Abstract
Background:
Functional neurological disorder (FND) is a condition at the intersection of neurology and psychiatry. Individuals with FND exhibit corticolimbic abnormalities, yet little is known about the role of white matter tracts in the pathophysiology of FND. This study characterized between-group differences in microstructural integrity, and correlated fiber bundle integrity with symptom severity, physical disability and illness duration.
Methods:
A diffusion tensor imaging (DTI) study was performed in 32 patients with mixed FND compared to 36 healthy controls. Diffusion-weighted magnetic resonance images were collected along with patient-reported symptom severity, physical disability (Short Form Health Survery-36), and illness duration data. Weighted-degree and link-level graph theory and probabilistic tractography analyses characterized fractional anisotropy (FA) values across cortico-subcortical connections. Results were corrected for multiple comparisons.
Results:
Compared to controls, FND patients showed reduced FA in the stria terminalis / fornix, medial forebrain bundle, extreme capsule, uncinate fasciculus, cingulum bundle, corpus callosum, and striatal-postcentral gyrus projections. Except for the stria terminalis / fornix, these differences remained significant adjusting for depression and anxiety. In within-group analyses, physical disability inversely correlated with stria terminalis / fornix and medial forebrain bundle FA values; illness duration negatively correlated with stria terminalis / fornix white matter integrity. A FND symptom severity composite score did not correlate with FA in patients.
Conclusions:
In this first DTI study of mixed FND, microstructural differences were observed in limbic and associative tracts implicated in salience, defensive behaviors and emotion regulation. These findings advance our understanding of neurocircuit pathways in the pathophysiology of FND.
Keywords: conversion disorder, somatization, psychogenic nonepileptic seizures, functional movement disorder, DTI
Introduction
For much of the 20th century, functional neurological (conversion) disorder (FND) was marginalized across neurology and psychiatry despite being the second most common reason for neurological referral and incurring significant healthcare costs(Barsky et al., 2005, Hallett, 2006, Stone et al., 2010a). Patients with FND have symptoms not explained by traditional neurological conditions, including limb weakness, tremor, gait abnormalities, seizures, sensory deficits, fatigue, pain, and cognitive difficulties(Espay et al., 2018a). FND has received renewed interest following increased recognition of physical examination signs and semiologic features that are specific for the diagnosis(Avbersek and Sisodiya, 2010, Daum et al., 2014). Improved diagnostic specificity offers an opportunity to elucidate the neurobiology of FND, decrease stigma, and develop biologically-informed treatments.
Though in its early stages compared to other neuropsychiatric disorders, functional magnetic resonance imaging (MRI) studies of FND have characterized several differences compared to healthy subjects including: 1) amygdalar and periaqueductal gray (PAG) hyperreactivity to negatively-valenced stimuli(Aybek et al., 2015, Aybek et al., 2014b, Hassa et al., 2017, Voon et al., 2010a); 2) increased amygdalar and cingulo-insular connectivity to motor control areas(Diez et al., 2019, Espay et al., 2018c, van der Kruijs et al., 2012, Voon et al., 2010a); 3) right temporoparietal junction hypoactivation and altered connectivity with sensorimotor cortices(Baek et al., 2017, Maurer et al., 2016a, Voon et al., 2010b); and 4) motor deficits(Voon et al., 2011). Quantitative MRI has also elucidated gray matter abnormalities in FND, including increased amygdalar volume(Maurer et al., 2018), cingulo-insular atrophy(Labate et al., 2011, Perez et al., 2018, Perez et al., 2017b, Vasta et al., 2018), decreased pituitary volume(Atmaca et al., 2016), and sensorimotor and striato-thalamic alterations(Aybek et al., 2014a, Espay et al., 2018c, Maurer et al., 2018, Nicholson et al., 2014).
By contrast, white matter investigations in FND are in their infancy(Ding et al., 2013, Hernando et al., 2015, Lee et al., 2015, Tomic et al., 2018), despite non-specific white matter lesions being common in this population(Bolen et al., 2016) and reported links between subcortical hyperintensities and the development of somatic symptom disorders(Inamura et al., 2015). This literature gap, if clarified, can help contextualize the broadly-distributed, multi-network findings described in FND. By measuring the diffusion of water molecules, diffusion tensor imaging (DTI) allows for the in vivo characterization of white matter microstructural integrity. Fractional anisotropy (FA) is a global microstructural integrity measure, with reduced FA linked to decreased white matter integrity. In 44 patients with functional dystonia compared to healthy controls, FA reductions in the uncinate fasciculus, cingulum bundle, corpus callosum, corticospinal tract, anterior thalamic radiations and brainstem structures were identified using tract-based spatial statistics(Tomic et al., 2018); this study also showed robust white matter differences in those with fixed functional dystonia compared to mobile functional dystonia patients and healthy controls. DTI studies in 8(Hernando et al., 2015) and 16(Lee et al., 2015) individuals with psychogenic nonepileptic seizures (PNES, a.k.a. dissociative seizures) showed microstructural differences in the uncinate fasciculus compared to controls; the internal/external capsules and corona radiata also showed group-level differences in one PNES study(Lee et al., 2015). A study performed in 17 patients with PNES showed altered (more lattice-like) structural connectivity profiles compared to controls, including disruptions in sensorimotor, attentional, default mode, and subcortical networks(Ding et al., 2014). These studies, however, did not adjust for comorbid depression and anxiety, and FA profiles inconsistently correlated with clinical data.
In this DTI study, we performed graph theory and tractography analyses to examine white matter integrity in 32 patients with FND compared to 36 healthy controls. First, a white matter-based graph theory analysis was performed to identify the cortico-subcortical areas, and associated fiber bundles originating from these identified brain regions, exhibiting microstructural differences in patients compared to controls. Secondly, probabilistic tractography was employed to quantify within-tract FA differences in patients with FND compared to controls. Within-group tractography analyses also investigated relationships between FA profiles, patient-reported FND severity, physical disability, and illness duration. In this cohort, we previously characterized gray matter and resting-state salience network alterations(Diez et al., 2019, Perez et al., 2017a, Perez et al., 2018, Perez et al., 2017b), theorizing that impaired multimodal integration and emotion dysregulation play important roles in the pathophysiology of FND(Perez et al., 2015, Pick et al., 2019). Thus, we hypothesized that individuals with FND would exhibit reduced limbic white matter integrity compared to controls, and that reduced FA in these tracts would correlate with symptom severity, disability and illness duration.
