Abstract
Stem cell behavior is tightly regulated by spatiotemporal signaling from the niche, which is a four-dimensional microenvironment that can instruct stem cells to remain quiescent, self-renew, proliferate or differentiate. In this review, we discuss recent advances in understanding the signaling cues provided by the stem cell niche in two contrasting adult tissues, the rapidly cycling intestinal epithelium and the slowly renewing skeletal muscle. Drawing comparisons between these two systems, we discuss the effects of niche-derived growth factors and signaling molecules, metabolic cues, the extracellular matrix and biomechanical cues, and immune signals on stem cells. We also discuss the influence of the niche in defining stem cell identity and function in both normal and pathophysiologic states.
Introduction
Tissue homeostasis is maintained throughout an organism’s lifespan by adult stem cells (SCs) whose activity is tightly regulated, depending on the function and proliferative requirements of the tissue. Epithelia, such as the intestinal lining or the epidermis, are subject to frequent damage because they act as a barrier between the organism and its environment. Therefore, they generally require rapid turnover to replace lost or damaged cells [1]. In contrast, low turnover tissues, like skeletal muscle or the brain, tend to maintain SCs in a quiescent state until regeneration is stimulated [2,3]. The proliferative potential of different adult SCs is not solely defined by their intrinsic properties, but also relies on the SC niche, a four-dimensional microenvironment where the SCs reside and respond to spatially and temporally coordinated biochemical and biophysical signals provided in an autocrine, juxtracrine, paracrine, or systemic manner.
Decades of studies have provided insight into the highly dynamic molecular communications between SCs and their niches. Here, we review recent advances in our understanding of the niche signals that regulate quiescence, self-renewal and differentiation of SCs, focusing as examples on the niche of intestinal SCs (ISCs) as a model for fast-turnover tissue SCs and muscle SCs (MuSCs), also called satellite cells, as a model for slow-turnover tissue SCs (Figure 1).
Figure 1. Fast- and slow- turnover tissue SC niches.
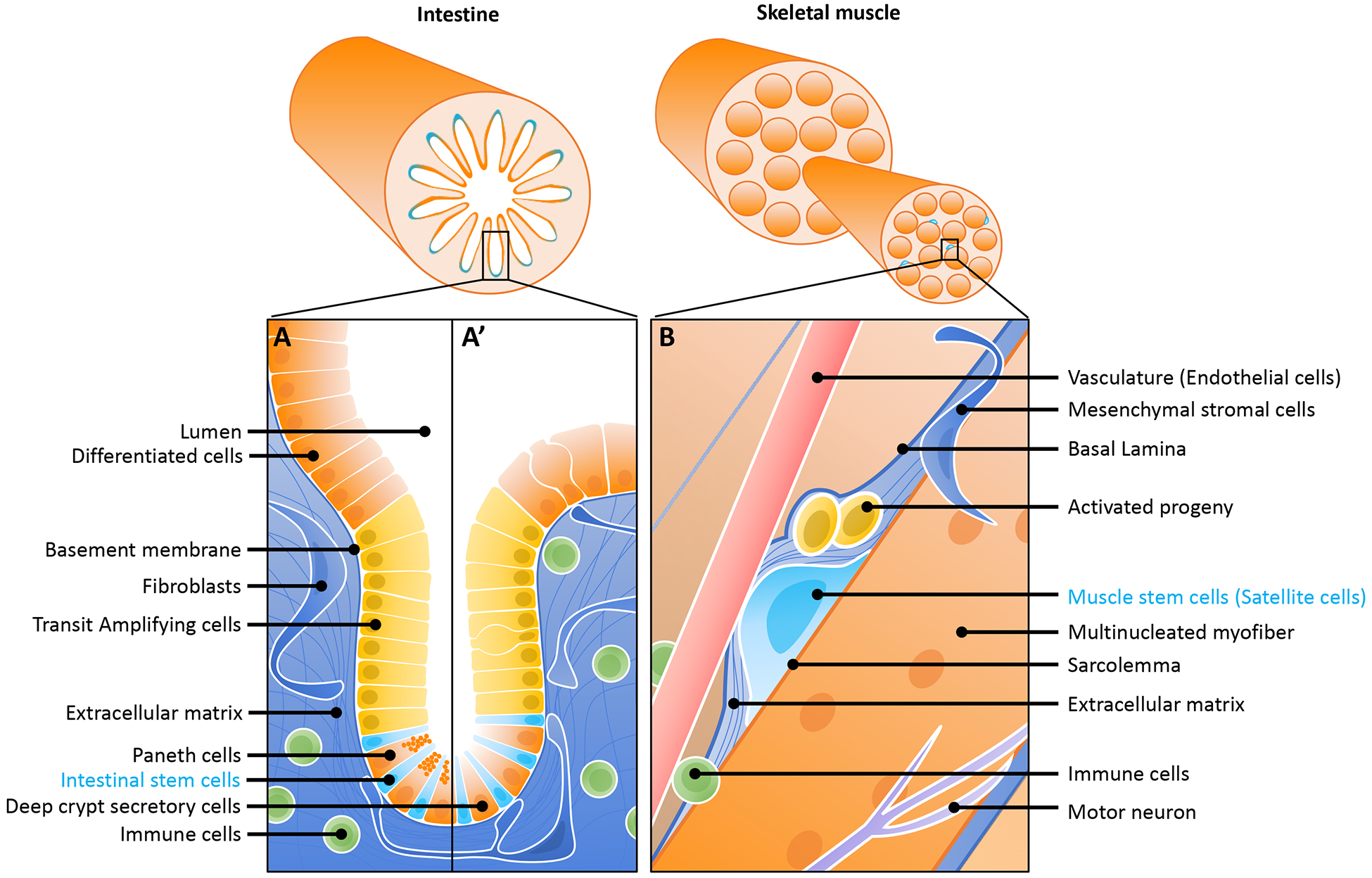
(A) The small intestinal SC niche. The single-layered intestinal epithelium is folded upon itself, creating invaginations into the underlying mesenchyme, called crypts of Lieberkuhn. Three to sixteen (depending on the study) ISCs [4], which express the R-spondin receptor LGR5 [5], reside at the bottom these crypts. Each ISC divides daily to give rise to proliferating transit amplifying cells that further divide and differentiate as they migrate up the crypt and into the overlying villus compartment in the small intestine (A) or into the intercrypt epithelium in the colon (A’). Sandwiched between the ISCs at the bottom of the crypt, terminally differentiated Paneth secretory cells produce several of the key growth factors required for the maintenance and proliferation of ISC. In the small intestine, these are the Paneth cells (A) whereas in the colon, these are deep crypt secretory cells (A’). Niche signals also come from the underlying mesenchyme, most notably from stromal fibroblasts. The stiffness of the basement membrane and underlying extracellular matrix act as key signals for ISC maintenance, and immune cells and cytokines also contribute to the ISC niche.
(B) The skeletal muscle SC niche. MuSCs are enclosed in a membrane compartment between the basal lamina (a thin sheet-like layer of proteoglycans, collagen, laminin) and the myofiber plasma membrane. In this microenvironment, MuSCs are surrounded by extracellular matrix, where they respond to a diversity of biochemical and biophysical signals that regulate SC function and tissue homeostasis [55,138]. These signals come from the circulation as well as from the MuSCs themselves, endothelial cells, myofibers, fibroblasts and pericytes, fibroadipogenic progenitors, immune cells, and also from adjacent motor neurons through neuromuscular junctions [139]. Upon activation, MuSCs divide symmetrically for self-renewal and expansion or asymmetrically for differentiation. Self-renewal also can occur by asymmetric division, producing one quiescent daughter and one myoblast daughter. Activated satellite cells proliferate as myoblasts, eventually differentiating through a process that involves expression of the myogenic transcription factors MYF5 and MYOD, followed by expression of the differentiation factor myogenin (MYOG), and later yet, loss of expression of PAX7, setting up a myogenic program in committed myoblasts to migrate and fuse with multinucleated existing or damaged myofibers [139].
Homeostatic Signals in the SC Niche
The single-layered intestinal epithelium is continuously renewed by a pool of actively dividing ISCs located at the bottom of epithelial cavities called crypts of Lieberkuhn. Each ISC divides daily to give rise to transit amplifying (TA) progenitors that further divide and give rise to differentiated lineages (absorptive or secretory) as they migrate up the crypt and into the villus compartment, in the small intestine, or intercrypt epithelium, in the colon (Figure 1a) [4]. Both this compartmentalization and the establishment of unique markers for ISCs, most notably the R-spondin receptor LGR5 [5], make this an ideal model to study fast-cycling stem cells. Alongside the ISCs at the bottom of small intestinal crypts are Paneth cells, secretory progenitors that produce not only antimicrobial peptides that protect the crypt environment but also key ISC niche signals. In the colon, ISCs are intercalated between secretory cells called deep crypt secretory (DCS) cells that play a similar niche function [6]. Beneath this epithelial layer is a basement membrane and then the lamina propria populated with stromal fibroblasts, immune cells, vasculature, nerve cells, and smooth muscle (Figure 1a).
Similar cellular components exist in the muscle niche, as myofibers, the structural and functional elements of the skeletal muscle, are surrounded by complex vascular networks, motor neurons and connective tissue with various stromal populations. The quiescent MuSCs reside in a distinct enclosed membrane compartment on the periphery of the myofiber [3] (Figure 1b). Upon muscle injury or myofiber degeneration, healthy MuSCs activate the myogenic program and migrate towards the center of the myofiber to complete differentiation by fusing within the myofiber, thereby repairing and maintaining the myofiber contractile unit. While head muscles are derived from cranial mesoderm, all body muscles arise from somites from the paraxial mesoderm and, in the adult, all MuSCs express the canonical transcription factor paired box 7 (PAX7) [3]. In this review, we focus primarily on the most recent studies on MuSCs from adult skeletal muscle.
Drawing comparisons between these two systems, we will discuss the effects of niche-derived growth factors, metabolic cues, biomechanical cues, and immune signals on SC function.
Growth factors and secreted signals in the ISC and MuSC niche
The best-established pathways in the ISC niche are the Wnt/β-catenin and Notch signaling cascades [7,8] (Figure 2). Canonical Wnt signals, notably WNT3 from Paneth cells and WNT2b from the mesenchyme, create a gradient starting at the crypt bottom. In canonical Wnt signaling, these ligands bind Frizzled receptors and activate downstream signaling that results in β-catenin accumulation and translocation to the nucleus, where it can interact with transcription factors, in this case TCF/LEF [9], driving a pro-proliferative transcriptional program as well as expression of the ISC marker and R-spondin receptor LGR5 in ISCs. Wnt signals were also recently shown to play an essential role in the MuSC niche (Figure 3), where WNT4 in particular is essential to keep MuSCs quiescent [10], as deletion of WNT4 from myofibers induced activation and proliferation of MuSCs. In this context, WNT4 signaling acts non-canonically through Rho GTPase and cytoskeletal remodeling [10]. In addition, Wnt signaling through β-catenin plays a role during myoblast differentiation [11–13], although this is once again independent of TCF/LEF transcription factors [9] and instead involves cooperation with myoblast determination protein 1 (MYOD) and α-catenin-dependent membrane functions of β-catenin [11].
