Graphical abstract
Keywords: Virus detection, Biosensor, Electrochemical sensor, Immunosensor, Biomarker detection, Point of care device
Highlights
-
•
Highly sensitive electrochemical sensors for viral DNA enables early detection of disease.
-
•
Antibody testing allows the extent of disease and the immunity status of recovered individuals to be assessed.
-
•
Sample-to-answer devices open up testing in the community.
-
•
Integration of sensors, and wireless technologies enables autonomous, point-of-care applications.
-
•
Challenges remain in terms of selectivity, shelf-life, manufacturability and the use of sustainable materials.
Abstract
Near patient detection of viral infection represents a powerful approach for the control of emerging threats to global health. Moreover, the ability to identify individuals who have contracted the disease and developed antibodies that confer immunity is central to a return to normal daily activities. This review presents some of the recent advances in electrochemical sensors for the detection of viruses and their associated antibody profiles. Given the speed, portability, sensitivity and selectivity achieved using electrochemical detection, these sensor systems hold the promise of transformative change in clinical practice.
1. Introduction
The threat to global health from viral infections, such as COVID-19 [1], influenza, HIV, hepatitis, Zika, chikungunya, Ebola etc., has brought into sharp focus the need for rapid, sensitive and selective detection of viruses as well as post-infection antibodies. In particular, halting the severe acute respiratory syndrome-related coronavirus-2 (SARS–CoV-2) pandemic depends critically on the use of diagnostic testing on an immense scale. In-community testing could successfully identify cases enabling contact tracing and containment without lockdowns [2]. A particular challenge is to identify infected, but asymptomatic individuals (incubation phase), and to measure the extent of live virus shedding so as to enable isolation to end at the earliest safe time [3]. The speed of this testing process could be significantly increased using Point Of Care, POC, devices that would allow testing to be carried out in the community [4]. Blood based commercial POC nucleic acid and immunoassays for COVID-19 are rapidly emerging. While fluorescence based detection dominates these devices (approximately 85% of 76 assays that have received emergency use clearance by the Food and Drug Administration of the USA at time of writing), some electrochemical approaches have been developed. For example, those based on electrochemiluminescence and changes in the rate of electron transfer to a redox active probe in solution upon binding of the target. However, achieving the required performance, especially with respect to selectivity, e.g., high false positive rate for antibodies due to past exposure to the common cold coronavirus rather than SARS-CoV-2, has proven challenging for some devices. Current knowledge suggests the detection of SARS–CoV-2 and its associated antibodies is not significantly different than other viruses. This observation suggests that accelerating the deployment of these devices may have impacted on the usual processes of characterisation, verification and validation. Under these time constraints, validation is especially challenging, i.e., the monitoring of specifications that will consistently produce results fit for purpose. This part of the product development cycle is crucial to assuring that a high test sensitivity (the ability to correctly identify those with the disease, true positive rate) and test specificity (ability to correctly identify those without the disease, true negative rate), are achieved. Moreover, the analytical sensitivity may not be adequate for early detection, or for confirming non-communicable levels of disease post-infection. The development of POC devices for high performance viral and antibody detection is clearly a very challenging goal [5]. For example, sample collection should be minimally invasive, e.g., nose/throat swab or saliva, rather than blood. The concentration of virus present can be very low especially early in the disease cycle when testing is most effective at preventing community transmission, placing extreme demands on analytical sensitivity. The sample to answer time needs to be short, ideally less than 20 min. This mini- review reports on virus and antibody detection using electrochemical methods, focusing on recent key innovations that drive the development of portable, high performance point-of-care technologies [6].
2. Enhancing analytical sensitivity
Decreasing the limit of detection allows infections to be detected earlier and ensures that individuals are not infectious before returning to normal life. DNA methods represent a powerful approach to definitively identifying the causative entity and, since the target can be chemically amplified, e.g., using PCR, can offer extremely high sensitivity.
For electrochemical sensors, the choice of electrode material is important since it can influence many aspects of the performance of electrochemical sensors. These include the double layer capacitance (influences limit of detection, S/N), the rate of heterogeneous electron transfer (influences response time, sensitivity), the nature of the coupling chemistry required to immobilise the bioreceptors, the propensity towards non-specific binding and quenching in electrochemiluminescent sensors. Electrode materials that are commonly used as platforms for sensor development include carbon [7], [8], [9], [10], [11], gold [12], [13], [14], platinum [15], silicon (oxide) [16] and Fluorine Doped Tin Oxide (FDTO) [17].