Materials and Methods
Participants and questionnaires
Thirty-two subjects with FND (22 women, 10 men; mean age=40.9±13.1; average illness duration=3.5±4.5 years) were recruited from the Massachusetts General Hospital FND Clinic between 2014 and 2018 following a “rule-in” FND diagnosis in accord with the Diagnostic and Statistical Manual of Mental Disorders 5th Edition criteria(American Psychiatric Association, 2013, Stone et al., 2010b). Four additional patients were enrolled but excluded following image acquisition and preprocessing (see Supplementary Methods). Given the overlap across the FND spectrum(Perez et al., 2015), we used a transdiagnostic approach that included clinically-established functional movement disorders (n=17; 5 tremor, 5 gait, 1 jerky movements, 1 paroxysmal truncal/head movements, 5 mixed (including one with functional dystonia)), functional weakness (n=13), and documented (n=13) or clinically-established (n=1) psychogenic nonepileptic seizures (PNES). Eleven of 32 subjects had mixed phenotypes. Exclusion criteria included major neurological comorbidities with magnetic resonance imaging (MRI) abnormalities (e.g. encephalomalacia), epilepsy, poorly controlled medical problems with known central nervous system consequences, active substance dependence, history of mania or psychosis, and/or active suicidality. Comorbid psychiatric diagnoses assessed using the Structured Clinical Interview (SCID-I) for DSM-IV-TR were present in 29 of 32 participants. Fourteen were on selective serotonin reuptake inhibitors (SSRIs) and/or serotonin-norepinephrine reuptake inhibitors (SNRIs). See Supplementary Table 1 for clinical information. Thirty-six healthy controls (24 women, 12 men; mean age=39.2±11.8) were recruited through local advertisements, and all screened negative for SCID-I major psychiatric comorbidities (one had past depression not-otherwise-specified). Four additional controls were enrolled but excluded due to lifetime major psychiatric comorbidities. All subjects signed informed consent and the Partners Human Research Committee approved this study.
Patients completed the Conversion Disorder subscale of the Screening for Somatoform Symptoms-7 scale (SOMS:CD)(Rief and Hiller, 2003) and the Patient Health Questionnaire-15 (PHQ15)(Kroenke et al., 2002) as patient-reported FND symptom severity measures. The SOMS:CD is a 14-item measure of FND symptoms within the past 7 days scored on a 5-point scale. The PHQ15 is a 15-item measure of somatic complaints within the past 4 weeks scored on a 3-point scale. To reduce the number of statistical tests, a SOMS:CD-PHQ15 composite was created by averaging the z-scores of the two scales consistent with our previously published approach(Diez et al., 2019). Patients also completed the Short Form Health Survey 36 (SF-36) as a questionnaire of health-related quality of life, and the composite physical health score was used as a physical disability measure(Ware and Sherbourne, 1992). Subjects also completed the Beck Depression Inventory-II (BDI) and the Spielberger State-Trait Anxiety Inventory (STAI).
Data Acquisition and Preprocessing
See Supplementary Methods for T1-weighted and diffusion-weighted MRI scan acquisition parameters. Data preprocessing is also outlined in the Supplementary Methods. Note: the total head motion index was computed as a covariate of non-interest to account for possible head motion confounds(Yendiki et al., 2014).
Weighted-Degree and Link-Level Graph Theory Analyses
Graph theory was used to delineate white matter integrity profiles in relation to cortico-subcortical structures(Rubinov and Sporns, 2010, van den Heuvel and Sporns, 2011). The first step to study the structural connectome is to create an individual-subject graph. These graphs are composed of nodes (brain regions of interest) and links (defining the connectivity property of interest). For the nodes, an 87 region brain parcellation was used: 68 cortical and 16 subcortical regions from the Desikan atlas, the midbrain and pons structures from the brainstem parcellation(Iglesias et al., 2015), and the PAG from a probabilistic map(Keuken et al., 2014).
The next step to generate an individual-subject structural connectome is to select the metric defining the link connectivity measure between each pair of 87 regions(van den Heuvel and Sporns, 2011). Commonly used DTI metrics are the mean FA of all the fiber connections between each pair of nodes and fiber density (how many fibers connect each pair of nodes). We focused on the mean FA (a proxy of microstructural integrity) to identify less efficacious connectivity patterns(van den Heuvel and Sporns, 2011), as well as to allow the graph theory and within-tract analyses (see below) to use the same diffusion parameter. To complement the FA graph analysis, the fiber density (streamline) metric was also used as an additional link connectivity measure in a secondary analysis.
To compute the path of the fibers connecting each pair of regions (nodes), PROBTRACKX2 tool was used taking 100 samples from the range of possible principal diffusion directions within each voxel. To remove low probability voxels from the density map (a map defining how many fibers pass through each voxel connecting two regions), we eliminated those voxels in the path containing less than 0.1% of all the fibers starting in the seed region. To compute the mean FA of the above defined path at the individual-subject level, the FA values in each voxel were weighted by the probability of fibers passing through that voxel when connecting a pair of regions. Thus, FA values of the voxels with highest probability were given a greater weight when computing the FA values defining the connectivity of two regions. An 87×87 connectivity matrix was obtained for each subject.
While different graph analysis metrics are available(Konigs et al., 2017, Rubinov and Sporns, 2010, van den Heuvel and Sporns, 2011), weighted-degree is an index that sums all the weights of the links starting in a given node (i.e. one of the 87 regions-of-interest). The weighted-degree calculation is defined as:
Where the weighted-degree for the node i is computed as the sum of all the FA values of the links starting in node i and ending in the rest of the n nodes of the brain. Thus, the weighted-degree FA value is a proxy of the integrity of white matter originating from a given node (brain area).
To examine between-group differences in the weighted-degree FA maps, a general linear model was performed controlling for age, gender and head motion, and findings were corrected for multiple comparisons using a false-discovery-rate (FDR) q=0.05. This identified cortical-subcortical brain areas showing differential fiber integrity in patients with FND compared to controls.
To identify altered links, a general linear model was again performed examining between-group differences at the link-level. These analyses controlled for age, gender and head motion, and findings were corrected for multiple comparisons using a false-discovery-rate (FDR) q=0.05 considering all possible links. To restrict the results to the weighted-degree findings, only the resulting links starting in regions that showed group differences in the weighted-degree FA analysis were displayed.
To label altered links in patients with FND vs. controls onto anatomically-defined fiber bundles, the following procedures were performed: first, we created a probabilistic white matter atlas of the 36 healthy controls by computing all the tracts defined in the query language for each of the healthy subjects(Wassermann et al., 2016), and then the resulting probabilistic density maps were transformed to MNI space. Thereafter, the mean of these density maps across all controls was computed for each fiber bundle. Secondly, to map statistically significant link-level findings in relation to the created fiber bundle probabilistic atlas, we computed the density map of the fibers passing through both the starting and end points for all controls, transformed the data to MNI space, and computed the mean density map containing fibers connecting two regions. Finally, this link-level probability map was compared with the created atlas to label these links as belonging to the highest probability fiber bundle.
