Abstract
The development of a functional nervous system entails establishing connectivity between appropriate synaptic partners. During axonal pathfinding the developing axon navigates through the extracellular environment, extending toward postsynaptic targets. In the early 1900s, Ramon y Cajal suggested that the growth cone, a specialized, dynamic, and cytoskeletal-rich structure at the tip of the extending axon, is guided by chemical cues in the extracellular environment. A century of work supports this hypothesis and introduced myriad guidance cues and receptors that promote a variety of growth cone behaviors including extension, pause, collapse, retraction, turning, and branching. Here we highlight research from the last two years regarding pathways implicated in axon pathfinding.
Introduction
Formation of appropriate neural networks requires that axons navigate to and connect with appropriate targets. Defining how the growth cone at the tip of the extending axon accomplishes its vast repertoire of responses associated with establishing neuronal connectivity remains an area of intense research. Growth cone motility and axonal pathfinding involve spatial and temporal remodeling of growth cone architecture resulting in mechanotransduction and motility (Fig. 1). Attractive and repulsive guidance cues interact with receptors on the axon surface and promote or constrain growth, respectively1. Diffusion of soluble chemotactic cues influence axon dynamics over long distances, whereas interaction of the axon with adherent, haptotactic guidance cues influences dynamics locally. These cues activate a host of diverse signaling pathways, which results in cytoskeletal and membrane remodeling within the growth cone. Here we review recent work on axonal pathfinding, with attention to the combinatorial synergy of pathways and parallels to morphogenesis of the dendritic spine.
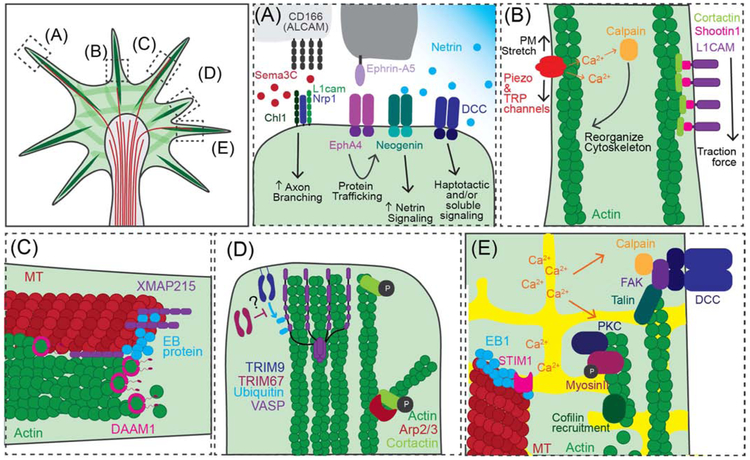
Figure 1. Recent research in growth cone motility.
Although guidance cues, mechanotransduction, cytoskeleton reorganization, and calcium signaling holistically contribute to growth cone motility, we’ve selected several new discoveries in each of these categories to highlight here.
(A) New work highlights contribution of adhesion molecules (CD166 enhances Sema3C-mediated axon branching), combinatorial effects (Ephrin-A5 signaling increases Neogenin surface expression and downstream Netrin-1 signaling) and both haptotactic and soluble signaling (Netrin-1) in axon guidance. (B) Mechanotransduction influences motility, both by the generation of traction force on the ECM (L1CAM) and the activation of Piezo and TRP channels by stretching of the plasma membrane. (C) Coupling of the microtubule and actin cytoskeletons in the growth, newly shown to be mediated by XMAP215 and DAAM1. (D) Post-translational modifications facilitate rapid changes in filopodial formation and stability, as indicated by phosphorylation of cortactin and VASP ubiquitination. (E) Calcium signaling is required for growth cone remodeling. Recent work includes: microtubule organization is regulated by STIM1, calpain cleavage of FAK, talin and DCC, and PKC-mediated Myosin-II activation promotes cofilin recruitment and actin severing.
Guidance cues direct the growth cone
Axon pathfinding begins with the guidance cues that direct growth. Over the last 30+ years, a host of guidance cues have been identified, classically divided into four families: semaphorins, ephrins, netrins, and slits. Identification of additional cues such as sonic hedgehog (Shh), Wnt, and growth factors provides additional complexity and control to axonal wiring2. This list continues to grow: for example, recent work showed that the extracellular domains of the trans-synaptic adhesion molecule lasso (teneurin-2) are cleaved in rat brain, releasing soluble lasso3. As this suggested a novel, pre-synaptic role for lasso, subsequent work in cultured hippocampal neurons demonstrated soluble lasso binds growth cone localized latrophilin-1, initiating calcium signaling and attractive axon turning4. Identification of additional guidance cues and receptors will continue to illuminate how precise directional growth cone extension is tuned.
Beyond identification of cues and receptors, our understanding of axon pathfinding complexity continues to increase. Numerous cues originally proposed to promote a single axonal behavior are now known to promote multiple behaviors based on context, such as target cell receptors and extracellular factors (Fig. 1A). For example, netrin acts as an attractive or repulsive cue based on receptors, concentration, and the extracellular environment5-10. Furthermore, while netrin was historically described as a soluble cue that promotes growth over long distances11,12, recent work suggests netrin exhibits local, haptotactic influences10,13-16 These new studies may suggest that netrin has only an adherent haptotactic role, or alternatively that there is production of distinct pools of netrin in different brain regions; with netrin produced in the ventral zone promoting haptotaxis17 and floor plate netrin diffusing, promoting chemotaxis18. The diverse responses to a single guidance cue, the rich variety of guidance cues and receptors, and the variable downstream signaling cascades together highlight the combinatorial computation occurring within the neuron. The output of this computation is exquisite axon pathfinding tunability.
