Abstract
Today, we are witnessing a revolution in the treatment of cancer through the use of immunotherapy. In the last decade, work from many laboratories and clinicians have unequivocally demonstrated that the immune system can eradicate established cancers and enhance patient survival. However, immunotherapies have distinct tumor response-to-toxicity profiles due to distinct mechanisms of action. We have previously termed immunotherapies that activate a general, systemic immune response as “enhancement cancer immunotherapy” and those that target a specific dysfunctional immune response, especially within the tumor microenvironment, as “normalization cancer immunotherapy”. In this perspective, we provide a framework for normalization cancer immunotherapy in the context of melanoma.
INTRODUCTION
The history of cancer immunology is interwoven with the history of melanoma research, sharing in mutual triumphs and failures. One reason why melanoma’s history is so inextricably linked to cancer immunology’s is that melanoma is often considered one of the most immunogenic of human cancers, evidenced by significant infiltration of hematopoietic cells including various types of lymphocytes and myeloid cells, and this has made it a favored target on the forefront of burgeoning immunotherapies. Thus, for the past 40 years, breakthroughs in the field of cancer immunology were often accomplished through the study of melanoma, ultimately resulting in successful immunotherapies that target melanoma cells. The promise of immunotherapy began with great enthusiasm with the discovery of interferons (Isaacs and Lindenmann, 1957) and the cancer trials that followed shortly thereafter. Treatment of melanoma with interferon-alpha 2b (IFN-α 2b) was one of the first human cancers to achieve moderate success with immunotherapy (Goldstein and Laszlo, 1986), eventually becoming the first immunotherapy for melanoma to be approved by the Food and Drug Administration (FDA) in 1996 (Kirkwood et al., 1996). Several years after the discovery of interluekin-2 (IL-2) (Morgan et al., 1976, Taniguchi et al., 1983), melanoma patients administered high dose IL-2 had durable responses in ~10–15% of patients (Atkins et al., 1999), clearly demonstrating that immunomodulation was indeed a viable weapon in the oncologist’s armamentarium. Subsequently, IL-2 therapy for the treatment of metastatic melanoma was approved in 1998 by the FDA (Atkins et al., 1999). The early successes of IFN-α and IL-2 therapy against human cancers, although limited, prompted scientists to identify the targets of immunotherapy—cancer antigens. Perhaps not surprisingly, the first human cancer antigen identified was the melanoma antigen, MAGE-1, which provided the much needed evidence that immunity could specifically target cancer cells (van der Bruggen et al., 1991). Melanoma has also served as critical target for the development of cancer vaccines (Rosenberg et al., 1998), adoptive cell therapy (ACT) (Rosenberg et al., 1988), and the first FDA-approved oncolytic virus, talimogene laherparepvec (Andtbacka et al., 2015). Perhaps most importantly, melanoma was the first cancer to be FDA-approved for immunotherapies targeting T-cell regulation including cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) (Hodi et al., 2010) and programmed death-1 receptor (PD-1) (Topalian et al., 2012, Topalian et al., 2014).
However, each of these immunotherapies has very distinct efficacies and toxicity profiles due to distinct mechanisms of action. We recently proposed the concept of “enhancement immunotherapy” and “normalization cancer immunotherapy” (Sanmamed and Chen, 2018) to better understand tumor response-to-toxicity profiles and guide future therapeutic targets for cancer immunotherapy. Enhancement cancer immunotherapies attempt to boost underlying immune responses while normalization cancer immunotherapies seek to repair a dysfunctional immune response within the tumor microenvironment (TME). The archetypal normalization cancer immunotherapy is PD-1 or programmed death receptor-ligand 1 (PD-L1 or B7-H1) blockade (collectively called anti-PD therapy) which more selectively targets a dysfunctional T cell response within the TME, resulting in greater tumor response and reduction of systemic toxicities. In this perspective, we do not attempt to provide a comprehensive review of immune responses to melanoma, but rather provide a conceptual framework for normalization cancer immunotherapy to be adopted for further immunotherapeutic discovery and rational clinical trial design.
IMMUNE RESPONSES TO CANCER AND SUBSEQUENT IMMUNE ESCAPE
There is unequivocal evidence that endogenous immune responses to cancer occur (Kaplan et al., 1998, Shankaran et al., 2001, Vesely et al., 2011). Furthermore, cancer antigens are recognized by T cells, leading to tumor cell destruction and sculpting by immunological mechanisms in a process called cancer immunoediting (Matsushita et al., 2012, Schreiber et al., 2011). Ultimately, immune control of cancers may become broken, leading to immune escape where cancers progressively grow and eventually become clinically apparent (Khong and Restifo, 2002). This latter event frequently leads to formation of an immunosuppressive tumor microenvironment that inhibits subsequent naturally occurring immune responses and resistance to immunotherapy (Sharma et al., 2017a, Zitvogel et al., 2006). Thus, escape from immune control is considered a hallmark of cancer (Hanahan and Weinberg, 2011).
Adaptive immune resistance
Upon tumor cell recognition and immune activation, tumor cells often counter an ongoing immune attack with upregulation or recruitment of immunosuppressive moieties, resulting in a dysfunctional immune response locally within the TME (Figure 1a). This concept has been termed adaptive immune resistance or adaptive resistance (Kim et al., 2018, Taube et al., 2012). The first demonstration of adaptive immune resistance in human patients identified colocalization of tumor-infiltrating lymphocytes (TILs) expressing IFN-γ and melanoma cells expressing B7-H1 (PD-L1) (Taube et al., 2012). Furthermore, activated T cells express immune inhibitory receptor PD-1 and subsequent engagement of PD-1 on activated T cells with B7-H1 (PD-L1) expressed on tumor cells results in T cell dysfunction and impaired immune responses to cancer (Dong et al., 2002, Dong et al., 1999). Overcoming this dysfunctional immune response is critical to obtaining effective immunotherapies.
