Abstract
Background
Limited studies have been published on practices and management of Coronavirus Disease 2019 (COVID-19) in children. Despite the fact that COVID-19 rarely caused any severe disease in children, the asymptomatic children might be playing an important role for spreading COVID-19 in healthcare facilities. This review aimed at sharing our experience of how to handle patients with COVID-19 in a pediatric referral and tertiary care hospital to prevent the possible transmissions to the healthcare workers (HCWs).
Methods
This review sought to identify infection control practices measures during COVID-19 pandemic comes from our daily practice combined with the most recent guidelines with the new experience and information.
Results
Prevention the transmission of COVID-19 to the HCWs, 4 primary themes should be taken into consideration; (1) ongoing education and importance of the organization of the healthcare facility, (2) proper clinical triage and isolation of the suspected or confirmed COVID-19 patients in the outpatient clinics and in the emergency departments, (3) necessity of the organization of the COVID-19 wards, and (4) utilization of personal protective equipment.
Conclusions
Infection control precautions to prevent the possible transmissions to HCWs as well as the other patients and their caregivers from children with COVID-19 are very critical. If sufficient precautions are not taken, healthcare settings may serve as additional source of transmission and spread of COVID-19 in the society.
Key Words: Coronavirus, Healthcare workers, Experience in children
In December 2019, a number of patients with pneumonia of unknown etiology were identified in Wuhan City, Hubei province in China.1 A new type of coronavirus was isolated by the Chinese authorities on January 7, 2020.2 Subsequently, the 2019 novel coronavirus was named by the World Health Organization (WHO) as the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and on February 11, 2020, WHO named the disease associated with 2019 novel coronavirus as the Coronavirus Disease 2019 (COVID-19).3
Since the outbreak of the COVID-19, and with its rapid spread worldwide, the number of infected cases and mortality rates have grown expeditiously. As a result of this rapid spread of COVID-19, WHO announced COVID-19 outbreak as a public health emergency of international concern on March 11, 2020.4 Within less than 3 months since the first set of identified cases, the infection spread to at least 200 countries. As of May 18, 2020, globally, there are 4,857,311 confirmed cases and at least 318,419 deaths due to COVID-19.5
Human coronaviruses (HCoVs), which are enveloped positive sense single-stranded RNA viruses have caused worldwide outbreaks with very high numbers of hospitalized cases.6 Before the identification of SARS-CoV-2, 6 types of HCoVs were known to infect humans. While 4 of these are endemic in humans (HCoV 229E, OC43, NL63, and HKU1), the remaining 2 are epidemic (severe acute respiratory syndrome coronavirus [SARS-CoV] and Middle East respiratory syndrome Coronavirus [MERS-CoV]).7 Generally, HCoVs cause milder respiratory diseases, whereas SARS-CoV and MERS-CoV, due to their high pathogenicity for humans, result in severe respiratory diseases and high mortality rates as in the case of SARS-CoV-2.8 , 9
In Turkey, the first case of COVID-19 was announced on March 11, 2020 and the first death was reported on March 17, 2020.10 While reported fatality rate remains relatively low, Turkey has one of the rapidly rising numbers of confirmed cases in the world. The Turkish Ministry of Health confirmed that the total number of documented cases increased to 150,593 and the numbers of deaths reached 4,171 on May 18, 2020.5 However, there is still no sufficient data on pediatric population in Turkey.
Although some clinical studies of adult COVID-19 exist,11, 12, 13 very limited studies have been published on COVID-19 in children so far. Currently, the data on children is accessible in studies on adult population with limited information on children.14, 15, 16, 17, 18, 19, 20, 21, 22 In a review of 45 relevant scientific papers and letters, it was observed that only 1%-5% of diagnosed COVID-19 cases were children, who generally had milder disease than adults and deaths had been extremely rare.23
Transmission of SARS-CoV-2 seems to be primarily through respiratory secretions on droplets of an infected individual by coughing or sneezing.24 , 25 There is not any available evidence of airborne spread for COVID-19, however this possibility must be taken into consideration while conducting certain aerosol-generating procedures in health care facilities.26 , 27 Recent studies showed the viability of SARS-CoV-2 on environmental surfaces26 , 27 that may indicate that the transmission of SARS-CoV-2 through contaminated surfaces might be possible. And although viable virus associated with fecal shedding has been demonstrated in a limited number of cases,28 yet, its role in transmission of COVID-19 is still controversial.
