Abstract
Bone is a critically important part of the skeletal system that is essential for body support and locomotion. The immune system protects against pathogens and is active in host defense. These two seemingly distinct systems in fact interact with each other, share molecules and create a collaborative regulatory system called the “osteoimmune system”. The most representative osteoimmune molecule is receptor activator of NF-κB ligand (RANKL), which plays multiple roles in the osteoimmune system under both physiological and pathological conditions such as rheumatoid arthritis and cancer metastasis to bone. Based on accumulating evidence for such mutual dependence, it is concluded that the relationship between bone and the immune system did not develop by accident but as a necessary consequence of evolution. Here I describe the history of and recent advances in osteoimmunology, providing a perspective in the contexts of both science and medicine.
Keywords: osteoimmunology, bone metabolism, immunity, rheumatoid arthritis, bone marrow microenvironment, inter-organ crosstalk
Introduction
Bone is composed of hard extracellular matrix along with a small number of crucially important bone cells. The extracellular matrix is mainly made up with type I collagen and hydroxyapatite (calcium phosphate crystal). Bone cells are comprised of bone-forming osteoblasts and bone-resorbing osteoclasts, both of which are effector cells that act on the surface of bone.1,2) Osteocytes are embedded in the bone matrix and are terminally differentiated cells of osteoblast lineage, functioning as a command cell for osteoblasts and osteoclasts in response to stimuli such as mechanical stress and hormones.1,2) Old and damaged bone, which is detected by poorly understood mechanisms, is resorbed by osteoclasts, after which osteoblasts make an equivalent amount of resorbed bone. If the balance between resorption and formation is shifted to resorption, the bone mass decreases, leading to osteoporosis. If osteoclastic bone resorption is impaired, the bone mass increases (osteopetrosis) but the bones become susceptible to fracture.1,2) Thus, bone should be constantly renewed by osteoclasts and osteoblasts in a proper balance in this remodeling process. In addition to osteoporosis, excessive osteoclastic bone resorption beyond osteoblastic bone formation is seen in various pathological conditions such as rheumatoid arthritis, periodontitis and congenital bone diseases as well as primary and metastatic bone tumors.1,2) Thus, understanding the mechanism of differentiation and activation of osteoclasts and osteoblasts is vitally important for developing future therapeutic strategies for bone and joint diseases.
The immune system detects and eradicates non-self entities such as pathogens for the purpose of host defense. The invasion of pathogens induces the activation of the innate immune system followed by the antigen-specific response of the acquired immune system. The bone marrow is one of the two primary lymphoid organs. It is the primary site of hematopoiesis, harboring hematopoietic stem cells (HSCs) and myeloid and lymphoid progenitors, as well as mature immune cells, including B cells, neutrophils, macrophages and T cells.1,2) Therefore, bone and immune cells share the same microenvironment in the bone marrow. It is highly likely that bone and immune cells interact with each other and elicit mutual influence.1,2) However, until fairly recently, little attention has been paid to the interaction and shared molecular mechanisms of the bone and immune systems.
Discovery of osteoclast differentiation factor
Osteoclasts are formed in co-cultures of osteoblastic cells and osteoclast precursor cells of the monocyte/macrophage lineage. Since direct contact with osteoblastic cells is required, it was expected that the osteoclast differentiation factor should be expressed by osteoclastogenesis-supporting cells, such as osteoblastic cells in a membrane-bound form.3) Osteoprotegerin (OPG) was first identified as a factor that inhibits osteoclastogenesis.4,5) Subsequently, osteoclast differentiation factor was discovered as the ligand of OPG in 1998.6,7) Surprisingly, osteoclast differentiation factor was found to be identical to the receptor activator of NF-κB (RANKL) already reported by immunologists as a factor expressed by T cells.8,9) The essential role of RANKL in osteoclast differentiation was proven by the finding that RANKL-deficient mice lack osteoclasts completely.10) RANKL-deficient mice exhibit osteopetrosis with no tooth eruption and a defect in the immune system: no lymph node formation.10) RANKL was found independently by bone biologists and immunologists, and plays crucial roles in both the bone and immune systems, and is thus regarded as one of the most representative osteoimmune molecules. In humans, loss-of-function mutations of RANKL are reported to cause autosomal recessive osteopetrosis.11) Anti-RANKL antibodies have thus been developed that target osteoclasts in the treatment of metastatic bone tumors and osteoporosis, with fairly successful clinical outcomes.12,13)
The mechanism of bone destruction in rheumatoid arthritis
