Abstract
Background
The endothelial glycocalyx (EG) is involved in critical regulatory mechanisms that maintain endothelial vascular integrity. We hypothesized that prolonged cardiopulmonary bypass (CPB) may be associated with EG degradation. We performed an analysis of soluble syndecan-1 levels in relation to duration of CPB, as well as factors associated with cell stress and damage, such as mitochondrial DNA (mtDNA) and inflammation.
Methods
Blood samples from subjects undergoing cardiac surgery with CPB (n=54) were obtained before and during surgery, 4–8 hours and 24 hours after completion of CPB, and on post-operative day 4. Flow cytometry was used to determine subpopulations of white blood cells. Plasma levels of mtDNA were determined using quantitative PCR and plasma content of shed syndecan-1 was measured. In order to determine whether syndecan-1 was signaling white blood cells, the effect of recombinant syndecan-1 on mobilization of neutrophils from bone marrow was tested in mice.
Results
CPB is associated with increased mtDNA during surgery, increased syndecan-1 blood levels at 4–8hrs, and increased white blood cell count at 4–8hrs and 24hrs. Correlation analysis revealed significant positive associations between time on CPB and syndecan-1 (rs=0.488, p<0.001), and level of syndecan-1 and neutrophil count (rs=0.351, p=0.038) at 4–8 hrs. Intravenous administration of recombinant syndecan-1 in mice resulted in a 2.5 fold increase in the number of circulating neutrophils, concurrent with decreased bone marrow neutrophil number.
Conclusions
Longer duration of CPB is associated with increased plasma levels of soluble syndecan-1, a signal for EG degradation, which can induce neutrophil egress from the bone marrow. Development of therapy targeting endothelial glycocalyx shedding may be beneficial in patients with prolonged CPB.
Keywords: Cardiac Surgery, Cardiopulmonary Bypass, Endothelial Glycocalyx, Damage Associated Molecular Patterns (DAMPs), Neutrophils
INTRODUCTION
While cardiopulmonary bypass (CPB) is considered a safe means to facilitate cardiac surgery, however, prolonged CPB is associated with complications 1, 2. Circulating a patient’s blood volume through the extracorporeal circuit of CPB is associated with physiologic derangements 3. Blood exposure to abnormal shear stress and contact with the artificial surface of the bypass circuit results in the activation of the coagulation and complement systems 4,6, pro-inflammatory activation, endothelial cell death 5, 6 and platelet activation 7, followed by release of damage associated molecular patterns (DAMPs) 8, 9, which may contribute to leukocyte activation 10, 11. Elucidation of interactions between different pathways triggered by extracorporeal circulation may help develop new strategies to reduce post-CPB complications.
The endothelial cell (EC) monolayer is protected by the endothelial glycocalyx (EG) which is composed mainly of transmembrane syndecans and bound heparan sulfates. Syndecan-1, a major component of EG, is expressed as membrane-bound protein on epithelial cells and endothelial cells 12, 13. During stress, activated metalloproteinases mediate shedding of syndecan-1 from the cell surface 14. Both membrane-bound and soluble syndecan-1 play a role in the regulation of inflammation and tissue damage 12. Membrane-bound syndecan-1 reduces pro-inflammatory cytokine secretion and neutrophil infiltration, and prevention of syndecan-1 shedding has been shown to be protective against intestinal inflammation 15. Inhibition of syndecan-1 shedding in a mouse model of lung injury by ablation of matrix metalloproteinase-7 gene expression is associated with significantly reduced inflammation and mouse death 16, 17. Moreover, soluble syndecan-1 induces neutrophil activation 18 and chemotaxis of monocytes 19.
Cardiac surgery using CPB is associated with a release of DAMPs and syndecan-1 shedding 20, 21. Both DAMPs and shed EG components may induce immune cell activation and amplification of the inflammatory response22. Although CPB-induced stress and inflammation-related changes in blood are well described 23, 24, the dynamics of these changes and correlations with prolonged CPB time have not been investigated. We analyzed the post-CPB presence of: circulating mtDNA level, a DAMP associated with cellular death and platelet activation, the shed fragment of the glycocalyx glycoprotein syndecan-1, leukocytes and pro-inflammatory cytokine IL6.
METHODS
Study subject enrollment
Research was performed in accordance with study protocols approved by Maine Medical Center Institutional Review Board (NCT 02820233), accredited by the Association for the Accreditation of Human Research Protection Programs (AAHRPP). The study includes female and male patients having cardiac surgery supported by cardiopulmonary bypass (CPB). Exclusion criteria included active myocarditis, hypertrophic cardiomyopathy, constrictive pericarditis, significant pericardial disease, severe pulmonary hypertension, severe ventricular arrhythmias, significant hypotension, hepatic disease, renal impairment, pregnancy, known malignancy other than non-melanoma skin cancers, emergency surgery, and patients with expected survival less than one year. Of the sixty patients were enrolled in this study six were missing three or more time points and were excluded leaving 54 patients for the data analysis.
Pertinent clinical data was collected from the electronic medical record for all study participants. Demographic and clinical characteristics of the 54 study subjects is provided in Table 1.
