Abstract
Methotrexate (MTX) is used to treat autoimmune and oncologic diseases in children with Down syndrome (DS). However, increased MTX-related toxicity is reported in this population. We evaluated differences in the concentrations and distribution of erythrocyte folates in children with DS as a potential basis for this enhanced toxicity.
Keywords: Folates, Down Syndrome, Pediatrics, Precision Medicine, Toxicity
Down syndrome, or trisomy of chromosome 21, is the most frequent genetic cause of intellectual disability and occurs at an estimated incidence of 14.5 per 10,000 individuals in the United States (1, 2). Children with DS are known to have an increased incidence of oncologic and autoimmune conditions, specifically juvenile idiopathic arthritis (JIA), acute lymphoblastic and acute myeloid leukemia, hypothyroidism, and celiac disease, among other conditions that often require chronic drug therapies (3). In particular, inflammatory arthritis, observed at a 3-fold higher incidence in children with DS compared with the general population (4) can be a diagnostic and therapeutic challenge in children with DS. Children with DS who develop arthritis have a delay in diagnosis of their arthritis, coupled with a more severe polyarticular course, which puts them at increased risk for long-term joint damage and physical disability (5, 6). Moreover, therapeutic challenges unique to children with DS compound this risk, including enhanced sensitivity to the toxic effects of commonly used drugs to treat juvenile arthritis, including methotrexate) (5).
As a potent anti-folate drug, MTX has several enzymatic targets in the folate pathway, leading to the accumulation of anti-inflammatory agents (such as adenosine) and the interruption of purine and pyrimidine synthesis, which are pivotal for DNA synthesis (7, 8). Reduction in serum and erythrocyte folate are observed after MTX administration (9,10), and folic acid supplementation is thus often used to ameliorate common clinical side effects to the drug (11).
Folates are water-soluble B vitamins consisting of a reduced pteroic acid ring system conjugated to a polyglutamate chain. Ingested folates are deglutamated to their folate monoglutamte (folateGlu1) form in the gastrointestinal tract to facilitate absorption (12). Circulating folateGlu1 is transported into the cell where glutamyl chain accumulation (folate polyglutamation) occurs (folateGlun). Polyglutamation is an important process as it facilitates intracellular retention (13) and leads to bioactivation, by enhancing the interaction between folate and folate metabolizing enzymes (7). Once within the cell, folates convert between intermediates that differ by oxidation state and one-carbon substitution to participate in downstream biochemical reactions. The distributions of folate intermediates within the cell maintain an important balance of one carbon derivatives for use in DNA synthesis and methylation. A deficiency or disruption in cellular folate distribution results in aberrant DNA methylation, genome instability, and abnormal gene expression. There are data to suggest there is cellular adaptation to folate deficiency by an increased expression of FPGS (14) and higher concentrations of long chain folateGlun in in vitro and animal models (15, 9).
Individuals with DS have a well-documented clinical sensitivity to high-dose MTX for the treatment of leukemia (16) as well as to low-dose MTX for the treatment of arthritis (5). These sensitivities may be related to unique metabolic alterations associated with the extra chromosome 21 that may influenceMTX biotransformation. For example, several enzymes in the folate pathway are encoded on chromosome 21, some highly suspected to affect MTX metabolism. SLC19A1 is a transmembrane protein that transports intracellular reduced folates as well as MTX (17, 18). It has been documented that in DS patients, hyperdiploid acute lymphoblastic leukemia (ALL) cells with extra copies of chromosome 21 generate higher levels of active MTX polyglutamates, proposed to be due to increased intracellular transport of MTX via SLC19A1 (18). Additional folate enzymes encoded on chromosome 21 include cystathionine beta synthase (CBS) (19) whose overexpression in DS results in altered homocysteine metabolism and a subsequent “folate trap” phenomenon, and phosphoribosylglycinamide transformylase (GART), a multifunctional protein integral for purine synthesis (20).
Although serum and total concentrations of erythrocyte folates have been characterized in subjects with DS, prior research has not characterized the distribution of erythrocyte folate intermediates and the polyglutamated states of erythrocyte folate within this population of patients. We hypothesize that differences in intracellular folate homeostasis may contribute to the MTX sensitivity observed clinically in patients with DS. Thus, in this pilot study we compared intracellular folate isoforms and their polyglutamated states in a sample of pediatric patients who have DS with a previously-collected cohort of pediatric patients with JIA, with neither group receiving MTX therapy at the time of sampling (21). By establishing improved methods for folate analysis we aimed to clarify our knowledge of folate metabolism and folate intermediates in patients with DS to enable future larger studies to quantify the effects of folate deprivation or supplementation in children with DS.