Figure 2. Signaling pathways in the intestinal SC niche.
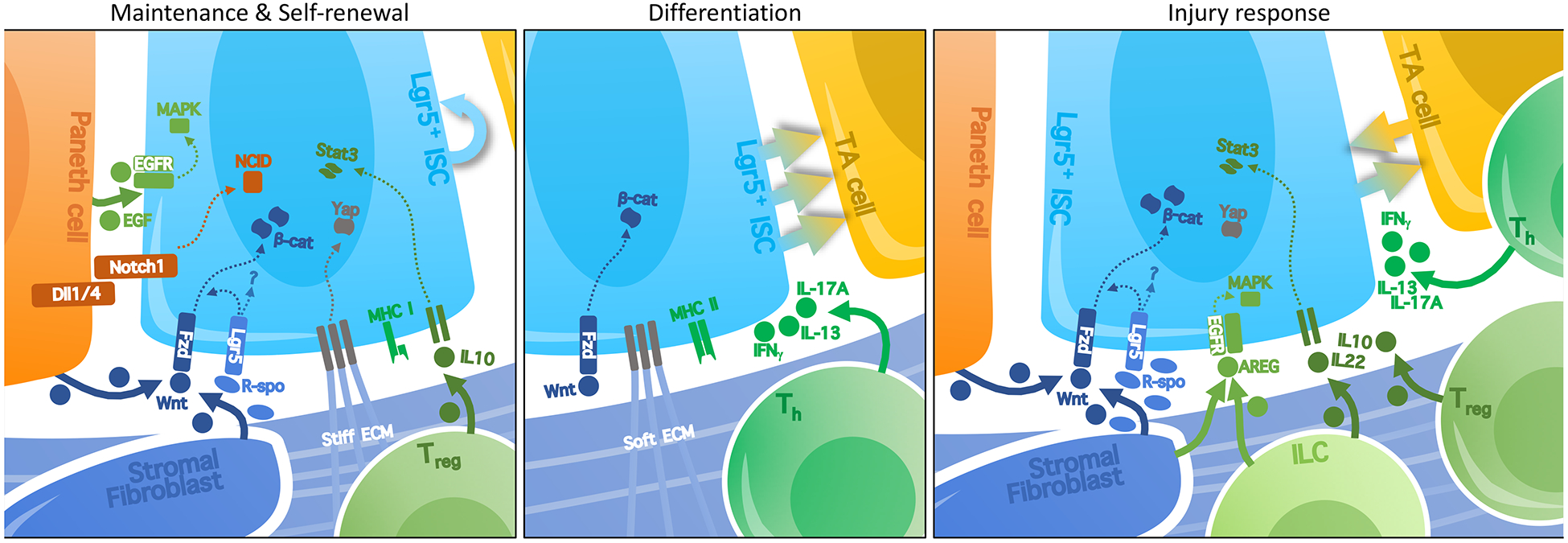
Localized signaling pathways in the ISC niche regulate their maintenance, self-renewal, and engagement towards differentiation. ISCs express NOTCH1, which directly interacts with its ligands, notably DLL1 and DLL4, on neighboring Paneth cells [18]. The resulting activation of Notch signaling in ISCs is important for the maintenance and proliferation of ISCs. Paneth cells also produce EGF ligands that stimulate pro-proliferative MAPK signaling in ISC. Short-range Wnt signals from Paneth cells and stromal fibroblasts are crucial in this niche, driving proliferation throughout the crypt as well as maintenance of ISC through canonical Wnt/β-catenin signaling. Wnt/β-catenin signaling drives expression of the ISC marker Lgr5, the protein product of which receives R-spondin ligands secreted by stromal fibroblasts. R-spondin stimulates Wnt/β-catenin signaling, but also independently primes the ISC state. Recent studies have demonstrated an effect of extracellular matrix (ECM) stiffness in regulating ISC self-renewal, notably through YAP signaling, whereas softer matrices stimulate differentiation. Signals from immune cells can also affect the balance between self-renewal and differentiation in the crypt, with anti-inflammatory IL-10 promoting self-renewal, while pro-inflammatory IL-17, IL-13, or IFNγ promote proliferation and differentiation. Additionally, ISCs are subject to immune clearance, as they express high levels of MHC class I (unlike quiescent MuSCs). Conversely, ISCs can modulate immunity by acting as a non-conventional antigen-presenting cell that expresses high levels of MHC class II compared to the rest of the epithelium. In the context of injury response, some of these signals are upregulated: stromal cells upregulate expression of R-spondins and express EGF family ligands like AREG. Immune signaling in the niche is also increased during injury response.
Figure 3. Signaling Pathways in the Muscle SC niche.
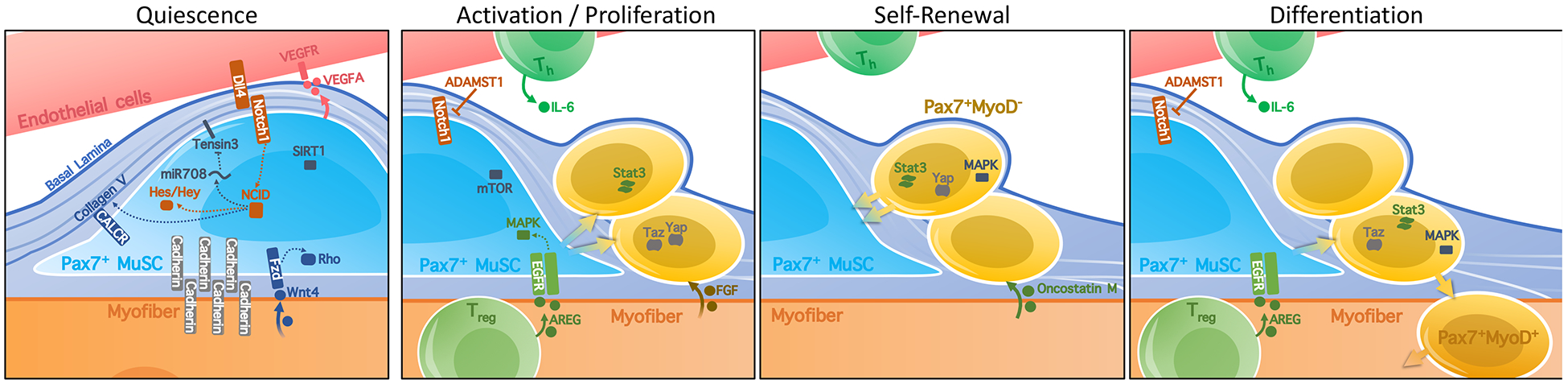
Signaling in the MuSC niche regulates the maintenance of quiescence or activation towards self-renewal or differentiation of the MuSC. MuSCs produce VEGFA that promotes direct contact with DLL4-expressing endothelial cells. This stimulates Notch signaling, involving the mirtron miR708, which antagonizes cell migration by targeting the focal adhesion protein TENSIN3, as well as transcriptional repressors from the Hes/Hey family. Notch activation has thus been associated with maintenance of quiescence of MuSCs. Inhibition of Notch signaling, notably by the secretion of the metalloprotease ADAMST1 during inflammation, can promote exit from quiescence and differentiation of MuSC. Interactions of MuSCs with the surrounding extracellular matrix regulate maintenance of quiescence or activation towards self-renewal or differentiation of the MuSCs within the niche [139–141]. Additionally, the integrity of cell adhesion molecules such as Cadherins, and interactions with Collagen V through the Calcitonin receptor CALCR are believed to maintain the quiescent MuSC pool. Recent work has additionally shown a role for myofiber-derived WNT4 in MuSC quiescence through Rho GTPase and the cytoskeletal remodeling. On the other hand, FGF signals from myofibers, satellite cells and fibroblasts [21] are known to promote activation of MuSC, while MAPK signaling has been implicated in both self-renewal and differentiation. IL-6 promotes proliferation and, along with its derivative Oncostatin M, can signal through the JAK-STAT pathway to promote the re-establishment of MuSC quiescence following injury. The transcriptional regulators YAP and TAZ stimulate myoblast proliferation differentially; while TAZ enhances myogenic differentiation, YAP promotes self-renewal. Lastly, regulatory T cells in the muscle stimulate myoblast differentiation from MuSCs during regeneration by secreting the EGF-like growth factor amphiregulin (AREG).
Direct contact between cells expressing Notch ligands and their neighbors expressing a NOTCH receptor results in cleavage of the NOTCH intracellular domain (NICD), which can directly act as a transcription factor in the nucleus [7,14]. Notch signaling is crucial for the maintenance of quiescence in MuSCs, which express the NOTCH1 receptor. Notch signals in MuSC were recently shown to occur through transcriptional regulation of the mirtron miR-708, which antagonizes cell migration by targeting the transcripts of the focal-adhesion-associated protein Tensin3 [15,16] (Figure 3). In the intestine, ISCs also express NOTCH1, which upon ligand binding leads to repression of secretory lineage commitment by inhibiting expression of the transcription factor ATOH1 [17,18] and is also crucial for ISC maintenance and proliferation in the crypt [17,18], although the downstream mechanisms of the latter function remains unclear (Figure 2). Thus, Notch signaling is important for the maintenance of the SC pool in both of these fast- and slow- turnover tissues.
In addition to Wnt and Notch ligands, other signals also play important roles in these two systems. Ex-vivo intestinal organoid systems, where ISCs or whole crypts are cultured without mesenchyme, allow the exploration of the minimal niche signals required for proper ISC function [19]. The requirement for exogenous EGF (epidermal growth factor), BMP (bone morphogenic protein) inhibitors and R-spondins for ISC maintenance in this system suggests a crucial role of these signals in the ISC niche. Likewise, using activated MuSCs co-cultured with myoblast progenitors, growth factors such as heparin binding EGF like growth factor and vascular endothelial growth factor A (VEGFA) were identified as key players in the process of muscle organoid formation [20]. Furthermore, in skeletal muscle, FGF (fibroblast growth factor) and MAPK (mitogen-activated protein kinase) signaling also regulate MuSC proliferation and myogenic commitment: FGF signaling from myofibers, satellite cells and fibroblasts promotes proliferation and self-renewal [21], whereas p38 MAPK has been implicated in both self-renewal and differentiation [21–24].
In addition to understanding the downstream effects of these pathways, a major focus has been identifying the signal sources within the SC niche. Notch signaling involves direct membrane contact between neighboring cells, and secreted Wnt signals act within a restricted range in the ISC niche [25]. In the MuSC niche, the source of Notch ligands was unclear until 3D imaging in mice recently showed that up to 80% of MuSCs were in direct contact with the capillaries [26], and blocking either global or MuSC-derived vascular endothelial growth factor A (VEGFA) downregulated Notch signaling in MuSCs. Indeed, endothelial cells express the Notch ligand DLL4 that is required for self-renewal and maintenance of the PAX7+MYOD− quiescent MuSC in culture [26].