Table 1.
Baseline Characteristics
| Characteristic | N=54 |
|---|---|
| Procedure, n (%) | |
| CABG | 40 (74%) |
| Isolated Valve (AVR/MVR replacement or repair) | 6 (11%) |
| CABG/Valve | 6 (11%) |
| Other | 2 (4%) |
| Urgent Cases, n (%) | 21 (38.9%) |
| Age years, mean (SD) | 64 (10.4) |
| Sex, n(%) female | 25 (35.2%) |
| Diabetic, n (%) | 35 (64.8%) |
| Preoperative HbA1C, mean (SD) | 7.2 (1.8) |
| Peripheral Arterial Disease, n (%) | 12 (22.2%) |
| Congestive Heart Failure, n (%) | 14 (25.9%) |
| Previous Myocardial Infarction, n (%) | 26 (47.3%) |
| 1–7days prior to surgery, n (%) | 7 (12.7%) |
| 8–21days prior to surgery, n (%) | 2 (3.6%) |
| >21days prior to surgery, n (%) | 17 (30.9%) |
| Cardiopulmonary bypass time minutes, mean (SD) | 112 (37) |
| Cross clamp time minutes, mean (SD) | 87 (27) |
Blood sample collection
Blood samples from subjects were obtained immediately before surgery, post anesthesia induction but prior to skin incision, 15 minutes after initiation of CPB, 4–8 hours after separation from CPB, 24 hours after separation from CPB and 4 days after surgery. Due to clinical circumstances or unavailability of personnel, blood samples were missing in 11% of patientspre-operatively, 11% at surgery, 19% at 4–8 hrs, 19% at 24 hrs and 11% at post-operative day 4. Venous blood (10 ml) was collected using BD Vacutainer ACD tubes. Blood plasma was prepared at room temperature using two-step centrifugation, each at 2,000g for 20 minutes.
Animals
All studies were conducted in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the US National Institutes of Health. Animal studies were reviewed and approved by the institutional animal care and use committee of Maine Medical Center Research Institute. FVB/NJ mice were obtained from the Jackson Laboratory (Bar Harbor, ME).
Administration of syndecan-1
Human recombinant syndecan-1 (11429-H08H, Sino Biologicals Wayne, PA) in phosphate buffered saline (PBS) was intravenously injected to FVB/NJ mice at a dose of 120 μg/kg body weight. Control mice were given the same volume of PBS. Blood plasma samples were obtained from the submandibular region, and subpopulations of WBC were analyzed using flow cytometry. Bone marrow cells were isolated from mouse femurs.
Flow cytometric analysis
Subpopulations of WBC were analyzed using a combination of side scatter (SSC) characteristics and CD45-APC/Cy7 (HI30) (purchased from BioLegend, San Diego, CA). Murine peripheral blood cells and bone marrow cells were stained with CD11b conjugated with FITC (clone: M1/70), CD45-APC/Cy7 (clone: 30-F11) and Ly6G-PeCy7(clone: 1A8) (all purchased from BioLegend, San Diego, CA). Viable and non-viable cells were distinguished using DAPI.
mtDNA analysis
mtDNA was isolated from plasma using Zymo plasma mini kit (No.D4016). Plasma levels of mtDNA were determined using quantitative PCR. Plasma DNA was analyzed using Biorad iQ Sybr Green Supermix and primers: sense (5’-CACCCAAGAACAGGGTTTGT); anti-sense (5’-TGGCCATGGGTATGTTGTTA); and cycled 40 times on a Biorad CFX real time machine. Absolute numbers were calculated from the isolated human mtDNA internal standard curve.
Analysis of circulating syndecan-1 and IL-6
The shedding of syndecan-1 was determined using ELISA (Abcam). Plasma level of IL-6 was measured using ELISA kits (Bio-techne/R&D Systems).
Statistical analysis
Comparisons between two groups were performed using two-tailed unpaired t-tests (normal distribution). Comparisons between three or more groups were performed using Friedman test with Dunn’s multiple comparisons post-test for skewed distribution. Repeated measures two-way ANOVA with Sidak’s multiple comparisons test was used to determine the differences between the levels of mtDNA and syndecan-1 in groups with shorter and longer duration of CPB before and after CABG surgery. Patients were divided into two groups with shorter and longer duration of CPB based on the median value of CPB time (102 minutes) in the current study. For continuous variables, correlation analysis was performed using Spearman’s rank test (skewed distribution correlation). A P-value < 0.05 was considered significant.
RESULTS
Patients
All patients underwent cardiac operations with CPB as planned. Seventy-four percent of patients underwent isolated CABG, 11% isolated valve, and 8% other types of cardiac operations. There were no mortalities. Although the study was not powered for clinical outcomes, a comparison of patients who had prolonged CPBhad no differences in complication rates, specifically stroke, low output cardiac failure, prolonged intubation, acute kidney injury or reoperation for bleeding. (P >0.05 for all).
