Abstract
The hybrid 0.35T magnetic resonance imaging (MRI) and radiotherapy (RT) system functions in part as a simulation platform for treatment planning. We have found that the images generated are particularly helpful for planning of stereotactic radiotherapy for spinal metastases. Advantages include the following: (1) Low-field MRI mitigates magnetic susceptibility artifacts caused by spinal hardware. (2) Volumetric pulse sequence provides isotropic images for improved target delineation. (3) Wide bore MRI in the radiation oncology department allows for easy simulation in treatment position for accurate fusion across imaging modalities. (4) When patients are treated on the MRI-RT device, adaptive radiotherapy is available for special situations to avoid mobile organs at risk.
Keywords: Spinal Metastases, Stereotactic Radiosurgery, Stereotactic Ablative Radiotherapy, Stereotactic Body Radiation Therapy, Image Guided Radiation Therapy, Adaptive Radiation Therapy, Radiation Toxicity
Introduction
Hybrid magnetic resonance imaging and radiotherapy (MRI-RT) platforms provide high contrast volumetric imaging for use in treatment planning and patient setup at time of therapy [1]. The 0.35T hybrid MRI-RT cobalt-60 system and linear accelerator (linac) variant received Food and Drug Administration (FDA) 510(k) clearance in 2012 and 2017, respectively[2] [3].
In part to offset the use of a relatively low magnetic field strength, the system employs a balanced steady state free-precession (bSSFP, also known as true FISP or “TRUFI”) sequence [4]. This provides high signal-to-noise with high temporal resolution, and is commonly used in diagnostic body MRI [5]. Spinal cord imaging with bSSFP gives bright CSF contrast, resistance to inhomogeneity artifacts, rapid image acquisition, and relatively high spatial resolution [6]. As employed on the MRIdian system (ViewRay, Oakwood Village, OH), the bSSFP sequence uses a high flip angle which increases the T2-weighting and can be acquired isotropically (equal voxel size in x, y, and z dimensions) allowing for reconstruction into all planes [7].
For selected patients with localized spinal metastases (SM), stereotactic ablative radiotherapy (SABR) offers advantages over conventional fractionation including more rapid and durable pain control, high clinical and radiographic response rates irrespective of histology, and improved patient quality of life [8–10]. Spinal SABR planning depends on accurate fusion of CT with MRI for target and organ-at-risk (OAR) delineation [11].
However, the use of diagnostic MRI for spine SABR planning presents practical challenges. In high strength magnetic fields associated with diagnostic MR machines, field inhomogeneities from implanted metal hardware can result in dephasing, signal loss and geometric distortion at the metal/tissue interface, obscuring nearby structures such as tumor and spinal cord and preventing accurate delineation [12]. Further, diagnostic MRIs are often performed outside of radiation oncology departments, and radiology protocols can be inconsistent with RT planning preferences [13]. For example, radiotherapy treatment planning often requires interpolation of thick slice diagnostic imaging onto thinner cut CT simulation images making tumor target and spinal cord difficult to identify due to partial volume effects. Variations in patient set-up and table shape between diagnostic MRI and CT simulation can alter spinal orientation and curvature that further complicate the image co-registration.
Given these challenges, we found through experience that visualization of the target and OARs with bSSFP on the MRIdian 0.35T tri-cobalt-60 system can be very useful as an MRI simulation platform for spinal SABR planning irrespective of whether the treatment is later delivered with MRI-RT or an x-ray guided system. These findings are translatable to the Viewray MR-linac variant, which has comparable sub-millimeter three-dimensional (3D) spatial accuracy [14]. We also present a special case where MRI verification at the time of treatment could be useful to detect changes between planning and delivery phases of patient care.