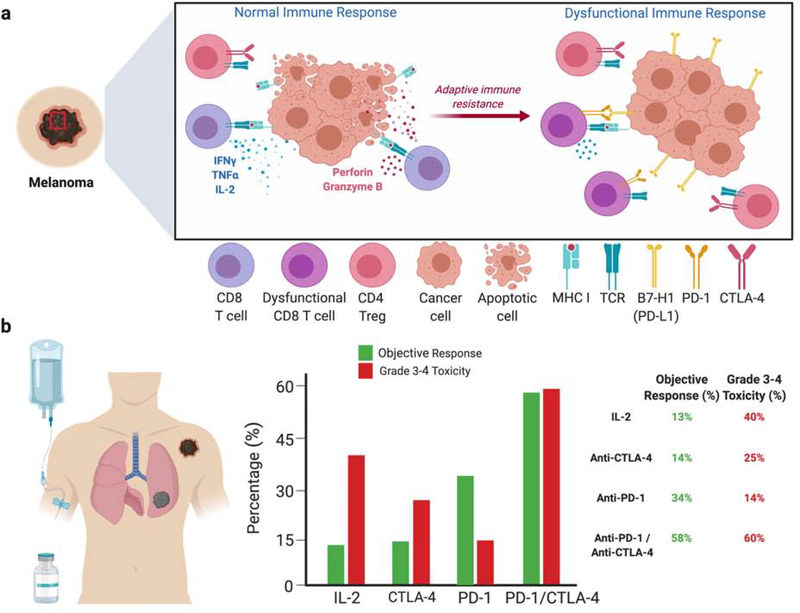
Figure 1: Adaptive immune resistance of cancers to endogenous immune attack and the distinct tumor response rate-to-toxicity rate of FDA-approved immunotherapies for melanoma.
(a) Within the melanoma TME, T cells recognize and destroy cancers cells through various mechanisms including IFN-γ, TNF-α, perforin and granzyme B (left). Over time, tumors develop immune escape mechanisms such as increased expressing of B7-H1 (PD-L1) in a process called adaptive immune resistance, resulting in a dysfunctional immune response (right). (b) The right panel shows a schematic of a patient with metastatic melanoma receiving immunotherapy. The middle panel displays the percentage of patients who achieve objective responses (green) and Grade 3–4 toxicities (red) treated with distinct immunotherapies. The right panel is a table showing the percentages of objective responses and Grade 3–4 toxicities.
NORMALIZATION vs. ENHANCEMENT CANCER IMMUNOTHERAPY
Melanoma therapies have advanced rapidly in the past decade and may be broadly divided into tumor-targeting therapies and immune-modulating therapies. Tumor-targeted therapies include more conventional chemotherapy and radiation as well as kinase inhibitors such as vemurafenib, dabrafenib, and trametinib which target the tumor cell for death. In contrast, immunotherapies are immune modulating where the target is a component of the immune system and the effective response is tumor detection and eradication or control by anti-tumor immunity. Distinct immunotherapies have distinct tumor response-to-toxicity profiles (Figure 1b). For example, among the previously FDA-approved immunotherapies for metastatic melanoma, only anti-PD therapy has a favorable objective tumor response-to-systemic toxicity ratio. While IFN-α, IL-2, and anti-CTLA-4 boost general immune activation (enhancement), anti-PD-1 therapies more selectively target a dysfunctional immune response triggered within the TME (normalization).
Enhancement Cancer Immunotherapies
Most cancer immunotherapeutic strategies attempt to boost the anti-tumor immune response by targeting the fundamental cellular and molecular mechanisms which are physiologically required or necessary in generating an immune response: antigen-uptake, -processing, and -presentation by antigen presenting cells (APCs); migration of APCs to lymphoid organs; T cell activation and co-stimulation; trafficking of activated T cells to the TME; T cell recognition of tumor cells and tumor cell death; generation of antigen-specific memory T cells. We will limit our discussion to FDA-approved immunotherapies for metastatic melanoma including IL-2, anti-CTLA-4, and anti-PD therapy. However, there is great promise of emerging immunotherapies against melanoma such as adoptive cell transfer (ACT) (Chandran et al., 2017), chimeric antigen-receptor T cells (CAR-T) (Wiesinger et al., 2019), melanoma neo-antigen vaccines (Ott et al., 2017), and oncolytic viruses (Andtbacka et al., 2015). Of note, vaccines would be characterized as enhancement immunotherapy while ACT, CAR-T, and oncolytic viruses uniquely perform both tumor-targeting and immunomodulatory functions that predominately enhance on ongoing immune response.
The major limiting factor for cancer immunotherapies, especially enhancement immunotherapies, is immune-related adverse events (irAEs) (Postow et al., 2018). Rare objective responses and significant systemic toxicity limited the success of IFN-α and IL-2 therapy. Type I IFNs are known to be important for DC maturation, activation, migration and survival, resulting in enhanced adaptive immune responses (Decker et al., 2005). IFN-α 2b was FDA-approved as an adjuvant therapy in high-risk resected melanoma in 1996 (Kirkwood et al., 1996). As IFN-α 2b was approved as adjuvant therapy after surgical resection there are no objective responses to be measured. Meta-analysis of phase III randomized clinical trials did show a modest increase in overall survival (Wheatley et al., 2003). However, patients receiving IFN-α 2b had significant systemic toxicity, limiting its use as an adjuvant therapy.
High dose IL-2 therapy was met with initial excitement as it provided a response rate of 16% and long-term survival in some patients with metastatic melanoma (Atkins et al., 1999). However, the toxicity from IL-2 therapy was severe, often requiring admission to medical intensive care units to manage life-threatening organ failure (Schwartz et al., 2002). A pooled analysis from multiple phase II and phase III clinical trials of patients with metastatic melanoma treated with high-dose IL-2 shows an objective response rate of 14% (92/722 patients) and a rate of Grade 3–4 toxicities of 40% (286/722 patients) (Figure 1B) (Agarwala et al., 2002, Atkins et al., 1999, Parkinson et al., 1990, Rosenberg et al., 1994, Sanmamed and Chen, 2018, Schwartzentruber et al., 2011, Tarhini et al., 2007). IL-2 is a critical growth factor for T cells, allowing for expansion of T cells after antigen stimulation. Therefore, recombinant IL-2 therapy generates a broad stimulation of the immune system with significant toxicity and relatively poor anti-tumor responses. This is most likely due to the greater number of self-reactive or non-tumor-specific T cells that expand as compared to the fewer tumor-specific T cells that expand in response to IL-2 therapy.