Due to their localization alongside ISCs at the crypt bottom in the small intestine, Paneth cells were long thought to constitute the ISC cellular niche, and these cells produce not only Wnt signals, but also Notch ligands DLL1 and DLL4 and EGF-family ligands [27,28]. In the colon, DCS cells have a similar transcriptomic signature and also provide Notch ligands and EGF ligands to ISC, although they notably do not produce any Wnts [6,29] Recently, stromal fibroblast populations identified by markers including PDGFRα, CD34, FOXL1, and GLI1 were found to serve as additional, mesenchymal sources of Wnt signals for ISC maintenance [30–33]. These markers label heterogeneous, partially overlapping stromal populations; all markers are present on telocytes, cells with long processes that can directly interact with ISCs, consistent with the idea of short-range Wnt signaling. In addition to Wnt, R-spondins are also produced by stromal fibroblasts labelled by either PDGFRα or GLI1 [32,34] (Figure 2). Although R-spondins were initially described to amplify Wnt signals by regulating receptor turnover at the membrane [35,36], an additional role of R-spondins in ISC self-renewal, distinct from the proliferative effects of Wnt signaling, was recently brought to light: blocking R-spondin signaling either systemically or specifically from GLI1+ stromal cells disrupts ISC numbers, even in the presence of a strong Wnt signal [34,37,38]. R-spondin signals also play a role in the regeneration of both fast- and slow-turnover tissues, including the gastric epithelium and liver [39–41], where some SC populations also express Lgr5, although whether the effects of R-spondin act strictly by amplifying Wnt signaling in these tissues remains to be explored. R-spondins also play a role in myogenic cell differentiation during regeneration in the muscle, acting both on canonical Wnt/β-catenin activation and non-canonical Wnt signaling through cytoskeletal remodeling networks in myocytes, but not in MuSCs directly [42]. The presence of multiple, seemingly redundant cellular niche components (e.g., both Paneth cells and stromal fibroblasts producing Wnt) creates a robust SC niche where tissue renewal can be ensured even after disruption of individual niche components, and also allows for fine-tuning of SC behavior through diverse but cooperative signaling pathways (e.g., Wnt and R-spondin signals).
Metabolic interplay in the ISC and MuSC niche
It has long been thought that stem cells, in order to prevent the accumulation of mutations, should favor an anaerobic glycolytic metabolism that maintains low levels of reactive oxygen species (ROS). This has been described in hematopoietic stem cells, where oxidative metabolism, specifically fatty acid oxidation (FAO), is induced only upon activation [43,44]. However, this scheme does not seem to be as widespread in adult SCs as previously thought.
Upon muscle demands to regenerate, healthy MuSCs can be primed for regeneration by shifting from fatty acid oxidation to glucose catabolism [3]. This metabolic reprogramming is under the control of mammalian target of rapamycin (mTOR) signaling, which in turn controls mitochondrial metabolism (Table 1) [45]. This transition decreases intracellular NAD+ levels and, in turn, decreases the activity of the NAD-dependent protein deacetylase SIRT1, ultimately resulting in epigenetic alterations that further drive activation and differentiation (Table 1) [46]. Excessive fatty acid oxidation, however, results in high levels of ROS and myofiber degeneration in cachectic cancer models [47]. In this pathological context, pharmacologic inhibition of fatty acid oxidation leads the MuSCs towards glycolic metabolism and myogenic activation, resulting in myofiber formation and increased muscle mass [47]. Whether and how niche factors are involved in inducing this metabolic switch in MuSCs remains an open question.
Table 1.
Signaling Pathways and their role in the ISC and MuSC niche.
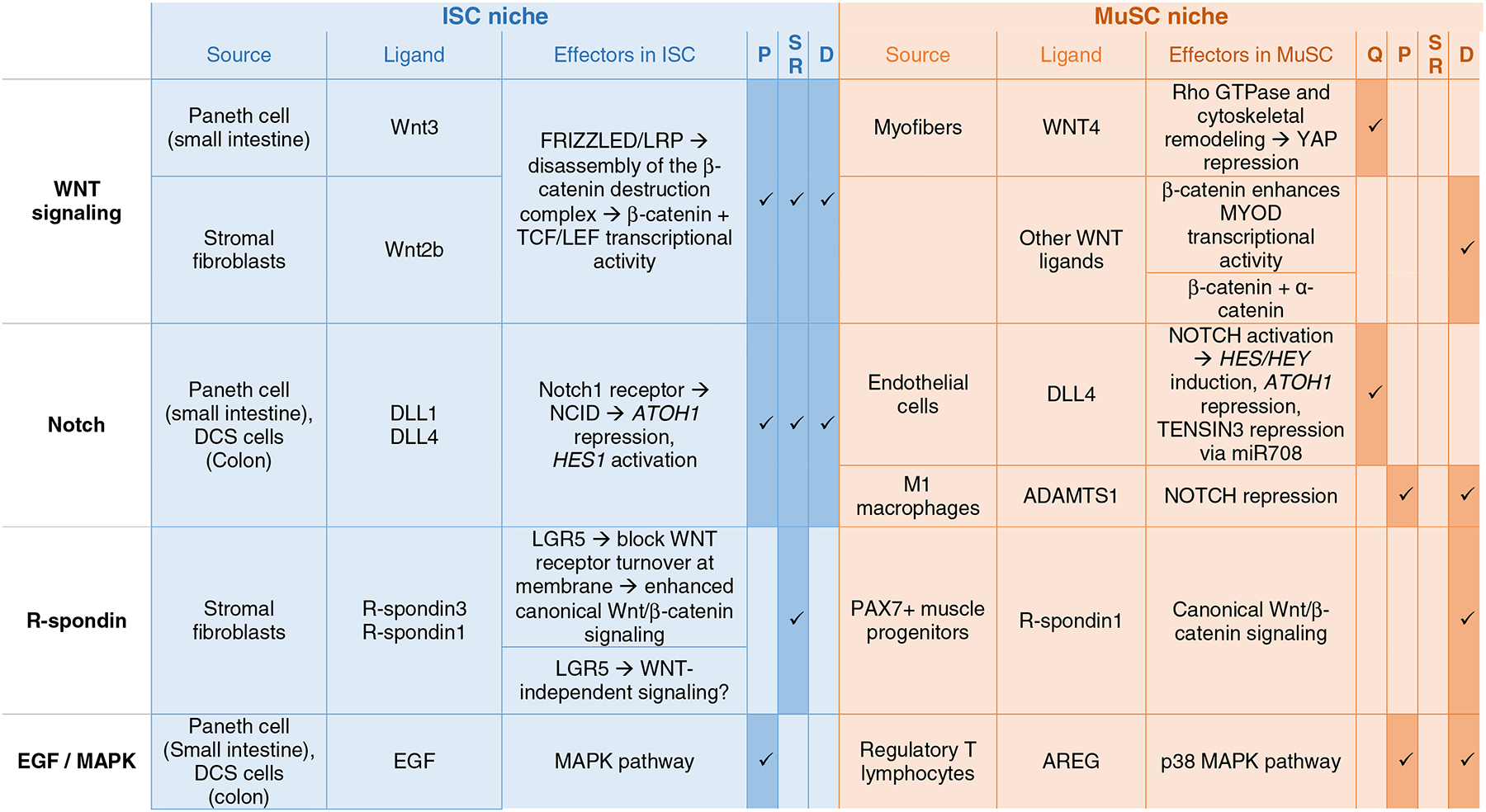

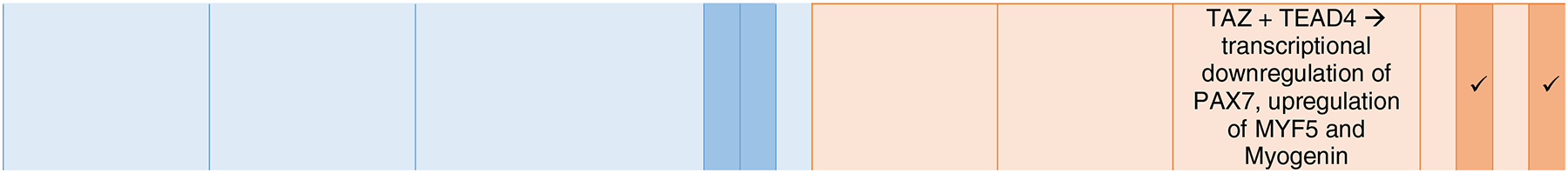
|
Q: quiescence, P: proliferation, SR: self-renewal, D: differentiation
Likewise, ISCs have high mitochondrial activity, and the ROS generated by their metabolism can drive ISC proliferation through the p38 MAPK pathway [48]. Interestingly, the high glycolytic activity of neighboring Paneth cells supports the mitochondrial metabolism and function of ISCs, presumably by providing them with lactate [48]. ISC function does not, however, depend on pyruvate oxidation; in fact, blocking pyruvate transport into mitochondria enhanced ISC function by stimulating fatty acid oxidation (FAO) [49]. Two recent studies have since supported the notion that FAO could act as a primary fuel for ISC metabolism [50,51] and that regulators of this pathway, like PPARδ and HNF4, play key roles in regulating ISC function [50,51]. It comes as no surprise that changes in diet and therefore in the luminal levels of metabolic substrates should affect ISC function. A high fat diet increases the availability of fatty acids and results in an expansion of ISC independently of other niche cells in both the small intestine and colon [52]. Surprisingly, a short-term fast also results in enhanced ISC function by stimulating PPARδ in ISC [52]. While prolonged caloric restriction also promotes ISC self-renewal in the small intestine, it does so indirectly by modulating Paneth cell secretion of cyclic ADP ribose, which in turn increases intracellular NAD+ and stimulates SIRT1 and mTOR in ISC [53,54]. These studies show that while ISCs have the intrinsic ability to respond to acute changes in nutrient availability, niche cells can ultimately override nutrient sensing in ISC during prolonged nutrient-low conditions.
Thus, fatty acid oxidation and metabolic switches are emerging as key regulators of SC maintenance in several adult tissues, highlighting the role of local metabolites in the SC niche.
Biomechanical cues and extracellular matrix regulate SC behavior
Within their niche, MuSCs are asymmetrically exposed to microenvironmental signals, with factors associated with the myofiber plasma membrane presented to the apical surface of MuSCs and factors associated with the basal lamina presented to the basal surface of MuSCs [55] (Figure 1B). This basal lamina is a network of extracellular matrix (ECM) components, including type IV collagen, laminin, fibronectin, and proteoglycans that can bind an array of soluble glycoproteins, including bFGF and Wnt ligands derived from MuSCs themselves, the myofiber or systemically [55]. One key ECM signal affecting MuSC function is Collagen V, which is produced by the MuSC and deposited under the basement membrane [56]. This signal is received by the plasma membrane calcitonin receptor (CALCR) on the MuSC, in order to maintain quiescence and prevent exhaustion of the MuSC pool [56,57] (Figure 3, Table 1).
