Abstract
Background:
Obesity is a risk factor for total knee replacement (TKR). Randomized trials such as LookAHEAD (Action for Health in Diabetes) have shown long-term successful weight loss with an intensive lifestyle intervention (ILI). It is unknown however, if intentional weight loss can reduce the risk of TKR.
Methods:
LookAHEAD randomized persons with type 2 diabetes who were overweight or obese to either an ILI to achieve/maintain 7% weight loss or to standard diabetes support and education (DSE). Knee pain was assessed using the Visual Analog Scale (VAS) and Western Ontario McMaster University Osteoarthritis Index (WOMAC) questionnaire in 5,125 participants without previous TKR. Cox proportional hazard regression was used to model differences in risk of TKR in relation to randomization group assignment (ILI vs. DSE) along with baseline BMI and baseline knee pain.
Results:
Baseline WOMAC knee pain scores did not differ by treatment assignment (ILI: 3.6±2.9, DSE: 3.9±3.0, p=0.08). Six-hundred-thirty-one (12%) participants who reported having a TKR were more likely to have been heavier (p<0.001) and older (p<0.001), but risk of TKR did not differ by treatment group assignment (HR [95%CI] 1.07 [0.91, 1.25]; P=0.43). In persons without knee pain at baseline, there was a 29% reduced risk of TKR in ILI compared to DSE (HR [95%CI]: 0.71[0.52, 0.96]). However, in persons with knee pain at baseline, there was no statistically significant association of treatment assignment (HR [95%CI]: 1.11[0.92, 1.33]).
Conclusion:
Findings suggest that ILI and behavioral changes to achieve weight loss may be most effective in preventing TKR.
Keywords: Knee, Arthroplasty, Obesity, weight loss
Introduction
There is overwhelming evidence establishing the link between obesity and many adverse health consequences such as hypertension, diabetes and heart disease, as well as early knee osteoarthritis. It has also been well established that obesity increases the risk for end stage osteoarthritis (OA) of the knee and that individuals with obesity have a three-fold increased risk of acquiring OA of the knee [1]. Total knee replacement (TKR) remains one of the most commonly performed and successful interventions for patients with significant knee pain from OA, with over 700,000 procedures performed in 2010 in the United States including nearly 50,000 revision procedures [2–5]. In the last several years across the United States, the TKR CPT (Current Procedural Terminology) medical code is linked to over $11 billion in hospital reimbursed costs [6, 7]. Moreover, individuals who remain overweight or obese following TKR have lower self-reported quality of life, such as reduced mobility and ability to perform activities of daily living, and a greater likelihood of need for future revision of the current TKR [8–13], in addition to undergoing TKR of the opposite limb [14]. This unfortunately presents an accumulating health disparity for the TKR patient population who are overweight or obese. Despite the growing health care cost and challenge facing providers, there remains a void in the current evidence indicating whether intentional long-term weight loss in individuals with overweight and obesity can prevent TKR and associated functional concerns. Therefore, there is a strong need to determine if intentional and sustained weight loss in persons with overweight and obesity is associated with TKR along with what, if any, factors are associated with and possibly protect against reported knee pain escalating to TKR [15].
Look AHEAD was a multi-center, randomized controlled trial designed to test the effects on cardiovascular mortality of an intensive lifestyle intervention intended to produce 5-10% weight loss. All Look AHEAD participants alive at the end of the trial when the intervention was stopped, were invited to join a follow-up observational study to determine the longer-term effects of the intervention on a number of outcomes. Large clinical trials, such as Look AHEAD, have shown that multicomponent weight loss interventions combining diet, physical activity, and behavior modification are effective in achieving long-term weight reduction [16, 17] and that weight loss can prevent the development of knee pain [18]. As a result, clinical practice guidelines recommend weight loss interventions for numerous health conditions, including knee pain [19, 20]. Using the data from Look AHEAD, we examined the relationship of assignment to ILI with risk of TKR during follow-up. This paper reports on the entire Look AHEAD population (N=5,145) from baseline randomization through Look AHEAD Extension with a median follow-up of 14 years.
Methods
Study Population
Look AHEAD was a multicenter, randomized controlled trial that enrolled 5,145 adults with Type 2 diabetes who were 45-76 years of age, with a BMI>25 kg/m2 (>27 kg/m2 if taking insulin) from 16 research sites across the US. Study participants were randomized into either an Intensive Lifestyle Intervention (ILI) with the goals to lose >7% of body weight and participate in >175 minutes/week of moderate to vigorous physical activity or a Diabetes Support and Education (DSE) comparison group. Additional exclusionary criteria included HbA1c > 11%, blood pressure > 160/100mmHg, triglycerides >600 mg/dL, weight >350 lbs, cardio- or cerebrovascular events in the previous 3-months, significant recent weight loss or inability to walk two blocks [21, 22]. The Look AHEAD study design, recruitment, baseline characteristics and primary outcomes have been published [17, 22].
Data from baseline randomization through Look AHEAD Extension (median follow-up = 14 years) was examined for self-report of TKR was obtained from follow-up visit questionnaires collected approximately every 6 months in both groups. Additionally, chart review was performed to determine if TKR was a partial knee replacement or revision and serious adverse event reports were consulted for which knee was evaluated (left or right).
During the baseline visit, all participants were asked “Have you had any pain or discomfort in your knees in the past month?” (yes or no). For those participants responding “yes”, the Visual Analog Scale-Knee Pain [23] (VAS-Knees) and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) knee pain [24] questionnaires were used to assess knee pain severity. Specifically, the VAS-knee pain questionnaire asked participants “On a scale of 0 to 100 how bad was the pain in your left or right knee in the past two weeks” and the WOMAC knee pain and function questionnaire had participants rate their knee pain during five different activities on a scale of “none (0), mild (1), moderate (2), severe (3), and extreme (4).” All five values were then summed across the questions to yield WOMAC level of knee pain score. The Medical Outcomes Study Short Form 36 (SF-36) [25], physical functioning and bodily pain subscales were also examined.
