Abstract
Rationale & Objective:
Studies using a single measurement of fibroblast growth factor 23 (FGF-23) suggest that elevated FGF-23 levels are associated with increased risk for requirement for kidney replacement therapy (KRT) in patients with chronic kidney disease. However, the data do not account for changes in FGF-23 levels as chronic kidney disease progresses.
Study Design:
Case-cohort study.
Setting & Participants:
To evaluate the association between serial FGF-23 levels and risk for requiring KRT, our primary analysis included 1,597 individuals in the Chronic Renal Insufficiency Cohort Study who had up to 5 annual measurements of carboxy-terminal FGF-23. There were 1,135 randomly selected individuals, of whom 266 initiated KRT, and 462 individuals who initiated KRT outside the random subcohort.
Exposure:
Serial FGF-23 measurements and FGF-23 trajectory group membership.
Outcomes:
Incident KRT.
Analytical Approach:
To handle time-dependent confounding, our primary analysis of time-updated FGF-23 levels used time-varying inverse probability weighting in a discrete time failure model. To compare our results with prior data, we used baseline and time-updated FGF-23 values in weighted Cox regression models. To examine the association of FGF-23 trajectory subgroups with risk for incident KRT, we used weighted Cox models with FGF-23 trajectory groups derived from group-based trajectory modeling as the exposure.
Results:
In our primary analysis, the HR for the KRT outcome per 1 SD in the mean of natural log-transformed (ln)FGF-23 in the past was 1.94 (95% CI, 1.51–2.49). In weighted Cox models using baseline and time-updated values, elevated FGF-23 level was associated with increased risk for incident KRT (HRs per 1 SD lnFGF-23 of 1.18 [95% CI, 1.02–1.37] for baseline and 1.66 [95% CI, 1.49–1.86] for time-updated). Membership in the slowly and rapidly increasing FGF-23 trajectory groups was associated with ~3- and ~21-fold higher risk for incident KRT compared to membership in the stable FGF-23 trajectory group.
Limitations:
Residual confounding and lack of intact FGF-23 values.
Conclusions:
Increasing FGF-23 levels are independently associated with increased risk for incident KRT.
Progressive loss of kidney function in patients with chronic kidney disease (CKD) culminates in kidney failure. Reduced estimated glomerular filtration (eGFR) and elevated urinary albumin-creatinine ratio are strong and well-established predictors of kidney failure requiring kidney replacement therapy (KRT).1–5 Mechanisms that contribute to CKD progression include hypertension, activation of the renin-angiotensin-aldosterone system, inflammation, and interstitial injury.6 Numerous investigations are focused on identifying additional modifiable mechanisms for accelerated decline in kidney function.
Fibroblast growth factor 23 (FGF-23), an osteocyte-derived hormone, regulates phosphate homeostasis by stimulating urinary phosphate excretion and lowering 1,25-dihydroxyvitamin D levels.7 Plasma FGF-23 level increases early in CKD and may be thousands-fold higher than baseline by the time individuals approach the need for KRT.8 Although definitive evidence linking elevated FGF-23 level to kidney function decline is lacking, several proposed mechanisms that require further testing have emerged. By promoting phosphaturia and increasing intratubular phosphate concentrations, elevated FGF-23 levels may induce renal tubular damage and tubulointerstitial fibrosis.9,10 Elevated FGF-23 levels may also promote CKD progression by directly stimulating renal fibrosis and indirectly by sup-pressing 1,25-dihydroxyvitamin D levels and stimulating production of proinflammatory cytokines.11–13 Observational studies that measured FGF-23 at a single time point reported independent associations between elevated FGF-23 levels and increased risk for requirement for KRT in some but not in all studies.14–16 Data for the association of serial FGF-23 levels with risk for CKD progression are limited.17,18 Using up to 5 annual serial FGF-23 measurements, we conducted a prospective observational case-cohort study within the Chronic Renal Insufficiency Cohort (CRIC) Study to test the hypothesis that serial plasma FGF-23 levels are independently associated with increased risk for requirement for KRT in patients with mild to moderate CKD.
Methods
Study Population
The CRIC Study is a longitudinal prospective cohort study of 3,939 individuals aged 21 to 74 years with CKD stages 2 to 4.19,20 Participants were recruited between June 2003 and September 2008 from 7 clinical centers across the United States.19,20 The study design and methods have previously been published.19,20 The institutional review board at each site approved the study protocol, and all participants provided written informed consent.
Study Design
We performed a case-cohort study.21–26 We measured FGF-23 at 2 to 5 annual time points in 1,135 individuals who were included in the randomly selected CRIC Study longitudinal mineral metabolism subcohort.27 All 1,135 individuals had FGF-23 measured at enrollment and at least at 1 additional annual visit. We then sampled outside the subcohort an additional 462 individuals who initiated KRT within 5 years of the baseline visit. All included individuals had FGF-23 levels measured at 2 to 5 annual time points, including baseline FGF-23.
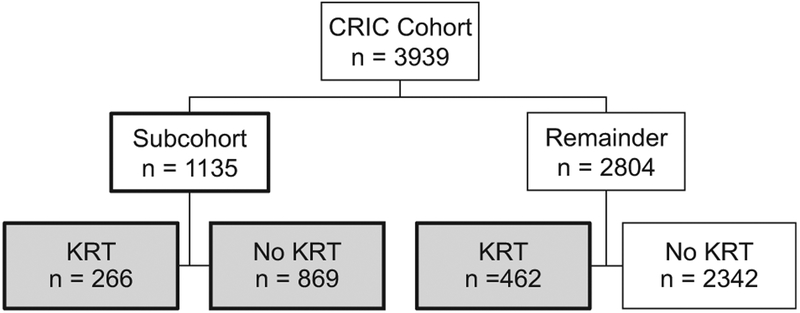
We examined the relationship between serial FGF-23 measurements and risk for KRT requirement using multiple integrative approaches. As our primary approach, we used time-varying inverse probability weighting (IPW) in a discrete time failure model, which allowed us to investigate longitudinal risk relationships with appropriate adjustment for time-dependent confounders.28 To bench-mark our results against prior findings on the relationship between baseline FGF-23 level and risk for KRT requirement, we also performed weighted Cox proportional hazards models using baseline and time-updated FGF-23 levels. The total analytic population for models using baseline and time-updated FGF-23 levels and the time-varying IPW discrete time failure models included 1,597 individuals, of whom 728 initiated KRT, with 266 cases within the subcohort and 462 cases outside the subcohort (Fig 1). To examine the association of distinct FGF-23 trajectory subgroups with risk for KRT requirement, we used weighted Cox models with FGF-23 trajectory groups derived from group-based trajectory modeling as the exposure.27 For our trajectory-based analyses, individuals needed to have a baseline FGF-23 measurement and at least 1 other FGF-23 measurement available and have survived beyond their fifth annual study visit without initiating KRT. Our total analytic population for our trajectory-based analyses included 1,163 individuals.