Methods
Fifteen pediatric patients were recruited as a convenience sample from the multidisciplinary Down syndrome clinic at Children’s Mercy, Kansas City. None of the patients were on MTX therapy at the time of sampling. If clinical laboratory tests were required at the visit, parental written informed consent was obtained to draw an additional blood sample for research purposes. Age in months and folate supplementation/multivitamin (MVI) use were recorded for the DS cohort at the time of the sample collection. Erythrocyte folate concentrations in patients with DS were compared with a cross-sectional cohort of juvenile arthritis patients reported previously (21). Work described was carried out in accordance with the Code of Ethics of the World Medical Association and written informed consent was obtained from the parents or guardians of the children who served as subjects and, when appropriate, assent from the subjects themselves. Studies were conducted under the approval of the Children’s Mercy, Kansas City Institutional Review Board.
Preparation of erythrocyte lysates and folate analyses:
During phlebotomy for routine laboratory monitoring, a 6 mL sample of blood for folate assessment was collected and processed immediately upon receipt. During processing, two 300 μL aliquots of blood were immediately frozen at −80°C, and the remaining sample was centrifuged at low speed (600g) to pellet the erythrocytes. The plasma and buffy coat were removed and the remaining erythrocytes were suspended 4:1 in Phosphate Buffered Saline, and respun. The wash procedure was repeated two more times. Packed erythrocytes and plasma were divided into aliquots and stored at −80°C. Concentrations of 5-methyl-tetrahydrofolate (5-CH3-THF) and 5,10-methenyl- tetrahydrofolate (5,10-methenyl-THF) in whole blood and plasma were determined by LC-MS/MS utilizing the endogenous plasma enzyme gamma-glutamylhydrolase to deglutamate the folates into their monoglutamate form as described by Huang et al. (22) Commercially available, isotopically labeled folate monoglutamates were used as internal standards. The folate concentration in erythrocytes was calculated from whole blood folate values, using hematocrit levels and correcting for plasma folate. As a complete set of standards for 5-CH3-THFGlun is not commercially available, we measured the relative proportion of each polyglutamate 5-CH3-THFGlu3 to 5-CH3-THFGlu10. Relative concentrations of 5-CH3-THF polyglutamates (5-CH3-THFGlun) were measured in erythrocyte lysates using an ion-pair reversed phase chromatography (IPRPC) method modified from our previously published method (23).
Statistical Analysis:
Descriptive statistics were employed to characterize variability in the concentrations of each individual folate isoform and polyglutamate pattern. Univariate analyses were performed between clinical variables, including age and folate supplementation, and intracellular erythrocyte folate isoforms 5-CH3-THF and 5,10-methenyl-THF, using two-sample t-test, analysis of variance, or simple linear regression as appropriate. For data that were not normally distributed (ie, 5,10 methenyl-THF concentrations), Wilcoxon rank-sum analysis or Spearman correlation analysis was used as appropriate. In the multivariable regression analyses, we chose age, folate supplementation, and presence of DS as explanatory variables for variation in total erythrocyte folate levels. Analyses were performed using JMP 11 (Cary, NC).
Results
For the 15 children with DS recruited to this study, median (IQR) age was 52 (24, 63) months. Six (40%) were on a MVI that contained folate, with doses that ranged from 200–400 mcg/day.
Samples from the previously reported cohort of 93 juvenile arthritis patients had been collected in a cross-sectional manner and included all children who presented with arthritis not currently receiving anti-folate medications such as methotrexate (MTX) (21). None of the patients carried the diagnosis of DS. The median (IQR) age of this cohort was 144 (100.5, 192) months. Twenty-five (27%) were on a MVI that contained folate, with doses that ranged from 200–400 mcg/day.
Mean total erythrocyte folate concentrations (ie, sum of erythrocyte 5-CH3-THF and 5,10-methenyl-THF) in patients with DS were 25% lower than the JIA comparator group (p=0.03) (Table). The lower erythrocyte folate concentrations represented disproportionately lower erythrocyte 5,10-methenyl-THF concentrations (P = .005) compared with erythrocyte 5-CH3-THF concentrations (p=0.05), and represented 65% and 23% reductions in the DS population, respectively.
