Abstract
Background:
Research to date has largely conceptualized irritability in terms of intra-individual differences. However, the role of inter-personal dyadic processes has received little consideration. Nevertheless, difficulties in how parent-child dyads synchronize during interactions may be an important correlate of irritably in early childhood. Innovations in developmentally-sensitive neuroimaging methods now enable the use of measures of neural synchrony to quantify synchronous responses in parent-child dyads and can help clarify the neural underpinnings of these difficulties. We introduce the Disruptive Behavior Diagnostic Observation Schedule: Biological Synchrony (DB-DOS:BioSync) as a paradigm for exploring parent-child neural synchrony as a potential biological mechanism for interpersonal difficulties in preschool psychopathology.
Methods:
Using functional near-infrared spectroscopy (fNIRS) 4–5-year-olds (N = 116) and their mothers completed the DB-DOS:BioSync while assessing neural synchrony during mild frustration and recovery. Child irritability was measured using a latent irritability factor that was calculated from four developmentally-sensitive indicators.
Results:
Both the mild frustration and the recovery contexts resulted in neural synchrony. However, less neural synchrony during the recovery context only was associated with more child irritability.
Conclusion:
Our results suggest that recovering after a frustrating period might be particularly challenging for children high in irritability and offer support for the use of the DB-DOS:BioSync task to elucidate interpersonal neural mechanisms of developmental psychopathology.
Keywords: Neural Sycnhrony, irritability, PFC, parent-child synchrony, recovery
Introduction
Irritability is defined as the tendency to experience dysregulated mood and temper outbursts when a goal is blocked (Wakschlag et al., 2015; Brotman et al., 2017). While irritability is common, high levels of irritability in early childhood are considered a transdiagnostic marker of psychopathology (e.g., Dougherty et al., 2013; Pagliaccio et al., 2018; Stringaris et al., 2009; Vidal-Ribas et al., 2016; Wakschlag et al., 2018). One mechanism for how early irritability influences maladaptive behavioral patterns is through disrupted recruitment of the brain networks associated with emotion regulation. Difficulties with the regulation of frustration in irritability have been linked with variability in the function of regions like the prefrontal cortex (PFC), anterior cingulate cortex (ACC), ventral striatum, anterior insula, and the amygdala (Deveney et al., 2013; Perlman et al., 2015; Roy et al., 2018). Among these regions, the lateral PFC appears to be a particularly important component of this network (Grabell et al., 2018; Leibenluft, 2017). Studies with preschool-aged children have shown that high but non-clinical levels of irritability are associated with increased activation of the lateral PFC (e.g., Fishburn et al., 2019a; Perlman et al., 2014). This has been hypothesized to serve as a compensatory mechanism for the regulation of their frustration, allowing these children to effectively regulate their emotions even when experiencing high levels of frustration. Interestingly, this association seems to flip at the clinical level suggesting that the association between PFC activation and irritability can be better described using an inverted U shape (Grabell et al., 2018). What remains a question, however, is how child irritability might shape the interaction of children with their parents from both a behavioral and neural perspective.
During the first few years of life, effective regulation of frustration transitions from externally to internally mediated largely via parent-child interactions (Kochanska, Coy, & Murray, 2001; Morris et al., 2017). Although dyadic processes have received little attention in irritability, this is a priority area for elucidating mechanisms that shape the likelihood that irritability will result in psychopathology (Wakschlag et al., 2018). There is substantial research demonstrating the bi-directional influence of young children’s negative emotionality and parenting (e.g., Kiff et al., 2011). There is also evidence that children’s irritability has an aversive influence on the way parents interact with their children (Crockenberg & McCluskey, 1986; Lengua, 2006), and that less responsive parenting is associated with an increased likelihood that negative emotionality escalates to psychopathology (Wakschlag & Hans, 2002). These studies, however, have largely examined dyadic processes as statistical interaction effects, rather than during real time interactions.
High dyadic behavioral synchrony, defined as contingent social responding through mutually responsive and co-regulated interactions has been linked with better self-control, greater communicative competence, and fewer behavioral problems both concurrently and longitudinally (Feldman, Greenbaum, & Yirmiya, 1999; Harrist & Waught 2002; Im-Bolter, Anam, & Cohen, 2015; Kochanska et al., 2008; Lindsey et al., 2009). Parent-child behavioral synchrony has been found as early as in infancy (Feldman, Greenbaum, Yirmiya, 1999; Ham & Tronick, 2009) and remains a useful index of adaptive social interactions throughout the lifespan (e.g., Helm, Sbarra, & Ferrer, 2013). Given the role of high parent-child behavioral synchrony in healthy development, low parent-child synchrony might serve as a risk factor for later psychopathology. Indeed, one study found that 6–10-year-old children with clinical levels of behavioral problems had significantly lower behavioral synchrony during play compared to a non-clinical group (Im-Bolter, Anam, & Cohen, 2015), suggesting a negative association between parent-child behavioral synchrony and clinical levels of behavioral problems. Another study found that more behavioral synchrony during the discussion of family conflicts in 10-year-olds was associated with less antisocial behavior even when controlling for antisocial behavior at age 8 (Criss, Shaw, & Ingoldsby, 2003), suggesting that being able to maintain reciprocal interactions during taxing or potentially frustrating/negative situations is associated with better outcomes. While most studies suggest that behavioral synchrony in parent-child dyads is generally adaptive, frequent contingent responding of negative emotion and verbal exchanges will likely result in negative child outcomes. In earlier ages, research on mother-infant dyads suggests that returning to synchrony during periods of recovery after a stressful interaction is a particularly important indicator of adaptive parent-child interactions (Ham & Tronick, 2009). As difficulties with the regulation of frustration are a defining feature of irritability (Perlman et al., 2014), it follows that dyads in which a child is high in irritability might have difficulty achieving dyadic synchrony, further exacerbating clinical risk. Moreover, because irritability has been associated with sustained negative mood (Brotman et al., 2017) it is likely that irritable children will require continued emotion regulation support to recover from frustrating events. In fact, previous work has shown recovery phases to be particularly relevant for the regulation of anger in healthy children (Kahle et al., 2016; Miller et al., 2013). Thus, it is possible that in the context of irritability, sustained synchrony might be particularly crucial in periods of recovery post-frustration as these children are likely to take longer to recover from their negative mood.
Advances in neurodevelopmental science now allow for the examination of dyadic synchrony at both neurobiological and behavioral levels (Feldman, 2012). In a study with infants, increases in mother-child behavioral synchrony, evidenced by increases in affective and vocal matching, were reflected in an increase in the coordination of heart rhythms between the mothers and their infants (Feldman et al., 2011) offering important evidence of the links between biological and behavioral synchrony. In another study with preschoolers and their mothers, child and parent cardiac autonomic reactivity during collaborative drawing was linked to greater behavioral synchrony and better child self-regulation (Suveg et al., 2016). Further serving as evidence that increased parent-child behavioral and physiological synchrony are linked with positive outcomes, Lunkenheimer and colleagues (2018) found that decreased concordance of autonomic regulation between preschoolers and their mothers was associated with a higher risk for psychopathological symptoms.