Tractography Analyses
As a complementary analysis, we used query language informed tractography to characterize the location of FA alterations within specific fiber bundles(Colby et al., 2012, Wassermann et al., 2016). This approach incorporates information from the starting and end points of each white matter tract as well as brain areas that the tract passes through (i.e. way points). Two limbic tracts not defined in the previously published query language were constructed as follows: stria terminalis / fornix – starting in the amygdala/hippocampus and passing superior to the thalamus and through the ventral diencephalon(Kamali et al., 2015); medial forebrain bundle / PAG – fibers starting in the PAG as defined from a 7T probabilistic atlas(Ezra et al., 2015). A nonlinear transformation was subsequently applied to the tracts to project them to MNI152 space using MRtrix3 software. To generate templates for each tract to project the results, fibers from healthy controls were combined into group-based fiber bundles. Thereafter, to account for false positives we eliminated fibers with low probability (<0.1%) and high curvature (>70%). To remove crossing fibers, we computed a histogram for each voxel with the probability of the passing fibers going through each possible direction. Fibers with a probability lower than 5% of going within ±15 degrees of the highest probability direction were removed. Additionally, fibers passing through voxels with a probability lower than 0.1% were removed.
To investigate between-group within-tract differences in FA values at the voxel-level, a general linear model was performed. All between-group analyses controlled for age, gender, and head motion. Separate secondary analyses also adjusted between-group findings for: 1) BDI and STAI-trait anxiety scores; 2) SSRI and/or SNRI use (yes/no); and 3) motor FND subtypes (PNES, functional movement disorders, or functional weakness). Correction for multiple comparisons used Monte Carlo simulation cluster-wise correction with 10,000 iterations and α value of 0.05, applied to a whole-brain white matter mask.
In addition, within-group tractography analyses were performed using a general linear model to characterize relationships between fiber tract FA values and indices of FND symptom severity (SOMS:CD-PHQ15 composite scores), physical disability (SF-36 physical health component scores), and illness duration. Within-group analyses controlled for age, gender, and head motion, and separate secondary analyses also adjusted for: 1) BDI and STAI-trait anxiety scores; 2) SSRI and/or SNRI use; and 3) motor FND subtypes. Correction for multiple comparisons used Monte Carlo simulation cluster-wise correction with 10,000 iterations and α value of 0.05, applied to a whole-brain white matter mask.
Results
Weighted-Degree and Link-Level Graph Theory
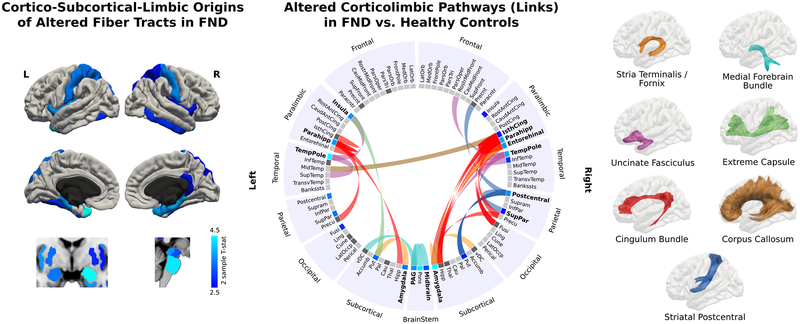
Patients with FND compared to controls showed white matter differences originating from the following brain areas: bilateral amygdala, insula, parahippocampal gyri, temporal poles, precentral gyri, superior parietal lobules, putamen, periaqueductal gray, midbrain, pons and right hippocampus, entorhinal cortex, and the isthmus of the cingulate gyrus. Based on link-level analyses, 7 fiber bundles showed reduced FA in patients with FND compared to controls: 1) stria terminalis / fornix; 2) medial forebrain bundle / PAG projections; 3) uncinate fasciculus; 4) extreme capsule; 5) cingulum bundle; 6) corpus callosum; and 7) striatal-postcentral gyrus projections (See Figure. 1). See Supplementary Figure. 1 for a description of all links surviving multiple comparison correction. No statistically significant links with higher FA in FND vs. control cohorts were found. Using the fiber density (streamline) metric, there were no statistically-significant group-level weighted-degree differences.
Figure 1. Weighted-degree and link-level graph theory white matter analyses in patients with functional neurological disorder (FND).
Left panel shows cortico-subcortico-limbic-brainstem origins of the fiber bundles showing decreased fractional anisotropy in patients with FND compared to healthy controls (HC). Only results surviving to FDR q=0.05 adjusting for age, gender and head motion are displayed. The origins of the regions showing microstructural alterations in FND included the bilateral amygdala, insula, parahippocampal gyri, temporal poles, precentral gyri, superior parietal lobules, putamen, periaqueductal gray, midbrain, pons and right hippocampus, entorhinal cortex, and the isthmus of the cingulate gyrus. The middle panel displays the connectogram identifying the altered fiber bundles starting in the regions previously identified in the initial weighted-degree graph theory analyses and connecting to other brain areas; only results surviving to FDR q=0.05 adjusting for age, gender and head motion are displayed. Each tract was labeled based on the maximum probability of fiber identified using query language. The seven fiber bundles showing between-group alterations included: the stria terminalis / fornix, medial forebrain bundle, uncinate fasciculus, extreme capsule, cingulum bundle, corpus callosum, and striatal to postcentral gyrus projections.
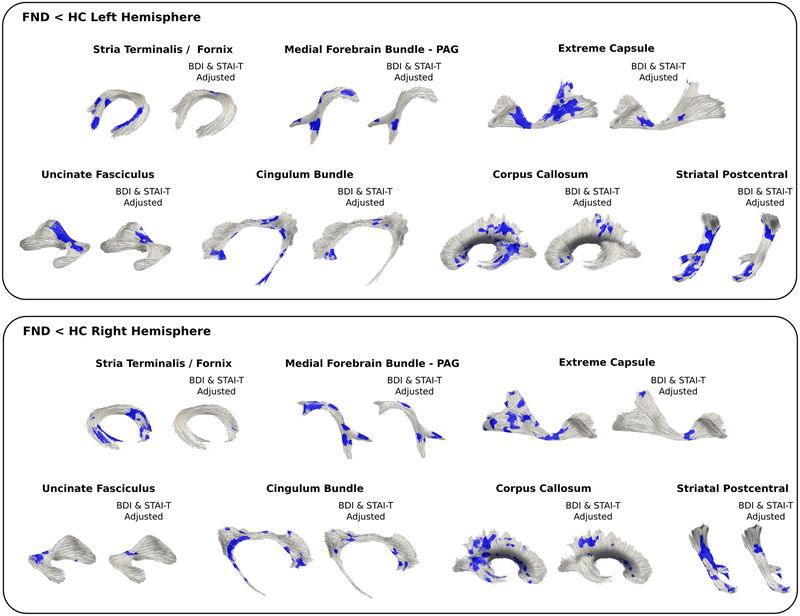
Between-Group Tractography
Probabilistic tractography also showed reduced FA in the above identified 7 white matter tracts (See Figure. 2). In secondary analyses adjusting separately for depression and anxiety scores, antidepressant use, and motor FND subtypes, all tracts remained significant except for the stria terminalis / fornix FA reductions that were no longer significant after adjusting for group-level depression and anxiety scores.
Figure 2. Probabilistic tractography differences in patients with functional neurological disorder (FND) compared to healthy controls (HC).
