Abstract
Salmonella Typhimurium (ST) infection in chickens inhibits their growth and can lead to food-borne diseases in humans. Probiotics are expected to enhance the function of host intestinal barrier against pathogen infection. The aim of our study was to determine the effect of viable Lactobacillus reuteri (LR) on the response of the mucosal barrier function to antigen stimulation in broiler chicks. Day-old male (n=8) and female (n=4) broiler chicks were orally administered either 1 × 108 LR or a water-only control, every day for 7 days. After 7 days, either 1 × 108 heat-killed ST (k-ST), or a buffer-only control, was administered via intra-cardiac injection. The ileum and cecum were collected 3 h post-injection, and paraffin sections were prepared for either mRNA extraction (males), or gut permeability tests (females). Villus and crypt lengths were determined via histological analysis. Real-time PCR was used to calculate expression levels of Toll-like receptors (TLRs), pro-inflammatory cytokines, anti-inflammatory cytokines, avian β-defensins, and tight-junction-associated molecules. Gut permeability was assessed using the inverted intestine method. We found that (1) expression of TLR2-1, TLR21, TGF-β2 and TGF-β3 were reduced following k-ST stimulation, but were unaffected by LR-treatment; (2) oral administration of LR led to increased Claudin1, Claudin5, ZO2, and JAM2 expression following k-ST stimulation; (3) cecal permeability was reduced by co-treatment with LR and k-ST, but not by treatment with LR or k-ST alone. These results suggest that LR, as used in this study, may enhance the intestinal mucosal physical barrier function, but not the expression of other immune-related factors in newly hatched chicks.
Keywords: broiler chick, cytokine, intestine, probiotics, Salmonella Typhimurium, tight junction
Introduction
Chicken intestine is continuously exposed to pathogenic bacteria via contaminated feed and water. Salmonella Typhimurium (ST), a major serotype of Salmonella, can infect broiler chickens, leading to decrease in their growth and food-borne diseases in humans (Rajani, et al., 2016). ST infects intestinal cells and macrophages in the small and large intestinal mucosa. It can pass through the lamina propria and subsequently migrate to other organs via the bloodstream (Ashayerizadeh, et al., 2017; Dar, et al., 2017). Adaptive immune function, such as T and B cell production, does not develop until approximately 2 weeks after hatching; however, chicks are often infected with ST as early as 3 days after hatching (Barrow, et al., 1987; Beal, et al., 2004). Therefore, the enhancement of immune function in the intestinal tract of newly hatched chicks is important for their protection from ST infection.
The mucosal barrier consists of a mucus layer, immune-related factors secreted in the mucus, and a tight junction of mucosal epithelium. The mucosal barrier is the first line of protection against pathogens invading the intestinal tissue. Avian β-defensins (AvBDs) are anti-microbial peptides that have been identified in avian species, and 14 AvBDs (AvBD1–14) have been detected in the intestinal tissue of chickens (Lynn, et al., 2007; Ramasamy, et al., 2012). It is reported that AvBDs directly kill pathogenic bacteria, fungi, and viruses (van Dijk, et al., 2008; Cuperus, et al., 2013) and AvBD1, 2, 6–9, and 12, in particular, have antibacterial activity against ST (Higgs, et al., 2007; van Dijk, et al., 2008; Derache, et al., 2009; Zhao, et al., 2014). The tight junction is a paracellular structure consisting of multi-protein complexes, including claudins, junctional adhesion molecules (JAMs), and zonula occludens (ZO) proteins, that regulate the physical barrier and ion permeability (Awad, et al., 2017).
Pathogen-associated molecular patterns (PAMPs) can be recognized using Toll-like receptors (TLRs). Ten subfamilies of TLRs (TLR1-1, 1-2, 2-1, 2-2, 3, 4, 5, 7, 15, and 21) have been detected in chickens, and all subfamilies except TLR1-2 have been found in their intestinal tissue (Brownlie and Allan, 2011). These TLRs may work to recognize pathogens that pass through the mucosal barrier. TLR2 recognizes Gram-positive bacterial cell wall components such as peptidoglycan, lipoteichoic acids, and lipoproteins; TLR4 recognizes lipopolysaccharide (LPS), which is a Gram-negative bacterial component; and TLR21 recognizes unmethylated CpG oligodeoxynucleotide (CpG-ODN), a microbe-specific DNA sequence (Keestra, et al., 2007; Brownlie and Allan, 2011; St Paul, et al., 2013). Production of cytokines and anti-microbial peptides is enhanced after the recognition of PAMPs by TLRs. The pro-inflammatory cytokines, interleukin (IL-) 1β and tumor necrosis factor (TNF)-α, activate immunocompetent cells such as macrophages and T and B cells, leading to inflammation and thus, the elimination of pathogens (Kushner, 1993; VanCott, et al., 1998; Ayres and Vance, 2012). CXCLi2 (homologous to human IL-8) is a chemokine involved in recruiting macrophages and monocytes (Sick, et al., 2000; Poh, et al., 2008). Additionally, anti-inflammatory cytokines, such as transforming growth factors (TGF)β-2, TGFβ-3, and TGFβ-4 (TGFβ-4 is the same as TGFβ-1) suppressing excess inflammation (Babyatsky, et al., 1996; Chen and Wahl, 1999).
Probiotic bacteria are known as beneficial bacteria because they regulate the host intestinal microflora. An example of probiotic organisms are lactic acid bacteria, which perform immune regulatory functions, such as increasing macrophage and T cell influx into the cecal tonsil and regulating cytokine expression in the ileum and cecum of chickens (Haghighi, et al., 2008; Fajardo, et al., 2012; Hu, et al., 2015; Penha Filho, et al., 2015). In addition, feeding chickens with probiotics, including Lactobacillus sp., reduces infection of the cecum with ST and Salmonella Enteritidis (SE) (Haghighi, et al., 2008; Higgins, et al., 2010; Penha Filho, et al., 2015). Therefore, probiotics are expected to aid in safe and stable poultry production by increasing host intestinal mucosal immune and barrier function. However, it is unknown whether treatment with probiotics affects the mucosal barrier function against antigens in the intestine of broiler chicks.