Similarly, while the apical side of intestinal epithelial cells is exposed to the intestinal lumen, their basal side is in direct contact with a network of type IV collagen, laminin, and proteoglycans that form the basement membrane [58] (Figure 1A), and beneath it, the ECM and mesenchymal cells that make up the lamina propria. Both the composition of this ECM and consequent mechanical cues have recently emerged as integral to the maintenance of ISCs. For example, the need to embed intestinal organoids in Matrigel highlights the importance of the basement membrane and the laminin-enriched ECM underlying the intestinal crypts in vivo. In fact, embedding organoids in matrices of different compositions revealed that while soft, laminin-based matrices favor differentiation and organoid formation, stiff, fibronectin-based matrices favor ISC expansion, resulting in spheroid formation [59]. Similarly, MuSCs and muscle organoids have been cultured in 2D and 3D Matrigel [20] as well as over fibrin hydrogels [60] and under uniaxial tension to stimulate healthy and dystrophic muscle models [61].
Another theme emerging in parallel to the integral role of the ECM and mechanical cues has been the role of the Hippo pathway and its downstream effectors YAP and TAZ in SC self-renewal and early regeneration following injury [62–66]. Although the upstream effectors of this are still poorly understood, Hippo signaling in mammals acts through kinases MST1/2 and LATS1/2 to regulate phosphorylation of YAP and TAZ and thereby their nuclear translocation, where they can interact with TEAD transcription factors [67]. This pathway regulates growth in several organs, notably by modulating Wnt signaling, and it has also been shown to serve as a relay of mechanical stresses [68]. Nuclear localization of YAP was directly linked to ISC self-renewal in response to matrix stiffness [59] (Figure 2, Table 1). Altering the matrix composition can also shift intestinal organoids to a more regenerative profile associated with the re-acquisition of fetal ISC properties, and this process was shown to involve YAP relocalization [69]. In skeletal muscle, YAP/TAZ signaling is increased upon MuSC activation [10,70,71], but the two effectors of the Hippo pathway play complementary roles in this tissue: while YAP stimulates MuSC activation and self-renewal, TAZ seems to enhance myogenic differentiation (Figure 3, Table 1) [70]. YAP signaling was also recently linked to upstream biomechanical signals in MuSCs, as YAP nuclear expression is repressed downstream of WNT4-Rho GTPases in quiescent MuSCs.
In addition to tension from the underlying matrix, forces from adhesion to neighboring cells, proliferation and crowding [72], or tissue contractility [73,74] can also act as important mechanical cues for SC maintenance and differentiation in various tissues. Cadherins are a major class of cell-cell adhesion molecules, expressed in the muscle niche at sites of direct contact between MuSCs and myofibers [75]. Cadherin-deficient MuSCs (N-cadherin and M-cadherin double mutants) have increased committed (PAX+MYOD+) and differentiated (PAX7-Myogenin+) progenitors and fibers with centrally located nuclei indicating regeneration. This suggests that cadherin-mediated adhesion is required for MuSC quiescence (Figure 3, Table 1) and that partial disruption of these adhesive junctions leads to MuSC activation [75].
Cytokines and other immune cell-derived signals
Over the past few years, the interaction between SCs and immune cells has generated increasing interest, with immune cells emerging as key components of the niche and strong regulators of SC behavior [76]. Using a murine model in which GFP is expressed by various tissue SCs and T cells are engineered to specifically recognize GFP loaded on MHC class I, it was revealed that although fast-cycling epithelial SCs (including ISCs, ovarian SCs, and mammary SCs) are subject to T cell clearance, quiescent SCs (like MuSCs or hair follicle bulge SCs) escape immune clearance by downregulating their antigen presentation machinery [77]. Interestingly, once quiescent SCs are activated and proliferate, they upregulate antigen presentation and in turn become subject to immune surveillance. Thus, the proliferative status of SCs helps determine their interaction with immune cells in their microenvironment.
Conversely, ISCs express high levels of MHC class II (MHCII) compared to the rest of the intestinal epithelium and can act as non-conventional antigen-presenting cells [78]. This ISC-immune crosstalk affects not only the recruitment of immune cells in the mesenchyme surrounding the crypts but also SC number, as deletion of either ISC-specific MHCII or of T-cells results in an expansion of the ISC pool. The ISC-immune cell interactions are in part mediated by cytokine signaling: pro-inflammatory T helper cell subsets and cytokines like Interferon-γ (IFNγ) reduce ISC numbers by favoring TA cell proliferation and ISC apoptosis [78,79], whereas regulatory T cell signals like IL-10 enhance ISC self-renewal [78] (Figure 2, Table 1).
Recent work has also demonstrated the importance of circulating cytokines in regulating MuSC and myofiber function. The pro-inflammatory IL-6 pathway has been a particular focus, because the cytokine is upregulated following exercise, and studies with IL-6-deficient animals indicated a role for IL-6 in MuSC and myoblast proliferation via STAT3 signaling [23,80]. Another member of the IL-6 family of cytokines secreted by myofibers, Oncostatin M, is key in the reestablishment of MuSC quiescence following activation [23]. Regulatory T cells in the muscle stimulate myoblast differentiation from MuSCs during regeneration by secreting the EGF-like growth factor amphiregulin (AREG) [76,81] (Figure 3, Table 1). As regulatory T cells also affect SC behavior in other tissues, notably in the hair follicle and hematopoietic SC niche [82,83], such crosstalk could be a common feature of SC niches.
In addition to T cells, innate immune cells also play an important role in SC niches. As repair progresses in the muscle after injury, inflammatory M1 macrophages first promote MuSC activation by secreting the metalloprotease ADAMTS1, which suppresses Notch signaling in MuSC [84], and then are replaced by regenerative M2 macrophages, which stimulate differentiation into myoblasts [76,85]. Likewise, upon intestinal damage, macrophages [86,87] as well as innate lymphoid cells (ILCs) [88,89] and stromal fibroblasts [31,90–92] upregulate expression of growth factors like WNT and AREG and of pro-inflammatory cytokines and wound repair factors that promote ISC expansion and regeneration. For example, in response to genotoxic injury, tissue-resident group 3 ILCs produce IL-22, a cytokine of the IL-10 superfamily that acts through STAT3 signaling to both drive ISC expansion [88] and stimulate the DNA damage response in ISCs [93]. Thus, this innate immune signal favors intestinal regeneration while protecting the genomic integrity of the ISC pool. Altogether, these findings highlight the role of both innate and adaptive immune cells and their interaction with SCs and their niche to promote optimal tissue repair.
The niche can define SC identity and heterogeneity
Historically, SCs have been postulated to either divide asymmetrically, giving rise to both a new stem cell and a differentiating daughter cell, or symmetrically, favoring either expansion of the SC pool or regeneration of the differentiated tissue. Over the past decade, various studies have supported the idea that daughter cell fate can be regulated at the level of the entire SC population rather than in terms of individual stem cells. Studies in the intestine strongly support this model: at the crypt bottom, the niche space within which ISCs continuously divide is defined by a gradient of signals from Paneth and mesenchymal cells, and exiting this defined niche leads cells to differentiate [94–96]. Symmetrically dividing ISCs therefore stochastically compete for niche occupancy and, over time, ISCs originating from a single clone will neutrally come to fill the niche space in a process called neutral drift.
Although several cell-intrinsic mechanisms have been described to regulate cell fate upon asymmetric divisions of MuSCs, cell adhesion and the polarized niche architecture play a central role in determining the fate of asymmetrically dividing MuSC daughters (reviewed in [97]). Moreover, soluble niche signals can also favor symmetric divisions to drive either MuSC self-renewal or increased differentiation for repair. Therefore, as in the intestine, niche signals can determine MuSC fate. Studies in other epithelia like the hair follicle or lung alveoli also support this notion of niche-defined SC identity, further implying that functional and transcriptional heterogeneity within SC populations are directly linked to position within the niche or proximity to mesenchymal micro-niches [98–100].
Recent evidence also supports the idea of neutral drift in the MuSC niche: although slow turnover of these SCs makes the process more difficult to study, examination of MuSC clonal complexity during aging and injury revealed that, whereas clonal heterogeneity was conserved with homeostatic aging, under repetitive injury, MuSCs undergo symmetric expansion during tissue repair resulting in a reduction of clonal complexity [101]. Uninjured muscles may preserve MuSCs in a highly polarized niche with repeated asymmetric division whereas, upon injury, disruption of this niche with loss of polarization and non-uniform contact with the neighboring matrix could favor serial rounds of symmetric division [101]. Thus, in the context of successive muscle injury, neutral drift dynamics are observed in MuSCs and are likely driven by the niche.
In addition to the rapidly-cycling, Lgr5-expressing ISC population, a relatively quiescent [102], label-retaining [103] population of ISCs has been extensively described in the literature as residing at the +4 position from the crypt bottom and being capable of replenishing the fast-cycling ISC pool after injury [104]. However, whether this is a unique stem cell pool or a subpopulation of the same Lgr5+ ISC pool has sparked much debate and controversy [105,106]. It is possible that cells at this particular position in the crypt – towards the border of the ISC niche – are exposed to different levels of niche signals that results in slower cycling or stronger resistance to injury [25,107]. Another possibility is that these “reserve stem cells” are actually committed progenitors capable of reverting back into ISCs. In fact, over the past few years, studies have demonstrated a high degree of plasticity within the intestinal crypt, such that upon injury and loss of Lgr5+ ISCs, committed progenitors and even terminally-differentiated Paneth cells can de-differentiate and replenish the Lgr5+ ISC pool [108–112]. Mechanistically, such dedifferentiation has been shown to involve not only cell-intrinsic events like chromatin remodeling [110] but also microenvironmental cues including activation of Notch signaling [113,114], and a transient re-acquisition of a fetal ISC phenotype [69,115] involving pro-inflammatory IFNγ signaling [116] or ECM remodeling [69]. Interestingly, regeneration of the Lgr5+ SC pool in the hair follicle is also associated with inflammatory responses [117]. These studies highlight a contribution of the niche to driving the dedifferentiation of committed progenitor cells back into multipotent SCs; how these signals reprogram differentiated cells into SCs and whether similar microenvironmental cues are involved in plasticity in other tissues, including slow-cycling tissues, remains to be determined.
Niche Dysfunction in Aging and Pathology
In the slow-turnover skeletal muscle, aged MuSC function decreases over time, suggesting a dysregulation of MuSC quiescence, activation, or self-renewal. Recent studies have asked whether the niche plays a role in this process [23,118]. In aged muscle, the Notch ligand Delta1 level was decreased in myofibers, and MuSCs failed to induce Notch signaling during regeneration [23,118]. Defects in JAK-STAT as well as FGF and TGF signaling are also dysregulated in the aged niche, with detrimental effects on the maintenance and proliferation of aged MuSCs [23,118]. Recent evidence from human cells points to increased histone H3K27ac (an active enhancer mark) in aged muscle, resulting in up-regulation of ECM genes and thereby alterations in the MuSC niche environment that lead to decreased myogenic potential and increased fibrogenic conversion of MuSC [119]. Decline of MuSC number and function during aging has been reported in post-menopausal women, where loss of estradiol and estrogen receptors result in decreased MuSC survival due to mitochondrial caspase-induced apoptosis and impaired self-renewal and muscle regeneration [120]. Further elucidation of the niche signaling pathways will provide detailed information about the spatiotemporal instructions that drive MuSC decisions and might be compromised in a variety of diseases, including aging sarcopenia and muscular dystrophy.
