Abstract
Purpose
To assess the additional value of quantitative tCho evaluation to diagnose malignancy and lymph node metastases in suspicious lesions on multiparametric breast MRI (mpMRI, BI-RADS 4, and BI-RADS 5).
Methods
One hundred twenty-one patients that demonstrated suspicious multiparametric breast MRI lesions using DCE, T2w, and diffusion-weighted (DW) images were prospectively enrolled in this IRB-approved study. All underwent single-voxel proton MR spectroscopy (1H-MRS, point-resolved spectroscopy sequence, TR 2000 ms, TE 272 ms) with and without water suppression. The total choline (tCho) amplitude was measured and normalized to millimoles/liter according to established methodology by two independent readers (R1, R2). ROC-analysis was employed to predict malignancy and lymph node status by tCho results.
Results
One hundred three patients with 74 malignant and 29 benign lesions had full 1H-MRS data. The area under the ROC curve (AUC) for prediction of malignancy was 0.816 (R1) and 0.809 (R2). A cutoff of 0.8 mmol/l tCho could diagnose malignancy with a sensitivity of > 95%. For prediction of lymph node metastases, tCho measurements achieved an AUC of 0.760 (R1) and 0.788 (R2). At tCho levels < 2.4 mmol/l, no metastatic lymph nodes were found.
Conclusion
Quantitative tCho evaluation from 1H-MRS allowed diagnose malignancy and lymph node status in breast lesions suspicious on multiparametric breast MRI. tCho therefore demonstrated the potential to downgrade suspicious mpMRI lesions and stratify the risk of lymph node metastases for improved patient management.
Key Points
• Quantitative tCho evaluation can distinguish benign from malignant breast lesions suspicious after multiparametric MRI assessment.
• Quantitative tCho levels are associated with lymph node status in breast cancer.
• Quantitative tCho levels are higher in hormonal receptor positive compared to hormonal receptor negative lesions.
Electronic supplementary material
The online version of this article (10.1007/s00330-020-06678-z) contains supplementary material, which is available to authorized users.
Keywords: Magnetic resonance spectroscopy, Magnetic resonance imaging, Breast neoplasms, Prognosis, Sensitivity and specificity
Introduction
Breast cancer is a major burden on the female population and subsequently for the socioeconomic system. Consequently, most industrialized nations have introduced nation-wide mammography screening programs that are supported by the majority of specialist societies [1]. Screening with mammography and additional evaluations with ultrasound and MRI add in cancer diagnosis and assessment of disease [2–4].
Currently, breast cancer is treated by a combination of surgery, pharmaceutical therapy, and radiation therapy tailored to the individual patient based on risk factors including cancer type, lymph node metastases, and menopausal status [5]. To select the best treatment option, breast cancer needs to be diagnosed and accurately characterized. Although breast cancer type can be determined using image-guided biopsies, biopsies only provide information on the biopsied part of the lesion which is subject to selection bias, potentially leading to inaccurate diagnoses. It has been demonstrated that imaging characteristics, in particular those derived from breast MRI, can help to identify prognostically relevant information [6–11]. Lymph node status is more challenging to assess: metastatic lymph nodes might be present even if suspicious imaging characteristics are absent [12–14], and in some cases of lymph nodes with uncertain morphology it might be difficult to perform a biopsy and confirm or exclude metastasis. Thus, there is a need for more accurate and non-invasive methods to determine lymph nodes status before treatment.
Proton MR spectroscopy (1H-MRS) allows the assessment of total choline (tCho), a compound resonance that is connected to multiple enzymatic changes involved in oncogenesis, tumor progression, and metastasis [15]. 1H-MRS has therefore been proposed as an additional tool to improve lesion characterization, but its use has been restricted due to the technical difficulties related to data acquisition and interpretation, yielding heterogeneous results [16]. Nevertheless, 1H-MRS is able to give information on a molecular level without the use of contrast agents, and its feasibility is highly improved owing to the improved performance of magnets and coils. Accurate breast cancer diagnosis including lymph node status is pivotal in determining treatment and currently requires biopsies and open surgery. We hypothesize that the additional molecular information provided by 1H-MRS can be used to facilitate breast lesion workup by potentially avoiding invasive diagnostic procedures to diagnose breast cancer and lymph node metastases.
The aim of this study was to evaluate the additional value of quantitative tCho evaluation to diagnose malignancy, breast cancer type, and lymph node status in suspicious lesions on multiparametric breast MRI (mpMRI, BI-RADS 4, and BI-RADS 5).
Methods
Study design, participants, and reference standard
This prospective, IRB-approved single-center cross-sectional study was performed at the university hospital of Jena, Germany, a certified tertiary care academic breast center. The study aimed to evaluate the additional value of tCho measurements from 1H-MRS to diagnose malignancy, breast cancer type, and lymph node status in MRI BI-RADS 4 and BI-RADS 5 lesions. A summary of the study design is given in Supplementary figure 1. Eligible were consecutive patients undergoing breast MRI for further workup of equivocal and suspicious conventional imaging (digital mammography, MG; ultrasound, US) findings (BI-RADS 0, BI-RADS 4, and BI-RADS 5). To be further eligible for this study, they had to present with a contrast-enhancing lesion of at least 8 mm in size on the MRI scan that was rated as suspicious (MRI BI-RADS 4 and BI-RADS 5) according to pre-defined criteria: non-circumscribed or spiculated margins, type II or III curve, non-circumscribed, non-hyperintense T2w correlate, and intermediate to low apparent diffusion coefficient (ADC) values < 1.5 10−3 mm2/s were considered suspicious, the combined appearance of several of these criteria as highly suspicious. This initial assessment was done by one of two breast MRI experts (W.A.K., P.A.B.) while the patient was placed in the scanner and MR spectroscopy was performed in eligible patients in the same breast MRI examination. Exclusion criterion was missing informed consent. Note that ineligibility for contrast-enhanced MRI was not counted as an exclusion criterion as patients were recruited before the MRI examination at the day of their visit while claustrophobia, presence of allergies, and metallic implants were excluded when scheduling the MRI appointment. Patients were excluded from the final analysis in case of termination of the examination before completion, incomplete imaging data, or non-interpretable imaging data due to artifacts (e.g., caused by motion).
Histopathologic workup of the patients served as the reference standard and was always performed after the breast MRI scan. Spectroscopy results were not used to guide patient management decisions. Biopsies were performed either US-guided by 14-gauge core needle biopsy in case of US visibility or MRI-guided 9G console-mounted vacuum-assisted biopsy in case the lesion was not visible on conventional imaging. All malignant lesions underwent surgery. If there was a discordance between biopsy and surgery, the latter was used as reference standard. However, if the patient was treated by neoadjuvant therapy, the histopathological result from the biopsy was used as reference standard. Benign results were checked for consistency within weekly interdisciplinary meetings and re-biopsied or operated on when deemed necessary (inconclusive results, e.g., B1 or unspecific B2 in case of circumscribed lesions). Lesions with uncertain malignant potential (B3) upon histopathology were surgically removed [17]. Benign B2 findings deemed consistent with histopathology were followed up by the imaging test best suited to visualize the lesion (either MG, US, or MRI) over 24 months as has been done in prior studies (e.g., listed in [18]). At the time of the study, sentinel lymph node biopsy (SNLB) was performed in all surgeries of malignant lesions. In case of positive SNLB, axillary lymph node dissection was performed. For the lymph node analysis in this study, only patients that underwent axillary surgery without prior neoadjuvant therapy were used. The study design is summarized in a flowchart (Supplementary Fig. 1).
