Abstract
Background:
The risk of BIA-ALCL for patients with textured breast implants has been estimated between 1/2832 and 1/30,000 women. Existing studies estimating the numbers exposed and at risk, may have under reported cases, and/or lacked comprehensive follow-up. Our objective is to determine the risk of BIA-ALCL in a defined cohort of patients reconstructed with macro-textured breast implants and consistently followed long-term.
Methods:
A prospective cohort study was conducted in patients who underwent breast reconstruction by a single surgeon at Memorial Sloan Kettering Cancer Center (MSKCC) from December 1992 to December 2017. Major events related to implants were prospectively recorded. We identified cases of BIA-ALCL by cross-checking clinical, pathology and external records data. Patients were followed until lymphoma occurrence or last follow-up. The primary outcomes were incidence rate per person-years and cumulative incidence.
Results:
From 1992 to 2017, 3546 patients underwent 6023 breast reconstructions, mainly after breast cancer removal, or contralateral prophylactic mastectomy, using macro-textured surface expanders and implants. All reconstructions were performed by a single surgeon (PGC). Median follow-up was 8.1 years (range, 3 months – 30.9 years). Ten women, 1/354, developed ALCL after a median exposure of 11.5 years (range, 7.4–15.8 years). Overall risk of BIA-ALCL in our cohort was is 0.311 cases per 1000 person-years (95% CI 0.118 to 0.503).
Discussion:
This study, the first to evaluate the risk of macro-textured breast implants from a prospective database with long term follow-up, demonstrates that the incidence rate of BIA-ALCL may be higher than previously reported. These results can help inform implant choice for women undergoing breast reconstruction.
Introduction
The first case of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) was reported in 1997.[1] The U.S. Food and Drug Administration (FDA) released safety communications in 2011 and 2016 on the risk of developing BIA-ALCL for women with breast implants. An increased understanding of the unique clinical presentation and pathology led BIA-ALCL to be listed as a separate entity in the World Health Organization (WHO) classification of lymphoid malignancies,[2, 3] and in 2017 the first treatment guidelines were defined.[4]
Estimation of the risk of developing BIA-ALCL from exposure to breast implants is largely based on approximations for numbers at risk and may be impacted by under reporting of cases. Some studies evaluating this risk, approximated the denominator of women with implants based on sales registries of the implant manufactures, or on complex population estimates. Some recent studies are also limited by short follow-up time of the patients with implants, since BIA-ALCL is a disease that usually occurs after a median of 6–13 years of exposure. The highest risk of BIA-ALCL described so far (1/2832) has been associated with macro-textured breast implants.[5] Patients who underwent breast implant surgery for breast cancer, performed by Peter G. Cordeiro (PGC), at Memorial Sloan Kettering Cancer Center (MSKCC), have been routinely followed and clinical outcomes prospectively recorded for 27 years. This database was referenced to determine the risk of BIA-ALCL occurrence related to implant exposure.
Methods
A cohort analysis was conducted using data collected from 1992 to 2019. All patients underwent two staged prosthesis-based breast reconstructions performed by PGC and the macro-textured expanders and permanent implants were placed in the submuscular position. All the patients in this cohort underwent mastectomy for breast cancer, and/or prophylactic contralateral mastectomy. Standard aseptic techniques specific for implant procedures were utilized: these included perioperative intravenous antibiotic, betadine skin preparation, irrigation of the implant pocket with bacitracin, re-draping prior to device placement, and oral antibiotics while drains remained in place.
Women were monitored from the time of tissue expander placement, followed at MSKCC, and referred to the lymphoma service in case of clinical suspicion (late seroma, mass, lymphadenopathy) and/or suspicious histology. 134 women who received smooth-surface implants were excluded from the analysis, since BIA-ALCL has been associated primarily or exclusively in those with textured surface[6–8]. No cases of BIA-ALCL were recorded in these subjects. An IRB waiver was approved to evaluate outcomes of patients undergoing breast reconstruction procedures.