Cardiac surgery is associated with an increase in levels of circulating mtDNA, syndecan-1, WBC, and IL-6
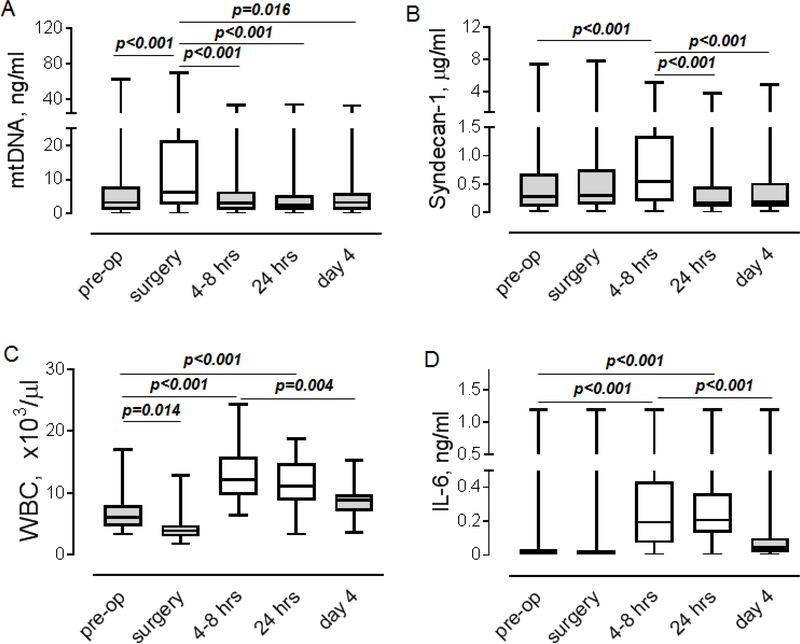
Our analysis of time-dependent changes revealed different dynamics in the elevation of mtDNA, syndecan-1, and proinflammatory factors (Figure 1). The level of mtDNA was significantly elevated immediately after the onset of CPB followed by its return to pre-operative levels at 4–8 hrs (Figure 1A). Syndecan-1 level was increased at 4–8 hrs (Figure 1B). More complex dynamics characterized changes in the number of WBC compared to mtDNA or syndecan-1. As shown in Figure 1C, initially, the number of WBC was decreased after the onset of CPB, reflecting a dilution of blood with perfusate, following an increase at 4–8 hrs and 24 hrs. The number of WBC returned to the preoperative level on post-operative day 4. IL-6 was significantly elevated at 4–8 hrs and 24 hrs compared to the pre-operative level (Figures 1C and 1D).
Figure 1. Time-dependent changes in levels of mtDNA, soluble syndecan-1, WBC and IL-6.
A-D. Levels of mtDNA (A, n=50), syndecan-1 (B, n=44), WBC (C, n=44), and IL-6 (D, n=44) were measured in blood plasma obtained from cardiac surgery patients before (pre-op), during surgery and at 4–8 hours, 24 hours and 96 hours (day 4) after surgery. Friedman test with Dunn’s multiple comparisons test. P values are indicated.
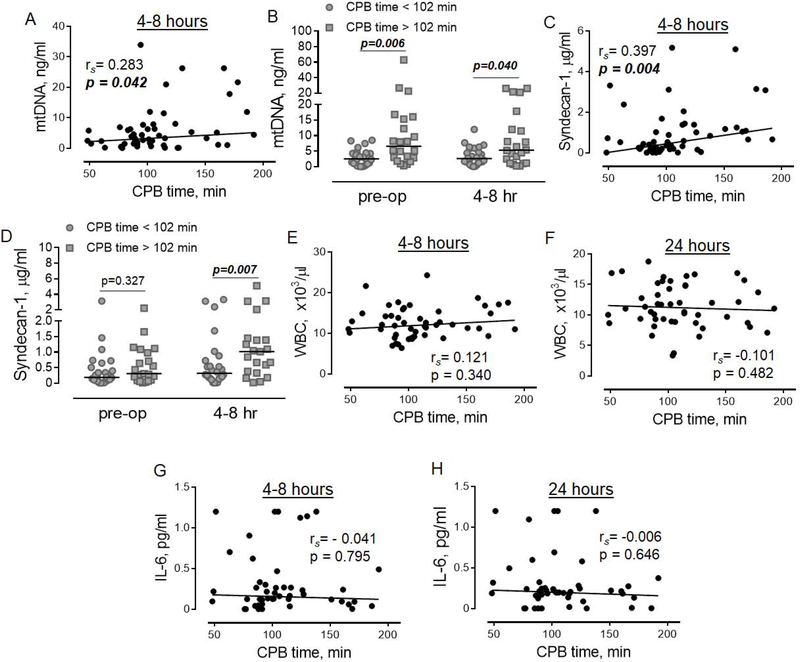
Circulating levels of mtDNA and syndecan-1 correlate with longer duration of CPB but do not correlate with each other
Our data demonstrated that prolonged CPB positively correlated with circulating mtDNA at 4–8 hrs (Figure 2A). Given that CPB induces rapid elevation of circulating mtDNA (Figure 1A), a positive correlation between CPB and mtDNA at 4–8 hrs may be due to higher accumulation of mtDNA in patients with prolonged CPB time. To determine if the latter is true, we performed an analysis of mtDNA levels in two groups of patients with shorter and longer CPB time, divided based on the median value of CPB time, which equals 102 minutes in our study. As shown in Figure 2B, the level of mtDNA was significantly elevated in the group of patients with longer CPB time at both time points, before and after the surgery. These data demonstrated that longer duration of CPB did not induce the elevation of the level of mtDNA, and rather reflects the pre-existing slightly elevated level of mtDNA before surgery. We also found a moderately positive correlation between the duration of CPB and syndecan-1 (Figure 2C). The analysis in groups of patients with shorter and longer CPB time revealed a significant effect of longer duration of CPB on the elevation of circulating syndecan-1 at 4 to 8 hours after, but not before surgery (Figure 2D). No associations were found between levels of syndecan-1 and mtDNA during surgery or at 4–8 hrs, indicating that the elevation of circulating syndecan-1 is not related to the levels of mtDNA. No correlations were found between the duration of CPB and markers of the systemic inflammatory response, WBC (Figure 2E and 2F) or IL-6 (Figure 2G and 2H).