Synergy between axon guidance cues was first observed nearly two decades ago. Work in Xenopus spinal axons demonstrated netrin-1 attraction is mitigated by the binding of the repulsive cue Slit to its receptor Robo. Hindering response to netrin-1 is critical for midline crossing in the brain19. Since this discovery, a multitude of guidance cue combinations have been observed to coordinate axon pathfinding20. In recent years, a synergistic effect between netrin-1 and the repulsive cue Ephrin-A5 was observed in both mouse and chick spinal lateral motor column (LMC) axons, yet the mechanism of this cooperation was unknown21. Subsequent work demonstrated explant treatment with Ephrin-A5 led to increased surface expression of the netrin receptor neogenin, therefore enhancing netrin-1 attractive axon turning22. Future work will question if this convergence between netrin and ephrin signaling impacts other stages of development, including synaptogenesis.
With the expanding repertoire of guidance cues, receptors, cell adhesion molecules and intersecting downstream signaling pathways, there are ample opportunities to investigate potential synergy and discord between diverse molecular pathways, as well as potential context dependence (i.e. cell type, receptor expression, or extracellular environment), that combine to direct the axon. For example, cell adhesion molecules can further sculpt pathfinding. A recent example in cultured murine midbrain dopamine (mDA) neurons demonstrated that the cell adhesion molecule CD166 (ALCAM) interacts in trans with the semaphorin receptor complex (L1cam, Chl1 and Nrp1). Interestingly, this interaction blocked Sema3A-induced axon growth and enhanced Sema3C-induced axon branching, likely by altering receptor availabilities23 (Fig. 1A). Additional work is required to understand the convergence of numerous signaling events downstream of extracellular cues. For example, Shh-mediated guidance of commissural axons toward the floorplate requires activation of unconventional guanine exchange factors (GEFs) Dock3 and 4, their target Rac, and subsequent cytoskeletal remodeling, as well as Shh stimulated endocytosis of its receptor Boc and subsequently Ptch124,25. All these inputs eventually converge on production of mechanical forces that drive motility.
The growth cone transduces mechanical force
The classic “clutch hypothesis” of motility was first proposed three decades ago26,27. In this model, neurons are linked to the extracellular matrix (ECM) by adhesion molecules. In turn, adhesion molecules are connected to the acto-myosin network by “clutch proteins”. Contraction of the actin cytoskeleton by myosin motors generates force, which is then transmitted to the ECM, facilitates motility (Fig. 1B). In non-neuronal cells, talin and FAK are understood as the clutch between integrins and the actin cytoskeleton. However, it is well appreciated that neuronal clutch molecules can differ between adhesion types, cell types, and extracellular substrates. For example, the cell adhesion molecule (CAM) L1-CAM is critical for adhesion during laminin-based haptotaxis in rat hippocampal neurons. Interestingly, L1-CAM mobility in the growth cone inversely correlates with traction force generated by the growth cone, indicating tunable adhesion and force generation. Human L1-CAM mutations associated with neurological syndromes and projection defects reduce L1-CAM adhesion to laminin and growth cone mechanotransduction28, highlighting physiological importance of force generation. Shootin1a and cortactin serve as clutch molecules linking F-actin retrograde flow in the growth cone to L1-CAM29,30 to promote axon outgrowth. Notably, netrin-1 treatment of hippocampal neurons enhances L1-CAM and shootin1a interactions, facilitating growth cone motility and highlighting the irrevocable link between guidance cue signaling and mechanotransduction30.
Transmembrane adhesion receptors are not alone in mechanotransduction of growth cones; stimulation of mechanosensitive ion channels, such as Piezo or TRP channels, feeds back into signaling pathways to promote outgrowth31-33. In particular, membrane stretch triggers TRPV2 activation, increasing calcium signaling and enhancing axon outgrowth in cultured murine DRGs34. To understand how TRPV2 is activated, subsequent studies in PC12 cells demonstrated TRPV2 localizes to the growth cone and clusters in response to mechanically applied force. TRPV2 also interacts with actin, facilitating cytoskeletal remodeling, increased filopodial length, and ultimately axonal outgrowth35 (Fig. 1B). Critical questions remain unanswered in neuronal mechanotransduction. Although new research has identified clutch molecules between various adhesion molecules and the cytoskeleton, the conservation of these interactions between cell types or with other receptors is unknown. Furthermore, while soluble netrin-1 treatment enhanced L1-CAM-shootin interactions, it is unknown if haptotactic netrin-1 acts through this same pathway. As netrin-1 has numerous known receptors, this research also opens the door to discovery of new mechanotransduction pathways.
Cytoskeleton reorganization drives growth cone motility
Growth cone morphology, and in turn motility, are controlled by reorganization of the actin and microtubule cytoskeletons. Although neuronal studies have focused on Actβ, six mammalian genes encode actin36. Actα, Actβ, and Actγ all localize to growth cone filopodia in cultured murine motorneurons. Knockdown of any isoform decreases filopodia initiation. Loss of Actα or Actγ decreases filopodial dynamics whereas Actβ knockdown decreases growth cone size and motility37. Actin-based myosin motors play numerous roles in growth cones, including traction force generation (myosin II) and vesicle/cargo transport (myosin V, VI and X). New work in cultured murine cortical neurons shows Myo1b localizes to the growth cone membrane and regulates actin dynamics, filopodia stability, and growth cone morphology, potentially via propagation of actin waves proposed to supply cytoplasmic material for growth cone motility38. Additional work investigating the interplay of different actin genes and myosin motors in growth cone motility is warranted.