In 2011, the modern revolution of cancer immunotherapy began with the approval of anti-CTLA-4 (ipilimumab) monoclonal antibody (mAb) for metastatic melanoma (Hodi et al., 2010, Robert et al., 2011). The function of CTLA-4 is to control self-reactive T cells, typically through regulatory T cells (Tregs) (Kuehn et al., 2014, Tivol et al., 1995, Waterhouse et al., 1995, Wing et al., 2008). Accumulated data now supports that the main effect of anti-CTLA-4 therapy in melanoma patients is through modulation of Treg function, including Treg depletion (Arce Vargas et al., 2018, Romano et al., 2015). Pooled data analysis from multiple phase II and phase III clinical trials of patients with metastatic melanoma treated with anti-CTLA-4 shows an objective response rate of 14% (222/1561 patients) and a rate of Grade 3–4 toxicities of 25% (392/1561 patients) (Figure 1b) (Ascierto et al., 2017, Hodi et al., 2016, Hodi et al., 2010, Larkin et al., 2015, Ribas et al., 2015, Robert et al., 2015b, Sanmamed and Chen, 2018, Weber et al., 2009). Similar to IL-2, the toxicity of anti-CTLA-4 exceeds that of tumor responses likely due to targeting of Tregs in peripheral lymph organs rather than within the TME.
Normalization Cancer Immunotherapies
In contrast to high-dose IL-2 and anti-CTLA-4 therapy, blockade of the PD-1/PD-L1 pathway results in greater tumor efficacy with reduced systemic side effects, suggesting a very distinct mechanism of action (Figure 1B). Since its FDA-approval for melanoma in 2014 (Topalian et al., 2014), it is now well-established that the PD-1/B7-H1 (PD-L1) axis is a major pathway resulting in dysfunctional immune responses within the local TME (Zou et al., 2016). Pooled data analysis from multiple phase II and phase III clinical trials of patients with metastatic melanoma treated with anti-PD-1 shows an objective response rate of 34% (598/1773 patients) and a rate of Grade 3–4 toxicities of 14% (256/1773 patients) (Figure 1B) (Larkin et al., 2015, Larkin et al., 2018, Ribas et al., 2015, Robert et al., 2015a, Robert et al., 2015b, Sanmamed and Chen, 2018, Topalian et al., 2014). For the first time in the history of oncology, a medical therapy had greater tumor efficacy than systemic toxicity. Importantly, anti-PD therapy appears to be targeting a mechanism widely shared by cancers as it has been approved for 18 different cancer indications and shows efficacy in more than 25 different malignancies which will likely result in future FDA-approvals (Ribas and Wolchok, 2018). In contrast, anti-CTLA-4 monotherapy has failed to show efficacy or gain FDA-approval for cancers beyond melanoma (Beer et al., 2017, Lynch et al., 2012). This is likely due to minimal expression of B7-H1 in non-inflamed tissues and more selective upregulation of B7-H1 within the local TME, allowing for a more precise, tumor-specific immune response with less systemic immune activation when targeting the PD-pathway.
Combination anti-PD-1 and anti-CTLA-4 is also approved for melanoma and shows an increased objective response rate as well as significant toxicity (Hodi et al., 2016, Larkin et al., 2015). Pooled analysis from phase I, II, and III clinical trials of metastatic melanoma patients treated with combination anti-PD-1/anti-CTLA-4 show a response rate of 58% (679/1171 patients) and a rate of Grade 3–4 toxicity of 60% (699/1171 patients) (Figure 1B) (Hodi et al., 2016, Hodi et al., 2018, Larkin et al., 2015, Sznol et al., 2017). A recent analysis showed benefit of 4-year overall survival in melanoma patients treated with combination nivolumab/ipilimumab vs. nivolumab alone (Hodi et al., 2018). It is unclear if the increased toxicity with combination blockade limits the benefit of increased objective responses. Careful dosing and sequential therapy may help maximize objective responses while ameliorating systemic toxicity.
Normalization Cancer Immunotherapy Beyond Melanoma
Melanoma has long been considered an “immunogenic” tumor due to evidence that a significant subset of melanoma (~40–50%) is infiltrated with lymphocytes and that some melanomas, albeit rare, spontaneously regress (Ferradini et al., 1993, van Houdt et al., 2008). Therefore, at least two types of immune dysfunctions in melanoma could be identified; one with infiltration of lymphocytes (“hot tumor”) whereas others are not (“cold tumor”). The immune evasion mechanisms in the “hot” tumors may be caused by the over-expression of B7-H1 as well as well as other immune suppressors. The “cold tumors” seem to restrict T cell access and therefore appear non-inflamed.
This concept of “hot” versus “cold” tumors extends to all solid cancers. Importantly, anti-PD therapy appears to be targeting a mechanism widely shared by cancers as it has been approved for 18 different cancer indications and shows efficacy in many more malignancies (Ribas and Wolchok, 2018). For most of these FDA-approved indications, anti-PD therapy shows a significant tumor response-to-toxicity profile (Table 1). Listed in this table are pooled analysis from landmark clinical trials with FDA-approved PD-therapies as monotherapy. Additional anti-PD mAbs that are currently in clinical trials, have been approved in combination with other therapies, or have not yet been approved by the FDA are excluded. This is a rapidly expanding therapeutic landscape with more than 3,000 active cancer clinical trials involving PD-therapy (Tang et al., 2018). The current analysis is a single snapshot in time that will undoubtedly change with updated objective response rates and toxicity rates due to longer follow-up and additional data from ongoing clinical trials. Nevertheless, these data underscore the benefit of targeted local immune dysfunction within the TME.