Figure 2. Associations between the duration of CPB and levels of mtDNA, soluble syndecan-1, WBC, and IL-6.
The correlation between CPB time and mtDNA (A, n=50), syndecan-1 (C, n=44), WBC (E-F, n=44), and IL-6 (G-H, n=44). The correlation analysis was performed at 4–8 hours (A, C, E and G) and 24 hours (F and H) after cardiac surgery. Spearman’s rank correlation coefficient and p values are indicated. The comparisons of mtDNA (B) and syndecan-1 (D) levels between groups of patients with shorter (grey-shaded circles) and longer (gray-shaded quadrants) CPB time. The statistical significance was calculated using repeated measures two-way ANOVA with Sidak’s multiple comparisons test. P values are indicated.
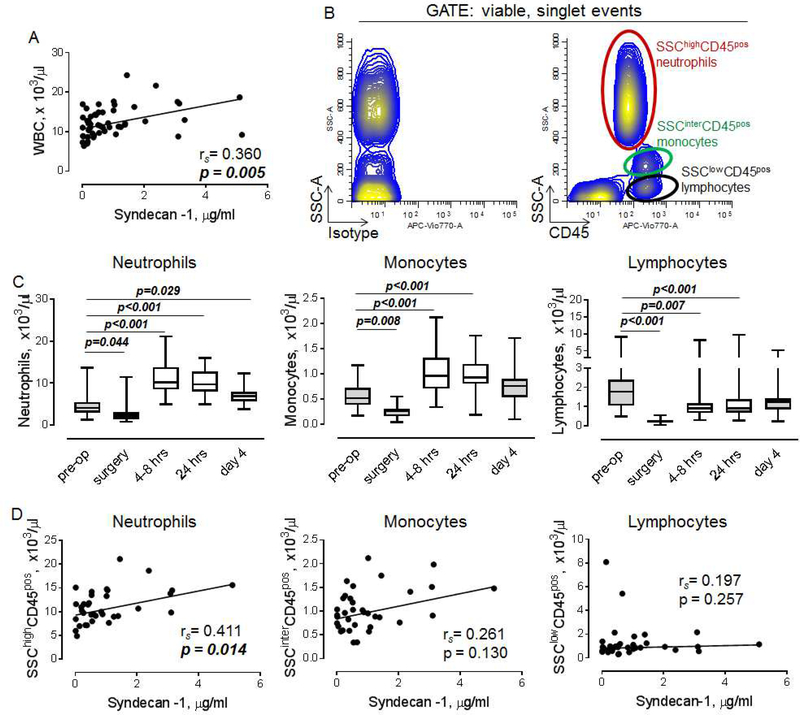
Level of syndecan-1 correlates with the number of peripheral blood neutrophils
Our data revealed a positive association between the level of syndecan-1 and WBC at 4–8 hrs (Figure 3A). To determine the subpopulation of WBC which correlates with elevated syndecan-1, we analyzed freshly isolated blood cells using flow cytometry. Major subpopulations of CD45 positive WBC including SSChigh neutrophils, SSCintermediate monocytes and SSClow lymphocytes were identified as shown in Figure 3B. As expected, the number of circulating neutrophils after the decrease during the surgery was significantly elevated during all time points, including 4–8 hrs, 24hrs and post-operative day 4 (Figure 3C, left). The number of monocytes was increased at 4–8 hrs and 24hrs (Figure 3C, middle). In contrast to myeloid cells, the number of lymphocytes was significantly decreased at 4–8 hrs and 24hrs (Figure 3C, right).
Figure 3. Level of circulating syndecan-1 correlates with the number of neutrophils.
A. The correlation between level of soluble syndecan-1 and WBC at 4–8 hours after the surgery (n=44). B. Flow cytometry showing subpopulations of circulating WBC, stained with isotype-matched control (left) or antibody against CD45 (right). C. Number of neutrophils (left), monocytes (middle) and lymphocytes (right). Friedman test with Dunn’s multiple comparisons test. D. The relationships between levels of syndecan-1 and neutrophils (left), monocytes (middle), and lymphocytes (right). Spearman’s rank correlation coefficient and p values are indicated.