Data on individuals aged 18 years old and under suggest that there is a relatively low attack rate in this age group (about 2% of all reported cases).29 Published data from Italy reported that only 1.2% of COVID-19 cases were children.30 And early reported cases in the United States demonstrated that 1.7% occurred in children.31 In addition to the previous reports, a recent study from pediatric intensive care units at United States and Canada, including 48 COVID-19 patients hospitalized at pediatric intensive care units, 18 (38%) required invasive ventilation, and the hospital mortality rate was reported to be 4.2%, supporting the favorable outcomes of the children compared to adults.32 The largest child case series published previously demonstrated that 5.2% had severe disease and 0.6% had critical disease with respiratory failure, signs of multi-organ failure, encephalopathy, heart failure, abnormal coagulation, or acute renal failure.33 However, it should be noted that approximately 40% of the children with critical COVID-19 in this study were less than 5 years of age.33
Based on available data, it is possible to conclude that COVID-19 has been less severe among children compared to adults. However, these findings should be interpreted with caution as a high percentage of children might have remained undiagnosed due to the asymptomatic pattern of the disease in this age of group,22 , 33 which makes it not feasible to determine the full extent of infection among children.
A recent study reported that 86% of all early COVID-19 infections in China remained undiagnosed.34 According to the same study, despite the lower transmission rate of such asymptomatic cases, their greater number suggested that these might have been the source of 79% of all early cases.34 This may pose a problem since the undiagnosed children can be a major source of transmission and spread of COVID-19 in the society.
Since the onset of this pandemic, health care workers (HCWs) have been exposed to SARS-CoV-2 in different clinical settings. The Chinese Center for Disease Control and Prevention reported that among the 44,672 confirmed cases in mainland China, 3,019 HCWs were infected and 14.8% of the infected HCWs had severe and critical disease which led to 5 deaths.35 The initial infections of HCWs might be due to the contact with a new contagious disease, and thus can be attributed to limited knowledge. However, approximately 3 months after the beginning of COVID-19 outbreak, 94 doctors and 26 nurses had died due to SARS-CoV-2 in Italy, and a total of 12,681 HCWs got infected with SARS-CoV-2 according to the Italian Institute of Health.36 Currently, up to 10% of reported cases in China37and up to 9% of overall cases in Italy are among HCWs.38 A study from the Netherlands, where the first COVID-19 cases were reported on 27th February, state that 86 (6%) of the HCWs tested for COVID-19 were found to be positive.39 All of these reports reveal the vulnerability of the HCWs to COVID-19. Moreover, Centers for Disease Control and Prevention (CDC) COVID-19 response team reported that 9,282 HCWs were infected with COVID-19 in the United States,40 and it will not be surprising to expect more COVID-19 infected HCWs all around the World as long as this pandemic remains uncontrolled.
The COVID-19 pandemic has several aspects that need to be addressed. These include the patients’ health and treatment, the risk for HCWs and their protection, and the public health aspects and measures to reduce the impact of the pandemic. Despite the fact that COVID-19 rarely caused any severe disease in children, as mentioned above, the asymptomatic children might be playing an important role in spreading COVID-19 in health facilities as well as in outpatient clinics.
In this review, we aimed at sharing our experience of how to handle patients with COVID-19 at the University of Health Sciences Dr. Behçet Uz Children's Hospital and of the infection control committee measures to prevent the possible transmission to the healthy children and HCWs from the children with COVID-19.