Participants with TKR (n=2), revisions (n=3) or partial knee replacements (n=15) prior to randomization were excluded from these analyses, resulting in 5,125 of the 5,145 randomized participants included in our analytic sample. In the secondary analysis of the effect of year 1 weight change on time to TKR, 67 participants who censored or had a TKR in the first year of Look AHEAD were excluded (ILI=33, DSE=34).
Statistical Analysis
Demographic characteristics and baseline knee pain were compared between groups who subsequently had a TKR and those who did not using chi-square for categorical and Student’s t-test for continuous measures (Table 1). Cox proportional hazard models were used to analyze differences in time to first TKR by randomization group adjusting for baseline BMI category (<30, ≥30 kg/m2), baseline knee pain (yes, no), gender, age, and race. Participants without TKR were censored at either their last available Study Outcomes form collection date, date of death, date of study close-out visit(if refused to continue), date lost to follow-up, or at date of last known status change (completed final follow-up, refused contact, moved away, or terminated for other reasons). Models testing interaction for differential effect of treatment group on time to TKR for by baseline BMI category and baseline knee pain were then examined. Interactions that were not statistically significant were dropped from the final model.
Table 1.
Baseline Characteristics of Participants by Total Knee Replacement (TKR) Status During Follow-up
| Had TKR (n=631) | No TKR (n=4494) | P-value | |
|---|---|---|---|
| Treatment Assignment, No. (%) | 0.5985 | ||
| Diabetes Support and Education (DSE) | 322 (51.0%) | 2243 (49.9%) | |
| Intensive Lifestyle Intervention (ILI) | 309 (49.0%) | 2251 (50.1%) | |
| Age, mean ± SD, years | 60.2 ± 6.0 | 58.5 ± 6.9 | <.0001 |
| Age Category, No. (%), years | <.0001 | ||
| 44 – 55 | 133 (21.1%) | 1480 (32.9%) | |
| 56 – 65 | 374 (59.3%) | 2266 (50.4%) | |
| 66 – 76 | 124 (19.7%) | 748 (16.6%) | |
| Gender, No. (%) | 0.0273 | ||
| Male | 230 (36.5%) | 1845 (41.1%) | |
| Female | 401 (63.5%) | 2649 (58.9%) | |
| Race, No. (%) | <.0001 | ||
| African American / Black (not Hispanic) | 81 (12.8%) | 721 (16.0%) | |
| White | 453 (71.8%) | 2786 (62.0%) | |
| Hispanic | 62 (9.8%) | 614 (13.7%) | |
| Other/Mixed | 35 (5.5%) | 372 (8.3%) | |
| Body Mass Index, mean ± SD, kg/m2 | 37.6 ± 5.7 | 35.7 ± 5.9 | <.0001 |
| Body Mass Index Group, No. (%), kg/m2 | <.0001 | ||
| Overweight: Less than 30 | 44 (7.0%) | 720 (16.0%) | |
| Class I: 30-34 | 183 (29.0%) | 1625 (36.2%) | |
| Class II: 35-39 | 204 (32.3%) | 1205 (26.8%) | |
| Class III: ≥ 40 | 200 (31.7%) | 944 (21.0%) | |
| Baseline Knee Pain, No. (%) | <.0001 | ||
| Yes | 451 (72.4%) | 1720 (38.9%) | |
| No | 172 (27.6%) | 2697 (61.1%) | |
| Visual Analog Scale Knee Pain | 39.5 ± 23.2 | 32.5 ± 20.9 | <.0001 |
| WOMAC* Knee Pain | 4.5 ± 3.2 | 3.6 ± 2.8 | <.0001 |
| Short Form-36 Health Related Quality of Life, mean ± SD | |||
| Physical Component Score | 45.3 ± 8.5 | 48.3 ± 7.8 | <.0001 |
| Bodily Pain | 48.1 ± 8.6 | 51.0 ± 8.7 | <.0001 |
| Physical Functioning | 45.9 ± 8.4 | 48.8 ± 7.7 | <.0001 |
Western Ontario McMaster Universities Osteoarthritis Index
In secondary analyses that were parallel to the main analyses, the effect of year 1 weight loss on time to TKR was examined. Year 1 weight loss was categorized as lost >5%, stable weight change, and gained > 2% of baseline weight. As in the primary analyses, tests for heterogeneity in randomization groups and baseline knee pain as well as between randomization group and year 1 weight change categories were investigated after adjusting for gender, race, age, and baseline BMI. Alpha of 0.05 was used as the significance level for all tests.
Results
Of the 5,125 included participants (N=2560 in ILI, 2565 in DSE), no statistically significant difference between intervention groups existed in any baseline characteristic including age, gender, race, education, smoking status, alcohol use, insulin use, HbA1c group, or BMI (as expected due to equality of groups by randomization; data not shown). A total of 2,171 participants reported knee pain (43%) while 2,869 reported no knee pain (57%) at the baseline visit with no difference between ILI and DSE groups (p=0.81). In addition, neither WOMAC knee pain score nor VAS knee pain differed by random assignment (WOMAC mean±SD ILI:3.6±2.9, DSE:3.9±3.0, p=0.08; VAS knee pain ILI:33.8±20.9, DSE: 34.1±22.1, p=0.69). A complete list of baseline characteristics by group assignment has been published elsewhere [16, 17].
During a median follow up of 14.0 years 631 participants reported having a TKR. Of those, the 309 in the ILI group had a median time to TKR of 7.3 years while the 322 in the DSE group had a median time of 6.8 years to TKR. Participants who had a TKR post-randomization were more likely to have been older at enrollment, heavier, female, and Caucasian. In addition, they were more likely to have reported significant knee pain at baseline, for pain to be greater and more severe with activities, and to have lower scores on the SF-36 Physical Component Score and subscales for physical function and bodily pain (Table 1). There was no statistically significant difference in the incidence of TKR between the ILI and DSE group (HR [95%CI] 1.07 [0.91, 1.25]) p= 0.43 (Figure 1) in either unadjusted or fully adjusted models.
Fig. 1.