Magnetic susceptibility artifacts
In order to provide a quality MRI simulation for stereotactic radiotherapy of spinal metastases for eligible patients, it is necessary to be able to visualize the location of the spinal cord. Otherwise, a conventional radiotherapy approach is required which may not be optimal for radioresistant histologies. Spinal cord visualization is complicated by the fact that patients with spinal tumors who undergo surgical stabilization or decompression may present for SABR with hardware in the treatment field. Near implanted hardware, the use of high field diagnostic MRI even with recommended parameters for in vivo 2D fast-spin-echo (FSE) has been shown to create in-plane distortion of 4.5 mm and through-plane distortion of 20.5 mm [15]. In comparison, recent studies demonstrate that low-field MRI can measure the relative position of implant and surrounding tissue with a translational error of ≤ 0.13 mm and a rotational error of ≤ 0.15°[16]. In high field MRI, the use of fat-saturated slice encoding for metal artifact correction (SEMAC)-corrected T2-weightedsequences, multiacquisition variable-resonance image combination (MAVRIC) sequences or a fusion of the two (MAVRIC-SL) can mitigate signal loss caused by spinal prostheses [17]. These sequences have only recently entered clinical practice and are not yet standard-of-care magnetic artifact reduction sequences (MARS) outside of orthopedic centers. At our National Cancer Institute (NCI)-designated cancer center, radiology reports regularly describe areas of spinal hardware-associated MR distortion as uninterpretable, evidence of the imperfection of current MARS. Imaging alternatives to MRI include CT myelogram, an invasive procedure [18].
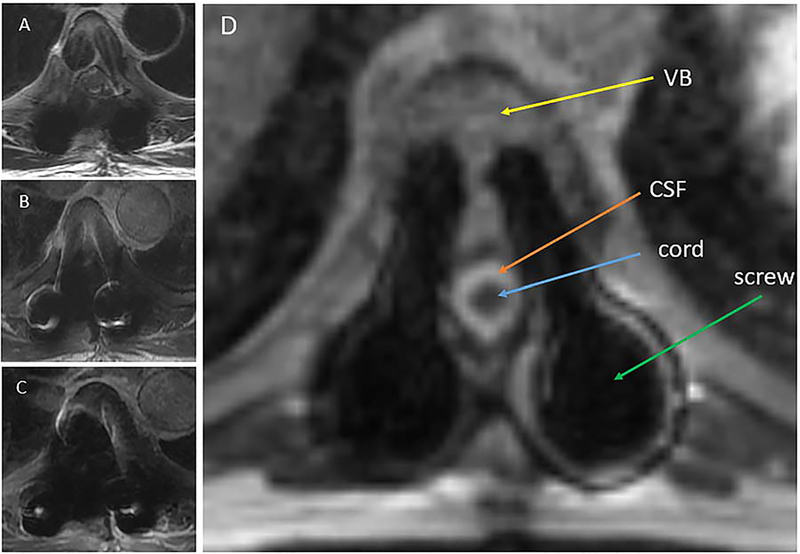
With magnetic susceptibility artifact directly proportional to field strength, the low field 0.35T MRI platform reduces distortion, improving detection and delineation of target and OARs. This is further enhanced by the inhomogeneity resistant bSSFP sequence [19]. An example is shown in Figure 1 where the spinal cord could not be visualized for several slices on any of the provided axial diagnostic MRI images but could be visualized on all axial slices of the 0.35 T images.
Figure 1.
Magnetic susceptibility artifacts. Patients with spinal tumors who undergo surgical stabilization or decompression may present for RT with hardware in the treatment field. In high strength magnetic fields associated with diagnostic MR machines, field inhomogeneities can result in dephasing, signal loss and geometric distortion at the metal/tissue interface, obscuring nearby structures such as tumor and spinal cord and preventing accurate delineation (Images A, B & C). The bSSFP (Image D) sequence uses a high readout bandwidth within a comparatively low strength magnetic field (0.35 T). This minimizes magnetic susceptibility artifacts and improves detection and delineation of nearby soft tissue. Diagnostic 3T MRI images, T2 frFSE (Image A), T1 SE (Image B) and T1 SE + contrast (Image C), of the same axial slice are presented for comparison.
