Abstract
Ischemia heart disease is the leading cause of death world-widely and has increased prevalence and exacerbated myocardial infarction with aging. Sestrin2, a stress-inducible protein, declines with aging in the heart and the rescue of Sestrin2 in the aged mouse heart improves the resistance to ischemic insults caused by ischemia and reperfusion. Here, through a combination of transcriptomic, physiological, histological, and biochemical strategies, we found that Sestrin2 deficiency shows an aged-like phenotype in the heart with excessive oxidative stress, provoked immune response, and defected myocardium structure under physiological condition. While challenged with ischemia and reperfusion stress, the transcriptomic alterations in Sestrin2 knockout mouse heart resembled aged wild type mouse heart. It suggests that Sestrin2 is an age-related gene in the heart against ischemia reperfusion stress. Sestrin2 plays a crucial role in modulating inflammatory response through maintaining the intracellular redox homeostasis in the heart under ischemia reperfusion stress condition. Together, the results indicate that Sestrin2 is a potential target for treatment of age-related ischemic heart disease.
Keywords: Aging, Ischemic insults, Redox homeostasis, Inflammation
Graphical abstract

Highlights
-
•
Sestrin2 regulates cardiac redox homeostasis under ischemic stress.
-
•
Alterations in substrate metabolism with aging cause impaired inflammatory response.
-
•
Sestrin2 is a potential target for treatment of ischemic heart disease.
-
•
Sestrin2 modulates cardiac inflammatory response during ischemia and reperfusion.
Abbreviations
- AMPK
AMP-activated protein kinase
- CCL2/3
C–C motif chemokine ligand 2/3
- CD14/40
cluster of differentiation 14/40
- CVD
cardiovascular disease
- DEG
differently expressed gene
- E/A
early (E) to late (A) ventricular filling velocities ratio
- EF
ejection fraction
- FS
fractional shortening
- GEO
gene expression omnibus
- GO
gene ontology
- KEGG
kyoto encyclopedia of genes and genomes
- LAD
left anterior descending coronary artery
- MAP2K
mitogen activated protein kinase kinase
- MAP3K
mitogen activated protein kinase kinase kinase
- MAPK
mitogen activated protein kinase
- MGI
mouse genome informatics
- REACTOME
open source manually curated pathway database
- SAPK/JNK
c-Jun N-terminal protein kinase
- SV
stroke volume
- WGA
wheat germ agglutinin
1. Introduction
Coronary heart disease (CHD), also known as ischemic heart disease is the leading cause of death world widely which killed 0.365 million people in 2017 [1]. The prevalence of CHD is 48% overall in adults from 2016 data and its prevalence increases with aging [1,2]. Ischemia heart disease is referring to a breakdown of blood flow to the myocardium because of coronary atherosclerosis, coronary thrombosis, and the narrowing of arterioles in the heart [3]. Thus, depleted oxygen and energy supply induces various intracellular damages, such as disturbance of cellular ion homeostasis, increased production of reactive oxygen species (ROS) and lactate accumulation, and increase of permeability of cellular membrane in myocardium [4,5]. The treatment of choice is called reperfusion by restoring blood flow to the heart immediately using either percutaneous coronary intervention or thrombolytic therapy [6,7]. However, the process of reperfusion can itself coaxes cardiomyocyte death through further damage intracellular proteins and DNA by releasing of extensive free radicals and increasing oxidative stress [8]. Subsequently, damaged myocardium from ischemia reperfusion (I/R) injury initiate an inflammatory response including macrophages, neutrophils, lymphocytes, as well as the complement system, and pro- and anti-inflammatory cytokines [2,4,[7], [8], [9], [10], [11], [12], [13]]. Other cellular components, such as ROS and cytokine TNF-α, released during the inflammatory response may produce cytotoxicity and induce additional cell injury, ultimately leading to cell death [4,10].
Mounting evidence suggested that aged heart sustains greater injury during I/R compared to the adult heart over the augmented production and release of ROS from mitochondria in aged heart [14]. Sestrin2 (Sesn2) was known to be an antioxidant by repressing the ROS and provide cardioprotection against several noxious stimuli, such as endoplasmic reticulum stress, oxidative stress, and I/R stress [[15], [16], [17]]. Moreover, previous studies reported that Sestrin depletion in Drosophila (dSesn) leads to age-associated pathologies indicating that Sesn2 might be an important age-related cardioprotective effectors and a new therapeutic target in cardiovascular disease for aging population [[17], [18], [19]]. However, the correlation of Sesn2 and aging in heart is unconfirmed and the cardioprotective mechanism of Sesn2 in protecting heart especially aged heart from I/R injury remains unclear.