A critical relationship exists between microtubule stability—and consequently microtubule reorganization—and growth cone motility39,40. This was highlighted in studies of both regulator of presynaptic morphology 1 (RPM-1), an E3 ubiquitin ligase, and the scaffolding protein WDR47. RPM-1 localizes to the growth cone of C. elegans PLM mechanosensory neurons and promotes collapse, potentially by opposing the function of microtubule stabilizers41. Similarly, WDR47 interacts with the microtubule destabilizing protein, superior cervical ganglion-10 (SCG10). Wdr47−/− cortical and hippocampal neurons exhibit decreased growth cone area, which can be partially rescued by Epothilone D, a pharmacological microtubule stabilizer42. Wdr47−/− mice exhibit microcephaly and corpus callosum agenesis, highlighting the critical function of microtubule stability in pathfinding.
Emerging motility research highlights links between microtubules and actin via Xenopus microtubule associated protein 215 (XMAP215) and Dishevelled-associated activator of morphogenesis (DAAM) (Fig. 1C). The microtubule polymerase XMAP215 regulates growth cone morphology and facilitates microtubule invasion into filopodia. This involves an interaction of XMAP215 with both actin and microtubules and likely contributes to growth cone remodeling during guidance43. Likewise, the actin-nucleating formin DAAM also binds both microtubules and tip localized EB1. In Drosophila primary neurons, DAAM binds pioneer microtubules and nucleates new actin filaments to promote filopodial stability44.
The rapid remodeling of the growth cone cytoskeleton in response to guidance cues highlights the demand for precise cytoskeletal regulation. Post-translational modification of cytoskeletal elements poises the cytoskeleton for rapid regulation, and examples continue to emerge. For example, cortactin, an activator of the actin branch nucleating Arp2/3 complex, is a Src kinase substrate. Phosphorylation of cortactin promotes the formation of new growth cone filopodia and increased filopodial length45. As Src family kinases act downstream of guidance cues, cortactin phosphorylation may initiate rapid filopodial changes in response to extracellular stimuli (Fig. 1D).
Ubiquitination also confers rapid signaling and changes in protein function. Classically, ubiquitination is considered a modification marking proteins for proteasomal degradation. Proper protein turnover is essential for neuronal function, and numerous neurodevelopmental and neurodegenerative diseases are associated with defective protein degradation46. New work demonstrates the Siah1-mediated ubiquitination and degradation of protein kinase Akt3 (also known as PKBγ) is required for appropriate axon extension and branching. Furthermore, an Akt3 mutation that reduces Siah1 binding and ubiquitination is linked to focal malformations of cortical development47. In addition to its degradative purposes, ubiquitination—particularly mono-ubiquitination—has emerged as a mechanism to regulate protein localization and activity. For example, reversible, non-degradative ubiquitination of the actin polymerase VASP regulates filopodial stability and axon guidance in response to netrin48. New work also suggests competition between two class I TRIM E3 ubiquitin ligases, TRIM9 and TRIM67, that controls VASP ubiquitination49 (Fig. 1D). Interestingly previous work demonstrating Madd-2 , the single C. elegans class I TRIM ortholog, interacts with UNC-40 (DCC homolog) and is required for proper axon guidance50,51. How duplication of TRIM genes adds additional complexity to signaling pathways integral to axon guidance will be an interesting avenue of future research. As TRIM9 and TRIM67 both localize to the growth cone, the identification of new ubiquitination targets—both regulatory and degradative—will further illuminate complex signaling pathways. Furthermore, with over 600 E3 ligases encoded in the mammalian genome, the role of ubiquitination remains a rich and understudied area of neuronal biology.
In neurons, the dynamic cytoskeleton also includes septins and neurofilaments. SEPT6 and SEPT7 function in filopodia formation and axon branching52, yet the role of septin family is unknown in the growth cone. Likewise, neurofilaments are present in the growth cone and filopodia53, yet their precise role is understudied. For example, the intermediate filament protein nestin was previously thought only to be expressed in neuronal progenitor cells. Surprisingly, new work demonstrates maintenance of nestin expression in immature cortical neurons sensitizes neurons to Sema3A-induced collapse and retraction54. The inclusion of both septins and neurofilaments in future growth cone studies is a necessary measure to fully understand axon guidance.
Calcium regulates growth cone signaling
Calcium signaling is essential for regulating the actin and microtubule cytoskeletons, and ultimately axon guidance55,56 (Fig. 1E). For example, the actin severing protein cofilin is activated downstream of the calcium dependent phosphatase calcineurin in response to serotonin57. In serotonin-treated Aplysia bag neurons, cofilin activation is enhanced downstream of PKC and myosin II, which modulates actin density, traction stress in the growth cone, and neurite outgrowth. This suggests myosin II regulates cofilin-mediated actin depolymerization downstream of calcium58. The microtubule cytoskeleton is regulated via Stromal interacting molecule 1 (STIM1), which localizes to the ER and regulates calcium levels59. STIM1 colocalizes with End binding protein 3 (EB3) and promotes microtubule invasion into filopodia and growth cone turning in cultured neurons, and proper axon guidance in zebrafish caudal primary (CaP) neurons60.