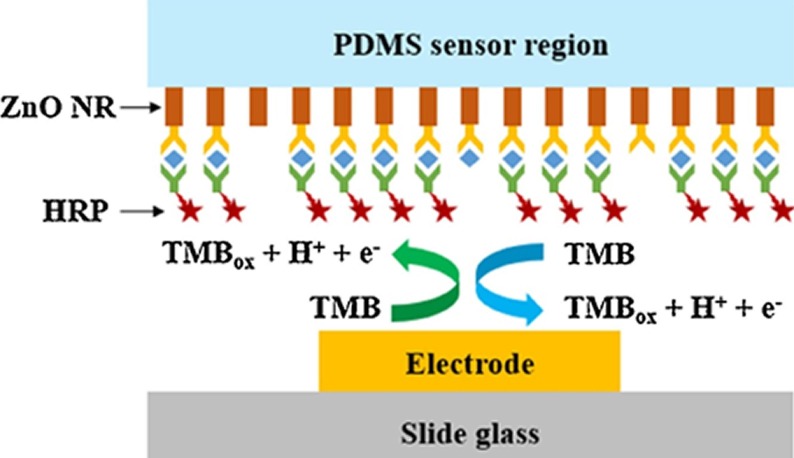
Provided that the background current does not increase proportionally, increasing the active electrode area can improve the detection sensitivity since the faradaic current depends directly on the active area of the electrode. Nanomaterials and nanocomposites, can increase the surface area so that more bioreceptors, e.g., antibodies or nucleic acid capture strands, can be immobilized giving a wider dynamic range and perhaps increasing the sensitivity [18]. Significantly, the porosity of these materials can be controlled so that the relatively large (2 k to 500 kdaltons) target can access a large fraction of the immobilised bioreceptors. As shown in Fig. 1 , Han et al. used this strategy for the detection of influenza H1N1, H5N1, and H7N9 viruses [12]. In this case, ZnO nanorods were grown in PDMS to give a higher density of immobilized antibodies. This system produced an LOD of 1 pg/mL, a linear dynamic range between 1 and 10 ng/ml and a good ability to discriminate between the individual viruses. However, further testing with real samples would be needed to demonstrate its real world performance.
Fig. 1.
Schematic of the PDMS substrate and ZnO nanowires functionalised with the primary capture antibody. The secondary antibody is labelled with horseradish peroxidase, HRP, which oxidises 3,3′,5,5′-tetramethylbenzidine, TMB, generating a current whose magnitude depends on the concentration of the viral target.
Irrespective of the virus, identifying infectious individuals is paramount for stopping community transmission. For example, Hepatitis B is a viral disease that attacks the liver and may cause jaundice (yellow skin and eyes). While most people clear the virus within 6 months and become immune, about 10% remain infectious and may go on to develop cirrhosis or liver cancer. Hepatitis B e-antigen (HBeAg), is a protein found in a patient’s blood when the virus is actively replicating and can be spread.
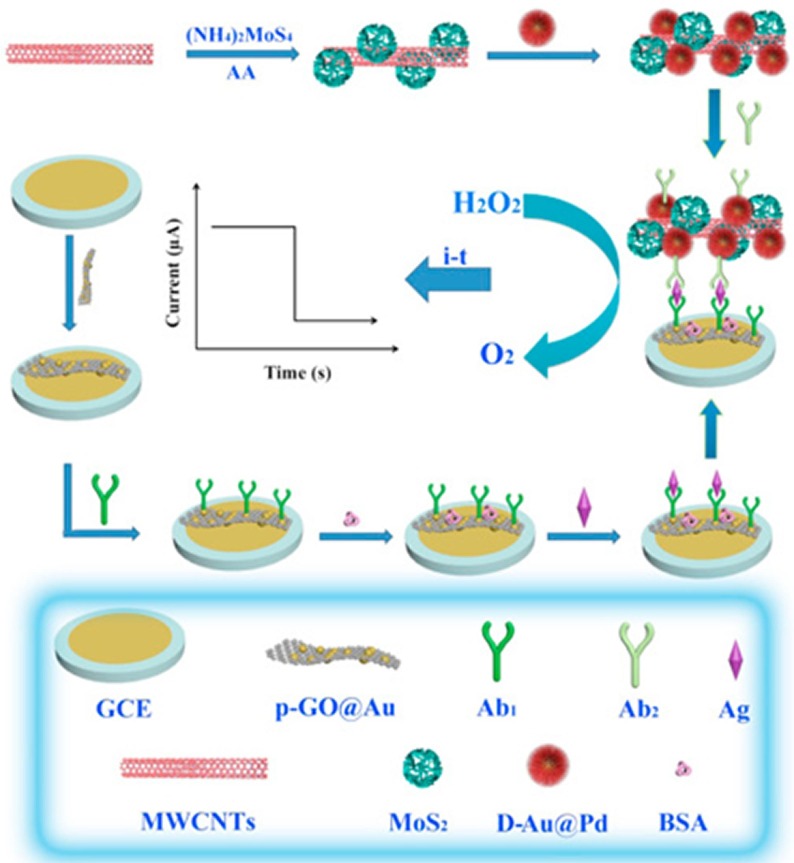
Fig. 2 shows an immunoassay that immobilises secondary antibodies, electrocatalysts, metal nanoparticles and molybdenum disulphide, on multiwalled carbon nanotubes [13]. This label can amplify the current response associated with the electrocatalytic reduction of hydrogen peroxide when the HBeAg is present. The immunosensor has a dynamic range from 0.1 pg mL−1 to 500 pg mL−1 and an LOD of 26 fg mL−1 (approximately 1.5 fM). The selectivity of the response for HBeAg over other proteins including carcinoembryonic antigen, human IgG, prostate specific antigen, BSA and HBsAg was investigated with the RSD being less than 5% when the interfering substances were added. This highly sensitive nanocomposite-based immunoassay opens up the possibility of rapidly identifying individuals who are capable of transmitting disease.