Recently, hyperscanning—the concurrent measurement of more than one person’s brain activity (Montague et al., 2002)—has made it possible to study the neural concordance of interacting partners or “neural synchrony” (Cui et al., 2012; Fishburn et al., 2018; Miller et al., 2019; Reindl et al., 2018). This synchronization of brain activity has been hypothesized to facilitate bond formation and shared mental states (Redcay & Schilbach, 2019; Wheatley et al., 2012), and is likely to play an important role in children’s healthy development. A study of 5–9-year-olds found that higher parent-child neural synchrony in the PFC during cooperation was associated with better emotion regulation in both the parent and the child (Reindl et al., 2018). Moreover, higher neural synchrony mediated the link between parent and child emotion regulation, supporting the role of neural synchrony as an underlying biological mechanism for the co-regulation of emotion (Reindl et al., 2018). While the meaning of synchrony may be different across systems (e.g. physiological synchrony may be a better index of synchronous arousal while neural synchrony may be a better measure of synchronous cognitions), research across biological and behavioral levels offers evidence of the crucial role of a dyads ability to synchronize behaviors, cognitions, and neurophysiology on a child’s healthy development.
If neural synchrony within the lateral PFC is, as we hypothesize, a biological mechanism for the parent-child co-regulation of emotion, deficits in neural synchrony may increase the likelihood of clinically salient psychopathology symptoms later in life for children who are high in irritability. We propose that variations in neural synchrony may be a biological marker of disruptions in the parent-child relationship that could explain the increased risk of psychopathology in irritable children. The goal of this study was to introduce a novel paradigm, the Disruptive Behavior Diagnostic Observation Schedule-Biological Synchrony (DB-DOS:BioSync) that melds developmentally-sensitive behavioral and physiological methods specifically designed to sharpen characterization and elucidate mechanisms during this age period. We validate the DB-DOSBioSync for use with preschoolers, its utility in relation to behavioral measures, and examine whether patterns of synchrony varied based on child irritability. We used Functional Near-Infrared Spectroscopy (fNIRS) to assess parent-child neural synchrony during DB-DOS:BioSync and explored associations with irritability in preschoolers using a latent child irritability factor, allowing us to comprehensively assess irritability through temperamental, clinically-relevant, and impairment measures.
Method
Participants
One hundred and fifty-one preschoolers and a caregiver (144 mothers; referred from here on as “mothers”) participated in a study designed to assess variability in preschool irritability and its neural underpinnings (Fishburn et al., 2019a; Quiñones-Camacho et al., 2019). As part of the initial screening procedures, children were excluded from participating in the study if their parents reported having already sought clinical services for the child or if they had any current or past psychiatric diagnosis. Children were also excluded if they had a neurological disorder, a history of loss of consciousness, or sensory impairments, such as epilepsy, cerebral palsy, ASD, or significantl intellectual disability. The study was approved by the Institutional Review Board and all families were consented before participation in the study. Because of the subject-compliance challenges of imaging preschoolers, 117 parent-child dyads had usable fNIRS data for both subjects in both conditions of the DB-DOS:BioSync. Loss of data was due to computer errors, poor contact of the sensors with the scalp, or too much movement in the parent or child. The mean age for the 117 children was 4.86 years (SD=0.60; 54 females). Children were identified as 71% Caucasian, 23% African-American, 3% Asian, and 3% Biracial (96% Non-Hispanic, and 4% Hispanic). Household income varied widely from $0–20,000 (14%), $21,000–60,000 (29%), $61,000–100,000 (25%), $101,000+ (32%).
Disruptive Behavior Diagnostic Observation Schedule: Biological Synchrony (DB-DOS: BioSync)
The DB-DOS (Wakschlag et al., 2008) was developed as a behavioral paradigm designed to elicit variations in children’s regulation of irritable affect and behavior and the dyads ability to co-regulate across contexts with varying demands, as this has proven to be clinically informative (Petitclerc et al., 2015). We modified the DB-DOS to fit task requirements of fNIRS and other biological measures, such as minimization of movement, use of a block design with repeated trials to maximize power of biological signals, and reduction of overall task time to increase preschoolers’ engagement. We refer to this new version as the DB-DOS- Biological Synchrony (DB-DOS:BioSync), which aimed to leverage the efficient elicitation of variations in co-regulation with integration of biological measures. During the first ‘Frustration’ context (10 minutes) dyads were left alone, seated at a table with attractive toys and instructed not to touch them while completing tangram puzzles. These puzzles consist of 7 flat geometric shapes that are combined to form larger shapes (an object or animal). This Frustration context consisted of 4 blocks of solving 5 puzzles within 2 minutes, followed by a 15-second inter-block interval. Dyads are told that they will receive a prize if they complete the task. However, the puzzles were too difficult for the child’s age, time was cut short (they are given 1:45 instead of 2:00 minutes), and the dyads saw a countdown clock indicating how much time they had left.
After the frustration context ended, the experimenter came in and explained the next task to the dyad, during this time the experimenter also took the puzzle blocks away and placed the toys within reach of both members of the dyad, allowing for some time to pass between the two task contexts. Following this, dyads were allowed to play with the attractive toys (10 minutes). The ‘Recovery’ context served as a recovery period during a low demand context. To mirror the ‘Frustration’ context, ‘Recovery’ consisted of 4 blocks of 2 minutes followed by a 15-second inter-block interval. A new toy was added to play after each block.
Behavioral synchrony coding
Parent-child behavioral synchrony, defined as the amount of time the parent-child dyad spent engaged in mutually responsive and co-regulated interactions during each of the contexts, was coded using a scheme developed in-house. Synchrony was defined as reciprocal, coordinated engagement through shared attention, topic, and contingent responding. Exchanges demonstrating synchrony showed reciprocal communication, eye contact, and coordinated behaviors with directed gaze. Every second of the interaction was coded as being either synchronous or asynchronous. These individual measures were then used to calculate a general synchrony score (i.e., the total time spent in synchrony during each context) and were not used as a dichotomous (synchrony/asynchrony) variable in the primary analyses. Before an experimenter gave a code of synchrony, the parent-child dyad had to exchange three verbal or behavioral turns, as reciprocal interactions are necessary to establish synchrony. Synchrony continues to be coded until there is a break in reciprocal exchanges (e.g., more than three seconds passed since the dyad had showed reciprocal responding). The same procedures were used to code for synchrony in the ‘Frustration’ and ‘Recovery’ contexts. Synchrony was coded by six trained research assistants who did not interact with the dyad during the visit and were blind to the irritability scores of children and parent. Training consisted of conceptual grounding and coding for eight master tapes to .80 reliability (kappa) of the master codes. Of the original 151 participants, 127 videos were codable (this missingness was due to problems with the video camera and audio of the interaction). Reliability was coded on 20% of data (K = .807) for all codable videos. Because some children had fNIRS data but not codable videos, the sample for analyses with behavioral coding are smaller. From the 117 dyads with usable fNIRS data included in the main analyses, 98 had data for both behavioral coding and neural synchrony, thus, analyses looking at associations with these two variables have a sample size of 98. For analyses, we summed all seconds spent in synchrony to create a single behavioral synchrony variable for each context.