Rheumatoid arthritis is a prevalent autoimmune disease characterized by the inflammation of multiple synovial joints followed by joint destruction and deformity. In the 1990s it was not well understood why bone is affected by autoimmunity. Certain researchers proposed that bone damage can be caused by the proteinases produced by inflammatory cells, but from the standpoint of bone biology, it was difficult to construe such severe bone loss occurring without the involvement of osteoclasts.14) We accordingly undertook an investigation of the mechanism of the bone destruction that occurs in arthritis by focusing on the contribution of osteoclasts.14) Numerous osteoclasts were observed at the interface of the inflammatory synovium and bone. Furthermore, it was found that osteoclasts were formed in cultures of the synovial cells derived from rheumatoid arthritis patients.14) We attributed the bone destruction to pathological synovial fibroblasts, since these cells have the ability to support osteoclastogenesis similar to osteoblastic cells in bone.14) After RANKL was cloned, we tested the expression of RANKL in rheumatoid arthritis patients and found that synovial fibroblasts express RANKL and induce osteoclastogenesis.15)
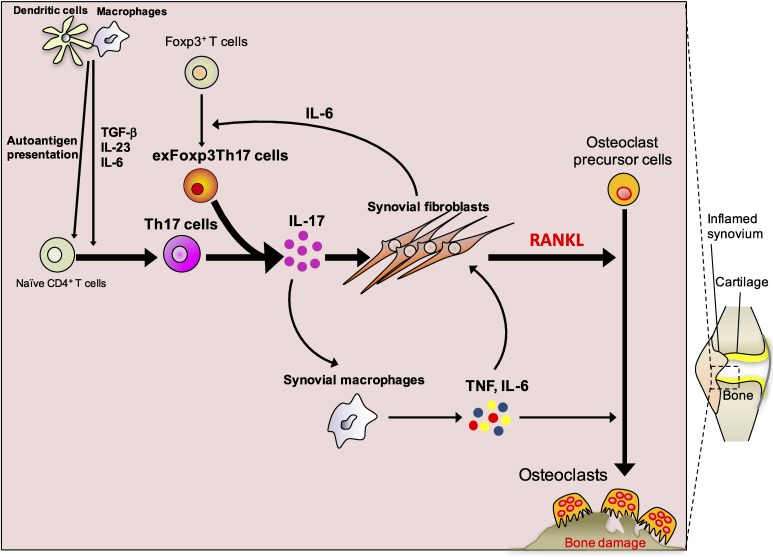
Because T cells also produce RANKL, there was a possibility that T cells infiltrate into the synovium and induce osteoclasts by expressing RANKL.16) We thus explored the effect of T cells on osteoclastogenesis in greater detail. Despite surface expression of RANKL, activated cells that produce interferon-γ suppressed osteoclastogenesis.17) We found that T cells produce various membrane-bound and soluble factors that influence osteoclastogenesis, and the effect of T cells on osteoclastogenesis is dependent on the balance between and among cytokines that T cells produce.17) This study explicitly dealt with the molecular mechanisms underlying the interaction between immune and bone cells.17) Dr. Yongwon Choi (Howard Hughes Medical Institute, Rockefeller University, currently University of Pennsylvania) coined the term “osteoimmunology” to describe this interdisciplinary research.18) If interferon-γ-producing T cells suppress osteoclastogenesis, which T cells are osteoclastogenic? We examined the effect of helper T cell subsets on osteoclasts and revealed interleukin-17 (IL-17) producing Th17 cells to be the exclusive osteoclastogenic subset.19) In arthritis, Th17 cells accumulate in the synovium and produce IL-17A, IL-17F and IL-21. IL-17A and IL-17F stimulate RANKL expression on synovial fibroblasts and at the same time make synovial macrophages secrete inflammatory cytokines such as tumor necrosis factor (TNF) and IL-6.19,20) Inflammatory cytokines further increase RANKL expression and stimulate osteoclast precursor cells. As a result, RANKL-induced osteoclastogenesis is powerfully stimulated, leading to excessive osteoclastic bone resorption and bone damage (Fig. 1). Osteoblastic bone formation was shown to be suppressed by TNF and TNF target molecules, such as dickkopf-1 (DKK1).21)
Figure 1.
(Color online) Molecular mechanisms of bone destruction in autoimmune arthritis. The autoimmune response starts with the presentation of autoantigens by antigen-presenting cells such as dendritic cells and macrophages. Naïve CD4+ T cells are activated by antigen-presenting cells and differentiate into Th17 cells, a process which is supported by various cytokines, including IL-6, IL-23 and TGF-β. Synovial fibroblast-derived IL-6 induces the conversion of Foxp3+ T cells into exFoxp3Th17 cells. Th17 cells and exFoxp3Th17 cells produce IL-17, which stimulates the expression of RANKL and inflammatory cytokines such as IL-6 in synovial fibroblasts. IL-17 also induces the production of inflammatory cytokines by synovial macrophages, including IL-6 and TNF. These inflammatory cytokines further upregulate RANKL on synovial fibroblasts and synergistically enhance RANKL-induced osteoclastogenesis.
Biologic disease-modifying antirheumatic drugs such as anti-TNF and anti-IL-6R antibodies are widely used in the clinic and brought about epoch-making change in the prognosis of rheumatoid arthritis.1,2) TNF or IL-6 blockage is considered to inhibit not only local inflammation but also the induction of RANKL and the osteoclast precursor cell activation, exerting a powerful suppressive effect on bone destruction.1,2) Anti-RANKL antibodies, the most direct method to suppress bone destruction, were approved for rheumatoid arthritis in Japan in 2017. It is worth noting that all drugs for rheumatoid arthritis other than anti-RANKL antibodies were developed to inhibit inflammation. Initiating the targeting of osteoclasts in the treatment of rheumatoid arthritis is one of the important fruits of osteoimmunology.
The origin of bone-damaging T cells
Regulatory T (Treg) cells suppress unnecessary, excessive and/or prolonged immune responses. We examined the fate of Treg cells in arthritis and found that a certain population of Tregs with a low expression of CD25 convert to Th17 cells in the inflammatory synovium.22) The Treg cells that lose the expression of the hallmark transcription factor Foxp3 and produce IL-17 are called exFoxp3Th17 cells.22) exFoxp3Th17 cells are more highly pathogenic than naïve-T cell-derived Th17 cells in terms of both inflammatory and osteoclastogenic capability, playing a critical role in the acceleration of arthritis (Fig. 1).
