi: 10.1128/IAI.67.8.3703-3713.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin W, Wang L, Lei L. New findings on the function and potential applications of the trimeric autotransporter adhesin. Antonie Van Leeuwenhoek. 2015;108:1–14. doi: 10.1007/s10482-015-0477-4. [DOI] [PubMed] [Google Scholar]
- 24.Kaiser PO, Linke D, Schwarz H, et al. Analysis of the BadA stalk from Bartonella henselae reveals domain-specific and domain-overlapping functions in the host cell infection process. Cell Microbiol. 2012;14:198–209. doi: 10.1111/j.1462-5822.2011.01711.x. [DOI] [PubMed] [Google Scholar]
- 25.Comanducci M, Bambini S, Brunelli B, et al. NadA, a novel vaccine candidate of Neisseria meningitidis. J Exp Med. 2002;195:1445–1454. doi: 10.1084/jem.20020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Łyskowski A, Leo JC, Goldman A. Structure and biology of trimeric autotransporter adhesins. In: Linke D, Goldman A, editors. Bacterial adhesion: chemistry, biology and physics. Dordrecht: Springer Netherlands; 2011. pp. 143–158. [DOI] [PubMed] [Google Scholar]
- 27.Henderson IR, Navarro-Garcia F, Desvaux M, et al. Type V protein secretion pathway: the autotransporter story. Microbiol Mol Biol Rev. 2004;68:692–744. doi: 10.1128/MMBR.68.4.692-744.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nummelin H, Merckel MC, Leo JC, et al. The Yersinia adhesin YadA collagen-binding domain structure is a novel left-handed parallel β-roll. EMBO J. 2004;23:701–711. doi: 10.1038/sj.emboj.7600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Rourke F, Schmidgen T, Kaiser PO, et al. Adhesins of Bartonella spp. In: Linke D, Goldman A, et al., editors. Bacterial adhesion: chemistry, biology and physics. Dordrecht: Springer Netherlands; 2011. pp. 51–70. [DOI] [PubMed] [Google Scholar]
- 30.Ackermann N, Tiller M, Anding G, et al. Contribution of trimeric autotransporter C-terminal domains of oligomeric coiled-coil adhesin (Oca) family members YadA, UspA1, EibA, and Hia to translocation of the YadA passenger domain and virulence of Yersinia enterocolitica. J Bacteriol. 2008;190:5031–5043. doi: 10.1128/JB.00161-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roggenkamp A, Ackermann N, Jacobi CA, et al. Molecular analysis of transport and oligomerization of the Yersinia enterocolitica adhesin YadA. J Bacteriol. 2003;185:3735–3744. doi: 10.1128/JB.185.13.3735-3744.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leo CJ, Linke D. A unified model for BAM function that takes into account type Vc secretion and species differences in BAM composition. AIMS Microbiol. 2018;4:455–468. doi: 10.3934/microbiol.2018.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bernstein HD. Looks can be deceiving: recent insights into the mechanism of protein secretion by the autotransporter pathway. Mol Microbiol. 2015;97:205–215. doi: 10.1111/mmi.13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leo JC, Grin I, Linke D. Type V secretion: mechanism(s) of autotransport through the bacterial outer membrane. Philos Trans R Soc B Biol Sci. 2012;367:1088–1101. doi: 10.1098/rstb.2011.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rollauer SE, Sooreshjani MA, Noinaj N, Buchanan SK. Outer membrane protein biogenesis in Gram-negative bacteria. Philos Trans R Soc B Biol Sci. 2015;370:20150023. doi: 10.1098/rstb.2015.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chauhan N, Hatlem D, Orwick-Rydmark M, et al. Insights into the autotransport process of a trimeric autotransporter, Yersinia Adhesin A (YadA) Mol Microbiol. 2019;111:844–862. doi: 10.1111/mmi.14195. [DOI] [PubMed] [Google Scholar]
- 37.Pavlova O, Peterson JH, Ieva R, Bernstein HD. Mechanistic link between β barrel assembly and the initiation of autotransporter secretion. Proc Natl Acad Sci. 2013 doi: 10.1073/pnas.1219076110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grosskinsky U, Schütz M, Fritz M, et al. A conserved glycine residue of trimeric autotransporter domains plays a key role in Yersinia adhesin A autotransport. J Bacteriol. 2007;189:9011. doi: 10.1128/JB.00985-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lehr U, Schütz M, Oberhettinger P, et al. C-terminal amino acid residues of the trimeric autotransporter adhesin YadA of Yersinia enterocolitica are decisive for its recognition and assembly by BamA. Mol Microbiol. 2010;78:932–946. doi: 10.1111/j.1365-2958.2010.07377.x. [DOI] [PubMed] [Google Scholar]
- 40.Knowles TJ, Scott-Tucker A, Overduin M, Henderson IR. Membrane protein architects: the role of the BAM complex in outer membrane protein assembly. Nat Rev Microbiol. 2009;7:206. doi: 10.1038/nrmicro2069. [DOI] [PubMed] [Google Scholar]
- 41.Jong WSP, Soprova Z, de Punder K, et al. A structurally informed autotransporter platform for efficient heterologous protein secretion and display. Microb Cell Fact. 2012;11:85. doi: 10.1186/1475-2859-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zinner KM, van Ulsen P, Luirink J, Jong WSP. On display: autotransporter secretion and application. FEMS Microbiol Lett. 2018 doi: 10.1093/femsle/fny165. [DOI] [PubMed] [Google Scholar]
- 43.Meuskens I, Saragliadis A, Leo JC, Linke D. Type V secretion systems: an overview of passenger domain functions. Front Microbiol. 2019;10:1163. doi: 10.3389/fmicb.2019.01163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wells TJ, Tree JJ, Ulett GC, Schembri MA. Autotransporter proteins: novel targets at the bacterial cell surface. FEMS Microbiol Lett. 2007;274:163–172. doi: 10.1111/j.1574-6968.2007.00833.x. [DOI] [PubMed] [Google Scholar]
- 45.Yang X, Wyatt R, Sodroski J. Improved elicitation of neutralizing antibodies against primary human immunodeficiency viruses by soluble stabilized envelope glycoprotein trimers. J Virol. 2001;75:1165. doi: 10.1128/JVI.75.3.1165-1171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pantophlet R, Burton DR. Immunofocusing: antigen engineering to promote the induction of HIV-neutralizing antibodies. Trends Mol Med. 2003;9:468–473. doi: 10.1016/j.molmed.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Kramer U, Rizos K, Apfel H, et al. Autodisplay: development of an efficacious system for surface display of antigenic determinants in Salmonella vaccine strains. Infect Immun. 2003;71:1944–1952. doi: 10.1128/IAI.71.4.1944-1952.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rizos K, Lattemann CT, Bumann D, et al. Autodisplay: efficacious surface exposure of antigenic UreA fragments from Helicobacter pylori in Salmonella vaccine strains. Infect Immun. 2003;71:6320–6328. doi: 10.1128/IAI.71.11.6320-6328.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jose J, Meyer TF. The autodisplay story, from discovery to biotechnical and biomedical applications. Microbiol Mol Biol Rev. 2007;71:600. doi: 10.1128/MMBR.00011-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jong WS, Daleke-Schermerhorn MH, Vikström D, et al. An autotransporter display platform for the development of multivalent recombinant bacterial vector vaccines. Microb Cell Fact. 2014;13:162. doi: 10.1186/s12934-014-0162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.den Berg Van, van Saparoea HB, Houben D, de Jonge MI, et al. Display of recombinant proteins on bacterial outer membrane vesicles by using protein ligation. Appl Environ Microbiol. 2018;84:e02567-17. doi: 10.1128/AEM.02567-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mukherjee O, Singh B, Bayrak B, et al. A fusion protein derived from Moraxella catarrhalis and Neisseria meningitidis aimed for immune modulation of human B cells. Hum Vaccine Immunother. 2015;11:2223–2227. doi: 10.1080/21645515.2015.1034917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feavers IM, Pizza M. Meningococcal protein antigens and vaccines. Vaccine. 2009;27:B42–B50. doi: 10.1016/j.vaccine.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 54.Pizza M, Scarlato V, Masignani V, et al. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science (80-) 2000;287:1816. doi: 10.1126/science.287.5459.1816. [DOI] [PubMed] [Google Scholar]