MR imaging and proton MR spectroscopy
All imaging was performed on a 1.5-T scanner (Siemens Magnetom Sonata, Siemens Healthineers) using the dedicated vendor-supplied 4-channel double breast coil. The standardized protocol was in accordance with international recommendations and employed an axial 2D T2-w turbo spin echo (TSE, TR 8900 ms, TEeff 207 ms, field of view 340 mm, matrix 512 mm, slice thickness 3 mm) and a dynamic T1-weighted spoiled gradient echo sequence (fast low angle shot, FLASH, GRAPPA factor 2, TR 113 ms, TE 5 ms, FOV 340 mm, matrix 384, slice thickness 3 mm). Afterwards, 0.1 mmol/l body weight of gadopentetate dimeglumine (Gd-DTPA, Magnevist) was administered intravenously as a rapid bolus (3 ml/s), performed by an automatic injector (Spectris, Medrad), followed by 20 ml saline solution. The dynamic scan had a temporal resolution of 1 min and was repeated 8 times. An injection delay of 30 s between scans 1 (precontrast) and 2 (first postcontrast acquisition) was applied. In addition, a diffusion-weighted Echo Planar Imaging (EPI) sequence (GRAPPA factor 2, TR 3500, TEeff 80, echo distance 0.95 ms, six averages, three b-values: 0, 750, 1000 s/mm2, spectral fat saturation, in plane resolution 1.8 × 1.8 mm, slice thickness 6 mm) was acquired. Apparent diffusion coefficient (ADC) maps were calculated in-line by the scanner software.
Proton MR spectroscopy (1H-MRS, vendor-supplied standard single-voxel point-resolved spectroscopy sequence PRESS, TR 2000 ms, TE 272 ms, vector size 1024, weak water suppression with a bandwidth of 35 Hz, 128 averaged acquisitions with an acquisition time of 4:16 min) was acquired after automatic and manual first- and second-order shim gradient adjustments. The full width at half maximum was usually below 25 Hz. An additional scan without water suppression using 32 averages and the same adjustments was subsequently acquired as an internal reference. Including planning and shimming, spectroscopy was performed within 8–10 min. All spectra acquisitions were performed by an experienced breast radiologist trained in MR spectroscopy and acquisition (referred to as investigator, > 10 years of experience, P.A.B.) and supervised by two dedicated spectroscopists (> 25 and > 10 years of experience, A.G., R.Z.).
Data analysis
All acquired spectra were technically reviewed by the investigator after acquisition using the vendor-supplied software and then exported as raw data (.rda file) for further analysis. Consecutive analysis by two independent readers (both radiologists with > 2 years of training, supervised by the investigator) was done using v 4.0 of the free java Magnetic Resonance User Interface (jMRUI) software (www.jmrui.eu). The readers knew the study design but were not aware of any specific patient-related data. FID data were zero filled to 2048 data points and a Gaussian apodization of 5 Hz was applied. Fourier transformation and manual phase correction followed. The spectra were referenced using the water and methylene peaks as reference (4.74 and 1.33 ppm). Quantification was done by the AMARES algorithm [19] using prior knowledge on peak position (soft constraints ± 0.15 ppm) and tCho peak amplitude (soft constraints 0–200 arbitrary units). The fitting result was compared to the original spectrum by subtraction and was considered acceptable if systematic baseline deviations > 1.5-fold higher the baseline noise were absent. In case of an insufficient quantitation of tCho at 3.23 ppm, soft constraints for peak amplitude were adapted based on overall noise level and visual inspection of visible tCho peaks. The fitting procedure was repeated until the subtracted peak yielded a zero baseline as defined above. Absolute tCho concentrations were quantified in mmol/l by using the following equation:
While the water concentration was approximated to 55,600 mmol/l, the hydrogen quantities in water and choline molecules were set to 2 and 9, respectively. Finally, T1 and T2 relaxation times of water and choline were adapted from a review article of Haddadin et al ( = 0.441 s; = 1.513 s; = 0.075 s; = 0.269 s) [20, 21].
In addition, lesion size was measured volumetrically by assessing lesion dimensions in all three plains on early DCE images acquired 2 min after CM injection. The lesion volume was calculated in milliliters by multiplying the lesion size in all three plains in centimeters with 0.52 and expressed in milliliters.
Statistical analysis
Continuous variables are presented by median, interquartile range, and range. Quantitative tCho values were calculated according to the formula above and compared between lesion subgroups using non-parametric statistics (Mann-Whitney U test in case of two independent samples, Kruskal-Wallis test in case of > 2 independent samples). Bland-Altman statistics were performed to calculate mean differences and limits of agreement between both tCho measurements of R1 and R2.
The area under the receiver operating characteristics (ROC) curve (AUC) was used as a measure of diagnostic performance for tCho measurements. Two independent sets of ROC analyses were performed for R1 and R2, respectively: diagnosis of benign vs malignant and diagnosis of lymph node status (negative vs positive) in malignant lesions. The null hypothesis was defined as AUC = 0.5. To determine exploratory tCho cutoff values, we followed the approach of identifying an exploratory “rule-out” criterion for malignancy [22, 23]. Such a criterion was considered present if the corresponding tCho cutoff yielded a sensitivity of ≥ 95% in both readers. As all lesions in this study were considered suspicious by mpMRI (T2w; diffusion-weighted imaging, DWI; dynamic contrast enhanced, DCE), the specificity value at this cutoff indicates the rate of benign lesions that may not have required biopsy if the tCho value was below this exploratory threshold. P values of < 0.05 were considered statistically significant.
All statistical analyses were performed using Medcalc 18.10.2 software.
Results
General
One hundred twenty-one patients were examined with the study protocol. Eighteen of these (14.9%) had to be excluded due to technically insufficient spectra (bulk motion during acquisition and wrongly applied water suppression in the reference scan being the most common reasons). Finally, 103 patients had full diagnostic and reference data and were eligible for further analysis (median age 55 years, range 23–83 years) with 103 lesions (74 malignant, 29 benign, lymph node status was positive in 17 and negative in 46 breast cancers; in 11 patients no reliable reference standard for axillary lymph node status was available due to neoadjuvant therapy before surgery. See Table 1 for histological details and Supplementary figure 1 for the patient selection flowchart). Lesion volumes ranged from 0.45 to 274.6 ml with a median volume of 3.98 ml. No significant differences were found between median volumes of benign (3.59 ml, range 0.47–61.92 ml) and malignant (4.06, range 0.45–274.6 ml) lesions (p = 0.401, Mann-Whitney U test). Spectroscopy voxel sizes ranged between 1.73 and 27 ml with a median volume of 5.83 ml.
Table 1.