We found that the number of neutrophils is positively associated with the level of syndecan-1 (Figure 3D, left). While not statistically significant, a positive correlation trend between monocytes and syndecan-1 has been identified (Figure 3D, middle). No correlation was found between syndecan-1 and lymphocytes (Figure 3D, right).
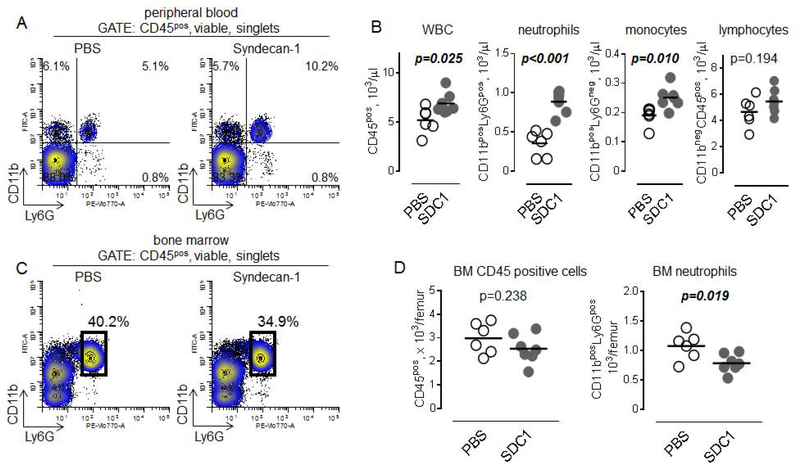
Recombinant syndecan-1 induces mobilization of myeloid cells from bone marrow in mice
To test whether or not syndecan-1 induces egress of neutrophils from bone marrow, we performed experiments with intravenous injections of recombinant human syndecan-1 in mice. The extracellular domain of mouse syndecan-1 shares 70% amino acid identity with the extracellular domain of human syndecan-1. Blood and bone marrow were analyzed for changes in the major subpopulations of WBC. As shown in Figure 4A and 4B, syndecan-1 induced accumulation of neutrophils and monocytes but not lymphocytes in the peripheral circulation. In parallel, total numbers of CD45 positive WBC and neutrophils were significantly decreased in bone marrow (Figure 4C), indicating that accumulation of peripheral blood neutrophils is due to syndecan-1-induced mobilization of neutrophils from bone marrow.
Figure 4. Syndecan-1 induces mobilization of murine bone marrow myeloid cells.
A. Flow cytometry plots showing percentage of CD11bposLy6Gpos circulating neutrophils (upper right quadrant), CD11bposLy6Gneg monocytes (upper left quadrant) and lymphocytes (lower left quadrant) 6 hours later after injection of PBS (control, left) or 120 μg/kg human recombinant syndecan-1 (right). B. Graphical representation of data from flow cytometry showing number of (left to right): WBC, neutrophils, monocytes, and lymphocytes after administration of PBS (n=6) or syndecan-1 (SDC1, n=7). Unpaired t test. C. Flow cytometry plots showing percentage of CD11bposLy6Gpos neutrophils (black gate) in bone marrow 6 hours later after injection of PBS (control, left) or 120 μg/kg human recombinant syndecan-1 (right). D. Number of CD45pos cells (left) and neutrophils (right) in murine bone marrow after administration of PBS (n=6) or syndecan-1 (SDC1, n=7). Unpaired t test.
COMMENT
Our major findings are that a longer duration of CPB is associated with an increased level of soluble syndecan-1, which is associated with an increased number of peripheral blood neutrophils in human patients. Furthermore, we found that syndecan-1 can directly induce neutrophil mobilization from the bone marrow of uninjured mice. Syndecan-1 may set up a vicious cycle of leukocytosis with large numbers of WBC’s getting through the EG defenses and damaging the endothelial monolayer, destroying cellular architecture, violating adherens junctions, then transmigrating extravascularly.
Recently, it has been demonstrated that shedding of endothelial glycocalyx, including syndecan-1, is associated with microcirculation perfusion disorder after coronary artery bypass grafting surgery 25, highlighting the necessity of better understanding of factors contributing to the degradation of EG. Our data demonstrated that significantly higher levels of soluble syndecan-1 characterize patients with longer duration of CPB compared to those patients with shorter CPB time, identifying the prolonged CPB time as an independent risk factor for the shedding of EG. In agreement with our data, the promoting effect of longer duration of CPB on syndecan-1 shedding has been demonstrated in infants after CABG surgery 26. The level of soluble syndecan-1 after the coronary artery bypass grafting surgery with and without CPB has been investigated previously by Svennevig et al. 27, who demonstrated that initiation of CPB induced a rapid increase in the level of syndecan-1. No significant differences were found between on-pump and off-pump patients, suggesting that surgical trauma but not CPB is a major contributor to the degradation of EG. This is in contradistinction to this work and that of Rhem et al. 14, who showed that induction of anesthesia and initiation of surgery did not result in significant increases in syndecan-1 shedding. In our study, we investigated the level of syndecan-1 in on-pump patients only and, therefore, cannot comment on the contribution of CPB versus surgery trauma to glycocalyx shedding. Our data, however, indicate that the duration of CPB could be an additional risk factor that either increases the rate of EG damage or limits the clearance of soluble syndecan-1 from the circulation during the late recovery period after the operation. The latter can be due to kidney damage, which is more common in patients with prolonged CPB time28.