Patients with FND in tractography analyses showed decreased fractional anisotropy in the following fiber bundles: stria terminalis / fornix, medial forebrain bundle, uncinate fasciculus, extreme capsule, cingulum bundle, corpus callosum, and striatal to postcentral gyrus projections. Only results surviving multiple comparisons are shown adjusting for age, gender and head motion. Results from secondary analyses adjusting for between-group differences in depression (Beck Depression Inventory-II (BDI)) and trait anxiety (Spielberger State Trait Anxiety Inventory (STAI-T)) are also displayed.
Within-Group Tractography
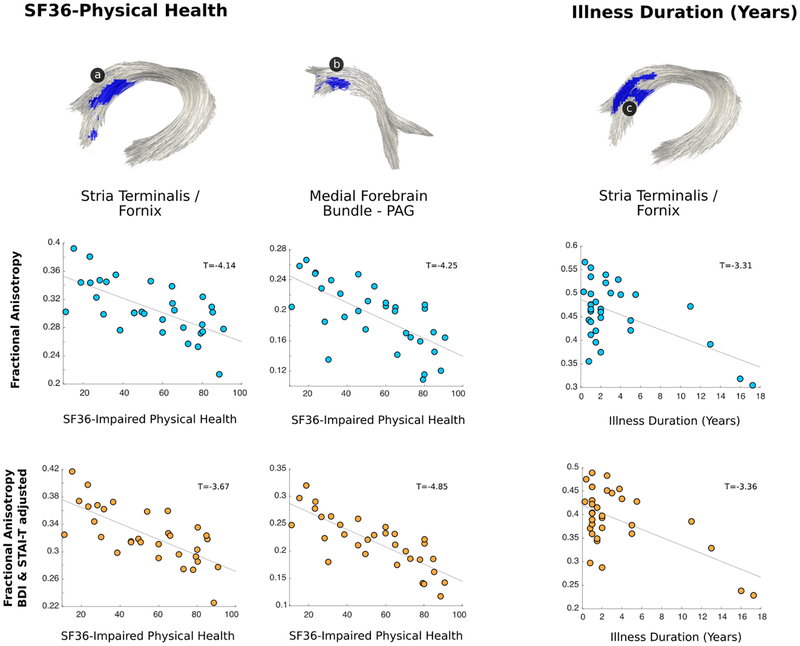
In patients with FND, probabilistic tractography identified that physical disability and longer illness durations correlated with reduced stria terminalis / fornix fiber bundle integrity (See Figure. 3). Decreased FA in the medial forebrain bundle / PAG projections also correlated with physical disability. These within-group findings remained statistically significant when adjusting separately for individual differences in depression and anxiety scores, antidepressant use, and motor FND subtypes. There were no statistically significant findings associated with the SOMS:CD-PHQ15 composite score.
Figure 3. Individual differences in stria terminals / fornix and medial forebrain bundle microstructural integrity correlated with physical disability and illness duration in patients with functional neurological disorder (FND).
In within-group analyses, the magnitude of patient-reported physical disability (impaired SF-36 physical health) inversely correlated with stria terminalis / fornix and medial forebrain bundle FA values; illness duration also negatively correlated with stria terminalis / fornix white matter integrity. Only results surviving multiple comparisons are shown adjusting for age, gender and head motion; all within-group findings also remained significant in secondary analyses adjusting separately for depression and anxiety, antidepressant medication use, and motor FND subtypes. Note: SF-36 scores were inverted so that higher scores represent greater physical disability.
Discussion
Consistent with hypotheses, individuals with FND compared to controls showed reduced white matter integrity in the stria terminalis / fornix, medial forebrain bundle, extreme capsule, uncinate fasciculus, cingulum bundle, corpus callosum, and striatal-postcentral gyrus projections. With the exception of the stria terminalis / fornix, these differences remained significant adjusting for group-level depression and anxiety scores. In within-group analyses, the magnitude of patient-reported physical disability (SF-36 physical health) correlated with reduced stria terminalis / fornix and medial forebrain bundle FA values; illness duration also negatively correlated with stria terminalis / fornix white matter integrity. The SOMS:CD-PHQ15 composite score did not show any statistically significant relationship with fiber bundle integrity. All significant within-group findings held adjusting for individual differences in depression and anxiety, antidepressant medication use, and motor FND subtypes. Notably, weighted-degree and link-level graph theory analyses connected the identified white matter alterations to cortico-subcortical-limbic-brainstem areas commonly implicated in the pathophysiology of FND including the amygdala, insula, parahippocampus, precentral gyrus, putamen and PAG among other regions(Pick et al., 2019, Voon et al., 2016).
The stria terminalis, carrying efferent amygdalar projections to the hypothalamus and septal nuclei, and the fornix, connecting the diencephalon and basal forebrain to the hippocampus, showed reduced microstructural integrity in patients with FND compared to controls. Disruptions in the integrity of the stria terminalis / fornix fiber bundle also correlated with greater physical disability and longer illness durations. These novel, clinically relevant findings can be contextualized based on the neuroimaging, neuroendocrine and autonomic literature in FND. Across several functional MRI studies, patients with FND exhibited heightened amygdalar reactivity to affective-valenced stimuli(Aybek et al., 2015, Hassa et al., 2017, Voon et al., 2010a), suggesting a neurobiological correlate of emotional dysregulation. Increased amygdalar volume was also observed in an FND cohort compared to controls(Maurer et al., 2018), and individual differences in amygdalar gray matter volume positively correlated with elevated trait anxiety and mental health disability in our FND cohort(Perez et al., 2017b). Furthermore, some patients with FND exhibit exaggerated startle responses(Seignourel et al., 2007) and heightened sympathetic tone in cardiac-related autonomic measures(Maurer et al., 2016b). Studies have also characterized elevated skin conductance(Pick et al., 2016) and cortisol profiles in patients with FND, including links between heightened salary cortisol levels and negative attentional biases(Bakvis et al., 2009). Similarly, reduced hippocampal activity has been reported during stressful life event processing in individuals with FND(Aybek et al., 2014b), and adverse life event burden and maladaptive coping styles correlated with decreased hippocampal volumes(Perez et al., 2017a, Williams et al., 2018). While important for declarative memory, the fornix and hippocampus are also implicated in anxiety(Bannerman et al., 2004), and fornix integrity inversely correlated with somatization scores in individuals exposed to verbal abuse(Choi et al., 2009). Our findings, together with the literature, suggest convergent gray matter, white matter and activity-based amygdalar-hippocampal abnormalities in patients with FND. More research is needed to determine if the stria terminalis / fornix findings are FND specific or more closely tied to psychiatric comorbidities.