Thus, the aim of the study was to determine the effects of probiotics on the response of the intestinal mucosal barrier function to antigen stimulation in broiler chicks. Day-old broiler chicks were given viable Lactobacillus reuteri (LR) using oral gavage, followed by intra-cardiac injection of heat-killed ST (k-ST) on day 6. Then, we examined the following factors: villus height, crypt depth, gut permeability, and gene expression of TLRs (TLR2-1, 4, and 21), proinflammatory cytokines (IL-15, CXCLi2, and TNFSF15), Anti-inflammatory cytokines (TGF-β2, 3, and 4), AvBDs (AvBD2 and 7, because these two genes were highly expressed in the intestine of broiler chicks in our preliminary experiment), and tight junction-related factors (Claudin1, Claudin5, ZO2, and JAM2).
Materials and Methods
Treatment of Chicks and Tissue Collection
Experiment 1 Day-old male broiler chicks (Chunky broilers) were separated into four groups: the no-LR administered and no-k-ST stimulated group (CC), no-LR administered and k-ST stimulated group (CS), LR administered and no-k-ST stimulated group (LC), and LR administered and k-ST stimulated group (LS) (n = 8, for each of the groups). Chicks were kept under a lighting schedule of 23 h light/1 h dark and were provided with feed (commercial starter diet: NICHIWA SANGYO Co. Ltd., Kobe, Japan) and water ad libitum. The commercial starter diet contained two types of antibiotics (lasalocid sodium and enramycin). Chicks were orally administered with 300 µL sterilized water either with or without 1 × 108 cfu of viable Lactobacillus reuteri (5 mg FINELACT, Asahi Calpis Wellness Co. Ltd.), every morning for 7 days (day-0 to -6). On day-7, chicks received an intracardiac injection of 100 µL sterilized PBS/100 g body weight (BW), either with or without 1 × 108 cfu of k-ST (ATCC 13311). Chicks were euthanized using CO2, which was administered 3 h after injection, and the ileum and cecum were collected for preparation of paraffin sections for total RNA extraction. Oral administration of k-ST did not cause an immune reaction in the intestine during the preliminary experiment; therefore, in this study, k-ST stimulation was achieved using intra-cardiac injection in order to mimic ST infection via the bloodstream.
Experiment 2 Day-old female broiler chicks (Chunky broilers) were separated into four groups (CC, CS, LC, and LS; n=4, respectively), and were maintained in the same conditions as Experiment 1 and treated either with or without LR and k-ST, in the same way as Experiment 1. Three hours after intra-cardiac injection, chicks were euthanized and the ileum and cecum were removed and subjected to an intestinal permeability test. BWs were recorded on day 0 and 7 in both Experiments 1 and 2.
All experiments in this study were approved by Hiroshima University Animal Research Committee (Approval No. C15-17). All animal handling was carried out in accordance with its regulations.
Histological Analysis of Mucosal Structure
The ileum and cecum were fixed using 10% (v/v) formalin in phosphate-buffered saline (PBS), embedded in paraffin and the 4 µm-thick sections were stained with Hansen's hematoxylin and eosin. Light microscopy images were analyzed using NIS-Elements software (Nikon, Tokyo, Japan). The villus height and crypt depth of the ileum and cecum were measured, and these measurements were performed in quintuple on each section, and an average was derived.
Analysis of Gene Expression of Mucosal Barrier Function-related Molecules
Total RNA was extracted from the mucosa of the ileum and cecum using Sepasol RNA I Super G (Nacalai Tesque Inc., Kyoto, Japan) according to the manufacturer's instructions, dissolved in TE buffer (10 mM Tris-HCl, pH 8.0, 1 mM EDTA), and stored at −80°C until use.
Total RNA concentration was measured in each sample using Nano Drop Lite (Thermo Fisher Scientific, Waltham, MA, USA). Reverse transcription was performed using ReverTra Ace qPCR RT Master Mix with gDNA Remover (Toyobo Co. Ltd., Osaka, Japan) on a PTC-100 programmable thermal controller (MJ Research, Waltham, MA, USA), which was programmed according to the manufacturer's instructions. Real-time PCR was performed using the Aria MX real time PCR system (Agilent Technologies, Santa Clara, CA, USA) with Brilliant III Ultra-Fast SYBR Green QPCR Master Mix (Agilent Technologies). Table 1 shows the primer sequences used for PCR in this study. The cycle parameters for the amplification step of the PCR program were denaturation at 95°C for 5 s and followed by annealing at either 58°C (TNFSF15, TGF-β1, TGF-β2, and ZO2), 60°C (TLR2-1, TLR4, TLR21, CXCLi2, TGF-β, Claudin1, and RPS17), 62°C (AvBD2 and Claudin5), 63°C (IL-1β and AvBD7), or 64°C (JAM2) for 10 s, and these steps were repeated for 50 cycles. The cycle parameters for the melt step were 95°C for 30 s, 65°C for 30 s and 95°C for 30 s. RNA expression levels were calculated using the relative quantification method and a standard curve for each target gene. Relative target mRNA expression in each sample was normalized as a ratio between the RPS17 housekeeping gene, and the mean fold-change in gene expression from one standard sample in the control group.
Table 1. Primer sequences used in PCR analysis.