More important, shed syndecan-1 is a biologically active proteoglycan, which forms complexes with cytokines and growth factors, and increased levels of soluble syndecan-1 can contribute to the shift in the circulatory function 12. Our data revealed the presence of a correlation between the level of soluble syndecan-1 and neutrophils. This correlation and the observed increase of neutrophil count in mice injected with recombinant syndecan-1 allow us to suggest that shed syndecan-1 is a factor involved in CPB-related inflammation. This suggestion is supported by the results of a study showing that syndecan-1 shedding is important for neutrophil activation and invasion during lung injury 18. Interestingly, the soluble form of a similar glycocalyx protein, syndecan-4, has been shown to induce the upregulation of cell surface inflammation markers ICAM and VCAM, and proinflammatory cytokines in cardiovascular cells, suggesting a positive regulation of neutrophil recruitment by shed syndecans 29. The neutrophils directly or indirectly mobilized by shed syndecan-1 could further enhance EG shedding because they are characterized by elevated production of secreted proteases, reactive oxygen species and myeloperoxidase 30. It would be interesting to determine whether neutrophils can mediate syndecan shedding, which could represent a positive feedback mechanism contributing to the amplification of inflammatory responses after surgery. Previous work has demonstrated an association between circulating cell-free DNA and the level of soluble syndecan-1, suggesting the contribution of DNA released from cells to EG degradation 31.
We found that initiation of CPB causes an immediate increase in the level of plasma mtDNA. However, we did not observe a significant correlation between the immediate post-operative mtDNA content in plasma and later increase of syndecan-1. In our recent in vitro study 32, the reliable destabilization of the endothelial monolayer by mtDNA occurred when it was applied at the concentrations higher than 300 ng/ml. In the present study, the mtDNA content in patients plasma never exceeded 70 ng/ml. Our results show that while there are elevated levels of mtDNA soon after the initiation of CPB, the content is not sufficient to induce shedding of syndecan-1 and may be a consequence of the activation of platelets rather than cellular damage.
Proinflammatory cytokines, such as IL-6, are known to activate the expression of matrix metalloproteinases 33, 34, including MMP-7 35, which are involved in the shedding of syndecan-1 17. Our data demonstrated that the level of IL-6 was significantly increased in patients after the operation. However, we did not find an association between levels of IL-6 and syndecan-1, suggesting that IL-6 is not a major factor contributing to CPB-induced elevation of soluble syndecan-1.
With regard to limitations, the small heterogeneous sample size can cause difficulty in finding correlations and demonstrating causation. Other correlations may exist in the complex post-CPB milieu that we did not recognize. The operations were performed by five surgeons, which results in heterogeneity in intra- and post-operative patient management. We hypothesize that shedding of the EG can lead to EC damage and inflammation contributing to post-operative complications and worse patient outcomes; however, this study was not powered to identify clinical endpoints, and the patients were low risk. In our study, we purposefully excluded patients with comorbidities to exclude confounding factors related to pre-existing inflammation that could contribute to the degradation of the EG glycocalyx.
In summary, we found that prolonged CPB contributes to an elevated level of syndecan-1, which may promote mobilization of neutrophils from the bone marrow with a resulting leukocytosis. Neutrophils are equipped with a vast variety of bioactive factors that can contribute to the amplification of local inflammation 36. Those neutrophils can access the vulnerable endothelial cells, getting past the compromised protective EG and transmigrate into the extracellular space via intracellular gaps. Therefore, we speculate that cardiac surgery with prolonged CPB is associated with EG shedding and mobilization of neutrophils from the bone marrow, contributing to and amplifying a systemic inflammatory response. It has been shown that heparin biocompatible coating can significantly prevent the increase in the level of circulating syndecan-1 37. In future studies, it would be interesting to study the effect of treatments that prevent the degradation of EG on systemic inflammatory response after cardiovascular surgery with prolonged CPB time.
Acknowledgments
We are grateful to Joanne S. Burgess and Susan Bosworth-Farrell for subject recruitment and providing logistical support during sample collection.