Calpain is a calcium-dependent protease implicated in adhesion and motility. In immortalized cell lines, cleavage of numerous focal adhesion related proteins—including talin61 and FAK62—by calpain is well appreciated to promote adhesion turnover during cell migration. New work shows talin and FAK are also targets of calpain in Xenopus spinal cord neurons, and their cleavage is required for repulsive growth cone turning in vitro and appropriate extension of Rohon–Beard peripheral axons in vivo63. Furthermore, netrin-1 promotes calpain activity and cleavage of the netrin receptor DCC in rodent cortical neurons64. This suggests DCC cleavage may present a negative feedback mechanism to regulate growth cone motility. Furthermore, if DCC is binding substrate adherent netrin as opposed to diffusible netrin, DCC proteolysis may highlight another role for calpain in adhesion. Whether cleavage of other guidance receptors is linked to calpain activation65 is an open question.
Membrane trafficking delivers plasma membrane material during axon outgrowth
Unlike cytoskeleton remodeling or calcium signaling, membrane trafficking plays an underappreciated role in axon guidance. As highly polarized cells, neurons face the unique challenge of directionally targeting cargo to the growing axon. In particular, growth cone motility during neurite outgrowth is concomitant with increased plasma membrane surface area66. Classic hypotheses propose insertion and remodeling of membrane material occurs via calcium regulated exocytosis and membrane flow67,68. Recent empirical measurements of VAMP2 and VAMP7-mediated exocytosis in cultured neurons along with computational simulations of exocytosis, endocytosis, and membrane expansion suggest that VAMP2-mediated exocytosis supplies sufficient membrane material for neurite outgrowth early in murine cortical neuron development. Surprisingly, few exocytic events were observed in the growth cone, suggesting that membrane flow toward the tips of axons may play a role in axon outgrowth69, yet this remains to be tested. However, this hypothesis contradicts earlier work that proposes asymmetric exocytosis as a mechanism for growth cone turning in chick dorsal root ganglion neurons70. To understand these disparities, further work on sites of membrane addition and flow, as well as exocytic vesicle trafficking in the growth cone, are needed.
In addition to exocytic vesicles, other membrane bound compartments contribute to membrane addition and protein trafficking in the growth cone71. For example, lysosomes promote degradation of plasma membrane and extracellular substrates, exocytosis, and plasma membrane repair. Recent work indicates lysosomes are directionally transported to the axonal growth cone via kinesin-1 and a large ensemble of proteins including the BLOC-1 related complex72. This biased motility maintains growth cone size and outgrowth in rat hippocampal neurons. Mitochondria also localize to the growth cone and facilitate axon outgrowth, presumably by generating ATP for actin polymerization73. Mitochondria dynamics within the zebrafish growth cones in vivo suggested that a subset of mitochondria dock in the central zone to microtubules in a syntaphilin-dependent manner and a subset localize to growth cone filopodia, although the mechanism of transport is unclear74. Future work is still required to fully connect membrane trafficking to guidance cue and mechanotransduction signaling to create a holistic understanding of growth cone motility.
Parallels between growth cones and dendritic spines
As cytoskeletal rearrangements dominate both growth cone motility and synaptogenesis, we asked whether pathways in the growth cone are conserved in dendritic spines (Fig 2). During synapse formation, filopodia grow and retract from dendrites, with the potential to form contacts with axons, and mature into actin-rich dendritic spines that receive synaptic transmission75. Like in the growth cone, dendritic filopodia are dynamic structures that must respond to signals in the local environment. However, they also contain the proper machinery to develop into long-lived dendritic spines if they find the correct pre-synaptic partner. These distinct outcomes for dendritic filopodia may exploit parallel combinatorial usage of pathways for diverse outcomes as highlighted in distinct growth responses.
Figure 2. Dendritic filopodia and dendritic spine.
(A) Dendritic filopodia. Like in the growth cone, formin proteins have been observed at the tips of filopodia, elongating actin filaments. However, unlike in the growth cone, dendritic filopodia are known to contain the branching Arp2/3 complex and capping protein, while lacking the actin-bundling protein fascin. Sustained Eph-B signaling (following axonal EphrinB binding) facilitates synaptogenesis.
(B) Dendritic spine. Numerous and diverse cell adhesion molecules facilitate synaptic maturation and plasticity, by connecting the pre- and post-synapse (along with the ECM) and contributing to signaling pathway activation. Netrin signaling via the DCC receptor leads to downstream Src signaling required for long-term potentiation. NMDAR signaling results in the calcium-dependent activation of CaMKII, activating numerous cytoskeleton proteins including cofilin (leading to actin severing and depolymerization), RhoA (leading to myosin activation) and Cdc42 (ultimately leading to Arp2/3 mediated actin branching). The actin cytoskeleton of the dendritic spine is predominantly branched, akin to the lamellipodia, with formin and VASP-mediated protrusions.