Table 1.
Erythrocyte folate levels in subjects with DS compared with a cohort of 93 JIA patients.
| Folate | DS | JIA | Difference (%) | p-value |
|---|---|---|---|---|
| 5-CH3-THF, Mean (±SD) | 790.1 (±404.2) | 1028.9 (±487.6) | −23% | 0.05 |
| 5,10-methenyl-THF, Median (IQR) | 23.1 (15.6, 66.4) | 66.9 (36.1, 102.0) | −65% | 0.005 |
| Total folate, Mean (±SD) | 845.5 (±403.5) | 1121.6 (±512.3) | −25% | 0.03 |
Because the age of the subjects was quite different between groups, we assessed the impact of age upon erythrocyte folate concentrations (5-CH3-THF, 5,10-methenyl-THF, and the total erythrocyte folate concentrations) and no significant association was observed statistically between age and cellular folate concentrations within the group as a whole nor specifically in the cohort of patients with DS. We also assessed if the consumption of MVI containing folic acid would affect erythrocyte folate concentrations, and in the combined group, there was a non-statistically-significant trend towards an increase in erythrocyte folate concentrations in patients who concurrently took MVI containing folic acid (p=0.07).
In light of the differences in baseline characteristics between groups and existing data to suggest that intracellular folate concentrations may decrease with age (24), we adjusted for the presence of DS, age, and folate supplementation in a multivariable linear regression model using total erythrocyte folate concentrations as the dependent variable. Controlling for these additional variables in the model, the presence of DS (p=0.02) and the consumption of MVI with folate supplementation (p=0.05) remained statistically significantly associated with total erythrocyte folate concentrations.
In previous work, regression analysis and hierarchical clustering demonstrated an internal correlation among folate polyglutamates that revealed long chain and short chain folate polygluatamates clustered together and prompted us to evaluate whether the observed reductions in erythrocyte folates in children with DS was associated in a change in the folate polyglutamation profile (21). Examination of the relative contributions of individual 5-CH3-THFGlun revealed a proportional shift towards longer chain 5-CH3-THF polyglutamates in subjects with DS, resulting from a predominantly lower relative proportion of short chain 5-CH3-THFGlu3–6 (Figure). As a result, subjects with DS had a decrease in mean % (±SD) short chain 5-CH3-THFGlu3–6 (84.2% [± 3.4] vs. 86.5% [± 2.8], p= 0.03) and a corresponding increase in longer chain 5-CH3-THFGlu7–10 (13.5% [± 2.8] vs. 15.8% [± 3.4], p= 0.03).
Figure 1.
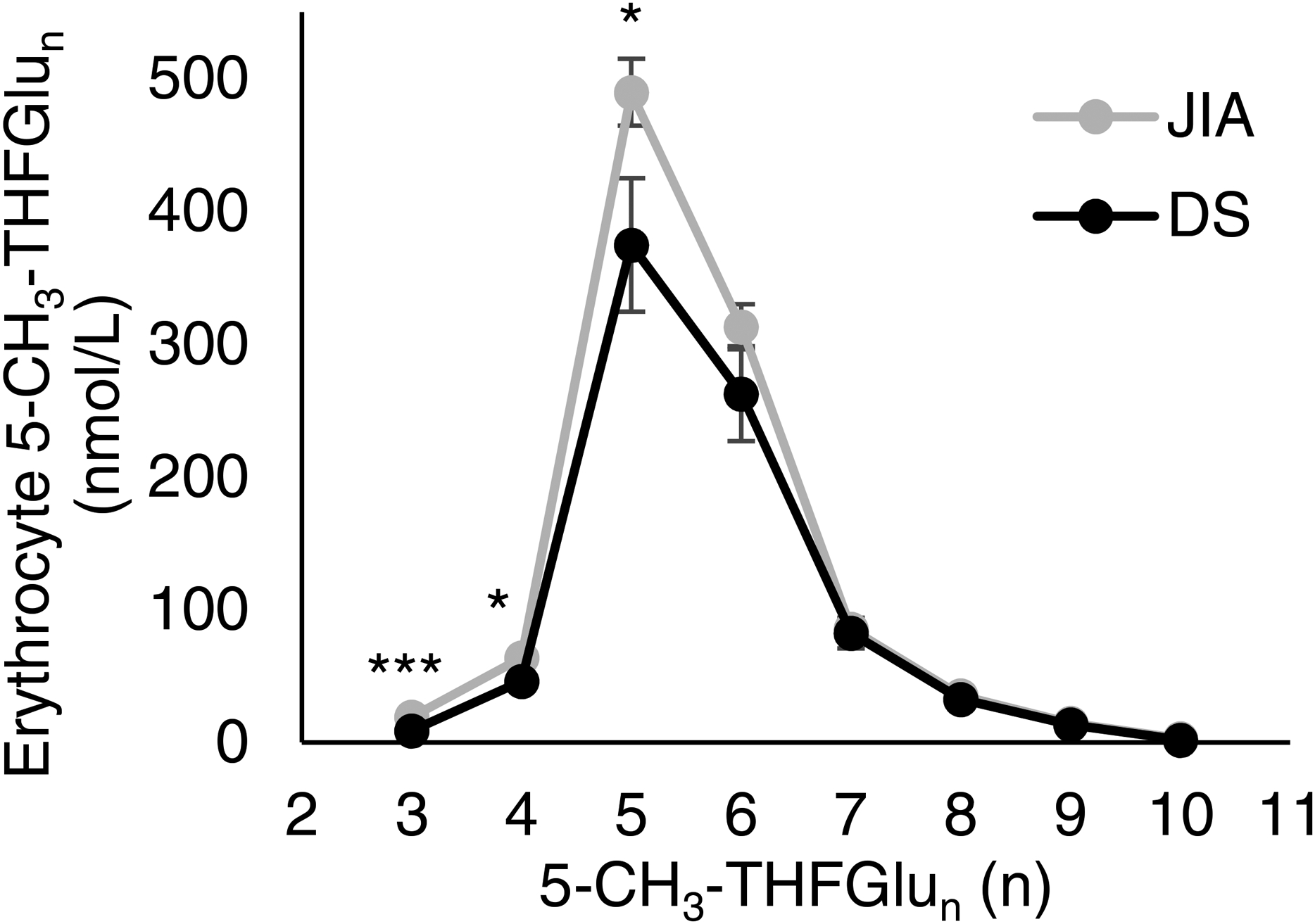