Funding Statement: This work was supported by the Maine Medical Center Cardiovascular Research Institute 2015 Pilot Project Program, the National Heart, Lung, and Blood Institute of the National Institutes of Health under grants U01 HL100398 and R01 HL136560, R01 HL 139887, and the American Heart Association under grant 17POST33410474. We utilized Maine Medical Center’s Progenitor Cell Analysis Core facility which is supported by NIH/NIGMS grants P30GM106391, COBRE in Stem and Progenitor Cell Biology and Regenerative Medicine and U54GM115516, Northern New England Clinical and Translational Research Network (Translational Technologies Core). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Welsby IJ, Bennett-Guerrero E, Atwell D, White WD, Newman MF, Smith PK and Mythen MG. The association of complication type with mortality and prolonged stay after cardiac surgery with cardiopulmonary bypass. Anesth Analg. 2002;94:1072–8. [DOI] [PubMed] [Google Scholar]
- 2.Salis S, Mazzanti VV, Merli G, Salvi L, Tedesco CC, Veglia F and Sisillo E. Cardiopulmonary Bypass Duration Is an Independent Predictor of Morbidity and Mortality After Cardiac Surgery. Journal of Cardiothoracic and Vascular Anesthesia. 2008;22:814–822. [DOI] [PubMed] [Google Scholar]
- 3.Butler J, Rocker GM and Westaby S. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 1993;55:552–9. [DOI] [PubMed] [Google Scholar]
- 4.Hunt BJ, Parratt RN, Segal HC, Sheikh S, Kallis P and Yacoub M. Activation of coagulation and fibrinolysis during cardiothoracic operations. Ann Thorac Surg. 1998;65:712–8. [DOI] [PubMed] [Google Scholar]
- 5.Verrier ED and Morgan EN. Endothelial response to cardiopulmonary bypass surgery. Ann Thorac Surg. 1998;66:S17–9; discussion S25–8. [DOI] [PubMed] [Google Scholar]
- 6.Schmid FX, Vudattu N, Floerchinger B, Hilker M, Eissner G, Hoenicka M, Holler E and Birnbaum DE. Endothelial apoptosis and circulating endothelial cells after bypass grafting with and without cardiopulmonary bypass. Eur J Cardiothorac Surg. 2006;29:496–500. [DOI] [PubMed] [Google Scholar]
- 7.Weerasinghe A and Taylor KM. The platelet in cardiopulmonary bypass. Ann Thorac Surg. 1998;66:2145–52. [DOI] [PubMed] [Google Scholar]
- 8.Sandler N, Kaczmarek E, Itagaki K, Zheng Y, Otterbein L, Khabbaz K, Liu D, Senthilnathan V, Gruen RL and Hauser CJ. Mitochondrial DAMPs Are Released During Cardiopulmonary Bypass Surgery and Are Associated With Postoperative Atrial Fibrillation. Heart, Lung and Circulation. 2018;27:122–129. [DOI] [PubMed] [Google Scholar]
- 9.Cognasse F, Laradi S, Berthelot P, Bourlet T, Marotte H, Mismetti P, Garraud O and Hamzeh-Cognasse H. Platelet Inflammatory Response to Stress. Front Immunol. 2019;10:1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pittman K and Kubes P. Damage-associated molecular patterns control neutrophil recruitment. J Innate Immun. 2013;5:315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakayama H and Otsu K. Mitochondrial DNA as an inflammatory mediator in cardiovascular diseases. Biochem J. 2018;475:839–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teng YH, Aquino RS and Park PW. Molecular functions of syndecan-1 in disease. Matrix Biol. 2012;31:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palaiologou M, Delladetsima I and Tiniakos D. CD138 (syndecan-1) expression in health and disease. Histol Histopathol. 2014;29:177–89. [DOI] [PubMed] [Google Scholar]
- 14.Endo K, Takino T, Miyamori H, Kinsen H, Yoshizaki T, Furukawa M and Sato H. Cleavage of syndecan-1 by membrane type matrix metalloproteinase-1 stimulates cell migration. J Biol Chem. 2003;278:40764–70. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Wang Z, Liu J, Zhang S, Fei J, Li J, Zhang T, Wang J, Park PW and Chen Y. Cell surface-anchored syndecan-1 ameliorates intestinal inflammation and neutrophil transmigration in ulcerative colitis. J Cell Mol Med. 2017;21:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swee M, Wilson CL, Wang Y, McGuire JK and Parks WC. Matrix metalloproteinase-7 (matrilysin) controls neutrophil egress by generating chemokine gradients. J Leukoc Biol. 2008;83:1404–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Q, Park PW, Wilson CL and Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111:635–46. [DOI] [PubMed] [Google Scholar]
- 18.Gill SE, Nadler ST, Li Q, Frevert CW, Park PW, Chen P and Parks WC. Shedding of Syndecan-1/CXCL1 Complexes by Matrix Metalloproteinase 7 Functions as an Epithelial Checkpoint of Neutrophil Activation. Am J Respir Cell Mol Biol. 2016;55:243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mosheimer BA, Kaneider NC, Feistritzer C, Djanani AM, Sturn DH, Patsch JR and Wiedermann CJ. Syndecan-1 Is Involved in Osteoprotegerin-Induced Chemotaxis in Human Peripheral Blood Monocytes. The Journal of Clinical Endocrinology & Metabolism. 2005;90:2964–2971. [DOI] [PubMed] [Google Scholar]