Infection control practices experience in children with COVID-19
The current hospital is a 400-bed pediatric referral and tertiary care hospital in İzmir, Turkey. The hospital is a dedicated pediatric hospital with 24-bed pediatric intensive care unit, 80-bed neonatal intensive care unit, 30-bed pediatric hematology unit, 12-bed hematopoietic bone marrow transplantation unit, 6-bed pediatric cardiovascular, 12-bed pediatric surgery intensive care units in addition to general pediatric wards, pediatric orthopedics, otorhinolaryngology, and ophthalmology units. A total of 1,484 HCWs, including 287 doctors, 537 nurses, and 660 administrative staff work in the hospital. Moreover, there are several outpatient clinics and hemodialysis unit as well as other clinics. The emergency department serves about a half of the population of the third biggest city in Turkey. As of May 12, 2020, we had 55 confirmed COVID-19 pediatric cases, and additionally 230 suspected COVID-19 cases were hospitalized at the center. To the date this review was submitted, 4 of the HCWs were diagnosed with COVID-19, while no one was attributable to hospital exposure (the source of infection was generally their spouse or roommates).
The following are the main infection control measures that were taken during the COVID-19 pandemic which came from our daily practice combined with the most recent guidelines. Keeping in mind that with more experience and newly available information, the practices as well as guidelines will eventually change.
Education and importance of the organization of the healthcare facility
Since the start of the COVID-19 outbreak and later the declaration of a pandemic by the WHO, the infection control committee of the hospital has been providing continuing education workshops for all HCWs. Early infection prevention and control recommendations about SARS-CoV-2 were mostly adapted from the experience of MERS-CoV infection, and former severe acute respiratory diseases caused by other respiratory viruses.41 , 42 Updated information and management practices on COVID-19 were shared with the staff through ongoing workshops when necessary. Training videos with detailed instructions for HCWs were prepared on how to put on and remove personal protective equipment (PPE), procedures for using N95 masks, hand hygiene practices, etc. Moreover, all procedures were modified according to the changes in the national guidelines of the Ministry of Health including diagnosis and treatment strategies.
Following the announcement of the first COVID-19 case in Turkey, the hospital administration took the necessary precautions and updated and increased the PPE stocks under the guidance of the infection control committee. Initially, the number of N95 and N99 masks was increased in the early phase of the pandemic in Turkey; 2 weeks later the number of surgical masks and some other PPEs were also increased in line with the new recommendations of healthcare authorities requiring all HCWs to adopt in the healthcare facilities.
Furthermore, after the first case in Turkey, the hospital staff was reorganized into different shifts and the number of HCWs in the wards was increased considering the potential of children with COVID-19. For instance, a single pediatric infectious diseases ward was initially considered to be adequate for hospitalization of possible or confirmed COVID-19 cases. However, as the number of cases began to increase within ten days, additional wards as well as a room with ventilation system separated from the rest of the rooms in the pediatric intensive care unit were allocated. The shifts of the staff working at the COVID-19 wards and other HCWs were strictly separated from each other in order to reduce the HCWs contact with COVID-19 patients and indirect transmission and to reduce PPE usage. Moreover, the elective surgeries and outpatient appointments were postponed with the exceptions of appointments for retinopathy of prematurity in ophthalmology unit, daily chemotherapies for pediatric hematology-oncology patients and also the intravenous immunoglobulin replacement therapies for congenital immunodeficiency patients.
Clinical triage and isolation of the suspected COVID-19 patients
Under these unusual circumstances, potential transmission was a crucial problem considering the relatively high rate of asymptomatic children or children with milder presentation of COVID-19 and additionally the potentially asymptomatic parents.31 , 43 Therefore, reducing the number of the HCWs exposed to possible or confirmed COVID-19 patients was prioritized by the hospital management and organizing a more proper triage was a great challenge for the infection control committee.44
The clinical triage, especially in the case of COVID-19 pandemic, is an evolving process requiring a high level of institutional preparedness and includes evaluating outpatients during the first admission to the hospital, the pediatric emergency admissions and the referrals from other hospitals as well.