Lesion characteristics
| Lesions characteristics | tCho (R1, mmol/kg)# |
tCho (R2, mmol/kg)# |
p value | ||
|---|---|---|---|---|---|
| Malignant lesion histological subtypes | n = 74 | 3.22 (2.07, 7.17; 0.23–15.89) | 3.29 (1.83, 7.28; 0.22–16.00) | ||
| Invasive ductal carcinoma NST | 57 | 2.82 (2.01, 7.32; 0.23–15.89) | 2.95 (1.78, 7.32; 0.22–16.00) |
0.346 (R1), 0.301 (R2) |
|
| Invasive lobular carcinoma | 12 | 4.36 (2.66, 7.51; 1.04–11.19) | 4.20 (2.94, 8.45; 1.41–11.01) | ||
| Ductal carcinoma in situ | 2 | 3.57 (3.03, 4.12; 3.03–4.12) | 3.29 (3.26, 3.32; 3.26–3.32) | ||
| Other§ | 3 | 1.79 (0.99, 2.87; 0.73–3.23) | 1.96 (1.03, 2.68; 0.72–2.93) | ||
| Malignant lesion grading | n = 74 | ||||
| G1 | 5 | 1.65 (1.07, 4.79; 0.62–7.79) | 1.74 (1.01, 5.50; 0.49–9.05) |
0.200 (R1), 0.264 (R2) |
|
| G2 | 26 | 3.57 (2.65, 7.41; 0.23–15.89) | 3.70 (2.67, 7.62; 0.22–16.00) | ||
| G3 | 43 | 3.11 (1.82, 6.30; 0.54–13.22) | 3.10 (1.75, 6.33; 0.83–13.17) | ||
| Malignant lesion receptor status | n = 73* | ||||
| HR+, her2neu− | 38 | 4.42 (2.62, 7.94; 0.23–15.89) | 4.25 (2.66, 4.27; 0.22–16.0) |
0.051 (R1), 0.056 (R2)x |
|
| HR+, her2neu+ | 15 | 3.20 (2.67, 4.09; 1.70–7.32) | 2.95 (2.66, 4.27; 1.41–8.73) | ||
| HR−, her2neu+ | 6 | 2.41 (1.58, 2.48; 1.33–4.00) | 2.37 (1.51, 2.42; 1.29–3.71) | ||
| HR−, her2neu− | 14 | 1.95 (1.69, 6.47; 0.84–11.19) | 1.81 (1.70, 6.74; 0.83–11.01) | ||
| Lymph node status | n = 63* | ||||
| LN+ | 17 | 7.17 (3.78, 8.43; 2.48–13.22) | 8.19 (3.81, 8.81; 2.42–13.17) | 0.002 (R1), < 0.001 (R2) | |
| LN− | 46 | 2.79 (1.75, 2.66; 0.23–15.89) | 2.86 (1.71, 4.92; 0.22–16.00) | ||
| Benign lesions | n = 29 | 0.93 (0.27, 2.55; 0.00–6.00) | 1.13 (0.27, 2.57; 0.00–6.06) | ||
| Epithelial proliferations | 15 | 1.07 (0.13, 2.94; 0.00–6.00) | 1.10 (0.11, 2.89; 0.00–6.06) |
0.919 (R1), 0.872 (R2) |
|
| Fibroadenoma | 8 | 1.13 (0.09, 3.37; 0.00–4.78) | 1.13 (0.09, 3.59; 0.00–5.03) | ||
| Solid B3$ | 3 | 1.93 (0.30, 2.16; 0.30–2.16) | 2.11 (0.41, 2.22; 0.41–2.22) | ||
| Inflammation | 3 | 0.73 (0.26, 0.93; 0.26–0.93) | 0.84 (0.25, 1.13; 0.25–1.13) |
* in 11 patients, no reliable reference standard for axillary lymph node status was available due to neoadjuvant treatment before surgery and in one patient no receptor status was available in a DCIS; #given as: median (interquartile range Q25, Q75, range); §one invasive papillary, mucinous, and tubular carcinoma, respectively; $two papilloma, one phyllodes; xHR+ breast cancers differed significantly from all others (p = 0.018 (R1) and p = 0.020 (R2), respectively
tCho levels in benign and malignant lesions
tCho levels were significantly higher in malignant compared to benign lesions (p < 0.001 for both R1 and R2, respectively, see Table 1 and supplementary figure 2). While highest tCho levels were observed in ILC (see Table 1), these differences compared to other cancer subgroups did not prove statistically significant (p = 0.346, R1; p = 0.301, R2). Lower tCho was observed in G1 as compared to G2 and G3 cancers (Table 1), again without demonstrating statistical significance (p = 0.200, R1; p = 0.264, R2).
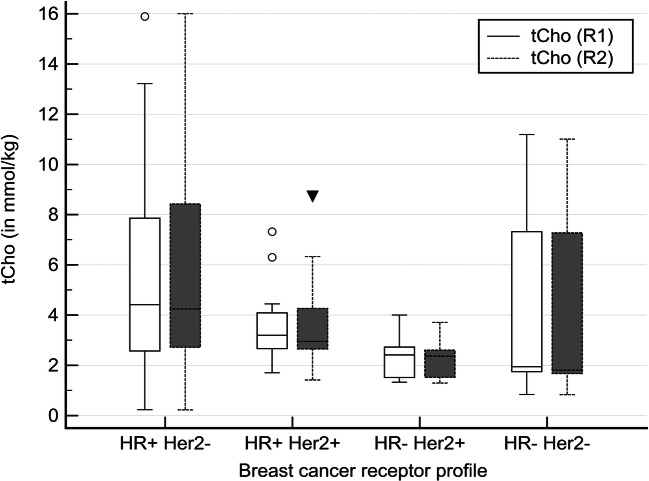
Full receptor status was available in 73 of 74 malignant lesions (Table 1). Figure 1 shows tCho levels stratified by breast cancer receptor status. The tCho levels in hormonal receptor positive breast cancers were significantly higher than those in hormonal receptor negative lesions (p = 0.018, R1; p = 0.020, R2). No further subgroup differences between breast cancer subgroups as distinguished by receptor status were found (p = 0.051, R1 and p = 0.056, R2).
Fig. 1.
Boxplots of tCho results stratified by breast cancer receptor status (HR: hormonal receptors, Her2+: her2neu receptor status, + indicating positivity, − negativity). The observed tCho levels were significantly higher in hormonal receptor positive breast cancers (p = 0.018, R1; p = 0.020, R2)
In 63 cancer patients with available lymph node status (11 without reference standard after neoadjuvant breast cancer treatment), tCho levels were significantly higher in those with positive as compared to negative lymph nodes (p = 0.002, R1; p < 0.001, R2, Table 1).
In benign lesions, no significant subgroup differences were found (p = 0.919, R1; p = 0.872, R2; see Table 1).