Fig. 2.
Schematic illustration of the preparation of the multiwalled carbon nanotubes initially functionalised with gold-palladium nanoparticles loaded with molybdenum disulphide followed by the secondary antibodies. This nanocomposite is an efficient electrocatalyst thus enhancing the current response in the immunoassay.
Fu and co-workers [19] have reported a self-sacrificial label for the detection of the avian influenza virus H5N1. The secondary antibody is immobilised on magnetic nanoparticles and used in a sandwich immunoassay based sensor. The magnetic nanoparticles, MNP, are converted to an electroactive Prussian blue, PB, analogue by electrochemically generating protons from water that release Fe3+ from the MNPs. A second electrochemical step at a lower potential drives the reduction of deposited K3Fe(CN)6 and Fe3+ to K4Fe(CN)6 and Fe2+, respectively, which then react to form a Prussian Blue, PB, analogue. The approach produces a porous PB and the assay requires significantly lower reactant concentration compared to conventional methods. The detection is highly sensitive with an LOD of 0.0022 hemagglutination units.
A very significant contemporary issue is the detection of viruses that cause severe respiratory illness in humans including MERS and SARS, but most especially the COVID-19 causing virus, SARS-CoV-2 [20]. For example, an immunosensor using voltammetric detection has been developed to detect coronavirus type virus, MERS-CoV [21]. The immunosensor is based on disposable carbon microarray electrodes that are functionalised with electrodeposited gold nanoparticles which enhance the sensitivity of the sensor. In a competition assay, the current observed in square wave voltammetry for ferrocyanide/ferricyanide in solution decreases linearly with increasing target concentration. The linear dynamic range is from 0.001 to 100 ng mL−1 for MERS-CoV and from 0.01 to 10,000 ng mL−1 for HCoV. The assay can be performed in 20 min with limits of detection of 0.4 and 1.0 pg mL−1 for HCoV and MERS-CoV, respectively. In spiked nasal samples, non-specific adsorption was minimal and a good level of selectivity was achieved for the target over possible interferences such as Influenza A and B.
Label Free Detection. Using bioreceptors that are functionalised with labels, such as electrocatalytic nanoparticles, 2D layered materials, electroactive metal complexes etc., can deliver excellent analytical sensitivity. However, they add complexity and cost to the assay and may increase the sample to answer time by requiring an additional step where the labelled bioreceptor is bound. The general features of strategies that use labelled capture / target / probe vs. direct, unlabelled detection are shown in Table 1 .
Table 1.