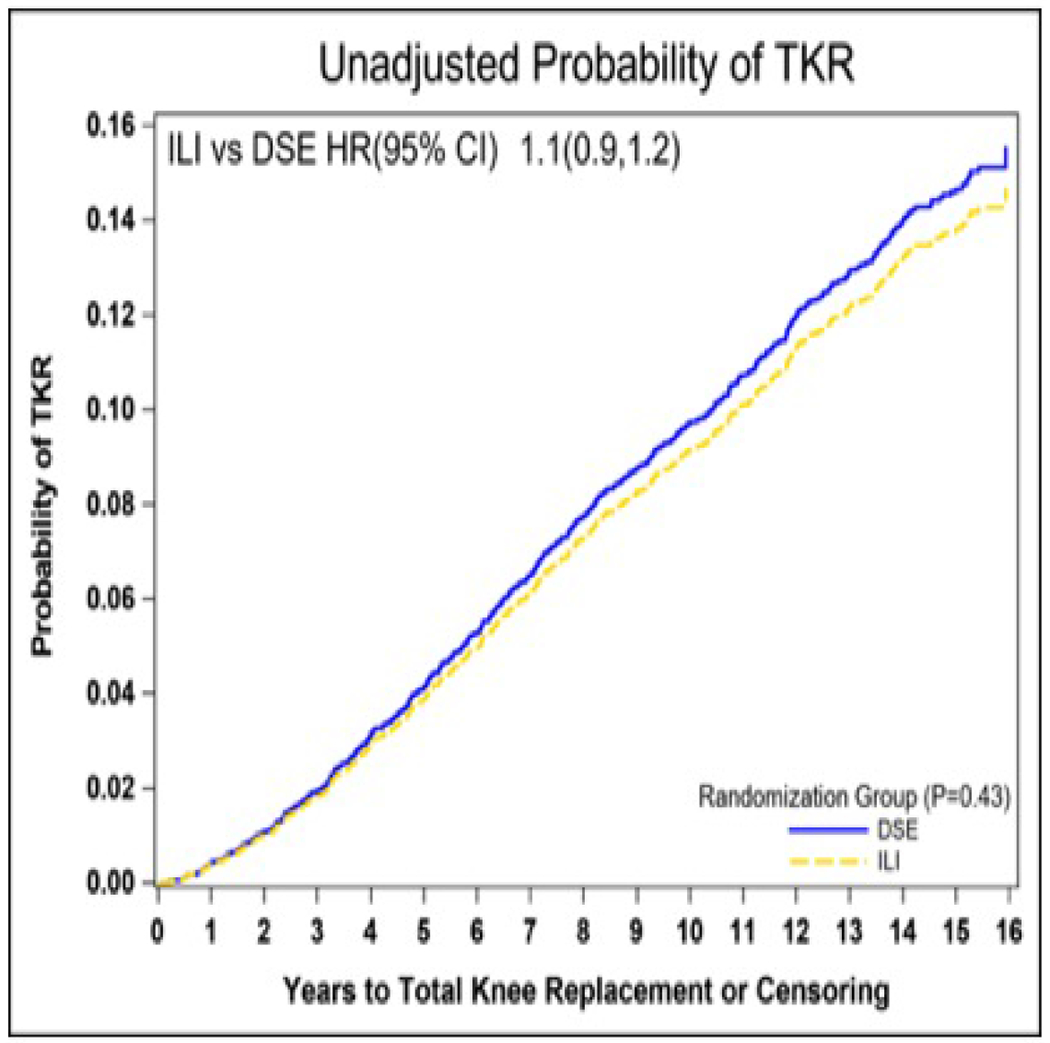
Unadjusted probability of total knee replacement by randomization group. TKR, total knee replacement; ILI, intensive lifestyle intervention; DSE, diabetes support and education; HR, hazard ratio; CI, confidence interval.
On further analyses that considered interaction effects, heterogeneity of treatment effect by baseline knee pain was observed for TKR (interaction p = 0.0143) (Figure 2). In participants without knee pain at baseline, there was a 29% reduction in risk of TKR in the ILI group compared to the DSE group (HR [95%CI] 0.71 [0.52,0.96]; p = 0.02) (Table 2), whereas in participants with knee pain at baseline there was no statistically significant difference between randomization groups (HR [95%CI] 1.09 [0.91,1.31]; p = 0.36). In this same model, test for heterogeneity of treatment effect by baseline BMI category as well as baseline BMI category by baseline knee pain were investigated. While baseline BMI category did not differ by randomization assignment or baseline knee pain, participants with baseline BMI ≥ 30 kg/m2 had 222% increase in risk of TKR compared to participants with BMI < 30 kg/m2 (HR[95%CI]: 2.22[1.63,3.02], p<.0001), even after adjusting for randomization group by knee pain interaction, age, gender, and race.
Fig. 2.

Probability of total knee replacement by randomization group and baseline knee pain after adjusting for age, gender, race, and baseline BMI. BMI, body mass index.
In parallel secondary analyses of year 1 weight change with the reduced sample, results were similar to the primary analyses. There was no significant heterogeneity of treatment effect by year 1 weight change category or year 1 weight change category by baseline knee pain. After removing non-significant interactions and adjusting for randomization group by knee pain interaction, age, gender, and race, year 1 weight change category was not significantly associated with risk of TKR (gained vs stable HR[95%CI] = 1.21 [0.95,1.55]; lost vs stable 1.13[0.92,1.39]; p=0.2145).
Discussion:
Multicomponent intensive weight loss interventions such as the Look AHEAD ILI have shown promise for long-term weight loss in people with Type 2 diabetes [16, 26]. The Look AHEAD trial was successful in achieving and maintaining long-term intentional weight loss through use of an ILI program. To date, however, the effect of a long-term multicomponent behavioral weight loss intervention from a randomized clinical trial such as Look AHEAD on TKR risk has not been reported. This paper fills a critical research gap by examining the relationship between intentional weight loss and incidence of TKR.