Numerous guidance cues and receptors discussed above are also linked to synaptogenesis and synaptic plasticity. For example, Netrin and DCC are enriched in the synapse and netrin increases cortical dendritic filopodia number, synapse number, and synapse strength76. Netrin-1 facilitates synaptic plasticity by increasing surface localization of Glutamate receptor-177. DCC and downstream Src signaling are also required for long-term potentiation and spatial memory78,79. Likewise, recent reviews highlight the role of semaphorins80, wnt81, and ephrins82 in synaptogenesis. Furthermore, adhesion molecules implicated in axon guidance also localize to synapses and modulate cytoskeletal dynamics. For example, downstream of integrins, CaMKII is critical for actin reorganization during synaptic plasticity83. Cadherins are also required for long-term potentiation in both the hippocampus84 and ventral tagmental area85. Notable recent work focuses on the binding of filopodial EphB (a receptor tyrosine kinase) to axonal ephrinB during synaptogenesis. Interestingly, the kinetics of EphB activation determined filopodial fate—whereas fast activation led to filopodial retraction, slower activation facilitated persistent binding86. Finally, mechanotransduction, generated by linking these proteins to the cytoskeleton, is hypothesized to play a role in dendritic spine formation and plasticity, and is an avenue of future research87.
The dendritic spine actin cytoskeleton has been equated to the branched actin network of lamellipodia88,89, with formin and VASP-mediated protrusions contributing to motility90. As a multitude of proteins can regulate actin polymerization—enhancing nucleation, elongation, bundling, severing, etc—it is notable that similar proteins play similar roles in both structures. This suggests mechanisms observed in growth cones may be conserved in spines, with the potential to inform future hypotheses regarding spine maturation and plasticity. For example, as our lab has found that TRIM9-mediated ubiquitination of VASP regulates growth cone filopodia stability, we hypothesize that VASP ubiquitination may also impact dendritic spine morphology or function. Although key differences between these two structures are obvious—namely size and longevity—both structures must be poised to react rapidly remodel their cytoskeleton in response to extracellular cues, albeit the outcome leading to axon turning or plasticity.
Similar to the growth cone, additional research is required to understand the transient dendritic filopodia that preface spine development. However, interesting differences between growth cone and dendritic filopodia have been noted; unlike the growth cone, Arp2/3 and capping protein are enriched in dendritic filopodia that lack the actin-bundler fascin91. These disparities hint at the combinatorial power of actin regulators—the slight tweaking of the molecular makeup results in structures with distinct architecture or dynamics.
Conclusions
Although significant, unanswered questions remain regarding growth cone signaling and motility, a multitude of knowledge has accumulated over the past century. A theme of increasing complexity, specificity, and synergy emerges, likely lending exquisite tunability to growth cone navigation. As research continues, we expect additional convergence and specificity of pathways and cytoskeletal regulation to be revealed. Additional parallels and differences between the development of spines and growth cones will emerge, accounting for the dynamic tuning yet unique maturation and morphological changes of these neuronal structures.
ACKNOWLEDGMENTS
Funding from the National Institutes of Health supported this research: including R01GM108970 (SLG), and F31NS113381 (LEM).
Footnotes
Disclosures:
The authors declare no conflict of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
**outstanding interest
- 1.Lowery LA & Van Vactor D The trip of the tip: understanding the growth cone machinery. Nat. Rev. Mol. cell Biol 10, 332–343 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolodkin AL & Tessier-Lavigne M Mechanisms and Molecules of Neuronal Wiring: A Primer. Cold Spring Harb. Perspect. Biol 3, a001727–a001727 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vysokov NV et al. The mechanism of regulated release of lasso/teneurin-2. Front. Mol. Neurosci 9, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vysokov NV et al. Proteolytically released Lasso/teneurin-2 induces axonal attraction by interacting with latrophilin-1 on axonal growth cones. Elife 7, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor AM, Menon S & Gupton SL Passive microfluidic chamber for long-term imaging of axon guidance in response to soluble gradients. Lab Chip 15, 2781–2789 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki TT et al. Netrin Signaling Defines the Regional Border in the Drosophila Visual Center. iScience 8, 148–160 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun KLW et al. Netrins: versatile extracellular cues with diverse functions. Development 138, 2153–2169 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Ahmed G et al. Draxin Inhibits Axonal Outgrowth through the Netrin Receptor DCC. J. Neurosci 31, 14018–14023 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song H et al. Conversion of Neuronal Growth Cone Responses from Repulsion to Attraction by Cyclic Nucleotides | Lisa McKerracher - Academia.edu. Science (80-. ). 281, 1515–1518 (1998). [DOI] [PubMed] [Google Scholar]
- 10.Boyer NP & Gupton SL Revisiting Netrin-1: One Who Guides (Axons). Front. Cell. Neurosci 12, 337 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kennedy TE et al. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell 78, 425–435 (1994). [DOI] [PubMed] [Google Scholar]
- 12.Serafini T et al. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell 87, 1001–1014 (1996). [DOI] [PubMed] [Google Scholar]
- **13.Varadarajan SG et al. Netrin1 Produced by Neural Progenitors, Not Floor Plate Cells, Is Required for Axon Guidance in the Spinal Cord. Neuron 94, 790–799.e3 (2017).By specifically deleting netrin-1 in the ventricular zone, this paper demonstrates haptotactic netrin-1 is required for proper guidance of commissural axons.
- 14.Dominici C et al. Floor-plate-derived netrin-1 is dispensable for commissural axon guidance. Nature 545, 350–354 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- **15.Moreno-Bravo JA, Roig Puiggros S, Mehlen P & Chédotal A Synergistic Activity of Floor-Plate- and Ventricular-Zone-Derived Netrin-1 in Spinal Cord Commissural Axon Guidance. Neuron 101, 625–634.e3 (2019).In recent years, netrin has been debated as a soluble cue or a haptotactic cue. This paper provides evidence that both forms of netrin contribute to proper axon guidance.