Recent studies in ISCs have similarly reported a decrease in regenerative capacity of ISCs with age [50,121], linked both to cell-autonomous defects [50] and non-cell-autonomous mechanisms like loss of niche-derived Wnt signals [121]. Dysregulation of the ISC niche has also been assessed in the context of colorectal cancer (CRC) and inflammatory bowel disease (IBD). For example, whereas increases in signals like Wnt or R-spondin that will enhance ISC numbers or proliferation generally increase the risk for CRC, the opposite can surprisingly have the same effect, by decreasing the size of the ISC pool and thereby enhancing the mutation fixation rate [122,123]. Just as niche signals can affect tumorigenesis, both the clonogenicity of CRC cells and their resistance to treatment depends on signals from the surrounding mesenchymal niche [124,125]. Furthermore, intestinal tumors display plasticity akin to that observed in the homeostatic crypt, as cancer SCs are rapidly replenished after ablation by remaining tumor cells [126,127]. Understanding which niche signals are required for this phenomenon may open the door to new therapeutic targets, with the potential complication that, as tumor cells accumulate mutations, their dependence on niche signals gradually diminishes [128,129]. Likewise, in IBD, the stromal cell populations are altered, with the notable expansion of fibroblast populations known to sustain the ISC niche [31,91,92,130]. Thus, the pro-inflammatory environment in IBD likely affects the regenerative capacity of ISC.
Conclusions and future directions
Recent advances have expanded our understanding of SC niche signals and components, and of how the niche mediates tissue regeneration, in both homeostasis and disease. Although this review focused on the ISC and MuSC niches, parallels to other fast- and slow- turnover tissues are increasingly apparent in the literature, revealing common trends in how SC function is regulated. One such trend is the convergence of multiple pathways to maintain niche robustness while also allowing context-dependent modulation of SC behavior. Another is the importance of heterogeneous niche components in defining the identity and behavior of SCs. These findings raise many questions regarding how cross-talk modulates SCs in their tissue-specific niches: What is the importance of the spatial and temporal distribution of niche signals on SC behavior? How do SCs integrate simultaneous cues from the niche? Do analogous cell populations play similar roles in different tissue SC niches? Are common signaling pathways involved? How does such cross-talk affect pathology? Recent technical developments, for example single-cell RNA profiling of niche cells, will help to shed light on the niche components in the muscle, gut [32,33,131] and other tissues [98,132–135]. Another exciting development in the SC niche field is the in vitro differentiation of tissues designed to recreate as much as possible the niche’s naturally occurring cell to cell and ECM interactions [60,61,136,137]. For instance, human muscle organoids fully derived from induced pluripotent SCs contain multilineage, isogenic cellular constituents of the tissue. As has been done previously for the gut [136], this was recently established for skeletal muscle, including vascular endothelial cells, pericytes and motor neurons [61]. These new organoid models are laying the foundation for developmental studies, understanding SC-niche interactions in pathology, and therapy development [137].
Acknowledgments
We thank Drs. Rachel Zwick and Laura Weichselbaum for their feedback on this manuscript. Work in the Klein Laboratory was supported by NIH R35-DE026602 and in the Pomerantz Laboratory by CIRM New Faculty Physician Scientist Award RN3-06504 and NIH R01AR072638-03.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE
The authors declare no conflict of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Chacón-Martínez CA, Koester J, Wickström SA: Signaling in the stem cell niche: regulating cell fate, function and plasticity. Development 2018, 145:dev165399. [DOI] [PubMed] [Google Scholar]
- 2.Llorens-Bobadilla E, Zhao S, Baser A, Saiz-Castro G, Zwadlo K, Martin-Villalba A: Single-Cell Transcriptomics Reveals a Population of Dormant Neural Stem Cells that Become Activated upon Brain Injury. Cell Stem Cell 2015, 17:329–340. [DOI] [PubMed] [Google Scholar]
- 3.Evano B, Tajbakhsh S: Skeletal muscle stem cells in comfort and stress. npj Regen Med 2018, 3:75015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker N: Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol 2014, 15:19–33. [DOI] [PubMed] [Google Scholar]
- 5.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. : Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007, 449:1003–1007. [DOI] [PubMed] [Google Scholar]
- 6.Sasaki N, Sachs N, Wiebrands K, Ellenbroek SIJ, Fumagalli A, Lyubimova A, Begthel H, van den Born M, van Es JH, Karthaus WR, et al. : Reg4 + deep crypt secretory cells function as epithelial niche for Lgr5 + stem cells in colon. Proc Natl Acad Sci 2016, 113:E5399–E5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gehart H, Clevers H: Tales from the crypt: new insights into intestinal stem cells. Nat Rev Gastroenterol Hepatol 2019, 16:19–34. [DOI] [PubMed] [Google Scholar]
- 8.Clevers H, Loh KM, Nusse R: Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 2014, 346:1248012. [DOI] [PubMed] [Google Scholar]
- 9.van Es JH, Haegebarth A, Kujala P, Itzkovitz S, Koo B-K, Boj SF, Korving J, van den Born M, van Oudenaarden A, Robine S, et al. : A Critical Role for the Wnt Effector Tcf4 in Adult Intestinal Homeostatic Self-Renewal. Mol Cell Biol 2012, 32:1918–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eliazer S, Muncie JM, Christensen J, Sun X, D’Urso RS, Weaver VM, Brack AS: Wnt4 from the Niche Controls the Mechano-Properties and Quiescent State of Muscle Stem Cells. Cell Stem Cell 2019, doi: 10.1016/j.stem.2019.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study links niche Wnt signaling and mechanical properties of MuSCs to their quiescent state. Wnt4, produced by myofibers at homeostasis, maintains MuSCs in a quiescent state through non-canonical signaling via RhoA, which in turn represses YAP. In the case of injury, Wnt4 from myofibers is downregulated and YAP is induced in MuSCs, leading to their activation.
- 11.Cui S, Li L, Yu RT, Downes M, Evans RM, Hulin JA, Makarenkova HP, Meech R: β-Catenin is essential for differentiation of primary myoblasts via cooperation with MyoD and α-catenin. Dev 2019, 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim C-H, Neiswender H, Baik EJ, Xiong WC, Mei L: β-Catenin Interacts with MyoD and Regulates Its Transcription Activity. Mol Cell Biol 2008, 28:2941–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudolf A, Schirwis E, Giordani L, Parisi A, Lepper C, Taketo MM, Le Grand F: β-Catenin Activation in Muscle Progenitor Cells Regulates Tissue Repair. Cell Rep 2016, 15:1277–1290. [DOI] [PubMed] [Google Scholar]
- 14.Sueda R, Kageyama R: Regulation of active and quiescent somatic stem cells by Notch signaling. Dev Growth Differ 2019, doi: 10.1111/dgd.12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baghdadi MB, Firmino J, Soni K, Evano B, Di Girolamo D, Mourikis P, Castel D, Tajbakhsh S: Notch-Induced miR-708 Antagonizes Satellite Cell Migration and Maintains Quiescence. Cell Stem Cell 2018, 23:859–868.e5. [DOI] [PubMed] [Google Scholar]
- 16.Bjornson CRR, Cheung TH, Liu L, Tripathi PV., Steeper KM, Rando TA: Notch signaling is necessary to maintain quiescence in adult muscle stem cells. Stem Cells 2012, 30:232–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis-Tsakonas S: Notch signals control the fate of immature progenitor cells in the intestine. Nature 2005, 435:964–8. [DOI] [PubMed] [Google Scholar]
- 18.Pellegrinet L, Rodilla V, Liu Z, Chen S, Koch U, Espinosa L, Kaestner KH, Kopan R, Lewis J, Radtke F: Dll1- and Dll4-mediated notch signaling are required for homeostasis of intestinal stem cells. Gastroenterology 2011, 140:1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, et al. : Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459:262–5. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y xiao, Wu B bing, Gong L, An C rui, Lin J xin, Li Q kai, Jiang D ming, Jin K xiu, Mechakra A, Bunpetch V, et al. : Dissecting cell diversity and connectivity in skeletal muscle for myogenesis. Cell Death Dis 2019, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pawlikowski B, Vogler TO, Gadek K, Olwin BB: Regulation of skeletal muscle stem cells by fibroblast growth factors. Dev Dyn 2017, 246:359–367. [DOI] [PubMed] [Google Scholar]
- 22.Palacios D, Mozzetta C, Consalvi S, Caretti G, Saccone V, Proserpio V, Marquez VE, Valente S, Mai A, Forcales SV., et al. : TNF/p38α/polycomb signaling to Pax7 locus in satellite cells links inflammation to the epigenetic control of muscle regeneration. Cell Stem Cell 2010, doi: 10.1016/j.stem.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sampath SC, Sampath SC, Ho ATV, Corbel SY, Millstone JD, Lamb J, Walker J, Kinzel B, Schmedt C, Blau HM: Induction of muscle stem cell quiescence by the secreted niche factor Oncostatin M. Nat Commun 2018, 9:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]; *By screening for secreted protein regulators of MuSC proliferation in the niche, the authors identify Oncostatin M, a member of the IL-6 family of cytokines, as capable of suppressing MuSC activation and proliferation in vitro, while maintaining the ability of these cells to engraft following transplantation in vivo.
- 24.Bernet JD, Doles JD, Hall JK, Kelly Tanaka K, Carter TA, Olwin BB: P38 MAPK signaling underlies a cell-autonomous loss of stem cell self-renewal in skeletal muscle of aged mice. Nat Med 2014, doi: 10.1038/nm.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farin HF, Jordens I, Mosa MH, Basak O, Korving J, Tauriello DVF, De Punder K, Angers S, Peters PJ, Maurice MM, et al. : Visualization of a short-range Wnt gradient in the intestinal stem-cell niche. Nature 2016, 530:340–343. [DOI] [PubMed] [Google Scholar]
- 26.Verma M, Asakura Y, Murakonda BSR, Pengo T, Latroche C, Chazaud B, McLoon LK, Asakura A: Muscle Satellite Cell Cross-Talk with a Vascular Niche Maintains Quiescence via VEGF and Notch Signaling. Cell Stem Cell 2018, 23:530–543.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]; * In vivo 3D imaging reveals that the majority of MuSCs are closely associated with the surrounding microvasculature. By gene ontology, they look into the most enriched ligand-receptor interactions between these cells, finding most of them related to angiogenesis, ECM organization, regulation of migration and VEGF signaling, the latter being required for Notch activation and induction of MuSC quiescence.