| Target genes | Forward Primer | Revers Primer | Product size | Accession no. |
|---|---|---|---|---|
| TLR2-1 | ACATGTGTGAATGGCCTGAA | TTGAGAAATGGCAGTTGCAG | 151 | NM_204278.1 |
| TLR4 | AGTCTGAAATTGCTGAGCTCAAAT | GCGACGTTAAGCCATGGAAG | 190 | NM_001030693.1 |
| TLR21 | TGCCCCTCCCACTGCTGTCCACT | AAAGGTGCCTTGACATCCT | 113 | NM_001030558.1 |
| IL-1β | GTGAGGCTCAACATTGCGCTGTA | TGTCCAGGCGGTAGAAGATGAAG | 214 | NM_204524.1 |
| CXCLi2 | CTGTCCTGGCCCTCCTCCTGGTT | TGGCGTCAGCTTCACATCTTG | 146 | NM_205498.1 |
| TNFSF15 | CCTGAGTTATTCCAGCAACGCA | ATCCACCAGCTTGATGTCACTAAC | 292 | NM_001024578.1 |
| TGFβ-2 | AGGAATGTGCAGGATAATT | ATTTTGGGTGTTTTGCCAA | 269 | NM_001031045.3 |
| TGFβ-3 | CAGATCCTGGCGCTCTACA | GAGGCCCTGGATCATGTCA | 141 | NM_205454.1 |
| TGFβ-4 | ATGAGTATTGGGCCAAAG | ACGTTGAACACGAAGAAG | 109 | NM_001318456.1 |
| AvBD2 | GTTCTGTAAAGGAGGGTCCTGCCAC | ACTCTACAACACAAAACATATTGC | 238 | NM_204992.2 |
| AvBD7 | ACCTGCTGCTGTCTGTCCTC | TGCACAGCAAGAGCCTATTC | 173 | NM_001001194.1 |
| Claudin1 | GACTCGCTGCTTAAGCTGGA | AAATCTGGTGTTAACGGGTG | 276 | NM_001013611.2 |
| Claudin5 | GTCCCGCTCTGCTGGTTC | CCCTATCTCCCGCTTCTGG | 84 | NM_204201.1 |
| ZO2 | GAAGCAGAGGTCGTAGTAGG | CTGTCCATAGCCACCATCC | 140 | NM_204918.1 |
| JAM2 | AGCCTCAAATGGGATTGGATT | CATCAACTTGCATTCGCTTCA | 59 | NM_001006257.1 |
| RPS17 | AAGCTGCAGGAGGAGGAGAGG | GGTTGGACAGGCTGCCGAAGT | 136 | NM_204217.1 |
Gut Permeability Test
Permeability in the ileum and cecum was examined using fluorescein isothiocyanate (FITC) -labeled dextran with a molecular weight of 4,000 (FD-4, Sigma Aldrich, St Louis MO, USA) and a modified version of the protocol outlined by Murai et al. (2018). The ileum and cecum were cut into sections of approximately 3 cm, inverted, closed with a string on one side, and filled with oxygen aerated Tyrode's balanced salt solution [0.02% (v/v) CaCl2, 0.02% (v/v) KCl, 0.005% (v/v) MgCl2, 0.8% (v/v) NaCl, 0.0048% (v/v) NaH2 PO4, 0.119% (v/v) HEPES, and 0.1% (v/v) glucose]. The open end was then sealed using a clip and the inverted intestine was incubated in Tyrode's solution supplemented with 100 µg/mL FITC-dextran for 30 min at 39°C in a shaking water bath (60 rpm). After incubation, the inner solution was collected and the fluorescence of the FITC-dextran that permeated was measured using a 2030 ARVO X4 Multilabel Reader spectrofluorometer (PerkinElmer, Waltham, MA, USA; excitation 485 nm, emission 535 nm) in order to calculate the concentration of FITC-dextran. Gut permeability was evaluated using the FITC concentration in the inner solution in the inverted intestinal sac and high FITC concentration suggested high-permeability and poor tight-junction strength.
Statistical Analysis
Values are expressed as mean±SEM. The differences in villus height, crypt depth, mRNA expression level, and permeability of each tissue, between the 4 groups were analyzed using two-way ANOVA and the Tukey-Kramer test (differences were considered significant when P<0.05).
Results
BW of Experimental Birds
Table 2 shows BWs of the chicks on days 0 and 7 in both experiments. BW did not change upon oral administration of LR and k-ST stimulation.
Table 2. Body weights (g) of chicks at day 0 and 7.
| CC | CS | LC | LS | ||
|---|---|---|---|---|---|
| Experiment 1 | day 0 | 43.2±0.9 | 43.2±0.9 | 43.5±0.9 | 43.7±1.0 |
| day 7 | 176.0±3.9 | 173.8±7.3 | 166.0±4.7 | 163.3±8.7 | |
| Experiment 2 | day 0 | 43.4±1.7 | 43.0±1.1 | 42.8±1.0 | 42.6±0.8 |
| day 7 | 168.8±6.4 | 172.3±6.1 | 170.8±5.4 | 166.8±5.7 | |
CC=no-LR and no-ST; CS=no-LR and ST; LC=LR and no-ST; LS=LR and ST.
LR: Lactobacillus reuteri, ST: Salmonella Typhimurium.
Experiment 1
Histological analysis
Ileal and cecal epithelial mucosa were covered with epithelial cells, including goblet cells. The villi and crypts in the ileum were well developed in all experimental groups (data not shown). On the other hand, the cecum had short and wide villi and crypts, and they were not clearly distinguished (data not shown). The height of each villus and depth of each crypt in the ileum was measured, and sum of villus height and crypt depth was calculated in the cecum. The villus height in the ileum of the LC group was significantly higher than that of the CC group (P<0.05; Fig. 1A). However, there was no significant difference in the ileal crypt depth between any of the experimental groups (Fig. 1B). The sum of villus height and crypt depth in the cecum of the CS group was lower than of the CC group (P<0.05; Fig. 1C). The apparent histological damage and increase in leukocyte infiltration in the ileal and cecal mucosal tissue were not found in all experimental groups (data not shown).
Fig. 1.
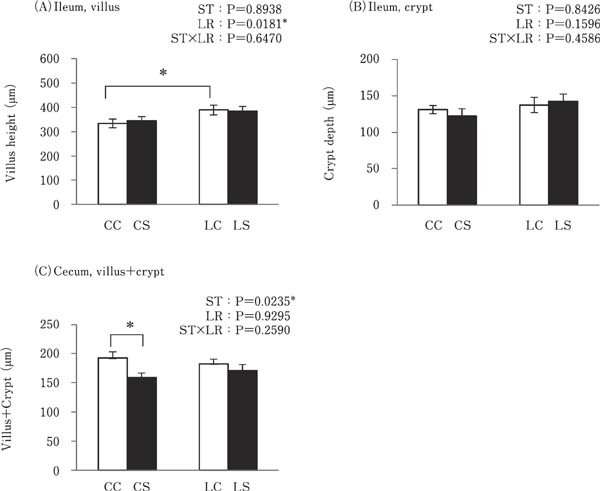
Effects of oral administration of Lactobacillus reuteri (LR) and stimulation of heat-killed Salmonella Typhimurium (k-ST) on the length of the ileal villi (a), ileal crypts (b) and cecal villi and crypts (c). Values are expressed as mean±SEM (n=8). CC=no-LR and no-ST; CS=no-LR and ST; LC=LR and no-ST; LS=LR and ST. □=no-ST stimulated group, ■=ST stimulated groups. * P<0.05.