fNIRS data acquisition and preprocessing
fNIRS data were collected using a continuous-wave NIRScout fNIRS system (NIRx Medical Technologies LLC, Glen Head, NY). The light was emitted at 760 nm, and 850 nm from a total of 8 LED light sources and measured from 4 photodiode light detectors, yielding ten measurement channels per wavelength. The optical signals were collected at 15.625 Hz. Sensors were placed on a neoprene head cap, with a source-detector distance of 2.9–3.1 cm. For each participant, the fNIRS head cap was positioned according to the international 10–20 coordinate system with the dorsomedial sources over AF3/AF4, and the ventromedial sources over Fp1/Fp2. Hair was manually parted under the optodes to improve signal detection. The probe extended over middle frontal gyrus (MFG) and inferior frontal gyrus (IFG) of each hemisphere of the PFC and were registered to the Colin27 Brain Atlas (Holmes et al., 1998).
Preprocessing and activation analyses were carried out using NIRS Brain AnalyzIR toolbox (Santosa, Zhai, Fishburn, & Huppert, 2018). First, the fNIRS raw intensity signals were converted to changes in optical density. Optical density signals were then corrected for motion artifacts using the temporal derivative distribution repair (TDDR) method (Fishburn, Ludlum, Vaidya, & Medvedev, 2019). Corrected optical density signals were then resampled to 4 Hz to reduce the computational overhead of the synchrony calculations. Slow drifts were removed from the signals using a high-pass Butterworth filter with a cutoff of 0.01 Hz and filter order of 4. Signals were then converted to oxygenated hemoglobin concentration using the modified Beer-Lambert law.
Quantification of neural synchrony
In this section, we describe the procedures to calculate parent-child neural synchrony, which we defined as the association between concurrent lateral PFC activation of the parent and the child during the ‘Frustration’ and ‘Recovery’ contexts separately. Before calculating neural synchrony, timings were standardized across all participants. Signals were whitened by removing temporal autocorrelations using an autoregressive model as serial correlations are a common source of noise in fNIRS data that can inflate correlation estimates (Santosa, Aarabi, Perlman, & Huppert, 2017). The order of the AR model was chosen using the Bayesian Information Criterion from a minimum value of 1 to a maximum of 32. Previous studies have shown a model order of 20 to be sufficient for whitening signals (Santosa, Aarabi, Perlman, & Huppert, 2017). The robust correlation coefficients were calculated between participants using the robust regression approach (Shevlyakov & Smirnov, 2011), in which the geometric mean is taken of the robust regression coefficients obtained from regressing channel X onto channel Y and vice-versa, e.g., . Synchronization was then quantified using the Fisher r-to-z transform of the absolute value of the robust correlation coefficient. Synchrony was assessed in this way for all possible channel-pairs. Given that we had no hypotheses regarding non-reciprocal connections (e.g., channel A of the child connected with channel B of the parent, but not vice-versa), reciprocal connections were enforced to reduce the number of unique connections and thus prevent multiple comparisons corrections from being overly-conservative. This was done by taking the mean of the z-value, e.g., .
Statistical analysis of neural synchrony
In this section, we describe the procedures to calculate the significance of our neural synchrony findings, we did this via permutation testing with random dyads (e.g., parent of dyad A with child of dyad B) which allowed us to confirm that the synchrony was due to a child actively interacting with their parent during the task rather than being driven simply by two people completing the same task. To determine the appropriate null distribution of synchrony values, synchrony was calculated between all possible subject pairs. For each channel-pair, there were synchrony values for 117 concurrent (observed) parent-child dyads and 27,144 non-concurrent (null) parent-child dyads (). The p-value associated with each observed synchrony value was computed via a permutation test by determining the proportion of values from null-pairings that were equal to or greater than the observed value, e.g. . The constant terms were selected to ensure that the resulting p-values would be between 0 and 1. Adjusted z-values were then derived from the estimated p-values using the inverse cumulative density function for the standard normal distribution. One dyad had adjusted Z-values over 4 SD and were removed from analyses, bringing the sample to 116 pairs. These values were then submitted to a mixed effects model with task condition modeled as a fixed effect and dyad ID modeled as a random effect. The presence of synchrony was assessed for each condition by applying the t-contrast corresponding to a 1-sample t-test. Differences between conditions were assessed with the ‘Frustration – Recovery’ t-contrast. The corresponding p-values were corrected for multiple comparisons by calculating the Benjamini-Hochberg FDR-corrected p-value (Benjamini & Hochberg, 1995) (denoted throughout as ‘q-value’) across all unique channel pairs. The mean of the adjusted z-values was computed across significant (q<.05) channel-pairs for each dyad and extracted for further analyses.
Child irritability
Temperamental irritability.
Caregivers completed the Children’s Behavior Questionnaire (CBQ; Rothbart et al., 2001). The CBQ is a widely used assessment of 15 temperamental dimensions in children 3–7 years-old. Given our interest in exploring links between parent-child neural synchrony and child irritability, we focused on the anger/frustration dimension which has been successfully used to assess temperamental irritability (e.g., Fishburn et al., 2019a; Perlman et al., 2014). Reliability of this subscale was good (α =.81) and scores varied widely from 1.50 – 6.67 (M = 4.296, SD = 1.110).
Dimensional spectrum of irritability.
Caregivers also completed the Temper Loss scale of the Multidimensional Assessment Profile of Disruptive Behavior questionnaire (MAP-DB; Wakschlag et al., 2012). This questionnaire measures the full dimension of normative to clinical levels of irritability and has shown good reliability and validity in previous studies (Wakschlag et al., 2012; Wakschlag et al., 2018). The Temper Loss subscale consists of 22 items that assess variations in quality, intensity, and context of irritable moods and tantrums. The maximum possible score is 110, with scores in our sample ranging from 0–89 (M = 22.121, SD = 15.177). Reliability of the scale in our sample was excellent (α =.96).
Irritability-related impairment.
Parents were interviewed about their children’s irritability by a trained researcher using the Early Childhood Irritability Impairment Interview (E-CRI; Wakschlag et al., under review). This semi-structured interview was designed to assess meaningful variations in impairment associated with irritable mood and tantrums across various contexts (i.e., home, out and about, with peers, siblings, non-parental adults, and school/childcare). The interview has been shown to have good inter-rater, test-retest, and longitudinal reliability (Wakschlag et al., under review). A total of 12 scores were derived from this interview, six tantrum impairment scores (for each of the six social contexts) and six irritable mood impairment scores. During validation, multi-method, multi-trait modeling (MTMM) was used to generate a two-factor model with tantrum-related and mood-related impairment factors with excellent fit (CFIs > .999, RMSEAs = .015−.22) (Wakschlag et al., under review), supporting the creation of independent sum scores of mood and tantrum impairment to be used here (Mood: 0–14, M = 3.888, SD = 2.593; Tantrums: 1–16, M = 4.621, SD = 2.583).