-
i
Outpatient clinics: A front desk was set up for triage on the ground floor at the only entrance of the hospital (the other entrances were closed) with 2 fully-equipped nurses and 1 pediatrician who measured the temperature of the child and the parents or caregivers and also who applied a specially designed questionnaire to screen on the symptoms of COVID-19 (eg, fever, cough, difficulty to breath), information on whether the patient had any relatives infected with COVID-19 and also the symptoms that occurred within the last 2 days. In the first 20 days of the pandemic in Turkey, medical masks were only given to the parents and children with fever, whereas later everyone entering the outpatient clinics was requested to wear medical masks. Additionally, only 1 caregiver with medical mask was allowed to enter the examination room.
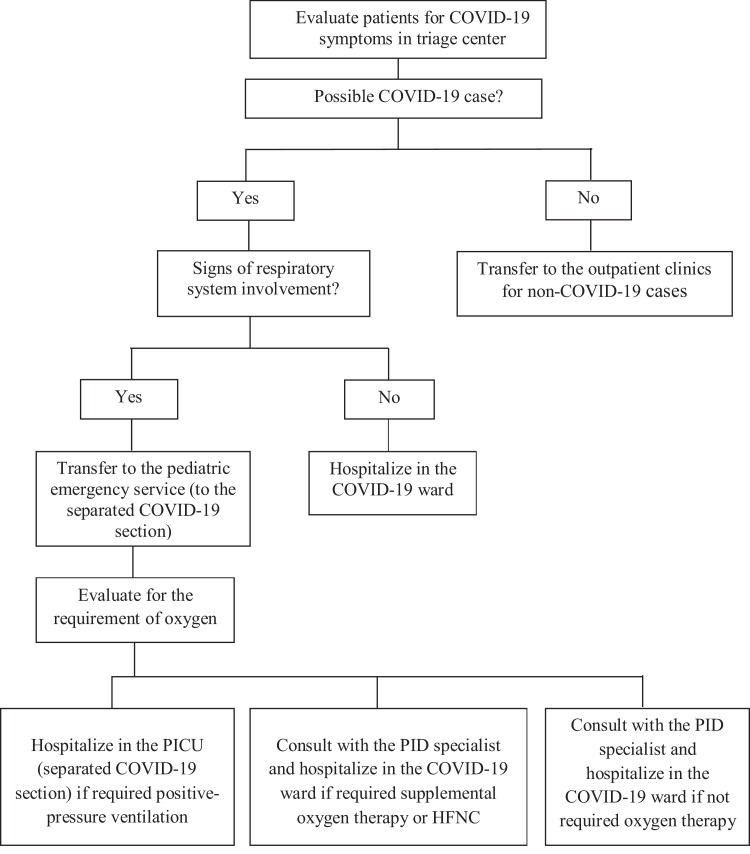
The triage was performed according to the algorithm demonstrated in Figure 1 , where the possible COVID-19 patients were examined by fully equipped clinicians (pediatricians and a pediatric infectious diseases specialist as a supervisor) on the ground floor. The patients who did not meet the COVID-19 criteria were transferred to the upper floors. The radiology (chest x-ray) and phlebotomy team was placed also on the ground floor and dedicated only to COVID-19 patients. This shift continued from 08.00 A.M. until to 04.00 P.M.
-
i
Emergency departments: The ground floor front desk for triage described above from 04.00 P.M. until 08.00 A.M. in line with the same criteria except 1; during this shift the patients without fever and/or cough were directly transferred to the emergency room for physical examination. The COVID-19 patients who needed hospitalization were immediately admitted to inpatient clinics and the patients who required monitoring for transient duration were transferred to isolation rooms arranged in emergency department. The emergency department was divided into 2 separate sections according to probable COVID-19 positive or negative patients. The patients with suspected COVID-19, who required high-flow nasal cannula oxygen therapy, were immediately transferred to the inpatient clinics.
-
ii
Referral from other centers: Since the current hospital is a referral center for pediatric COVID-19 patients, nearly half of the COVID-19 patients were referred from other hospitals. Depending on the clinical status of the patient, these patients were directly transferred to the COVID-19 ward without entering any other part of the hospital. The staff would get ready with their PPE following their direct communication with the transfer ambulance before admission.