Figure 1.
Flow chart for study population in the case-cohort study design. Fibroblast growth factor 23 (FGF-23) was measured at 2 to 5 annual time points in 1,135 individuals who were included in the randomly selected Chronic Renal Insifficiency Cohort (CRIC) Study longitudinal mineral metabolism subcohort. We then sampled outside the subcohort an additional 462 individuals who reached the kidney replacement therapt (KRT) outcome in whom we also measured FGF-23 at 2 to 5 annual time points. The total analytic population for all analyses, except for FGF-23 trajectory analyses, included 1,597 individuals, of whom 728 reached the KRT outcome, with 266 cases within the subcohort and 462 cases outside the subcohort.
Primary Exposure
The primary exposures were baseline and time-updated FGF-23 levels and FGF-23 trajectory group membership. The CRIC Central Laboratory measured FGF-23 after a single thaw of frozen plasma samples from the baseline study visit and from up to 5 annual visits.16 On average, participants had 4.0 ± 1.2 annual FGF-23 measurements. FGF-23 was measured in duplicate using a second-generation carboxy-terminal assay (Quidel). The mean intra-assay coefficient of variation for paired assays was <6.5% and the lower limit of detection was 3 RU/mL.16
Primary Outcomes
The primary outcome was requirement of KRT, defined as initiation of maintenance dialysis or receipt of a kidney transplant. Participants were followed up until KRT initiation, death, withdrawal from the study, loss to follow-up, or mid-2013, when the database was locked for analysis. Given that individuals needed to survive to initiate KRT, we also incorporated a secondary composite outcome of KRT or death.
Covariates
We used demographic and laboratory data collected at baseline and at up to 5 annual time points. Serum creatinine was measured using standard assays in real time at the annual study visits. eGFR was calculated using the CKD Epidemiology Collaboration (CKD-EPI) equation.29 Data for other measured covariates and assays are provided in Item S1.
Statistical Analysis
Time-Varying IPW Discrete Time Failure Models
For our primary analyses, we performed time-varying IPW discrete time failure models using time-updated covariates (Item S1).28,30,31 We used the time-varying IPW method to appropriately adjust for the effects of eGFR as a time-dependent confounder, which occurs when a covariate affects both the exposure and outcome but also affects prior exposure. In the risk relationship between FGF-23 level and KRT, FGF-23 level may affect eGFR but eGFR can affect prior FGF-23 levels. eGFR is a both a confounder affecting FGF-23 levels and KRT risk and a mediator on the causal pathway between FGF-23 and KRT risk. Standard methods that adjust for eGFR would adjust for both the confounding and mediator effects and may underestimate the true association between FGF-23 level and KRT. The time-varying IPW method allows for adjustment of confounding by time-dependent eGFR without incorrectly adjusting for its mediator effects.32
The IPW method is a 2-step approach that uses as the first step the calculation of the FGF-23 exposure weights and as the second step the fitting of a discrete time failure model for the outcomes (KRT and KRT or death) by applying the final weight derived in the first step.
In the first step of performing the IPW method, we calculated the FGF-23 exposure IPW-stabilized weights. Because FGF-23 levels were not normally distributed, we modeled FGF-23 level at each visit in quartiles, as was done in a prior CRIC Study analysis using time-varying IPW models.28,30,31,33 To compute conditional probabilities, we performed multinomial logistic regression on FGF-23 quartiles with adjustment for time-varying covariates including eGFR with the exception of C-reactive protein and interleukin 6, which were only measured at baseline. The unstabilized weight for a specific study visit was calculated as one over the cumulative probabilities of the observed FGF-23 level history up to that visit (the denominator of stabilized weights). To allow for valid inference, we next stabilized the weights by multiplying the unstabilized weight by the unconditional probability of observed FGF-23 level history adjusting for baseline-only predictors without conditioning on time-varying covariates (the numerator of stabilized weights).34,35 Given the small number of loss to follow-up, we did not calculate the censoring weight.36 The final stabilized IPW weight is the ratio of the unconditional probability (the numerator) to the conditional probability (denominator). In the second step of performing the IPW method, inverse probability weighted structural models were fit using a discrete time failure model for the KRT outcome by applying the final stabilized weight calculated in the first step to the study visit level data, as previously done (Item S1).28,34,35 FGF-23 level history was modeled in the final model as the mean FGF-23 level in the past.
Assuming that the assumptions of positivity, exchangeability, and correct specification of the weight models are met37 (Item S1), the hazard ratio (HR) estimated from the time-varying IPW discrete time failure model can be interpreted as the HR estimate per 1–standard deviation (SD) increase in mean ln(FGF-23) in the past under conditions of proper adjustment for time-dependent confounding.
Baseline and Time-Updated Analyses
We used the Barlow weighting method for the case-cohort design in the weighted Cox proportional hazards models24 for our baseline and time-updated analyses. We analyzed time to KRT initiation according to baseline FGF-23 level expressed as a continuous variable with HRs calculated per 1-SD increase in ln(FGF-23). Next, we analyzed time to KRT initiation according to time-updated ln(FGF-23) per the same 1-SD increments used for baseline ln(FGF-23). For baseline and time-updated analyses, follow-up time began at the baseline CRIC Study visit. We hierarchically adjusted for possible confounders, including demographics (age, sex, race, and ethnicity), CKD-specific risk factors (eGFR, urinary protein-creatinine ratio, serum albumin, hemoglobin, C-reactive protein, and interleukin 6 values), cardiovascular risk factors (diabetes, smoking, systolic blood pressure, body mass index, history of coronary artery disease, heart failure, stroke, peripheral vascular disease, number of blood pressure medications, use of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker), and markers of mineral metabolism (calcium, phosphate, and parathyroid hormone [PTH] levels). All adjusted models included a stratification term by study site. For analyses that examined baseline FGF-23 levels, we adjusted for baseline covariates. For analyses that examined time-updated FGF-23 levels, we adjusted for time-updated covariates when appropriate, except for C-reactive protein and interleukin 6, which were only available at baseline. We calculated Schoenfeld residuals in the fully adjusted model for both the KRT and the composite outcome to confirm that the proportionality assumption for ln(FGF-23) was not violated. We also performed correlation tests for our primary KRT outcome between Schoenfeld residuals and event time, rank order of the event times, and Kaplan-Meier estimates to test for the proportionality assumption for ln(FGF-23).38
Trajectory-Based Analyses
We performed group-based trajectory modeling to identify subpopulations with distinct patterns of FGF-23 exposure.27,39 Our total analytic population for the trajectory-based analyses included 1,163 individuals. Similar to prior analyses, we identified 3 FGF-23 trajectory categories: stable, slowing increasing, and rapidly increasing (Item S1).27 We derived eGFR trajectories using analogous methods.