Key properties of labelled and unlabelled detection strategies.
| Wet Lab Complexity | Instrumentation Cost (time) | Quantitative | High Throughput | Comparability of Samples | Suitability for POC | |
|---|---|---|---|---|---|---|
| Labelled Detection | ||||||
| Electrochemiluminescent probe | Moderate | Moderate (short) | Yes | Yes | Some challenges | Moderate |
| Redox probe | Little | Inexpensive (medium) | Yes | Yes | Straightforward | High |
| Label Free | ||||||
| Resistance | Little | Inexpensive (short) | Yes | Yes | Challenging | Moderate |
| Capacitance | Little | Inexpensive (medium) | Semi-quantitative | Moderate | Challenging | Low |
| Stripping Voltammetry/Potentiometry | Little | Inexpensive (short) | Semi-quantitative | Moderate | Challenging | Low/Moderate |
| Direct Electrochemistry | Little | Inexpensive (short) | Yes | Yes | Some challenges | Moderate/High |
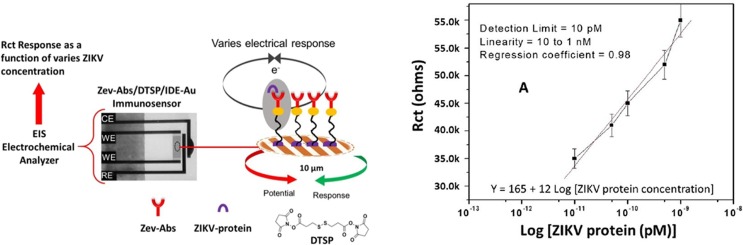
Impedance immunosensors are designed to monitor in real-time the change in the interfacial impedance (capacitance or resistance) before and after binding of the target analyte (label free), or the change in charge transfer resistance to an electroactive probe dissolved in solution (avoids the need to functionalise bioreceptor). Fig. 3 illustrates a miniaturized POC immunosensor for Zika virus detection [7]. The principle of operation is that binding of the ZIKV-envelop protein target to antibodies immobilised on a gold microelectrode decreases the rate of electron transfer to a Fe(II)/(III) couple in solution avoiding the need to label the capture or target. This causes a corresponding increase in the charge transport resistance that is measured using electrochemical impedance.
Fig. 3.
(A) Schematic of the impedance based immunosensor (B) Dependence of the charge transfer resistance on the ZIKV concentration from10 pM to 1 nM.
The resistance to charge transfer measured using an interdigitated microelectrode gave a high sensitivity (12 kΩ M−1), a dynamic range of over two orders of magnitude and a detection limit of 10 pM. Somewhat unusually, the selectivity of the response was investigated by adding potentially interfering viruses such as dengue virus, chikungunya virus and Nile West virus only after adding the ZIKV target. However, under these circumstances the addition of the interferences did not significantly change the impedance. The shelf-life was investigated which is critically important for successful real-world implementation and generally deserves more attention in the literature. Significantly, the results confirm that the sensor is stable for 30 days when stored at 4 °C, but beyond that time the magnitude of electrochemical response reduced significantly.
3. Point-of-care devices
Point of Care technologies seek to dramatically reduce the sample-to-answer time enabling diagnosis and monitoring to be carried out close to the patient in their own homes or a secondary care setting at lower overall cost [22], [23]. Other potential advantages include small sample volumes (noting that when the target analyte is low, there is a minimum volume of sample required so that a detectable quantity is present), reduced reagent use and small physical size [24]. Within the context of the Internet-of-Things, IoT, data generated can be uploaded to the cloud for meta-analysis [25], e.g., close to real-time outbreak tracking and hospital consultant assessment. These approaches are especially applicable to low resource environments where the hospital system is less well developed which is especially relevant to the current COVID pandemic [26]. Devices incorporating electrochemical detection are well suited for creating flexible biosensing devices [27].
POC diagnostic devices integrate two key processes, i.e., sample preparation and target analyte detection. The quality of the final analysis is highly dependent on the sample preparation which is particularly challenging for complex samples such as blood, saliva, urine and even breath condensate. These samples contain a significant number of interferences, e.g., proteins, antibodies, DNA, cells etc., that can interfere with the detection of the target analyte. Fundamentally, there are two limiting approaches to achieving a selective response. First, the target analyte can be isolated and pre-concentrated using an instrumental approach, e.g., magnetic nanoparticles. For example, separating plasma from whole blood can help to minimise interference [28] noting that some approaches could induce in vitro haemolysis that could interfere with the analysis [29]. A recent interesting innovation was superhydrophobic plasma separators [30]. Second, the selectivity of the bioreceptor with the target, e.g., the difference in association constant of an antibody for the target relative to interferences, can be maximised. This can be achieved through antibody selection and by carefully controlling the local microenvironment within the immobilising film so as to maximise the association constant.