-
iii
The organization of the COVID-19 wards: Three specific wards were arranged and classified according to the risks of COVID-19 patients as high, moderate and low. The other remaining wards were designated COVID-19 free pediatric wards where only patients without fever and any respiratory symptoms were admitted. Pediatric infectious diseases ward was the only ward that confirmed or suspected COVID-19 cases were hospitalized. The patients with respiratory symptoms and fever in which COVID-19 possibility was nullified by molecular assays were transferred to the other 2 low-risk wards.
Fig 1.
Algorithm for the clinical triage and isolation of the suspected COVID-19 patients at the center. HFNC, high-flow nasal cannula; PICU, pediatric intensive care unit; PID, pediatric infectious diseases.
Precautions and specific applications in the pediatric wards following children with COVID-19
Despite the fact that most children with COVID-19 do not require hospitalization, certain precautions should be taken to prevent nosocomial infections in the wards. Unlike the general hospitals, the children's hospital may require certain specific measures worth mentioning:
-
i
Before the pandemic, there was only one entrance to the ward used both by the patients and the HCWs. A second entrance dedicated for use by HCWs was built.
-
ii
HCWs were made to wear scrubs which were washed at the hospital after use and they were not allowed to go out with scrubs on.
-
iii
In the clinical practice, after the first examination of the children with COVID-19, the parent/caregiver and the child were immediately transferred to the patient's room and all information required to fill the forms such as additional information about family history, complaints of the child, etc. were taken by the nurse on the phone.
-
iv
HCWs wore full PPE including N95 masks.
-
v
Before the pandemic, there was an open food court shared by all HCWs. However, during the pandemic phase, to observe the recommended social distancing and to prevent possible transmission from unknown viral spreaders, HCWs had food delivered in disposable closed lunch boxes at the locations they work.
-
vi
In every patient room, patient-specific stethoscopes were placed to prevent possible transmission via fomites and wall-clocks (for evaluating the patients’ heart rate as well as respiratory rate) were installed as HCWs were not allowed to use their personal belongings in the COVID-19 patient rooms.
-
vii
The children who require high-flow nasal cannula or conventional oxygen therapy were immediately transferred to negative pressure isolation room when available. As there was only 1 such room at the hospital, aerosol-generating procedures had to be conducted in regular rooms taking the necessary measures such as fully-equipped HCWs donning N95 or N99 masks.45
-
viii
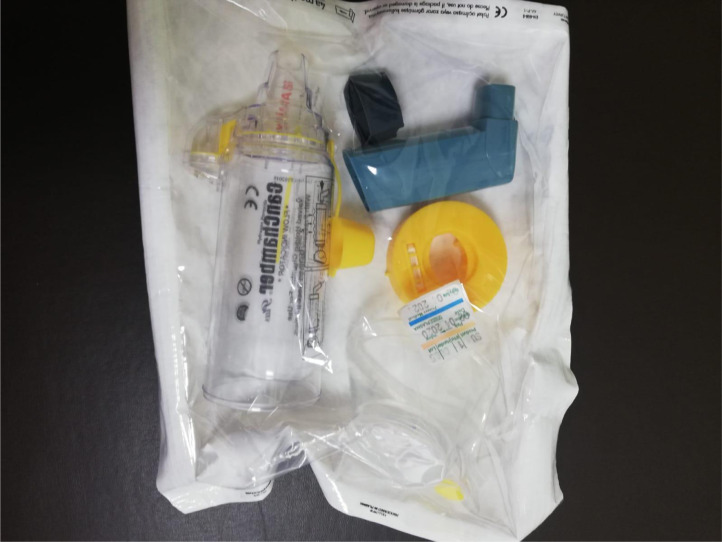
Before the COVID-19 pandemic, the young children and infants who required salbutamol inhalation via nebulizer were kept together in the same area in the emergency service. During the pandemic, this practice was discontinued and a metered dose inhaler via a holding chamber or spacer was used instead. The reusable holding chambers and the outer parts of the nebulizer were sterilized with vaporized hydrogen peroxide and stored as a sterile package (Image 1 ).