Table 1:
Approved anti-PD monotherapies for cancer
| Cancer | Objective Response | Grade 3–5 Toxicity | Drug (target; FDA-approval) | References |
|---|---|---|---|---|
| Melanoma | 34% | 14% | Pembrolizumab (PD-1; 2014) Nivolumab (PD-1; 2014) | (Larkin et al., 2015, Larkin et al., 2018, Ribas et al., 2015, Robert et al., 2015a, Robert et al., 2015b, Sanmamed and Chen, 2018, Topalian et al., 2014 |
| NSCLC | 26% | 23% | Pembrolizumab (PD-1; 2015) Nivolumab (PD-1; 2015) Atezolizumab (PD-L1; 2016) Durvalumab (PD-L1; 2018) | (Antonia et al., 2017, Borghaei et al., 2015, Brahmer et al., 2015, Fehrenbacher et al., 2016, Reck et al., 2016) |
| RCC | 25% | 19% | Nivolumab (PD-1; 2015) Avelumab (PD-L1; 2019)[combined with Axitinib] | (Motzer et al., 2015) |
| Hodgkin lymphoma | 70% | 18% | Nivolumab (PD-1; 2016) Pembrolizumab (PD-1; 2017) | (Armand et al., 2018, Chen et al., 2019) |
| Urothelial carcinoma | 20% | 12% | Atezolizumab (PD-L1; 2016) Nivolumab (PD-1; 2017) Durvalumab (PD-L1; 2017) Avelumab (PD-L1; 2017) Pembrolizumab (PD-1; 2017) | (Balar et al., 2017, Necchi et al., 2017, Patel et al., 2018, Powles et al., 2017, Sharma et al., 2017b) |
| HNSCC | 15% | 13% | Pembrolizumab (PD-1; 2016) Nivolumab (PD-1; 2016) | (Ferris et al., 2016, Mehra et al., 2018) |
| Merkel Cell Carcinoma | 41% | 20% | Avelumab (PD-L1; 2017) Pembrolizumab (PD-1; 2018) | (Kaufman et al., 2016, Nghiem et al., 2019) |
| MSI-hi | 33% | 15% | Pembrolizumab (PD-1; 2017) | (Le et al., 2019) |
| Colorectal carcinoma (MSI-hi) | 31% | 20% | Nivolumab (PD-1; 2017) | (Overman et al., 2017) |
| Hepatocellular carcinoma | 19% | 21% | Nivolumab (PD-1; 2017) Pembrolizumab (PD-1; 2018) | (El-Khoueiry et al., 2017, Zhu et al., 2018) |
| Gastric cancer | 12% | 18% | Pembrolizumab (PD-1; 2017) | (Fuchs et al., 2018) |
| Cervical cancer | 15% | 12% | Pembrolizumab (PD-1; 2018) | (Chung et al., 2019) |
| PMBCL | 45% | 23% | Pembrolizumab (PD-1; 2018) | (Armand et al., 2019) |
| SCLC | 14% | 11% | Nivolumab (PD-1; 2018) Atezolizumab (PD-L1; 2019) [combined with chemotherapy] Pembrolizumab (PD-1; 2019) | (Antonia et al., 2016), NCT02628067, NCT02054806 |
| cSCC | 47% | 12%* | Cemiplimab (PD-1; 2018) | (Migden et al., 2018) |
| Esophageal cancer | 10% | 12% | Pembrolizumab (PD-1; 2019) | (Shah et al., 2019) |
cSCC, cutaneous squamous cell carcinoma; HNSCC, head and neck squamous cell carcinoma; MSH-hi, microsatellite instability high tumors including colorectal, endomentrial and gastrointestinal cancers; NSCLC, non-small cell lung cancer; PMBCL, primary mediastinal large B-cell lymphoma; RCC, renal cell carcinoma; SCLC, small cell lung cancer. Not included are endometrial carcinoma (pembrolizumab; 2019) and triple negative breast cancer (atezolizumab; 2019) which are approved in combination with chemotherapy or targeted therapy.
overall Grade 3–5 toxicity for cemiplimab is 42% while treatment related Grade 3–5 toxicity was 12%.
Immune-related adverse events in cancer immunotherapy
In addition to distinct tumor responses, enhancement cancer immunotherapy (anti-CTLA-4) and normalization cancer immunotherapy (anti-PD) have distinct immune-related adverse events (iRAEs) (Postow et al., 2018). For example, the most common cutaneous iRAE from ipilimumab is a spongiotic, eczematous eruption (Lacouture et al., 2014) while lichenoid dermatoses are more prominent in patients treated with PD-therapy (Shi et al., 2016). For systemic iRAEs, anti-CTLA-4 therapy is more associated with colitis and hypophysitis, whereas anti-PD therapy is more associated with pneumonitis and thyroiditis (Abdel-Rahman et al., 2016, Naidoo et al., 2017). There is significant debate as to the underlying mechanism of iRAEs during cancer immunotherapy. One potential mechanism is the disruption of tissue-specific tolerance. In this manner, tissue-resident Tregs expressing CTLA-4 are frequently inhibiting tissue-specific autoreactive T cells to maintain homeostasis. Similarly, activated tissue-resident T cells expressing PD-1 are inhibited by expression of PD-L1 on inflamed tissues, thereby preventing autoimmunity. Disruption of this balance by targeting CTLA-4 on Tregs or PD-1/PD-L1 on inflamed tissues would result in tissue-specific autoimmunity. Another potential mechanism is the endogenous expression of CTLA-4 and PD-1/PD-L1 is unique to distinct tissues. For example, expression of endogenous CTLA-4 on normal pituitary may help explain the frequency of hypophysitis with anti-CTLA-4 therapy (Iwama et al., 2014). In contrast, expression of PD-1 on alveolar macrophages may contribute the development of pneumonitis during anti-PD therapy (Igarashi et al., 2016).
Melanoma is unique where development of vitiligo as an iRAEs is related to the cancer target as opposed to the mechanism of immunotherapy (Hua et al., 2016). Shared melanoma antigens with normal melanocytes predisposes the development of T cells recognizing and destroying normal melanocytes in patients with melanoma treated with cancer immunotherapy. Vitiligo may develop during immunotherapy to other cancers as well (Kosche et al., 2018), but with a much lower frequency than melanoma. Overall, the development of iRAEs do not confer any treatment benefit with the exception of vitiligo during melanoma treatment (Hua et al., 2016).