Lateral Flow Assays, LFA, are similar to enzyme-linked immunosorbent assays, ELISA, with antibodies or nucleic acid capture strands being immobilised on a membrane, often nitrocellulose. LFs for traditional molecular biomarkers, contaminants and infectious agents such as viruses have been developed [9]. Paper based devices have been important for qualitative or semi-quantitative detection of biomarkers, e.g., based on a visible colour change, but quantitative, paper based electrochemical devices are emerging. An electrochemical lateral flow device for the rapid immunomagnetic detection of myeloperoxidase, MPO, a general biomarker of infection, has been developed based on the use of antibody-modified magnetic beads and a detection (secondary) antibody labeled with horseradish peroxidase, HRP. The sample is first incubated with the magnetic beads, MBs, and detection antibody, Ab, for 5 min and then directly transferred onto the strip. The MBs are retained using a magnet and the current measured using TMB as the enzyme substrate, allows MPO to be detected in 1:100 diluted serum with an LOD of 0.18 ng mL−1 in less than 15 min.
Crooks and co-workers [9] have used paper folding methods to create an electrochemical sensor that is capable of detecting a 30-base nucleotide sequence characteristic of DNA from the hepatitis B virus (HBV) with a detection limit of 85 pM. A hollow-channel accommodates micrometre-scale particles and a highly innovative “slip layer” allows the individual incubation steps to be easily staged in time. Two stages of amplification were used with silver nanoparticle labels providing a maximum amplification factor of 250,000 while magnetic microbeads, functionalised with capture probes, can be pre-concentrated at a detection electrode to give an additional amplification of the signal by approximately 25-fold. Significantly, there are no enzymes or antibodies used in the assay, which increases its speed, stability, robustness and most likely shelf-life and tolerance of higher storage temperatures. Moreover, the approach requires only one sample incubation step before detection.
More recently, Crooks and co-workers recently developed a new hybrid microfluidic device based on a disposable paper electrode and a three-dimensional, 3D, printed plastic chip for the electrochemical detection of magnetic bead (MB)–silver nanoparticle (MB–AgNP) bioconjugates. By minimising entrapment/non-specific binding of the magnetic particles by the membrane, a detection limit for AgNPs of 12 pM was achieved, representing just 22 AgNPs per MB [10].
Loop-mediated isothermal amplification (LAMP) can amplify DNA at constant temperature and is highly selective as the target sequences are recognized by four different primers. LAMP is also less sensitive to compounds in the sample that can inhibit PCR. A portable electrochemical LAMP based device has been developed and demonstrated for the detection of hepatitis B virus [15]. The viral DNA, i.e., conserved sequences of the S gene and overlapping polymerase regions that are homologous to all the available genotypes of HBV, was monitored in real-time by measuring the current associated with methylene blue in the reaction mixture using square wave voltammetry. The peak height ratio and threshold time depend directly on the DNA concentration. The approach can detect viral DNA at concentrations as low as 6.18 fg ml−1 and shows excellent selectivity against human DNA. Significantly, HBV positive serum could be correctly identified following simple thermal pre-treatment. The integration of DNA amplification and robust electrochemical detection within a microfluidic device is an important strategy for the development of highly sensitive, high throughput, point-of-care devices for virus identification and quantification of viral load.
Antibody Seroprevalency: Understanding the extent of immunity within the community is essential for predicting the future course of viral outbreaks. Recovered patients have antibodies in their bloodstream for at least a time (especially IgG) after recovery from the infection. A wide range of serology immunoassays, IAs, have also been developed for testing blood samples including automated chemiluminescent, ELISA, and rapid lateral flow immunoassays [14], LFIA. These assays bind a specific target, e.g., the immunoglobulin M, IgM, or immunoglobulin G, IgG, that is found in individuals who have contracted a SARS-CoV-2 infection.
A significant innovation in the development of autonomous devices has been the integration of a paper-based microfluidic fuel cell inside a lateral flow device [31]. The key components of the bioanode were glucose oxidase, Nafion and tetrabutylammonium bromide deposited on a methylene blue electro-polymerized Toray paper. This electrode oxidizes glucose present in a serum or blood sample. The cathode comprises Pt/C on Toray paper that reduces oxygen from air. The paper-based microfluidic fuel cell exhibited power densities of 0.16 and 0.12 mW cm−2 using human serum and blood samples, respectively. While the current density decreases significantly over 30 min, the power is considered sufficient to power an assay and the fuel cell has been integrated within a two-piece HIV assay cassette.