- 55.Shahid F, Ashraf ST, Ali A. Reverse vaccinology approach to potential vaccine candidates against Acinetobacter baumannii. In: Biswas I, Rather PN, editors. Acinetobacter baumannii: methods and protocols. New York: Springer; 2019. pp. 329–336. [DOI] [PubMed] [Google Scholar]
- 56.Daleke-Schermerhorn MH, Felix T, Soprova Z, et al. Decoration of outer membrane vesicles with multiple antigens by using an autotransporter approach. Appl Environ Microbiol. 2014;80:5854–5865. doi: 10.1128/AEM.01941-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hatlem D, Trunk T, Linke D, Leo JC. Catching a SPY: using the SpyCatcher-SpyTag and related systems for labeling and localizing bacterial proteins. Int J Mol Sci. 2019 doi: 10.3390/ijms20092129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shirley M, Taha M-K. MenB-FHbp meningococcal group B vaccine (Trumenba®): a review in active immunization in individuals aged ≥ 10 years. Drugs. 2018;78:257–268. doi: 10.1007/s40265-018-0869-7. [DOI] [PubMed] [Google Scholar]
- 59.Van Alphen L, Riemens T, Poolman J, Zanen HC. Characteristics of major outer membrane proteins of Haemophilus influenzae. J Bacteriol. 1983;155:878. doi: 10.1128/JB.155.2.878-885.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moran EE, Burden R, Labrie JE, et al. Analysis of the bactericidal response to an experimental Neisseria meningitidis vesicle vaccine. Clin Vaccine Immunol. 2012;19:659–665. doi: 10.1128/CVI.00070-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liguori A, Dello Iacono L, Maruggi G, et al. NadA3 structures reveal undecad coiled coils and LOX1 binding regions competed by meningococcus B vaccine-elicited human antibodies. MBio. 2018;9:e01914–e01918. doi: 10.1128/mBio.01914-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Winter LE, Barenkamp SJ. Immunogenicity of nontypeable Haemophilus influenzae outer membrane vesicles and protective ability in the chinchilla model of otitis media. Clin Vaccine Immunol. 2017;24:e00138-17. doi: 10.1128/CVI.00138-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bentancor LV, Routray A, Bozkurt-Guzel C, et al. Evaluation of the trimeric autotransporter Ata as a vaccine candidate against Acinetobacter baumannii infections. Infect Immun. 2012;80:3381. doi: 10.1128/IAI.06096-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Raghunathan D, Wells TJ, Morris FC, et al. SadA, a trimeric autotransporter from Salmonella enterica serovar Typhimurium, can promote biofilm formation and provides limited protection against infection. Infect Immun. 2011;79:4342. doi: 10.1128/IAI.05592-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aebi C, Lafontaine ER, Cope LD, et al. Phenotypic effect of isogenic UspA1 and UspA2 mutations on Moraxella catarrhalis 035E. Infect Immun. 1998;66:3113. doi: 10.1128/IAI.66.7.3113-3119.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fusco WG, Choudhary NR, Routh PA, et al. The Haemophilus ducreyi trimeric autotransporter adhesin DsrA protects against an experimental infection in the swine model of chancroid. Vaccine. 2014;32:3752–3758. doi: 10.1016/j.vaccine.2014.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rosqvist R, Skurnik M, Wolf-Watz H. Increased virulence of Yersinia pseudotuberculosis by two independent mutations. Nature. 1988;334:522–525. doi: 10.1038/334522a0. [DOI] [PubMed] [Google Scholar]
- 68.Bottone EJ. Yersinia enterocolitica: the charisma continues. Clin Microbiol Rev. 1997;10:257. doi: 10.1128/CMR.10.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nörenberg D, Wieser A, Magistro G, et al. Molecular analysis of a novel Toll/interleukin-1 receptor (TIR)-domain containing virulence protein of Y. pseudotuberculosis among Far East scarlet-like fever serotype I strains. Int J Med Microbiol. 2013;303:583–594. doi: 10.1016/j.ijmm.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 70.Grassl GA, Bohn E, Müller Y, et al. Interaction of Yersinia enterocolitica with epithelial cells: invasin beyond invasion. Int J Med Microbiol. 2003;293:41–54. doi: 10.1078/1438-4221-00243. [DOI] [PubMed] [Google Scholar]
- 71.Sun W, Singh AK. Plague vaccine: recent progress and prospects. npj Vacc. 2019;4:11. doi: 10.1038/s41541-019-0105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Demeure CE, Derbise A, Carniel E. Oral vaccination against plague using Yersinia pseudotuberculosis. Chem Biol Interact. 2017;267:89–95. doi: 10.1016/j.cbi.2016.03.030. [DOI] [PubMed] [Google Scholar]
- 73.Galimand M, Guiyoule A, Gerbaud G, et al. Multidrug resistance in Yersinia pestis mediated by a transferable plasmid. N Engl J Med. 1997;337:677–681. doi: 10.1056/NEJM199709043371004. [DOI] [PubMed] [Google Scholar]
- 74.Welch TJ, Fricke WF, McDermott PF, et al. Multiple antimicrobial resistance in plague: an emerging public health risk. PLoS One. 2007;2:e309. doi: 10.1371/journal.pone.0000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Quenee LE, Ciletti NA, Elli D, et al. Prevention of pneumonic plague in mice, rats, guinea pigs and non-human primates with clinical grade rV10, rV10-2 or F1-V vaccines. Vaccine. 2011;29:6572–6583. doi: 10.1016/J.VACCINE.2011.06.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Noll A, Autenrieth IB. Immunity against Yersinia enterocolitica by vaccination with Yersinia HSP60 immunostimulating complexes or Yersinia HSP60 plus interleukin-12. Infect Immun. 1996;64:2955. doi: 10.1128/IAI.64.8.2955-2961.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Palmer LE, Hobbie S, Galán JE, Bliska JB. YopJ of Yersinia pseudotuberculosis is required for the inhibition of macrophage TNF-α production and downregulation of the MAP kinases p38 and JNK. Mol Microbiol. 1998;27:953–965. doi: 10.1046/j.1365-2958.1998.00740.x. [DOI] [PubMed] [Google Scholar]