Antigen quality over antigen quantity for successful cancer immunotherapy
The development of vitiligo during cancer immunotherapy of melanoma is reflective of immune detection of cancer antigens. Without immune detection of tumor-specific antigens, there can be no immune response to cancers. Therefore, it has been hypothesized that cancers with greater tumor mutational burden will have a greater number of potential neoantigens and thereby a higher success rate for cancer immunotherapy (Snyder et al., 2014). For example, cutaneous malignancies such as melanoma and cutaneous squamous cell carcinoma (cSCC) have high mutational load due to UV exposure and also have a high response rate to anti-PD therapy. However, tumor mutational burden does not always predict response to normalization cancer immunotherapy (Cristescu et al., 2018). Among the 18 cancers indications FDA-approved with PD-therapy, the two cancers with the lowest mutational burden have some of the highest response rates (Hodgkin’s lymphoma and PMBCL) (Table 1) (Armand et al., 2018, Armand et al., 2019). In addition to hematologic malignancies, tumor mutational burden as a predictive biomarker of response also does not correlate with virally induced solid cancers. For example, both head and neck squamous cell carcinoma (HNSCC) and Merkel cell carcinoma (MCC) can either be due viral transformation (lower mutational burden) or carcinogen-induction (higher mutational burden) (Goh et al., 2016, Stransky et al., 2011). Nevertheless, both subtypes show similar responses to anti-PD therapy (Ferris et al., 2016, Nghiem et al., 2019). Therefore, the quality of the antigens targeted is more important than the quantity of antigens. This underlies the importance of targeting the mechanism of immune escape within the TME with normalization cancer immunotherapy regardless of tumor mutational burden or cancer subtype.
PRINCIPLES OF NORMALIZATION CANCER IMMUNOTHERAPY
Because the mechanisms of immune suppression are largely developed within the TME, a deep understanding of the TME is critical to identify pathways selectively upregulated within the TME and resultant dysfunctional immunity against cancers. Therefore, the goal of normalization cancer immunotherapy is to reset or reprogram a previously effective immune response against cancers by targeting unique immune evasion mechanisms that predominately reside within the TME. The normalization effect includes at least two aspects – restoring existing dysfunctional immunity in the TME and preventing newly recruited immune cells from losing their functions (i.e., becoming dysfunctional).
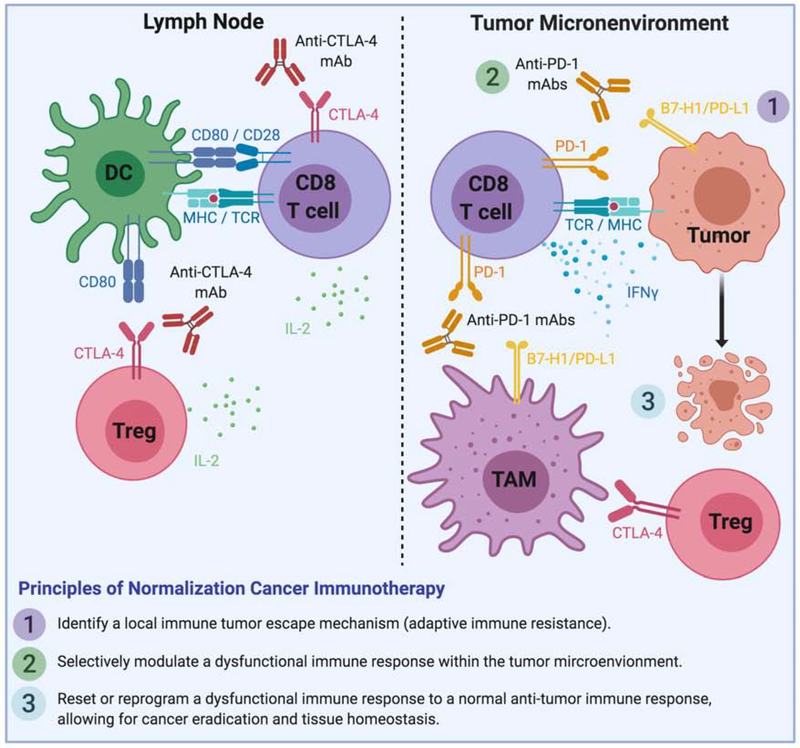
There are three major principles for normalization cancer immunotherapy to be successful (Figure 2). First, identify an immune tumor escape mechanism that occurs during tumor progression. This escape mechanism is largely developed and associated with tumor progression, but not part of normal physiological immune suppressive mechanisms required for control of immune responses. On such example is CTLA-4, which is required for the control of autoimmunity under physiological conditions. Genetic ablation or antibody blockade of CTLA-4 leads to severe autoimmune adverse events (Kuehn et al., 2014). Second, selectively modulate a dysfunctional immune response within the TME for therapeutic intervention. The majority of tumor evasion mechanism occurs in the TME. In addition, selective manipulation of immune responses in the TME could allow a more focused immune response so as to prevent severe adverse events. Third, manipulate only those mechanisms which are dominant in the TME. As multiple immune evasion mechanisms could occur simultaneously in the TME, it is obviously important to identify dominant mechanism or mechanisms in patients for therapeutic intervention. Currently, the only immunotherapy approved for melanoma or any other cancer that best fits the principles of normalization cancer immunotherapy is anti-PD therapy.
Figure 2: Targeting the tumor microenvironment: principles of normalization cancer immunotherapy.
The left panel represents T cell activation by dendritic cells in the lymph node where the function of anti-CTLA-4 and high-dose IL-2 acts to enhance T cell activation. The right panel represents T cells within the tumor microenvironment where anti-PD therapy acts to block PD-1/PD-L1 interactions. The three guiding principles of normalization cancer immunotherapy are listed at the bottom. TAM, tumor-associated macrophage.
However, anti-PD-therapy is far from perfect with only a subset of patients responding and some patients experiencing significant toxicity, albeit less than other immunotherapies. Clearly there are other mechanisms of adaptive immune resistance to be interrogated, including other immune inhibitory molecules (Wang et al., 2019a, Wang et al., 2019b, Yao et al., 2013). In addition to other immune inhibitory molecules expressed within the TME as a mechanism of resistance to PD-therapy, tumor intrinsic adaptations may occur which evade immune-mediated tumor cell destruction (Sharma et al., 2017a). For example, mutations in antigen processing and presentation machinery (Zaretsky et al., 2016) or interferon-gamma signaling pathways (Shin et al., 2017) results in resistance to PD-therapy. Despite these occurrences of tumor intrinsic resistance, normalization cancer immunotherapy is still a powerful approach to restore a normal anti-tumor immune response locally within the TME so as to maximize tumor eradication and limit systemic toxicity.