Electrochemiluminescence represents an attractive strategy to enhance detection sensitivity [11], [32]. An immunosensor was developed for HIV-1 p24 antigen, using a novel nanocomposite based on [Ru(bpy)3]2+-SiO2 and gold nanoparticles coupled to an anti-p24 antibody loaded on graphene. The p24 antigen is recognized first by the primary antibodies immobilized on the GCE. The nanocomposite carries a high load of the ruthenium electrochemiluminescent molecule which increases the ECL intensity and the analytical performance. The sensor delivers a low detection limit, 1 pg mL−1 and a wide dynamic range. The selectivity against different proteins, BSA, CEA and AFP, as well as human serum, was excellent. However, a long incubation time is needed, 150 min, which would ideally need to be reduced for many POC applications.
4. Conclusions and outlook
Electrochemical sensors continue to become more sophisticated exploiting novel nanomaterials, instrumental advances and microfluidics to achieve highly selective and sensitive detection, identification and quantification of viruses. Despite the latest advances in camera technologies that give smartphones increasingly sophisticated imaging capabilities, electrochemical detection continues to have significant advantages including design simplicity, physical robustness, low power needs, ease of integration within microfluidic devices, excellent analytical sensitivity, and low instrumentation costs. Recent outbreaks of respiratory illnesses in humans including MERS-CoV, SARS-CoV-1 and SARS-CoV-2 (COVID-19) viruses highlight the need for rapid, sample-to-answer, point-of-care devices that can be deployed in the community. As discussed here, the use of novel nanomaterials, labels, advanced instrumentation, and sample clean-up/nucleic acid amplification using microfluidics continue to drive improved sensitivity. The COVID-19 case poses particular challenges to the POC diagnostics industry. The massive scaling of testing (approximately 23 per 1000 population in OECD countries) has been achieved predominantly using PCR based, centralised laboratory testing rather than POC devices. Moreover, in many countries the sample-to-answer time has decreased significantly to the point where it can play a useful role in contact tracing. Thus, reliable, high throughput testing within a centralised laboratory is continuing to play the central role in containing the pandemic. The major opportunity for POC devices may now lie in identifying recovered patients who have an appropriate antibody profile to confer immunity to re-infection. Given the issues with validation of device performance so far, it appears likely that the successful POC devices will be adaptations of current, highly successful antibody based detection devices. Looking further forward to improving testing performance, including speed, reliability, and accuracy, as well as using minimally invasive samples such as saliva or exhaled breath, schemes, such as the NIH Rapid Acceleration of Diagnostics (RADx), will play important roles. Significantly, these networks will include experts in technology, regulatory approval, commercialization, and manufacturing so as to accelerate progress. While being increasingly considered, issues, such as ability to handle the diverse properties of real-world samples, shelf-life, cost of goods, adoption by end users, cost effectiveness and mass manufacturability, need to be considered early in the device development life cycle.
There are some important emerging trends that have significant potential to impact devices for virus diagnosis. First, the integration of wireless communication within the device so that sophisticated machine learning and AI approaches can be used to extract the maximum amount of information from the analytical responses or to allow the results to rapidly inform hospital consultant decision making and epidemiological models. Second, the use of biocompatible materials that enables wearable and even implantable devices to be developed. The complete life cycle including manufacturability, the use of sustainable, non-toxic materials and the ability to recycle/reuse the components after testing, all need to be carefully considered. Third, there is a need to use minimally invasive samples, such as saliva, breath, tears or urine.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie Grant Agreement No. 813439, Enterprise Ireland (Award CF-2019-1075-P) and the Irish Environmental Protection Agency (2019-W-MS-41).