- **16.Wu Z et al. Long-Range Guidance of Spinal Commissural Axons by Netrin1 and Sonic Hedgehog from Midline Floor Plate Cells. Neuron 101, 635–647.e4 (2019).In addition to supporting both soluble and haptotactic forms of netrin, this work demonstrates both floor plate-derived netrin-1 and Sonic hedgehog contribute to axon guidance.
- 17.Varadarajan SG et al. Netrin1 Produced by Neural Progenitors, Not Floor Plate Cells, Is Required for Axon Guidance in the Spinal Cord. Neuron 94, 790–799.e3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kennedy TE, Wang H, Marshall W & Tessier-Lavigne M Axon guidance by diffusible chemoattractants: A gradient of netrin protein in the developing spinal cord. J. Neurosci 26, 8866–8874 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stein E & Tessier-Lavigne M Hierarchical organization of guidance receptors: Silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science (80-. ). 291, 1928–1938 (2001). [DOI] [PubMed] [Google Scholar]
- 20.Dudanova I & Klein R Integration of guidance cues: Parallel signaling and crosstalk. Trends in Neurosciences 36, 295–304 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Poliak S et al. Synergistic integration of netrin and ephrin axon guidance signals by spinal motor neurons. Elife 4, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- *22.Croteau L-P, Kao T-J & Kania A Ephrin-A5 potentiates netrin-1 axon guidance by enhancing Neogenin availability. Sci. Rep 9, 12009 (2019).Treatment of chick lateral LMC explants with the repulsive cue Ephrin-A5 increases surface Neogenin levels at the growth cone, facilitating attractive netrin-1 axon guidance.
- **23.Bye CR, Rytova V, Alsanie WF, Parish CL & Thompson LH Axonal Growth of Midbrain Dopamine Neurons is Modulated by the Cell Adhesion Molecule ALCAM Through Trans-Heterophilic Interactions with L1cam, Chl1, and Semaphorins. J. Neurosci 39, 6656–6667 (2019).The cell adhesion molecule ALCAM tunes the Semaphorin receptor complex to enhance Sema3c-mediated branching.
- 24.Ferent J et al. Boc Acts via Numb as a Shh-Dependent Endocytic Platform for Ptch1 Internalization and Shh-Mediated Axon Guidance. Neuron 102, 1157–1171.e5 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Makihara S et al. Polarized Dock Activity Drives Shh-Mediated Axon Guidance. Dev. Cell 46, 410–425.e7 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Mitchison T & Kirschner M Cytoskeletal dynamics and nerve growth. Neuron 1, 761–772 (1988). [DOI] [PubMed] [Google Scholar]
- 27.Jay DG The clutch hypothesis revisited: Ascribing the roles of actin-associated proteins in filopodial protrusion in the nerve growth cone. J. Neurobiol 44, 114–25 (2000). [DOI] [PubMed] [Google Scholar]
- **28.Abe K et al. Grip and slip of L1-CAM on adhesive substrates direct growth cone haptotaxis. Proc. Natl. Acad. Sci 115, 2764–2769 (2018).The adhesion molecule L1CAM interacts with both the extracellular matrix and F-actin bound clutch molecules. Utilizing cultured hippocampal neurons plated on laminin-striped PDL, the authors demonstrate L1CAM movement decreases (“grippage”) when encountering laminin, increasing traction force and promoting haptotaxis.
- 29.Shimada T et al. Shootin1 interacts with actin retrograde flow and L1-CAM to promote axon outgrowth. J. Cell Biol 181, 817–829 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kubo Y et al. Shootin1-cortactin interaction mediates signal-force transduction for axon outgrowth. J. Cell Biol 210, 663–76 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shibasaki Koji, Murayama Namie, Ono Katsuhiko, Ishizaki Yasuki, Tominaga M TRPV2 Enhances Axon outgrowth through its activation by membrane stretch in developing sesnory and motor neurons. J. Neurosci 30, 4601–4612 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sugio S, Nagasawa M, Kojima I, Ishizaki Y & Shibasaki K Transient receptor potential vanilloid 2 activation by focal mechanical stimulation requires interaction with the actin cytoskeleton and enhances growth cone motility. FASEB J. 31, 1368–1381 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Patrick C Kerstein RHNIVTMG, Kerstein PC, Nichol IV RH & Gomez TM Mechanochemical regulation of growth cone motility. Front. Cell. Neurosci 9, 1–16 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shibasaki K, Murayama N, Ono K, Ishizaki Y & Tominaga M TRPV2 enhances axon outgrowth through its activation by membrane stretch in developing sensory and motor neurons. J. Neurosci 30, 4601–4612 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sugio S, Nagasawa M, Kojima I, Ishizaki Y & Shibasaki K Transient receptor potential vanilloid 2 activation by focal mechanical stimulation requires interaction with the actin cytoskeleton and enhances growth cone motility. FASEB J. 31, 1368–1381 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Perrin BJ & Ervasti JM The actin gene family: function follows isoform. Cytoskeleton (Hoboken). 