FUTURE CONSIDERATIONS FOR CANCER IMMUNOTHERAPY
Careful interrogation of the TME is critical to addressing the numerous defects or dysfunction of anti-tumor immunity to help determine the mechanisms. As new potential therapeutics are developed and future clinical trials embarked, it is critical to focus efforts on those that try to restore an already powerful anti-tumor immune response within the TME based on mechanisms of tumor-specific immune evasion rather than general immune activation.
Acknowledgments
M.D.V. is supported by a Physician-Scientist Career Development Award from the Dermatology Foundation, a Dermatology Fellow Award from the Melanoma Research Alliance, and KL2 TR001862 from National Center for Advancing Translational Sciences (NCATS) through Yale Center for Clinical Investigation. The study is also partially funded by NIH grants CA016350, CA186689, CA121974 and an endowment from the United Technologies Corporation (L.C.). Figures generated using Biorender.com with academic license.
Conflict of Interest: M.D.V. serves as the Resident/Fellow representative on the Board of Directors for the Society of Investigative Dermatology. Spouse of M.D.V. is an employee at Regeneron Pharmaceuticals. L.C. is a consultant/advisory board member for NextCure, Junshi, Zai Lab, Tayu, Vcanbio and GenomiCare; is a scientific founder of NextCure and Tayu and has sponsored research grants from NextCure and DynamiCure in the past 12 months.
Abbreviations
- APC
antigen-presenting cells
- CTLA-4
cytotoxic T-lymphocyte-associated protein 4
- DC
dendritic cell
- IFN
interferon
- IL
interleukin
- irAEs
immune-related adverse events
- PD-1
programmed death-1 receptor
- PD-L1
programmed death receptor ligand 1
- TILs
tumor-infiltrating lymphocytes
- TME
tumor microenvironment
- Treg
regulatory T cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abdel-Rahman O, ElHalawani H, Fouad M. Risk of endocrine complications in cancer patients treated with immune check point inhibitors: a meta-analysis. Future Oncol 2016;12(3):413–25. [DOI] [PubMed] [Google Scholar]
- Agarwala SS, Glaspy J, O’Day SJ, Mitchell M, Gutheil J, Whitman E, et al. Results from a randomized phase III study comparing combined treatment with histamine dihydrochloride plus interleukin-2 versus interleukin-2 alone in patients with metastatic melanoma. J Clin Oncol 2002;20(1):125–33. [DOI] [PubMed] [Google Scholar]
- Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J Clin Oncol 2015;33(25):2780–8. [DOI] [PubMed] [Google Scholar]
- Antonia SJ, Lopez-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016;17(7):883–95. [DOI] [PubMed] [Google Scholar]
- Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377(20):1919–29. [DOI] [PubMed] [Google Scholar]
- Arce Vargas F, Furness AJS, Litchfield K, Joshi K, Rosenthal R, Ghorani E, et al. Fc Effector Function Contributes to the Activity of Human Anti-CTLA-4 Antibodies. Cancer Cell 2018;33(4):649–63 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armand P, Engert A, Younes A, Fanale M, Santoro A, Zinzani PL, et al. Nivolumab for Relapsed/Refractory Classic Hodgkin Lymphoma After Failure of Autologous Hematopoietic Cell Transplantation: Extended Follow-Up of the Multicohort Single-Arm Phase II CheckMate 205 Trial. J Clin Oncol 2018;36(14):1428–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armand P, Rodig S, Melnichenko V, Thieblemont C, Bouabdallah K, Tumyan G, et al. Pembrolizumab in Relapsed or Refractory Primary Mediastinal Large B-Cell Lymphoma. J Clin Oncol 2019;37(34):3291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascierto PA, Del Vecchio M, Robert C, Mackiewicz A, Chiarion-Sileni V, Arance A, et al. Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma: a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol 2017;18(5):611–22. [DOI] [PubMed] [Google Scholar]
- Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 1999;17(7):2105–16. [DOI] [PubMed] [Google Scholar]
- Balar AV, Castellano D, O’Donnell PH, Grivas P, Vuky J, Powles T, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol 2017;18(11):1483–92. [DOI] [PubMed] [Google Scholar]
- Beer TM, Kwon ED, Drake CG, Fizazi K, Logothetis C, Gravis G, et al. Randomized, Double-Blind, Phase III Trial of Ipilimumab Versus Placebo in Asymptomatic or Minimally Symptomatic Patients With Metastatic Chemotherapy-Naive Castration-Resistant Prostate Cancer. J Clin Oncol 2017;35(1):40–7. [DOI] [PubMed] [Google Scholar]
- Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373(17):1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373(2):123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran SS, Somerville RPT, Yang JC, Sherry RM, Klebanoff CA, Goff SL, et al. Treatment of metastatic uveal melanoma with adoptive transfer of tumour-infiltrating lymphocytes: a single-centre, two-stage, single-arm, phase 2 study. Lancet Oncol 2017;18(6):792–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Zinzani PL, Lee HJ, Armand P, Johnson NA, Brice P, et al. Pembrolizumab in relapsed or refractory Hodgkin lymphoma: 2-year follow-up of KEYNOTE-087. Blood 2019;134(14):1144–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HC, Ros W, Delord JP, Perets R, Italiano A, Shapira-Frommer R, et al. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Cervical Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol 2019;37(17):1470–8. [DOI] [PubMed] [Google Scholar]
- Cristescu R, Mogg R, Ayers M, Albright A, Murphy E, Yearley J, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 2018;362(6411). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker T, Muller M, Stockinger S. The yin and yang of type I interferon activity in bacterial infection. Nat Rev Immunol 2005;5(9):675–87.0 [DOI] [PubMed] [Google Scholar]
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002;8(8):793–800. [DOI] [PubMed] [Google Scholar]
- Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 1999;5(12):1365–9. [DOI] [PubMed] [Google Scholar]
- El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389(10088):2492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387(10030):1837–46. [DOI] [PubMed] [Google Scholar]