Erythrocyte 5-CH3-THF polyglutamate distribution in patients with DS. Concentrations of 5-CH3-THF-Glu3–10 in circulating erythrocytes from patients with DS are presented and compared with the reference JIA cohort. Data represents the mean concentration of 5-CH3-THFGlun in nmol/L based on the number of attached glutamic acid residues (n). Error bars represent the standard error of the mean. Statistical significance was evaluated by Student’s t-test analysis (*,p≤0.05; ***,p≤0.0001).
Discussion
Patients with DS have often not been included in clinical trials, yet they have an increased risk of comorbid conditions that require management with chronic pharmacologic therapies.
With respect to methotrexate, one main target of the drug is the endogenous folate pathway, and in DS specifically, this pathway has been investigated on several levels. Alterations in maternal folate homeostasis have been interrogated as potential risk factors for fetal chromosome 21 nondisjunction with inconsistent results (25) and folate supplementation has been investigated as a therapeutic intervention to improve cognition (26).
In this study, we demonstrated the ability to quantify specific cellular redox states of intracellular folate and the relative polyglutamation status of 5-CH3-THF, the most abundant reduced form. We are able to illustrate the folate depleted state of children with DS, manifested as both a decline in intracellular folate isoforms and a shift in polyglutamation status in favor of longer chain polyglutamates. This may indicate a compensatory effect to retain cellular folate, which plays an important role as a one-carbon donor for many critical downstream cellular activities. The relative greater decline in 5,10-methenyl-THF in our DS cohort is potentially meaningful as this folate isoform is a critical substrate for enzymes involved in de novo purine and pyrimidine biosynthesis. The introduction of MTX could result in further folate depletion in this key folate isoform, which could explain the extreme drug toxicity in children with DS treated with MTX. In fact, a maximal rescue effect in vitro has been shown in trisomy 21 fibroblast cell lines after supplementation with a combination of reduced folate isoforms compared with the oxidized isoform, folic acid. (27) Quantifying this degree of detail in folate homeostasis may prove useful as a pharmacodynamic tool to understand better MTX disposition and response at the level of an individual patient. Because of its widespread use, future studies have the potential to investigate the alterations of erythrocyte folate concentrations subsequent to MTX in several other conditions that use the drug chronically including psoriasis, inflammatory bowel disease, and various additional rheumatologic and oncologic disorders.