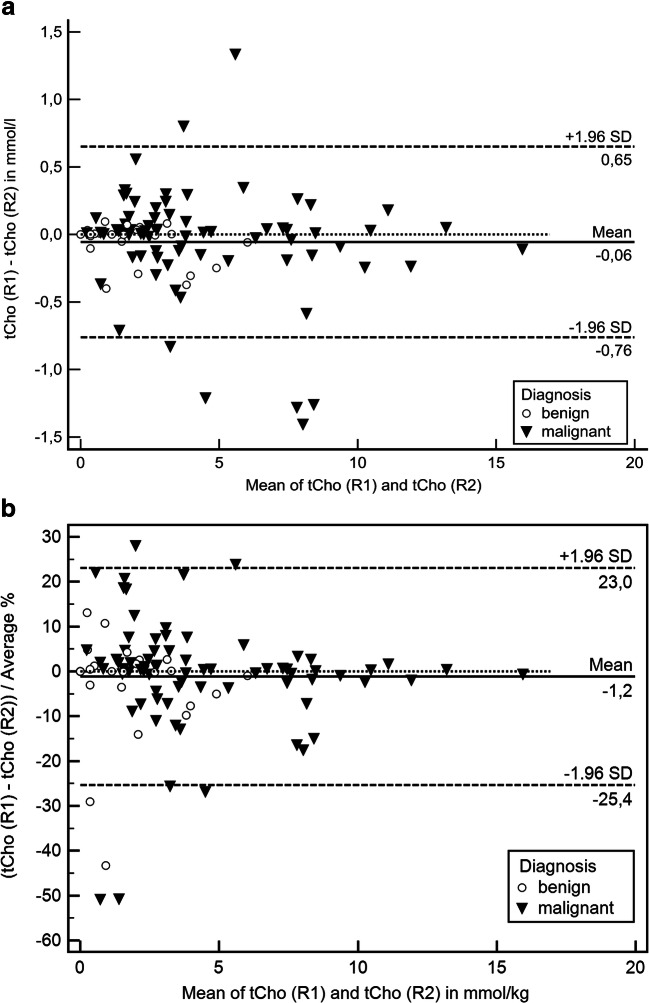
Reproducibility of tCho quantification on acquired spectroscopy data
Figure 2 demonstrates Bland-Altman plots for differences and limits of agreement between R1 and R2 as absolute (Fig. 2a) and percentage (Fig. 2b). The absolute mean difference between R1 and R2 was − 0.06 mmol/l, the limits of agreement ranging between − 0.76 and 0.65 mmol/l. The relative mean difference between R1 and R2 was − 1.2%, the limits of agreement ranging between − 25.4 and 23.0%.
Fig. 2.
Bland-Altman plots of tCho differences between R1 and R2. a Absolute differences; b relative differences
tCho to distinguish benign from malignant breast lesions
Figure 3 and Table 2 display the results of the ROC analysis. tCho showed a good area under the curve to distinguish benign from malignant lesions as measured by the AUC (0.816 (R1) and 0.809 (R2), p < 0.0001 for R1 and R2, respectively). The ROC curve analysis revealed that if tCho was ≤ 0.8 mmol/l, sensitivity for detection of breast cancer exceeded 95% for both readers (also see supplementary figure 2). Applying this threshold, could have potentially avoided n = 14/29 (48.3%, R1) or n = 13/29 (44.8%, R2) unnecessary biopsies in the investigated cohort while yielding n = 3/74 (4.1%, R1) or n = 2/74 (2.7%, R2) false negative diagnoses (two T1b hormonal receptor positive NST G2, one T1c hormonal receptor positive invasive tubular carcinoma G1).
Fig. 3.
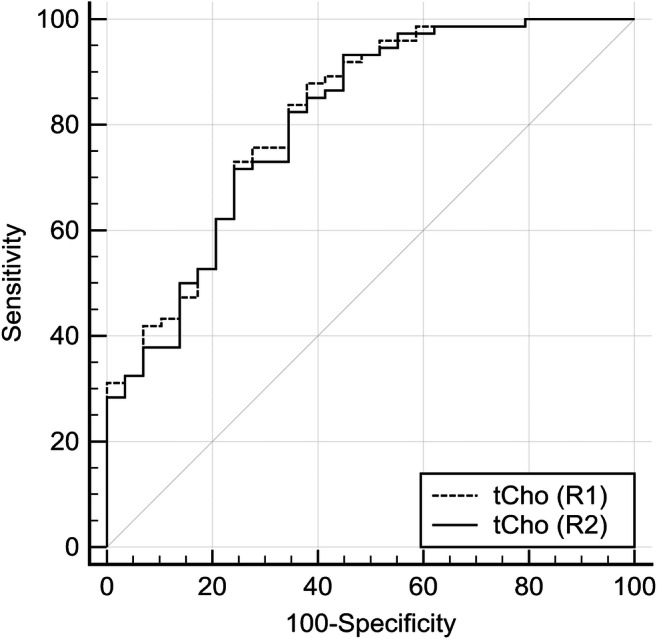
Receiver operating characteristic (ROC) curves estimating diagnostic performance of tCho to distinguish benign from malignant breast lesions (n = 103). Lesion volume serves as a reference and did not predict the presence of cancer (p > 0.05)
Table 2.
Diagnostic performance estimates and cutoff values for tCho to distinguish benign (n = 29) from malignant (n = 74) breast lesions
| Parameter | AUC | p value | tCho cutoff in mmol/l | Sensitivity (95% CI) in % |
Specificity (95% CI) in % |
|---|---|---|---|---|---|
| tCho (R1) | 0.816 | < 0.0001 | 0.8 |
96.0 (88.6–99.2) |
48.3 (29.4–67.5) |
| tCho (R2) | 0.809 | < 0.0001 | 0.8 |
97.3 (90.6–99.7) |
44.8 (26.4–64.3) |
tCho to predict positive lymph node metastasis
Figure 4 and Table 3 summarize ROC analysis results. Primary lesion tCho could significantly predict lymph node status with an AUC of 0.760 (R1, p < 0.0001) and 0.788 (R2, p < 0.0001). Below a tCho of 2.4 mmol/l, no metastatic lymph nodes where observed, thus achieving a sensitivity of 100% in both readers (also see supplementary figure 3). This condition applied to 39.1% (18/46) of all non-metastatic cancers in both R1 and R2. Details are given in Table 3. Clinical example cases are shown in Fig. 5 and Fig. 6.
Fig. 4.
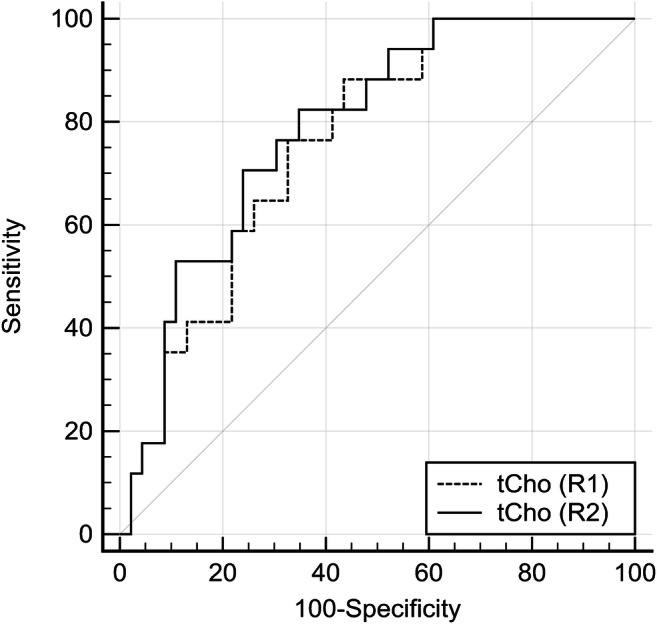
Receiver operating characteristics (ROC) curves estimating diagnostic performance of tCho to predict the presence or absence of metastatic lymph nodes (n = 63). Lesion volume serves as a reference and did not predict the presence of lymph node metastases (p > 0.05)
Table 3.