- 27.Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H: Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 2011, 469:415–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pellegrinet L, Rodilla V, Liu Z, Chen S, Koch U, Espinosa L, Kaestner KH, Kopan R, Lewis J, Radtke F: Dll1- and Dll4-mediated notch signaling are required for homeostasis of intestinal stem cells. Gastroenterology 2011, 140:1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rothenberg ME, Nusse Y, Kalisky T, Lee JJ, Dalerba P, Scheeren F, Lobo N, Kulkarni S, Sim S, Qian D, et al. : Identification of a cKit + colonic crypt base secretory cell that supports Lgr5 + stem cells in mice. Gastroenterology 2012, 142:1195–1205.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aoki R, Shoshkes-Carmel M, Gao N, Shin S, May CL, Golson ML, Zahm AM, Ray M, Wiser CL, Wright CVE, et al. : Foxl1-Expressing Mesenchymal Cells Constitute the Intestinal Stem Cell Niche. Cell Mol Gastroenterol Hepatol 2016, 2:175–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stzepourginski I, Nigro G, Jacob J, Dulauroy S, Sansonetti PJ, Eberl G, Peduto L: CD34 + mesenchymal cells are a major component of the intestinal stem cells niche at homeostasis and after injury. Proc Natl Acad Sci U S A 2017, 114:E506–E513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Degirmenci B, Valenta T, Dimitrieva S, Hausmann G, Basler K: GLI1-expressing mesenchymal cells form the essential Wnt-secreting niche for colon stem cells. Nature 2018, 558:449–453. [DOI] [PubMed] [Google Scholar]; *This work, along with several others published in the past two years, identifies a heterogeneous population of pericryptal mesenchymal cells that provide Wnt signals, which maintain colon stem cells and act as a complement to epithelial Wnt in the small intestine. These cells express the Hedgehog-responsive marker Gli1.
- 33.Shoshkes-Carmel M, Wang YJ, Wangensteen KJ, Tóth B, Kondo A, Massassa E, Itzkovitz S, Kaestner KH: Subepithelial telocytes are an important source of Wnts that supports intestinal crypts. Nature 2018, 557:242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]; **This study describes compartmentalized production of Wnt from subepithelial Foxl1+ telocytes, which is essential both in the colonic and small intestinal stem cell niche.
- 34.Greicius G, Kabiri Z, Sigmundsson K, Liang C, Bunte R, Singh MK, Virshup DM: PDGFRα + pericryptal stromal cells are the critical source of Wnts and RSPO3 for murine intestinal stem cells in vivo. Proc Natl Acad Sci 2018, 115:E3173–E3181. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Here, mesenchymal R-spondin expression from PdgfRα+ pericryptal myofibroblasts is shown to be important for ISC function, including in crypt development and response to chemically-induced colitis in adults.
- 35.Hao H-X, Xie Y, Zhang Y, Charlat O, Oster E, Avello M, Lei H, Mickanin C, Liu D, Ruffner H, et al. : ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature 2012, 485:195–200. [DOI] [PubMed] [Google Scholar]
- 36.de Lau W, Barker N, Low TY, Koo B-K, Li VSW, Teunissen H, Kujala P, Haegebarth A, Peters PJ, van de Wetering M, et al. : Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 2011, 476:293–297. [DOI] [PubMed] [Google Scholar]
- 37.Storm EE, Durinck S, de Sousa e Melo F, Tremayne J, Kljavin N, Tan C, Ye X, Chiu C, Pham T, Hongo J-A, et al. : Targeting PTPRK-RSPO3 colon tumours promotes differentiation and loss of stem-cell function. Nature 2016, 529:97–100. [DOI] [PubMed] [Google Scholar]
- 38.Yan KS, Janda CY, Chang J, Zheng GXY, Larkin KA, Luca VC, Chia LA, Mah AT, Han A, Terry JM, et al. : Non-equivalence of Wnt and R-spondin ligands during Lgr5+ intestinal stem-cell self-renewal. Nature 2017, 545:238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This study points to the differential roles of Wnt and R-spondin signals in ISC function, highlighting the pro-proliferative and “priming” role of Wnt, which drives Lgr5 expression, as compared to the self-renewal effect of R-spondin.
- 39.Lin Y, Fang ZP, Liu HJ, Wang LJ, Cheng Z, Tang N, Li T, Liu T, Han HX, Cao G, et al. : HGF/R-spondin1 rescues liver dysfunction through the induction of Lgr5+ liver stem cells. Nat Commun 2017, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sigal M, Logan CY, Kapalczynska M, Mollenkopf HJ, Berger H, Wiedenmann B, Nusse R, Amieva MR, Meyer TF: Stromal R-spondin orchestrates gastric epithelial stem cells and gland homeostasis. Nature 2017, 548:451–455. [DOI] [PubMed] [Google Scholar]
- 41.Rocha AS, Vidal V, Mertz M, Kendall TJ, Charlet A, Okamoto H, Schedl A: The Angiocrine Factor Rspondin3 Is a Key Determinant of Liver Zonation. Cell Rep 2015, 13:1757–1764. [DOI] [PubMed] [Google Scholar]
- 42.Lacour F, Vezin E, Bentzinger CF, Sincennes MC, Giordani L, Ferry A, Mitchell R, Patel K, Rudnicki MA, Chaboissier MC, et al. : R-spondin1 Controls Muscle Cell Fusion through Dual Regulation of Antagonistic Wnt Signaling Pathways. Cell Rep 2017, 18:2320–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kohli L, Passegué E: Surviving change: The metabolic journey of hematopoietic stem cells. Trends Cell Biol 2014, 24:479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ito K, Carracedo A, Weiss D, Arai F, Ala U, Avigan DE, Schafer ZT, Evans RM, Suda T, Lee CH, et al. : A PML-PPAR-δ pathway for fatty acid oxidation regulates hematopoietic stem cell maintenance. Nat Med 2012, 18:1350–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodgers JT, King KY, Brett JO, Cromie MJ, Charville GW, Maguire KK, Brunson C, Mastey N, Liu L, Tsai CR, et al. : MTORC1 controls the adaptive transition of quiescent stem cells from G 0 to GAlert. Nature 2014, 510:393–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ryall JG, Dell’Orso S, Derfoul A, Juan A, Zare H, Feng X, Clermont D, Koulnis M, Gutierrez-Cruz G, Fulco M, et al. : The NAD+-dependent sirt1 deacetylase translates a metabolic switch into regulatory epigenetics in skeletal muscle stem cells. Cell Stem Cell 2015, 16:171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fukawa T, Yan-Jiang BC, Min-Wen JC, Jun-Hao ET, Huang D, Qian CN, Ong P, Li Z, Chen S, Mak SY, et al. : Excessive fatty acid oxidation induces muscle atrophy in cancer cachexia. Nat Med 2016, 22:666–671. [DOI] [PubMed] [Google Scholar]
- 48.Rodríguez-Colman MJ, Schewe M, Meerlo M, Stigter E, Gerrits J, Pras-Raves M, Sacchetti A, Hornsveld M, Oost KC, Snippert HJ, et al. : Interplay between metabolic identities in the intestinal crypt supports stem cell function. Nature 2017, 543:424–427. [DOI] [PubMed] [Google Scholar]
- 49.Schell JC, Wisidagama DR, Bensard C, Zhao H, Wei P, Tanner J, Flores A, Mohlman J, Sorensen LK, Earl CS, et al. : Control of intestinal stem cell function and proliferation by mitochondrial pyruvate metabolism. Nat Cell Biol 2017, 19:1027–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mihaylova MM, Cheng C-W, Cao AQ, Tripathi S, Mana MD, Bauer-Rowe KE, Abu-Remaileh M, Clavain L, Erdemir A, Lewis CA, et al. : Fasting Activates Fatty Acid Oxidation to Enhance Intestinal Stem Cell Function during Homeostasis and Aging. Cell Stem Cell 2018, 22:769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen L, Vasoya R, Toke N, Parthasarathy A, Luo S, Chiles E, Flores J, Gao N, Bonder E, Su X, et al. : HNF4 Regulates β-Oxidation and is Indispensable for Intestinal Stem Cell Renewal. SSRN Electron J 2019, doi: 10.2139/ssrn.3403334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beyaz S, Mana MD, Roper J, Kedrin D, Saadatpour A, Hong S, Bauer-rowe KE, Xifaras ME, Akkad A, Arias E, et al. : High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature 2016, 531:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yilmaz H, Katajisto P, Lamming DW, Gu Y, Birsoy K, Dursun A, Yilmaz VO, Selig M, Nielsen GP, Mino-kenudson M, et al. : mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature 2012, 486:490–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Igarashi M, Guarente L: mTORC1 and SIRT1 Cooperate to Foster Expansion of Gut Adult Stem Cells during Calorie Restriction. Cell 2016, 166:436–450. [DOI] [PubMed] [Google Scholar]
- 55.Cosgrove BD, Sacco A, Gilbert PM, Blau HM: A home away from home: Challenges and opportunities in engineering in vitro muscle satellite cell niches. Differentiation 2009, 78:185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baghdadi MB, Castel D, Machado L, Fukada S, Birk DE, Relaix F, Tajbakhsh S, Mourikis P: Reciprocal signalling by Notch–Collagen V–CALCR retains muscle stem cells in their niche. Nature 2018, 557:714–718. [DOI] [PMC free article] [PubMed] [Google Scholar]; **Satellite cells autonomously produce various collagens. Of these, only Collagen V sustains PAX7 expression, indicative of stemness, and represses differentiation. In addition, MuSC response to Collagen V requires expression of the calcitonin receptor CALCR. Through various approaches, the authors elegantly dissect the interaction of Collagen V, CALCR and Notch signaling and how it affects MuSC quiescence and self-renewal.
- 57.Yamaguchi M, Watanabe Y, Ohtani T, Uezumi A, Mikami N, Nakamura M, Sato T, Ikawa M, Hoshino M, Tsuchida K, et al. : Calcitonin Receptor Signaling Inhibits Muscle Stem Cells from Escaping the Quiescent State and the Niche. Cell Rep 2015, 13:302–314. [DOI] [PubMed] [Google Scholar]
- 58.Lee S-E, Massie I, Meran L, Li VSW: Extracellular Matrix Remodeling in Intestinal Homeostasis and Disease. Elsevier Inc.; 2018. [Google Scholar]
- 59.Gjorevski N, Sachs N, Manfrin A, Giger S, Bragina ME, Ordóñez-Morán P, Clevers H, Lutolf MP: Designer matrices for intestinal stem cell and organoid culture. Nature 2016, 539:560–564. [DOI] [PubMed] [Google Scholar]; *This study reveals the importance of matrix stiffness in ISC behavior, showing that soft matrices favor differentiation while stiff matrices favor self-renewal. The authors not only demonstrate the importance of the basement membrane and ECM composition in the ISC niche but also provide a new in vitro system for organoid culture.