We estimated the risk for KRT for each FGF-23 trajectory group compared to the referent FGF-23 trajectory group.24 The participants’ fifth annual visit was classified as time 0 (onset of longitudinal follow-up) for our survival analyses. We hierarchically adjusted for confounding by demographics, CKD-specific risk factors, cardiovascular disease risk factors, and markers of mineral metabolism. The multivariable adjustment was completed based on covariate values at time 0 (fifth annual study visit) and we also adjusted for baseline eGFR and eGFR trajectory group. In secondary analyses, we derived the FGF-23 trajectory groups using only 3 annual time points, including only those who survived without reaching the KRT outcome by their third annual study visit. We graphed individual observed FGF-23 values according to predicted FGF-23 trajectory group memberships using spaghetti plots.40
Additional Analyses
Given experimental evidence linking total-body phosphate load with increases in FGF-23 levels,28 in additional analyses we separately adjusted our model using baseline FGF-23 level as the exposure for baseline 24-hour urinary phosphate and fractional excretion of phosphate. FGF-23 inhibits production of 1,25-dihydroxyvitamin D,7 and low calcitriol levels are associated with CKD progression.41 In our model using time-updated FGF-23 levels, we additionally adjusted for time-updated 1,25-dihydroxyvitamin D levels. Serum bicarbonate levels are also associated with CKD progression.42 In our model using time-updated FGF-23 levels, we additionally adjusted for time-updated serum bicarbonate levels.
All analyses were performed using SAS, version 9.4. Two-sided P < 0.05 was considered statistically significant.
Results
Baseline characteristics were similar in cases within the subcohort and cases outside the subcohort (Table 1). Participants who went on to require KRT had higher systolic blood pressures, were more likely be black and Hispanic and have diabetes, heart failure, peripheral vascular disease, and coronary artery disease; and had lower baseline eGFR and higher urinary protein-creatinine ratio, PTH, and FGF-23 values than individuals who did not reach the KRT outcome (Table 1). Baseline characteristics of individuals from the entire CRIC Study cohort, individuals within the random subcohort, and those outside the random subcohort are presented in Table S1. Table S2 demonstrates baseline characteristics of the case cohort and trajectory analyses population compared with the entire CRIC population.
Table 1.
Baseline Characteristics of the Study Population
| Random Subcohort | Added Cases | ||
|---|---|---|---|
| Baseline | No KRT (n = 869) | KRT (n = 266) | Added Cases of KRT (n = 462) |
| Age, y | 58.3 ± 10.4 | 56.0 ± 11.9 | 55.5 ± 11.5 |
| Female sex | 46.0% | 33.8% | 41.8% |
| Black | 39.8% | 51.1% | 52.6% |
| Hispanic | 10.2% | 17.3% | 16.7% |
| Current smoking | 10.2% | 11.7% | 17.1% |
| Body mass index, kg/m2 | 32.0 ± 7.7 | 33.2 ± 9.0 | 32.4 ± 7.6 |
| Systolic blood pressure, mm Hg | 124.2 ± 20.2 | 136.3 ± 22.4 | 139.5 ± 24.4 |
| Hypertension | 83.9% | 93.6% | 93.9% |
| Diabetes | 44.9% | 60.9% | 64.9% |
| Heart failure | 6.6% | 11.7% | 12.3% |
| Stroke | 8.9% | 9.4% | 11.7% |
| Peripheral vascular disease | 4.8% | 7.5 % | 10.6% |
| Coronary artery disease | 19.6% | 23.7% | 22.7% |
| No. of hypertension medications | 2.3 ± 1.3 | 2.8 ± 1.3 | 3.0 ± 1.1 |
| ACEi/ARB use | 68.6% | 72.4% | 73.3% |
| IL-6, pg/mL | 1.8 [1.0–2.9] | 2.4 [1.5–3.8] | 2.1 [1.4–3.3] |
| CRP, mg/L | 2.5 [1.1–6.3] | 2.6 [1.0–7.1] | 2.7 [1.0–6.5] |
| eGFR, mL/min/1.73 m2 | 48.2 ± 14.0 | 33.9 ± 10.6 | 34.8 ± 11.2 |
| UPCR (g/g) | 0.09 [0.05–0.28] | 1.13 [0.35–2.48] | 1.28 [0.44–3.36] |
| Hemoglobin, g/dL | 12.9 ± 1.7 | 11.9 ± 1.8 | 11.9 ± 1.8 |
| Serum albumin, g/dL | 4.0 ± 0.4 | 3.8 ± 0.5 | 3.7 ± 0.5 |
| Calcium, mg/dL | 9.3 ± 0.5 | 9.1 ± 0.5 | 9.0 ± 0.5 |
| Phosphate, mg/dL | 3.6 ± 0.6 | 3.9 ± 0.7 | 4.0 ± 0.8 |
| PTH, pg/mL | 47.0 [32.0–72.7] | 89.0 [55.7–138.0] | 81.8 [49.1–135.0] |
| FGF-23, RU/mL | 126.1 [87.2–189.7] | 189.4 [130.7–305.5] | 202.9 [136.4–315.3] |
Note: Results are reported as proportion, mean ± standard deviation, or median [interquartile range].
Abbreviations: ACEi/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; FGF-23, fibroblast growth factor 23; IL-6, interleukin 6; KRT, kidney replacement therapy; PTH, parathyroid hormone; UPCR, urinary protein-creatinine ratio.