- 78.Singh AK, Kingston JJ, Gupta SK, Batra HV. Recombinant bivalent fusion protein rVE induces CD4 + and CD8 + T-Cell mediated memory immune response for protection against Yersinia enterocolitica infection. Front Microbiol. 2015;6:1407. doi: 10.3389/fmicb.2015.01407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Andersson JA, Sha J, Erova TE, et al. Identification of new virulence factors and vaccine candidates for Yersinia pestis. Front Cell Infect Microbiol. 2017;7:448. doi: 10.3389/fcimb.2017.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bölin I, Wolf-Watz H. Molecular cloning of the temperature-inducible outer membrane protein 1 of Yersinia pseudotuberculosis. Infect Immun. 1984;43:72. doi: 10.1128/IAI.43.1.72-78.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bancerz-Kisiel A, Pieczywek M, Łada P, Szweda W. The most important virulence markers of Yersinia enterocolitica and their role during infection. Genes (Basel) 2018 doi: 10.3390/genes9050235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bölin I, Norlander L, Wolf-Watz H. Temperature-inducible outer membrane protein of Yersinia pseudotuberculosis and Yersinia enterocolitica is associated with the virulence plasmid. Infect Immun. 1982;37:506. doi: 10.1128/IAI.37.2.506-512.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Portnoy DA, Moseley SL, Falkow S. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect Immun. 1981;31:775. doi: 10.1128/IAI.31.2.775-782.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heise T, Dersch P. Identification of a domain in Yersinia virulence factor YadA that is crucial for extracellular matrix-specific cell adhesion and uptake. Proc Natl Acad Sci USA. 2006;103:3375. doi: 10.1073/pnas.0507749103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Han YW, Miller VL. Reevaluation of the virulence phenotype of the inv yadA double mutants of Yersinia pseudotuberculosis. Infect Immun. 1997;65:327. doi: 10.1128/IAI.65.1.327-330.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mühlenkamp MC, Hallström T, Autenrieth IB, et al. Vitronectin binds to a specific stretch within the head region of Yersinia adhesin A and thereby modulates Yersinia enterocolitica host interaction. J Innate Immun. 2017;9:33–51. doi: 10.1159/000449200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roggenkamp A, Ruckdeschel K, Leitritz L, et al. Deletion of amino acids 29 to 81 in adhesion protein YadA of Yersinia enterocolitica serotype O:8 results in selective abrogation of adherence to neutrophils. Infect Immun. 1996;64:2506. doi: 10.1128/IAI.64.7.2506-2514.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schutz M, Weiss EM, Schindler M, et al. Trimer stability of YadA is critical for virulence of Yersinia enterocolitica. Infect Immun. 2010;78:2677–2690. doi: 10.1128/IAI.01350-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schmid Y, Grassl GA, Buhler OT, et al. Yersinia enterocolitica adhesin A induces production of interleukin-8 in epithelial cells. Infect Immun. 2004;72:6780–6789. doi: 10.1128/IAI.72.12.6780-6789.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bohn E, Müller S, Lauber J, et al. Gene expression patterns of epithelial cells modulated by pathogenicity factors of Yersinia enterocolitica. Cell Microbiol. 2004;6:129–141. doi: 10.1046/j.1462-5822.2003.00346.x. [DOI] [PubMed] [Google Scholar]
- 91.Hanski C, Naumann M, Hahn H, Riecken EO. Determinants of invasion and survival of Yersinia enterocolitica in intestinal tissue. Med Microbiol Immunol. 1989;178:289–296. doi: 10.1007/BF00191063. [DOI] [PubMed] [Google Scholar]
- 92.Sory MP, Tollenaere J, Laszlo C, et al. Detection of pYV + Yersinia enterocolitica isolates by P1 slide agglutination. J Clin Microbiol. 1990;28:2403. doi: 10.1128/JCM.28.11.2403-2408.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.El Tahir Y. YadA, the multifaceted adhesin. Int J Med Microbiol. 2001;291:209–218. doi: 10.1078/1438-4221-00119. [DOI] [PubMed] [Google Scholar]
- 94.El Tahir Y, Kuusela P, Skurnik M. Functional mapping of the Yersinia enterocolitica adhesin YadA. Identification of eight NSVAIG-S motifs in the amino-terminal half of the protein involved in collagen binding. Mol Microbiol. 2000;37:192–206. doi: 10.1046/j.1365-2958.2000.01992.x. [DOI] [PubMed] [Google Scholar]
- 95.Tsugo K, Nakamura S, Yamanaka H, Une Y. A study on the efficacy of the recombinant Yersinia adhesin A vaccine against yersiniosis in the early phase. J Vet Med Sci. 2017;79:855–863. doi: 10.1292/jvms.16-0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fagnocchi L, Biolchi A, Ferlicca F, et al. Transcriptional regulation of the nadA gene in Neisseria meningitidis impacts the prediction of coverage of a multicomponent meningococcal serogroup B vaccine. Infect Immun. 2013;81:560–569. doi: 10.1128/IAI.01085-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Peak IR, Srikhanta YN, Weynants VE, et al. Evaluation of truncated NhhA protein as a candidate meningococcal vaccine antigen. PLoS One. 2013;8:1–9. doi: 10.1371/journal.pone.0072003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Malito E, Biancucci M, Faleri A, et al. Structure of the meningococcal vaccine antigen NadA and epitope mapping of a bactericidal antibody. Proc Natl Acad Sci. 2014;111:17128–17133. doi: 10.1073/pnas.1419686111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sjölinder H, Eriksson J, Maudsdotter L, et al. Meningococcal outer membrane protein NhhA is essential for colonization and disease by preventing phagocytosis and complement attack. Infect Immun. 2008;76:5412. doi: 10.1128/IAI.00478-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tzeng Y-L, Stephens DS. Epidemiology and pathogenesis of Neisseria meningitidis. Microbes Infect. 2000;2:687–700. doi: 10.1016/S1286-4579(00)00356-7. [DOI] [PubMed] [Google Scholar]
- 101.Tikhomirov E, Santamaria M, Esteves K. Meningococcal disease: public health burden and control. World Health Stat Q. 1997;50:170–177. [PubMed] [Google Scholar]
- 102.Pollard AJ. Global epidemiology of meningococcal disease and vaccine efficacy. Pediatr Infect Dis J. 2004;23:274–279. doi: 10.1097/01.inf.0000147642.85129.05. [DOI] [PubMed] [Google Scholar]
- 103.Dbaibo G, Khinkarly R, Hedari C. Meningococcal serogroups A, C, W-135, and Y tetanus toxoid conjugate vaccine: a new conjugate vaccine against invasive meningococcal disease. Infect Drug Resist. 2014;7:85. doi: 10.2147/IDR.S36243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Harrison LH, Pelton SI, Wilder-Smith A, et al. The Global Meningococcal Initiative: Recommendations for reducing the global burden of meningococcal disease. Vaccine. 2011;29:3363–3371. doi: 10.1016/j.vaccine.2011.02.058. [DOI] [PubMed] [Google Scholar]
- 105.Borrow R, Alarcón P, Carlos J, et al. The global meningococcal initiative: global epidemiology, the impact of vaccines on meningococcal disease and the importance of herd protection. Expert Rev Vacc. 2017;16:313–328. doi: 10.1080/14760584.2017.1258308. [DOI] [PubMed] [Google Scholar]
- 106.Finne J, Bitter-Suermann D, Goridis C, Finne U. An IgG monoclonal antibody to group B meningococci cross-reacts with developmentally regulated polysialic acid units of glycoproteins in neural and extraneural tissues. J Immunol. 1987;138:4402. [PubMed] [Google Scholar]
- 107.Unkmeir A, Kämmerer U, Stade A, et al. Lipooligosaccharide and polysaccharide capsule: virulence factors of Neisseria meningitidis that determine meningococcal interaction with human dendritic cells. Infect Immun. 2002;70:2454. doi: 10.1128/IAI.70.5.2454-2462.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Simões MJ, Bettencourt C, De Paola R, et al. Predicted strain coverage of a meningococcal multicomponent vaccine (4CMenB) in Portugal. PLoS One. 2017;12:e0176177. doi: 10.1371/journal.pone.0176177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Perez JL, Absalon J, Beeslaar J, et al. From research to licensure and beyond: clinical development of MenB-FHbp, a broadly protective meningococcal B vaccine. Expert Rev Vacc. 2018;17:461–477. doi: 10.1080/14760584.2018.1483726. [DOI] [PubMed] [Google Scholar]