In arthritis, the conversion of Treg cells to Th17 cells is exclusively pathogenic, but we also considered the role of exFoxp3Th17 cells in periodontitis, since Th17 cells are important for the host defense against bacteria in mucosal immunity.23) exFoxp3Th17 cells are frequently identified in the periodontal tissues and have a critical role in the induction of alveolar bone loss.23) However, Th17 cells have also been shown to be important for the elimination of bacteria in periodontitis. Moreover, systemic bacterial dissemination from periodontal tissue is suppressed by the removal of infected teeth.23) This is a rare observation of a beneficial effect of bone and tooth loss on host defense. We infer that the bone loss mechanism exerted by Th17 cells might be due to a primitive host defense against pathogens that arose in polyphyodont vertebrates.23)
Osteoclastogenic signal transduction
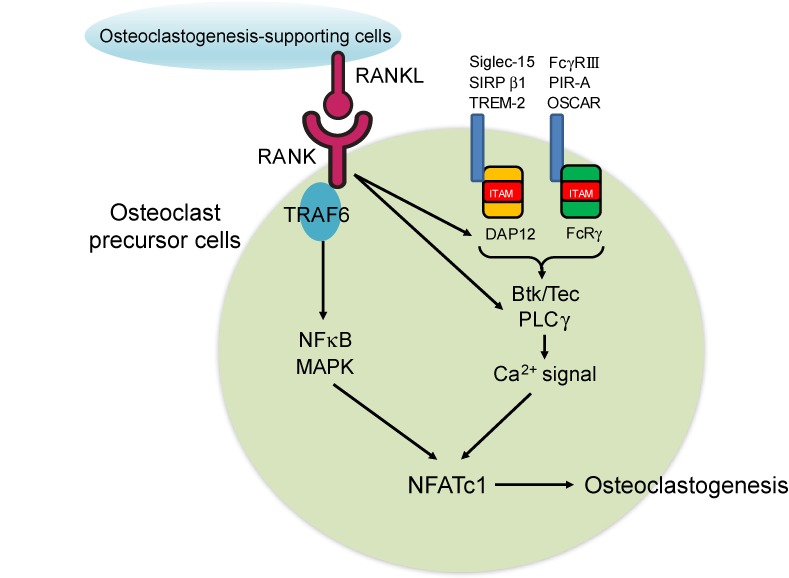
Before the discovery of RANKL, the knowledge about the molecules involved in osteoclast differentiation was obtained from naturally occurring or genetically modified mice that develop osteopetrosis.1,2) NF-κB, tumor necrosis factor receptor-associated factor 6 (TRAF6) and c-Fos were among the essential mediators of osteoclastogenic signaling, but none of these factors represented the specific master regulator of osteoclast differentiation.1,2) We performed a genome-wide screening of RANKL-inducible genes and identified nuclear factor of activated T cells c1 (NFATc1) as the master transcription factor for osteoclastogenesis.24) NFATc1 is markedly and specifically induced by RANKL in osteoclast precursor cells and is necessary and sufficient for osteoclast fate commitment.24) NFATc1 activation requires calcium signaling, which is dependent on the immunoreceptor tyrosine-based activation motif (ITAM) signaling mediated by Fc receptor γ-chain (FcRγ) and DNAX-activating protein of 12 kDa (DAP12).25) These adaptor molecules associate with immunoglobulin-like receptors such as FcγRIII, osteoclast-associated receptor (OSCAR), paired immunoglobulin-like receptor A (PIR-A), signal-regulatory protein β-1 (SIRPβ1), sialic acid binding immunoglobulin-like lectin 15 (Siglec-15) and triggering receptor expressed on myeloid cells 2 (TREM-2) (Fig. 2). These receptors convey costimulatory signals for RANKL. The ligands of the costimulatory receptors are not yet fully identified, but include immunglobulin G (IgG) immune complex, collagen and major histocompatibility complex (MHC) class I molecules.1,2,26) RANKL binding to its receptor RANK results in the recruitment and trimerization of TRAF6, leading to the activation of NF-κB and the induction of NFATc1 (Fig. 2). NFATc1 is persistently stimulated by the calcium signaling activated by Tec family kinases and phospholipase Cγ (PLCγ), which are phosphorylated downstream of RANK and ITAM signaling.27,28) NFATc1 and c-Fos cooperatively bind the NFATc1 promoter and this induces the autoamplification of NFATc1, which is a hallmark event of osteoclast differentiation (Fig. 2).29) It is notable that most of the molecules utilized in osteoclast signal transduction were originally identified and have been closely studied in the immunology field, highlighting the importance of the molecules and regulatory mechanisms shared by the bone and the immune systems.
Figure 2.
(Color online) Osteoclastogenic signal transduction. Osteoclastogenesis-supporting cells, such as osteoblasts and osteocytes, express RANKL. RANKL binding to the RANK expressed by osteoclast precursors leads to the activation of signaling cascades, including the NF-κB and MAPK pathways, via the adapter protein TRAF6. RANKL also stimulates the ITAM signaling mediated by immunoglobulin-like receptors such as TREM-2, SIRPβ1, Siglec-15, OSCAR, PIR-A and FcγRIII, which associate with DAP12 or FcRγ. RANK and ITAM signaling activate Btk/Tec and PLCγ, which in turn induce calcium signaling. These signaling cascades ultimately lead to the induction and activation of NFATc1, the master transcriptional factor of osteoclastogenesis.
Which cells express RANKL?
Even after the anti-RANKL antibodies had been introduced into the clinic, it remained unclear which cells were the major source of RANKL under physiologic and pathological conditions. In vitro experiments suggested that osteoblastic cells support osteoclastogenesis, but the in vivo source remained obscure, since osteoblastic cells include mesenchymal progenitor cells, osteoblasts and osteocytes. We and others provided in vivo evidence for osteocyte regulation of osteoclastogenesis through RANKL expression in postnatal bone remodeling.30,31) It was also shown that osteoblasts and chondrocytes are the major source of RANKL during embryonic development. RANKL flox mice also helped determine the RANKL-expressing cells in arthritis. Bone destruction was ameliorated in mice lacking RANKL in synovial fibroblasts but not in mice lacking RANKL in T cells, indicating that it is the synovial fibroblasts that are the main source of RANKL in arthritis.32) Although T cells are deeply involved in the inflammatory responses and the initiation of bone damage, T cells do not induce osteoclastogenesis by expressing RANKL, but rather stimulate the surrounding synovial fibroblasts to express RANKL and indirectly induce bone damage. Consistent with this, a recent report on single cell analysis of arthritic fibroblasts indicated that RANKL-expressing synovial fibroblasts are the fibroblast subset responsible for bone destruction.33,34) In periodontitis, RANKL was shown to be expressed by periodontal ligament cells and osteoblasts rather than T cells or B cells.23)
Immunological function of RANKL
As mentioned above, RANKL is regarded as a key osteoimmune molecule and drug target in bone metastasis, osteoporosis and rheumatoid arthritis. RANKL was first identified in T cells and suggested to regulate dendritic cells, but the function of RANKL in acute immune response has not been emphasized in the literature, partly because of the redundant function of CD40.1,2) When T cell-specific RANKL conditional knockout mice were examined in experimental autoimmune encephalomyelitis, we noticed that Th17 migration to the central nervous system was reduced in spite of a normal accumulation of Th17 in the periphery.35) RANKL expressed on T cells was involved in the T cell-astrocyte interaction and induction of chemokine (C-C motif) ligand (CCL)-20 required for Th17 migration across the blood brain barrier, suggesting RANKL blockade may have beneficial effect on diseases characterized by inflammation of the central nervous system, such as multiple sclerosis.35)