Gene Expression of Mucosal Barrier Function-related Factors
Cecal TLR2-1 was significantly lower in the k-ST stimulated groups (CS and LS) when compared to both the CC and LC groups (P<0.05; Fig. 2B). TLR21 expression in the ileum and cecum were significantly lower in the CS group than in the CC group (P<0.05), but no such difference was observed between the LS and LC groups (Fig. 2E and F). TLR21 expression in the ileum was lower in the LC group than in the CC group (P<0.01; Fig. 2E). However, there was no significant difference in the expression of TLR2-1 in the ileum and TLR4 in the ileum and cecum between any of the experimental groups (Fig. 2A, C and D).
Fig. 2.
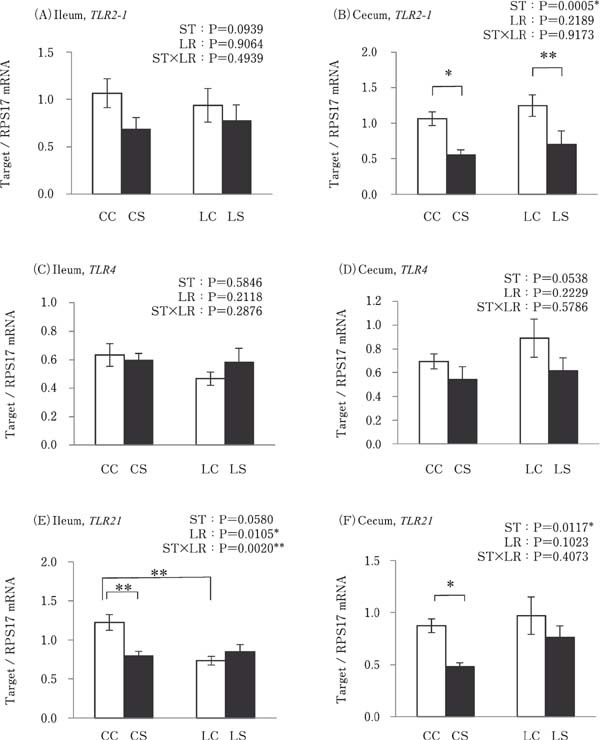
Effects of oral administration of Lactobacillus reuteri (LR) and stimulation of heat-killed Salmonella Typhimurium (ST) on the mRNA expression levels of TLRs in the intestinal tract. Values are mean± SEM (n=8) of fold-change in target gene expression, calculated from a standard sample of each segment in the control group. CC=no-LR and no-ST; CS=no-LR and ST; LC=LR and no-ST; LS=LR and ST. □=no-ST stimulated group, ■=ST stimulated groups. *P<0.05 and **P<0.01.
Cecal IL-1β expression was significantly higher in the LS group than in the CS and LC groups (P<0.05; Fig. 3B). Cecal TNFSF15 expression was significantly higher in the LS group than in the CS group (P<0.05; Fig. 3F). However, the expressions of these pro-inflammatory cytokines in the ileum and the expression of CXCLi2 in the ileum and cecum were similar in all experimental groups (Fig. 3A, C, D and E).
Fig. 3.
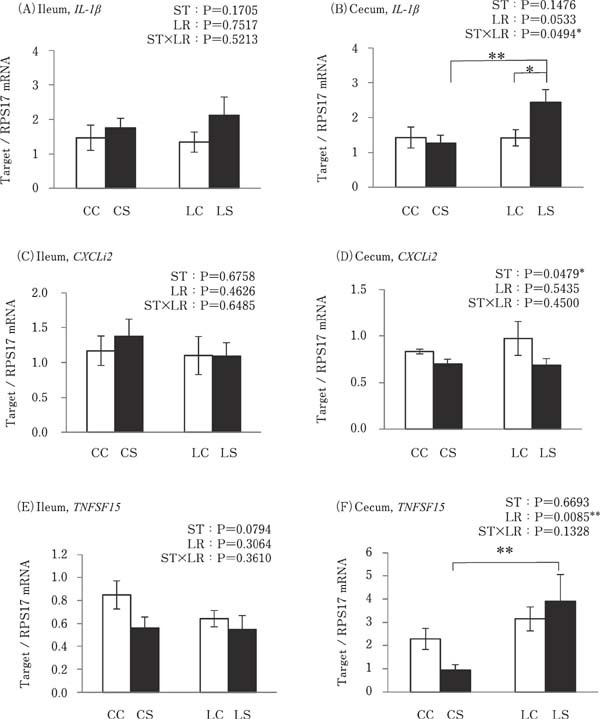
Effects of oral administration of Lactobacillus reuteri (LR) and stimulation of heat killed Salmonella Typhimurium (ST) on the mRNA expressions of pro-inflammatory cytokines in the intestinal tract. Values are mean±SEM (n=8) of fold change in the target gene expression, calculated from a standard sample of each segment in the control group. CC=no-LR and no-ST; CS=no-LR and ST; LC=LR and no-ST; LS=LR and ST. □=no-ST stimulated group, ■=ST stimulated groups. *P<0.05 and **P<0.01.
Cecal expression of TGFβ-2 was significantly lower in the k-ST stimulated groups compared to that in the no-k-ST stimulated groups (P<0.05; Fig. 4B). TGFβ-3 expression in the CS group was significantly lower than that in the CC group in both the ileum and cecum. The LC group showed lower TGFβ-3 expression than the CC group in the ileum, while the LS group showed higher TGFβ-3 expression levels than the CS group (P<0.05; Fig. 4C and D). Furthermore, no differences were observed in the expression of TGFβ-2 in the ileum and TGFβ-4 in the ileum and cecum between all of the experimental groups (Fig. 4A and E).
Fig. 4.