The medial forebrain bundle is a brainstem and subcortical fiber tract connecting the deep cerebellar nuclei, PAG, hypothalamus and ventral striatum, among other structures. PAG tractography studies have verified that the medial forebrain bundle connects the PAG to the hypothalamus(Ezra et al., 2015). Our finding of group-level reductions in medial forebrain bundle microstructural integrity, and correlations between decreased FA in this tract and greater physical disability, are consistent with the literature implicating the PAG in the pathophysiology of FND. Neurobiologically, the PAG is involved in defensive behaviors, pain modulation, stress responses, and homeostasis(Roelofs, 2017). In FND, increased PAG activity was identified during negative emotion processing(Aybek et al., 2015), and our laboratory has characterized enhanced laterobasal amygdala-to-PAG resting state connectivity(Diez et al., 2019). Individual differences in PAG volume also correlated with mental health disability in patients with FND(Perez et al., 2017b). By measuring postural sway, a marker of the freezing response, one study identified impaired automatic defensive behaviors in patients with FND compared to controls(Zito et al., 2018). Together with the autonomic and neuroendocrine literature(Pick et al., 2019), amygdala–hypothalamus and PAG–hypothalamus interactions appear to play roles in the pathophysiology of FND.
Our FND cohort also showed decreased microstructural integrity in the uncinate fasciculus, cingulate bundle, and extreme capsule, fiber bundles implicated in top-down emotion regulation and language functions(Bubb et al., 2018, Makris and Pandya, 2009, Von Der Heide et al., 2013). Decreased FA in the uncinate fasciculus and cingulum bundle is consistent with white matter profiles previously reported in functional dystonia(Tomic et al., 2018). Reduced integrity of the uncinate fasciculus, a limbic fiber tract connecting the ventromedial and orbitofrontal prefrontal regions to medial temporal structures including the amygdala, has been reported across mood, anxiety, and trauma-related disorders(Jenkins et al., 2016). Interestingly, uncinate fasciculus microstructure disruptions are linked to neuroticism(McIntosh et al., 2013), psychopathologic vulnerability to stress(Hanson et al., 2015), and exaggerated fear-potentiated startle responses(Fani et al., 2015); abnormal ventromedial prefrontal cortex engagement is reported in FND populations(Voon et al., 2016). Patients with FND also exhibited reduced cingulum bundle FA, particularly in the subgenual and parahippocampal/retrospenial subsections, which have been linked to affective and nociceptive functions (anterior) and memory performance (posterior). The extreme capsule connects fronto-insular areas to the inferior parietal lobule and superior temporal gyrus(Makris and Pandya, 2009). It is also notable that the most affected white matter sites in the uncinate fasciculus and extreme capsule were adjacent to the insular cortices. Given the functional and gray matter neuroimaging literature identifying insular alterations in FND populations(Espay et al., 2018b, Perez et al., 2017a, Perez et al., 2017b, van der Kruijs et al., 2012, Vasta et al., 2018, Voon et al., 2011), we speculate that peri-insular structural and functional alterations relate to deficits in multimodal integration, interoception, and emotional awareness(Perez et al., 2015).
Other findings included microstructural alterations in the corpus callosum and striatal radiations to the post-central gyrus. Notably, temporoparietal (posterior) aspects of the corpus callosum showed FA reductions in patients with FND compared to controls. This finding may relate to impaired hemispheric transfer of information and altered bodily processing and self-agency perceptions that have been implicated in the pathophysiology of FND(Devinsky, 2000, Voon et al., 2010b). Disruptions in striatal–postcentral gyrus connections also warrant more inquiry in relation to sensory gating and attentional mechanisms.
An unanswered question relates to how to optimally contextualize the fiber bundle differences identified in this study, particularly whether the findings relate to disease mechanisms, predisposing vulnerabilities, psychiatric comorbidities, and/or compensatory processes(Begue et al., 2019). While studies with larger sample sizes and longitudinal neuroimaging data collection are needed to clarify these issues, it is notable that the integrity of two specific fiber tracts, the stria terminalis / fornix and the medial forebrain bundle, were implicated in patient-reported physical disability and illness duration. Given the high frequency of uncinate fasciculus alterations characterized in studies of mood and anxiety disorders, we speculate that this fiber tract disruption relates more broadly to emotional dysregulation. A particularly important theme warranting additional research is the role of experience and activity-dependent white and gray matter neuroplasticity. Such research in FND populations should consider antenatal and genetic-epigenetic influences, developmental trajectories, life experiences, psychiatric comorbidities, mobility levels, number of relapses, and prior treatments among other factors(Keynejad et al., 2018, Kozlowska et al., 2017, Ludwig et al., 2018, Sampaio-Baptista and Johansen-Berg, 2017).
Limitations include modest sample size, psychiatric comorbidities, psychotropic medication use, phenotypic heterogeneity, and sole reliance on patient-reported symptom severity scales. While between-group and within-group tractography findings were adjusted for depression and anxiety scores which is a study strength, future studies with psychiatric and neurological controls, other FND populations, and functional somatic disorders (e.g. somatic symptom disorder with predominant pain, fibromyalgia, chronic pain disorders ect.) are needed to further contextualize our DTI findings. This is particularly needed given that several of the identified white matter tracts (and associated cortico-subcortical brain areas) are also components of the central pain matrix, suggesting the considerably more research is needed at the intersection of FND and functional somatic disorders(Begue et al., 2019, Denk et al., 2014, Ploner et al., 2011). In addition, while individuals with FND commonly exhibit mixed symptoms and/or develop new symptoms over the course of their illness, the use of a transdiagnostic research approach remains debated. Here, adjusting for FND subtypes did not robustly influence the between-group and within-group findings providing support for a transdiagnostic approach. Furthermore, it is difficult to directly compare the results of our present study with the 4 prior DTI studies in FND(Ding et al., 2014, Hernando et al., 2015, Lee et al., 2015, Tomic et al., 2018) given phenotypic and methodological differences, underscoring the need for additional patient cohorts with larger sample sizes and multimodal methodology. Future DTI studies should also seek to combine multiple diffusion parameters to clarity the specific white matter pathology implicated in FND, particularly given that we did not find statistically significant findings using the fiber density (streamline) metric. More research is also needed to combine patient-reported scales with objective performance measures, as well as combining DTI with behavioral, neuroendocrine, and autonomic data. Methodological limitations also included a 2mm voxel size and low B values which limited our ability to differentiate the stria terminalis and fornix. Similarly, given the proximity of the extreme and external capsules, we cannot exclude external capsule involvement. Higher resolution diffusion-weighted sequences are needed to replicate and further characterize the microstructural alterations in FND populations.
In conclusion, complementary graph theory and tractography analyses identified microstructural alterations in limbic and associative fiber bundles implicated in salience, defensive behaviors and emotion regulation. Reduced stria terminalis / fornix and medial forebrain bundle integrity were linked to physical disability and illness duration, advancing our understanding of neurocircuit pathways in the pathophysiology of FND.