RANKL expressed by CD4 T cells is important for the establishment of central immune tolerance by fostering the thymic medullary mociroenvironments.36–38) It is interesting that RANKL also critically contributes to the development of another primary lymphoid organ, the thymus. RANKL together with CD40 regulates the differentiation of medullary thymic epithelial cells expressing autoimmune regulator (AIRE). AIRE is one of the factors that regulate the expression of tissue-restricted antigens that are required for the negative selection of autoreactive T cells.36,37)
RANKL has also been shown to be indispensable for the differentiation of microfold (M) cells, which are mainly found in gut-associated lymphoid tissue (GALT). M cells are specialized epithelial cells which take up and transport bacterial antigens.39) We found that RANKL-expressing mesenchymal cells (called M cell-inducer cells) directly interact with the gut epithelium to control CCL20 expression and M cell differentiation.39) The deletion of mesenchymal RANKL impairs M cell-dependent antigen sampling and B cell-dendritic cell interaction, which results in a reduction in IgA production and a decrease in microbial diversity. RANKL and OPG have emerged as critical regulators of the immune response in the gut mucosa.39)
RANKL biology beyond the bone and immune systems
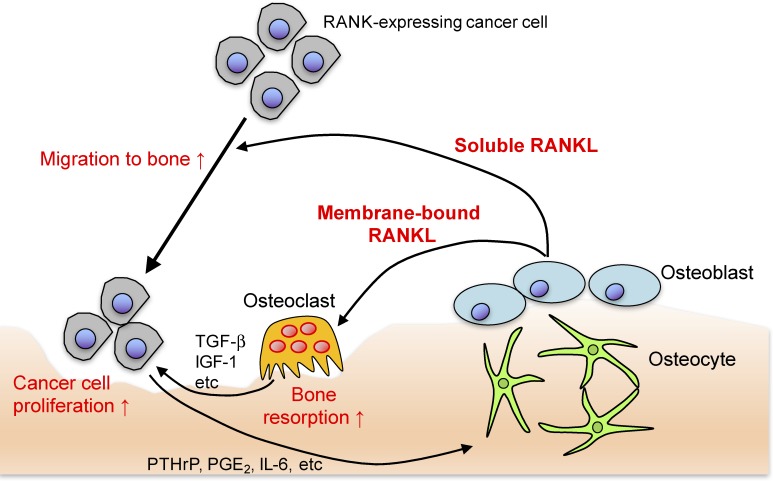
RANKL has also been shown to be indispensable for mammary gland formation during pregnancy and involved in the central nervous system control of fever and female body temperature.40,41) Anti-RANKL antibodies have been applied to control the skeletal related events in metastatic bone tumors, mainly because tumor cells and osteoclasts form a vicious circle: metastasized tumor cells produce parathyroid hormone-related protein (PTHrP) and IL-6, inducing RANKL, which stimulates osteoclastic bone resorption, leading to the release of growth factors such as transforming growth factor (TGF)-β and insulin-like growth factor-1 (IGF-1), thus promoting tumor growth (Fig. 3).1,2) If RANKL function was limited to the activation of osteoclasts, anti-RANKL treatment would be effective in only the osteolytic tumors that stimulate osteoclasts. In fact, anti-RANKL therapy suppresses non-osteolytic tumors in models of bone metastasis. This shows that the role of RANKL is not limited to osteoclast activation in the process of bone metastasis.1,2) It has been demonstrated that RANKL functions as a chemotactic factor which recruits RANK-expressing tumors to bone.42) It has also been shown that tumors that favor bone metastasis frequently express RANK.42)
Figure 3.
(Color online) The mechanisms of bone metastasis. The soluble RANKL released by osteogenic cells functions as a chemotactic factor which recruits RANK-expressing tumors to bone. Metastasized tumor cells produce PTHrP, prostaglandin E2 (PGE2) and IL-6, which stimulate osteoclastic bone resorption via the induction of membrane-bound RANKL on osteogenic cells. Accelerated bone resorption leads to the release of growth factors such as TGF-β and IGF-1, promoting tumor growth. Thus, tumor cells and osteoclasts form a vicious circle. RANKL represents a promising target for the prevention and treatment of bone metastasis.
The RANKL expressed in the membrane-bound form is cleaved by proteinases into the soluble form. It has been suggested that the membrane-bound form functions more efficiently than the soluble form, but there was no clear in vivo data to support this difference.3,43,44) To address this question, we generated mice in which the cleavage site of RANKL was deleted.39,45) The mice had no detectable soluble form of RANKL but a normal level of membrane-bound RANKL.39,45) Surprisingly, the soluble RANKL-deficient mice exhibited no abnormalities in bone development, osteoclastogenesis, lymph node organogenesis, thymic epithelial cells, M cells or mammary gland development.45) We examined certain disease models including ovariectomy-induced osteoporosis and periodontitis, but there was no significant difference in the severity of the symptoms.23,45) The only difference found was in bone metastasis cancer models.45) Bone metastasis of B16F10 melanoma cells injected into the heart was markedly reduced in cases of soluble RANKL deficiency.45) Therefore, soluble RANKL is physiologically dispensable but involved in the acceleration of tumor metastasis to bone (Fig. 3).45) In humans, the serum level of soluble RANKL has been shown to correlate with the prognosis of bone metastasis. RANKL has also been shown to be involved in the onset of certain malignant tumors, including mammary tumors, and in the regulation of anti-tumor immunity.1,2) Thus, RANKL has attracted considerable attention from researchers in a variety of physiological and pathological conditions, which together might be called “RANKL biology”, extending outward from its initial role in the osteoimmune system.1,2)
Regulation of immune cells by bone
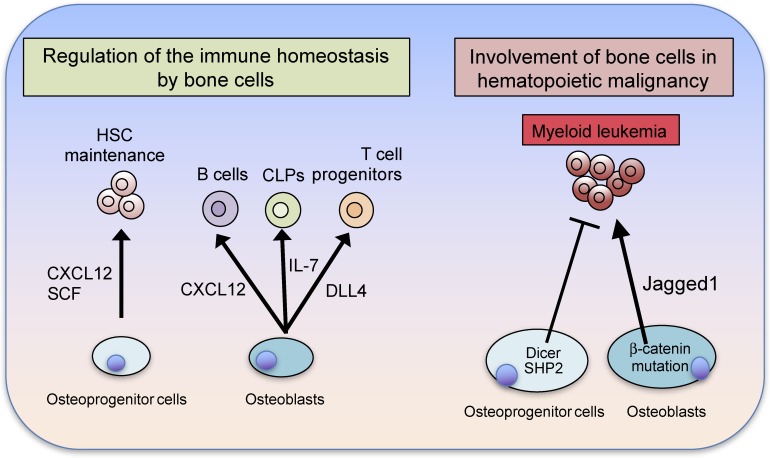
Since immune cells are able to infiltrate into any organ and exert effects in all tissues, the special relationship between bone and the immune system is not complete without a demonstration of bidirectional regulation. The regulation of immune cells by bone is particularly important in this context. HSCs are known to reside in the bone marrow after birth and the mesenchymal osteoprogenitor cells that express CXC chemokine ligand (CXCL)-12 and leptin receptors function as the HSC niche.1,2) Mature osteoblasts mainly support lymphoid progenitor cells via IL-7 production.1,2,46) In addition to physiological maintenance of hematopoietic progenitors, bone cells play a role in restraining HSCs from transformation into malignancy. The function of osteoclasts in HSC regulation is still controversial and needs further study, but osteogenic cells play a crucial role in the regulation of HSCs, shedding light on the bidirectional dialogue between the bone and immune systems (Fig. 4).1,2) Bone functions as a part of the skeletal system, a calcium reservoir and lymphoid organ. In light of the fact that all of the cells in the bone marrow share the same microenvironment, the time has come to recognize that all bone functions take place in the context of the osteoimmune system.