Latent irritability factor.
We used factor analysis to combine the four indicators of irritability—temperamental irritability, dimensional spectrum of irritability, tantrum-related impairment, and irritable mood-related impairment—into a single score. All four variables were significantly correlated (rs > .315, p < .001). A factor analysis using a principal axis factor extraction was conducted. A single factor accounted for most of the variance 61.40%, with an eigenvalue of 2.456. All four indicators had good factor loadings: temperamental irritability .685, dimensional spectrum of irritability.791, tantrum-related impairment .761, and irritable mood-related impairment .548.
Next, we used a Barlett approach to compute factor scores from the factor solution. We chose this approach because of its advantages in producing unbiased estimates of the true factor scores (Hershberger, 2005). Two children had factor scores values that were more than 4 SD above the mean. Their scores were winsorized to the value for 3 SD above the mean (a value of 3.24) to improve the normality of this variable (Wilcox, 2011).
Maternal irritability.
Mothers completed the Affective Reactivity Index (ARI; Stringaris et al., 2012) to assess their own irritability. This short questionnaire consists of seven items, six of which assess irritability severity and one assessing impairment. The mean of the six severity items was used in analyses, as a three-level gradation (0–2) of irritability severity. Analyses including the related construct of neuroticism (NEO-FFI-3; McCrae & Costa, 2010) are included in Appendix S1 in the Supporting Information.
Results
Differences in behavioral synchrony between conditions
Descriptive statistics can be found in Table 1. On average, dyads spent 292.36 seconds (SD=127.73 seconds) in synchrony during Frustration and 306.50 seconds (SD=105.94 seconds) in synchrony during Recovery. A paired-sample t-test revealed no differences in behavioral synchrony between contexts, t(97)=−1.156, p=.251.
Table 1.
Descriptive statistics and correlations among predictors
| Mean | SD | Range | 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|---|---|---|
| 1. Child irritability* | −0.026 | 1.007 | −1.69–3.33 | - | |||||
| 2. Neural Synchrony Frustration | .431 | .482 | −.71–1.56 | −.107 | - | ||||
| 3. Neural Synchrony Recovery | .432 | .471 | −.72–1.96 | -.206 | .039 | - | |||
| 4. Behavioral Synchrony Frustration | 292.36+ | 127.73 | 0–488 | -.349 | .209 | .018 | - | ||
| 5. Behavioral Synchrony Recovery | 306.50+ | 105.94 | 18–517 | -.269 | −.049 | −.094 | .475 | - | |
| 6. Maternal Irritability | .349 | .442 | 0–2 | .340 | −.038 | −.059 | −.113 | −.097 | - |
Note. Bold = p < .05.
Factor scores extracted from the FA (winsorized).
Values correspond to sum of seconds in synchrony.
Differences in neural synchrony between conditions
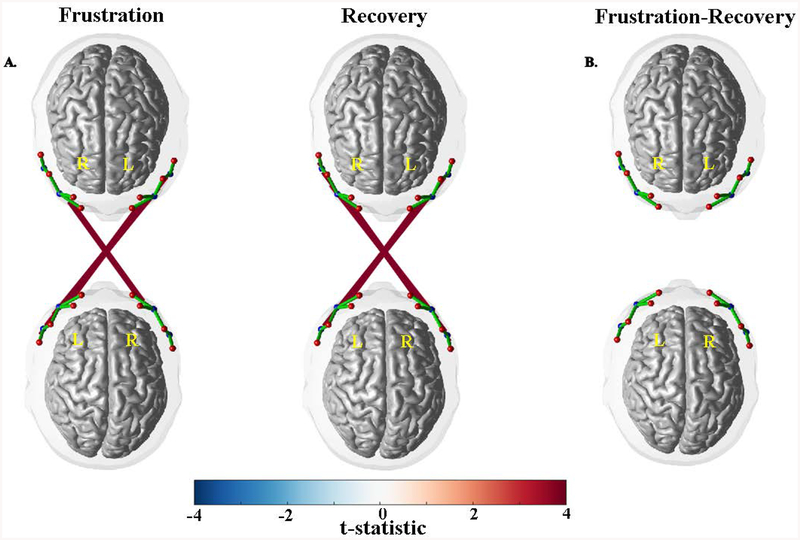
Parent-child inter-subject connectivity for the ‘Frustration’ context was significant; there was significant neural synchrony for 12 channel-pairs (peak connection: t(115)=4.759, q=.0002) compared to the null distribution (Figure 1). For the ‘Recovery’ context, there was significant neural synchrony in 14 channel-pairs (peak connection: t(115)=4.934, q=.0001). There were no differences in neural synchronization between contexts (peak connection: t(115)=2.298, q=.445).
Figure 1.
(A) Mean inter-subject synchronization for the ‘Frustration’ and ‘Recovery’ conditions relative to the null distribution derived from permutation testing. (B) Comparisons of inter-subject synchronization between the two conditions.
Correlations between behavioral and neural synchrony
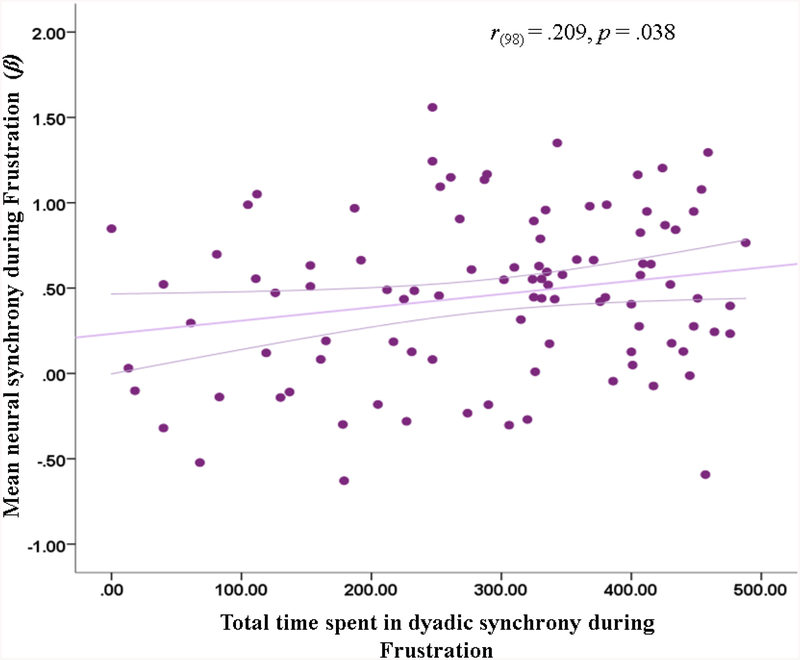
Behavioral and neural synchrony for were significantly associated in the ‘Frustration’ context (r(98)=.209, p=.038 (Figure 2). Having stronger mean levels of synchrony was associated with more behavioral synchrony during the ‘Frustration’ context. Behavioral and neural synchrony were not correlated in the ‘Recovery’ context (, r(98)=−.094, p=.358).