Target delineation
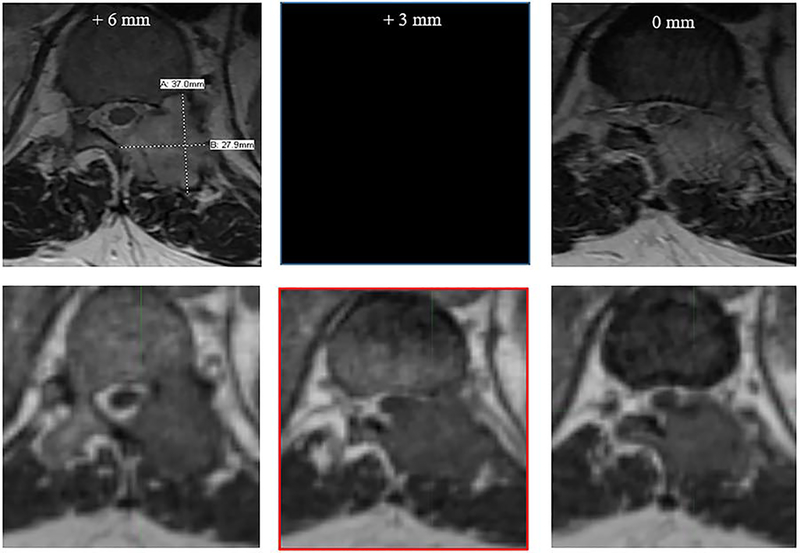
Diagnostic MRI studies include thick slices for best in plane resolution, but details of lesions with thin axial dimensions can escape detection. On the MRI-RT system, the treating radiation oncologist can select a preferred slice width with isotropic 1.5 mm resolution obtained in about a minute and easily transferred and reconstructed in multiple planes with contouring software. Figure 2 demonstrates a case where diagnostic scan and MRI-RT scan were performed one day apart, and the 0.35T bSSFP thin cut scan identified tumor abutting the spinal cord that was not visualized on any of the thick cut diagnostic MRI sequences. This anatomic detail and precision delivery is critical to spine SABR, as translational positioning errors may lead to radiation-induced myelopathy while tumor underdosing leads to treatment failure [20, 21].
Figure 2.
Deficiencies of diagnostic MRI studies include wider than ideal slice thickness. In spinal SABR, selection of slice thickness is critical to safe and effective treatment. In the figure above, the top row represents a diagnostic axial MR image (T2 sequence), with left and rightmost images reflecting 6 mm cranio-caudal slice thickness (neighboring slices on PACS viewer). The bottom row demonstrates the 0.35T bSSFP sequence with 1.5 mm isotropic resolution. The diagnostic MRI was read as T11 nerve root compression and tumor extension into the epidural space without clear cord abutment. In the Viewray simulation, 90-degree cord abutment is readily observed (lower-central image outlined in red) between the diagnostic MRI slices.
MRI and CT simulation alignment
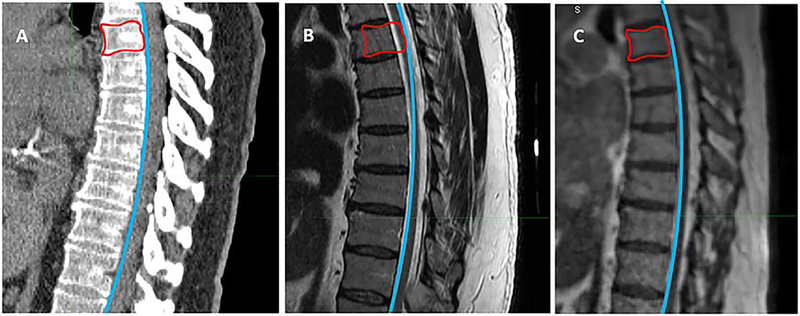
For diagnostic spinal MRIs performed without radiation oncology supervision, different setup protocols can result in variable flexion, extension and rotation of the vertebral column. Co-registration is often needed to correlate imaging for treatment planning, introducing a potential source of error. With in-house MRI-RT systems, immobilization conditions for each patient can be supervised and reproduced, making spinal curvature comparable between MR and CT scans and fusion minimal or unnecessary for accurate target delineation. Wide bore MR enhances patient comfort and tolerance of fastidious setup. Examples of simulation alignment are shown in Figure 3.