Figure 4.
(Color online) Immune regulation by bone cells. Bone cells critically contribute to the immune homeostasis that takes place in the bone marrow microenvironment. Osteoprogenitors, including leptin receptor-expressing (LepR+) cells and CXCL12-abundant reticular (CAR) cells support HSCs by producing SCF and CXCL12. Osteoblasts produce IL-7, delta-like 4 (DLL4) and CXCL12 to support common lymphoid progenitors (CLPs), T cell progenitors and B cells, respectively. Alterations in osteogenic cells predispose them toward the development of hematologic malignancy. Deletion of Dicer in osteoprogenitors leads to the development of myeloid leukemia. The constitutive activation of β-catenin in osteoblasts increases the expression of the Notch ligand Jagged1 in osteoblasts, leading to the development of myeloid leukemia. The activating mutations of Src homology phosphatase 2 (SHP2) in osteoprogenitor cells result in the development of myeloid leukemia. Thus, osteoblast lineage cells importantly contribute to the suppression of hematologic malignancy.
Bone cell communication factors
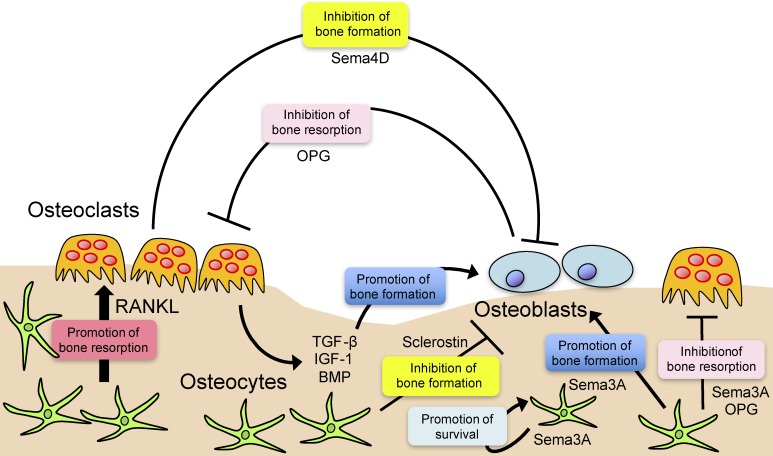
If all of the cells in the bone marrow interact with each other and regulate bone function in cooperation, it is of critical importance to identify the molecular mechanisms involved in bone cell communication. Since bone is formed in a magnitude that coordinated with the level of bone resorption, it has long been expected that this coupling process (i.e., the transition from resorption to formation) is linked by certain molecules called “coupling factors”.1,2) IGF-1 and TGF-β are among the classical coupling factors that are released by bone resorption and increase bone formation.1,2) More recently, factors such as sphingosine-1-phosphate (S1P), ephrins, semaphorins and Wnt family proteins have been suggested to play a role in bone cell communication, but the interacting effect is not always the same as classical coupling mechanisms.1,2) For example, the semaphorin (Sema)-4D produced by osteoclasts suppresses bone formation, suggesting that this molecule is working in the bone resorption phase (Fig. 5).47)
Figure 5.
(Color online) Bone cell communication. Bone cells secrete factors that modulate bone resorption and formation. Osteoclast formation is induced by RANKL, which is secreted from osteoblastic cells, including osteocytes. Osteoblastic cells produce OPG, a decoy receptor for RANKL, in order to inhibit osteoclastic bone resorption. Osteoclasts inhibit bone formation through the production of Sema4D. TGF-β, IGF-1 and bone morphogenetic protein (BMP) are positive regulators of bone formation stored in the bone matrix that are released as soluble factors by osteoclastic bone resorption. Sema3A produced by osteocytes contributes to bone homeostasis by autoregulation of osteocyte survival in addition to osteoclast suppression and osteoblast stimulation. Osteocytes produce sclerostin, a potent inhibitor of osteoblastic bone formation.