- 110.Tavano R, Franzoso S, Cecchini P, et al. The membrane expression of Neisseria meningitidis adhesin A (NadA) increases the proimmune effects of MenB OMVs on human macrophages, compared with NadA–OMVs, without further stimulating their proinflammatory activity on circulating monocytes. J Leukoc Biol. 2009;86:143–153. doi: 10.1189/jlb.0109030. [DOI] [PubMed] [Google Scholar]
- 111.Pizza M, Rappuoli R. Neisseria meningitidis: pathogenesis and immunity. Curr Opin Microbiol. 2015;23:68–72. doi: 10.1016/j.mib.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 112.Capecchi B, Adu-Bobie J, Di Marcello F, et al. Neisseria meningitidis NadA is a new invasin which promotes bacterial adhesion to and penetration into human epithelial cells. Mol Microbiol. 2004;55:687–698. doi: 10.1111/j.1365-2958.2004.04423.x. [DOI] [PubMed] [Google Scholar]
- 113.Nägele V, Heesemann J, Schielke S, et al. Neisseria meningitidis adhesin NadA targets β1 integrins. J Biol Chem. 2011;286:20536–20546. doi: 10.1074/jbc.M110.188326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mazzon C, Baldani-Guerra B, Cecchini P, et al. IFN- and R-848 dependent activation of human monocyte-derived dendritic cells by Neisseria meningitidis adhesin A. J Immunol. 2007;179:3904–3916. doi: 10.4049/jimmunol.179.6.3904. [DOI] [PubMed] [Google Scholar]
- 115.Tavano R, Capecchi B, Montanari P, et al. Mapping of the Neisseria meningitidis NadA cell-binding site: relevance of predicted α-helices in the NH2-terminal and dimeric coiled-coil regions. J Bacteriol. 2011;193:107. doi: 10.1128/JB.00430-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bowe F, Lavelle EC, McNeela EA, et al. Mucosal vaccination against serogroup B meningococci: induction of bactericidal antibodies and cellular immunity following intranasal immunization with NadA of Neisseria meningitidis and mutants of Escherichia coli heat-labile enterotoxin. Infect Immun. 2004;72:4052–4060. doi: 10.1128/IAI.72.7.4052-4060.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Comanducci M, Bambini S, Caugant DA, et al. NadA diversity and carriage in Neisseria meningitidis. Infect Immun. 2004;72:4217–4223. doi: 10.1128/IAI.72.7.4217-4223.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bambini S, De Chiara M, Muzzi A, et al. Neisseria adhesin A variation and revised nomenclature scheme. Clin Vaccine Immunol. 2014;21:966–971. doi: 10.1128/CVI.00825-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lucidarme J, Comanducci M, Findlow J, et al. Characterization of fHbp, nhba (gna2132), nadA, porA, sequence type (ST), and genomic presence of IS1301 in group B Meningococcal ST269 clonal complex isolates from England and Wales. J Clin Microbiol. 2009;47:3577–3585. doi: 10.1128/JCM.00936-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bambini S, Muzzi A, Olcen P, et al. Distribution and genetic variability of three vaccine components in a panel of strains representative of the diversity of serogroup B meningococcus. Vaccine. 2009;27:2794–2803. doi: 10.1016/j.vaccine.2009.02.098. [DOI] [PubMed] [Google Scholar]
- 121.Shahsavani N, Sheikhha MH, Yousefi H, Sefid F. In silico homology modeling and epitope prediction of NadA as a potential vaccine candidate in Neisseria meningitidis. Int J Mol Cell Med. 2018;7:53–68. doi: 10.22088/IJMCM.BUMS.7.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bertoldi I, Faleri A, Galli B, et al. Exploiting chimeric human antibodies to characterize a protective epitope of Neisseria adhesin A, one of the Bexsero vaccine components. FASEB J. 2016;30:93–101. doi: 10.1096/fj.15-273813. [DOI] [PubMed] [Google Scholar]
- 123.Litt DJ, Savino S, Beddek A, et al. Putative vaccine antigens from Neisseria meningitidis recognized by serum antibodies of young children convalescing after meningococcal disease. J Infect Dis. 2004;190:1488–1497. doi: 10.1086/424464. [DOI] [PubMed] [Google Scholar]
- 124.Giuliani MM, Adu-Bobie J, Comanducci M, et al. A universal vaccine for serogroup B meningococcus. Proc Natl Acad Sci. 2006;103:10834–10839. doi: 10.1073/pnas.0603940103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Scarselli M, Serruto D, Montanari P, et al. Neisseria meningitidis NhhA is a multifunctional trimeric autotransporter adhesin. Mol Microbiol. 2006;61:631–644. doi: 10.1111/j.1365-2958.2006.05261.x. [DOI] [PubMed] [Google Scholar]
- 126.Weynants VE, Feron CM, Goraj KK, et al. Additive and synergistic bactericidal activity of antibodies directed against minor outer membrane proteins of Neisseria meningitidis. Infect Immun. 2007;75:5434–5442. doi: 10.1128/IAI.00411-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gasparini R, Panatto D, Bragazzi NL, et al. How the knowledge of interactions between Meningococcus and the human immune system has been used to prepare effective Neisseria meningitidis vaccines. J Immunol Res. 2015;2015:1–26. doi: 10.1155/2015/189153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Echenique-Rivera H, Brunelli B, Scarselli M, et al. A naturally occurring single-residue mutation in the translocator domain of Neisseria meningitidis NhhA affects trimerization, surface localization, and adhesive capabilities. Infect Immun. 2011;79:4308–4321. doi: 10.1128/IAI.00198-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Singh B, Al Jubair T, Mörgelin M, et al. Haemophilus influenzae surface fibril (Hsf) is a unique twisted hairpin-like trimeric autotransporter. Int J Med Microbiol. 2015;305:27–37. doi: 10.1016/j.ijmm.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 130.Barenkamp SJ, St. Geme JW., III Identification of a second family of high-molecular-weight adhesion proteins expressed by non-typable Haemophilus influenzae. Mol Microbiol. 1996;19:1215–1223. doi: 10.1111/j.1365-2958.1996.tb02467.x. [DOI] [PubMed] [Google Scholar]
- 131.Singh B, Su Y-C, Al-Jubair T, et al. A fine-tuned interaction between trimeric autotransporter Haemophilus surface fibrils and vitronectin leads to serum resistance and adherence to respiratory epithelial cells. Infect Immun. 2014;82:2378–2389. doi: 10.1128/IAI.01636-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.St. Geme JW, Cutter D, Barenkamp SJ. Characterization of the genetic locus encoding Haemophilus influenzae type b surface fibrils. J Bacteriol. 1996;178:6281. doi: 10.1128/jb.178.21.6281-6287.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.St. Geme JW, Kumar VV, Cutter D, Barenkamp SJ. Prevalence and distribution of the hmw and hia genes and the HMW and Hia adhesins among genetically diverse strains of nontypeable Haemophilus influenzae. Infect Immun. 1998;66:364. doi: 10.1128/IAI.66.1.364-368.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Phillips ZN, Brizuela C, Jennison AV, et al. Analysis of invasive nontypeable Haemophilus influenzae isolates reveals selection for the expression state of particular phase-variable lipooligosaccharide biosynthetic genes. Infect Immun. 2019 doi: 10.1128/IAI.00093-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Peltola H, Käythy H, Sivonen A, Mäkelä PH. Haemophilus influenzae type b capsular polysaccharide vaccine in children: a double-blind field study of 100,000 vaccinees 3 months to 5 years of age in Finland. Pediatrics. 1977;60:730. [PubMed] [Google Scholar]