Figure 3.
MRI and CT simulation alignment. Different setup protocols for spinal diagnostic MRI can result in variable flexion, extension and rotation of the vertebral column, leading to misalignment of tumor and cord between diagnostic MRI and CT simulation. With in-house MRI-RT systems, immobilization conditions for each patient can be supervised and reproduced, making spinal curvature comparable between MR and CT scans. Image A represents a CT simulation scan, with the curve of the anterior border of the spinal canal delineated in blue and the gross tumor volume (GTV) delineated in red. Identical curves are fitted to both the diagnostic MRI (Image B) and the 0.35T MR/RT set-up scan (Image C). While the curve conforms to the spinal curvature of the MR/RT scan (Image C), subtle kyphosis in the diagnostic MRI (Image B) causes the curve to transect the spinal cord at the level of the GTV, demonstrating cord misalignment.
Given the examples above, our group frequently performs a 0.35T MRI scan at each follow upvisit in select patients after spine SABR to evaluate treatment response since measuring repeat lesion dimensions on the diagnostic scans can be impossible due to metal artifacts or variations in slice thickness and orientation across studies.
Adaptive planning
For patients undergoing radiotherapy to the pelvis, studies have shown significant variation in rectal filling, with the radius of the rectal lumen ranging from 1 to 3+ cm from fraction to fraction even among patients receiving bowel preparation [24]. In cases of sacral metastases treated with SABR, variability in rectal diameter can unexpectedly position the rectal wall in regions of higher radiation dose than anticipated on initial planning, despite reasonable precautions. Daily MR set up imaging with the option of adaptive replanning can mitigate this risk of rectal toxicity.
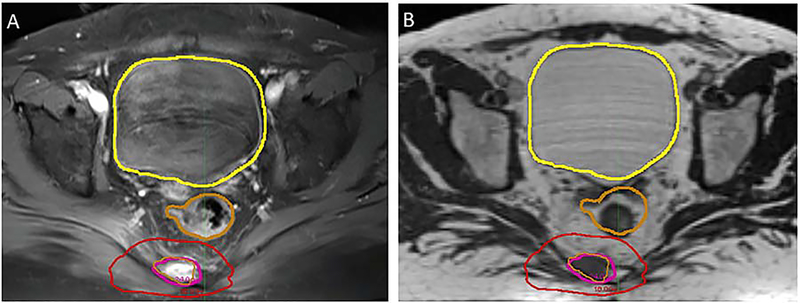
During our institutional experience of MRI-guided spine SABR, we found a case where we strongly considered adaptive radiotherapy. A patient with a painful sacral metastasis from oligometastatic renal cell carcinoma was observed to have variable rectal filling between diagnostic MRI scan and 0.35T MRI-RT simulation scan (Figure 4). The patient was planned to 24 Gy in a single fraction [25]. However, concern was noted for the relationship of mobile, radiosensitive rectum to the radioresistant tumor. With adaptive planning, the original RT plan can be modified and optimized based on real-time anatomy, sparing nearby normal tissues while preserving target coverage [26]. Treatment was delivered on the MRI-RT system, though adaptation was not necessary since the rectum was verified at the time of treatment to be safely away from the high dose radiotherapy field.
Figure 4.