We did not find significant differences in the incidence of TKR between participants in the ILI and DSE study arms, and also did not find evidence for a relationship of weight loss within the first year of the study with subsequent TKR. However, in participants with no knee pain at baseline, ILI study arm membership was associated with a reduced risk of TKR, while in the group with knee pain, there was no difference between arms. Because both groups were of comparable size, this difference in significant/non-significant association cannot be explained by appreciably different detectable effect sizes that would be a consequence of unequal group sizes.
The Look AHEAD trial was successful in maintaining significant weight loss in participants who were randomized to the ILI. The Look AHEAD ILI utilized walking as its primary exercise modality. However standard exercise prescription guidelines suggest the importance of including non-weight bearing exercises for individuals with overweight and type 2 diabetes, particularly those with knee pain [27]. In individuals with increased BMI, higher impact activities producing loads ≥ 5-10 times bodyweight may increase knee pain and complications from OA when present (we do assume that OA is the primary cause of knee pain but see limitations below). Loads across the knee are multifactorial [28] and may play a role in progression of OA as well as TKR risk in persons with obesity. Specifically, lower extremity alignment, along with weight and the adductor moment magnitude, all play a significant role in applied loads across the knee and hence the progression of OA [29]. Henrikson et al, reported significant OA progression of an exercise intervention group compared to diet only and control groups, at 1 year in a study of 192 participants with OA and obesity [29] . Messier et al, reported in similar findings in their weight loss investigation of 454 community-dwelling adults with radiographic diagnosed knee OA, self-reported knee pain and overweight and obesity. In that study, knee compressive force (joint loading) decreased the most, by 10%, in the diet only group compared to a 9% decrease in the diet + exercise group, while the exercise only group showed a decrease of 5% [28]. Our analyses are limited by the lack of knee loading, knee imaging and knee pain etiology data in the Look AHEAD trial.
We examined the Look AHEAD data from baseline to Look AHEAD-Extension to determine if there were significant differences in the incidence of TKR between treatment group assignments. Our analysis revealed that although ILI reduced the risk of TKR compared to DSE in participants who did not report having knee pain at baseline, there was no difference for those reporting having knee pain at baseline. Rather, in participants who reported having knee pain at baseline and who received ILI, as compared to those reporting baseline knee pain who received DSE, the ILI group demonstrated an increased risk of TKR approaching significance. Although it did not reach significance in our analyses, we still believe this trend is important to report as it is a modifiable lifestyle behavior. Specifically, one possible explanation for this observation could be that once knee pain manifests in individuals with overweight or Class I obesity, increased weight bearing activity, such as the walking intervention prescribed to ILI participants in Look AHEAD, may contribute to, rather than lessen, the progression of OA and ultimately incidence of TKR. Although the weight loss achieved at one year was significantly greater for ILI, it may have not been enough to offset the load incurred by increasing weight bearing physical activity to 175 minutes per week, potentially resulting in increased knee pain.
The major strength of this investigation is the observation that intentional weight loss has a reduction of risk for TKR in this large study population in persons who are not experiencing knee pain (N=2,869). This investigation examined the incidence of TKR prospectively in participants willing to lose weight by reducing their caloric intake and increasing their physical activity to 175 minutes per week. Therefore, future studies designed to investigate weight loss approaches, in particular physical activity modalities in populations with overweight and obesity, are warranted. Moreover, this study suggests that physical function, pain, and quality of life are important areas to define intervention tailoring. Limitations of this analysis include reduced sample size in subgroups when modeled for baseline knee pain, obesity group, and treatment arm, as well as limited use of actual weight change during extended follow-up times. Knee imaging or other data to determine the etiology of the pain was not performed in the Look AHEAD study, therefore in this post hoc exploratory analyses we are unable to determine if knee pain was indeed a result of OA. Further investigations, with larger samples of these combined characteristics is warranted. Future analyses could also include pre-intervention fitness level, exercise history, and family history of knee OA.
In summary, in persons with overweight or obesity reporting no knee pain prior to beginning an intensive lifestyle intervention, those participating in the ILI group demonstrated significantly reduced weight and the risk of TKR compared to those receiving only diabetes support and education. However, in persons with overweight or obesity reporting they did have knee pain prior to beginning an intensive lifestyle intervention, there was no significantly reduced risk of TKR demonstrated in ILI group participants compared to those receiving only diabetes support and education.
These findings suggest that a weight loss intervention for prevention of TKR, which includes a physical activity component, initiated prior to the development of significant knee pain, may be more effective. Further investigation of non-weight bearing activities to sustain weight loss in populations at risk for OA with overweight/obesity is warranted.