Diagnostic performance estimates and cutoff values for tCho to predict the presence (n = 17) or absence (n = 43) of metastatic lymph nodes
| Parameter | AUC | p value | tCho cutoff mmol/l |
Sensitivity (95% CI) in % |
Specificity (95% CI) in % |
|---|---|---|---|---|---|
| tCho (R1) | 0.760 | < 0.0001 | 2.4 |
100 (80.5–100) |
39.1 (25.1–54.6) |
| tCho (R2) | 0.788 | < 0.0001 | 2.4 |
100 (80.5–100) |
39.1 (25.1–54.6) |
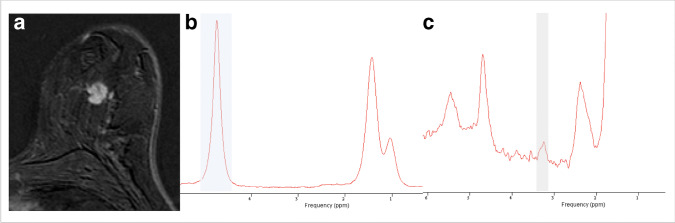
Fig. 5.
Thirty-four-year-old woman presenting with an enhancing suspicious mass in the early contrast enhanced subtraction image (a). b presents the water resonance peak at 4.74 ppm in the unsuppressed water reference spectrum (blue bar), weaker resonance peaks can be depicted at 3.23 (corresponding to total Choline tCho) and 1.3 and 0.9 ppm (methylene and methyl groups from lipids). The magnified water suppressed spectrum reveals the distinct tCho resonance peak at 3.23 ppm (gray bar). Using water as an internal reference, tCho was calculated as 13 mmol/l. Histopathology revealed an invasive ductal cancer NST G3 with ipsilateral lymph node metastases
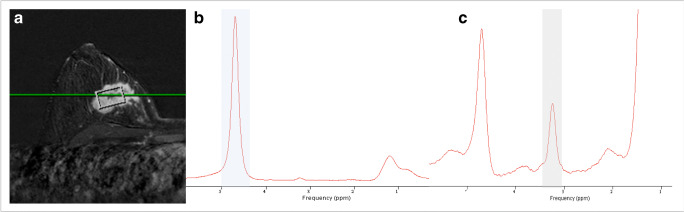
Fig. 6.
Forty-six-year-old woman presenting with an enhancing suspicious mass in the early contrast enhanced subtraction image (a). b presents the water resonance peak at 4.74 ppm in the unsuppressed water reference spectrum (blue bar). The magnified water suppressed spectrum reveals a weak tCho resonance peak at 3.23 ppm (gray bar). Using water as an internal reference, tCho was calculated as 1.9 mmol/l. Histopathology revealed an invasive ductal cancer NST G2 and negative lymph nodes
Discussion
Quantitative tCho evaluation from 1H-MRS allowed to diagnose malignancy and lymph node status in breast lesions that were considered suspicious with multiparametric breast MRI assessment using DCE, T2w and diffusion-weighted (DW) images. Specifically, low tCho concentrations associated with a low risk of breast cancer and lymph node metastasis. If confirmed by prospective studies, these findings would be of high clinical relevance as positive findings on MRI require invasive workup in order to avoid unnecessary surgery [24]. Further, the availability of MRI-guided interventions is limited [25], stressing the need for non-invasive tools to avoid unnecessary biopsies. While lymph node status is one of the most important predictors of breast cancer outcome, surgical management is increasingly conservative, avoiding axillary lymph node dissection in case of limited disease [26–28]. Again, a non-invasive tool to accurately assess the risk of present lymph node metastases could facilitate the clinical workup of breast cancer patients.
We were not the first group to investigate quantitative tCho measurements using an internal reference to distinguish benign from malignant breast lesions (see Table 4). Prior reports differed regarding their results, either reporting very high sensitivity and specificity [29, 30], very high specificity but moderate sensitivity [31], and only one group reported moderate sensitivity and specificity [32]. These different results can both be explained by the application of different thresholds and different patient and lesion groups. All groups examined lesions above 0.7 cm in size [29–32] and two reports pre-defined a minimum size of the investigated lesions and focused on mass lesions only [29, 31]. Our specificity is seemingly lower than previously reported (see Table 4). This is due to the different study design: opposed to prior reports, our study investigated whether MR spectroscopy can further improve diagnosis of suspicious mpMRI findings. The specificity values at the tCho threshold associated with a high sensitivity can thus be directly translated into the rate of avoidable biopsies of benign lesions [23]. Our results are possibly best compared to those of Dorrius et al, who investigated a comparable setting. In their study, the authors used 1H-MRS as an additional test to rule out cancer in a problem-solving setting using a three-dimensional spatially resolved spectroscopy technique [33]. We did not in detail investigate the effects of single techniques on diagnostic outcomes as reported by Pinker et al [34]. This was due to the fact that the study rationale was using MR spectroscopy only in case of suspicious multiparametric MRI using T2w, DWI, and DCE sequences. While several formal approaches of integrating these data into a diagnosis have been reported [35–38], our study protocol relied on the classical empirical approach of assigning BI-RADS categories. Our study design, however, allows to estimate the potential downgrade rates of benign breast lesions appearing suspicious upon triparametric (T2w, DWI, DCE) MRI as > 40%.
Table 4.