Adaptive planning. In the treatment of sacral tumors, variable rectal filling may result in organ deformation, driving normal tissue into regions of radiation exposure. This anatomic variability may escape notice on x-ray-based setup techniques, while the enhanced soft tissue visualization with MRI guidance is likely to detect such changes. In adaptive planning on Viewray, the original RT plan can be modified and optimized based on real-time anatomy, sparing nearby normal tissues while preserving target coverage. Image A is the pretreatment diagnostic MR image, with the gold contour delineating the rectum. Image B shows the Viewray simulation MR several days later at the same axial level. Change in rectal diameter is clearly visible when compared to the transposed rectal contour from the diagnostic MRI. Adaptive radiotherapy was available at the time of treatment in case the rectum violated constraints, however it remained at the border of the red 18 Gy isodose line, and so treatment was delivered without adaptation in this case.
Conclusion
Hybrid 0.35T MRI RT systems function in part as simulation platforms for treatment planning and can be particularly helpful for simulation of spine SABR. Advantages include the following: (1) Low field strength MRI is resistant to magnetic susceptibility artifacts caused by spinal hardware. (2) Volumetric pulse sequence provides isotropic images for improved target delineation. (3) Wide bore MRI in the radiation oncology department allows for easy simulation in treatment position for accurate fusion across imaging modalities. (4) When patients are treated on the MRI-RT device, adaptive radiotherapy is available for special situations to avoid mobile organs at risk.
Acknowledgments
Financial support and sponsorship: There was no specific funding for this work.
Footnotes
Conflicts of interest: Eric A Mellon received funding from ViewRay in January 2017 for travel to the American Society of Clinical Oncology (ASCO) Gastrointestinal Cancer (GI) Symposium.
Patient consent: All patients signed consent to participate in IRB-approved MRI/RT database.
Ethics approval: N/A
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Noel CE, Parikh PJ, Spencer CR et al. Comparison of onboard low-field magnetic resonance imaging versus onboard computed tomography for anatomy visualization in radiotherapy. Acta Oncol 2015; 54: 1474–1482. [DOI] [PubMed] [Google Scholar]
- 2.Mutic S, Dempsey JF. The ViewRay system: magnetic resonance-guided and controlled radiotherapy. Semin Radiat Oncol 2014; 24: 196–199. [DOI] [PubMed] [Google Scholar]
- 3.FDA 510(k) medical device clearance. In. https://www.fda.gov/medical-devices/510k-clearances/february-2017-510k-clearances: 2017.
- 4.Scheffler K, Lehnhardt S. Principles and applications of balanced SSFP techniques. Eur Radiol 2003; 13: 2409–2418. [DOI] [PubMed] [Google Scholar]
- 5.Chavhan GB, Babyn PS, Jankharia BG et al. Steady-state MR imaging sequences: physics, classification, and clinical applications. Radiographies 2008; 28: 1147–1160. [DOI] [PubMed] [Google Scholar]
- 6.Buch K, Caruso P, Ebb D, Rincon S. Balanced Steady-State Free Precession Sequence (CISS/FIESTA/3D Driven Equilibrium Radiofrequency Reset Pulse) Increases the Diagnostic Yield for Spinal Drop Metastases in Children with Brain Tumors. AJNR Am J Neuroradiol 2018; 39: 1355–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernstein MA. Handbook of MRI Pulse Sequences. Elsevier Academic Press,2004. [Google Scholar]
- 8.Nguyen QN, Shiu AS, Rhines LD et al. Management of spinal metastases from renal cell carcinoma using stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 2010; 76: 1185–1192. [DOI] [PubMed] [Google Scholar]
- 9.Gerszten PC, Burton SA, Quinn AE et al. Radiosurgery for the treatment of spinal melanoma metastases. Stereotact Funct Neurosurg 2005; 83: 213–221. [DOI] [PubMed] [Google Scholar]
- 10.Ryu S, Jin R, Jin JY et al. Pain control by image-guided radiosurgery for solitary spinal metastasis. J Pain Symptom Manage 2008; 35: 292–298. [DOI] [PubMed] [Google Scholar]