Figure 2.
Correlation between neural and behavioral synchrony during Frustration. Magenta lines represent 95% confidence interval of the prediction line.
Correlations between child and maternal irritability and behavioral synchrony
Parent and child irritability were significantly correlated (r(116)=.344, p<.001) (see Appendix S1 for alternative analyses using maternal neuroticism instead of maternal irritability). Pearson correlations revealed that less dyadic synchrony during both Frustration (r(98)=−.349, p<.001) and Recovery (r(98)=−.269, p=.007) were associated with more child irritability. Maternal irritability was not associated with behavioral synchrony (rs(116)<−.113, ps>.268).
Correlations between child and maternal irritability and neural synchrony
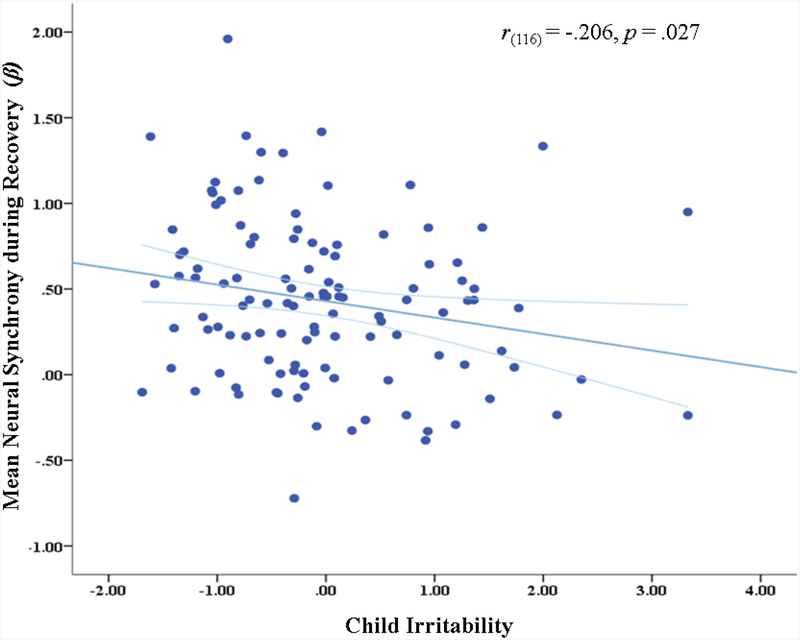
Child irritability was associated with neural synchrony during Recovery, r(116)=−.206, p=.027, such that having a child with high irritability was associated with greater difficulty achieving neural synchrony during the ‘Recovery’ context only (Figure 3). Maternal irritability was not associated with neural synchrony (rs(116)<−.059, ps>.531) and controlling for maternal irritability did not change the nature of the association between neural synchrony and child irritability (r(113)=−.198, p=.034).
Figure 3.
Correlation between child irritability factor scores and mean neural synchrony during Recovery. Light blue lines represent 95% confidence interval of the prediction line.
Lastly, to assess if this link between irritability and neural synchrony during ‘Recovery’ was a methodologic artifact (i.e., if it could be the result of decreases in neural synchrony from Frustration to Recovery), we created a difference score from our neural measures and correlated this with child irritability. This correlation was not significant, r(116)=.069, p=.463, suggesting that child irritability was not associated with a marked decrease in synchrony from the ‘Frustration’ to ‘Recovery’.
Discussion
The current study validates the DB-DOS: BioSync, a paradigm that builds upon a developmentally-sensitive behavioral paradigm by demonstrating the utility of using biological indicators of synchrony to understand parent-child interactions and its implications for child temperament and psychopathology. As expected, higher child irritability was associated with less neural synchrony in the lateral PFC. These findings offer a neural explanation for why some parents with highly irritable children may have difficulties supporting the development of their children’s regulatory skills, namely because of problems establishing the social reciprocity that would enable co-regulation. Our finding that children with higher levels of irritability had difficulties achieving synchrony both at the behavioral and neural level offers novel evidence of a neurobiological pathway by which difficulties in the co-regulation of frustration contribute to impaired development of self-regulation and increased risk for psychopathology.
Our finding that neural synchrony during recovery related to irritability is consistent with previous work that has shown recovery phases to be particularly relevant for the regulation of anger in children (Kahle et al., 2016). Moreover, it builds on work by Tronick and colleagues (Ham & Tronick, 2009; Tronick, 2007) by showing that recovery periods may be more important for predicting positive outcome than the general amount of time spent in synchrony. Relative reductions in parent-child dyadic synchrony during recovery may suggest that children with higher levels of irritability have difficulty recovering from frustration extending previous work on synchrony during recovery periods. Additionally, our findings suggest that this might be primarily driven by child and not parent characteristics. It also extends work on frustration and irritability by exploring periods of post-frustration (i.e., recovery) in addition to periods of frustration, something that is often overlooked in irritability work. Moreover, the absence of a relationship between maternal irritability and parent-child synchrony could be taken as evidence that child factors are particularly strong drivers of dyadic synchrony. It is also possible, however, that the lack of findings were due to the relatively low levels of irritability reported by the mothers in our sample (although findings examining the related construct of neuroticism suggest this might not be the case; For results with neuroticism (NEO; McCrae & Costa, 2010) see Appendix S1).
Another explanation for our finding that neural synchrony during recovery only relates to irritability could be differences in task demands. While the recovery context was a low demand period of unstructured play, the frustration context was a structured goal-oriented task. It is possible that the structured nature of the frustration context might have constrained the types of interactions that occurred during this context, potentially obscuring differences in the way parents of more irritable subjects interacted with their children. Although our main findings were with the recovery period, both contexts resulted in significant neural synchrony and did not differ in the mean level of synchrony elicited, suggesting that our findings were not due to differences in synchrony between contexts. This lack of differences in level of neural synchrony, however, are not particularly surprising as neural synchrony is thought to emerge from shared mental states and is considered a mechanism for the facilitation of bond formation (Redcay & Schilbach, 2019; Wheatley et al., 2012), which were both important aspects of our frustration and recovery contexts.