Summary of prior reports on tCho quantification based on single-voxel acquisitions and comparison with the results reported in this paper
| First author/year | Field strength | Cases | Cancer prevalence (%) | Sensitivity (%) | Specificity (%) | tCho* range |
|---|---|---|---|---|---|---|
| Thakur/2011 [1] | 1.5 T | 88 | 64.8 | 96.5 | 93.5 | 0–47.1 |
| Baek/2012 [2] | 1.5 T | 112 | 88.4 | 65.7 | 92.3 | 0.9–10 |
| Sah/2012 [3] | 1.5 T | 189 | 79.9 | 76.2 | 76.3 | +0.58/1.6/4.2–5.4 |
| Suppiah/2012 [4] | 1.5 T | 57 | 73.7 | 95.2 | 93.3 | 0.1–6.9 |
| This study/2018 | 1.5 T | 103 | 71.8 | 95.9 | 48.3# | 0–16 |
* in mmol/l; +[1–4] no range given. Values indicate mean values for healthy tissue, benign and malignant lesions. Malignant lesions were reported in subgroups. #This study investigated only lesions classified as BI-RADS 4 or BI-RADS 5 after full triparametric (T2w, DWI, DCE) breast MRI assessment. Thus, the reported specificity does only apply to tCho quantification as investigated in this specific situation
We observed higher tCho levels in hormonal receptor–positive breast cancers. This seems to be in line with reports on choline kinase deregulation promoting estrogen receptor-driven proliferation [39]. Similar findings have been reported by Sah et al [32]. While the significance of this finding, e.g., on the general applicability of the exploratory tCho thresholds provided within this study cannot be estimated due to the limited sample size of our study (all FN findings were hormonal receptor positive), implications arise regarding the potential prediction of antihormonal treatment response. The tCho concentrations in our study fall within the range reported in prior studies (see Table 4). Note, however, that though using water as an internal reference addresses several normalization aspects such as voxel size and coil sensitivity differences, the method applied here and in the referenced papers relies on fixed assumptions regarding the real water concentration in tissue as well as the relaxation times of water and tCho in vivo [20]. Individual variations of these parameters, in particular tissue water concentration that might vary due to patient hydration, age, treatment and hormonal status, likely explain differences regarding tCho concentrations reported in the individual studies. We demonstrated a good reproducibility of tCho quantitation using the AMARES method. Though important in the field of imaging biomarker research, this aspect has only been investigated in other techniques such as breast DWI [40]. Due to the clinical study design, we did not analyze the repeatability of 1H-MRS data acquisition and inter-scanner variability. In this context, individual measurements of tCho and water relaxation times might be desirable in order to assess their potential impact and weigh it against the additional measurement time required. Therefore, all quantitative thresholds must be regarded as exploratory only until independently validated. Another issue is the potential interaction of tCho with contrast media that is further outlined below.
Our study results further indicate that the primary breast cancer tCho levels are predictive for lymph node metastases. Specifically, a low tCho concentration indicated the absence of lymph node metastases in a substantial proportion of our breast cancer cases. If prospectively validated, sentinel node biopsies (SNB) could be potentially avoided in patients classified as being at low risk for lymph node metastasis. While anecdotal and encouraging evidence exists on direct 1H-MRS in axillary lymph nodes [41], a direct measurement would require visibly enlarged lymph nodes and a time-consuming additive measurement. Indirect axillography based on an imaging phenotype of the index breast cancer has been shown by Dietzel et al in a radiomic approach using artificial neural networks on semantic lesion features [42]. Whether 1H-MRS data can contribute to prognostic breast cancer models or adds to morphological lymph node assessment remains to be investigated.
Our analysis is limited regarding several aspects: first, our spectroscopy technique is not spatially resolved. Though prior reports have reported encouraging results on spatially resolved 1H-MRS, quantification is still not established and difficult to achieve within a non-research setting. In addition, while theoretically of benefit, a spatially resolved spectroscopy still can only cover a region of interest and not two breasts. In the investigated application of 1H-MRS, that is the downgrading of lesions suspicious on conventional MRI assessment, it is of limited relevance to yield spatially resolved images as the area of interest is directly investigated. In this respect, our study is unique as we decided to use 1H-MRS on the spot, that is while the patient was still in the scanner. We are well aware that in many institutions MRI scans are acquired and interpreted at different time points and even different physical locations. While our approach may thus be difficult to employ in some institutions, the results do still apply: 1H-MRS has an additional diagnostic value in mpMRI of the breast and provides additional, prognostically relevant information on lymph node status. The PRESS sequence used had a long echo time. While shorter echo times may increase the tCho signal, TE is considered in the quantitation procedure. Besides, a meta-analysis reported no influence of TE on diagnostic performance of Cho assessment by 1H-MRS [16]. One potential drawback is the need for IV contrast. Gd-based contrast media show an interaction with the tCho signal in vivo [43], an effect that has been reported as being more pronounced in ionic contrast media and thus must be considered when comparing different study results [44]. Acquisition time is a further limitation of the used approach. Though aiming at screening, there is a trend to reduce magnet times in breast MRI and an additional test must prove its value also regarding magnet time efficiency [45]. Modern 3-T systems in combination with multichannel coils could substantially reduce acquisition times due to SNR gain that is more than fourfold [46] while a reduction of averages by 50% would only reduce the SNR by square root of 2. Consequently, the acquisition times reported here could potentially be reduced to 3 min or less while yielding the same SNR.
In conclusion, quantitative tCho evaluation from 1H-MRS allowed to diagnose malignancy and lymph node status in breast lesions that are considered suspicious with multiparametric breast MRI assessment using DCE, T2w, and diffusion-weighted (DW) images. tCho demonstrated the potential to improve diagnosis of malignancy in breast MRI lesions suspicious on mpMRI and stratify the risk of lymph node metastases with the aim of improved patient management.
Electronic supplementary material
(DOCX 317 kb)
Abbreviations
- 1H-MRS, MRS
Proton MR spectroscopy
- ADC
Apparent diffusion coefficient
- AUC
Area under the curve
- BI-RADS
Breast imaging–reporting and data system
- DCE
Dynamic contrast enhanced
- DWI
Diffusion-weighted imaging
- FLASH
Fast low angle shot
- GRAPPA
GeneRalized Autocalibrating Partial Parallel Acquisition
- IRB
Institutional Review Board
- MRI, mpMRI
Magnetic resonance imaging, multiparametric MRI
- PRESS
Point Resolved Spectroscopy Sequence
- ROC
Receiver operating characteristics
- tCho
Total choline
- TE
Echo time
- TR
Repetition time
- TSE
Turbo spin echo
Funding Information
Open access funding provided by Medical University of Vienna. This study has received funding by the Austrian National Bank Jubiläumsfonds project number 17186.
Compliance with ethical standards
Guarantor
The scientific guarantor of this publication is Pascal A.T. Baltzer.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Prospective
• Cross-sectional study
• Performed at one institution
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Claudia Sodano and Paola Clauser contributed equally to this work.