- 11.Ryu S, Pugh SL, Gerszten PC et al. RTOG 0631 phase 2/3 study of image guided stereotactic radiosurgery for localized (1–3) spine metastases: phase 2 results. Pract Radiat Oncol 2014; 4: 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stradiotti P, Curti A, Castellazzi G, Zerbi A. Metal-related artifacts in instrumented spine. Techniques for reducing artifacts in CT and MRI: state of the art. Eur Spine J 2009; 18 Suppl 1 : 102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paulson ES, Erickson B, Schultz C, Allen Li X. Comprehensive MRI simulation methodology using a dedicated MRI scanner in radiation oncology for external beam radiation treatment planning. Med Phys 2015; 42: 28–39. [DOI] [PubMed] [Google Scholar]
- 14.Ginn JS, Agazaryan N, Cao M et al. Characterization of spatial distortion in a 0.35 T MRI-guided radiotherapy system. Phys Med Biol 2017; 62: 4525–4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koff MF, Burge AJ, Koch KM, Potter HG. Imaging near orthopedic hardware. J Magn Reson Imaging 2017; 46: 24–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schroder FF, Verdonschot NJJ, Ten Haken B et al. Low-field magnetic resonance imaging offers potential for measuring tibial component migration. J Exp Orthop 2018; 5: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee YH, Lim D, Kim E et al. Feasibility of fat-saturated T2-weighted magnetic resonance imaging with slice encoding for metal artifact correction (SEMAC) at 3T. Magn Reson Imaging 2014; 32: 1001–1005. [DOI] [PubMed] [Google Scholar]
- 18.Sudha SP, Gopalakrishnan MS, Saravanan K. The role of CT myelography in sparing the spinal cord during definitive radiotherapy in vertebral hemangioma. J Appl Clin Med Phys 2017; 18: 174–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park SH, Han PK, Choi SH. Physiological and Functional Magnetic Resonance Imaging Using Balanced Steady-state Free Precession. Korean J Radiol 2015; 16: 550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H, Shiu A, Wang C et al. Dosimetric effect of translational and rotational errors for patients undergoing image-guided stereotactic body radiotherapy for spinal metastases. Int J Radiat Oncol Biol Phys 2008; 71: 1261–1271. [DOI] [PubMed] [Google Scholar]
- 21.Lovelock DM, Zhang Z, Jackson A et al. Correlation of local failure with measures of dose insufficiency in the high-dose single-fraction treatment of bony metastases. Int J Radiat Oncol Biol Phys 2010; 77: 1282–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teyssonneau D, Gross-Goupil M, Domblides C et al. Treatment of spinal metastases in renal cell carcinoma: A critical review. Crit Rev Oncol Hematol 2018; 125: 19–29. [DOI] [PubMed] [Google Scholar]
- 23.Bernardino ME, Green B, Goldstein HM. Ultrasonography in the evaluation of post-nephrectomy renal cancer patients. Radiology 1978; 128: 455–458. [DOI] [PubMed] [Google Scholar]
- 24.Yahya S, Zarkar A, Southgate E et al. Which bowel preparation is best? Comparison of a high-fibre diet leaflet, daily microenema and no preparation in prostate cancer patients treated with radical radiotherapy to assess the effect on planned target volume shifts due to rectal distension. Br J Radiol 2013; 86: 20130457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zelefsky MJ, Greco C, Motzer R et al. Tumor control outcomes after hypofractionated and single-dose stereotactic image-guided intensity-modulated radiotherapy for extracranial metastases from renal cell carcinoma. Int J Radiat Oncol Biol Phys 2012; 82: 1744–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henke L, Kashani R, Yang D et al. Simulated Online Adaptive Magnetic Resonance-Guided Stereotactic Body Radiation Therapy for the Treatment of Oligometastatic Disease of the Abdomen and Central Thorax: Characterization of Potential Advantages. Int J Radiat Oncol Biol Phys 2016; 96: 1078–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]