- Ferradini L, Mackensen A, Genevee C, Bosq J, Duvillard P, Avril MF, et al. Analysis of T cell receptor variability in tumor-infiltrating lymphocytes from a human regressive melanoma. Evidence for in situ T cell clonal expansion. J Clin Invest 1993;91(3):1183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris RL, Blumenschein G Jr., Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med 2016;375(19):1856–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol 2018;4(5):e180013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh G, Walradt T, Markarov V, Blom A, Riaz N, Doumani R, et al. Mutational landscape of MCPyV-positive and MCPyV-negative Merkel cell carcinomas with implications for immunotherapy. Oncotarget 2016;7(3):3403–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein D, Laszlo J. Interferon therapy in cancer: from imaginon to interferon. Cancer Res 1986;46(9):4315–29. [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144(5):646–74. [DOI] [PubMed] [Google Scholar]
- Hodi FS, Chesney J, Pavlick AC, Robert C, Grossmann KF, McDermott DF, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol 2016;17(11):1558–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Cowey CL, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol 2018;19(11):1480–92. [DOI] [PubMed] [Google Scholar]
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N Engl J Med 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua C, Boussemart L, Mateus C, Routier E, Boutros C, Cazenave H, et al. Association of Vitiligo With Tumor Response in Patients With Metastatic Melanoma Treated With Pembrolizumab. JAMA Dermatol 2016;152(1):45–51. [DOI] [PubMed] [Google Scholar]
- Igarashi T, Teramoto K, Ishida M, Hanaoka J, Daigo Y. Scoring of PD-L1 expression intensity on pulmonary adenocarcinomas and the correlations with clinicopathological factors. ESMO Open 2016;1(4):e000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs A, Lindenmann J. Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci 1957;147(927):258–67. [DOI] [PubMed] [Google Scholar]
- Iwama S, De Remigis A, Callahan MK, Slovin SF, Wolchok JD, Caturegli P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med 2014;6(230):230ra45. [DOI] [PubMed] [Google Scholar]
- Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, et al. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci U S A 1998;95(13):7556–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman HL, Russell J, Hamid O, Bhatia S, Terheyden P, D’Angelo SP, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol 2016;17(10):1374–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khong HT, Restifo NP. Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nat Immunol 2002;3(11):999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TK, Herbst RS, Chen L. Defining and Understanding Adaptive Resistance in Cancer Immunotherapy. Trends Immunol 2018;39(8):624–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, Blum RH. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol 1996;14(1):7–17. [DOI] [PubMed] [Google Scholar]
- Kosche C, Mohindra N, Choi JN. Vitiligo in a patient undergoing nivolumab treatment for non-small cell lung cancer. JAAD Case Rep 2018;4(10):1042–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn HS, Ouyang W, Lo B, Deenick EK, Niemela JE, Avery DT, et al. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science 2014;345(6204):1623–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacouture ME, Wolchok JD, Yosipovitch G, Kahler KC, Busam KJ, Hauschild A. Ipilimumab in patients with cancer and the management of dermatologic adverse events. J Am Acad Dermatol 2014;71(1):161–9. [DOI] [PubMed] [Google Scholar]
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015;373(1):23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin J, Minor D, D’Angelo S, Neyns B, Smylie M, Miller WH Jr., et al. Overall Survival in Patients With Advanced Melanoma Who Received Nivolumab Versus Investigator’s Choice Chemotherapy in CheckMate 037: A Randomized, Controlled, Open-Label Phase III Trial. J Clin Oncol 2018;36(4):383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le DT, Kim TW, Van Cutsem E, Geva R, Jager D, Hara H, et al. Phase II Open-Label Study of Pembrolizumab in Treatment-Refractory, Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: KEYNOTE-164. J Clin Oncol 2019:JCO1902107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch TJ, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol 2012;30(17):2046–54. [DOI] [PubMed] [Google Scholar]
- Matsushita H, Vesely MD, Koboldt DC, Rickert CG, Uppaluri R, Magrini VJ, et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature 2012;482(7385):400–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra R, Seiwert TY, Gupta S, Weiss J, Gluck I, Eder JP, et al. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: pooled analyses after long-term follow-up in KEYNOTE-012. Br J Cancer 2018;119(2):153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migden MR, Rischin D, Schmults CD, Guminski A, Hauschild A, Lewis KD, et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N Engl J Med 2018;379(4):341–51. [DOI] [PubMed] [Google Scholar]
- Morgan DA, Ruscetti FW, Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science 1976;193(4257):1007–8. [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015;373(19):1803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, et al. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J Clin Oncol 2017;35(7):709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Necchi A, Joseph RW, Loriot Y, Hoffman-Censits J, Perez-Gracia JL, Petrylak DP, et al. Atezolizumab in platinum-treated locally advanced or metastatic urothelial carcinoma: post-progression outcomes from the phase II IMvigor210 study. Ann Oncol 2017;28(12):3044–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nghiem P, Bhatia S, Lipson EJ, Sharfman WH, Kudchadkar RR, Brohl AS, et al. Durable Tumor Regression and Overall Survival in Patients With Advanced Merkel Cell Carcinoma Receiving Pembrolizumab as First-Line Therapy. J Clin Oncol 2019;37(9):693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017;547(7662):217–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 2017;18(9):1182–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson DR, Abrams JS, Wiernik PH, Rayner AA, Margolin KA, Van Echo DA, et al. Interleukin-2 therapy in patients with metastatic malignant melanoma: a phase II study. J Clin Oncol 1990;8(10):1650–6. [DOI] [PubMed] [Google Scholar]
- Patel MR, Ellerton J, Infante JR, Agrawal M, Gordon M, Aljumaily R, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol 2018;19(1):51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med 2018;378(2):158–68. [DOI] [PubMed] [Google Scholar]
- Powles T, O’Donnell PH, Massard C, Arkenau HT, Friedlander TW, Hoimes CJ, et al. Efficacy and Safety of Durvalumab in Locally Advanced or Metastatic Urothelial Carcinoma: Updated Results From a Phase 1/2 Open-label Study. JAMA Oncol 2017;3(9):e172411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375(19):1823–33. [DOI] [PubMed] [Google Scholar]
- Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015;16(8):908–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018;359(6382):1350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015a;372(4):320–30. [DOI] [PubMed] [Google Scholar]
- Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med 2015b;372(26):2521–32. [DOI] [PubMed] [Google Scholar]
- Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364(26):2517–26. [DOI] [PubMed] [Google Scholar]
- Romano E, Kusio-Kobialka M, Foukas PG, Baumgaertner P, Meyer C, Ballabeni P, et al. Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc Natl Acad Sci U S A 2015;112(19):6140–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med 1988;319(25):1676–80. [DOI] [PubMed] [Google Scholar]
- Rosenberg SA, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med 1998;4(3):321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA 1994;271(12):907–13. [PubMed] [Google Scholar]
- Sanmamed MF, Chen L. A Paradigm Shift in Cancer Immunotherapy: From Enhancement to Normalization. Cell 2018;175(2):313–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 2011;331(6024):1565–70. [DOI] [PubMed] [Google Scholar]
- Schwartz RN, Stover L, Dutcher JP. Managing toxicities of high-dose interleukin-2. Oncology (Williston Park) 2002;16(11 Suppl 13):11–20. [PubMed] [Google Scholar]
- Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med 2011;364(22):2119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MA, Kojima T, Hochhauser D, Enzinger P, Raimbourg J, Hollebecque A, et al. Efficacy and Safety of Pembrolizumab for Heavily Pretreated Patients With Advanced, Metastatic Adenocarcinoma or Squamous Cell Carcinoma of the Esophagus: The Phase 2 KEYNOTE-180 Study. JAMA Oncol 2019;5(4):546–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 2001;410(6832):1107–11. [DOI] [PubMed] [Google Scholar]
- Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017a;168(4):707–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2017b;18(3):312–22. [DOI] [PubMed] [Google Scholar]
- Shi VJ, Rodic N, Gettinger S, Leventhal JS, Neckman JP, Girardi M, et al. Clinical and Histologic Features of Lichenoid Mucocutaneous Eruptions Due to Anti-Programmed Cell Death 1 and Anti-Programmed Cell Death Ligand 1 Immunotherapy. JAMA Dermatol 2016;152(10):1128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DS, Zaretsky JM, Escuin-Ordinas H, Garcia-Diaz A, Hu-Lieskovan S, Kalbasi A, et al. Primary Resistance to PD-1 Blockade Mediated by JAK1/2 Mutations. Cancer Discov 2017;7(2):188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014;371(23):2189–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, et al. The mutational landscape of head and neck squamous cell carcinoma. Science 2011;333(6046):1157–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sznol M, Ferrucci PF, Hogg D, Atkins MB, Wolter P, Guidoboni M, et al. Pooled Analysis Safety Profile of Nivolumab and Ipilimumab Combination Therapy in Patients With Advanced Melanoma. J Clin Oncol 2017;35(34):3815–22. [DOI] [PubMed] [Google Scholar]
- Tang J, Yu JX, Hubbard-Lucey VM, Neftelinov ST, Hodge JP, Lin Y. Trial watch: The clinical trial landscape for PD1/PDL1 immune checkpoint inhibitors. Nat Rev Drug Discov 2018;17(12):854–5. [DOI] [PubMed] [Google Scholar]
- Taniguchi T, Matsui H, Fujita T, Takaoka C, Kashima N, Yoshimoto R, et al. Structure and expression of a cloned cDNA for human interleukin-2. Nature 1983;302(5906):305–10. [DOI] [PubMed] [Google Scholar]
- Tarhini AA, Kirkwood JM, Gooding WE, Cai C, Agarwala SS. Durable complete responses with high-dose bolus interleukin-2 in patients with metastatic melanoma who have experienced progression after biochemotherapy. J Clin Oncol 2007;25(25):3802–7. [DOI] [PubMed] [Google Scholar]
- Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012;4(127):127ra37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 1995;3(5):541–7. [DOI] [PubMed] [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366(26):2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014;32(10):1020–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 1991;254(5038):1643–7. [DOI] [PubMed] [Google Scholar]
- van Houdt IS, Sluijter BJ, Moesbergen LM, Vos WM, de Gruijl TD, Molenkamp BG, et al. Favorable outcome in clinically stage II melanoma patients is associated with the presence of activated tumor infiltrating T-lymphocytes and preserved MHC class I antigen expression. Int J Cancer 2008;123(3):609–15. [DOI] [PubMed] [Google Scholar]
- Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol 2011;29:235–71. [DOI] [PubMed] [Google Scholar]
- Wang J, Sanmamed MF, Datar I, Su TT, Ji L, Sun J, et al. Fibrinogen-like Protein 1 Is a Major Immune Inhibitory Ligand of LAG-3. Cell 2019a;176(1–2):334–47 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sun J, Liu LN, Flies DB, Nie X, Toki M, et al. Siglec-15 as an immune suppressor and potential target for normalization cancer immunotherapy. Nat Med 2019b;25(4):656–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 1995;270(5238):985–8. [DOI] [PubMed] [Google Scholar]
- Weber J, Thompson JA, Hamid O, Minor D, Amin A, Ron I, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res 2009;15(17):5591–8. [DOI] [PubMed] [Google Scholar]
- Wheatley K, Ives N, Hancock B, Gore M, Eggermont A, Suciu S. Does adjuvant interferon-alpha for high-risk melanoma provide a worthwhile benefit? A meta-analysis of the randomised trials. Cancer Treat Rev 2003;29(4):241–52. [DOI] [PubMed] [Google Scholar]
- Wiesinger M, Marz J, Kummer M, Schuler G, Dorrie J, Schuler-Thurner B, et al. Clinical-Scale Production of CAR-T Cells for the Treatment of Melanoma Patients by mRNA Transfection of a CSPG4-Specific CAR under Full GMP Compliance. Cancers (Basel) 2019;11(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science 2008;322(5899):271–5. [DOI] [PubMed] [Google Scholar]
- Yao S, Zhu Y, Chen L. Advances in targeting cell surface signalling molecules for immune modulation. Nat Rev Drug Discov 2013;12(2):130–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N Engl J Med 2016;375(9):819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018;19(7):940–52. [DOI] [PubMed] [Google Scholar]
- Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol 2006;6(10):715–27. [DOI] [PubMed] [Google Scholar]
- Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med 2016;8(328):328rv4. [DOI] [PMC free article] [PubMed] [Google Scholar]