- 20.Torres LN, Chung KK, Salgado CL, Dubick MA and Torres Filho IP. Low-volume resuscitation with normal saline is associated with microvascular endothelial dysfunction after hemorrhage in rats, compared to colloids and balanced crystalloids. Crit Care. 2017;21:160–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gouverneur M, Berg B, Nieuwdorp M, Stroes E and Vink H. Vasculoprotective properties of the endothelial glycocalyx: effects of fluid shear stress. J Intern Med. 2006;259:393–400. [DOI] [PubMed] [Google Scholar]
- 22.Zeng Y Endothelial glycocalyx as a critical signalling platform integrating the extracellular haemodynamic forces and chemical signalling. Journal of cellular and molecular medicine. 2017;21:1457–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paparella D, Yau TM and Young E. Cardiopulmonary bypass induced inflammation: pathophysiology and treatment. An update. Eur J Cardiothorac Surg. 2002;21:232–44. [DOI] [PubMed] [Google Scholar]
- 24.Bronicki RA and Hall M. Cardiopulmonary Bypass-Induced Inflammatory Response: Pathophysiology and Treatment. Pediatr Crit Care Med. 2016;17:S272–8. [DOI] [PubMed] [Google Scholar]
- 25.Wu Q, Gao W, Zhou J, He G, Ye J, Fang F, Luo J, Wang M, Xu H and Wang W. Correlation between acute degradation of the endothelial glycocalyx and microcirculation dysfunction during cardiopulmonary bypass in cardiac surgery. Microvascular Research. 2019;124:37–42. [DOI] [PubMed] [Google Scholar]
- 26.Bruegger D, Brettner F, Rossberg I, Nussbaum C, Kowalski C, Januszewska K, Becker BF and Chappell D. Acute degradation of the endothelial glycocalyx in infants undergoing cardiac surgical procedures. Ann Thorac Surg. 2015;99:926–31. [DOI] [PubMed] [Google Scholar]
- 27.Svennevig K, Hoel T, Thiara A, Kolset S, Castelheim A, Mollnes T, Brosstad F, Fosse E and Svennevig J. Syndecan-1 plasma levels during coronary artery bypass surgery with and without cardiopulmonary bypass. Perfusion. 2008;23:165–71. [DOI] [PubMed] [Google Scholar]
- 28.Boldt J, Brenner T, Lehmann A, Suttner SW, Kumle B and Isgro F. Is kidney function altered by the duration of cardiopulmonary bypass? The Annals of Thoracic Surgery. 2003;75:906–912. [DOI] [PubMed] [Google Scholar]
- 29.Strand ME, Aronsen JM, Braathen B, Sjaastad I, Kvaloy H, Tonnessen T, Christensen G and Lunde IG. Shedding of syndecan-4 promotes immune cell recruitment and mitigates cardiac dysfunction after lipopolysaccharide challenge in mice. J Mol Cell Cardiol. 2015;88:133–44. [DOI] [PubMed] [Google Scholar]
- 30.Chistiakov DA, Bobryshev YV and Orekhov AN. Neutrophil’s weapons in atherosclerosis. Exp Mol Pathol. 2015;99:663–71. [DOI] [PubMed] [Google Scholar]
- 31.Johansson PI, Windelov NA, Rasmussen LS, Sorensen AM and Ostrowski SR. Blood levels of histone-complexed DNA fragments are associated with coagulopathy, inflammation and endothelial damage early after trauma. J Emerg Trauma Shock. 2013;6:171–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prudovsky I, Carter D, Kacer D, Palmeri M, Soul T, Kumpel C, Pyburn K, Barrett K, DeMambro V, Alexandrov I, Brandina I, Kramer R and Rappold J. Tranexamic acid suppresses the release of mitochondrial DNA, protects the endothelial monolayer and enhances oxidative phosphorylation. J Cell Physiol. 2019;234:19121–19129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kothari P, Pestana R, Mesraoua R, Elchaki R, Khan KMF, Dannenberg AJ and Falcone DJ. IL-6-mediated induction of matrix metalloproteinase-9 is modulated by JAK-dependent IL-10 expression in macrophages. Journal of immunology (Baltimore, Md : 1950). 2014;192:349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kossakowska AE, Edwards DR, Prusinkiewicz C, Zhang MC, Guo D, Urbanski SJ, Grogan T, Marquez LA and Janowska-Wieczorek A. Interleukin-6 regulation of matrix metalloproteinase (MMP-2 and MMP-9) and tissue inhibitor of metalloproteinase (TIMP-1) expression in malignant non-Hodgkin’s lymphomas. Blood. 1999;94:2080–9. [PubMed] [Google Scholar]
- 35.Pini M, Rhodes DH, Castellanos KJ, Hall AR, Cabay RJ, Chennuri R, Grady EF and Fantuzzi G. Role of IL-6 in the resolution of pancreatitis in obese mice. J Leukoc Biol. 2012;91:957–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright HL, Moots RJ, Bucknall RC and Edwards SW. Neutrophil function in inflammation and inflammatory diseases. Rheumatology (Oxford). 2010;49:1618–31. [DOI] [PubMed] [Google Scholar]
- 37.Dekker NAM, Veerhoek D, van Leeuwen ALI, Vonk ABA, van den Brom CE and Boer C. Microvascular Alterations During Cardiac Surgery Using a Heparin or Phosphorylcholine-Coated Circuit. Journal of Cardiothoracic and Vascular Anesthesia. 2019. [DOI] [PubMed] [Google Scholar]