OPG, one of the earliest identified bone cell communication factors, was thought to explain the inhibitory effect of osteoblast conditioned medium on osteoclast formation. We found such inhibitory activity even in the conditioned medium of OPG-deficient osteoblasts and purified the molecule related to this activity.48) Sema3A was thus shown to be a potent osteoprotective factor, inhibiting osteoclast differentiation and promoting osteoblastic bone formation.48) Since Sema3A is produced by neuronal cells and acts as a repulsive factor for neurite growth, we investigated the in vivo source of Sema3A using Sema3A conditional knockout mice.49) In conclusion, Sema3A produced by osteocytes plays a major role in bone homeostasis in the postnatal stage by autoregulation of osteocyte survival in addition to osteoclast suppression and osteoblast stimulation (Fig. 5).49) Notably, RANKL and sclerostin (an inhibitor of Wnt), both of which are osteocyte products, have turned out to be auspicious drug targets, while Sema3A utilizes RANKL and Wnt signaling to regulate bone cells (Fig. 5).49)
Evolutionary insight into the interplay of the bone and the immune systems
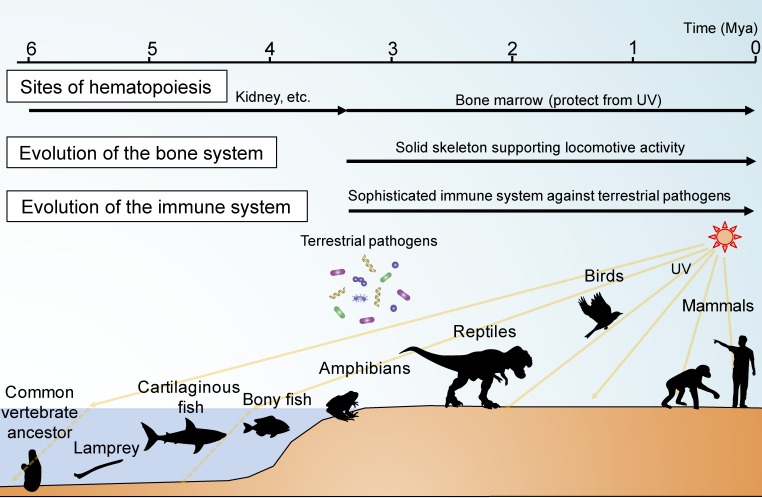
Bone has apparently unrelated functions: locomotion, calcium reservoir and hematopoiesis. During the course of vertebrate evolution, the skeletal system needed to be strengthened when aquatic vertebrates moved onto land and had to cope with the resistance of gravity in the new environment.1) At the same time, the calcium which had previously been readily taken up from sea water now needed to be stored in the body.1) The development of the acquired immune system also accelerated after exposure to the complexity of microorganisms in the terrestrial conditions.1) Furthermore, the exposure to a high level of ultraviolet light necessitated the housing of HSCs in a shielded place.1) The reason why so many different functions came to center on bone can be speculatively attributed to the simultaneous requirements of a strong skeleton, calcium storage, an advanced immune system and a hiding place for HSCs (Fig. 6).
Figure 6.
(Color online) Origin of the osteoimmune system in evolution. The bone and immune systems became linked during the course of vertebrate evolution. When aquatic vertebrates moved onto land, the skeletal system adapted to the need to support locomotive activity in the terrestrial environment. At the same time, the immune system rapidly evolved so as to cope with the plethora of terrestrial pathogens, and the location of hematopoiesis moved to the bone marrow to protect HSCs against the higher level of ultraviolet (UV) light exposure on land. Thus, the bone and immune systems developed synchronously, ultimately comprising an osteoimmune system, functioning as a locomotor organ and mineral reservoir as well as a primary lymphoid organ.
Future perspectives
Twenty years have passed since the term osteoimmunology was first coined. Since osteoimmunology deals with subjects beyond the scope of traditional immunology or bone research, it was at first not readily accepted as a discipline. However, the last two decades have witnessed the emergence of certain critically important insights from the osteoimmunological approach that have made it difficult for either immunologists or bone biologists to adequately understand their own fields without osteoimmunological input. The osteoimmune system is now one of the critical concepts in biology. Ultimately, it was human beings who separated the bone and the immune systems into distinct fields of inquiry, which nature did not. The time has come to go beyond the conventional paradigm and investigate biological systems from the viewpoint of multi-organ linkages. The clinical application of anti-osteoclast therapy to rheumatoid arthritis has been one of the important achievements of the osteoimmunological approach. The future discovery of bone cell communication factors would be expected to provide valuable new therapeutic targets in bone and immune diseases. Thus, the dynamic field of osteoimmunology will provide a thought-provoking paradigm for understanding vertebrate biology as a whole as well as providing a molecular basis for critically needed new treatments.
Acknowledgements
I thank all the lab members including M. Tsukasaki for critical reading of the manuscript, fruitful discussion and assistance in preparing the manuscript. I also appreciate the kind support from K. Okamoto and A. Terashima, who belong to Department of Osteoimmunology, Graduate School of Medicine and Faculty of Medicine, The University of Tokyo. This work was supported in part by a grant for the Grants-in-Aid for Specially Promoted Research (15H05703) from the Japan Society for the Promotion of Science (JSPS).
Profile
Hiroshi Takayanagi was born in Tokyo in 1965. He graduated from The University of Tokyo and became an orthopaedic surgeon and rheumatologist in the Department of Orthopaedic Surgery, Graduate School of Medicine, The University of Tokyo Hospital. After serving as a surgeon for 7 years, he started his basic research career in the Department of Orthopaedic Surgery. He studied the mechanism of bone destruction in rheumatoid arthritis, with a specific focus on the RANKL protein. He then moved to the Department of Immunology in the same university. After being awarded his Ph.D. degree in 2001, he became an Assistant Professor in the Department of Immunology. He explored the role of immune molecules such as interferons in the regulation of bone metabolism. He revealed that the key factors NFATc1 and immunoglobulin-like receptors in osteoclast development and pioneered a new interdisciplinary field called “osteoimmunology.” From 2003 through 2012, he was a Professor at the Tokyo Medical and Dental University. Since 2012, he has served as a Professor, Department of Immunology, Graduate School of Medicine and Faculty of Medicine, The University of Tokyo, focusing on osteoimmunology and the pathogenesis of autoimmune disease as well as the mechanism of immune tolerance established in the thymus. He has been President of the Japanese Society of Osteoimmunology since 2014. He was awarded the Japan Academy Prize in 2019.