Supplementary Material
Supplementary Figure 1. Link-level structural connectogram showing all the statistically significant links across all 87×87 regions-of-interest. Links shown in gray identify those links that connect regions not otherwise identified in the weighted-degree graph theory analysis (See Figure. 1). These additional links include: L_entorhinal to L_precuneus; L_isthmuscingulate to L_entorhinal; L_isthmuscingulate to L_parahippocampal; L_precuneus to L_fusiform; L_precuneus to L_superiortemporal; L_precuneus to L_insula; L_superiortemporal to L_temporalpole; L_transversetemporal to L_precentral; R_parsopercularis to R_parsorbitalis; R_transversetemporal to R_precentral; R_Thalamus_Proper to R_postcentral; R_Thalamus_Proper to R_temporalpole; R_Hippocampus to R_entorhinal; R_Hippocampus to R_isthmuscingulate; R_Hippocampus to R_precuneus; R_Hippocampus to R_Amygdala. L indicates left; R, right.
Supplementary Table 1. Demographic characteristics of patients with functional neurological disorder.
Acknowledgements / Funding:
D.L.P. was funded by the National Institute of Mental Health Grant K23MH111983-02, Massachusetts General Hospital Physician-Scientist Development Award, and the Sidney R. Baer Jr. Foundation. I.D. was supported by postdoctoral fellowship program from the Basque Country Government. This study was also supported by the NIH shared instrument grant S10RR023043.
Footnotes
Conflicts of Interest/Disclosures:
D.L.P. received honoraria from Harvard Medical School, American Academy of Neurology, Movement Disorder Society, and Toronto Western Hospital. All other authors report no conflicts of interests/disclosures.
References
- American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders (DSM-5). American Psychiatric Pub: Washington, DC. [Google Scholar]
- Atmaca M, Baykara S, Mermi O, Yildirim H & Akaslan U (2016). Pituitary volumes are changed in patients with conversion disorder. Brain Imaging Behav 10, 92–5. [DOI] [PubMed] [Google Scholar]
- Avbersek A & Sisodiya S (2010). Does the primary literature provide support for clinical signs used to distinguish psychogenic nonepileptic seizures from epileptic seizures? J Neurol Neurosurg Psychiatry 81, 719–25. [DOI] [PubMed] [Google Scholar]
- Aybek S, Nicholson TR, Draganski B, Daly E, Murphy DG, David AS & Kanaan RA (2014a). Grey matter changes in motor conversion disorder. J Neurol Neurosurg Psychiatry 85, 236–8. [DOI] [PubMed] [Google Scholar]
- Aybek S, Nicholson TR, O’Daly O, Zelaya F, Kanaan RA & David AS (2015). Emotion-motion interactions in conversion disorder: an FMRI study. PLoS One 10, e0123273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aybek S, Nicholson TR, Zelaya F, O’Daly OG, Craig TJ, David AS & Kanaan RA (2014b). Neural correlates of recall of life events in conversion disorder. JAMA Psychiatry 71, 52–60. [DOI] [PubMed] [Google Scholar]
- Baek K, Donamayor N, Morris LS, Strelchuk D, Mitchell S, Mikheenko Y, Yeoh SY, Phillips W, Zandi M, Jenaway A, Walsh C & Voon V (2017). Impaired awareness of motor intention in functional neurological disorder: implications for voluntary and functional movement. Psychol Med 47, 1624–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakvis P, Spinhoven P & Roelofs K (2009). Basal cortisol is positively correlated to threat vigilance in patients with psychogenic nonepileptic seizures. Epilepsy Behav 16, 558–60. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH & Feldon J (2004). Regional dissociations within the hippocampus--memory and anxiety. Neurosci Biobehav Rev 28, 273–83. [DOI] [PubMed] [Google Scholar]
- Barsky AJ, Orav EJ & Bates DW (2005). Somatization increases medical utilization and costs independent of psychiatric and medical comorbidity. Arch Gen Psychiatry 62, 903–10. [DOI] [PubMed] [Google Scholar]
- Begue I, Adams C, Stone J & Perez DL (2019). Structural alterations in functional neurological disorder and related conditions: a software and hardware problem? Neuroimage Clin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolen RD, Koontz EH & Pritchard PB 3rd (2016). Prevalence and distribution of MRI abnormalities in patients with psychogenic nonepileptic events. Epilepsy Behav 59, 73–6. [DOI] [PubMed] [Google Scholar]
- Bubb EJ, Metzler-Baddeley C & Aggleton JP (2018). The cingulum bundle: Anatomy, function, and dysfunction. Neurosci Biobehav Rev 92, 104–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Jeong B, Rohan ML, Polcari AM & Teicher MH (2009). Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biol Psychiatry 65, 227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby JB, Soderberg L, Lebel C, Dinov ID, Thompson PM & Sowell ER (2012). Along-tract statistics allow for enhanced tractography analysis. Neuroimage 59, 3227–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum C, Hubschmid M & Aybek S (2014). The value of ‘positive’ clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review. J Neurol Neurosurg Psychiatry 85, 180–90. [DOI] [PubMed] [Google Scholar]
- Denk F, McMahon SB & Tracey I (2014). Pain vulnerability: a neurobiological perspective. Nat Neurosci 17, 192–200. [DOI] [PubMed] [Google Scholar]
- Devinsky O (2000). Right Cerebral Hemisphere Dominance for a Sense of Corporeal and Emotional Self. Epilepsy & Behavior 1, 60–73. [Google Scholar]
- Diez I, Ortiz-Teran L, Williams B, Jalilianhasanpour R, Ospina JP, Dickerson BC, Keshavan MS, LaFrance WC Jr., Sepulcre J & Perez DL (2019). Corticolimbic fast-tracking: enhanced multimodal integration in functional neurological disorder. J Neurol Neurosurg Psychiatry 90, 929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, An D, Liao W, Wu G, Xu Q, Zhou D & Chen H (2014). Abnormal functional connectivity density in psychogenic non-epileptic seizures. Epilepsy Res 108, 1184–94. [DOI] [PubMed] [Google Scholar]
- Ding JR, An D, Liao W, Li J, Wu GR, Xu Q, Long Z, Gong Q, Zhou D, Sporns O & Chen H (2013). Altered functional and structural connectivity networks in psychogenic non-epileptic seizures. PLoS One 8, e63850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espay AJ, Aybek S, Carson A, Edwards MJ, Goldstein LH, Hallett M, LaFaver K, LaFrance WC Jr., Lang AE, Nicholson T, Nielsen G, Reuber M, Voon V, Stone J & Morgante F (2018a). Current Concepts in Diagnosis and Treatment of Functional Neurological Disorders. JAMA Neurol 75, 1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espay AJ, Maloney T, Vannest J, Norris MM, Eliassen JC, Neefus E, Allendorfer JB, Chen R & Szaflarski JP (2018b). Dysfunction in emotion processing underlies functional (psychogenic) dystonia. Mov Disord 33, 136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espay AJ, Maloney T, Vannest J, Norris MM, Eliassen JC, Neefus E, Allendorfer JB, Lang AE & Szaflarski JP (2018c). Impaired emotion processing in functional (psychogenic) tremor: A functional magnetic resonance imaging study. Neuroimage Clin 17, 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezra M, Faull OK, Jbabdi S & Pattinson KT (2015). Connectivity-based segmentation of the periaqueductal gray matter in human with brainstem optimized diffusion MRI. Hum Brain Mapp 36, 3459–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N, King TZ, Brewster R, Srivastava A, Stevens JS, Glover EM, Norrholm SD, Bradley B, Ressler KJ & Jovanovic T (2015). Fear-potentiated startle during extinction is associated with white matter microstructure and functional connectivity. Cortex 64, 249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M (2006). Psychogenic movement disorders: a crisis for neurology. Curr Neurol Neurosci Rep 6, 269–71. [DOI] [PubMed] [Google Scholar]