References
- 1.Sardanelli F, Aase HS, Álvarez M, et al. Position paper on screening for breast cancer by the European Society of Breast Imaging (EUSOBI) and 30 national breast radiology bodies from Austria, Belgium, Bosnia and Herzegovina, Bulgaria, Croatia, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Israel, Lithuania, Moldova, The Netherlands, Norway, Poland, Portugal, Romania, Serbia, Slovakia, Spain, Sweden, Switzerland and Turkey. Eur Radiol. 2017;27:2737–2743. doi: 10.1007/s00330-016-4612-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mann RM, Kuhl CK, Kinkel K, Boetes C. Breast MRI: guidelines from the European Society of Breast Imaging. Eur Radiol. 2008;18:1307–1318. doi: 10.1007/s00330-008-0863-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sardanelli Francesco, Boetes Carla, Borisch Bettina, Decker Thomas, Federico Massimo, Gilbert Fiona J., Helbich Thomas, Heywang-Köbrunner Sylvia H., Kaiser Werner A., Kerin Michael J., Mansel Robert E., Marotti Lorenza, Martincich Laura, Mauriac Louis, Meijers-Heijboer Hanne, Orecchia Roberto, Panizza Pietro, Ponti Antonio, Purushotham Arnie D., Regitnig Peter, Del Turco Marco Rosselli, Thibault Fabienne, Wilson Robin. Magnetic resonance imaging of the breast: Recommendations from the EUSOMA working group. European Journal of Cancer. 2010;46(8):1296–1316. doi: 10.1016/j.ejca.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 4.American College of Radiology (ACR) (2014) ACR practice parameter for the performance of contrast-enhanced magnetic resonance imaging (MRI) of the breast. Available via https://www.acr.org/-/media/ACR/Files/Practice-Parameters/mr-contrast-breast.pdf
- 5.Senkus E, Kyriakides S, Ohno S, et al. Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v8–v30. doi: 10.1093/annonc/mdv298. [DOI] [PubMed] [Google Scholar]
- 6.Kim Mi Young, Choi Nami. Mammographic and ultrasonographic features of triple-negative breast cancer: a comparison with other breast cancer subtypes. Acta Radiologica. 2013;54(8):889–894. doi: 10.1177/0284185113488580. [DOI] [PubMed] [Google Scholar]
- 7.Costantini M, Belli P, Distefano D, et al. Magnetic resonance imaging features in triple-negative breast cancer: comparison with luminal and HER2-overexpressing tumors. Clin Breast Cancer. 2012;12:331–339. doi: 10.1016/j.clbc.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Youk Ji Hyun, Son Eun Ju, Chung Jin, Kim Jeong-Ah, Kim Eun-kyung. Triple-negative invasive breast cancer on dynamic contrast-enhanced and diffusion-weighted MR imaging: comparison with other breast cancer subtypes. European Radiology. 2012;22(8):1724–1734. doi: 10.1007/s00330-012-2425-2. [DOI] [PubMed] [Google Scholar]
- 9.Dietzel M, Baltzer PA, Vag T, et al. Potential of MR mammography to predict tumor grading of invasive breast cancer. Rofo. 2011;183:826–833. doi: 10.1055/s-0031-1273244. [DOI] [PubMed] [Google Scholar]
- 10.Baltzer PA, Vag T, Dietzel M, et al. Computer-aided interpretation of dynamic magnetic resonance imaging reflects histopathology of invasive breast cancer. Eur Radiol. 2010;20:1563–1571. doi: 10.1007/s00330-010-1722-x. [DOI] [PubMed] [Google Scholar]
- 11.Dietzel M, Zoubi R, Vag T, et al. Association between survival in patients with primary invasive breast cancer and computer aided MRI. J Magn Reson Imaging. 2013;37:146–155. doi: 10.1002/jmri.23812. [DOI] [PubMed] [Google Scholar]
- 12.Schipper R.J., van Roozendaal L.M., de Vries B., Pijnappel R.M., Beets-Tan R.G.H., Lobbes M.B.I., Smidt M.L. Axillary ultrasound for preoperative nodal staging in breast cancer patients: Is it of added value? The Breast. 2013;22(6):1108–1113. doi: 10.1016/j.breast.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 13.van Nijnatten TJA, Ploumen EH, Schipper RJ, et al. Routine use of standard breast MRI compared to axillary ultrasound for differentiating between no, limited and advanced axillary nodal disease in newly diagnosed breast cancer patients. Eur J Radiol. 2016;85:2288–2294. doi: 10.1016/j.ejrad.2016.10.030. [DOI] [PubMed] [Google Scholar]
- 14.Baltzer PA, Dietzel M, Burmeister HP, et al. Application of MR mammography beyond local staging: is there a potential to accurately assess axillary lymph nodes? Evaluation of an extended protocol in an initial prospective study. AJR Am J Roentgenol. 2011;196:W641–W647. doi: 10.2214/AJR.10.4889. [DOI] [PubMed] [Google Scholar]
- 15.Glunde Kristine, Penet Marie-France, Jiang Lu, Jacobs Michael A, Bhujwalla Zaver M. Choline metabolism-based molecular diagnosis of cancer: an update. Expert Review of Molecular Diagnostics. 2015;15(6):735–747. doi: 10.1586/14737159.2015.1039515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baltzer PA, Dietzel M. Breast lesions: diagnosis by using proton MR spectroscopy at 1.5 and 3.0 T--systematic review and meta-analysis. Radiology. 2013;267:735–746. doi: 10.1148/radiol.13121856. [DOI] [PubMed] [Google Scholar]
- 17.Rageth Christoph J., O’Flynn Elizabeth A. M., Pinker Katja, Kubik-Huch Rahel A., Mundinger Alexander, Decker Thomas, Tausch Christoph, Dammann Florian, Baltzer Pascal A., Fallenberg Eva Maria, Foschini Maria P., Dellas Sophie, Knauer Michael, Malhaire Caroline, Sonnenschein Martin, Boos Andreas, Morris Elisabeth, Varga Zsuzsanna. Second International Consensus Conference on lesions of uncertain malignant potential in the breast (B3 lesions) Breast Cancer Research and Treatment. 2018;174(2):279–296. doi: 10.1007/s10549-018-05071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennani-Baiti B, Bennani-Baiti N, Baltzer PA. Diagnostic performance of breast magnetic resonance imaging in non-calcified equivocal breast findings: results from a systematic review and meta-analysis. PLoS One. 2016;11:e0160346. doi: 10.1371/journal.pone.0160346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson. 1997;129:35–43. doi: 10.1006/jmre.1997.1244. [DOI] [PubMed] [Google Scholar]
- 20.Haddadin IS, McIntosh A, Meisamy S, et al. Metabolite quantification and high-field MRS in breast cancer. NMR Biomed. 2009;22:65–76. doi: 10.1002/nbm.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baik Hyun-Man, Su Min-Ying, Yu Hon, Nalcioglu Orhan, Mehta Rita. Quantification of Choline-containing Compounds in Malignant Breast Tumors by 1H MR Spectroscopy Using Water as an Internal Reference at 1.5 T. Magnetic Resonance Materials in Physics, Biology and Medicine. 2006;19(2):96–104. doi: 10.1007/s10334-006-0032-4. [DOI] [PubMed] [Google Scholar]
- 22.Woitek R, Spick C, Schernthaner M, et al. A simple classification system (the tree flowchart) for breast MRI can reduce the number of unnecessary biopsies in MRI-only lesions. Eur Radiol. 2017;27:3799–3809. doi: 10.1007/s00330-017-4755-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spick Claudio, Pinker-Domenig Katja, Rudas Margaretha, Helbich Thomas H., Baltzer Pascal A. MRI-only lesions: application of diffusion-weighted imaging obviates unnecessary MR-guided breast biopsies. European Radiology. 2014;24(6):1204–1210. doi: 10.1007/s00330-014-3153-6. [DOI] [PubMed] [Google Scholar]