Supplementary Material
Acknowledgements:
Look AHEAD Research Group at End of Continuation
Clinical Sites
The Johns Hopkins University Frederick L. Brancati, MD, MHS1,*; Jeanne M. Clark, MD, MPH1 (Co-Principal Investigators); Lee Swartz2; Jeanne Charleston, RN3; Lawrence Cheskin, MD3; Richard Rubin, PhD3 ; Jean Arceci, RN; David Bolen; Danielle Diggins; Mia Johnson; Joyce Lambert; Sarah Longenecker; Kathy Michalski, RD; Dawn Jiggetts; Chanchai Sapun; Maria Sowers; Kathy Tyler
Pennington Biomedical Research Center George A. Bray, MD1; Allison Strate, RN2; Frank L. Greenway, MD3; Donna H. Ryan, MD3; Donald Williamson, PhD3; Timothy Church, MD3 ; Catherine Champagne, PhD, RD; Valerie Myers, PhD; Jennifer Arceneaux, RN; Kristi Rau; Michelle Begnaud, LDN, RD, CDE; Barbara Cerniauskas, LDN, RD, CDE; Crystal Duncan, LPN; Helen Guay, LDN, LPC, RD; Carolyn Johnson, LPN, Lisa Jones; Kim Landry; Missy Lingle; Jennifer Perault; Cindy Puckett; Marisa Smith; Lauren Cox; Monica Lockett, LPN
The University of Alabama at Birmingham Cora E. Lewis, MD, MSPH1; Sheikilya Thomas, PhD,MPH2; Monika Safford, MD3; Stephen Glasser, MD3; Vicki DiLillo, PhD3; Gareth Dutton, PhD, Charlotte Bragg, MS, RD, LD; Amy Dobelstein; Sara Hannum; Anne Hubbell, MS; Jane King, MLT; DeLavallade Lee; Andre Morgan; L. Christie Oden; Janet Wallace, MS; Cathy Roche, PhD, RN, BSN; Jackie Roche; Janet Turman
Harvard Center
Massachusetts General Hospital. David M. Nathan, MD1; Enrico Cagliero, MD3; Heather Turgeon, RN, BS, CDE2; Barbara Steiner, EdM; Valerie Goldman, MS, RDN2; Linda Delahanty, MS, RDN3; Ellen Anderson, MS, RDN3; Laurie Bissett, MS, RDN; Christine Stevens, RN; Mary Larkin, RN; Kristen Dalton, BS, Roshni Singh, BS
Joslin Diabetes Center: Edward S. Horton, MD1; Sharon D. Jackson, MS, RD, CDE2; Osama Hamdy, MD, PhD3; A. Enrique Caballero, MD3; Sarah Bain, BS; Elizabeth McKinney, BSN, RN; Barbara Fargnoli, MS,RD; Jeanne Spellman, BS, RD; Kari Galuski, RN; Ann Goebel-Fabbri, PhD; Lori Lambert, MS, RD; Sarah Ledbury, MEd, RD; Maureen Malloy, BS; Kerry Ovalle, MS, RCEP, CDE
Beth Israel Deaconess Medical Center: George Blackburn, MD, PhD1,* Christos Mantzoros, MD, DSc3; Ann McNamara, RN
University of Colorado Anschutz Medical Campus James O. Hill, PhD1; Marsha Miller, MS RD2; Holly Wyatt, MD3 , Brent Van Dorsten, PhD3; Judith Regensteiner, PhD3; Debbie Bochert; Gina Claxton-Malloy RD Ligia Coelho, BS; Paulette Cohrs, RN, BSN; Susan Green; April Hamilton, BS, CCRC; Jere Hamilton, BA; Eugene Leshchinskiy; Loretta Rome, TRS; Terra Thompson, BA, Kirstie Craul, RD, CDE; Cecilia Wang, MD
Baylor College of Medicine John P. Foreyt, PhD1; Rebecca S. Reeves, DrPH, RD2; Molly Gee, MEd, RD2; Henry Pownall, PhD3; Ashok Balasubramanyam, MBBS3; Chu-Huang Chen, MD, PhD3; Peter Jones, MD3; Michele Burrington, RD, RN; Allyson Clark Gardner, MS, RD; Sharon Griggs; Michelle Hamilton; Veronica Holley; Sarah Lee; Sarah Lane Liscum, RN, MPH; Susan Cantu-Lumbreras; Julieta Palencia, RN; Jennifer Schmidt; Jayne Thomas, RD; Carolyn White; Charlyne Wright, RN; Monica Alvarez, PCT
The University of Tennessee Health Science Center
University of Tennessee East. Karen C. Johnson, MD, MPH1; Karen L. Wilson, BSN2; Mace Coday, PhD3; Beate Griffin, RN, BS; Donna Valenski; Polly Edwards; Brenda Fonda; Kim Ward
University of Tennessee Downtown. Helmut Steinburg, MD3; Carolyn Gresham, BSN2; Moana Mosby, RN; Debra Clark, LPN; Donna Green RN; Abbas E. Kitabchi, PhD, MD (retired)
University of Minnesota Robert W. Jeffery, PhD1; Tricia Skarphol, MA2; John P. Bantle, MD3; J. Bruce Redmon, MD3; Richard S. Crow, MD3; Scott J. Crow, MD3; Manami Bhattacharya, BS; Cindy Bjerk, MS, RD; Kerrin Brelje, MPH, RD; Carolyne Campbell; Mary Ann Forseth, BA; Melanie Jaeb, MPH, RD; Philip Lacher, BBA; Patti Laqua, BS, RD; Birgitta I. Rice, MS, RPh, CHES; Ann D. Tucker, BA; Mary Susan Voeller, BA
St. Luke’s Roosevelt Hospital Center Xavier Pi-Sunyer, MD1; Jennifer Patricio, MS2; Carmen Pal, MD3; Lynn Allen, MD; Janet Crane, MA, RD, CDN; Lolline Chong, BS, RD; Diane Hirsch, RNC, MS, CDE; Mary Anne Holowaty, MS, CN; Michelle Horowitz, MS, RD; Les James; Raashi Mamtani, MS
University of Pennsylvania Thomas A. Wadden, PhD1; Barbara J. Maschak-Carey, MSN, CDE2 ; Robert I. Berkowitz, MD3; Gary Foster, PhD3; Henry Glick, PhD3; Shiriki Kumanyika, PhD RD, MPH3; Yuliis Bell, BA ; Raymond Carvajal, PsyD; Helen Chomentowski; Renee Davenport; Lucy Faulconbridge, PhD; Louise Hesson, MSN, CRNP; Sharon Leonard, RD; Monica Mullen, RD, MPH