References
- 1).Tsukasaki M., Takayanagi H. (2019) Osteoimmunology: Evolving concepts in bone-immune interactions in health and disease. Nat. Rev. Immunol. 19, 626–642. [DOI] [PubMed] [Google Scholar]
- 2).Okamoto K., Nakashima T., Shinohara M., Negishi-Koga T., Komatsu N., Terashima A., et al. (2017) Osteoimmunology: The conceptual framework unifying the immune and skeletal systems. Physiol. Rev. 97, 1295–1349. [DOI] [PubMed] [Google Scholar]
- 3).Takahashi N., Akatsu T., Udagawa N., Sasaki T., Yamaguchi A., Moseley J.M., et al. (1988) Osteoblastic cells are involved in osteoclast formation. Endocrinology 123, 2600–2602. [DOI] [PubMed] [Google Scholar]
- 4).Simonet W.S., Lacey D.L., Dunstan C.R., Kelley M., Chang M.S., Lüthy R., et al. (1997) Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell 89, 309–319. [DOI] [PubMed] [Google Scholar]
- 5).Yasuda H., Shima N., Nakagawa N., Mochizuki S.I., Yano K., Fujise N., et al. (1998) Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): A mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology 139, 1329–1337. [DOI] [PubMed] [Google Scholar]
- 6).Yasuda H., Shima N., Nakagawa N., Yamaguchi K., Kinosaki M., Mochizuki S., et al. (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl. Acad. Sci. U.S.A. 95, 3597–3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Lacey D.L., Timms E., Tan H.L., Kelley M.J., Dunstan C.R., Burgess T., et al. (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93, 165–176. [DOI] [PubMed] [Google Scholar]
- 8).Anderson D.M., Maraskovsky E., Billingsley W.L., Dougall W.C., Tometsko M.E., Roux E.R., et al. (1997) A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature 390, 175–179. [DOI] [PubMed] [Google Scholar]
- 9).Wong B.R., Rho J., Arron J., Robinson E., Orlinick J., Chao M., et al. (1997) TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells. J. Biol. Chem. 272, 25190–25194. [DOI] [PubMed] [Google Scholar]
- 10).Kong Y.Y., Yoshida H., Sarosi I., Tan H.L., Timms E., Capparelli C., et al. (1999) OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397, 315–323. [DOI] [PubMed] [Google Scholar]
- 11).Sobacchi C., Frattini A., Guerrini M.M., Abinun M., Pangrazio A., Susani L., et al. (2007) Osteoclast-poor human osteopetrosis due to mutations in the gene encoding RANKL. Nat. Genet. 39, 960–962. [DOI] [PubMed] [Google Scholar]
- 12).Takeuchi T., Tanaka Y., Ishiguro N., Yamanaka H., Yoneda T., Ohira T., et al. (2016) Effect of denosumab on Japanese patients with rheumatoid arthritis: a dose-response study of AMG 162 (Denosumab) in patients with RheumatoId arthritis on methotrexate to Validate inhibitory effect on bone Erosion (DRIVE)-a 12-month, multicentre, randomised, double-blind, placebo-controlled, phase II clinical trial. Ann. Rheum. Dis. 75, 983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Takeuchi T., Tanaka Y., Soen S., Yamanaka H., Yoneda T., Tanaka S., et al. (2019) Effects of the anti-RANKL antibody denosumab on joint structural damage in patients with rheumatoid arthritis treated with conventional synthetic disease-modifying antirheumatic drugs (DESIRABLE study): A randomised, double-blind, placebo-controlled phase 3 trial. Ann. Rheum. Dis. 78, 899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Takayanagi H., Oda H., Yamamoto S., Kawaguchi H., Tanaka S., Nishikawa T., et al. (1997) A new mechanism of bone destruction in rheumatoid arthritis: synovial fibroblasts induce osteoclastogenesis. Biochem. Biophys. Res. Commun. 240, 279–286. [DOI] [PubMed] [Google Scholar]
- 15).Takayanagi H., Iizuka H., Juji T., Nakagawa T., Yamamoto A., Miyazaki T., et al. (2000) Involvement of receptor activator of nuclear factor κB ligand/osteoclast differentiation factor in osteoclastogenesis from synoviocytes in rheumatoid arthritis. Arthritis Rheum. 43, 259–269. [DOI] [PubMed] [Google Scholar]
- 16).Kong Y.Y., Feige U., Sarosi I., Bolon B., Tafuri A., Morony S., et al. (1999) Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 402, 304–309. [DOI] [PubMed] [Google Scholar]
- 17).Takayanagi H., Ogasawara K., Hida S., Chiba T., Murata S., Sato K., et al. (2000) T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-γ. Nature 408, 600–605. [DOI] [PubMed] [Google Scholar]
- 18).Arron J.R., Choi Y. (2000) Bone versus immune system. Nature 408, 535–536. [DOI] [PubMed] [Google Scholar]
- 19).Sato K., Suematsu A., Okamoto K., Yamaguchi A., Morishita Y., Kadono Y., et al. (2006) Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J. Exp. Med. 203, 2673–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Kotake S., Udagawa N., Takahashi N., Matsuzaki K., Itoh K., Ishiyama S., et al. (1999) IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J. Clin. Invest. 103, 1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Diarra D., Stolina M., Polzer K., Zwerina J., Ominsky M.S., Dwyer D., et al. (2007) Dickkopf-1 is a master regulator of joint remodeling. Nat. Med. 13, 156–163. [DOI] [PubMed] [Google Scholar]
- 22).Komatsu N., Okamoto K., Sawa S., Nakashima T., Oh-hora M., Kodama T., et al. (2014) Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat. Med. 20, 62–68. [DOI] [PubMed] [Google Scholar]
- 23).Tsukasaki M., Komatsu N., Nagashima K., Nitta T., Pluemsakunthai W., Shukunami C., et al. (2018) Host defense against oral microbiota by bone-damaging T cells. Nat. Commun. 9, 701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Takayanagi H., Kim S., Koga T., Nishina H., Isshiki M., Yoshida H., et al. (2002) Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 3, 889–901. [DOI] [PubMed] [Google Scholar]