Effects of oral administration of Lactobacillus reuteri (LR) and stimulation of heat killed Salmonella Typhimurium (ST) on the mRNA expressions of anti-inflammatory cytokines in the intestinal tract. Values are mean±SEM (n=8) of fold change in the target gene expression, calculated from a standard sample of each segment in the control group. CC=no-LR and no-ST; CS=no-LR and ST; LC=LR and no-ST; LS=LR and ST. □=no-ST stimulated group, ■= ST stimulated groups. *P<0.05 and ** P<0.01.
No difference was observed between expressions of AvBD2 and AvBD7 in the ileum or cecum in any of the experimental groups (Fig. 5).
Fig. 5.
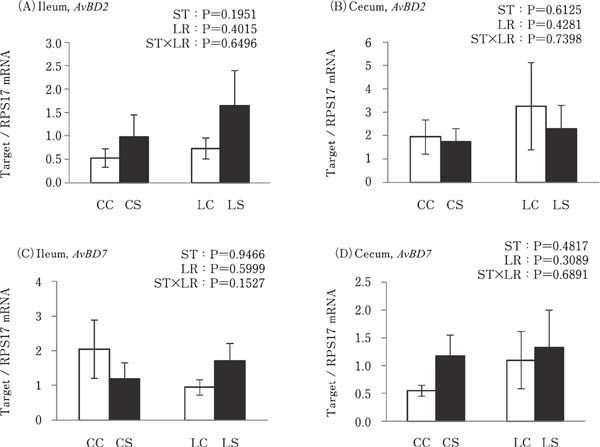
Effects of oral administration of Lactobacillus reuteri (LR) and stimulation of heat killed Salmonella Typhimurium (ST) on the mRNA expressions of avian β defensin (AvBD) in the intestinal tract. Values are mean±SEM (n=8) of fold change in the target gene expression, calculated from a standard sample of each segment in the control group. CC=no-LR and no-ST; CS=no-LR and ST; LC=LR and no-ST; LS=LR and ST. □=no-ST stimulated group, ■=ST stimulated groups.
Cecal expression of tight junction-related molecules (Claudin1, Claudin5, ZO2, and JAM2) was significantly higher in the LS group than in the CS group, and JAM2 expression was also higher in the LC group than in the CC group (P<0.01; Fig. 6B, D, F, and H). However, expression of these genes in the ileum was not altered by k-ST stimulation or LR treatment (Fig. 6A, C, E, and G).
Fig. 6.
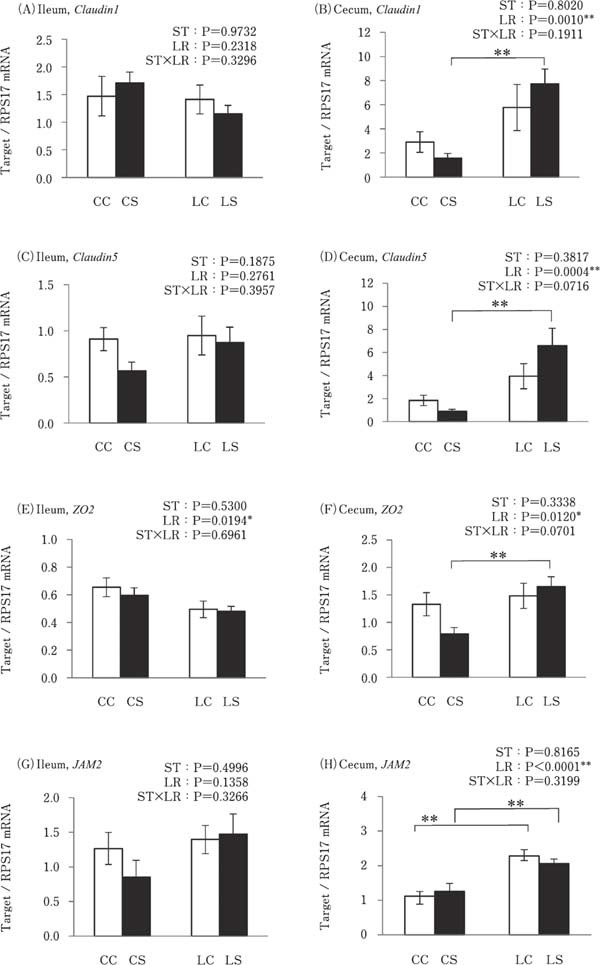
Effects of oral administration of Lactobacillus reuteri (LR) and stimulation of heat killed Salmonella Typhimurium (ST) on the mRNA expressions of tight junction related genes in the intestinal tract. Values are mean±SEM (n=8) of fold change in the target gene expression, calculated from a standard sample of each segment in the control group. CC=no-LR and no-ST; CS=no-LR and ST; LC=LR and no-ST; LS=LR and ST. □=no-ST stimulated group, ■=ST stimulated groups. **P<0.01.
Experiment 2
Gut permeability in the ileum and cecum
There was no significant difference between the concentrations of FITC in the ileum among all experimental groups; however, cecal FITC concentrations were significantly lower in the LS group than in the CS and LC groups (P<0.05; Fig. 7B).
Fig. 7.
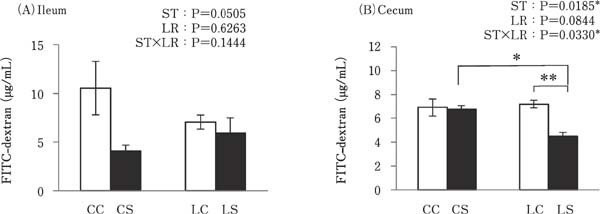
Effects of oral administration of Lactobacillus reuteri (LR) and stimulation of heat killed Salmonella Typhimurium (ST) on the gut permeability in the ileum and cecum. The concentration of the permeated FITC-dextran into everted gut sacs was examined using a spectrofluorometer. Values are mean±SEM (n=4). CC=no-LR and no-ST; CS=no-LR and ST; LC=LR and no-ST; LS=LR and ST. □=no-ST stimulated group, ■=ST stimulated groups. *P<0.05 and **P<0.01.
Discussion
This study showed the effects of oral administration of LR on the response of intestinal mucosal barrier function to k-ST antigen stimulation in broiler chicks. The significant findings of this study were that (1) TLR2-1, TLR21, TGF-β2, And TGF-β3 expression levels were decreased by k-ST stimulation, and there were no differences in their expression between the LR-treated groups; (2) oral administration of LR resulted in increased Claudin1, Claudin5, ZO2, JAM2 expression levels in the cecum following k-ST stimulation; (3) gut permeability was reduced in the cecum by co-treatment with both LR and k-ST, but not by LR or ST alone.