- 60.Gholobova D, Gerard M, Decroix L, Desender L, Callewaert N, Annaert P, Thorrez L: Human tissue-engineered skeletal muscle: a novel 3D in vitro model for drug disposition and toxicity after intramuscular injection. Sci Rep 2018, 8:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maffioletti SM, Sarcar S, Henderson ABH, Mannhardt I, Pinton L, Moyle LA, Steele-Stallard H, Cappellari O, Wells KE, Ferrari G, et al. : Three-Dimensional Human iPSC-Derived Artificial Skeletal Muscles Model Muscular Dystrophies and Enable Multilineage Tissue Engineering. Cell Rep 2018, 23:899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barry ER, Morikawa T, Butler BL, Shrestha K, de la Rosa R, Yan KS, Fuchs CS, Magness ST, Smits R, Ogino S, et al. : Restriction of intestinal stem cell expansion and the regenerative response by YAP SUPPLEMENT. Nature 2013, 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gregorieff A, Liu Y, Inanlou MR, Khomchuk Y, Wrana JL: Yap-dependent reprogramming of Lgr5+ stem cells drives intestinal regeneration and cancer. Nature 2015, 526:715–718. [DOI] [PubMed] [Google Scholar]
- 64.Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, Bresolin S, Frasson C, Basso G, Guzzardo V, et al. : YAP/TAZ Incorporation in the β-Catenin Destruction Complex Orchestrates the Wnt Response. Cell 2014, 158:157–170. [DOI] [PubMed] [Google Scholar]
- 65.Imajo M, Ebisuya M, Nishida E: Dual role of YAP and TAZ in renewal of the intestinal epithelium. Nat Cell Biol 2015, 17:7–19. [DOI] [PubMed] [Google Scholar]
- 66.Tremblay AM, Camargo FD: Hippo signaling in mammalian stem cells. Semin Cell Dev Biol 2012, 23:818–826. [DOI] [PubMed] [Google Scholar]
- 67.Varelas X: The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development 2014, 141:1614–1626. [DOI] [PubMed] [Google Scholar]
- 68.Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, Dupont S, Piccolo S: A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 2013, 154:1047–1059. [DOI] [PubMed] [Google Scholar]
- 69.Yui S, Azzolin L, Maimets M, Pedersen MT, Fordham RP, Hansen SL, Larsen HL, Guiu J, Alves MRP, Rundsten CF, et al. : YAP/TAZ-Dependent Reprogramming of Colonic Epithelium Links ECM Remodeling to Tissue Regeneration. Cell Stem Cell 2018, 22:35–49.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun C, De Mello V, Mohamed A, Ortuste Quiroga HP, Garcia-Munoz A, Al Bloshi A, Tremblay AM, von Kriegsheim A, Collie-Duguid E, Vargesson N, et al. : Common and Distinctive Functions of the Hippo Effectors Taz and Yap in Skeletal Muscle Stem Cell Function. Stem Cells 2017, 35:1958–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Judson RN, Tremblay AM, Knopp P, White RB, Urcia R, De Bari C, Zammit PS, Camargo FD, Wackerhage H: The hippo pathway member Yap plays a key role in influencing fate decisions in muscle satellite cells. J Cell Sci 2012, 125:6009–6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miroshnikova YA, Le HQ, Schneider D, Thalheim T, Rübsam M, Bremicker N, Polleux J, Kamprad N, Tarantola M, Wang I, et al. : Adhesion forces and cortical tension couple cell proliferation and differentiation to drive epidermal stratification. Nat Cell Biol 2018, 20:69–80. [DOI] [PubMed] [Google Scholar]
- 73.Broguiere N, Isenmann L, Hirt C, Ringel T, Placzek S, Cavalli E, Ringnalda F, Villiger L, Züllig R, Lehmann R, et al. : Growth of Epithelial Organoids in a Defined Hydrogel. Adv Mater 2018, 30. [DOI] [PubMed] [Google Scholar]
- 74.Sumigray KD, Terwilliger M, Lechler T: Morphogenesis and Compartmentalization of the Intestinal Crypt. Dev Cell 2018, 45:183–197.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goel AJ, Rieder MK, Arnold HH, Radice GL, Krauss RS: Niche Cadherins Control the Quiescence-to-Activation Transition in Muscle Stem Cells. Cell Rep 2017, 21:2236–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Naik S, Larsen SB, Cowley CJ, Fuchs E: Two to Tango: Dialog between Immunity and Stem Cells in Health and Disease. Cell 2018, 175:908–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Agudo J, Park ES, Rose SA, Alibo E, Sweeney R, Dhainaut M, Kobayashi KS, Sachidanandam R, Baccarini A, Merad M, et al. : Quiescent Tissue Stem Cells Evade Immune Surveillance. Immunity 2018, 48:271–285.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]; **Using a model that enables the study of antigen-dependent interactions between T cells and different tissue stem cells, the authors demonstrate a key difference in immunoregulation between fast-cycling and quiescent stem cells and provide evidence that this is regulated by proliferation-linked expression of Nlrc5, a transcriptional transactivator of the antigen presentation machinery.
- 78.Biton M, Haber AL, Rogel N, Burgin G, Beyaz S, Schnell A, Ashenberg O, Su CW, Smillie C, Shekhar K, et al. : T Helper Cell Cytokines Modulate Intestinal Stem Cell Renewal and Differentiation. Cell 2018, 175:1307–1320.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]; **In this study, the authors identify ISCs as non-conventional MHCII-expressing antigen presenting cells. They further screen the response of intestinal organoids to different T helper cell populations and T cell-produced cytokines and show that, while pro-inflammatory T cell subsets and cytokines modulate differentiation, anti-inflammatory T cells and cytokines promote ISC self-renewal.
- 79.Takashima S, Martin ML, Jansen SA, Fu Y, Bos J, Chandra D, O’Connor MH, Mertelsmann AM, Vinci P, Kuttiyara J, et al. : T cell–derived interferon-γ programs stem cell death in immune-mediated intestinal damage. Sci Immunol 2019, 4:eaay8556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tierney Matthew Timothy 1, 6, Aydogdu Tufan 2, 3, 6, Sala David 2, Malecova Barbora 2, Gatto Sole 2, Puri Pier Lorenzo 2, 4, Latella Lucia 4, 5 and S A: STAT3 signaling controls satellite cell expansion and skeletal muscle repair. Nat Med 2014, 20:1182–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, Sefik E, Tan TG, Wagers AJ, Benoist C, et al. : A Special Population of regulatory T Cells Potentiates muscle repair. Cell 2013, 155:1282–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ali N, Zirak B, Rodriguez RS, Pauli ML, Truong HA, Lai K, Ahn R, Corbin K, Lowe MM, Scharschmidt TC, et al. : Regulatory T Cells in Skin Facilitate Epithelial Stem Cell Differentiation. Cell 2017, 169:1119–1129.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fujisaki J, Wu J, Carlson AL, Silberstein L, Putheti P, Larocca R, Gao W, Saito TI, Lo Celso C, Tsuyuzaki H, et al. : In vivo imaging of T reg cells providing immune privilege to the haematopoietic stem-cell niche. Nature 2011, 474:216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Du H, Shih C-H, Wosczyna MN, Mueller AA, Cho J, Aggarwal A, Rando TA, Feldman BJ: Macrophage-released ADAMTS1 promotes muscle stem cell activation. Nat Commun 2017, 8:669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arnold L, Henry A, Poron F, Baba-Amer Y, Van Rooijen N, Plonquet A, Gherardi RK, Chazaud B: Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med 2007, 204:1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saha S, Aranda E, Hayakawa Y, Bhanja P, Atay S, Brodin NP, Li J, Asfaha S, Liu L, Tailor Y, et al. : Macrophage-derived extracellular vesicle-packaged WNTs rescue intestinal stem cells and enhance survival after radiation injury. Nat Commun 2016, 7:13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Quiros M, Nishio H, Neumann PA, Siuda D, Brazil JC, Azcutia V, Hilgarth R, O’Leary MN, Garcia-Hernandez V, Leoni G, et al. : Macrophage-derived IL-10 mediates mucosal repair by epithelial WISP-1 signaling. J Clin Invest 2017, 127:3510–3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lindemans CA, Calafiore M, Mertelsmann AM, O’Connor MH, Dudakov JA, Jenq RR, Velardi E, Young LF, Smith OM, Lawrence G, et al. : Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature 2015, 528:560–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Monticelli LA, Osborne LC, Noti M, Tran SV, Zaiss DMW, Artis D: IL-33 promotes an innate immune pathway of intestinal tissue protection dependent on amphiregulin-EGFR interactions. Proc Natl Acad Sci U S A 2015, 112:10762–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee JJ, Rothenberg ME, Seeley ES, Zimdahl B, Kawano S, Lu W-J, Shin K, Sakata-Kato T, Chen JK, Diehn M, et al. : Control of inflammation by stromal Hedgehog pathway activation restrains colitis. Proc Natl Acad Sci 2016, 113:E7545–E7553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Degirmenci B, Valenta T, Dimitrieva S, Hausmann G, Basler K: GLI1-expressing mesenchymal cells form the essential Wnt-secreting niche for colon stem cells. Nature 2018, 558:449–453. [DOI] [PubMed] [Google Scholar]
- 92.Kinchen J, Chen HH, Parikh K, Antanaviciute A, Jagielowicz M, Fawkner-Corbett D, Ashley N, Cubitt L, Mellado-Gomez E, Attar M, et al. : Structural Remodeling of the Human Colonic Mesenchyme in Inflammatory Bowel Disease. Cell 2018, 175:372–386.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]; **This study screens remodeling of the colonic mesenchymal cell populations in inflammatory bowel disease in both human patients and murine models. The authors notably identify variations in Wnt-secreting stem cell niche populations.