- 25).Koga T., Inui M., Inoue K., Kim S., Suematsu A., Kobayashi E., et al. (2004) Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature 428, 758–763. [DOI] [PubMed] [Google Scholar]
- 26).Negishi-Koga T., Gober H.J., Sumiya E., Komatsu N., Okamoto K., Sawa S., et al. (2015) Immune complexes regulate bone metabolism through FcRγ signalling. Nat. Commun. 6, 6637. [DOI] [PubMed] [Google Scholar]
- 27).Shinohara M., Koga T., Okamoto K., Sakaguchi S., Arai K., Yasuda H., et al. (2008) Tyrosine kinases Btk and Tec regulate osteoclast differentiation by linking RANK and ITAM signals. Cell 132, 794–806. [DOI] [PubMed] [Google Scholar]
- 28).Sato K., Suematsu A., Nakashima T., Takemoto-Kimura S., Aoki K., Morishita Y., et al. (2006) Regulation of osteoclast differentiation and function by the CaMK-CREB pathway. Nat. Med. 12, 1410–1416. [DOI] [PubMed] [Google Scholar]
- 29).Asagiri M., Sato K., Usami T., Ochi S., Nishina H., Yoshida H., et al. (2005) Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J. Exp. Med. 202, 1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Nakashima T., Hayashi M., Fukunaga T., Kurata K., Oh-Hora M., Feng J.Q., et al. (2011) Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 17, 1231–1234. [DOI] [PubMed] [Google Scholar]
- 31).Xiong J., Onal M., Jilka R.L., Weinstein R.S., Manolagas S.C., O’Brien C.A. (2011) Matrix-embedded cells control osteoclast formation. Nat. Med. 17, 1235–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Danks L., Komatsu N., Guerrini M.M., Sawa S., Armaka M., Kollias G., et al. (2016) RANKL expressed on synovial fibroblasts is primarily responsible for bone erosions during joint inflammation. Ann. Rheum. Dis. 75, 1187–1195. [DOI] [PubMed] [Google Scholar]
- 33).Mizoguchi F., Slowikowski K., Wei K., Marshall J.L., Rao D.A., Chang S.K., et al. (2018) Functionally distinct disease-associated fibroblast subsets in rheumatoid arthritis. Nat. Commun. 9, 789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Croft A.P., Campos J., Jansen K., Turner J.D., Marshall J., Attar M., et al. (2019) Distinct fibroblast subsets drive inflammation and damage in arthritis. Nature 570, 246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Guerrini M.M., Okamoto K., Komatsu N., Sawa S., Danks L., Penninger J.M., et al. (2015) Inhibition of the TNF family cytokine RANKL prevents autoimmune inflammation in the central nervous system. Immunity 43, 1174–1185. [DOI] [PubMed] [Google Scholar]
- 36).Hikosaka Y., Nitta T., Ohigashi I., Yano K., Ishimaru N., Hayashi Y., et al. (2008) The cytokine RANKL produced by positively selected thymocytes fosters medullary thymic epithelial cells that express autoimmune regulator. Immunity 29, 438–450. [DOI] [PubMed] [Google Scholar]
- 37).Akiyama T., Shimo Y., Yanai H., Qin J., Ohshima D., Maruyama Y., et al. (2008) The tumor necrosis factor family receptors RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity 29, 423–437. [DOI] [PubMed] [Google Scholar]
- 38).Rossi S.W., Kim M.Y., Leibbrandt A., Parnell S.M., Jenkinson W.E., Glanville S.H., et al. (2007) RANK signals from CD4+3− inducer cells regulate development of Aire-expressing epithelial cells in the thymic medulla. J. Exp. Med. 204, 1267–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Nagashima K., Sawa S., Nitta T., Tsutsumi M., Okamura T., Penninger J.M., et al. (2017) Identification of subepithelial mesenchymal cells that induce IgA and diversify gut microbiota. Nat. Immunol. 18, 675–682. [DOI] [PubMed] [Google Scholar]
- 40).Fata J.E., Kong Y.Y., Li J., Sasaki T., Irie-Sasaki J., Moorehead R.A., et al. (2000) The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell 103, 41–50. [DOI] [PubMed] [Google Scholar]
- 41).Hanada R., Leibbrandt A., Hanada T., Kitaoka S., Furuyashiki T., Fujihara H., et al. (2009) Central control of fever and female body temperature by RANKL/RANK. Nature 462, 505–509. [DOI] [PubMed] [Google Scholar]
- 42).Jones D.H., Nakashima T., Sanchez O.H., Kozieradzki I., Komarova S.V., Sarosi I., et al. (2006) Regulation of cancer cell migration and bone metastasis by RANKL. Nature 440, 692–696. [DOI] [PubMed] [Google Scholar]
- 43).Udagawa N., Takahashi N., Yasuda H., Mizuno A., Itoh K., Ueno Y., et al. (2000) Osteoprotegerin produced by osteoblasts is an important regulator in osteoclast development and function. Endocrinology 141, 3478–3484. [DOI] [PubMed] [Google Scholar]
- 44).Tsukasaki M., Hamada K., Okamoto K., Nagashima K., Terashima A., Komatsu N., et al. (2017) LOX fails to substitute for RANKL in osteoclastogenesis. J. Bone Miner. Res. 32, 434–439. [DOI] [PubMed] [Google Scholar]
- 45).Asano T., Nakai Y., Tsutsumi M., Muro R., Suematsu A., Hashimoto K., et al. (2019) Soluble RANKL is physiologically dispensable but accelerates tumour metastasis to bone. Nat. Metab. 1, 868–875. [DOI] [PubMed] [Google Scholar]
- 46).Terashima A., Okamoto K., Nakashima T., Akira S., Ikuta K., Takayanagi H. (2016) Sepsis-induced osteoblast ablation causes immunodeficiency. Immunity 44, 1434–1443. [DOI] [PubMed] [Google Scholar]
- 47).Negishi-Koga T., Shinohara M., Komatsu N., Bito H., Kodama T., Friedel R.H., et al. (2011) Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nat. Med. 17, 1473–1480. [DOI] [PubMed] [Google Scholar]
- 48).Hayashi M., Nakashima T., Taniguchi M., Kodama T., Kumanogoh A., Takayanagi H. (2012) Osteoprotection by semaphorin 3A. Nature 485, 69–74. [DOI] [PubMed] [Google Scholar]
- 49).Hayashi M., Nakashima T., Yoshimura N., Okamoto K., Tanaka S., Takayanagi H. (2019) Autoregulation of osteocyte Sema3A orchestrates estrogen action and counteracts bone aging. Cell Metab. 29, 627–637.e5. [DOI] [PubMed] [Google Scholar]