Higher levels of IL-1β and TNFSF15 expression were observed in the cecum in the LS group compared to the CS group (Fig. 3B and F). Although, histological damage and level of leukocyte infiltration did not differ between these groups, IL-1β was quickly released in response to bacterial and viral infection, and it contributed to the enhancement of host innate and acquired immune responses (Gibson, et al., 2014). Therefore, oral administration of the probiotic LR used in this study may increase host immune function by increasing the expression of pro-inflammatory cytokines against ST infection without mucosal damage and inflammation. Additionally, the sum of villus height and crypt depth in the cecum of the CS group was lower than that of the CC group, but the markers of inflammation, such as increased cytokine expression and leukocyte infiltration were not detected in the CS group. The inflammatory condition caused by E. coli and Salmonella infection typically decreases the ratio of villus height to crypt depth in the intestine of the broiler chicks (Borsoi, et al., 2011; Menconi, et al., 2015; Huang, et al., 2018). This data therefore suggests that k-ST stimulation did not cause heavy inflammation in the intestinal mucosa in this study, but did lead to mild inflammation, such as regression of mucosal villi. However, the lactic acid bacteria may reduce the mucosal damage caused by k-ST stimulation, since regression of cecal mucosa was not observed in the LR treated group in this study. Furthermore, ileal villus height was increased in the LR treated group in this study. These results suggest that the LR used in this study facilitated the development of the villus in the ileum. Based on previous findings, the positive effects on intestinal mucosal structure due to lactic acid bacteria in this study may reflect a common function (Deng, et al., 2017; Huang, et al., 2018; Li, et al., 2018).
TLR2-1, TGF-β2, and TGF-β3 expression levels in the cecum were reduced as a result of k-ST stimulation in both the LR and no-LR treatment groups. We believe that the LR treatment used in this study may not affect the gene expression of TLR2-1, TGF-β2, and TGF-β3 in the cecum. Meanwhile, TLR21 and TGF-β3 expressions were decreased by not only k-ST stimulation, but also by LR treatment in the ileum. The reason behind the decrease in TLR21 and TGF-β3 gene expression caused by LR treatment is still unknown. It is predicted that the change in the microbiome of the ileum caused as a result of LR oral administration affects the TLR21 and TGF-β expressions.
AvBD2 and AvBD7 mRNA expression was detected in the ileum and cecum. AvBD2 and AvBD7 exhibit antibacterial function against ST (Derache, et al., 2009; Zhao, et al., 2014), and hence their expression in the ileum and cecum may contribute to protection of the intestine of the broiler chicks from ST infection. However, their expression was not enhanced upon k-ST stimulation or LR treatment. Our previous results showed that AvBD2 and AvBD7 expression in the proventriculus of broiler chicks was not altered by the probiotic feed, that included Streptococcus faecalis, Clostridium buthricum, and Bacillus mesentericus (Mohammed, et al., 2015). Thus, oral administration of LR and ST stimulation, as in this study, may not affect the expression of AvBD2 and AvBD7 in the intestine.
Cecal Claudin1, Claudin5, ZO2, and JAM2 expression was higher in the LR treated group than in the group without LR treatment and under the k-ST stimulated condition. Moreover, cecal permeability was lower in the LS group than in the CS and LC groups. Wang et al., (2018) reported that ZO1 and Claudin5 expression levels were higher and gut permeability was lower in chicks co-treated with ST and Lactobacillus plantarum compared to chicks that were exposed to ST without probiotics. Our results also support this report, suggesting that an ST component may induce a decrease in the expression of tight junction related molecules, but that lactic acid bacteria enhance the function of tight junctions by increasing the reactivity of tight junction-related molecules against ST stimulation in the cecum, but not in the ileum. The bacterial abundance in chicken cecum contents is 10–1000-fold higher than in the ileal contents (Yeoman, et al., 2012). It is possible that probiotic bacteria have a stronger effect in the cecum than in the ileum and this effect may be relative to their respective bacterial populations.
Claudin1, Claudin5, and Claudin16 are interstitially located in the intestinal mucosal epithelial cells and together form a tight junction in chickens (Ozden, et al., 2010; Theerawatanasirikul, et al., 2017). In contrast, Claudin5 and JAM2 proteins are not only expressed in the mucosal epithelium, but also in the vascular endothelial cells of mice (Martin-Padura, et al., 1998; Morita, et al., 1999). Hence, the results of gene expressions of tight junction related molecules and gut permeability may reflect not only the function of tight junction in the mucosal epithelial cells, but also reflect the function of those in the vascular endothelium of the cecum, since we used intra-cardiac injection for k-ST stimulation in this study. It is assumed that the tight junctions of the cecal mucosal epithelium protect from invasion by bacteria, and similarly in the vascular endothelium prevent the diffusion of invading bacteria. Butyric acid promotes gut integrity by increasing the expression of tight junction-related molecules in piglets (Xiong, et al., 2019). Using feed with encapsulated organic acids and essential oils has been shown to increase the concentration of butyric acid and acetic acid in the ileal digesta and increase Claudin5 expression in the ileum of the broiler chicks (Yang, et al., 2019). Accordingly, it is possible that metabolites, such as organic acids derived from LR or other bacteria beneficial to the intestine may enhance the host mucosal barrier function, including immune function and tight junctions.
In conclusion, ST stimulation may decrease antigen recognition by TLR2-1 and 21 resulting in a decrease in cytokine production, whereas oral administration of LR may not affect their expression. However, the oral administration of LR enhanced the response of the physical barrier function of the tight junction by increasing the expression of Claudin1, Claudin5, JAM2, and ZO2 upon ST stimulation in the cecum. Thus, oral administration of LR may contribute to safe and sustainable poultry production through the enhancement of host intestinal mucosal physical barrier function in newly hatched chicks.