-
ix
Living spaces and resting areas of HCWs were strictly separated from the patients’ area and no personal belongings were allowed in the patients’ area.
-
x
When a child with suspected or confirmed COVID-19 was discharged from the ward, firstly disinfection was performed with a multifunctional UV disinfection robot with air-cleaning properties and then all surfaces in the room were physically cleaned (Image 2 ). All furniture, equipment, and frequently touched surfaces (e.g., door handles) were cleaned with a physical cleaning agent using 1,000 ppm bleach solution (2 in 1) made up daily from a concentrated solution. This cleaning technique was applied in all rooms where suspected or confirmed COVID-19 cases were hospitalized.
Image 1.
Sterile package of the reusable holding chambers and the outer parts of the nebulizer.
Image 2.
Multifunctional UV disinfection robot with air-cleaning properties used in addition to disinfection of the physically cleaned rooms.
Personal protective equipment (PPE)
All HCWs, including doctors, nursing and administrative staff having contact with patients were required to use PPE during the current pandemic. Moreover for every HCW, a form including possible exposure and presence of PPE during contact with COVID-19 was filled by the HCWs to determine the risk score.
-
i
All HCWs were required to follow standard precautions including rigorous hand-washing practices using water and soap or alcohol-based hand rub.
-
ii
Surgical masks were provided for all HCWs. Particularly for the HCWs performing aerosol generating procedures, N95 respirators were provided. Aerosol generating procedures were listed as procedures including collecting diagnostic respiratory samples (eg, nasopharyngeal swab), endotracheal intubation, bronchoscopy, open suctioning, administration of nebulized treatment, manual ventilation before intubation, physical prone positioning of the patient, disconnecting the patient from the ventilator, noninvasive positive pressure ventilation, tracheostomy, and cardiopulmonary resuscitation including tracheal intubation, bronchial suctioning, bronchoscopy, and sputum induction.45 According to the guidelines, these masks were allowed to be used for 8 hours for multiple patients without removing, unless the respirator is damaged, soiled or contaminated.46
-
iii
HCWs collecting diagnostic respiratory samples were required to wear gloves, gowns and N95 masks at COVID-19 wards. The HCWs at the other remaining wards were required to wear surgical masks while managing the COVID-19 free patients.
-
iv
Stock levels of PPEs were daily monitored by an inventory management software program. According to the use of PPEs, new products obtained immediately once the stocks declined to a certain level
-
v
Availability of PPEs for all HCWs was assessed at the point-of-care. Five sets of equipment of PPE including N95 masks, gowns, gloves, and face shields were prepared for the emergency department, outpatient clinics and wards that COVID-19 patients admitted. These sets are ready to use sets reserved for resuscitation.
-
vi
After an aerosol-generating procedure with a confirmed case, all PPEs were disposed off in a trash receptacle except the face shields which were sterilized for reuse.
In conclusion, the COVID-19 pandemic is an eventually changing and evolving circumstance. Infection control precautions to prevent the possible transmissions to HCWs as well as the other patients and their caregivers from children with COVID-19 are very critical. If sufficient precautions were not taken, healthcare settings may serve as additional source of transmission and spread of COVID-19 in the society. The process should be closely monitored, and the infection control precautions should be updated according to the updated information and new management practices/guidelines on COVID-19.
Acknowledgments
We thank to Işıl Demirakın for her help in language editing.