67, 630–4 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moradi M et al. Differential roles of α-, β-, and γ-actin in axon growth and collateral branch formation in motoneurons. J. Cell Biol 216, 793–814 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iuliano O et al. Myosin 1b promotes axon formation by regulating actin wave propagation and growth cone dynamics. J. Cell Biol 217, 2033 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williamson T, Gordon-Weeks PR, Schachner M & Taylor J Microtubule reorganization is obligatory for growth cone turning. Proc. Natl. Acad. Sci. U. S. A 93, 15221–6 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buck KB & Zheng JQ Growth cone turning induced by direct local modification of microtubule dynamics. J. Neurosci 22, 9358–67 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borgen MA, Wang D & Grill B RPM-1 regulates axon termination by affecting growth cone collapse and microtubule stability. Development 144, 4658–4672 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kannan M et al. WD40-repeat 47, a microtubule-associated protein, is essential for brain development and autophagy. Proc. Natl. Acad. Sci 114, E9308–E9317 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slater PG et al. XMAP215 promotes microtubule-F-actin interactions to regulate growth cone microtubules during axon guidance in Xenopuslaevis. J. Cell Sci 132, jcs224311 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szikora S et al. The formin DAAM is required for coordination of the actin and microtubule cytoskeleton in axonal growth cones. J. Cell Sci 130, 2506–2519 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Ren Y et al. A single tyrosine phosphorylation site in cortactin is important for filopodia formation in neuronal growth cones. Mol. Biol. Cell 30, 1817–1833 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tai HC & Schuman EM Ubiquitin, the proteasome and protein degradation in neuronal function and dysfunction. Nature Reviews Neuroscience 9, 826–838 (2008). [DOI] [PubMed] [Google Scholar]
- 47.Ko HR et al. SIAH1 ubiquitin ligase mediates ubiquitination and degradation of Akt3 in neural development. J. Biol. Chem jbc.RA119.009618 (2019). doi: 10.1074/jbc.RA119.009618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Menon S et al. The E3 Ubiquitin Ligase TRIM9 Is a Filopodia Off Switch Required for Netrin-Dependent Axon Guidance. Dev. Cell 35, 698–712 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boyer NP, McCormick LE, Urbina FL & Gupton S A pair of E3 ubiquitin ligases compete to regulate axon guidance and filopodial dynamics. bioRxiv 529222 (2019). doi: 10.1101/529222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alexander M et al. MADD-2, a homolog of the opitz syndrome protein MID1, regulates guidance to the midline through UNC-40 in Caenorhabditis elegans. Dev. Cell 18, 961–972 (2010). [DOI] [PubMed] [Google Scholar]
- 51.Hao JC et al. The tripartite motif protein MADD-2 functions with the receptor UNC-40 (DCC) in netrin-mediated axon attraction and branching. Dev. Cell 18, 950–960 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu J et al. Septin-driven coordination of actin and microtubule remodeling regulates the collateral branching of axons. Curr. Biol 22, 1109–15 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chan WK-H, Yabe JT, Pimenta AF, Ortiz D & Shea TB Growth cones contain a dynamic population of neurofilament subunits. Cell Motil. Cytoskeleton 54, 195–207 (2003). [DOI] [PubMed] [Google Scholar]
- 54.Bott CJ et al. Nestin in immature embryonic neurons affects axon growth cone morphology and Semaphorin3a sensitivity. Mol. Biol. Cell 30, 1214–1229 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Henley J & Poo MM Guiding neuronal growth cones using Ca2+ signals. Trends in Cell Biology 14, 320–330 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gasperini RJ et al. How does calcium interact with the cytoskeleton to regulate growth cone motility during axon pathfinding? Molecular and Cellular Neuroscience 84, 29–35 (2017). [DOI] [PubMed] [Google Scholar]
- 57.Zhang X-F, Hyland C, Van Goor D & Forscher P Calcineurin-dependent cofilin activation and increased retrograde actin flow drive 5-HT-dependent neurite outgrowth in Aplysia bag cell neurons. Mol. Biol. Cell 23, 4833–48 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- *58.Zhang X-F et al. Regulation of axon growth by myosin II–dependent mechanocatalysis of cofilin activity. J. Cell Biol. jcb.201810054 (2019). doi: 10.1083/jcb.201810054Following serotonin treatment, myosin II is activated by PKC and facilitates cofilin recruitment to actin filaments, leading to actin depolymerization.
- 59.Mitchell CB, Gasperini RJ, Small DH & Foa L STIM1 is necessary for store-operated calcium entry in turning growth cones. J. Neurochem 122, 1155–1166 (2012). [DOI] [PubMed] [Google Scholar]
- *60.Pavez M et al. STIM1 is required for remodelling of the endoplasmic reticulum and microtubule cytoskeleton in steering growth cones. J. Neurosci 2496–18 (2019). doi: 10.1523/jneurosci.2496-18.2019The ability of STIM1 to couple microtubule and ER remodeling influences filopodia dynamics and ultimately growth cone turning.
- 61.Franco SJ et al. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nat. Cell Biol 6, 977–983 (2004). [DOI] [PubMed] [Google Scholar]
- 62.Chan KT, Bennin DA & Huttenlocher A Regulation of adhesion dynamics by calpain-mediated proteolysis of focal adhesion kinase (FAK). J. Biol. Chem 285, 11418–11426 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- *63.Kerstein PC, Patel KM & Gomez TM Calpain-Mediated Proteolysis of Talin and FAK Regulates Adhesion Dynamics Necessary for Axon Guidance. J. Neurosci 37, 1568–1580 (2017).Transient calcium signaling in filopodia leads to calpain activation and negative regulation of focal adhesion formation via talin and FAK cleavage.