Baseline and Time-Updated FGF-23 and Risk for KRT Requirement
Within the case-cohort analytic sample (n = 1,597), 728 participants reached the KRT outcome and 859 experienced the composite outcome of KRT or death (131 death events, 196 KRT events before death, and 532 KRT events without death) during a median follow-up of 6.3 years.
In our time-varying IPW discrete time failure model, every 1-SD increase in the mean of ln(FGF-23) in the past was significantly associated with a 1.94-fold higher adjusted risk foe the KRT outcome (adjusted HR, 1.94; 95% confidence interval [CI], 1.51–2.49; Table 2). Results from our baseline and time-updated FGF-23 models demonstrated similar results. Every 1-SD increase in baseline ln(FGF-23) was significantly associated with 1.18-fold higher adjusted risk for the KRT outcome (95% CI, 1.02–1.37; Table 2). Time-updated FGF-23 level was associated with stronger adjusted risk for the KRT outcome (HR per 1-SD increase in ln[FGF-23], 1.66; 95% CI, 1.49–1.86; Table 2). Baseline, time-updated, and time-varying IPW discrete time failure models all demonstrated that plasma FGF-23 level was independently associated with the composite outcome of KRT or death (Table 2).
Table 2.
Baseline and Time-Updated FGF-23 and Risks for the KRT Outcome and the Composite Outcome of KRT or Death
| KRT (n = 728 events) | KRT or Death (n =859 events) | |||
|---|---|---|---|---|
| Weighted Cox Models | Baseline FGF-23a | Time-Updated FGF-23b | Baseline FGF-23a | Time-Updated FGF-23b |
| Unadjusted HR | 1.79 (1.64–1.96) | 2.21 (2.01–2.42) | 1.78 (1.63–1.94) | 2.13 (1.95–2.33) |
| Model 1 HR | 1.81 (1.62–2.03) | 2.29 (2.07–2.53) | 1.81 (1.62–2.02) | 2.18 (1.98–2.40) |
| Model 2 HR | 1.22 (1.07–1.40) | 1.54 (1.39–1.70) | 1.27 (1.12–1.43) | 1.52 (1.38–1.67) |
| Model 3 HR | 1.19 (1.04–1.37) | 1.57 (1.41–1.74) | 1.23 (1.09–1.39) | 1.54 (1.40–1.69) |
| Model 4 HR | 1.18 (1.02–1.37) | 1.66 (1.49–1.86) | 1.21 (1.06–1.38) | 1.62 (1.47–1.78) |
| Time-varying IPW discrete time failure models, fully adjustedb | Not estimated | 1.94 (1.51–2.49) | Not estimated | 2.12 (1.64–2.73) |
Note: All results are reported per 1-SD increase in natural log-transformed FGF-23 level with SD representing the ln(FGF-23) distribution at the CRIC Study baseline visit. Median duration of subsequent follow-up was 6.3 years in 1,597 total participants at risk. Model 1, stratified by center and adjusted for age, sex, race, and ethnicity. Model 2, model 1 plus estimated glomerular filtration rate, urine protein-creatinine ratio, serum albumin, hemoglobin, CRP (baseline), and IL-6 (baseline) values. Model 3, model 2 plus diabetes, smoking, systolic blood pressure, body mass index, history of coronary artery disease, history of heart failure, history of stroke, history of peripheral vascular disease, number of hypertension medications, and angiotensin-converting enzyme inhibitor/angiotensin receptor blocker use. Model 4: model 3 plus calcium, phosphate, and parathyroid hormone levels.
Abbreviations: CRIC, Chronic Renal Insufficiency Cohort; CRP, C-reactive protein; FGF-23, fibroblast growth factor 23; HR, hazard ratio; IL-6, interleukin 6; IPW, inverse probability weighting; KRT, kidney replacement therapy; SD, standard deviation.
Adjusted for covariates at the baseline visit.
Adjusted for time-updated covariates, except sex, race, ethnicity, and CRP and IL-6 levels.
FGF-23 Trajectories and Risk for KRT Requirement
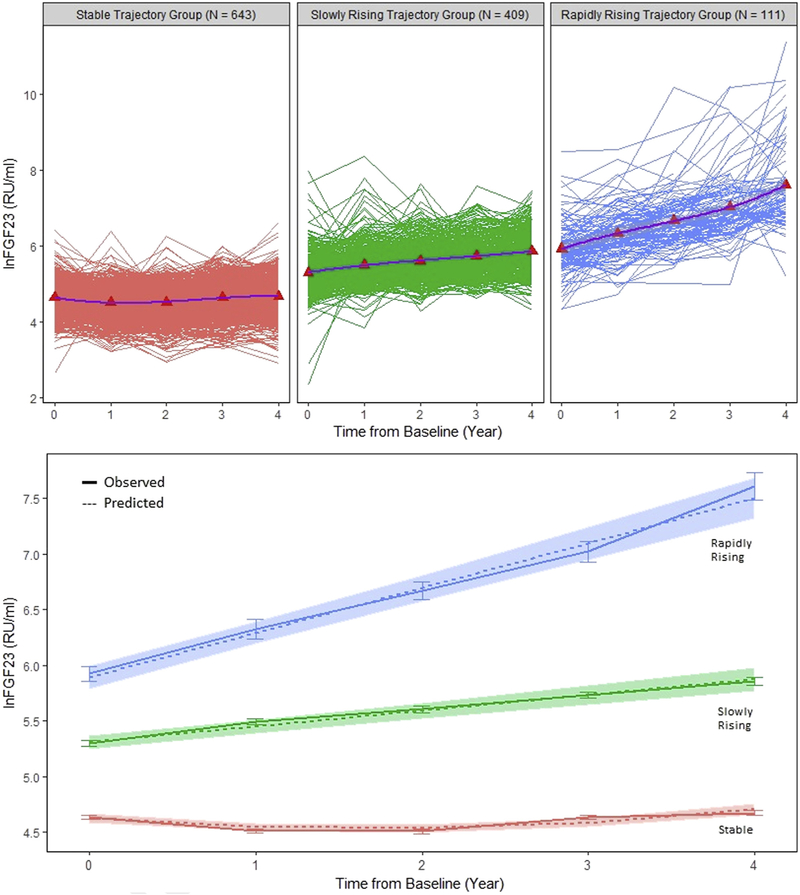
Group-based trajectory modeling among 1,163 individuals who survived beyond their fifth annual study visit without reaching the KRT outcome identified 3 distinct trajectory groups of FGF-23 change over time: stable (n = 643; mean group slope of 0.03 ln[FGF-23] per year), slowly increasing (n = 409; mean group slope of 0.14 ln[FGF-23] per year), and rapidly increasing (n = 111; mean group slope of 0.40 ln[FGF-23] per year; Fig 2). Through algebraic transformation ((exp(mean group slope) – 1) × 100), these mean group slopes can also be expressed as the following percent changes in mean FGF-23 level per year: +3%, +15%, and +49.2%.