- 136.Ladhani SN. Two decades of experience with the Haemophilus influenzae serotype b conjugate vaccine in the United Kingdom. Clin Ther. 2012;34:385–399. doi: 10.1016/j.clinthera.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 137.Morris SK, Moss WJ, Halsey N. Haemophilus influenzae type b conjugate vaccine use and effectiveness. Lancet Infect Dis. 2008;8:435–443. doi: 10.1016/S1473-3099(08)70152-X. [DOI] [PubMed] [Google Scholar]
- 138.Poolman JT, Bakaletz L, Cripps A, et al. Developing a nontypeable Haemophilus influenzae (NTHi) vaccine. Vaccine. 2000;19:S108–S115. doi: 10.1016/S0264-410X(00)00288-7. [DOI] [PubMed] [Google Scholar]
- 139.Ysebaert C, Denoël P, Weynants V, et al. A Protein E-PilA fusion protein shows vaccine potential against nontypeable Haemophilus influenzae in mice and chinchillas. Infect Immun. 2019 doi: 10.1128/IAI.00345-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Watson ME, Jr, Nelson KL, Nguyen V, et al. Adhesin genes and serum resistance in Haemophilus influenzae type f isolates. J Med Microbiol. 2013;62:514–524. doi: 10.1099/jmm.0.052175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Van Damme P, Leroux-Roels G, Vandermeulen C, et al. Safety and immunogenicity of non-typeable Haemophilus influenzae-Moraxella catarrhalis vaccine. Vaccine. 2019;37:3113–3122. doi: 10.1016/j.vaccine.2019.04.041. [DOI] [PubMed] [Google Scholar]
- 142.Hallström T, Trajkovska E, Forsgren A, Riesbeck K. Haemophilus influenzae surface fibrils contribute to serum resistance by interacting with vitronectin. J Immunol. 2006;177:430. doi: 10.4049/jimmunol.177.1.430. [DOI] [PubMed] [Google Scholar]
- 143.Yeo H, Cotter SE, Laarmann S, et al. Structural basis for host recognition by the Haemophilus influenzae Hia autotransporter. EMBO J. 2004;23:1245. doi: 10.1038/sj.emboj.7600142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Laarmann S, Cutter D, Juehne T, et al. The Haemophilus influenzae Hia autotransporter harbours two adhesive pockets that reside in the passenger domain and recognize the same host cell receptor. Mol Microbiol. 2002;46:731–743. doi: 10.1046/j.1365-2958.2002.03189.x. [DOI] [PubMed] [Google Scholar]
- 145.Winter LE, Barenkamp SJ. Antibodies specific for the Hia adhesion proteins of nontypeable Haemophilus influenzae mediate opsonophagocytic activity. Clin Vaccine Immunol. 2009;16:1040–1046. doi: 10.1128/CVI.00090-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Winter LE, Barenkamp SJ. Antibodies to the HMW1/HMW2 and Hia adhesins of nontypeable Haemophilus influenzae mediate broad-based opsonophagocytic killing of homologous and heterologous strains. Clin Vaccine Immunol. 2014;21:613. doi: 10.1128/CVI.00772-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fusco WG, Choudhary NR, Stewart SM, et al. Defining potential vaccine targets of Haemophilus ducreyi trimeric autotransporter adhesin DsrA. Monoclon Antib Immunodiagn Immunother. 2015;34:73–82. doi: 10.1089/mab.2014.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Griesenauer B, Tran TM, Fortney KR, et al. Determination of an interaction network between an extracellular bacterial pathogen and the human host. MBio. 2019 doi: 10.1128/mBio.01193-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Samo M, Choudhary NR, Riebe KJ, et al. Immunization with the Haemophilus ducreyi trimeric autotransporter adhesin DsrA with alum, CpG or imiquimod generates a persistent humoral immune response that recognizes the bacterial surface. Vaccine. 2016;34:1193–1200. doi: 10.1016/j.vaccine.2016.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.White CD, Leduc I, Olsen B, et al. Haemophilus ducreyi outer membrane determinants, including DsrA, define two clonal populations. Infect Immun. 2005;73:2387. doi: 10.1128/IAI.73.4.2387-2399.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Klotz P, Göttig S, Leidner U, et al. Carbapenem-resistance and pathogenicity of bovine Acinetobacter indicus-like isolates. PLoS One. 2017;12:1–18. doi: 10.1371/journal.pone.0171986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bagozzi D, Lindmeier C (2019) In the face of slow progress, WHO offers a new tool and sets a target to accelerate action against antimicrobial resistance. WHO Newsl
- 154.Davies L O, Bennett S (2017) Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. WHO Newsl 7
- 155.Garcia-Quintanilla M, McConnell MRPMJ. First steps towards a vaccine against Acinetobacter baumannii. Curr Pharm Biotechnol. 2013;14:897–902. doi: 10.2174/1389201014666131226123511. [DOI] [PubMed] [Google Scholar]
- 156.Ni Z, Chen Y, Ong E, He Y. Antibiotic resistance determinant-focused Acinetobacter baumannii vaccine designed using reverse vaccinology. Int J Mol Sci. 2017 doi: 10.3390/ijms18020458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.McConnell MJ, Domínguez-Herrera J, Smani Y, et al. Vaccination with outer membrane complexes elicits rapid protective immunity to multidrug-resistant Acinetobacter baumannii. Infect Immun. 2011;79:518–526. doi: 10.1128/IAI.00741-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bentancor LV, Camacho-Peiro A, Bozkurt-Guzel C, et al. Identification of Ata, a multifunctional trimeric autotransporter of Acinetobacter baumannii. J Bacteriol. 2012;194:3950. doi: 10.1128/JB.06769-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Weidensdorfer M, Ishikawa M, Hori K, et al. The Acinetobacter trimeric autotransporter adhesin Ata controls key virulence traits of Acinetobacter baumannii. Virulence. 2019;10:68–81. doi: 10.1080/21505594.2018.1558693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Weidensdorfer M, Chae JI, Makobe C, et al. Analysis of endothelial adherence of Bartonella henselae and Acinetobacter baumannii using a dynamic human ex vivo infection model. Infect Immun. 2016;84:711–722. doi: 10.1128/IAI.01502-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Helminen ME, Maciver I, Latimer JL, et al. A Large, antigenically conserved protein on the surface of Moraxella catarrhalis is a target for protective antibodies. J Infect Dis. 1994;170:867–872. doi: 10.1093/infdis/170.4.867. [DOI] [PubMed] [Google Scholar]
- 162.Blakeway LV, Tan A, Peak IRA, Seib KL. Virulence determinants of Moraxella catarrhalis: distribution and considerations for vaccine development. Microbiology. 2017;163:1371–1384. doi: 10.1099/mic.0.000523. [DOI] [PubMed] [Google Scholar]
- 163.Verhaegh SJC, Snippe ML, Levy F, et al. Colonization of healthy children by Moraxella catarrhalis is characterized by genotype heterogeneity, virulence gene diversity and co-colonization with Haemophilus influenzae. Microbiology. 2011;157:169–178. doi: 10.1099/mic.0.042929-0. [DOI] [PubMed] [Google Scholar]