Complications related to the implants (infections, explants, hematomas) were recorded and pathology of suspected cases of BIA-ALCL was analyzed at the hematopathology service at MSKCC. Flow cytometry, T cell receptor clonality and, when feasible, immunohistochemistry for T and B cell markers, CD30 and ALK, confirmed the diagnosis and ruled out other plausible histologies of lymphoproliferative processes, following the WHO classification. Cultures of the serous collection were performed in all patients presenting with a seroma.
Exposure was calculated from time of textured expander placement until lymphoma diagnosis or last follow-up. In cases of implant removal for reasons other than lymphoma, the optimal evaluation of exposure is uncertain. Therefore, in those cases, the time of exposure was calculated both from expander placement to last follow-up, which assumes risk of lymphoma remains after implant removal; and from expander placement to the date of removal, which assumes there is no risk after implant removal.
The incidence rate of BIA-ALCL was calculated by dividing the number of cases by the total follow-up years among all patients. The 95% confidence interval (CI) of the incidence rate was calculated assuming that the number of BIA-ALCL cases followed a Poisson distribution.
The cumulative incidence of BIA-ALCL was evaluated using the reverse Kaplan-Meier, with R 3.3.2[9]. Since the probability of developing BIA-ALCL and the probability of death are unlikely to be correlated, death was treated as a censoring event rather than a competing risk in the Kaplan-Meier analysis.
Results
This study includes 3546 women who underwent breast implant reconstructive surgery using macro-textured devices between December 1992 and December 2017. Table 1 summarizes patients characteristics. In our cohort, 2477 (69.9%) patients underwent bilateral mastectomy either for prophylaxis (BRCA positive) or for breast carcinoma with a contralateral prophylactic mastectomy. This follows a trend that has been described in the United States in the last decade.[10, 11] All women in this cohort had placement of a textured Biocell tissue expander and almost all, 3429 (96.7%) were reconstructed with Allergan Biocell permanent implants.
Table 1:
Characteristics of the cohort of women with breast implants (N=3546)
| Characteristic | n (%) |
|---|---|
| Median age at surgery, y (range) | 48 (18–89) |
| Type of reconstruction | |
| Unilateral | 1069 (30.1%) |
| Bilateral | 2477 (69.9%) |
| Type of textured implant | |
| Silicone | 1797 (50.6%) |
| Saline | 1749 (49.4%) |
| Surface of textured implant | |
| Biocell | 3429 (96.7%) |
| Siltex | 82 (2.3%) |
| True texture | 24 (0.7%) |
| unknown | 11 (0.3%) |
| Total implants inserted | 6023 |
| Reconstruction after mastectomy | 5821 (96.7%) |
| Cosmetic procedure for contralateral augmentation | 202 (3.3%) |
| Baseline breast cancer histology | |
| Lobular | 685 (19.3%), 117 in situ |
| Ductal | 2543 (71.7%), 593 in situ |
| Other | 60 (1.7%) |
| Unknown | 258 (7.2%) |
| Breast cancer management - other than surgery | |
| Chemotherapy only | 1025 (28.9%) |
| Radiotherapy only | 287 (8.1%) |
| Chemotherapy + radiotherapy | 714 (20.1%) |
| None | 1520 (42.9%) |
Median follow-up was 8.1 years (range, 3 months – 30.9 years). 98.3% of the patients were followed for at least 1 year, 59.3% were seen within the last year and 77.3% in the last 3 years. There were 358 deaths (10.1%). Of the 18 patients (0.5%) followed for less than 6 months, 1 died and 17 were lost to follow-up. These patients were included in the denominator of people exposed. Forty-seven patients (1.2%) had their implants removed for reasons other than lymphoma (mainly cellulitis, flap necrosis, and exposure of the implant). A total of 293 women underwent unilateral or bilateral implant exchange, with 43 undergoing one to two additional implant exchanges. The most common reason for revisionary surgery was rupture of the implant, followed by aesthetic revision, capsular contracture or asymmetry. In this case, since the implants were replaced with textured implants, the risk of exposure was assumed to be equivalent to the risk of people not undergoing revisionary surgery.