References
- 1.Zhou P., Yang X-.L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Cheng H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G.-F., Shi Z.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng M.-P., Papenburg J., Desjardins M., Kanjilal S., Quach C., Libman M., Dittrich S., Yansouni C.-P. Diagnostic testing for severe acute respiratory syndrome–related coronavirus-2: a narrative review. Ann. Intern. Med. 2020 doi: 10.7326/M20-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ricci F., Volpe G., Micheli L., Palleschi G. A review on novel developments and applications of immunosensors in food analysis. Anal. Chim. Acta. 2007;605:111–129. doi: 10.1016/j.aca.2007.10.046. [DOI] [PubMed] [Google Scholar]
- 4.Shrivastava S., Trung T.-Q., Lee N.-E. Recent progress, challenges, and prospects of fully integrated mobile and wearable point-of-care testing systems for self-testing. Chem. Soc. Rev. 2020;49:1812–1866. doi: 10.1039/c9cs00319c. [DOI] [PubMed] [Google Scholar]
- 5.Reddy B., Salm E., Bashir R. Electrical chips for biological point-of-care detection. Annu. Rev. Biomed. Eng. 2016;18:329–355. doi: 10.1146/annurev-bioeng-071813-104643. [DOI] [PubMed] [Google Scholar]
- 6.Hoekstra R., Blondeau P., Andrade F.-J. Distributed electrochemical sensors: recent advances and barriers to market adoption. Anal. Bioanal. Chem. 2018;410:4077–4089. doi: 10.1007/s00216-018-1104-9. [DOI] [PubMed] [Google Scholar]
- 7.Kaushik A., Yndart A., Kumar S., Jayant R.-D., Vashist A., Brown A.-N., Li C.-Z., Nair M. A sensitive electrochemical immunosensor for label-free detection of Zika-virus protein. Sci. Rep. 2018;8:9700. doi: 10.1038/s41598-018-28035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bandodkar A.-J., Imani S., Nunez-Flores R., Kumar R., Wang C., Mohan A.-M.-V., Wang J., Mercier P.-P. Re-usable electrochemical glucose sensors integrated into a smartphone platform. Biosens. Bioelectron. 2018;101:181–187. doi: 10.1016/j.bios.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X., Scida K., Crooks R.M. Detection of hepatitis B virus DNA with a paper electrochemical sensor. Anal. Chem. 2015;87:9009–9015. doi: 10.1021/acs.analchem.5b02210. [DOI] [PubMed] [Google Scholar]
- 10.Walgama C., Nguyen M.-P., Boatner L.-M., Richards I., Crooks R.-M. Hybrid paper and 3D-printed microfluidic device for electrochemical detection of Ag nanoparticle labels. Lab Chip. 2020 doi: 10.1039/d0lc00276c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou L., Huang J., Yu B., Liu Y., You T. A novel electrochemiluminescence immunosensor for the analysis of HIV-1 p24 antigen based on P-RGO@Au@Ru-SiO2 composite. ACS Appl. Mater. Interfaces. 2015;7:24438–24445. doi: 10.1021/acsami.5b08154. [DOI] [PubMed] [Google Scholar]
- 12.Han J.-H., Lee D., Chew C.-H.-C., Kim T., Pak J.-J. A multi-virus detectable microfluidic electrochemical immunosensor for simultaneous detection of H1N1, H5N1, and H7N9 virus using ZnO nanorods for sensitivity enhancement. Sens. Actuators B Chem. 2016;228:36–42. [Google Scholar]
- 13.Gao Z., Lia Y., Zhang X., Fenga J., Kong L., Wang P., Chen Z., Dong Y., Wei Q. Ultrasensitive electrochemical immunosensor for quantitative detection of HBeAg using Au@Pd/MoS2@MWCNTs nanocomposite as enzyme-mimetic labels. Biosens. Bioelectron. 2018;102:189–195. doi: 10.1016/j.bios.2017.11.032. [DOI] [PubMed] [Google Scholar]
- 14.Sinawang P.P.-D., Rai V., Ionescu R.-E., Marks R.-S. Electrochemical lateral flow immunosensor for detection and quantification of dengue NS1 protein. Biosens. Bioelectron. 2016;77:400–408. doi: 10.1016/j.bios.2015.09.048. [DOI] [PubMed] [Google Scholar]
- 15.Jayanath N., Nguyen L.-T., Vu T.-T., Lam T. Development of a portable electrochemical loop mediated isothermal amplification (LAMP) device for detection of hepatitis B virus. RSC Adv. 2018;8:34954–34959. doi: 10.1039/c8ra07235c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwon J., Lee Y., Lee T., Ahn J.-H. Aptamer-based field-effect transistor for detection of avian influenza virus in chicken serum. Anal. Chem. 2020;92:5524–5531. doi: 10.1021/acs.analchem.0c00348. [DOI] [PubMed] [Google Scholar]