University of Pittsburgh John M. Jakicic, PhD1; David E. Kelley, MD1; Jacqueline Wesche-Thobaben, RN, BSN, CDE2; Daniel Edmundowicz, MD3; Lin Ewing, PhD, RN3; Andrea Hergenroeder, PhD, PT, CCS3; Mary L. Klem, PhD, MLIS3; Mary Korytkowski, MD3; Andrea Kriska, PhD3; Lewis H. Kuller, MD, DrPH3; Amy D. Rickman, PhD, RD, LDN3; Rose Salata, MD3; Monica E. Yamamoto, DrPH, RD, FADA3; Janet Bonk, RN, MPH; Susan Copelli, BS, CTR; Rebecca Danchenko, BS; Tammy DeBruce, BA; Barbara Elnyczky; David O. Garcia, PhD; George A. Grove, MS; Patricia H. Harper, MS, RD, LDN; Susan Harrier, BS; Diane Heidingsfelder, MS, RD, CDE, LDN; Nicole L. Helbling, MS, RN; Diane Ives, MPH; Janet Krulia, RN, BSN, CDE; Juliet Mancino, MS, RD, CDE, LDN; Anne Mathews, PhD, RD, LDN; Lisa Martich, BS, RD, LDN; Meghan McGuire, MS; Tracey Y. Murray, BS; Anna Peluso, MS; Karen Quinn; Jennifer Rush, MPH; Joan R. Ritchea; Linda Semler, MS, RD, LDN; Karen Vujevich, RN-BC, MSN, CRNP; Kathy Williams, RN, MHA; Donna L. Wolf, PhD
The Miriam Hospital/Brown Medical School Rena R. Wing, PhD1; Renee Bright, MS2; Vincent Pera, MD3; Deborah Tate, PhD3; Amy Gorin, PhD3; Kara Gallagher, PhD3; Amy Bach, PhD; Barbara Bancroft, RN, MS; Anna Bertorelli, MBA, RD; Richard Carey, BS; Tatum Charron, BS; Heather Chenot, MS; Kimberley Chula-Maguire, MS; Pamela Coward, MS, RD; Lisa Cronkite, BS; Julie Currin, MD; Maureen Daly, RN; Caitlin Egan, MS; Erica Ferguson, BS, RD; Linda Foss, MPH; Jennifer Gauvin, BS; Don Kieffer, PhD; Lauren Lessard, BS; Deborah Maier, MS; JP Massaro, BS; Tammy Monk, MS; Rob Nicholson, PhD; Erin Patterson, BS; Suzanne Phelan, PhD; Hollie Raynor, PhD, RD; Douglas Raynor, PhD; Natalie Robinson, MS, RD; Deborah Robles; Jane Tavares, BS
The University of Texas Health Science Center at San Antonio Helen P. Hazuda, PhD1; Maria G. Montez, RN, MSHP, CDE2; Carlos Lorenzo, MD3; Charles F. Coleman, MS, RD; Domingo Granado, RN; Kathy Hathaway, MS, RD; Juan Carlos Isaac, RC, BSN; Nora Ramirez, RN, BSN
VA Puget Sound Health Care System / University of Washington Steven E. Kahn, MB, ChB1; Anne Kure, BS2; Edward J. Boyko, MD, MPH3; Edward Lipkin, MD, PhD3; Dace Trence, MD3; Subbulaxmi Trikudanathan, MD, MRCP, MMSc3; Elaine Tsai, MD3; Brenda Montgomery, RN, MS, CDE; Ivy Morgan-Taggart; Jolanta Socha, BS; Lonnese Taylor, RN, BS; Alan Wesley, BA
Southwestern American Indian Center, Phoenix, Arizona and Shiprock, New Mexico William C. Knowler, MD, DrPH1; Paula Bolin, RN, MC2; Tina Killean, BS2; Maria Cassidy-Begay, BSND, RND 2; Katie Toledo, MS, LPC2; Cathy Manus, LPN3; Jonathan Krakoff, MD3; Jeffrey M. Curtis, MD, MPH3; Sara Michaels, MD3; Paul Bloomquist, MD3; Peter H. Bennett, MB, FRCP3; Bernadita Fallis, RN, RHIT, CCS; Diane F. Hollowbreast; Ruby Johnson; Maria Meacham, BSN, RN, CDE; Christina Morris, BA; Julie Nelson, RD; Carol Percy, RN, MS; Patricia Poorthunder; Sandra Sangster; Leigh A. Shovestull, RD, CDE; Miranda Smart; Janelia Smiley; Teddy Thomas, BS
University of Southern California Anne Peters, MD1; Siran Ghazarian, MD2; Elizabeth Beale, MD3; Kati Konersman, RD, CDE; Brenda Quintero-Varela; Edgar Ramirez; Gabriela Rios, RD; Gabriela Rodriguez, MA; Valerie Ruelas MSW, LCSW; Sara Serafin-Dokhan; Martha Walker, RD
Coordinating Center
Wake Forest University Mark A. Espeland, PhD1; Judy L. Bahnson, BA, CCRP3; Lynne E. Wagenknecht, DrPH1; David Reboussin, PhD3; W. Jack Rejeski, PhD3; Alain G. Bertoni, MD, MPH3; Wei Lang, PhD3; David Lefkowitz, MD3,* Patrick S. Reynolds, MD3; Denise Houston, PhD3; Mike E. Miller, PhD3; Laura D. Baker, PhD3; Nicholas Pajewski, PhD3; Stephen R. Rapp, PhD3; Stephen Kritchevsky, PhD3; Haiying Chen, PhD, MM3; Valerie Wilson, MD3; Delia S. West, PhD3; Ron Prineas, MD3; Tandaw Samdarshi, MD3; Amelia Hodges, BS, CCRP2; Karen Wall2; Carrie C. Williams, MA, CCRP2; Andrea Anderson, MS; Jerry M. Barnes, MA; Tara D. Beckner; Delilah R. Cook; Valery S. Effoe, MD, MS; Melanie Franks, BBA; Katie Garcia, MS; Sarah A. Gaussoin, MS; Candace Goode; Michelle Gordon, MS; Lea Harvin, BS; Mary A. Hontz, BA; Don G. Hire, BS; Patricia Hogan, MS; Mark King, BS; Kathy Lane, BS; Rebecca H. Neiberg, MS; Julia T. Rushing, MS; Debbie Steinberg, BS; Jennifer Walker, MS; Michael P. Walkup, MS
Central Resources Centers
Central Laboratory, Northwest Lipid Metabolism and Diabetes Research Laboratories Santica M. Marcovina, PhD, ScD1; Jessica Hurting2; John J. Albers, PhD3, Vinod Gaur, PhD4
ECG Reading Center, EPICARE, Wake Forest University School of Medicine
Elsayed Z. Soliman MD, MSc, MS1; Charles Campbell 2; Zhu-Ming Zhang, MD3; Mary Barr; Susan
Hensley; Julie Hu; Lisa Keasler; Yabing Li, MD
Hall-Foushee Communications, Inc.