After a median exposure of 11.7 years (range, 7.4–15.8 years), 10 women developed BIA-ALCL. All 10 had macro-textured implants and the median age at diagnosis was 60 years (range, 53–73 years). Seven women presented with a unilateral peri-prosthetic fluid collection and were thus stage IA-IB, one with a localized mass and fluid collection (stage IIA), one with a mass invading the pectoralis muscle and axillary lymph nodes (stage III), and one with only internal mammary lymph node involvement without identifiable capsular involvement or fluid collection (stage IIB or III, T0N1M0). All specimens were CD30-positive and ALK-negative with histology consistent with ALCL. (Figure 1.) All cultures of the seromas were negative for bacteria. Characteristics of the BIA-ALCL cases are summarized in table 2.
Figure 1:
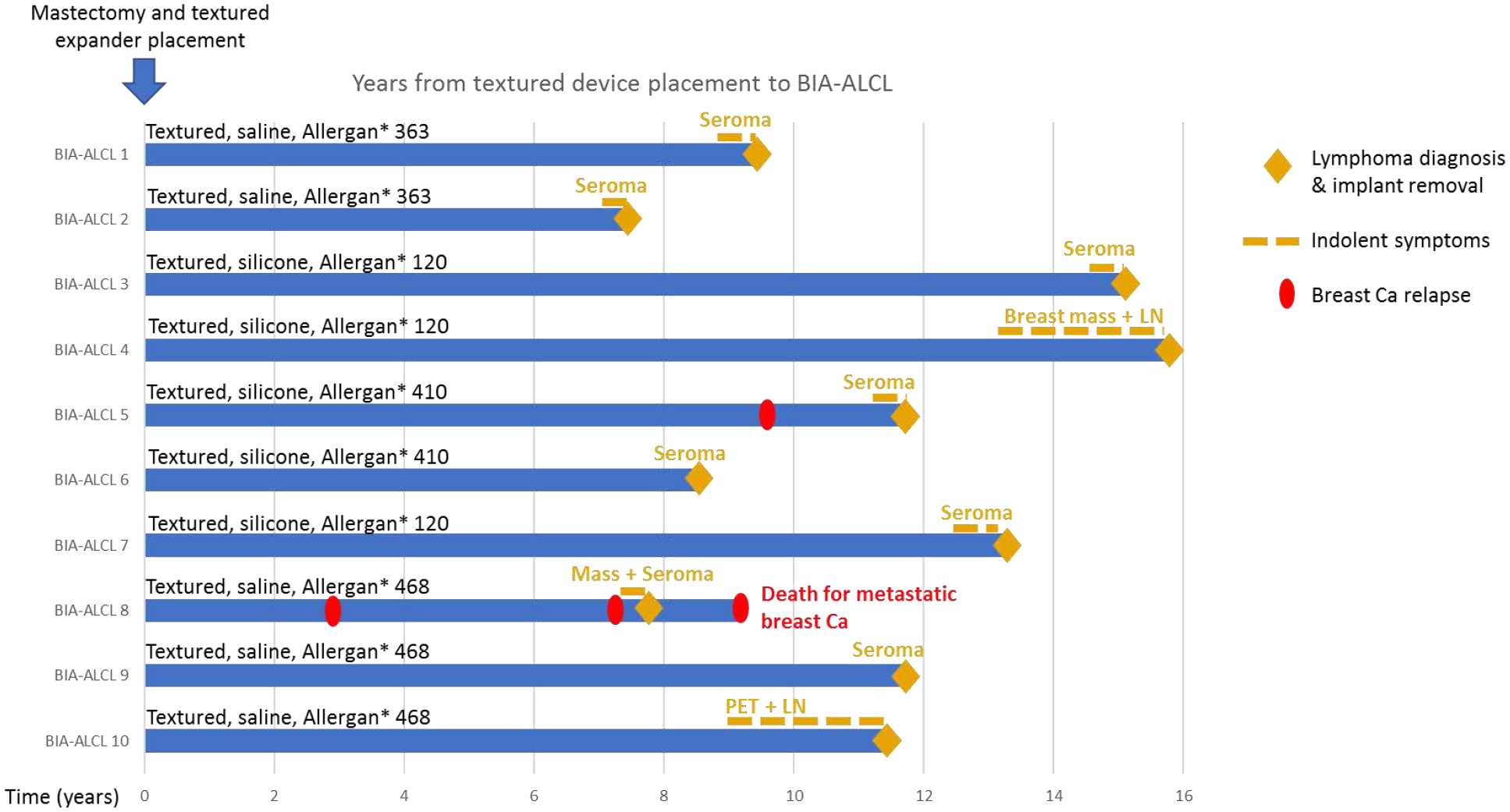
Swimmer’s plot of the BIA-ALCL cases (blue bars). BIA-ALCL, breast implant-associated anaplastic large cell lymphoma; LN, lymph node; PET, positron emission tomography.