- Hanson JL, Knodt AR, Brigidi BD & Hariri AR (2015). Lower structural integrity of the uncinate fasciculus is associated with a history of child maltreatment and future psychological vulnerability to stress. Dev Psychopathol 27, 1611–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassa T, Sebastian A, Liepert J, Weiller C, Schmidt R & Tüscher O (2017). Symptom-specific amygdala hyperactivity modulates motor control network in conversion disorder. NeuroImage: Clinical 15, 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernando KA, Szaflarski JP, Ver Hoef LW, Lee S & Allendorfer JB (2015). Uncinate fasciculus connectivity in patients with psychogenic nonepileptic seizures: a preliminary diffusion tensor tractography study. Epilepsy & Behavior 45, 68–73. [DOI] [PubMed] [Google Scholar]
- Iglesias JE, Van Leemput K, Bhatt P, Casillas C, Dutt S, Schuff N, Truran-Sacrey D, Boxer A, Fischl B & Alzheimer’s Disease Neuroimaging I (2015). Bayesian segmentation of brainstem structures in MRI. Neuroimage 113, 184–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamura K, Shinagawa S, Nagata T, Tagai K, Nukariya K & Nakayama K (2015). White matter hyperintensities are associated with the severity of late-life somatoform disorders and executive functions. Nord J Psychiatry, 1–8. [DOI] [PubMed] [Google Scholar]
- Jenkins LM, Barba A, Campbell M, Lamar M, Shankman SA, Leow AD, Ajilore O & Langenecker SA (2016). Shared white matter alterations across emotional disorders: A voxel-based meta-analysis of fractional anisotropy. Neuroimage Clin 12, 1022–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamali A, Yousem DM, Lin DD, Sair HI, Jasti SP, Keser Z, Riascos RF & Hasan KM (2015). Mapping the trajectory of the stria terminalis of the human limbic system using high spatial resolution diffusion tensor tractography. Neurosci Lett 608, 45–50. [DOI] [PubMed] [Google Scholar]
- Keuken MC, Bazin PL, Crown L, Hootsmans J, Laufer A, Muller-Axt C, Sier R, van der Putten EJ, Schafer A, Turner R & Forstmann BU (2014). Quantifying inter-individual anatomical variability in the subcortex using 7 T structural MRI. Neuroimage 94, 40–46. [DOI] [PubMed] [Google Scholar]
- Keynejad RC, Frodl T, Kanaan R, Pariante C, Reuber M & Nicholson TR (2018). Stress and functional neurological disorders: mechanistic insights. J Neurol Neurosurg Psychiatry 90, 813–821. [DOI] [PubMed] [Google Scholar]
- Konigs M, van Heurn LWE, Bakx R, Vermeulen RJ, Goslings JC, Poll-The BT, van der Wees M, Catsman-Berrevoets CE, Oosterlaan J & Pouwels PJW (2017). The structural connectome of children with traumatic brain injury. Hum Brain Mapp 38, 3603–3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowska K, Griffiths KR, Foster SL, Linton J, Williams LM & Korgaonkar MS (2017). Grey matter abnormalities in children and adolescents with functional neurological symptom disorder. NeuroImage Clin 15, 306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL & Williams JB (2002). The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med 64, 258–66. [DOI] [PubMed] [Google Scholar]
- Labate A, Cerasa A, Mula M, Mumoli L, Gioia MC, Aguglia U, Quattrone A & Gambardella A (2011). Neuroanatomic correlates of psychogenic nonepileptic seizures: a cortical thickness and VBM study. Epilepsia 53, 377–85. [DOI] [PubMed] [Google Scholar]
- Lee S, Allendorfer JB, Gaston TE, Griffis JC, Hernando KA, Knowlton RC, Szaflarski JP & Ver Hoef LW (2015). White matter diffusion abnormalities in patients with psychogenic non-epileptic seizures. Brain Res 1620, 169–76. [DOI] [PubMed] [Google Scholar]
- Ludwig L, Pasman JA, Nicholson T, Aybek S, David AS, Tuck S, Kanaan RA, Roelofs K, Carson A & Stone J (2018). Stressful life events and maltreatment in conversion (functional neurological) disorder: systematic review and meta-analysis of case-control studies. Lancet Psychiatry 5, 307–320. [DOI] [PubMed] [Google Scholar]
- Makris N & Pandya DN (2009). The extreme capsule in humans and rethinking of the language circuitry. Brain Struct Funct 213, 343–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer CW, LaFaver K, Ameli R, Epstein SA, Hallett M & Horovitz SG (2016a). Impaired self-agency in functional movement disorders: A resting-state fMRI study. Neurology 87, 564–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer CW, LaFaver K, Limachia GS, Capitan G, Ameli R, Sinclair S, Epstein SA, Hallett M & Horovitz SG (2018). Gray matter differences in patients with functional movement disorders. Neurology 91, e1870–e1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer CW, Liu VD, LaFaver K, Ameli R, Wu T, Toledo R, Epstein SA & Hallett M (2016b). Impaired resting vagal tone in patients with functional movement disorders. Parkinsonism Relat Disord 30, 18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AM, Bastin ME, Luciano M, Maniega SM, Del CVHM, Royle NA, Hall J, Murray C, Lawrie SM, Starr JM, Wardlaw JM & Deary IJ (2013). Neuroticism, depressive symptoms and white-matter integrity in the Lothian Birth Cohort 1936. Psychol Med 43, 1197–206. [DOI] [PubMed] [Google Scholar]
- Nicholson TR, Aybek S, Kempton MJ, Daly EM, Murphy DG, David AS & Kanaan RA (2014). A structural MRI study of motor conversion disorder: evidence of reduction in thalamic volume. J Neurol Neurosurg Psychiatry 85, 227–9. [DOI] [PubMed] [Google Scholar]
- Perez DL, Dworetzky BA, Dickerson BC, Leung L, Cohn R, Baslet G & Silbersweig DA (2015). An integrative neurocircuit perspective on psychogenic nonepileptic seizures and functional movement disorders: neural functional unawareness. Clin EEG Neurosci 46, 4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez DL, Matin N, Barsky A, Costumero-Ramos V, Makaretz SJ, Young SS, Sepulcre J, LaFrance WC Jr., Keshavan MS & Dickerson BC (2017a). Cingulo-insular structural alterations associated with psychogenic symptoms, childhood abuse and PTSD in functional neurological disorders. J Neurol Neurosurg Psychiatry 88, 491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez DL, Matin N, Williams B, Tanev K, Makris N, LaFrance WC Jr. & Dickerson BC (2018). Cortical thickness alterations linked to somatoform and psychological dissociation in functional neurological disorders. Hum Brain Mapp 39, 428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez DL, Williams B, Matin N, LaFrance WC Jr., Costumero-Ramos V, Fricchione GL, Sepulcre J, Keshavan MS & Dickerson BC (2017b). Corticolimbic structural alterations linked to health status and trait anxiety in functional neurological disorder. J Neurol Neurosurg Psychiatry 88, 1052–1059. [DOI] [PubMed] [Google Scholar]