- 93.Gronke K, Hernández PP, Zimmermann J, Klose CSN, Kofoed-Branzk M, Guendel F, Witkowski M, Tizian C, Amann L, Schumacher F, et al. : Interleukin-22 protects intestinal stem cells against genotoxic stress. Nature 2019, 566:249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD, et al. : Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 2010, 143:134–144. [DOI] [PubMed] [Google Scholar]
- 95.Lopez-Garcia C, Klein AM, Simons BD, Winton DJ: Intestinal Stem Cell Replacement Follows a Pattern of Neutral Drift. Science (80-) 2010, 330:822–825. [DOI] [PubMed] [Google Scholar]
- 96.Ritsma L, Ellenbroek SIJ, Zomer A, Snippert HJ, de Sauvage FJ, Simons BD, Clevers H, van Rheenen J: Intestinal crypt homeostasis revealed at single-stem-cell level by in vivo live imaging. Nature 2014, 507:362–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tierney MT, Sacco A: Satellite Cell Heterogeneity in Skeletal Muscle Homeostasis. Trends Cell Biol 2016, 26:434–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang H, Adam RC, Ge Y, Hua ZL, Fuchs E: Epithelial-Mesenchymal Micro-niches Govern Stem Cell Lineage Choices. Cell 2017, 169:483–496.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rompolas P, Deschene ER, Zito G, Gonzalez DG, Saotome I, Haberman AM, Greco V: Live imaging of stem cell and progeny behaviour in physiological hair-follicle regeneration. Nature 2012, 487:496–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nabhan AN, Brownfield DG, Harbury PB, Krasnow MA, Desai TJ: Single-cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science (80-) 2018, 359:1118–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tierney MT, Stec MJ, Rulands S, Simons BD, Sacco A, Jolla L, Program R, Burnham S, Medical P, Jolla L, et al. : To Tissue Repair and Homeostatic Aging. 2018, 22:119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sangiorgi E, Capecchi MR: Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet 2008, 40:915–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Potten CS, Hume WJ, Reid P, Cairns J: The segregation of DNA in epithelial stem cells. Cell 1978, 15:899–906. [DOI] [PubMed] [Google Scholar]
- 104.Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ: A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 2011, 478:255–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Muñoz J, Stange DE, Schepers AG, van de Wetering M, Koo B-K, Itzkovitz S, Volckmann R, Kung KS, Koster J, Radulescu S, et al. : The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent “+4” cell markers. EMBO J 2012, 31:3079–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Itzkovitz S, Lyubimova A, Blat IC, Maynard M, van Es J, Lees J, Jacks T, Clevers H, van Oudenaarden A: Single-molecule transcript counting of stem-cell markers in the mouse intestine. Nat Cell Biol 2011, 14:106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tao S, Tang D, Morita Y, Sperka T, Omrani O, Lechel A, Sakk V, Kraus J, Kestler HA, Kühl M, et al. : Wnt activity and basal niche position sensitize intestinal stem and progenitor cells to DNA damage. EMBO J 2015, 34:624–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Castillo-Azofeifa D, Fazio EN, Nattiv R, Good HJ, Wald T, Pest MA, de Sauvage FJ, Klein OD, Asfaha S: Atoh1 + secretory progenitors possess renewal capacity independent of Lgr5 + cells during colonic regeneration. EMBO J 2019, 38:e99984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tetteh PW, Basak O, Farin HF, Wiebrands K, Kretzschmar K, Begthel H, van den Born M, Korving J, de Sauvage F, van Es JH, et al. : Replacement of Lost Lgr5-Positive Stem Cells through Plasticity of Their Enterocyte-Lineage Daughters. Cell Stem Cell 2016, 18:203–213. [DOI] [PubMed] [Google Scholar]
- 110.Jadhav U, Saxena M, O’Neill NK, Saadatpour A, Yuan GC, Herbert Z, Murata K, Shivdasani RA: Dynamic Reorganization of Chromatin Accessibility Signatures during Dedifferentiation of Secretory Precursors into Lgr5+ Intestinal Stem Cells. Cell Stem Cell 2016, 21:65–77.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Metcalfe C, Kljavin NM, Ybarra R, De Sauvage FJ: Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell 2014, 14:149–159. [DOI] [PubMed] [Google Scholar]
- 112.van Es JH, Sato T, van de Wetering M, Lyubimova A, Yee Nee AN, Gregorieff A, Sasaki N, Zeinstra L, van den Born M, Korving J, et al. : Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat Cell Biol 2012, 14:1099–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yu S, Tong K, Zhao Y, Balasubramanian I, Yap GS, Ferraris RP, Bonder EM, Verzi MP, Gao N: Paneth Cell Multipotency Induced by Notch Activation following Injury. Cell Stem Cell 2018, 23:46–59.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jones JC, Brindley CD, Elder NH, Myers MG, Rajala MW, Dekaney CM, McNamee EN, Frey MR, Shroyer NF, Dempsey PJ: Cellular Plasticity of Defa4 Cre -Expressing Paneth Cells in Response to Notch Activation and Intestinal Injury. CMGH 2019, 7:533–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nusse YM, Savage AK, Marangoni P, Rosendahl-Huber AKM, Landman TA, De Sauvage FJ, Locksley RM, Klein OD: Parasitic helminths induce fetal-like reversion in the intestinal stem cell niche. Nature 2018, 559:109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nusse YM, Savage AK, Marangoni P, Rosendahl-Huber AKM, Landman TA, De Sauvage FJ, Locksley RM, Klein OD: Parasitic helminths induce fetal-like reversion in the intestinal stem cell niche. Nature 2018, 559:109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hoeck JD, Biehs B, Kurtova AV., Kljavin NM, De Sousa E Melo F, Alicke B, Koeppen H, Modrusan Z, Piskol R, De Sauvage FJ: Stem cell plasticity enables hair regeneration following Lgr5 + cell loss. Nat Cell Biol 2017, doi: 10.1038/ncb3535. [DOI] [PubMed] [Google Scholar]
- 118.Hwang AB, Brack AS: Muscle Stem Cells and Aging. Elsevier Inc.; 2018. [DOI] [PubMed] [Google Scholar]
- 119.Zhou J, So KK, Li Y, Li Y, Yuan J, Ding Y, Chen F, Huang Y, Liu J, Lee W, et al. : Elevated H3K27ac in aged skeletal muscle leads to increase in extracellular matrix and fibrogenic conversion of muscle satellite cells. Aging Cell 2019, doi: 10.1111/acel.12996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Collins B, Arpke R, Larson A, Baumann C, Cabelka C, Nash N, Juppi H, Laakkonen E, Sipila S, Kovanen V, et al. : Estrogen Regulates the Satellite Cell Compartment in Females. SSRN Electron J 2018, 28:368–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nalapareddy K, Nattamai KJ, Kumar RS, Karns R, Wikenheiser-Brokamp KA, Sampson LL, Mahe MM, Sundaram N, Yacyshyn MB, Yacyshyn B, et al. : Canonical Wnt Signaling Ameliorates Aging of Intestinal Stem Cells. Cell Rep 2017, 18:2608–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Huels DJ, Bruens L, Hodder MC, Cammareri P, Campbell AD, Ridgway RA, Gay DM, Solar-Abboud M, Faller WJ, Nixon C, et al. : Wnt ligands influence tumour initiation by controlling the number of intestinal stem cells. Nat Commun 2018, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Snippert HJ, Schepers AG, Van Es JH, Simons BD, Clevers H: Biased competition between Lgr5 intestinal stem cells driven by oncogenic mutation induces clonal expansion. EMBO Rep 2014, 15:62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lenos KJ, Miedema DM, Lodestijn SC, Nijman LE, van den Bosch T, Romero Ros X, Lourenço FC, Lecca MC, van der Heijden M, van Neerven SM, et al. : Stem cell functionality is microenvironmentally defined during tumour expansion and therapy response in colon cancer. Nat Cell Biol 2018, 20:1193–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Batlle E, Clevers H: Cancer stem cells revisited. Nat Med 2017, 23:1124–1134. [DOI] [PubMed] [Google Scholar]
- 126.de Sousa e Melo F, Kurtova AV, Harnoss JM, Kljavin N, Hoeck JD, Hung J, Anderson JE, Storm EE, Modrusan Z, Koeppen H, et al. : A distinct role for Lgr5+ stem cells in primary and metastatic colon cancer. Nature 2017, 543:676–680. [DOI] [PubMed] [Google Scholar]
- 127.Shimokawa M, Ohta Y, Nishikori S, Matano M, Takano A, Fujii M, Date S, Sugimoto S, Kanai T, Sato T: Visualization and targeting of LGR5+ human colon cancer stem cells. Nature 2017, 545:187–192. [DOI] [PubMed] [Google Scholar]
- 128.Drost J, van Jaarsveld RH, Ponsioen B, Zimberlin C, van Boxtel R, Buijs A, Sachs N, Overmeer RM, Offerhaus GJ, Begthel H, et al. : Sequential cancer mutations in cultured human intestinal stem cells. Nature 2015, 521:43–47. [DOI] [PubMed] [Google Scholar]
- 129.Fujii M, Shimokawa M, Date S, Takano A, Matano M, Nanki K, Ohta Y, Toshimitsu K, Nakazato Y, Kawasaki K, et al. : A Colorectal Tumor Organoid Library Demonstrates Progressive Loss of Niche Factor Requirements during Tumorigenesis. Cell Stem Cell 2016, 18:827–838. [DOI] [PubMed] [Google Scholar]
- 130.Greicius G, Kabiri Z, Sigmundsson K, Liang C, Bunte R, Singh MK, Virshup DM: PDGFRα + pericryptal stromal cells are the critical source of Wnts and RSPO3 for murine intestinal stem cells in vivo. Proc Natl Acad Sci 2018, 115:E3173–E3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kinchen J, Chen HH, Parikh K, Antanaviciute A, Jagielowicz M, Fawkner-Corbett D, Ashley N, Cubitt L, Mellado-Gomez E, Attar M, et al. : Structural Remodeling of the Human Colonic Mesenchyme in Inflammatory Bowel Disease. Cell 2018, 175:372–386.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tikhonova AN, Dolgalev I, Hu H, Sivaraj KK, Hoxha E, Cuesta-Domínguez Á, Pinho S, Akhmetzyanova I, Gao J, Witkowski M, et al. : The bone marrow microenvironment at single-cell resolution. Nature 2019, 569:222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Baryawno N, Przybylski D, Kowalczyk MS, Kfoury Y, Severe N, Gustafsson K, Kokkaliaris KD, Mercier F, Tabaka M, Hofree M, et al. : A Cellular Taxonomy of the Bone Marrow Stroma in Homeostasis and Leukemia. Cell 2019, 177:1915–1932.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zepp JA, Zacharias WJ, Frank DB, Cavanaugh CA, Zhou S, Morley MP, Morrisey EE: Distinct Mesenchymal Lineages and Niches Promote Epithelial Self-Renewal and Myofibrogenesis in the Lung. Cell 2017, 170:1134–1148.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee JH, Tammela T, Hofree M, Choi J, Marjanovic ND, Han S, Canner D, Wu K, Paschini M, Bhang DH, et al. : Anatomically and Functionally Distinct Lung Mesenchymal Populations Marked by Lgr5 and Lgr6. Cell 2017, 170:1149–1163.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, et al. : Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 2011, 470:105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kretzschmar K, Clevers H: Organoids: Modeling Development and the Stem Cell Niche in a Dish. Dev Cell 2016, 38:590–600. [DOI] [PubMed] [Google Scholar]
- 138.Dumont NA, Wang YX, Rudnicki MA: Intrinsic and extrinsic mechanisms regulating satellite cell function. Development 2015, doi: 10.1242/dev.114223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yin H, Price F, Rudnicki MA: Satellite Cells and the Muscle Stem Cell Niche. Physiol Rev 2013, doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rayagiri SS, Ranaldi D, Raven A, Mohamad Azhar NIF, Lefebvre O, Zammit PS, Borycki AG: Basal lamina remodeling at the skeletal muscle stem cell niche mediates stem cell self-renewal. Nat Commun 2018, 9:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Verma M, Asakura Y, Murakonda BSR, Pengo T, Latroche C, Chazaud B, McLoon LK, Asakura A: Muscle Satellite Cell Cross-Talk with a Vascular Niche Maintains Quiescence via VEGF and Notch Signaling. Cell Stem Cell 2018, 23:530–543.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]