- 164.Verduin CM, Hol C, Fleer A, et al. Moraxella catarrhalis: from emerging to established pathogen. Clin Microbiol Rev. 2002;15:125–144. doi: 10.1128/CMR.15.1.125-144.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Perez AC, Murphy TF. Potential impact of a Moraxella catarrhalis vaccine in COPD. Vaccine. 2017 doi: 10.1016/j.vaccine.2016.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Monasta L, Ronfani L, Marchetti F, et al. Burden of disease caused by otitis media: systematic review and global estimates. PLoS One. 2012;7:1–12. doi: 10.1371/journal.pone.0036226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370:765–773. doi: 10.1016/S0140-6736(07)61380-4. [DOI] [PubMed] [Google Scholar]
- 168.Perez AC, Murphy TF. A Moraxella catarrhalis vaccine to protect against otitis media and exacerbations of COPD: an update on current progress and challenges. Hum Vaccin Immunother. 2017;13:2322–2331. doi: 10.1080/21645515.2017.1356951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Murphy TF, Bartlett JG, File TM, Bond S (2019) Moraxella catarrhalis infections. In: UpToDate—Wolter Kluwen. https://www.uptodate.com/contents/moraxella-catarrhalis-infections#H432138274. Accessed 29 Jul 2019
- 170.Ruckdeschel EA, Kirkham C, Lesse AJ, et al. Mining the Moraxella catarrhalis genome: identification of potential vaccine antigens expressed during human infection. Infect Immun. 2008;76:1599–1607. doi: 10.1128/IAI.01253-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.McMichael JC. Vaccines for Moraxella catarrhalis. Vaccine. 2000;19:S101–S107. doi: 10.1016/S0264-410X(00)00287-5. [DOI] [PubMed] [Google Scholar]
- 172.Cope LD, Lafontaine ER, Slaughter CA, et al. Characterization of the Moraxella catarrhalis uspA1 and uspA2 genes and their encoded products. J Bacteriol. 1999;181:4026–4034. doi: 10.1128/JB.181.13.4026-4034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chen D, McMichael JC, VanDerMeid KR, et al. Evaluation of purified UspA from Moraxella catarrhalis as a vaccine in a murine model after active immunization. Infect Immun. 1996;64:1900–1905. doi: 10.1128/IAI.64.6.1900-1905.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Nordström T, Blom AM, Tan TT, et al. Ionic binding of C3 to the human pathogen Moraxella catarrhalis is a unique mechanism for combating innate immunity. J Immunol. 2005;175:3628. doi: 10.4049/jimmunol.175.6.3628. [DOI] [PubMed] [Google Scholar]
- 175.Murphy TF, Brauer AL, Aebi C, Sethi S. Antigenic specificity of the mucosal antibody response to Moraxella catarrhalis in chronic obstructive pulmonary disease. Infect Immun. 2005;73:8161–8166. doi: 10.1128/IAI.73.12.8161-8166.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Murphy TF, Brauer AL, Aebi C, Sethi S. Identification of surface antigens of Moraxella catarrhalis as targets of human serum antibody responses in chronic obstructive pulmonary disease. Infect Immun. 2005;73:3471–3478. doi: 10.1128/IAI.73.6.3471-3478.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Meier PS, Troller R, Grivea IN, et al. The outer membrane proteins UspA1 and UspA2 of Moraxella catarrhalis are highly conserved in nasopharyngeal isolates from young children. Vaccine. 2002;20:1754–1760. doi: 10.1016/S0264-410X(02)00030-0. [DOI] [PubMed] [Google Scholar]
- 178.Hays JP, van der Schee C, Loogman A, et al. Total genome polymorphism and low frequency of intra-genomic variation in the uspA1 and uspA2 genes of Moraxella catarrhalis in otitis prone and non-prone children up to 2 years of age: Consequences for vaccine design? Vaccine. 2003;21:1118–1124. doi: 10.1016/S0264-410X(02)00522-4. [DOI] [PubMed] [Google Scholar]
- 179.Brooks MJ, Sedillo JL, Wagner N, et al. Modular arrangement of allelic variants explains the divergence in Moraxella catarrhalis UspA protein function. Infect Immun. 2008;76:5330–5340. doi: 10.1128/IAI.00573-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hill DJ, Whittles C, Virji M. A novel group of Moraxella catarrhalis UspA proteins mediates cellular adhesion via CEACAMs and vitronectin. PLoS One. 2012;7:1–15. doi: 10.1371/journal.pone.0045452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sandt CH, Hill CW. Four different genes responsible for nonimmune immunoglobulin-binding activities within a single strain of Escherichia coli. Infect Immun. 2000;68:2205. doi: 10.1128/IAI.68.4.2205-2214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Paton AW, Srimanote P, Woodrow MC, Paton JC. Characterization of Saa, a novel autoagglutinating adhesin produced by locus of enterocyte effacement-negative shiga-toxigenic Escherichia coli strains that are virulent for humans. Infect Immun. 2001;69:6999–7009. doi: 10.1128/IAI.69.11.6999-7009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Valle J, Mabbett AN, Ulett GC, et al. UpaG, a new member of the trimeric autotransporter family of adhesins in uropathogenic Escherichia coli. J Bacteriol. 2008;190:4147. doi: 10.1128/JB.00122-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Totsika M, Wells TJ, Beloin C, et al. Molecular characterization of the EhaG and UpaG trimeric autotransporter proteins from pathogenic Escherichia coli. Appl Environ Microbiol. 2012;78:2179. doi: 10.1128/AEM.06680-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Nesta B, Pizza M. Vaccines against Escherichia coli. In: Frankel G, Ron EZ, editors. Escherichia coli, a versatile pathogen. Cham: Springer International Publishing; 2018. pp. 213–242. [Google Scholar]
- 186.Rojas-Lopez M, Monterio R, Pizza M, et al. Intestinal pathogenic Escherichia coli: insights for vaccine development. Front Microbiol. 2018 doi: 10.3389/fmicb.2018.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Leach S, Lundgren A, Carlin N, et al. Cross-reactivity and avidity of antibody responses induced in humans by the oral inactivated multivalent enterotoxigenic Escherichia coli (ETEC) vaccine ETVAX. Vaccine. 2017;35:3966–3973. doi: 10.1016/j.vaccine.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 188.Wagenlehner FM, Naber KG. A step further in a vaccine for Escherichia coli. Lancet Infect Dis. 2019;19:565–567. doi: 10.1016/S1473-3099(19)30069-6. [DOI] [PubMed] [Google Scholar]
- 189.Frenck RW, Ervin J, Chu L, et al. Safety and immunogenicity of a vaccine for extra-intestinal pathogenic Escherichia coli (ESTELLA): a phase 2 randomised controlled trial. Lancet Infect Dis. 2019;19:631–640. doi: 10.1016/S1473-3099(18)30803-X. [DOI] [PubMed] [Google Scholar]
- 190.Qadri F, Svennerholm A-M, Faruque ASG, Sack RB. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin Microbiol Rev. 2005;18:465. doi: 10.1128/CMR.18.3.465-483.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bourgeois AL, Wierzba TF, Walker RI. Status of vaccine research and development for enterotoxigenic Escherichia coli. Vaccine. 2016;34:2880–2886. doi: 10.1016/j.vaccine.2016.02.076. [DOI] [PubMed] [Google Scholar]
- 192.Walker RI. An assessment of enterotoxigenic Escherichia coli and Shigella vaccine candidates for infants and children. Vaccine. 2015;33:954–965. doi: 10.1016/j.vaccine.2014.11.049. [DOI] [PubMed] [Google Scholar]
- 193.Fleckenstein J, Sheikh A, Qadri F. Novel antigens for enterotoxigenic Escherichia coli vaccines. Expert Rev Vaccines. 2014;13:631–639. doi: 10.1586/14760584.2014.905745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Marandi BHG, Zolfaghari MR, Kazemi R, et al. Immunization against Vibrio cholerae, ETEC, and EHEC with chitosan nanoparticle containing LSC chimeric protein. Microb Pathog. 2019;134:103600. doi: 10.1016/j.micpath.2019.103600. [DOI] [PubMed] [Google Scholar]