References
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . World Health Organization; Geneva: 2020. WHO Director-General's Remarks at the Media Briefing on 2019-nCoV on 11 February 2020. Available at: https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020. Accessed April 12, 2020. [Google Scholar]
- 4.World Health Organization . World Health Organization; Geneva: 2020. WHO Director-General's Opening Remarks at the Media Briefing on COVID-19 - 11 March 2020 [Internet] Available at: https://www.who.int/dg/speeches/detail/who-directorgenerals-opening-remarks-at-the-media-briefing-on-covid-19-11-march-2020. Accessed April 12, 2020. [Google Scholar]
- 5.COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at John Hopkins University of Medicine. John Hopkins University of Medicine. Available at: https://coronavirus.jhu.edu/map.html. Accessed May 18, 2020
- 6.Jin Y, Yang H, Ji W, et al. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. 2020;12:E372. doi: 10.3390/v12040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Academy of Pediatrics . In: Red Book: 2018 Report of the Committee on Infectious Diseases. 31st ed. Kimberlin DW, Brady MT, Jackson MA, Long SS, editors. American Academy of Pediatrics; Itasca, IL: 2018. Coronaviruses, including SARS and MERS; pp. 297–301. [Google Scholar]
- 8.Petrosillo N, Viceconte G, Ergonul O, Ippolito G, Petersen E. COVID-19, SARS and MERS: are they closely related? Clin Microbiol İnfect. 2020;26:729–734. doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liya G, Yuguang W, Jian L, et al. Studies on viral pneumonia related to novel coronavirus SARS-CoV-2, SARS-CoV, and MERS-CoV: a literature review. APMIS. 2020;58:e00187-20. doi: 10.1111/apm.13047. [DOI] [PubMed] [Google Scholar]
- 10.Turkey Confirms First Case of Coronavirus. Available at:https://www.aa.com.tr/en/latest-on-coronavirus-outbreak/turkey-confirms-first-case-of-coronavirus/1761522
- 11.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 14.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bajema KL, Oster AM, McGovern OL, et al. Persons evaluated for 2019 novel coronavirus - United States, January 2020. MMWR Morb Mortal Wkly Rep. 2020;69:166–170. doi: 10.15585/mmwr.mm6906e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai J, Xu J, Lin D, et al. A case series of children with 2019 novel coronavirus infection: clinical and epidemiological features [e-pub ahead of print]. Clin Infect Dis. 10.1093/cid/ciaa198. Accessed June 18, 2020 [DOI] [PMC free article] [PubMed]
- 17.Cao Q, Chen YC, Chen CL, Chiu CH. SARS-CoV-2 infection in children: transmission dynamics and clinical characteristics. J Formos Med Assoc. 2020;119:670–673. doi: 10.1016/j.jfma.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehta NS, Mytton OT, Mullins EWS, et al. SARS-CoV-2 (COVID-19): What do we know about children? A systematic review [e-pub ahead of print]. Clin Infect Dis. 10.1093/cid/ciaa556. Accessed June 18, 2020 [DOI] [PMC free article] [PubMed]
- 19.Zhang B, Liu S, Zhang J, et al. Children hospitalized for coronavirus disease 2019 (COVID-19): a multicenter retrospective descriptive study [e-pub ahead of print] J Infect. 2020 doi: 10.1016/j.jinf.2020.04.045. Accessed June 18, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Zhu F, Wang C, et al. The risk of children hospitalized with severe COVID-19 in Wuhan. Pediatr Infect Dis J. 2020;39:e91–e94. doi: 10.1097/INF.0000000000002739. [DOI] [PubMed] [Google Scholar]
- 21.Song W, Li J, Zou N, Guan W, Pan J, Xu W. Clinical features of pediatric patients with coronavirus disease (COVID-19) J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhen-Dong Y, Gao-Jun Z, Run-Ming J, et al. Clinical and transmission dynamics characteristics of 406 children with coronavirus disease 2019 in China: a review [e-pub ahead of print]. J Infect. 10.1016/j.jinf.2020.04.030. Accessed June 18, 2020 [DOI] [PMC free article] [PubMed]