Acknowledgments
We thank Dr. A. Murai, Graduate School of Bioagricultural Science, Nagoya University, for the kind proffering his protocol and advice on gut permeability test in Experiment 2. This study was supported by a Grant-in-Aid for Scientific Research (B) from the Japan Society for the Promotion of Science (JSPS) (No. 17H03904) to Y. Y., and Grant-in-Aid for Early-Career Scientists from the JSPS (No. 18K14569) to T. N.
Conflict of Interest
The authors declare no conflict of interest.
References
- Ashayerizadeh A, Dastar B, Shams Shargh M, Sadeghi Mahoonak A, Zerehdaran S. Fermented rapeseed meal is effective in controlling Salmonella enterica serovar Typhimurium infection and improving growth performance in broiler chicks. Veterinary Microbiology, 201: 93-102. 2017. [DOI] [PubMed] [Google Scholar]
- Awad WA, Hess C, Hess M. Enteric Pathogens and Their Toxin-Induced Disruption of the Intestinal Barrier through Alteration of Tight Junctions in Chickens. Toxins, 9: E60 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayres JS, Vance RE. Cellular teamwork in antibacterial innate immunity. Nature Immunology, 13: 115-117. 2012. [DOI] [PubMed] [Google Scholar]
- Babyatsky MW, Rossiter G, Podolsky DK. Expression of transforming growth factors alpha and beta in colonic mucosa in inflammatory bowel disease. Gastroenterology, 110: 975-984. 1996. [DOI] [PubMed] [Google Scholar]
- Barrow PA, Tucker JF, Simpson JM. Inhibition of colonization of the chicken alimentary tract with Salmonella typhimurium gram-negative facultatively anaerobic bacteria. Epidemiology and Infection, 98: 311-322. 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal RK, Wigley P, Powers C, Hulme SD, Barrow PA, Smith AL. Age at primary infection with Salmonella enterica serovar Typhimurium in the chicken influences persistence of infection and subsequent immunity to re-challenge. Veterinary Immunology and Immunopathology, 100: 151-164. 2004. [DOI] [PubMed] [Google Scholar]
- Borsoi A, Ruschel do Santos L, Beatriz Rodrigues L, Luiz de Souza Moraes H, Tadeu Pippi Salle C, Pinheiro do Nascimento V. Behavior of salmonella heidelberg and salmonella enteritidis strains following broiler chick inoculation: evaluation of cecal morphometry, liver and cecum bacterial counts and fecal excretion patterns. Brazilian Journal of Microbiology, 42: 266-273. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlie R, Allan B. Avian toll-like receptors. Cell and Tissue Research, 343: 121-130. 2011. [DOI] [PubMed] [Google Scholar]
- Chen W, Wahl SM. Manipulation of TGF-beta to control autoimmune and chronic inflammatory diseases. Microbes and Infection, 1: 1367-1380. 1999. [DOI] [PubMed] [Google Scholar]
- Cuperus T, Coorens M, van Dijk A, Haagsman HP. Avian host defense peptides. Developmental and Comparative Immunology, 41: 352-369. 2013. [DOI] [PubMed] [Google Scholar]
- Dar MA, Ahmad S, Bhat SA, Ahmed R, Urwat U, Mumtaz PT, Bhat SA, Dar TA, Shah RA, Ganai NA. Salmonella typhimurium in poultry: a review. World's Poultry Science Journal, 73: 345-354. 2017. [Google Scholar]
- Deng B, Wu J, Li X, Men X, Xu Z. Probiotics and Probiotic Metabolic Product Improved Intestinal Function and Ameliorated LPS-Induced Injury in Rats. Current Microbiology, 74: 1306-1315. 2017. [DOI] [PubMed] [Google Scholar]
- Derache C, Labas V, Aucagne V, Meudal H, Landon C, Delmas AF, Magallon T, Lalmanach AC. Primary structure and antibacterial activity of chicken bone marrow-derived beta-defensins. Antimicrobial Agents and Chemotherapy, 53: 4647-4655. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajardo P, Pastrana L, Mendez J, Rodriguez I, Fucinos C, Guerra NP. Effects of feeding of two potentially probiotic preparations from lactic acid bacteria on the performance and faecal microflora of broiler chickens. Scientific World Journal, 2012: 562635 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson MS, Kaiser P, Fife M. The chicken IL-1 family: evolution in the context of the studied vertebrate lineage. Immunogenetics, 66: 427-438. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighi HR, Abdul-Careem MF, Dara RA, Chambers JR, Sharif S. Cytokine gene expression in chicken cecal tonsils following treatment with probiotics and Salmonella infection. Veterinary Microbiology, 126: 225-233. 2008. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Higgins SE, Wolfenden AD, Henderson SN, Torres-Rodriguez A, Vicente JL, Hargis BM, Tellez G. Effect of lactic acid bacteria probiotic culture treatment timing on Salmonella Enteritidis in neonatal broilers. Poultry Science, 89: 243-247. 2010. [DOI] [PubMed] [Google Scholar]
- Higgs R, Lynn DJ, Cahalane S, Alana I, Hewage CM, James T, Lloyd AT, O'Farrelly C. Modification of chicken avian beta-defensin-8 at positively selected amino acid sites enhances specific antimicrobial activity. Immunogenetics, 59: 573-580. 2007. [DOI] [PubMed] [Google Scholar]
- Hu JL, Yu H, Kulkarni RR, Sharif S, Cui SW, Xie MY, Nie SP, Gong J. Modulation of cytokine gene expression by selected Lactobacillus isolates in the ileum, caecal tonsils and spleen of Salmonella-challenged broilers. Avian Pathology, 44: 463-469. 2015. [DOI] [PubMed] [Google Scholar]
- Huang L, Luo L, Zhang Y, Wang Z, Xia Z. Effects of the Dietary Probiotic, Enterococcus faecium NCIMB11181, on the Intestinal Barrier and System Immune Status in Escherichia coli O78-Challenged Broiler Chickens. Probiotics and Antimicrobial Proteins, 10.1007/s12602-018-9434-7 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keestra AM, de Zoete MR, van Aubel RA, van Putten JP. The central leucine-rich repeat region of chicken TLR16 dictates unique ligand specificity and species-specific interaction with TLR2. Journal of Immunology, 178: 7110-7119. 2007. [DOI] [PubMed] [Google Scholar]