Table 2:
characteristics of the BIA-ALCL cases and history of the textured devices
| Characteristic | N (%) |
|---|---|
| Bilateral/Unilateral reconstruction | 8/10 (80%)/ 2/10 (20%) |
| Silicone/Saline filling | 5/10 (50%)/ 5/10 (50%) |
| Histology of breast Ca | |
| Lobular/Ductal | 3/10 (30%)/ 7/10 (70%) |
| In situ/Infiltrating | 5/10 (50%)/ 5/10 (50%) |
| Median time from tissue expander to permanent implant | 5.3 months (range 3.4 – 12 months) |
| Other implant exchanges (all replaced with textured devices) | 3/10 (30%) |
| Chemotherapy for breast Ca | 5/10 (50%) |
| Radiotherapy for breast Ca | 1/10 (10%) |
| Treatment of BIA-ALCL | |
| Implant removal and capsulectomy | 10/10 (100%) |
| Chemotherapy and radiation | 1/10 (10%) |
The incidence rate of BIA-ALCL was 0.311 cases per 1000 person-years (95% CI, 0.118–0.503) when considering patients to be at no risk after implant removal, and 0.308 cases per 1000 person-years (95% CI, 0.117–0.499) when considering the same patients at risk after implant removal. Cumulative risk analysis is shown in Figure 2.
Figure 2:
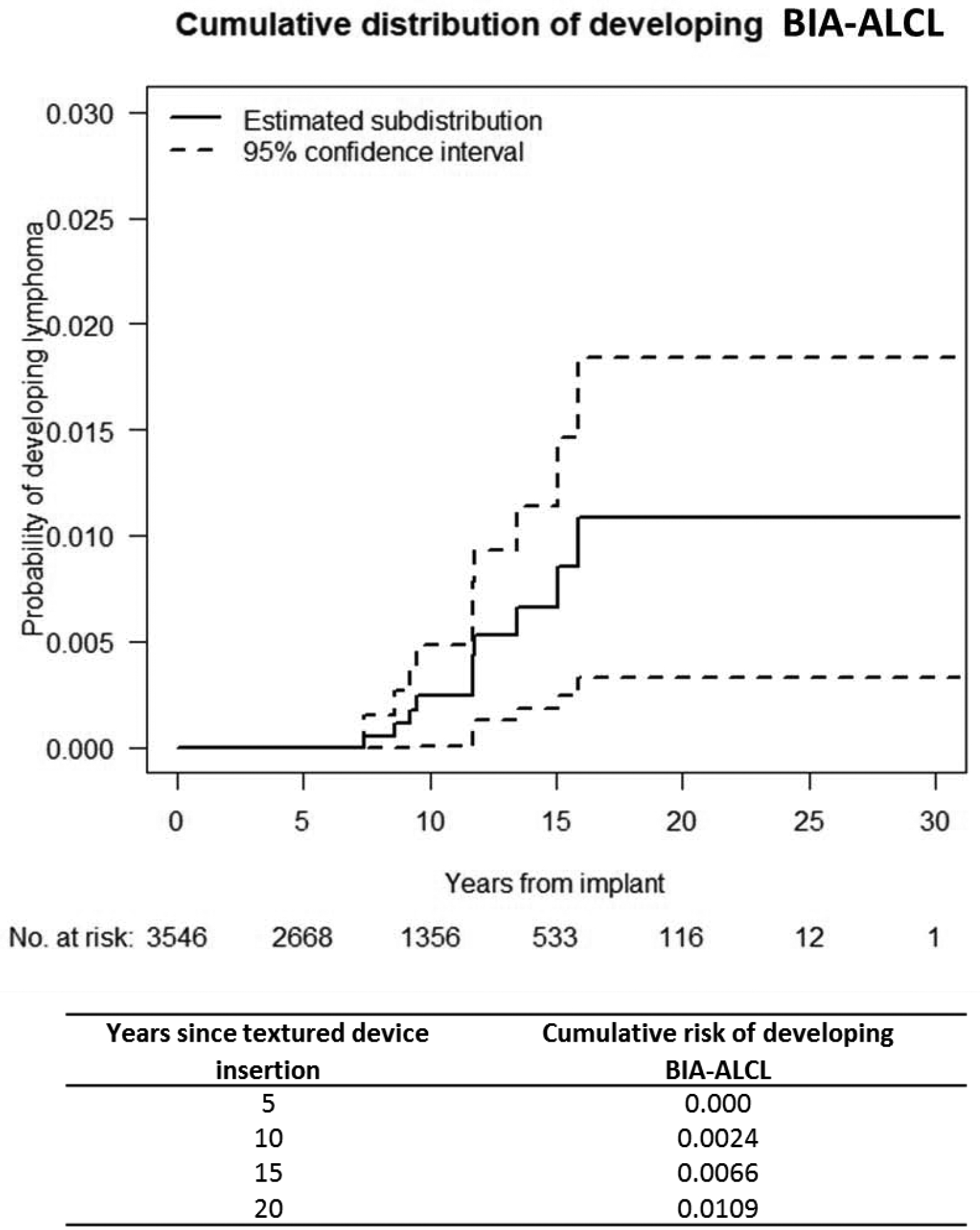
Cumulative risk of developing BIA-ALCL.
Discussion
Between 5 and 10 million women worldwide have breast implants, and approximately 550,000 implants are inserted each year in the United States[12]. This study is the first to report on a long-term, consistently and prospectively followed cohort of women with macro-textured tissue expanders and breast implants and evaluated for BIA-ALCL. In this cohort, 1/355 women developed BIA-ALCL (1/602 single devices), a greater risk than previously described[6, 12–14].
A 2011 report from the FDA estimated the risk of BIA-ALCL at 1/500,000 women/year. In recent years evidence has shown that the risk is associated exclusively or predominantly with textured implants[1, 6, 13–16]. Researchers have attempted to better define this risk, with complex extrapolations to determine the prevalence of breast implant exposure.