- 195.Durant L, Metais A, Soulama-Mouze C, et al. Identification of candidates for a subunit vaccine against extraintestinal pathogenic Escherichia coli. Infect Immun. 2007;75:1916. doi: 10.1128/IAI.01269-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Fingermann M, Avila L, De Marco MB, et al. OMV-based vaccine formulations against Shiga toxin producing Escherichia coli strains are both protective in mice and immunogenic in calves. Hum Vaccin Immunother. 2018;14:2208–2213. doi: 10.1080/21645515.2018.1490381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Leo JC, Lyskowski A, Hattula K, et al. The structure of E. coli IgG-binding protein D suggests a general model for bending and binding in trimeric autotransporter adhesins. Structure. 2011;19:1021–1030. doi: 10.1016/j.str.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 198.Merkel V, Ohder B, Bielaszewska M, et al. Distribution and phylogeny of immunoglobulin-binding protein G in shiga toxin-producing Escherichia coli and its association with adherence phenotypes. Infect Immun. 2010;78:3625–3636. doi: 10.1128/IAI.00006-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lu Y, Iyoda S, Satou H, et al. A new immunoglobulin-binding protein, EibG, is responsible for the chain-like adhesion phenotype of locus of enterocyte effacement-negative, shiga toxin-producing Escherichia coli. Infect Immun. 2006;74:5747. doi: 10.1128/IAI.00724-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Bardiau M, Grégoire F, Muylaert A, et al. Enteropathogenic (EPEC), enterohaemorragic (EHEC) and verotoxigenic (VTEC) Escherichia coli in wild cervids. J Appl Microbiol. 2010;109:2214–2222. doi: 10.1111/j.1365-2672.2010.04855.x. [DOI] [PubMed] [Google Scholar]
- 201.Touchon M, Hoede C, Tenaillon O, et al. Organised genome dynamics in the Escherichia coli species results in highly diverse adaptive paths. PLoS Genet. 2009;5:1–25. doi: 10.1371/journal.pgen.1000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Leo JC, Goldman A. The immunoglobulin-binding Eib proteins from Escherichia coli are receptors for IgG Fc. Mol Immunol. 2009;46:1860–1866. doi: 10.1016/j.molimm.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 203.Chaudhuri RR, Sebaihia M, Hobman JL, et al. Complete genome sequence and comparative metabolic profiling of the prototypical enteroaggregative Escherichia coli strain 042. PLoS One. 2010;5:1–19. doi: 10.1371/journal.pone.0008801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hartmann MD, Grin I, Dunin-Horkawicz S, et al. Complete fiber structures of complex trimeric autotransporter adhesins conserved in enterobacteria. Proc Natl Acad Sci. 2012;109:20907. doi: 10.1073/pnas.1211872110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hartmann MD, Ridderbusch O, Zeth K, et al. A coiled-coil motif that sequesters ions to the hydrophobic core. Proc Natl Acad Sci. 2009;106:16950. doi: 10.1073/pnas.0907256106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yue M, Schifferli D. Allelic variation in Salmonella: an underappreciated driver of adaptation and virulence. Front Microbiol. 2014;4:419. doi: 10.3389/fmicb.2013.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.European Food Safety Authority and European Centre for Disease Prevention and Control (EFSA and ECDC) The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018;16:e05500. doi: 10.2903/j.efsa.2018.5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Andino A, Hanning I. Salmonella enterica: survival, colonization, and virulence differences among serovars. Sci World J. 2015;2015:1–16. doi: 10.1155/2015/520179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Tennant SM, Levine MM. Live attenuated vaccines for invasive Salmonella infections. Vaccine. 2015;33:C36–C41. doi: 10.1016/j.vaccine.2015.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Bearson BL, Bearson SMD, Kich JD. A DIVA vaccine for cross-protection against Salmonella. Vaccine. 2016;34:1241–1246. doi: 10.1016/j.vaccine.2016.01.036. [DOI] [PubMed] [Google Scholar]
- 211.Kaiser PO, Riess T, O’Rourke F, et al. Bartonella spp.: throwing light on uncommon human infections. Int J Med Microbiol. 2011;301:7–15. doi: 10.1016/j.ijmm.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 212.Angelakis E, Raoult D. Pathogenicity and treatment of Bartonella infections. Int J Antimicrob Agents. 2014;44:16–25. doi: 10.1016/j.ijantimicag.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 213.Zhang P, Chomel BB, Schau MK, et al. A family of variably expressed outer-membrane proteins (Vomp) mediates adhesion and autoaggregation in Bartonella quintana. Proc Natl Acad Sci USA. 2004;101:13630. doi: 10.1073/pnas.0405284101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Abu Shtaya A, Perek S, Kibari A, Cohen S. Bartonella henselae endocarditis: an usual presentation of an unusual disease. Eur J Case Rep Intern Med. 2019;6:1. doi: 10.12890/2019_001038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Schulte B, Linke D, Klumpp S, et al. Bartonella quintana variably expressed outer membrane proteins mediate vascular endothelial growth factor secretion but not host cell adherence. Infect Immun. 2006;74:5003. doi: 10.1128/IAI.00663-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Raoult D, Roux V. The body louse as a vector of reemerging human diseases. Clin Infect Dis. 1999;29:888–911. doi: 10.1086/520454. [DOI] [PubMed] [Google Scholar]
- 217.Koehler JE, Quinn FD, Berger TG, et al. Isolation of Rochalimaea species from cutaneous and osseous lesions of bacillary angiomatosis. N Engl J Med. 1992;327:1625–1631. doi: 10.1056/NEJM199212033272303. [DOI] [PubMed] [Google Scholar]
- 218.MacKichan JK, Gerns HL, Chen Y-T, et al. A SacB mutagenesis strategy reveals that the Bartonella quintana variably expressed outer membrane proteins are required for bloodstream infection of the host. Infect Immun. 2008;76:788. doi: 10.1128/IAI.01174-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Müller NF, Kaiser PO, Linke D, et al. Trimeric autotransporter adhesin-dependent adherence of Bartonella henselae, Bartonella quintana, and Yersinia enterocolitica to matrix components and endothelial cells under static and dynamic flow conditions. Infect Immun. 2011;79:2544. doi: 10.1128/IAI.01309-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Boonjakuakul JK, Gerns HL, Chen Y-T, et al. Proteomic and immunoblot analyses of Bartonella quintana total membrane proteins identify antigens recognized by sera from infected patients. Infect Immun. 2007;75:2548–2561. doi: 10.1128/IAI.01974-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Minnick MF, Anderson BE, Lima A, et al. Oroya fever and verruga peruana: bartonelloses unique to South America. PLoS Negl Trop Dis. 2014;8:1–19. doi: 10.1371/journal.pntd.0002919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Karem KL. Immune aspects of Bartonella. Crit Rev Microbiol. 2000;26:133–145. doi: 10.1080/10408410008984173. [DOI] [PubMed] [Google Scholar]
- 223.Scherer DC, DeBuron-Connors I, Minnick MF. Characterization of Bartonella bacilliformis flagella and effect of antiflagellin antibodies on invasion of human erythrocytes. Infect Immun. 1993;61:4962–4971. doi: 10.1128/IAI.61.12.4962-4971.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Howe C, Hertig M. Prophylactic immunization against Carrión’s disease. J Immunol. 1943;47:471–482. [Google Scholar]