- 64.Duquette PM & Lamarche-Vane N The calcium-activated protease calpain regulates netrin-1 receptor deleted in colorectal cancer-induced axon outgrowth in cortical neurons. J. Neurochem jnc.14837 (2019). doi: 10.1111/jnc.14837 [DOI] [PubMed] [Google Scholar]
- 65.To KCW, Church J & O’Connor TP Combined activation of calpain and calcineurin during ligand-induced growth cone collapse. Mol. Cell. Neurosci 36, 425–434 (2007). [DOI] [PubMed] [Google Scholar]
- 66.Pfenninger KH Plasma membrane expansion: A neuron’s Herculean task. Nat. Rev. Neurosci 10, 251–261 (2009). [DOI] [PubMed] [Google Scholar]
- 67.Craig AM, Wyborski RJ & Banker G Preferential addition of newly synthesized membrane protein at axonal growth cones. Nature 375, 592–594 (1995). [DOI] [PubMed] [Google Scholar]
- 68.Winckler Bettina, Poo M No diffiusion barrior at the axon hillock. Nature 379, 213 (1996). [DOI] [PubMed] [Google Scholar]
- *69.Urbina FL, Gomez SM & Gupton SL Spatiotemporal organization of exocytosis emerges during neuronal shape change. J. Cell Biol 217, 1113–1128 (2018).Plasma membrane expansion during early neuronal development is facilitated by VAMP-2 mediated exocytosis.
- 70.Tojima T et al. Attractive axon guidance involves asymmetric membrane transport and exocytosis in the growth cone. Nat. Neurosci 10, 58–66 (2007). [DOI] [PubMed] [Google Scholar]
- 71.Sann S, Wang Z, Brown H & Jin Y Roles of endosomal trafficking in neurite outgrowth and guidance. Trends Cell Biol. 19, 317–324 (2009). [DOI] [PubMed] [Google Scholar]
- 72.Farías GG, Guardia CM, De Pace R, Britt DJ & Bonifacino JS BORC/kinesin-1 ensemble drives polarized transport of lysosomes into the axon. Proc. Natl. Acad. Sci. U. S. A 114, E2955–E2964 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith GM & Gallo G The role of mitochondria in axon development and regeneration. Dev. Neurobiol 78, 221–237 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Verreet T, Weaver CJ, Hino H, Hibi M & Poulain FE Syntaphilin-mediated docking of mitochondria at the growth cone is dispensable for axon elongation in vivo. eNeuro ENEURO.0026-19.2019 (2019). doi: 10.1523/ENEURO.0026-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ethell IM & Pasquale EB Molecular mechanisms of dendritic spine development and remodeling. Progress in Neurobiology 75, 161–205 (2005). [DOI] [PubMed] [Google Scholar]
- 76.Goldman JS et al. Netrin-1 promotes excitatory synaptogenesis between cortical neurons by initiating synapse assembly. J. Neurosci 33, 17278–89 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Glasgow SD et al. Activity-Dependent Netrin-1 Secretion Drives Synaptic Insertion of GluA1-Containing AMPA Receptors in the Hippocampus. Cell Rep. 25, 168–182.e6 (2018). [DOI] [PubMed] [Google Scholar]
- 78.Horn KE et al. DCC Expression by Neurons Regulates Synaptic Plasticity in the Adult Brain. Cell Rep. 3, 173–185 (2013). [DOI] [PubMed] [Google Scholar]
- 79.Glasgow SD et al. Pre- and post-synaptic roles for DCC in memory consolidation in the adult mouse hippocampus. bioRxiv 697631 (2019). doi: 10.1101/697631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Koropouli E & Kolodkin AL Semaphorins and the dynamic regulation of synapse assembly, refinement, and function. Curr. Opin. Neurobiol 27, 1–7 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.He C-W, Liao C-P & Pan C-L Wnt signalling in the development of axon, dendrites and synapses. Open Biol. 8, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Henderson NT & Dalva MB EphBs and ephrin-Bs: Trans-synaptic organizers of synapse development and function. Mol. Cell. Neurosci 91, 108–121 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shi Y & Ethell IM Integrins control dendritic spine plasticity in hippocampal neurons through NMDA receptor and Ca2+/calmodulin-dependent protein kinase II-mediated actin reorganization. J. Neurosci 26, 1813–1822 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Basu R et al. Heterophilic Type II Cadherins Are Required for High-Magnitude Synaptic Potentiation in the Hippocampus. Neuron 96, 160–176.e8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mills F et al. Cadherins mediate cocaine-induced synaptic plasticity and behavioral conditioning. Nat. Neurosci 20, 540–549 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mao Y-T et al. Filopodia Conduct Target Selection in Cortical Neurons Using Differences in Signal Kinetics of a Single Kinase. Neuron 98, 767–782.e8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kilinc D The Emerging Role of Mechanics in Synapse Formation and Plasticity. Front. Cell. Neurosci 12, 483 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hotulainen P et al. Defining mechanisms of actin polymerization and depolymerization during dendritic spine morphogenesis. J. Cell Biol 185, 323–39 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hotulainen P & Hoogenraad CC Actin in dendritic spines: Connecting dynamics to function. J. Cell Biol 189, 619–629 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chazeau A et al. Nanoscale segregation of actin nucleation and elongation factors determines dendritic spine protrusion. EMBO J. 33, 2745–2764 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Korobova F & Svitkina T Molecular Architecture of Synaptic Actin Cytoskeleton in Hippocampal Neurons Reveals a Mechanism of Dendritic Spine Morphogenesis. Mol. Biol. Cell 21, 165–176 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]