- 23.Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and better prognosis than adults. Acta Paediatr. 2020;109:1088–1095. doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sohrabi C, Alsafi Z, O'Neill N, et al. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int J Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ong SWX, Tan YK, Chia PY, et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020;323:1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Wang S, Xue Y. Fecal specimen diagnosis 2019 novel coronavirus-infected pneumonia. Med Virol. 2020;92:680–682. doi: 10.1002/jmv.25742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) — China, 2020. China CDC Weekly. 2020;2:113–122. [PMC free article] [PubMed] [Google Scholar]
- 30.Livingston E, Bucher K. Coronavirus disease 2019 (COVID-19) in Italy [e-pub ahead of print]. JAMA. 10.1093/cid/ciaa198. Accessed June 18, 2020 [DOI] [PubMed]
- 31.CDC COVID-19 Response Team Coronavirus disease 2019 in children - United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:422–426. doi: 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shekerdemian LS, Mahmood NR, Wolfe KK, et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units . JAMA Pediatr. 10.1001/jamapediatrics.2020.1948. Accessed June 18, 2020 [DOI] [PMC free article] [PubMed]
- 33.Dong Y, Mo X, Hu Y, et al. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics. 2020;16:16. [Google Scholar]
- 34.Li R, Pei S, Chen B, et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2) Science. 2020;368:489–493. doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19) — China, 2020 China CDC Weekly. Available at:http://weekly.chinacdc.cn/en/article/id/e53946e2-c6c4-41e9-9a9b-fea8db1a8f51. Accessed May 12, 2020 [PMC free article] [PubMed]
- 36.CNN World “94 Doctors and 26 Nurses in Italy Have Died of Coronavirus” Available at:https://edition.cnn.com/world/live-news/coronavirus-pandemic-04-07-20/h_b7583ec9fa05d053f6faca9b164817eb?utm_medium=social&. Accessed May 12, 2020.
- 37.World Health Organization (WHO). Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) 2020. Available at:https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf. Accessed May 12, 2020
- 38.Istituto Superiore di Sanità. Sorveglianza Integrata COVID-19 in Italia: AGGIORNAMENTO 22 Marzo 2020. Available at: https://www.epicentro.iss.it/coronavirus/bollettino/Infografica_22marzo%20ITA.pdf. Accessed May 12, 2020
- 39.Kluytmans M, Buiting A, Pas S, et al. SARS-CoV-2 infection in 86 healthcare workers in two Dutch hospitals in March 2020 [e-pub ahead of print]. BMJ. 10.1101/2020.03.23.20041913. Accessed June 18, 2020 [DOI]
- 40.CDC COVID-19 response team Characteristics of Health Care Personnel with COVID-19 — United States, February 12–April 9, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:477–481. doi: 10.15585/mmwr.mm6915e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.WHO . World Health Organization; Geneva, Switzerland: 2019. Infection Prevention and Control During Health Care for Probable or Confirmed Cases of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Infection. (WHO/MERS/IPC/15.1 Rev 1). Licence: CC BY-NC-SA 3.0 IGO. Available at: https://apps.who.int/iris/bitstream/handle/10665/174652/WHO_MERS_IPC_15.1_eng.pdf. jsessionid=D0567D3772FD88E83CEAA6E4E9D887EE?sequence=1. Accessed May 12, 2020. [Google Scholar]
- 42.WHO. Infection Prevention and Control of Epidemic- and Pandemic-Prone Acute Respiratory Infections in Health Care WHO Guidelines. Available at:https://apps.who.int/iris/bitstream/handle/10665/112656/9789241507134_eng.pdf?sequence=1. Accessed May 12, 2020 [PubMed]
- 43.Wei WE, Li Z, Chiew CJ, Yong SE, Toh MP, Lee VJ. Presymptomatic transmission of SARS-CoV-2—Singapore, January 23–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:411–415. doi: 10.15585/mmwr.mm6914e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20:689–696. doi: 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tran K, Cimon K, Severn M, Pessoa-Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7:e35797. doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pandemic planning. Recommended Guidance for Extended Use and Limited Reuse of N95 Filtering Facepiece Respirators in Healthcare Settings. Available at:https://www.cdc.gov/niosh/topics/hcwcontrols/recommendedguidanceextuse.html. Accessed May 12, 2020