- 24.Houssami N, Ciatto S, Macaskill P, et al. Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: systematic review and meta-analysis in detection of multifocal and multicentric cancer. J Clin Oncol. 2008;26:3248–3258. doi: 10.1200/JCO.2007.15.2108. [DOI] [PubMed] [Google Scholar]
- 25.Clauser P, Mann R, Athanasiou A, et al. A survey by the European Society of Breast Imaging on the utilisation of breast MRI in clinical practice. Eur Radiol. 2018;28:1909–1918. doi: 10.1007/s00330-017-5121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wright GP, Mater ME, Sobel HL, et al. Measuring the impact of the American College of Surgeons Oncology Group Z0011 trial on breast cancer surgery in a community health system. Am J Surg. 2015;209:240–245. doi: 10.1016/j.amjsurg.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Lim Geok Hoon, Upadhyaya Vidya S., Acosta Hannah Angela, Lim Jayne Michelley Adolfo, Allen John C., Leong Lester Chee Hao. Preoperative predictors of high and low axillary nodal burden in Z0011 eligible breast cancer patients with a positive lymph node needle biopsy result. European Journal of Surgical Oncology. 2018;44(7):945–950. doi: 10.1016/j.ejso.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Harris CK, Tran HT, Lee K, et al. Positive ultrasound-guided lymph node needle biopsy in breast cancer may not mandate axillary lymph node dissection. Ann Surg Oncol. 2017;24:3004–3010. doi: 10.1245/s10434-017-5935-y. [DOI] [PubMed] [Google Scholar]
- 29.Thakur SB, Brennan SB, Ishill NM, et al. Diagnostic usefulness of water-to-fat ratio and choline concentration in malignant and benign breast lesions and normal breast parenchyma: an in vivo (1) H MRS study. J Magn Reson Imaging. 2011;33:855–863. doi: 10.1002/jmri.22493. [DOI] [PubMed] [Google Scholar]
- 30.Suppiah S, Rahmat K, Mohd-Shah MN, et al. Improved diagnostic accuracy in differentiating malignant and benign lesions using single-voxel proton MRS of the breast at 3 T MRI. Clin Radiol. 2013;68:e502–e510. doi: 10.1016/j.crad.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Baek H-M. Diagnostic value of breast proton magnetic resonance spectroscopy at 1.5T in different histopathological types. ScientificWorldJournal. 2012;2012:508295. doi: 10.1100/2012/508295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sah Rani G., Sharma Uma, Parshad Rajinder, Seenu Vurthaluru, Mathur Sandeep R., Jagannathan Naranamangalam R. Association of estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 status with total choline concentration and tumor volume in breast cancer patients: An MRI and in vivo proton MRS study. Magnetic Resonance in Medicine. 2011;68(4):1039–1047. doi: 10.1002/mrm.24117. [DOI] [PubMed] [Google Scholar]
- 33.Dorrius MD, Pijnappel RM, van der Weide Jansen MC, et al. The added value of quantitative multi-voxel MR spectroscopy in breast magnetic resonance imaging. Eur Radiol. 2012;22:915–922. doi: 10.1007/s00330-011-2322-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinker K, Bogner W, Baltzer P, et al. Improved diagnostic accuracy with multiparametric magnetic resonance imaging of the breast using dynamic contrast-enhanced magnetic resonance imaging, diffusion-weighted imaging, and 3-dimensional proton magnetic resonance spectroscopic imaging. Invest Radiol. 2014;49:421–430. doi: 10.1097/RLI.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 35.Pinker K, Bickel H, Helbich TH, et al. Combined contrast-enhanced magnetic resonance and diffusion-weighted imaging reading adapted to the “Breast Imaging Reporting and Data System” for multiparametric 3-T imaging of breast lesions. Eur Radiol. 2013;23:1791–1802. doi: 10.1007/s00330-013-2771-8. [DOI] [PubMed] [Google Scholar]
- 36.Baltzer A, Dietzel M, Kaiser CG, Baltzer PA. Combined reading of contrast enhanced and diffusion weighted magnetic resonance imaging by using a simple sum score. Eur Radiol. 2016;26:884–891. doi: 10.1007/s00330-015-3886-x. [DOI] [PubMed] [Google Scholar]
- 37.Baltzer PA, Dietzel M, Kaiser WA. A simple and robust classification tree for differentiation between benign and malignant lesions in MR-mammography. Eur Radiol. 2013;23:2051–2060. doi: 10.1007/s00330-013-2804-3. [DOI] [PubMed] [Google Scholar]
- 38.Baum F, Fischer U, Vosshenrich R, Grabbe E. Classification of hypervascularized lesions in CE MR imaging of the breast. Eur Radiol. 2002;12:1087–1092. doi: 10.1007/s00330-001-1213-1. [DOI] [PubMed] [Google Scholar]
- 39.López-Knowles E, Wilkerson PM, Ribas R, et al. Integrative analyses identify modulators of response to neoadjuvant aromatase inhibitors in patients with early breast cancer. Breast Cancer Res. 2015;17:35. doi: 10.1186/s13058-015-0532-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bickel H, Pinker K, Polanec S, et al. Diffusion-weighted imaging of breast lesions: region-of-interest placement and different ADC parameters influence apparent diffusion coefficient values. Eur Radiol. 2017;27:1883–1892. doi: 10.1007/s00330-016-4564-3. [DOI] [PubMed] [Google Scholar]
- 41.Sharma U, Mehta A, Seenu V, Jagannathan NR. Biochemical characterization of metastatic lymph nodes of breast cancer patients by in vitro 1H magnetic resonance spectroscopy: a pilot study. Magn Reson Imaging. 2004;22:697–706. doi: 10.1016/j.mri.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 42.Dietzel M, Baltzer PA, Dietzel A, et al. Application of artificial neural networks for the prediction of lymph node metastases to the ipsilateral axilla - initial experience in 194 patients using magnetic resonance mammography. Acta Radiol Stockh Swed 1987. 2010;51:851–858. doi: 10.3109/02841851.2010.498444. [DOI] [PubMed] [Google Scholar]
- 43.Lenkinski RE, Wang X, Elian M, Goldberg SN. Interaction of gadolinium-based MR contrast agents with choline: implications for MR spectroscopy (MRS) of the breast. Magn Reson Med. 2009;61:1286–1292. doi: 10.1002/mrm.21937. [DOI] [PubMed] [Google Scholar]
- 44.Baltzer PA, Gussew A, Dietzel M, et al. Effect of contrast agent on the results of in vivo (1) H MRS of breast tumors - is it clinically significant? NMR Biomed. 2012;25:67–74. doi: 10.1002/nbm.1714. [DOI] [PubMed] [Google Scholar]
- 45.Kuhl Christiane K., Schrading Simone, Strobel Kevin, Schild Hans H., Hilgers Ralf-Dieter, Bieling Heribert B. Abbreviated Breast Magnetic Resonance Imaging (MRI): First Postcontrast Subtracted Images and Maximum-Intensity Projection—A Novel Approach to Breast Cancer Screening With MRI. Journal of Clinical Oncology. 2014;32(22):2304–2310. doi: 10.1200/JCO.2013.52.5386. [DOI] [PubMed] [Google Scholar]
- 46.Marshall H, Devine PM, Shanmugaratnam N, et al. Evaluation of multicoil breast arrays for parallel imaging. J Magn Reson Imaging. 2010;31:328–338. doi: 10.1002/jmri.22023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 317 kb)