Figure 2.
Fibroblast growth factor 23 (FGF-23) trajectories across 5 time points in 1,163 individuals at risk for end-stage kidney disease. (Top panel) Individual FGF-23 measurements across 5 time points (spaghetti-pots) and mean FGF-23 values (red triangle, means connected in purple line) within the predicted FGF-23 trajectory group. (Bottom panel) Mean and standard error of the observed FGF-23 trajectories (solid lines) and predicted trajectories (dashed lines) with 95% confidence intervals (shaded areas).
The rapidly increasing FGF-23 trajectory group had a higher percentage of blacks and Hispanics, higher percentage of women, higher systolic blood pressures, more comorbid conditions, lower eGFRs, and higher phosphate, PTH, and baseline and final FGF-23 values compared with the slowly increasing and stable FGF-23 trajectory groups (Table 3).
Table 3.
Clinical Characteristics of the FGF-23 Trajectory Groups at the Onset of the Survival Time (time 0, fifth annual study visit)
| FGF-23 Trajectory Group | |||
|---|---|---|---|
| Stable (n = 643) | Slowly Increasing (n = 409) | Rapidly Increasing (n = 111) | |
| Age, y | 61.7 ± 10.8 | 61.6 ± 11.1 | 59.6 ± 10.9 |
| Female sex | 39.2% | 51.8% | 55.0% |
| Black | 41.5% | 43.5% | 51.4% |
| Hispanic | 8.2% | 14.7% | 17.1% |
| Current smoking | 6.9% | 14.2% | 10.6% |
| Body mass index, kg/m2 | 31.8 ± 7.6 | 33.3 ± 8.7 | 32.7 ± 9.5 |
| Systolic blood pressure, mm Hg | 127.0 ± 21.8 | 132.2 ± 22.9 | 134.2 ± 21.1 |
| Diabetes | 48.5% | 63.9% | 61.1% |
| Heart failure | 9.7% | 17.7% | 27.7% |
| Stroke | 9.4% | 14.8% | 17.7% |
| Peripheral vascular disease | 7.2% | 14.4% | 17.3% |
| Coronary artery disease | 24.3% | 29.9% | 36.0% |
| No. of hypertension medications | 2.4 ± 1.3 | 2.8 ± 1.2 | 3.1 ± 1.0 |
| ACEi/ARB use | 66.7% | 63.3% | 53.5% |
| IL-6, pg/mLa | 1.5 [0.9–2.6] | 2.1 [1.3–3.4] | 2.1 [1.4–3.7] |
| CRP, mg/La | 2.2 [1.0–6.0] | 2.8 [1.2–6.9] | 3.4 [0.8–7.3] |
| UPCR, g/g | 0.11 [0.06–0.39] | 0.59 [0.15–1.75] | 1.11 [0.42–2.56] |
| Hemoglobin, g/dL | 12.8 ± 1.7 | 11.8 ± 1.7 | 11.2 ± 1.7 |
| Serum albumin, g/dL | 4.0 ± 0.4 | 3.8 ± 0.4 | 3.7 ± 0.4 |
| Calcium, mg/dL | 9.3 ± 0.5 | 9.2 ± 0.6 | 9.2 ± 0.6 |
| Phosphate, mg/dL | 4.0 ± 0.8 | 4.3 ± 0.9 | 4.7 ± 0.9 |
| PTH, pg/mL | 80.6 [43.3–155.9] | 133.0 [75.2–220.9] | 191.8 [107.0–281.8] |
| Baseline FGF-23, RU/mL | 103.0 [78.4–137.2] | 197.7 [145.4–273.1] | 334.8 [247.5–502.9] |
| Final FGF-23, RU/mL | 123.7 [83.2–202.5] | 402.2 [241.0–761.7] | 1,698.0 [995.0–3,085.1] |
| Baseline eGFR, mL/min/1.73 m2 | 50.4 ± 13.5 | 39.7 ± 12.0 | 34.9 ± 11.8 |
| Final eGFR, mL/min/1.73 m2 | 45.6 ± 17.1 | 31.4 ± 15.5 | 21.7 ± 12.3 |
Note: Results are reported as proportion. mean ± standard deviation, or median [interquartile range].
Abbreviations: ACEi/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; FGF-23, fibroblast growth factor 2; IL-6, interleukin 6; PTH, parathyroid hormone; UPCR, urine protein-creatinine ratio.
CRP and IL-6 are from baseline.
Among the 1,163 individuals, 354 participants reached the KRT outcome during a median follow-up time of 3.2 years. In all unadjusted and multivariable-adjusted analyses, membership to the rapidly increasing FGF-23 trajectory group was associated with significantly increased risk for requirement for KRT (Table 4). Despite adjustments for eGFR at baseline and time 0 and the eGFR trajectory groups, membership in the rapidly increasing FGF-23 trajectory group conferred an ~21-fold higher risk for the KRT outcome compared with membership in the stable FGF-23 trajectory group (HR, 21.41; 95% CI, 10.66–43.11). Individuals in the slowly increasing FGF-23 trajectory group were also at increased risk for the KRT outcome compared with those in the stable trajectory group (HR, 3.60; 95% CI, 2.35–5.53).
Table 4.
