Dear editor
Many questions regarding the use of corticosteroids in the treatment of COVID-19 remain unanswered, including the efficacy, the appropriate timing of initiation, and the dose and duration of treatment [[1], [2], [3]]. We describe a patient with COVID-19 and clinically severe ARDS with improvement after initial treatment but deterioration following abolition corticosteroids.
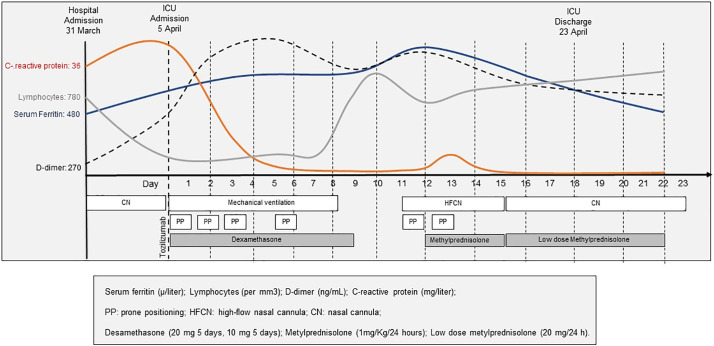
A 71-year-old woman with a history of hypertension and diabetes was admitted to the University Hospital of Santiago de Compostela on March 31, 2020, with symptoms of fever and fatigue for 10 days. COVID-19 was diagnosed based on RT-PCR testing. The initial treatments consisted of lopinavir plus ritonavir, hydroxychloroquine, azithromycin, and supplemental oxygen. Illness progressed requiring admission to the ICU on 5 April, with mechanical ventilation, sessions of prone positioning (PP), tocilizumab and dexamethasone. Chest X-ray showed multiple patchy shadows in both lungs. Laboratory test results during ICU admission are represented in Fig. 1 . Eight days after ICU admission, oxygenation and X-ray improved, antivirals and corticosteroids were suspended, and the patient was extubated. Two days after extubation, the patient's oxygenation and radiology worsened with an increase of laboratory inflammatory factors. We ruled out bacterial superinfection and restarted corticosteroid treatment for 3 days and low maintenance doses for one week. After four days with high-flow nasal cannula (HFNC) oxygen therapy (60% concentration, flow rate 40 L/min) and awake prone positioning sessions, the patient improved. She was discharged on 23 April after 18 days in ICU. Three other patients in our ICU displayed a similar course of disease after abolition of corticosteroids: two patients required reintubation and one patient needing HFNC. All three patients improved after medication with corticosteroids. We obtained informed written consent from the four patients.
Fig. 1.
Timeline of disease course according to days from Hospital admission, and days from ICU admission, from 31 March to 22 April.
Serum ferritin (μ/L); lymphocytes (mer mm3); D-dimer (ng/mL); C-reactive protein (mg/L); PP: prone positioning; HFCN: high-flow nasal cannula; dexamethasone (20 mg 5 days, 10 mg 5 days); methylprednisolone (1 mg/kg/day); low dose methylprednisolone (20 mg/day).
It appears that COVID-19 disease passes through three different stages: early infection phase, pulmonary phase and hyperinflammation phase [4]. The early phase appears to be due to the virus itself (5–7 days) whereas the two later phases are thought to be due to an inflammatory response (7–15 days from disease onset). Following admission to the ICU 8–15 days after symptom onset, it is likely that most critically ill patients are in the hyperinflammation phase, and it suggests that anti-inflammatory agents such as corticoids may have a beneficial effect. Pathological findings of pulmonary edema and hyaline membrane formation in patients who died by COVID-19 support this idea [5]. Several authors suggest that the effect of corticosteroids should vary depending on the course of the disease. Low dose of corticosteroids during the pulmonary phase may be beneficial to prevent a severe hyper-inflammation response whereas higher doses of corticosteroids or immunosuppressives agents such as tocilizumab might be necessary in the third stage to decrease an already existing hyperinflammatory state [2].
However, we do not know the duration of the hyper-inflammation stage. How long should we administer corticosteroids? There are several guidelines described [[1], [2], [3]], but no one knows how long this hyperinflammation will last. As clinical spectrum of patients with COVID-19 patients range from asymptomatic to severe symptoms, the duration and level of inflammation may vary also between patients. Thus, a rigid course of corticosteroids may not be adequate. In the patients described in present letter, we observed that discontinuation of corticosteroids was associated with an inflammatory response as shown by increased levels of inflammatory markers and subsequent clinical deterioration that resolved with reinstitution of treatment. Our findings support the necessity of corticosteroids during the hyper-inflammatory stage of the COVID-19 disease, but we don't know the optimal duration of the corticosteroid treatment. Rapid discontinuation may cause a disease relapse [4]. We propose gradual tapering to preserve the significant improvement achieved during corticosteroids administration.
Prior presentations
No
Funding statement
No funding provided.
Support
Support was provided solely from institutional and departmental sources.
Authors' contributions
-
1.
Conception of the study: Manuel Taboada.
-
2.
Study design: Manuel Taboada
-
3.
Data collection: Manuel Taboada, Valentín Caruezo.
-
4.
Drafting the manuscript: All authors helped to revise the draft of the manuscript
-
5.
Editing and approval of the manuscript: All authors
Declaration of competing interest
The authors declare the absence of conflict of interests.
Acknowledgments
The authors thank all physicians and nurses of the Hospital Clínico Universitario Santiago de Compostela, Spain.
References
- 1.Villar J., Ferrando C., Martínez D. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020 Mar;8(3):267–276. doi: 10.1016/S2213-2600(19)30417-5. [Epub 2020 Feb 7] [DOI] [PubMed] [Google Scholar]
- 2.Nicastri E., Petrosillo N., Bartoli T.A. National Institute for the infectious diseases “L. Spallanzani”, IRCCS. Recommendations for COVID-19 clinical management. Infect Dis Rep. 2020 Mar 16;12(1):8543. doi: 10.4081/idr.2020.8543. [eCollection 2020 Feb 25] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meduri G.U., Bridges L., Siemieniuk R.A.C., Kocak M. An exploratory reanalysis of the randomized trial on efficacy of corticosteroids as rescue therapy for the late phase of acute respiratory distress syndrome. Crit Care Med. 2018 Jun;46(6):884–891. doi: 10.1097/CCM.0000000000003021. [DOI] [PubMed] [Google Scholar]
- 4.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal [published online ahead of print, 2020 Mar 20] J Heart Lung Transplant. 2020 doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020 Apr;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [Epub 2020 Feb 18] [DOI] [PMC free article] [PubMed] [Google Scholar]