Richard Foushee, PhD; Nancy J. Hall, MA
Federal Sponsors
National Institute of Diabetes and Digestive and Kidney Diseases Mary Evans, PhD; Van S. Hubbard, MD, PhD; Susan Z. Yanovski, MD
National Heart, Lung, and Blood Institute Lawton S. Cooper, MD, MPH; Peter Kaufman, PhD, FABMR; Mario Stylianou, PhD
Centers for Disease Control and Prevention Edward W. Gregg, PhD; Ping Zhang, PhD
Funding and Support
Funded by the National Institutes of Health through cooperative agreements with the National Institute of Diabetes and Digestive and Kidney Diseases: DK57136, DK57149, DK56990, DK57177, DK57171, DK57151, DK57182, DK57131, DK57002, DK57078, DK57154, DK57178, DK57219, DK57008, DK57135, and DK56992. Additional funding was provided by the National Heart, Lung, and Blood Institute; National Institute of Nursing Research; National Center on Minority Health and Health Disparities; NIH Office of Research on Women’s Health; and the Centers for Disease Control and Prevention. This research was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. The Indian Health Service (I.H.S.) provided personnel, medical oversight, and use of facilities. The opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the I.H.S. or other funding sources.
Additional support was received from The Johns Hopkins Medical Institutions Bayview General Clinical Research Center (M01RR02719); the Massachusetts General Hospital Mallinckrodt General Clinical Research Center and the Massachusetts Institute of Technology General Clinical Research Center (M01RR01066); the Harvard Clinical and Translational Science Center (RR025758-04); the University of Colorado Health Sciences Center General Clinical Research Center (M01RR00051) and Clinical Nutrition Research Unit (P30 DK48520); the University of Tennessee at Memphis General Clinical Research Center (M01RR0021140); the University of Pittsburgh General Clinical Research Center (GCRC) (M01RR000056), the Clinical Translational Research Center (CTRC) funded by the Clinical & Translational Science Award (UL1 RR 024153) and NIH grant (DK 046204); the VA Puget Sound Health Care System Medical Research Service, Department of Veterans Affairs; and the Frederic C. Bartter General Clinical Research Center (M01RR01346).
The following organizations have committed to make major contributions to Look AHEAD: FedEx Corporation; Health Management Resources; LifeScan, Inc., a Johnson & Johnson Company; OPTIFAST® of Nestle HealthCare Nutrition, Inc.; Hoffmann-La Roche Inc.; Abbott Nutrition; and Slim-Fast Brand of Unilever North America.
Some of the information contained herein was derived from data provided by the Bureau of Vital Statistics, New York City Department of Health and Mental Hygiene.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
deceased
Principal Investigator
Program Coordinator
Co-Investigator
All other Look AHEAD staffs are listed alphabetically by site.
References
- 1.Gelber RPGJ, Orav EJ, Manson JE, Buring JE, Kurth T. Body mass index in young men and the risk of subsequent knee and hip osteoarthritis. Am J Med. 1999;107(6):7. [DOI] [PubMed] [Google Scholar]
- 2.Bozic KJDS, Berry DJ, Naessens JM, Rappaport K, Cisternas M, Saleh KJ, Rubash HE. Differences in patient and procedure characteristics and hospital resource use in primary and revision total joint arthroplasty: a multi center study. . J Arthroplasty. 2005;20:S17–S25. [DOI] [PubMed] [Google Scholar]
- 3.Coyte PCHG, Croxford R, Wright JG. Rates of revision knee replacement in Ontario, Canada. . J Bone Joint Surg Am. 1999;81(6):6. [DOI] [PubMed] [Google Scholar]
- 4.Kurtz SOK, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the US from 2005 to 2030. J Bone Joint Surg 2007;89A:780–5. [DOI] [PubMed] [Google Scholar]
- 5.Santaguida PLHG, Hudak PL, Glazier R, Mahomed NN, Kreder HJ, Coyte PC, Wright JG. Patient characteristics affecting the prognosis of total hip and knee joint arthroplasty: a systematic review. Can J Surg.51(6):9. [PMC free article] [PubMed] [Google Scholar]
- 6.Cutler DMGK. The potential for cost savings through bundled episode payments. The New England journal of medicine. 2012;366(12):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Risk Adjustment for Medicare T otal Knee Arthroplasty Bundled Payments. Orthopedics. 2016;39(5):5. [DOI] [PubMed] [Google Scholar]
- 8.Zeni JAS-ML Jr. Most patients gain weight in the 2 years after total knee arthroplasty: comparison to a healthy control group. Osteoarthritis Cartilage. 2010;18(4):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vasarhelyi EMMS. The influence of obesity on total joint arthroplasty. J Bone Joint Surg Br. 2013;94(11A):3. [DOI] [PubMed] [Google Scholar]
- 10.Naziri QIK, Malkani AL, Bonutti PM, Harwin SF, Mont MA. Bariatric orthopaedics: total knee arthroplasty in super-obese patients (GMI & gt; 50kg/m2). Survivorship and complications. Clin Orthop Relat Res. 2013;471(11):3532–0. Epub November 2013. doi: 10.1007/s11999-013-3154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mihalko WMBP, Kelly FB, Canale ST. Obesity, orthopaedics, and outcomes. J Am Acad Orthop Surg. 2014;22(11):8. [DOI] [PubMed] [Google Scholar]
- 12.McElroy MJPR, Issa K, Harwin SF, Mont MA. The effects of obesity and morbid obesity on outcomes in TKA. J Knee Surg. 2013;26(2):83–8. Epub April 2013. doi: 10.1055/s-0033-1341407 [DOI] [PubMed] [Google Scholar]