FGF-23 Trajectories and Risks for the KRT Outcome
| N | HR (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|
| FGF-23 Trajectory Groupa | Total | KRT | Unadj | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 |
| Stable | 643 | 70 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Slowly increasing | 409 | 193 | 7.45 (5.54–10.03) | 8.62 (6.22–11.93) | 3.69 (2.50–5.46) | 3.96 (2.65–5.90) | 4.17 (2.73–6.38) | 3.60 (2.35–5.53) |
| Rapidly increasing | 111 | 91 | 43.80 (26.57–72.20) | 52.25 (30.14–90.59) | 19.77 (11.10–35.22) | 23.54 (13.03–42.55) | 27.68 (13.68–56.15) | 21.41 (10.66–43.11) |
Note: Up to 5 annual time points, median duration of subsequent follow-up time 3.2 years in 1,163 total participants at risk. Results are reported as HRs compared to the referent group. Covariate adjustments are for fifth annual study visit (time 0) covariate values except when stated. Model 1, stratified by center and adjusted for age, sex, race, and ethnicity. Model 2, model 1 plus eGFR, urinary protein-creatinine ratio, serum albumin, hemoglobin, C-reactive protein (baseline), and interleukin 6 (baseline) values. Model 3, model 2 plus diabetes, smoking, systolic blood pressure, body mass index, history of coronary artery disease, history of heart failure, history of stroke, history of peripheral vascular disease, number of hypertension medications, and angiotensin-converting enzyme inhibitor/angiotensin receptor blocker use. Model 4, model 3 plus calcium, phosphate, parathyroid hormone, ln(FGF-23), and baseline eGFR values. Model 5, model 4 plus eGFR trajectory.
Abbreviations: CI, confidence interval; eGFR, estimated glomerular filtration rate; FGF-23, fibroblast growth factor 23; HR, hazard ratio; KRT, kidney replacement therapy.
The stable, slowly increasing, and rapidly increasing ln(FGF-23) groups increased by 0.03, 0.14, and 0.40 per year. Through algebraic transformation ((exp(mean group slope)-1) × 100), these mean group slopes can also be expressed as the following percent changes in mean FGF-23 levels per year: +3%, +15%, and +49.2%.
When we repeated our FGF-23 trajectory-based analyses using only 3 time points, our results were qualitatively similar (Table S3). In all analyses using FGF-23 trajectories defined by 3 time points, membership in the rapidly increasing FGF-23 group was associated with significantly increased risk for the KRT outcome (Table S3).
Additional Analyses
Our findings did not qualitatively change when we adjusted our model using baseline FGF-23 level as the exposure for 24-hour urine phosphate (HR per 1-SD greater ln[FGF-23], 1.17; 95% CI, 1.01–1.36) or fractional excretion of phosphate (HR per 1-SD greater ln [FGF-23], 1.21; 95%CI, 1.04–1.40). Our results also remained significant when we adjusted our model using time-updated FGF-23 level as the exposure for time-updated 1,25-dihydroxyvitamin D (HR per 1-SD increase in ln[FGF-23], 1.69; 95% CI, 1.52–1.88) and for time-updated bicarbonate level (HR per 1-SD increase in ln [FGF-23], 1.67; 95% CI, 1.49–1.86).
Discussion
In this large case-cohort study of individuals with moderate to severe CKD, we evaluated the relationship between serial FGF-23 levels and risk for requirement of KRT. For our primary analysis, we used the time-varying IPW method to account for time-updated covariates such as eGFR, which could affect our outcome of interest and which could be affected by preceding FGF-23 values as the exposure variable. Our primary results demonstrated independent associations between FGF-23 history and risk for the KRT outcome. Models that used baseline and time-updated FGF-23 levels as the exposure demonstrated similar results. In analyses based on group-based trajectory modeling, we found that individuals who belong to an increasing FGF-23 trajectory group are at substantially higher risk for progression to the KRT outcome than individuals who belong to the stable FGF-23 trajectory group. Taken together, our results demonstrate that increasing levels of FGF-23 are associated with increased risk for requiring KRT.
Prior work in nondiabetic CKD,14 advanced CKD,15 the pediatric population,43 and kidney transplant recipients44 suggests that FGF-23 level predicts progression of CKD. However, not all previous studies reported significant associations.16,45,46 Although inconsistent results from evaluations of single time-point FGF-23 measurements and risk for requiring KRT may be due to differences across studies in study design, sample size, participant case-mix, and differential ascertainment of covariates and duration of follow-up, identification of biomarkers for progression of CKD to kidney failure is also challenged by the excellent predictive performance of eGFR and albuminuria.2
Despite the potency of eGFR as a predictor, we were able to detect a significant risk for increasing FGF-23 levels associated with KRT initiation. Several characteristics of the models we deployed could help explain our findings. Assessment of exposure with increased precision afforded by our time-updated and trajectory-based analyses allowed for enhanced estimation of the relationship of serial FGF-23 levels with risk for the KRT outcome. The shortened interval between exposure ascertainment and the event of interest in our time-updated analyses also contributed to our ability to detect greater magnitude of risk compared with models that assessed baseline FGF-23 levels. By capturing the effects of baseline and time-updated FGF-23 exposure, time-varying IPW discrete time failure models more fully evaluated the total FGF-23 effect and demonstrated higher risk estimates than did baseline or time-updated models.37 Taken together, as we improved the ability to define FGF-23 exposure and take into account time-dependent confounders with our modeling approaches, we were able to reveal significant risk associations with growing magnitudes of effect.
Potential mechanisms by which FGF-23 excess may result in progression of CKD are not completely understood. Whether elevated FGF-23 level initiates kidney injury or propagates kidney injury in the setting of CKD is also unknown. Prior work suggests that FGF-23 may have profibrotic properties, which may contribute to cardiac fibrosis in individuals with CKD.47 Similar studies investigating FGF-23 and its impact on renal fibroblasts demonstrate that FGF-23 excess also may increase fibroblast activation during kidney injury and result in profibrotic signaling.12 FGF-23 may also indirectly result in CKD progression. By stimulating phosphaturia and promoting nephrocalcinosis, FGF-23 may induce renal tubular damage and tubulointersitital fibrosis.9,10 Other indirect effects of FGF-23 on the kidney could be mediated by 1,25-dihydroxyvitamin D deficiency, which has been implicated in CKD progression through multiple potential mechanisms, including failure to adequately suppress anti-inflammatory pathways, to downregulate the renin-angiotensin system, and to inhibit myofibroblast activation.13 Although we did not find independent associations between baseline 24-hour urine phosphate or fractional excretion of phosphate and risk for the KRT outcome, FGF-23 level may also be a surrogate of overall phosphate balance. Finally, FGF-23 level elevation may identify a subpopulation at highest risk for CKD progression. This possibility is supported by the distribution of clinical characteristics among individuals within the rapidly increasing FGF-23 trajectory group, who had more comorbid conditions, higher blood pressures, lower baseline eGFRs, and more abnormalities in markers of mineral metabolism than the slowly increasing and stable FGF-23 trajectory groups.