- Pick S, Goldstein LH, Perez DL & Nicholson TR (2019). Emotional processing in functional neurological disorder: a review, biopsychosocial model and research agenda. J Neurol Neurosurg Psychiatry 90, 704–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick S, Mellers JD & Goldstein LH (2016). Explicit Facial Emotion Processing in Patients With Dissociative Seizures. Psychosom Med 78, 874–85. [DOI] [PubMed] [Google Scholar]
- Ploner M, Lee MC, Wiech K, Bingel U & Tracey I (2011). Flexible cerebral connectivity patterns subserve contextual modulations of pain. Cereb Cortex 21, 719–26. [DOI] [PubMed] [Google Scholar]
- Rief W & Hiller W (2003). A new approach to the assessment of the treatment effects of somatoform disorders. Psychosomatics 44, 492–8. [DOI] [PubMed] [Google Scholar]
- Roelofs K (2017). Freeze for action: neurobiological mechanisms in animal and human freezing. Philos Trans R Soc Lond B Biol Sci 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M & Sporns O (2010). Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52, 1059–69. [DOI] [PubMed] [Google Scholar]
- Sampaio-Baptista C & Johansen-Berg H (2017). White Matter Plasticity in the Adult Brain. Neuron 96, 1239–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seignourel PJ, Miller K, Kellison I, Rodriguez R, Fernandez HH, Bauer RM, Bowers D & Okun MS (2007). Abnormal affective startle modulation in individuals with psychogenic [corrected] movement disorder. Mov Disord 22, 1265–71. [DOI] [PubMed] [Google Scholar]
- Stone J, Carson A, Duncan R, Roberts R, Warlow C, Hibberd C, Coleman R, Cull R, Murray G, Pelosi A, Cavanagh J, Matthews K, Goldbeck R, Smyth R, Walker J & Sharpe M (2010a). Who is referred to neurology clinics?--the diagnoses made in 3781 new patients. Clin Neurol Neurosurg 112, 747–51. [DOI] [PubMed] [Google Scholar]
- Stone J, LaFrance WC Jr., Levenson JL & Sharpe M (2010b). Issues for DSM-5: Conversion disorder. Am J Psychiatry 167, 626–7. [DOI] [PubMed] [Google Scholar]
- Tomic A, Agosta F, Sarasso E, Petrovic I, Basaia S, Pesic D, Kostic M, Fontana A, Kostic VS & Filippi M (2018). Are there two different forms of functional dystonia? A multimodal brain structural MRI study. Mol Psychiatry. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP & Sporns O (2011). Rich-club organization of the human connectome. J Neurosci 31, 15775–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kruijs SJ, Bodde NM, Vaessen MJ, Lazeron RH, Vonck K, Boon P, Hofman PA, Backes WH, Aldenkamp AP & Jansen JF (2012). Functional connectivity of dissociation in patients with psychogenic non-epileptic seizures. J Neurol Neurosurg Psychiatry 83, 239–47. [DOI] [PubMed] [Google Scholar]
- Vasta R, Cerasa A, Sarica A, Bartolini E, Martino I, Mari F, Metitieri T, Quattrone A, Gambardella A, Guerrini R & Labate A (2018). The application of artificial intelligence to understand the pathophysiological basis of psychogenic nonepileptic seizures. Epilepsy and Behavior 87, 167–172. [DOI] [PubMed] [Google Scholar]
- Von Der Heide RJ, Skipper LM, Klobusicky E & Olson IR (2013). Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain 136, 1692–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V, Brezing C, Gallea C, Ameli R, Roelofs K, LaFrance WC Jr. & Hallett M (2010a). Emotional stimuli and motor conversion disorder. Brain 133, 1526–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V, Brezing C, Gallea C & Hallett M (2011). Aberrant supplementary motor complex and limbic activity during motor preparation in motor conversion disorder. Mov Disord 26, 2396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V, Cavanna AE, Coburn K, Sampson S, Reeve A & LaFrance WC Jr. (2016). Functional Neuroanatomy and Neurophysiology of Functional Neurological Disorders (Conversion Disorder). J Neuropsychiatry Clin Neurosci 28, 168–90. [DOI] [PubMed] [Google Scholar]
- Voon V, Gallea C, Hattori N, Bruno M, Ekanayake V & Hallett M (2010b). The involuntary nature of conversion disorder. Neurology 74, 223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JE Jr. & Sherbourne CD (1992). The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 30, 473–83. [PubMed] [Google Scholar]
- Wassermann D, Makris N, Rathi Y, Shenton M, Kikinis R, Kubicki M & Westin CF (2016). The white matter query language: a novel approach for describing human white matter anatomy. Brain Struct Funct 221, 4705–4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B, Jalilianhasanpour R, Matin N, Fricchione GL, Sepulcre J, Keshavan MS, LaFrance WC, Dickerson BC & Perez DL (2018). Individual differences in corticolimbic structural profiles linked to insecure attachment and coping styles in motor functional neurological disorders. Journal of Psychiatric Research 102, 230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendiki A, Koldewyn K, Kakunoori S, Kanwisher N & Fischl B (2014). Spurious group differences due to head motion in a diffusion MRI study. Neuroimage 88, 79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zito GA, Apazoglou K, Paraschiv-Ionescu A, Aminian K & Aybek S (2018). Abnormal postural behavior in patients with functional movement disorders during exposure to stress. Psychoneuroendocrinology 101, 232–239. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Link-level structural connectogram showing all the statistically significant links across all 87×87 regions-of-interest. Links shown in gray identify those links that connect regions not otherwise identified in the weighted-degree graph theory analysis (See Figure. 1). These additional links include: L_entorhinal to L_precuneus; L_isthmuscingulate to L_entorhinal; L_isthmuscingulate to L_parahippocampal; L_precuneus to L_fusiform; L_precuneus to L_superiortemporal; L_precuneus to L_insula; L_superiortemporal to L_temporalpole; L_transversetemporal to L_precentral; R_parsopercularis to R_parsorbitalis; R_transversetemporal to R_precentral; R_Thalamus_Proper to R_postcentral; R_Thalamus_Proper to R_temporalpole; R_Hippocampus to R_entorhinal; R_Hippocampus to R_isthmuscingulate; R_Hippocampus to R_precuneus; R_Hippocampus to R_Amygdala. L indicates left; R, right.
Supplementary Table 1. Demographic characteristics of patients with functional neurological disorder.