- 13.Liljensoe ALJ, Soballe K, Mechlenburg I. Overweight preoperatively impairs clinical outcome after knee arthroplasty: a cohort study of 197 patients 3-5 years after surgery. Acta Orthop 2013;84(4):392–7. Epub August 2013. doi: 10.3109/17453674.2013.799419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noble PCSG, Brekke AC, Sikorskii A, Benjamin JB, Lonner JH, Chadha P, Daylamani DA, Scott WN, Bourne RB. Development of a new Knee Society scoring system. Clin Orthop Relat Res. 2012;470(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foy CGLC, Hairston KG, Miller GD, Lang W, Jakicic JM, Rejeski WJ, Ribisl PM, Walkup MP, Wagenknecht LE. Intensive lifestyle intervention improves physical function among obese adults with knee pain: Findings from the look AHEAD trial. Obesity. 2011;19(1):83–93. Epub June 17th, 2010. doi: 10.1038/oby.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Look Ahead Research Group, Pi-Sunyer X, Blackburn G, Brancati FL, Bray GA, Bright R, Clark JM, Curtis JM, Espeland MA, Foreyt JP, Graves K, Haffner SM, Harrison B, Hill JO, Horton ES, Jakicic J, Jeffery RW, Johnson KC, Kahn S, Kelley DE, Kitabchi AE, Knowler WC, Lewis CE, Maschak-Carey BJ, Montgomery B, Nathan DM, Patricio J, Peters A, Redmon JB, Reeves RS, Ryan DH, Safford M, Van Dorsten B, Wadden TA, Wagenknecht L, Wesche-Thobaben J, Wing RR, Yanovski SZ. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30(6):1374–83. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Look Ahead Research Group, Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, Crow RS, Curtis JM, Egan CM, Espeland MA, Evans M, Foreyt JP, Ghazarian S, Gregg EW, Harrison B, Hazuda HP, Hill JO, Horton ES, Hubbard VS, Jakicic JM, Jeffery RW, Johnson KC, Kahn SE, Kitabchi AE, Knowler WC, Lewis CE, Maschak-Carey BJ, Montez MG, Murillo A, Nathan DM, Patricio J, Peters A, Pi-Sunyer X, Pownall H, Reboussin D, Regensteiner JG, Rickman AD, Ryan DH, Safford M, Wadden TA, Wagenknecht LE, West DS, Williamson DF, Yanovski SZ. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. The New England journal of medicine. 2013;369(2):145–54. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White DK, Neogi T, Rejeski WJ, Walkup MP, Lewis CE, Nevitt MC, Foy CG, Felson DT, Look ARG. Can an intensive diet and exercise program prevent knee pain among overweight adults at high risk? Arthritis Care Res (Hoboken). 2015;67(7):965–71. doi: 10.1002/acr.22544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 7 ed Philadelphia, PA: Lippincott, Williams & Wilkins; 2006. [Google Scholar]
- 20.Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, Bierma-Zeinstra S, Brandt KD, Croft P, Doherty M, Dougados M, Hochberg M, Hunter DJ, Kwoh K, Lohmander LS, Tugwell P. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16(2):137–62. Epub 2008/02/19. doi: 10.1016/j.joca.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 21.Look Ahead Research Group, Wadden TA, West DS, Delahanty L, Jakicic J, Rejeski J, Williamson D, Berkowitz RI, Kelley DE, Tomchee C, Hill JO, Kumanyika S. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring). 2006;14(5):737–52. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryan DH, Espeland MA, Foster GD, Haffner SM, Hubbard VS, Johnson KC, Kahn SE, Knowler WC, Yanovski SZ, Look ARG. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Controlled clinical trials. 2003;24(5):610–28. [DOI] [PubMed] [Google Scholar]
- 23.Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis care & research. 2011;63 Suppl 11:S240–52. doi: 10.1002/acr.20543. [DOI] [PubMed] [Google Scholar]
- 24.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. The Journal of rheumatology. 1988;15(12):1833–40. [PubMed] [Google Scholar]
- 25.Rubin RR, Wadden TA, Bahnson JL, Blackburn GL, Brancati FL, Bray GA, Coday M, Crow SJ, Curtis JM, Dutton G, Egan C, Evans M, Ewing L, Faulconbridge L, Foreyt J, Gaussoin SA, Gregg EW, Hazuda HP, Hill JO, Horton ES, Hubbard VS, Jakicic JM, Jeffery RW, Johnson KC, Kahn SE, Knowler WC, Lang W, Lewis CE, Montez MG, Murillo A, Nathan DM, Patricio J, Peters A, Pi-Sunyer X, Pownall H, Rejeski WJ, Rosenthal RH, Ruelas V, Toledo K, Van Dorsten B, Vitolins M, Williamson D, Wing RR, Yanovski SZ, Zhang P, Look ARG. Impact of intensive lifestyle intervention on depression and health-related quality of life in type 2 diabetes: the Look AHEAD Trial. Diabetes Care. 2014;37(6):1544–53. Epub 2014/05/24. doi: 10.2337/dc13-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson KTF, Richey P, Tran Q, Tylavsky F, Miro D, Coday M. The primary results of the Treating Adult smokers at Risk for weight Gain with Interactive Technology (TARGIT) study. Obesity. 2017;25(10):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colberg SR, Albright AL, Blissmer BJ, Braun B, Chasan-Taber L, Fernhall B, Regensteiner JG, Rubin RR, Sigal RJ, American College of Sports M, American Diabetes A. Exercise and type 2 diabetes: American College of Sports Medicine and the American Diabetes Association: joint position statement. Exercise and type 2 diabetes. Med Sci Sports Exerc. 2010;42(12):2282–303. Epub 2010/11/19. doi: 10.1249/MSS.0b013e3181eeb61c. [DOI] [PubMed] [Google Scholar]
- 28.Messier SPPM, Beavers DP, Legault C, Loeser RF, Hunter DJ, DeVita P. Influences of alignment and obesity on knee joint loading in osteoarthritic gait. Osteoarthritis Cartilage. 2014;22(7):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henriksen MCR, Hunter DJ, Gudbergsen H, Boesen M, Lohmander LS, Bliddal H. Structural changes in the knee during weight loss maintenance after a significant weight loss in obese patients with osteoarthritis: a report of secondary outcome analyses from a randomized controlled trial. Osteoarthritis Cartilage. 2014;22(5):8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