An unexpected but important difference between these two contexts, however, pertains to the lack of correlations between behavioral and neural synchrony for Recovery. It is possible that our measure of behavioral synchrony was associated with neural synchrony during the Frustration task because it better captured the processes that would elicit synchrony during a goal-oriented task (e.g., actively working together towards a clear shared goal), but it did not completely capture the processes driving neural synchrony during our measure of recovery (i.e., a context without a clear goal). Though follow-up is needed, our study serves as evidence of the utility of neural synchrony for understanding biological risk for child psychopathology beyond what can be captured from behavioral synchrony alone. Moreover, our methodological decision to include a recovery context of play as well as a more structured but potentially frustrating context is a notable contribution to research on parent-child neural synchrony. While studies on parent-child neural synchrony have primarily used computer-based tasks (Millet et al., 2018; Reindl et al., 2018), we used two more ecologically valid contexts, allowing us to better capture the types of parent-child interactions that are likely to occur outside the laboratory.
Our study presents the DB-DOS: BioSync as a promising method for the assessment of neural synchrony in parent-child dyads with substantial implications for our understanding of early psychopathology. This task could be used as a potential outcome measure for studies examining biological mechanisms for treatment efficacy (e.g. Parent Child Interaction Therapy or PCIT). It also holds promise as a platform for yoked assessment using other imaging modalities and other physiological indicators of synchrony (e.g., shared arousal using autonomic measures), which would allow for a greater understanding of the biological processes underlying these interactions. Indeed, we are currently testing the utility of this paradigm using EEG methods in parent-infant dyads. Thus it provides a potentially robust biology:behavior linkage in the quest to elucidate mechanisms by which some irritable young children escalate to psychopathology while others develop adaptively over time. Specifically, using measures of parent-child neural synchrony could help clarify how difficulties in the co-regulation of frustration (as evidence by decreased neural synchrony between parent and child) potentiate risk for later psychopathology.
While our study has notable strengths, some limitations should be noted. First, while studies using dimensional approaches to irritability in community samples are important for clarifying trajectories towards psychopathology, few children in our study had clinical levels of irritability, however, temperament was quite variable. Additionally, while the use of a latent irritability construct that comprehensively captured the different expressions of irritability across contexts (which we could only do by capitalizing on parent’s knowledge of their child’s behavior across contexts) in a developmentally-sensitive manner is a notable strength of the study, future studies should aim to include non-parent-reported measures of irritability such as behavioral observations. Another limitation pertains to the lack of longitudinal data. Given evidence that how parents interact with their irritable children changes post-infancy (Crockenberg & McCluskey, 1986), it will be important to explore how neural synchrony matures across development beginning in infancy. We also acknowledge the limitations of our study design to fully disentangle the role of the structure and demands of each of the context on neural synchrony, future studies should more carefully consider the role of context on measures of neural synchrony to better parcel out what about the context is driving these associations. Moreover, our study focused on synchrony during interactions that were either positive or mildly frustrating, however, it is possible that a deeper exploration of synchrony during very negative interactions would also reveal important information about the parent-child relationship with vital implications for child psychopathology. When thinking about this in the context of neural synchrony, it is possible that high neural synchrony during very negative interactions would have a negative impact on child psychopathology, but this should be carefully tested. Relatedly, given that adaptive interactions are not always synchronous (Tronick, 2007), there are other types of adaptive behaviors that could have happened during the interaction that were not synchronous in nature. Lastly, because fNIRS only allows for the measurement of cortical regions, we were limited in how much we could probe the entirety of the emotion regulation network. Future work, however, should aim to complement our findings with measures of network connectivity and better spatially defined functional neuroimaging in children, in order to assess how parent-child synchrony might shape this network.
The DB-DOS:BioSync advances neurodevelopmental frameworks such as RDoC that aim to integrate brain:behavior mechanisms towards prevention at the earliest phase of the clinical sequence, but are underdeveloped in terms of neurodevelopmental operationalization and accounting for the role that the environment plays in shaping these pathways (Mittal & Wakschlag, 2017). Continued efforts along these lines are crucial to fully realize the promise of this approach for neuroscience-based prevention of mental disorders.
Supplementary Material
Appendix S1. Associations between maternal neuroticism (a closely related construct to irritability), child irritability, behavioral synchrony, and neural synchrony.
Key points.
There is some evidence that child irritability is associated with difficulties with the regulation of frustration and with less positive parenting. However, we know little about the biological mechanisms underlying this.
This study aimed to explore neural synchrony as a putative biological mechanism for co-regulation in the context of irritability in the preschool years
Neural synchrony was measured during a mildly frustrating goal-oriented context and an unstructured recovery play period. A latent irritability factor was calculated from four parent-report measures of child irritability.
Results showed that neural synchrony during play but not during a goal-oriented task was associated with child irritability.
Our study contributes new insight to our understanding of the biological underpinnings of difficulties in parent-child co-regulation of emotion in preschool irritability.
Acknowledgements
This work was supported by the National Institutes of Health grant R01 MH107540, PI: S.B.P.. L.E.Q-C. was supported by the National Institute of Mental Health training grant (NIMH T32 MH100019-06; PIs: Barch & Luby). M.C.C. was supported by the National Science Foundation Graduate Research Fellowship under grant No. 174745. The authors would like to thank Lauren Hampton for her contribution in the development of the synchrony coding scheme and Erica Anderson for her contributions in the development the DB-DOS- Biological Synchrony task. The authors would also like to thank Lisa M. Bemis and the undergraduate research assistants of the Laboratory for Child Brain Development for their help in data collection and management. Lastly, the authors woulf like to thank the children and families who participated in the study. The authors have declared that they have no competing or potential conflicts of interest.
Footnotes
Conflict of interest statement: No conflicts declared.