Although we tested our hypotheses using several complimentary strategies in a well-established and large CKD cohort with time-updated covariates, we acknowledge certain limitations. First, observational studies cannot prove causation or provide information on mechanistic links between increasing FGF-23 levels and the requirement for KRT. Second, although we adjusted for eGFR and urinary protein-creatinine ratio in numerous ways and through numerous approaches, residual confounding by reduced kidney function remains a possible explanation of our findings. We also do not have the cause of kidney disease, measurements of intact FGF-23, or repeated measurements of inflammatory markers or urinary phosphate excretion. Finally, findings of our case-cohort study will require confirmation in other prospective CKD cohorts.
We demonstrate that increasing FGF-23 levels are associated with increased risk for requiring KRT. However, before incorporating measurements of FGF-23 into clinical practice, additional clinical studies are needed to confirm our findings and further preclinical studies are needed to demonstrate whether elevated FGF-23 level has any mechanistic or causal role in the development of kidney failure.
Supplementary Material
Item S1: Supplemental methods.
Table S1: Baseline characteristics of individuals within and outside the random subcohort.
Table S2: Baseline characteristics of the total CRIC population, the case-cohort population, and trajectory analyses population.
Table S3: FGF-23 risks for the KRT outcome truncated to 3 time points.
Acknowledgements:
The authors thank the participants, investigators, and staff of the CRIC Study for their time and commitment.
Financial Disclosure: Dr Mehta has interest in Abbot Laboratories, AbbVie, Inc, and Teva Pharmaceuticals Industries Ltd. Ms Isakova has received honoraria from Bayer and grant support from Shire. Dr Wolf has received research support, honoraria, or consultant fees from Akebia, Amag, Amgen, Ardelyx, DiaSorin, Keryx, and Shire. The remaining authors declare that they have no relevant financial interests.
Support: This study was supported by grants P30DK114857, R01DK081374 (Dr Wolf), K24DK093723 (Dr Wolf), R01DK102438 (Ms Isakova), R01DK111952 (Dr Scialla), National Kidney Foundation of Illinois Young Investigator Grant (Dr Mehta), and a Strategically Focused Research Network Center Grant from the American Heart Association (Dr Wolf). Research reported in this publication was also supported in part by the National Institutes of Health’s (NIH’s) National Center for Advancing Translational Sciences (NCATS), grant number KL2TR001424. Funding for the CRIC Study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award (CTSA) NIH/NCATS UL1TR000003, Johns Hopkins University UL1TR-000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the NCATS component of the NIH and NIH Roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane COBRE for Clinical and Translational Research in Cardiometabolic Diseases P20 GM109036, and Kaiser Permanente NIH/NCRR UCSF-CTSI UL1 RR-024131. The funders had no role in the study design; data collection, analysis, or reporting; or the decision to submit for publication.
Footnotes
Publisher's Disclaimer: Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the position or policy of the Department of Veterans Affairs or the US government.
Peer Review: Received February 8, 2019. Evaluated by 3 external peer reviewers and a statistician, with editorial input from an Acting Editor-in-Chief (Editorial Board Member Bradley A. Warady, MD). Accepted in revised form September 16, 2019. The involvement of an Acting Editor-in-Chief to handle the peer-review and decision-making processes was to comply with AJKD’s procedures for potential conflicts of interest for editors, described in the Information for Authors & Journal Policies.
Contributor Information
CRIC Study Investigators:
Lawrence J. Appel, Alan S. Go, Jiang He, Panduranga S. Rao, Mahboob Rahman, and Raymond R. Townsend
References
- 1.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013;3:1–150. [Google Scholar]
- 2.Gansevoort RT, Matsushita K, van der Velde M, et al. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 2011;80(1):93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Chronic Kidney Disease Prognosis Consortium. Association of estimated glomerular filtration rate and albuminuria with mortality and end-stage renal disease: a collaborative meta-analysis of kidney disease cohorts. Kidney Int 2011;79(12): 1331–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grams ME, Sang Y, Ballew SH, et al. Predicting timing of clinical outcomes in patients with chronic kidney disease and severely decreased glomerular filtration rate. Kidney Int 2018;93(6):1442–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hemmelgarn BR, Manns BJ, Lloyd A, et al. ; Alberta Kidney Disease N. Relation between kidney function, proteinuria, and adverse outcomes. JAMA 2010;303(5):423–429. [DOI] [PubMed] [Google Scholar]
- 6.Fogo AB. Mechanisms of progression of chronic kidney disease. Pediatr Nephrol 2007;22(12):2011–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolf M Update on fibroblast growth factor 23 in chronic kidney disease. Kidney Int 2012;82(7):737–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isakova T, Wahl P, Vargas GS, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 2011;79(12):1370–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Markowitz GS, Stokes MB, Radhakrishnan J, D’Agati VD. Acute phosphate nephropathy following oral sodium phosphate bowel purgative: an underrecognized cause of chronic renal failure. J Am Soc Nephrol 2005;16(11):3389–3396. [DOI] [PubMed] [Google Scholar]
- 10.Goligorsky MS, Chaimovitz C, Rapoport J, Goldstein J, Kol R. Calcium metabolism in uremic nephrocalcinosis: preventive effect of verapamil. Kidney Int 1985;27(5):774–779. [DOI] [PubMed] [Google Scholar]
- 11.Leifheit-Nestler M, Kirchhoff F, Nespor J, et al. Fibroblast growth factor 23 is induced by an activated renin-angiotensin-aldosterone system in cardiac myocytes and promotes the pro-fibrotic crosstalk between cardiac myocytes and fibroblasts. Nephrol Dial Transplant 2018;33(10):1722–1734. [DOI] [PubMed] [Google Scholar]
- 12.Smith ER, Holt SG, Hewitson TD. FGF23 activates injury-primed renal fibroblasts via FGFR4-dependent signalling and enhancement of TGF-beta autoinduction. Int J Biochem Cell Biol 2017;92:63–78. [DOI] [PubMed] [Google Scholar]
- 13.Tian J, Liu Y, Williams LA, de Zeeuw D. Potential role of active vitamin D in retarding the progression of chronic kidney disease. Nephrol Dial Transplant 2007;22(2):321–328. [DOI] [PubMed] [Google Scholar]