- 17.Zhao C.-Q., Zhou J., Wu K.-W., Ding S.-N., Xu J.-J., Chen H.-Y. Plasmonic enhanced gold nanoclusters-based photoelectrochemical biosensor for sensitive alkaline phosphatase activity analysis. Anal. Chem. 2020;92:6886–6892. doi: 10.1021/acs.analchem.9b05432. [DOI] [PubMed] [Google Scholar]
- 18.McArdle H., Spaine E., Keyes T., Stallings R.L., Brennan-Fournet M. Triangular silver nanoplates: properties and ultrasensitive detection of miRNA. Electrochem. Commun. 2017;79:23–27. [Google Scholar]
- 19.Zhang Q., Li L., Qiao Z., Lei C., Fu Y., Xie Q., Yao S., Li Y., Ying Y. Electrochemical conversion of Fe3O4 magnetic nanoparticles to electroactive Prussian blue analogues for self-sacrificial label biosensing of avian influenza virus H5N1. Anal. Chem. 2017;89:12145–12151. doi: 10.1021/acs.analchem.7b02784. [DOI] [PubMed] [Google Scholar]
- 20.Sheridan C. Fast, portable tests come online to curb coronavirus pandemic. Nat. Biotechnol. 2020 doi: 10.1038/d41587-020-00010-2. [DOI] [PubMed] [Google Scholar]
- 21.Layqah L.A., Eissa S. An electrochemical immunosensor for the corona virus associated with the Middle East respiratory syndrome using an array of gold nanoparticle-modified carbon electrodes. Microchim. Acta. 2019;186:224. doi: 10.1007/s00604-019-3345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christodouleas D.C., Kaur B., Chorti P. From point-of-care testing to eHealth diagnostic devices (eDiagnostics) ACS Cent. Sci. 2018;4:1600–1616. doi: 10.1021/acscentsci.8b00625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nayak S., Blumenfeld N.-R., Laksanasopin T., Sia S.-K. Pointof-care diagnostics: recent developments in a connected age. Anal. Chem. 2017;89:102–123. doi: 10.1021/acs.analchem.6b04630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu J., Dong M., Rigatto C., Liu Y., Lin F. Lab-on-chip technology for chronic disease diagnosis. NPJ Digit. Med. 2018;1:7. doi: 10.1038/s41746-017-0014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Darwish N.-T., Sekaran S.-D., Khor S.-M. Point-of-care tests: a review of advances in the emerging diagnostic tools for dengue virus infection. Sens. Actuators. B. 2018;255:3316–3331. [Google Scholar]
- 26.Economou A., Kokkinos C., Prodromidis M. Flexible plastic, paper and textile lab-on-a chip platforms for electrochemical biosensing. Lab Chip. 2018;18:1812–1830. doi: 10.1039/c8lc00025e. [DOI] [PubMed] [Google Scholar]
- 27.Mielczarek W.-S., Obaje E.-A., Bachmann T.-T., Kersaudy-Kerhoas M. Microfluidic blood plasma separation for medical diagnostics: is it worth it? Lab Chip. 2016;16:3441–3448. doi: 10.1039/c6lc00833j. [DOI] [PubMed] [Google Scholar]
- 28.Son J.-H., Lee S.-H-., Hong S., Park S-.M., Lee J., Dickey A.-M., Lee L.-P. Hemolysis-free blood plasma separation. Lab Chip. 2014;14:2287–2292. doi: 10.1039/c4lc00149d. [DOI] [PubMed] [Google Scholar]
- 29.Liu C., Liao S.-C., Song J., Mauk M.-G., Li X., Wu G., Ge D., Greenberg R.-M., Yang S., Bau H.-H. A high-efficiency superhydrophobic plasma separator. Lab Chip. 2016;16:553–560. doi: 10.1039/c5lc01235j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ngom B., Guo Y., Wang X., Bi D. Development and application of lateral flow test strip technology for detection of infectious agents and chemical contaminants: a review. Anal. Bioanal. Chem. 2010;397:1113–1135. doi: 10.1007/s00216-010-3661-4. [DOI] [PubMed] [Google Scholar]
- 31.Dector A., Galindo-de-la-Rosa J., Amaya-Cruz D.-M., Ortiz-Verdın A., Guerra-Balcazar M., Olivares-Ramirez J.-M., Arriaga L.-G., Ledesma-Garcia J. Towards autonomous lateral flow assays: paper-based microfluidic fuel cell inside an HIV-test using a blood sample as fuel. Int. J. Hydrogen Energy. 2017;42:27979–27986. [Google Scholar]
- 32.E.J. O'Reilly, T. Keyes, R.J. Forster, L. Dennany, Deactivation of the ruthenium excited state by enhanced homogeneous charge transport: Implications for electrochemiluminescent thin film sensors, Electrochem. Commun. 86 (2018), 90–93.