Supporting information
Additional supporting information may be found online in the Supporting Information section at the end of the article:
References
- Benjamini Y, & Hochberg Y (1995). Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Methodological), 57(1), 289–300. [Google Scholar]
- Brotman MA, Kircanski K, Stringaris A, Pine DS, & Leibenluft E (2017). Irritability in youths: A translational model. American Journal of Psychiatry, 174(6), 520–532. 10.1176/appi.ajp.2016.16070839. [DOI] [PubMed] [Google Scholar]
- Criss m. M., Shaw DS & Ingoldsby EM (2003). Mother-son positive synchrony in middle childhood: Relation to antisocial behavior. Social Development, 12(3), 379–400. 10.1111/1467-9507.00239. [DOI] [Google Scholar]
- Crockenberg S, & McCluskey K (1986). Change in maternal behavior during the baby’s first year of life. Child Development, 57(3), 746–753. [Google Scholar]
- Cui X, Bryant DM, & Reiss AL (2012). NIRS-based hyperscanning reveals increased interpersonal coherence in superior frontal cortex during cooperation. Neuroimage, 59(3), 2430–2437. 10.1016/j.neuroimage.2011.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deveney CM, Connolly ME, Haring CT, Bones BL, Reynolds RC, Kim P, … & Leibenluft E (2013). Neural mechanisms of frustration in chronically irritable children. American Journal of Psychiatry, 170(10), 1186–1194. 10.1176/appi.ajp.2013.12070917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty LR, Smith VC, Bufferd SJ, Stringaris A, Leibenluft E, Carlson GA, & Klein DN (2013). Preschool irritability: longitudinal associations with psychiatric disorders at age 6 and parental psychopathology. Journal of the American Academy of Child & Adolescent Psychiatry, 52(12), 1304–1313. 10.1016/j.jaac.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R (2012). Parent-infant synchrony: a biobehavioral model of mutual influences in the formation of affiliative bonds. Monographs of the Society for Research in Child Development, 77, 42–51. 10.1111/j.1540-5834.2011.00660.x [DOI] [Google Scholar]
- Feldman R, Greenbaum CW, & Yirmiya N (1999). Mother–infant affect synchrony as an antecedent of the emergence of self-control. Developmental Psychology, 35(1), 223–231. https://psycnet.apa.org/doi/10.1037/0012-1649.35.1.223 [DOI] [PubMed] [Google Scholar]
- Feldman R, Magori-Cohen R, Galili G, Singer M, & Louzoun Y (2011). Mother and infant coordinate heart rhythms through episodes of interaction synchrony. Infant Behavior and Development, 34(4), 569–577. 10.1016/j.infbeh.2011.06.008 [DOI] [PubMed] [Google Scholar]
- Fishburn FA, Hlutkowsky CO, Bemis LM, Huppert TJ, Wakschlag LS, & Perlman SB, (2019a). Irritability uniquely predicts prefrontal cortex activation during preschool inhibitory control among all temperament domains: A LASSO approach. Neuroimage 184, 68–77. 10.1016/j.neuroimage.2018.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishburn FA, Ludlum RS, Vaidya CJ, & Medvedev AV (2019b). Temporal Derivative Distribution Repair (TDDR): A motion correction method for fNIRS. Neuroimage, 184, 171–179. 10.1016/j.neuroimage.2018.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishburn FA, Murty VP, Hlutkowsky CO, MacGillivray CE, Bemis LM, Murphy ME, … Perlman SB (2018). Putting our heads together: interpersonal neural synchronization as a biological mechanism for shared intentionality. Social Cognitive and Affective Neuroscience, 13(8), 841–849. 10.1093/scan/nsy060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabell AS, Li Y, Barker JW, Wakschlag LS, Huppert TJ, & Perlman SB (2018). Evidence of non-linear associations between frustration-related prefrontal cortex activation and the normal: abnormal spectrum of irritability in young children. Journal of Abnormal Child Psychology, 46(1), 137–147. 10.1007/s10802-017-0286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham J, & Tronick E (2009). Relational psychophysiology: Lessons from mother–infant physiology research on dyadically expanded states of consciousness. Psychotherapy Research, 19(6), 619–632. 10.1080/10503300802609672. [DOI] [PubMed] [Google Scholar]
- Harrist AW, & Waugh RM (2002). Dyadic synchrony: Its structure and function in children’s development. Developmental Review, 22(4), 555–592. 10.1016/S0273-2297(02)00500-2. [DOI] [Google Scholar]
- Helm JL, Sbarra DA, & Ferrer E (2014). Coregulation of respiratory sinus arrhythmia in adult romantic partners. Emotion, 14(3), 522–531. https://psycnet.apa.org/doi/10.1037/a0035960. [DOI] [PubMed] [Google Scholar]
- Hershberger SL (2005). Factor scores In Everitt BS and Howell DC (Eds.) Encyclopedia of Statistics in Behavioral Science. (pp. 636–644). New York: John Wiley. [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, & Evans AC (1998): Enhancement of MR images using registration for signal averaging. Journal of Computer Assisted Tomography, 22(2), 324–333. [DOI] [PubMed] [Google Scholar]
- Im-Bolter N, Anam M, & Cohen NJ (2015). Mother–child synchrony and child problem behavior. Journal of Child and Family Studies, 24(7), 1876–1885. 10.1007/s10826-014-9989-1. [DOI] [Google Scholar]
- Kahle S, Miller JG, Lopez M, & Hastings PD (2016). Sympathetic recovery from anger is associated with emotion regulation. Journal of Experimental Child Psychology, 142, 359–371. 10.1016/j.jecp.2015.10.004. [DOI] [PubMed] [Google Scholar]
- Kiff CJ, Lengua LJ, Zalewski M (2011). Nature and nurturing: Parenting in the context of child temperament. Clinical Child and Family Psychology Review, 14(3), 251–301. 10.1007/s10567-011-0093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanska G, Aksan N, Prisco TR, & Adams EE (2008). Mother-child and father-child mutually responsive orientation in the first 2 years and children’s outcomes at preschool age: Mechanisms of influence. Child Development, 79, 30–44. doi: 10.1111/j.1467-8624.2007.01109.x. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Coy K, Murray K (2001). The development of self-regulation in the first four years of life. Child Development, 72(4),1091–1111. 10.1111/1467-8624.00336. [DOI] [PubMed] [Google Scholar]
- Leibenluft E (2017). Pediatric irritability: A systems neuroscience approach. Trends in Cognitive Sciences, 21(4), 277–289. 10.1016/j.tics.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengua LJ (2006). Growth in temperament and parenting as predictors of adjustment during children’s transition to adolescence. Developmental Psychology, 42(5), 819 https://dx.doi.org/10.1037%2F0012-1649.42.5.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey EW, Cremeens PR, Colwell MJ, & Caldera YM (2009). The structure of parent–child dyadic synchrony in toddlerhood and children’s communication competence and self-control. Social Development, 18(2), 375–396. 10.1111/j.1467-9507.2008.00489.x. [DOI] [Google Scholar]
- Lunkenheimer E, Tiberio SS, Skoranski AM, Buss KA, & Cole PM (2018). Parent-child coregulation of parasympathetic processes varies by social context and risk for psychopathology. Psychophysiology, 55(2), e12985 10.1111/psyp.12985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae RR, & Costa PT (2010). NEO Inventories for the NEO Personality Inventory-3 (NEO-PI-3), NEO Five-Factor Inventory-3 (NEO-FFI-3), NEO Personality Inventory-Revised (NEO PI-R): Professional Manual. Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Miller JG, Chocol C, Nuselovici JN, Utendale WT, Simard M, & Hastings PD (2013). Children’s dynamic RSA change during anger and its relations with parenting, temperament, and control of aggression. Biological Psychology, 92(2), 417–425. 10.1016/j.biopsycho.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JG, Vrtička P, Cui X, Shrestha S, Hosseini SH, Baker JM, & Reiss AL (2019). Inter-brain synchrony in mother-child dyads during cooperation: An fNIRS hyperscanning study. Neuropsychologia, 124, 117–124. 10.1016/j.neuropsychologia.2018.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, & Wakschlag LS (2017). Research domain criteria (RDoC) grows up: Strengthening neurodevelopmental investigation within the RDoC framework. Journal of Affective Disorders, 216, 30–35. 10.1016/j.jad.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague PR, Berns GS, Cohen JD, McClure SM, Pagnoni G, Dhamala M, Wiest MC, Karpov I, King RD, Apple N, et al. , 2002. Hyperscanning: simultaneous fMRI during linked social interactions. Neuroimage 16, 1159–1164. 10.1006/nimg.2002.1150. [DOI] [PubMed] [Google Scholar]