[6, 13, 15] A case-control study derived from the Dutch pathology registry that compared the prevalence of breast implant exposure between women with primary breast ALCL and other breast lymphomas estimated the prevalence of breast implants in the population correcting by regional variations based on cancer screening data and implant sales in the Netherlands. The cumulative risk of BIA-ALCL for women with textured implants by the age of 75 years—was 1/6920.[13] Another study by implant manufacturers (Allergan) approximated the denominator of exposure using sales registries.[14] In that study the risk of BIA-ALCL (1/3500)[17] is likely underestimated as patient were followed for a median of 2–4 years, while BIA-ALCL usually occurs after a median of 6–13 years of exposure (range, 0.18–28 years).[8, 12, 15, 16, 18]
Similarly, an Australian series described one of the highest published risk of BIA-ALCL at 1/3817 women, with a denominator estimated from implant sales[6]. The highest risk to date (1/2832) has been described by the same group in Silimed high-textured polyurethane implants. This is a grade 4 surface on a new surface classification.[5, 19, 20] In recent months it appears evident that the risk of BIA-ALCL might differ depending on the extent of texturing of the implant surface. Women with Biocell macro-textured implants, which constitute the majority of the implants utilized in this paper, classified as surface grade 3 (intermediate roughness and surface area) by the same classification system[20] might be at higher risk than those with other surface types. In two different publications, a ratio of 16.5:16 and of 9:115 of Biocell to Siltex risk of BIA-ALCL was found.
To avoid overestimating the cases of BIA-ALCL, patients that had been followed for less than 6 months or not seen within the last 2 years, were included in the denominator of women at risk, with the assumption that BIA-ALCL cases during follow-up would likely be diagnosed at MSKCC and/or referred to the lymphoma service or to the patient’s primary oncologist.