- 14.Fliser D, Kollerits B, Neyer U, et al. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol 2007;18(9):2600–2608. [DOI] [PubMed] [Google Scholar]
- 15.Kendrick J, Cheung AK, Kaufman JS, et al. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol 2011;22(10):1913–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isakova T, Xie H, Yang W, et al. ; Chronic Renal Insufficiency Cohort Study Group. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 2011;305(23):2432–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bouma-de Krijger A, Bots ML, Vervloet MG, et al. Time-averaged level of fibroblast growth factor-23 and clinical events in chronic kidney disease. Nephrol Dial Transplant 2014;29(1): 88–97. [DOI] [PubMed] [Google Scholar]
- 18.Scialla JJ, Astor BC, Isakova T, Xie H, Appel LJ, Wolf M. Mineral metabolites and CKD progression in African Americans. J Am Soc Nephrol 2013;24(1):125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lash JP, Go AS, Appel LJ, et al. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol 2009;4(8): 1302–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feldman HI. The Chronic Renal Insufficiency Cohort (CRIC) Study: design and methods. J Am Soc Nephrol 2003;14(90002):148S–153S. [DOI] [PubMed] [Google Scholar]
- 21.Ohneberg K, Wolkewitz M, Beyersmann J, et al. Analysis of clinical cohort data using nested case-control and case-cohort sampling designs. a powerful and economical tool. Methods Inf Med 2015;54(6):505–514. [DOI] [PubMed] [Google Scholar]
- 22.Noma H, Tanaka S. Analysis of case-cohort designs with binary outcomes: improving efficiency using whole-cohort auxiliary information. Stat Methods Med Res 2014. [DOI] [PubMed] [Google Scholar]
- 23.Lu SE, Shih JH. Case-cohort designs and analysis for clustered failure time data. Biometrics 2006;62(4):1138–1148. [DOI] [PubMed] [Google Scholar]
- 24.Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. J Clin Epidemiol 1999;52(12):1165–1172. [DOI] [PubMed] [Google Scholar]
- 25.Prentice RL. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika 1986;73(1):1–11. [Google Scholar]
- 26.Wacholder S, Gail M, Pee D. Selecting an efficient design for assessing exposure-disease relationships in an assembled cohort. Biometrics 1991;47(1):63–76. [PubMed] [Google Scholar]
- 27.Isakova T, Cai X, Lee J, et al. Longitudinal FGF23 trajectories and mortality in patients with CKD. J Am Soc Nephrol 2018;29(2):579–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson AH, Yang W, Townsend RR, et al. Time-updated systolic blood pressure and the progression of chronic kidney disease: a cohort study. Ann Intern Med 2015;162(4):258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stephens-Shields AJ, Spieker AJ, Anderson A, et al. Blood pressure and the risk of chronic kidney disease progression using multistate marginal structural models in the CRIC Study. Stat Med 2017;36(26):4167–4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williamson T, Ravani P. Marginal structural models in clinical research: when and how to use them? Nephrol Dial Transplant 2017;32(suppl 2):ii84–ii90. [DOI] [PubMed] [Google Scholar]
- 32.Fukagawa M, Kido R, Komaba H, et al. Abnormal mineral metabolism and mortality in hemodialysis patients with secondary hyperparathyroidism: evidence from marginal structural models used to adjust for time-dependent confounding. Am J Kidney Dis 2014;63(6):979–987. [DOI] [PubMed] [Google Scholar]
- 33.Naimi AI, Moodie EE, Auger N, Kaufman JS. Constructing inverse probability weights for continuous exposures: a comparison of methods. Epidemiology 2014;25(2):292–299. [DOI] [PubMed] [Google Scholar]
- 34.Allison PD. Discrete-time methods for the analysis of event histories. Sociol Methodol 1982;13:61–98. [Google Scholar]
- 35.Joeng HK, Chen MH, Kang S. Proportional exponentiated link transformed hazards (ELTH) models for discrete time survival data with application. Lifetime Data Anal 2016;22(1):38–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howe CJ, Cole SR, Chmiel JS, Munoz A. Limitation of inverse probability-of-censoring weights in estimating survival in the presence of strong selection bias. Am J Epidemiol 2011;173(5):569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie D, Yang W, Jepson C, et al. Statistical methods for modeling time-updated exposures in cohort studies of chronic kidney disease. Clin J Am Soc Nephrol 2017;12(11):1892–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xue X, Xie X, Gunter M, et al. Testing the proportional hazards assumption in case-cohort analysis. BMC Med Res Methodol 2013;13:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol 2010;6:109–138. [DOI] [PubMed] [Google Scholar]
- 40.O’Hare AM, Batten A, Burrows NR, et al. Trajectories of kidney function decline in the 2 years before initiation of long-term dialysis. Am J Kidney Dis 2012;59(4):513–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ravani P, Malberti F, Tripepi G, et al. Vitamin D levels and patient outcome in chronic kidney disease. Kidney Int 2009;75(1):88–95. [DOI] [PubMed] [Google Scholar]
- 42.de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol 2009;20(9): 2075–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Portale AA, Wolf MS, Messinger S, et al. Fibroblast growth factor 23 and risk of CKD progression in children. Clin J Am Soc Nephrol 2016;11(11):1989–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolf M, Molnar MZ, Amaral AP, et al. Elevated fibroblast growth factor 23 is a risk factor for kidney transplant loss and mortality. J Am Soc Nephrol 2011;22(5):956–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Isakova T, Craven TE, Lee J, et al. Fibroblast growth factor 23 and incident CKD in type 2 diabetes. Clin J Am Soc Nephrol 2015;10(1):29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Drew DA, Katz R, Kritchevsky S, et al. Fibroblast growth factor 23: a biomarker of kidney function decline. Am J Nephrol 2018;47(4):242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leifheit-Nestler M, Kirchhoff F, Nespor J, et al. Fibroblast growth factor 23 is induced by an activated renin-angiotensin-aldosterone system in cardiac myocytes and promotes the pro-fibrotic crosstalk between cardiac myocytes and fibroblasts. Nephrol Dial Transplant 2018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Item S1: Supplemental methods.
Table S1: Baseline characteristics of individuals within and outside the random subcohort.
Table S2: Baseline characteristics of the total CRIC population, the case-cohort population, and trajectory analyses population.
Table S3: FGF-23 risks for the KRT outcome truncated to 3 time points.