- Morris AS, Criss MM, Silk JS, & Houltberg BJ (2017). The impact of parenting on emotion regulation during childhood and adolescence. Child Development Perspectives, 11(4), 233–238. 10.1111/cdep.12238. [DOI] [Google Scholar]
- Pagliaccio D, Pine DS, Barch DM, Luby JL, & Leibenluft E (2018). Irritability trajectories, cortical thickness, and clinical outcomes in a sample enriched for preschool depression. Journal of the American Academy of Child & Adolescent Psychiatry, 57(5), 336–342. 10.1016/j.jaac.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman SB, Jones BM, Wakschlag LS, Axelson D, Birmaher B, & Phillips ML (2015). Neural substrates of child irritability in typically developing and psychiatric populations. Developmental Cognitive Neuroscience, 14, 71–80. 10.1016/j.dcn.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman SB, Luna B, Hein TC, & Huppert TJ (2014). fNIRS evidence of prefrontal regulation of frustration in early childhood. Neuroimage 85, 326–334. 10.1016/j.neuroimage.2013.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitclerc A, Briggs-Gowan MJ, Estabrook R, et al. Contextual variation in young children’s observed disruptive behavior on the DB-DOS: Implications for early identification. Journal of Child Psychology and Psychiatry, 56(9), 1008–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiñones-Camacho LE, Fishburn FA, Camacho MC, Wakschlag LS, & Perlman SB (2019). Cognitive Flexibility-Related Prefrontal Activation in Preschoolers: A Biological Approach to Temperamental Effortful Control. Developmental Cognitive Neuroscience, 100651. 10.1016/j.dcn.2019.100651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E, & Schilbach L (2019). Using second-person neuroscience to elucidate the mechanisms of social interaction. Nature Reviews Neuroscience, 20, 495–505. 10.1038/s41583-019-0179-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reindl V, Gerloff C, Scharke W, Konrad K (2018). Brain-to-brain synchrony in parent-child dyads and the relationship with emotion regulation revealed by fNIRS-based hyperscanning. Neuroimage, 178, 493–502. 10.1016/j.neuroimage.2018.05.060. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Ahadi SA, Hershey KL, & Fisher P (2001). Investigations of Temperament at Three to Seven Years: The Children’s Behavior Questionnaire. Child Development, 72(5), 1394–1408. 10.1111/1467-8624.00355. [DOI] [PubMed] [Google Scholar]
- Roy AK, Bennett R, Posner J, Hulvershorn L, Castellanos FX, & Klein RG (2018). Altered intrinsic functional connectivity of the cingulate cortex in children with severe temper outbursts. Development and Psychopathology, 30(2), 571–579. 10.1017/S0954579417001080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santosa H, Aarabi A, Perlman SB, & Huppert T (2017). Characterization and correction of the false-discovery rates in resting state connectivity using functional near-infrared spectroscopy. Journal of Biomedical Optics, 22(5), 055002 10.1117/1.JBO.22.5.055002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santosa H, Zhai X, Fishburn F, & Huppert T (2018). The NIRS Brain AnalyzIR Toolbox. Algorithms, 11(5), 73 10.3390/a11050073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringaris A, Cohen P, Pine DS, & Leibenluft E (2009). Adult outcomes of youth irritability: a 20-year prospective community-based study. American Journal of Psychiatry, 166(9), 1048–1054. 10.1176/appi.ajp.2009.08121849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringaris A, Goodman R, Ferdinando S, Razdan V, Muhrer E, Leibenluft E, & Brotman MA (2012). The Affective Reactivity Index: a concise irritability scale for clinical and research settings. Journal of Child Psychology and Psychiatry, 53(11), 1109–1117. https://dx.doi.org/10.1111%2Fj.1469-7610.2012.02561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suveg C, Shaffer A, & Davis M (2016). Family stress moderates relations between physiological and behavioral synchrony and child self-regulation in mother–preschooler dyads. Developmental Psychobiology, 58(1), 83–97. 10.1002/dev.21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronick EZ (2007). The neurobehavioral and social-emotional development of infants and children. New York: Norton. [Google Scholar]
- Vidal-Ribas P, Brotman MA, Valdivieso I, Leibenluft E, & Stringaris A (2016). The status of irritability in psychiatry: a conceptual and quantitative review. Journal of the American Academy of Child & Adolescent Psychiatry, 55(7), 556–570. 10.1016/j.jaac.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag LS, Estabrook R, Petitclerc A, Henry D, Burns JL, Perlman SB, et al. (2015). Clinical implications of a dimensional approach: the normal:abnormal spectrum of early irritability. Journal of the American Academy of Child and Adolescent Psychiatry, 54, 626–634. 10.1016/j.jaac.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag LS, Hans SL (2002). Maternal smoking during pregnancy and conduct problems in high-risk youth: A developmental framework. Development & Psychopathology, 14(2), 351–369. https://psycnet.apa.org/doi/10.1017/S0954579402002092.. [DOI] [PubMed] [Google Scholar]
- Wakschlag LS, Henry DB, Tolan PH, Carter AS, Burns JL, & Briggs-Gowan MJ (2012). Putting theory to the test: modeling a multidimensional, developmentally-based approach to preschool disruptive behavior. Journal of the American Academy of Child and Adolescent Psychiatry, 51, 593–604. 10.1016/j.jaac.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag L, Hill C, Carter AS, Danis B, Egger HL, Keenan K, … & Briggs-Gowan MJ (2008). Observational Assessment of Preschool Disruptive Behavior, Part I: Reliability of the Disruptive Behavior Diagnostic Observation Schedule (DB-DOS). Journal of the American Academy of Child & Adolescent Psychiatry 47(6), 622–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag LS, Krogh-Jespersen S, Eastabrook CR, Hlutkowsky C, Anderson E, Burns J, Briggs-Gowan M, Petitclerc A, & Perlman SB (under review). The Early Childhood Irritability - Related Impairment Interview (E-CRI): A Novel Method for Assessing Clinical Significance of Young Children’s Irritability within Developmental Context.
- Wakschlag LS, Perlman SB, Blair JR, Leibenluft E, Briggs-Gowan MJ, Pine DS (2018). The neurodevelopmental basis of early childhood disruptive behavior: Irritable and callous phenotypes as exemplars. American Journal of Psychiatry, 175(2),114–130. 10.1176/appi.ajp.2017.17010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley T, Kang O, Parkinson C, & Looser CE (2012). From mind perception to mental connection: Synchrony as a mechanism for social understanding. Social and Personality Psychology Compass, 6(8), 589–606. 10.1111/j.1751-9004.2012.00450.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Associations between maternal neuroticism (a closely related construct to irritability), child irritability, behavioral synchrony, and neural synchrony.