The incidence of BIA-ALCL in this study could also be underestimated: BIA-ALCL tends to develop slowly and, in the earlier years of observation (prior to 2011) in this cohort, no lymphoma work-up was routinely performed on pericapsular fluid collections. The risk of BIA-ALCL occurrence after implant removal is also unclear. In this cohort, only 41 women without lymphoma had explantation of their implants. Perhaps most importantly the median follow-up of this cohort is 8.1 years, and the median time from surgery to occurrence of BIA-ALCL 11.7 years, suggesting that this already high incidence may increase over time.
Starting from July 2018, the plastic surgery team has been contacting all patients in this cohort with a letter outlining what is known about BIA-ALCL and a “frequently asked questions” sheet. Over 90% of the women contacted have returned for a follow-up and assessment in person by the surgeon. It is possible that increased awareness among our patients in addition to the letter could have prompted patients to return for follow-up and subsequently been diagnosed sooner than if they were not aware of this entity and waited until more significant symptoms developed.
It is uncertain whether these data can be extrapolated to the overall implant population. Most of the implants utilized for reconstruction had a Biocell macro-textured surface, to minimize rotation and capsular contractures.
Some of the most recent literature on BIA-ALCL, suggests that clustering might occur in certain cohorts, for uncertain reasons. Some authors theorized that biofilm by specific gram-negative bacteria might play a role[21]. All of the seromas in our cohort were negative for bacteria, though testing for the Ralstonia spp., the implicated pathogen species was not performed.
Our bias is that the high incidence described here is largely due to the consistent and long-term follow-up performed on this cohort. Moreover, since awareness of BIA–ALCL, our patients with clinical signs and/or symptoms have been carefully and thoroughly evaluated. However, we understand that our cohort includes women operated on by a single surgeon at a single cancer center raising the possibility of selection for an unappreciated risk factor or a possible degree of randomness. Ours is an exclusively breast cancer population, and nearly 60% of patients received chemotherapy or radiotherapy in addition to mastectomy. However, in other series, BIA-ALCL was not diagnosed more frequently in those with previous breast cancer or previous receipt of either chemotherapy or radiation therapy than in those who received implants for aesthetic and reconstructive procedures[6, 13, 15].
As outlined above in figure 2, our described incidence is accompanied by wide confidence intervals that may overlap with previous reports.
Conclusions
The incidence of BIA-ALCL in this prospectively followed cohort is higher than previously reported in the literature. If this risk is confirmed in larger series, the continued use of macro-textured implants in women for both aesthetic and reconstructive procedures as well as optimal follow-up for those currently with macro-textured implants needs to be carefully evaluated.
Acknowledgements
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Editorial support in the preparation of this article was provided by Hannah Rice, ELS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: Disclaimers: PGC reports grants from Allergan, Inamed and Acelity, outside of the submitted work, SMH reports grants and personal fees from Aileron, Seattle Genetics, Takeda, Kyowa Hakka Kirin, Verastem, Portola, Corvus, Celgene, Spectrum, Forty-Seven, outside of the submitted work, AN, AD, PG, NaG, NiG have no conflict of interest do declare.