- Kushner I. Regulation of the acute phase response by cytokines. Perspectives in Biology and Medicine, 36: 611-622. 1993. [DOI] [PubMed] [Google Scholar]
- Li CL, Wang J, Zhang HJ, Wu SG, Hui QR, Yang CB, Fang RJ, Qi GH. Intestinal Morphologic and Microbiota Responses to Dietary Bacillus spp. in a Broiler Chicken Model. Frontiers in Physiology, 9: 1968 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn DJ, Higgs R, Lloyd AT, O'Farrelly C, Herve-Grepinet V, Nys Y, Brinkman FS, Yu PL, Soulier A, Kaiser P, Zhang G, Lehrer RI. Avian beta-defensin nomenclature: a community proposed update. Immunology Letters, 110: 86-89. 2007. [DOI] [PubMed] [Google Scholar]
- Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A, Simmons D, Dejana E. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. Journal of Cell Biology, 142: 117-127. 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menconi A, Hernandez-Velasco X, Vicuna EA, Kuttappan VA, Faulkner OB, Tellez G, Hargis BM, Bielke LR. Histopathological and morphometric changes induced by a dextran sodium sulfate (DSS) model in broilers. Poultry Science, 94: 906-911. 2015. [DOI] [PubMed] [Google Scholar]
- Mohammed ESI, Okazaki A, Isobe N, Yoshimura Y. Effects of Probiotics on the Expression and Localization of Avian β-defensins in the Proventriculus of Broiler Chicks. Journal of Poultry Science, 52: 57-67. 2015. [Google Scholar]
- Morita K, Sasaki H, Furuse M, Tsukita S. Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. Journal of Cell Biology, 147: 185-194. 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai A, Kitahara K, Terada H, Ueno A, Ohmori Y, Kobayashi M, Horio F. Ingestion of paddy rice increases intestinal mucin secretion and goblet cell number and prevents dextran sodium sulfate-induced intestinal barrier defect in chickens. Poultry Science, 97: 3577-3586. 2018. [DOI] [PubMed] [Google Scholar]
- Ozden O, Black BL, Ashwell CM, Tipsmark CK, Borski RJ, Grubb BJ. Developmental profile of claudin-3, -5, and -16 proteins in the epithelium of chick intestine. Anatomical Record, 293: 1175-1183. 2010. [DOI] [PubMed] [Google Scholar]
- Penha Filho RA, Diaz SJ, Fernando FS, Chang YF, Andreatti Filho RL, Berchieri Junior A. Immunomodulatory activity and control of Salmonella Enteritidis colonization in the intestinal tract of chickens by Lactobacillus based probiotic. Veterinary Immunology and Immunopathology, 167: 64-69. 2015. [DOI] [PubMed] [Google Scholar]
- Poh TY, Pease J, Young JR, Bumstead N, Kaiser P. Reevaluation of chicken CXCR1 determines the true gene structure: CXCLi1 (K60) and CXCLi2 (CAF/interleukin-8) are ligands for this receptor. Journal of Biological Chemistry, 283: 16408-16415. 2008. [DOI] [PubMed] [Google Scholar]
- Rajani J, Dastar B, Samadi F, Karimi Torshizi MA, Abdulkhani A, Esfandyarpour S. Effect of extracted galactoglucomannan oligosaccharides from pine wood (Pinus brutia) on Salmonella typhimurium colonisation, growth performance and intestinal morphology in broiler chicks. British Poultry Science, 57: 682-692. 2016. [DOI] [PubMed] [Google Scholar]
- Ramasamy KT, Verma P, Reddy MR. Differential gene expression of antimicrobial peptides beta defensins in the gastrointestinal tract of Salmonella serovar Pullorum infected broiler chickens. Veterinary Research Communications, 36: 57-62. 2012. [DOI] [PubMed] [Google Scholar]
- Sick C, Schneider K, Staeheli P, Weining KC. Novel chicken CXC and CC chemokines. Cytokine, 12: 181-186. 2000. [DOI] [PubMed] [Google Scholar]
- St Paul M, Brisbin JT, Abdul-Careem MF, Sharif S. Immuno-stimulatory properties of Toll-like receptor ligands in chickens. Veterinary Immunology and Immunopathology, 152: 191-199. 2013. [DOI] [PubMed] [Google Scholar]
- Theerawatanasirikul S, Koomkrong N, Kayan A, Boonkaewwan CB. Intesitinal barrier and mucosal immunity in broilers, Thai Betong, and native Thai Praduhangdum chickens. Turkish Journal of Veterinary and Animal Sciences, 41: 357-364. 2017. [Google Scholar]
- van Dijk A, Veldhuizen EJ, Haagsman HP. Avian defensins. Veterinary Immunology and Immunopathology, 124: 1-18. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanCott JL, Chatfield SN, Roberts M, Hone DM, Hohmann EL, Pascual DW, Yamamoto M, Kiyono H, McGhee JR. Regulation of host immune responses by modification of Salmonella virulence genes. Nature Medicine, 4: 1247-1252. 1998. [DOI] [PubMed] [Google Scholar]
- Wang L, Li L, Lv Y, Chen Q, Feng J, Zhao X. Lactobacillus plantarum Restores Intestinal Permeability Disrupted by Salmonella Infection in Newly-hatched Chicks. Scientific Reports, 8: 2229 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X, Tan B, Song M, Ji P, Kim K, Yin Y, Liu Y. Nutritional Intervention for the Intestinal Development and Health of Weaned Pigs. Frontiers in Veterinary Science, 6: 46 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Liu Y, Yan F, Yang C. Effects of encapsulated organic acids and essential oils on intestinal barrier, microbial count, and bacterial metabolites in broiler chickens. Poultry Science, 10.3382/ps/pez031 2019. [DOI] [PubMed] [Google Scholar]
- Yeoman CJ, Chia N, Jeraldo P, Sipos M, Goldenfeld ND, White BA. The microbiome of the chicken gastrointestinal tract. Animal Health Research Reviews, 13: 89-99. 2012. [DOI] [PubMed] [Google Scholar]
- Zhao L, Yang M, Zhang M, Zhang S. Expression, purification, and in vitro comparative characterization of avian beta-defensin-2, -6, and -12. Avian Diseases, 58: 541-549. 2014. [DOI] [PubMed] [Google Scholar]