This pilot study describes the detailed folate phenotype in a small population of children with DS, and shows a potential role for the folate pathway as a pharmacodynamic focus in future studies. In DS specifically, the endogenous folate pathway is particularly vulnerable to metabolic alterations, and cellular folate phenotyping offers a quantifiable folate signature that could potentially be a biomarker of MTX response or toxicity. A detailed folate phenotype can be quantified and may be a starting point for future research to distiguish the effects of folate deprivation or supplementation in children with Down syndrome.
Acknowledgements
We thank Chelsey Smith, research coordinator at Children’s Mercy, Kansas City, for assistance in patient recruitment and sample collection for this study.
Support for this project was provided by a Patton Trust Grant awarded by the Kansas City Area Life Sciences Institute; a Young Investigator Award, awarded by Children’s Mercy Hospital, Kansas City, MO; and CTSA grant from NCATS awarded to the University of Kansas for Frontiers: University of Kansas Clinical and Translational Science Institute (# KL2TR002367).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
Portions of this study were presented at the Pediatric Academic Societies annual meeting, << >>, 2011, Denver, Colorado.
References
- 1.Parker SE, Mai CT, Canfield MA, Rickard R, Wang Y, Meyer RE, et al. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res A Clin Mol Teratol 2010; 88:1008–16. [DOI] [PubMed] [Google Scholar]
- 2.Pritchard M, Reeves RH, Dierssen M, Patterson D, Gardiner KJ. Down syndrome and the genes of human chromosome 21: current knowledge and future potentials. Report on the Expert workshop on the biology of chromosome 21 genes: towards gene-phenotype correlations in Down syndrome. Cytogenet Genome Res 2008; 121:67–77. [DOI] [PubMed] [Google Scholar]
- 3.Baum RA, Nash, Foster JE, Spader M, Ratliff-Schaub K, Coury DL. Primary care of children and adolescents with down syndrome: an update. Curr Probl Pediatr Adolesc Health Care 2008; 38: 241–61. [DOI] [PubMed] [Google Scholar]
- 4.Padmakumar B, Jones LG, Sills JA. Is arthritis more common in children with Down syndrome? Rheumatology 2002; 41:1191–93. [DOI] [PubMed] [Google Scholar]
- 5.Jones JT, Talib N, Lovell D, Becker ML. Clinical Features and Treatment of Arthropathy of Down syndrome. Pediatric Drugs 2019; 21:33–39. [DOI] [PubMed] [Google Scholar]
- 6.Juj H, Emery H. The arthropathy of Down syndrome: an underdiagnosed and under-recognized condition. J Pediatr 2009;154: 234–8. [DOI] [PubMed] [Google Scholar]
- 7.Chabner BA, Allegra CJ, Curt GA, Cleneninn NJ, Baram J, Koizumi S, et al. Polyglutamation of Methotrexate. Is Methotrexate a pro drug? J Clin Invest 1985;76:907–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cronstein BN, Naime D, Ostad E. The anti-inflammatory mechanism of Methotrexate: Increased Adenosine release at inflamed sites diminishes leukocyte accumulation in an in vivo model of inflammation. J Clin Invest 1993;92:2675–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Funk RS, van Haandel L, Leeder JS, Becker ML. Folate Depletion and Increased Glutamation in Juvenile Idiopathic Arthritis Patients Treated with Methotrexate. Arthritis Rheum 2014; 66:3476–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morgan SL, Baggott JE, Altz-Smith M. Folate status of rheumatoid arthritis patients receiving long term, low dose methotrexate therapy. Arthritis Rheum 1987; 30:1348–1356. [DOI] [PubMed] [Google Scholar]
- 11.Shea B, Swinden MV, Tanjong Ghogomu E, Ortiz Z, Katchamart W, Rader T, et al. Folic acid and folinic acid for reducing side effects in patient receiving methotrexate for rheumatoid arthritis. Cochrane Database Syst Rev. 2013;31:CD000951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pietrzik K. Folate deficiency: morphological and functional consequences. Bibl Nutr Dieta 1989;44:123–30 [DOI] [PubMed] [Google Scholar]