References
- 1.Keech JA Jr. and Creech BJ, Anaplastic T-cell lymphoma in proximity to a saline-filled breast implant. Plast Reconstr Surg, 1997. 100(2): p. 554–5. [DOI] [PubMed] [Google Scholar]
- 2.Srinivasa DR, et al. , Global Adverse Event Reports of Breast Implant-Associated ALCL: An International Review of 40 Government Authority Databases. Plast Reconstr Surg, 2017. 139(5): p. 1029–1039. [DOI] [PubMed] [Google Scholar]
- 3.Arber DA, et al. , The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood, 2016. 127(20): p. 2391–405. [DOI] [PubMed] [Google Scholar]
- 4.Clemens MW and Horwitz SM, NCCN Consensus Guidelines for the Diagnosis and Management of Breast Implant-Associated Anaplastic Large Cell Lymphoma. Aesthet Surg J, 2017. 37(3): p. 285–289. [DOI] [PubMed] [Google Scholar]
- 5.Collett DJ, et al. , Current Risk Estimate of Breast Implant-Associated Anaplastic Large Cell Lymphoma in Textured Breast Implants. Plast Reconstr Surg, 2019. 143(3S A Review of Breast Implant-Associated Anaplastic Large Cell Lymphoma): p. 30s–40s. [DOI] [PubMed] [Google Scholar]
- 6.Loch-Wilkinson A, et al. , Breast Implant-Associated Anaplastic Large Cell Lymphoma in Australia and New Zealand: High-Surface-Area Textured Implants Are Associated with Increased Risk. Plast Reconstr Surg, 2017. 140(4): p. 645–654. [DOI] [PubMed] [Google Scholar]
- 7.Clemens MW, et al. , Complete Surgical Excision Is Essential for the Management of Patients With Breast Implant-Associated Anaplastic Large-Cell Lymphoma. J Clin Oncol, 2016. 34(2): p. 160–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brody GS, Anaplastic Large Cell Lymphoma Occurring in Women with Breast Implants: Analysis of 173 Cases. Plast Reconstr Surg, 2015. 136(4): p. 553e–4e. [DOI] [PubMed] [Google Scholar]
- 9.R Core Team (2016). R: A language and environment for statistical computing R foundation for Statistical Computing, Vienna, Austria: 2016. [Google Scholar]
- 10.Tuttle TM, et al. , Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol, 2007. 25(33): p. 5203–9. [DOI] [PubMed] [Google Scholar]
- 11.Kummerow KL, et al. , Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg, 2015. 150(1): p. 9–16. [DOI] [PubMed] [Google Scholar]
- 12.Mehta-Shah N, Clemens MW, and Horwitz SM, How I treat breast implant-associated anaplastic large cell lymphoma. Blood, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Boer M, et al. , Breast Implants and the Risk of Anaplastic Large-Cell Lymphoma in the Breast. JAMA Oncol, 2018. 4(3): p. 335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGuire P, Reisman NR, and Murphy DK, Risk Factor Analysis for Capsular Contracture, Malposition, and Late Seroma in Subjects Receiving Natrelle 410 Form-Stable Silicone Breast Implants. Plast Reconstr Surg, 2017. 139(1): p. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doren EL, et al. , U.S. Epidemiology of Breast Implant-Associated Anaplastic Large Cell Lymphoma. Plast Reconstr Surg, 2017. 139(5): p. 1042–1050. [DOI] [PubMed] [Google Scholar]
- 16.Clemens MW, et al. , Understanding rare adverse sequelae of breast implants: anaplastic large-cell lymphoma, late seromas, and double capsules. Gland Surg, 2017. 6(2): p. 169–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGuire P, Reply: Risk Factor Analysis for Capsular Contracture, Malposition, and Late Seroma in Subjects Receiving Natrelle 410 Form-Stable Silicone Breast Implants. Plast Reconstr Surg, 2017. 140(3): p. 500e. [DOI] [PubMed] [Google Scholar]
- 18.Clemens MW, Discussion: Breast Implant-Associated Anaplastic Large Cell Lymphoma in Australia and New Zealand: High-Surface-Area Textured Implants Are Associated with Increased Risk. Plast Reconstr Surg, 2017. 140(4): p. 660–662. [DOI] [PubMed] [Google Scholar]
- 19.Magnusson M, et al. , SPECIAL UPDATE: The epidemiology of Breast Implant Associated Large Cell Lymphoma in Australia and New Zealand confirms the highest risk for grade 4 surface breast implants. Plast Reconstr Surg, 2019. [DOI] [PubMed] [Google Scholar]
- 20.Jones P, et al. , The Functional Influence of Breast Implant Outer Shell Morphology on Bacterial Attachment and Growth. Plast Reconstr Surg, 2018. 142(4): p. 837–849. [DOI] [PubMed] [Google Scholar]
- 21.Hu H, et al. , Bacterial Biofilm Infection Detected in Breast Implant-Associated Anaplastic Large-Cell Lymphoma. Plast Reconstr Surg, 2016. 137(6): p. 1659–69. [DOI] [PubMed] [Google Scholar]