- 225.Gomes C, Ruiz J. Carrion’s disease: the sound of silence. Clin Microbiol Rev. 2018;31:e00056-17. doi: 10.1128/CMR.00056-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Henriquez-Camacho C, Ventosilla P, Minnick MF, et al. Proteins of bartonella bacilliformis: candidates for vaccine development. Int J Pept. 2015 doi: 10.1155/2015/702784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Minnick MF. Identification of outer membrane proteins of Bartonella bacilliformis. Infect Immun. 1994;62:2644–2648. doi: 10.1128/IAI.62.6.2644-2648.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Morales SC, Breitschwerdt EB, Washabau RJ, et al. Detection of Bartonella henselae DNA in two dogs with pyogranulomatous lymphadenitis. J Am Vet Med Assoc. 2007;230:681–685. doi: 10.2460/javma.230.5.681. [DOI] [PubMed] [Google Scholar]
- 229.Dietrich F, Schmidgen T, Maggi RG, et al. Prevalence of Bartonella henselae and Borrelia burgdorferi Sensu Lato DNA in Ixodes ricinus ticks in Europe. Appl Environ Microbiol. 2010;76:1395–1398. doi: 10.1128/AEM.02788-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.McCool TL, Hoey JG, Montileone F, et al. Discovery and analysis of Bartonella henselae antigens for use in clinical serologic assays. Diagn Microbiol Infect Dis. 2008;60:17–23. doi: 10.1016/j.diagmicrobio.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 231.Wagner CL, Riess T, Linke D, et al. Use of Bartonella adhesin A (BadA) immunoblotting in the serodiagnosis of Bartonella henselae infections. Int J Med Microbiol. 2008;298:579–590. doi: 10.1016/j.ijmm.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 232.Eberhardt C, Engelmann S, Kusch H, et al. Proteomic analysis of the bacterial pathogen Bartonella henselae and identification of immunogenic proteins for serodiagnosis. Proteomics. 2009;9:1967–1981. doi: 10.1002/pmic.200700670. [DOI] [PubMed] [Google Scholar]
- 233.Saisongkorh W, Kowalczewska M, Azza S, et al. Identification of candidate proteins for the diagnosis of Bartonella henselae infections using an immunoproteomic approach. FEMS Microbiol Lett. 2010;310:158–167. doi: 10.1111/j.1574-6968.2010.02058.x. [DOI] [PubMed] [Google Scholar]
- 234.Chenoweth MR, Greene CE, Krause DC, Gherardini FC. Predominant outer membrane antigens of Bartonella henselae. Infect Immun. 2004;72:3097. doi: 10.1128/IAI.72.6.3097-3105.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Olsen CW. Vaccination of cats against emerging and reemerging zoonotic pathogens. Vet Vac Diagno. 1999;41:333–346. doi: 10.1016/S0065-3519(99)80025-1. [DOI] [PubMed] [Google Scholar]
- 236.Huwyler C, Heiniger N, Chomel BB, et al. Dynamics of co-infection with Bartonella henselae genotypes I and II in naturally infected cats: implications for feline vaccine development. Microb Ecol. 2017;74:474–484. doi: 10.1007/s00248-017-0936-8. [DOI] [PubMed] [Google Scholar]
- 237.Feng S, Kasten RW, Werner JA, et al. Immunogenicity of Bartonella henselae P26 in cats. Vet Immunol Immunopathol. 2009;132:251–256. doi: 10.1016/j.vetimm.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 238.Berghoff J, Viezens J, Guptill L, et al. Bartonella henselae exists as a mosaic of different genetic variants in the infected host. Microbiology. 2007;153:2045–2051. doi: 10.1099/mic.0.2007/006379-0. [DOI] [PubMed] [Google Scholar]
- 239.Kaiser PO, Riess T, Wagner CL, et al. The head of Bartonella adhesin A is crucial for host cell interaction of Bartonella henselae. Cell Microbiol. 2008;10:2223–2234. doi: 10.1111/j.1462-5822.2008.01201.x. [DOI] [PubMed] [Google Scholar]
- 240.Riess T, Andersson SGE, Lupas A, et al. Bartonella adhesin A mediates a proangiogenic host cell response. J Exp Med. 2004;200:1267–1278. doi: 10.1084/jem.20040500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Riess T, Raddatz G, Linke D, et al. Analysis of Bartonella adhesin A expression reveals differences between various B. henselae strains. Infect Immun. 2007;75:35–43. doi: 10.1128/IAI.00963-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Muñoz González F, Sycz G, Alonso Paiva IM, et al. The BtaF adhesin is necessary for full virulence during respiratory infection by Brucella suis and is a novel immunogen for nasal vaccination against Brucella infection. Front Immunol. 2019;10:1775. doi: 10.3389/fimmu.2019.01775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Gourlay LJ, Peano C, Deantonio C, et al. Selecting soluble/foldable protein domains through single-gene or genomic ORF filtering: structure of the head domain of Burkholderia pseudomallei antigen BPSL2063. Acta Crystallogr Sect D Biol Crystallogr. 2015;71:2227–2235. doi: 10.1107/S1399004715015680. [DOI] [PubMed] [Google Scholar]
- 244.Whitlock GC, Deeraksa A, Qazi O, et al. Protective response to subunit vaccination against intranasal Burkholderia mallei and B. pseudomallei challenge. Proc Vaccinol. 2010;2:73–77. doi: 10.1016/j.provac.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Adler NRL, Stevens JM, Stevens MP, Galyov EE. Autotransporters and their role in the virulence of Burkholderia pseudomallei and Burkholderia mallei. Front Microbiol. 2011 doi: 10.3389/fmicb.2011.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Daigneault MC, Lo RYC. Analysis of a collagen-binding trimeric autotransporter adhesin from Mannheimia haemolytica A1. FEMS Microbiol Lett. 2009;300:242–248. doi: 10.1111/j.1574-6968.2009.01786.x. [DOI] [PubMed] [Google Scholar]
- 247.Wang YP, Hsieh MK, Tan DH, et al. The haemagglutinin of Avibacterium paragallinarum is a trimeric autotransporter adhesin that confers haemagglutination, cell adherence and biofilm formation activities. Vet Microbiol. 2014 doi: 10.1016/j.vetmic.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 248.Araya-Hidalgo E, Gutiérrez-Jiménez C, Chaves-Ramírez M, et al. Sequence analysis of the hypervariable region in hmtp210 of Avibacterium paragallinarum. J Vet Med Sci. 2017;79:1210–1214. doi: 10.1292/jvms.16-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Wu J-R, Wu Y-R, Shien J-H, et al. Recombinant proteins containing the hypervariable region of the haemagglutinin protect chickens against challenge with Avibacterium paragallinarum. Vaccine. 2011;29:660–667. doi: 10.1016/j.vaccine.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 250.Qin W, Wang L, Zhai R, et al. Apa2H1, the first head domain of Apa2 trimeric autotransporter adhesin, activates mouse bone marrow-derived dendritic cells and immunization with Apa2H1 protects against Actinobacillus pleuropneumoniae infection. Mol Immunol. 2017;81:108–117. doi: 10.1016/j.molimm.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 251.Lattanzi Maria, Rappuoli Rino. Genomics, Proteomics and Vaccines. Chichester, UK: John Wiley & Sons, Ltd; 2005. Vaccination: Past, Present and Future; pp. 1–22. [Google Scholar]
- 252.Plotkin SL, Plotkin SA (2013) A short history of vaccination. In: Vaccines. Elsevier, pp 1–13
- 253.Yun S-I, Lee Y-M. Japanese encephalitis. Hum Vaccin Immunother. 2014;10:263–279. doi: 10.4161/hv.26902. [DOI] [PMC free article] [PubMed] [Google Scholar]