- 13.Kim JS, Lowe KE, Shane B. Regulation of folate and one-carbon metabolism in mammalian cells. IV. Role of folylpoly-gamma-glutamate synthetase in methotrexate metabolism and cytotoxicity. J Biol Chem 1993; 268: 21680–5 [PubMed] [Google Scholar]
- 14.Ifergan I, Shafran A, Jansen G, Hooijberg JH, Scheffer GL, Assaraf YG. Folate deprevation results in the loss of breast cancer resistance protein (BCRP/ABCG2) expression. A role for BCRP in cellular folate homeostasis. J Biol Chem 2004; 279: 25527–34. [DOI] [PubMed] [Google Scholar]
- 15.Varela-Moreiras G, Selhub J. Long term folate deficiency alters folate content and distribution differentially in rate tissues. The Journal of Nutrition 1992; 122: 986–991. [DOI] [PubMed] [Google Scholar]
- 16.Peeters M, Poon A. Down syndrome and leukemia: unusual clinical aspects and unexpected methotrexate sensitivity. Eur J Pediatr 1987; 146: p. 416–22. [DOI] [PubMed] [Google Scholar]
- 17.Yang-Feng TL, Ma YY, Liang R, Prasad PD, Leibach FH, Ganapathy V. Assignment of the human folate transporter gene to chromosome 21q22.3 by somatic cell hybrid analysis and in situ hybridization. Biochem Biophys Res Commun 1995; 210: 874–9. [DOI] [PubMed] [Google Scholar]
- 18.Taub JW, Ge Y. Down Syndrome, Drug Metabolism, and Chromosome 21. Pediatric Blood Cancer 2005; 44:33–39. [DOI] [PubMed] [Google Scholar]
- 19.Pogribna M, Melnyk S, Pogribny I, Chango A, Yi P, James SJ. Homocysteine metabolism in children with Down syndrome: in vitro modulation. Am J Hum Genet. 2001; 69:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knox AJ, Graham C, Bleskan J, Brodsky G, Patterson D. Mutations in the Chinese hamster ovary cell GART gene of de novo purine synthesis. Gene. 2009; 429: 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Becker ML, van Haandel L, Gaedigk R, Thomas B, Hoeltzel MF, Lasky A, et al. Red Blood Cell Folate Concentrations and Polyglutamate Distribution in JIA: Predictors of Folate Variability. Pharmacogenetics and Genomics 2012; 22:236–46. [DOI] [PubMed] [Google Scholar]
- 22.Huang Y, Khartulyari S, Morales ME, Stanislawska-Sachadyn A, Von Feldt JM, Whitehead AS, et al. Quantification of key red blood cell folates from subjects with defined MTHFR677C>T genotypes using stable isotope dilution liquid chromatography/mass spectrometry. Rapid Communications in Mass Spec 2008; 22:2403–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Haandel L, Becker ML, Williams TD, Stobaugh JF, Leeder JS, Comprehensive Quantitative Measurement of Folate Polyglutamates in Human Erythrocytes by Ion Pair UPLC-MS/MS. Rapid Communications in Mass Spec 2012; 26: 1617–1630. [DOI] [PubMed] [Google Scholar]
- 24.Kerr MA, Livingstone B, Bates CJ, Bradbury I, Scott JM, Ward M, et al. Folate, related B vitamins, and homocysteine in childhood and adolescence: potential implications for disease risk in later life. Pediatrics 2009; 123: 627–35. [DOI] [PubMed] [Google Scholar]
- 25.Hollis ND, Allen EG, Oliver TR, Tinker SW, Druschel C, Hobbs CA, et al. Preconception folic acid supplementation and risk for chromosome 21 nondisjunction: A report from the National Down Syndrome Project. Am J Med Genet A. 2013; 161: 438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blehaut H, Mircher C, Ravel A, Conte M, de Portzamparc V, Poret G, et al. Effect of Leucovorin (Folinic Acid) on the Developmental Quotient of Children With Down’s Syndrome (Trisomy 21) and Influence of Thyroid Status. PLos One. 2010; 5: e8394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vitale L, Serpieri V, Lauriola M, Piovesan A, Antonaros F, Cicchini E, et al. Human Trisomy 21 Fibroblasts Rescue Methotrexate Toxic Effect After Treatment With 5-methyl-tetrahydrofolate and 5-formyl-tetrahydrofolate L Vitale et al. J Cell Physiol. 2019; 234:15010–15024. [DOI] [PubMed] [Google Scholar]