In this study, we revealed the substantial association of Sesn2 and aging in the heart in terms of mediating inflammatory response and the mechanism of Sesn2 protecting heart against the intensive oxidative stress induced by I/R stress. We observed that Sesn2 deficiency induced disruption of redox homeostasis and provoked inflammation in heart leading to exacerbated myocardial cell death and cardiac dysfunction after I/R stress. Our results suggest that Sesn2 is a critical age-associated protein that is required to maintain redox homeostasis and regulate immune system in heart against I/R injury.
2. Materials and methods
2.1. Animals
Young (3–4 months) C57BL/6J mice (young-WT) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Aged (24–26 months) C57BL/6J mice (aged-WT) were supplied from Charles River Laboratories (Wilmington, MA, USA). Sesn2 knockout (Sesn2-KO) (3–4 months, C57BL/6J) were bred and supplied by our lab as the previous reports described [15,20,21]. All animal protocols in this study were approved by the Institutional Animal Care and Use Committee of the University of South Florida and conform to the NIH Guide for the care and use of laboratory animals.
2.2. In vivo regional ischemia/reperfusion surgery and samples collection
Mice were anesthetized, intubated and ventilated as we previously described [16,22]. After a left lateral thoracotomy, the left anterior descending coronary artery (LAD) was occulated for 45 min with an 8-0 nylon suture and polyethylene tubing to prevent arterial injury and subsequently reperfused for 24 h. The heart was then removed, and the left ventricle was isolated, and freeze clamped in liquid nitrogen for total RNA extraction, protein extraction, and histology analysis. Meanwhile, the whole-body blood was collected in a 1.5 ml Eppendorf tube, allowed to clot at the room temperature for 2 h and centrifuged at 2000g for 15 min to obtain approximately 200 μl serum.
2.3. Transcriptomic analysis
For RNA-Seq analysis, the left ventricle of heart was isolated using RNeasy® mini kit (Qiagen) with the manufacturer protocol. The quality of the total RNA was determined by agarose gel and analysis on an Agilent 2100 Bioanalyzer® (www.chem.agilent.com). Subsequently, 1 μg total RNA with the RNA integrity number (RIN) of 8 or higher was enriched for mRNA using Illumina TruSeq stranded mRNA Library Prep kit (Illumina, 20020594) according to the manufacturer instruction. Next, the Bioanalyzer was used to detect size distribution of the transcriptome after fragmentation and adapter ligation, and a Qubit® Fluorometer (InvitrogenTM, Waltham, MA) was used to quantify the yield post-PCR amplification. Finally, the pooled cDNA libraries with 10 μM concentration from each sample were sequenced on Illumina NextSeq 500-mRNA-Seq platform in a 2x100 bp paired-end (PE) at Molecular and Genomics Core of University of Mississippi Medical center (Jackson, MS).
2.4. Bioinformatics analysis
A customized bioinformatics pipeline was applied to process the output reads from RNA-Seq. FastQC was applied to inspect the reads quality of each RNA-Seq raw sequencing file by measuring the quality score distribution of nucleotides per read, the existence of adapters, the duplication level of specific reads et al. [23]. Tuxedo suite pipeline is widely used and one of the most popular analysis pipelines designed for RNA-Seq projects [[24], [25], [26], [27], [28]]. In this study, Bowtie2, TopHat2, Cufflinks2, and HTSeq-count of the pipeline were used to perform alignment, transcript assembly, and read-counting. In addition, Samtools was used for sorting the mapped reads and format conversion [29,30]. The reads count text file of each sample was fed into DESeq2, an R package providing the means to test differential expressed genes (DEGs) between each two groups using the negative binomial generalized linear models [31,32]. Multiple databases were utilized in this study to obtain comprehensive insights of the altered transcriptomic pattern in responding to I/R stress of different groups. Kyoto Encyclopedia of Genes and Genomes (KEGG) database and one of the clustering analysis and visualization package from R called clusterProfiler was used to outstand the significant regulated signaling pathways based on the integrated p-value from all related DEGs involved in the same pathways [33]. Besides, the Reactome pathway database was utilized as well to map the DEGs for clustering to broad and specific metabolism and biological processes pathways, such as immune system, apoptosis, and MAPK signaling pathway [34]. In addition, Mouse Genome Informatics (MGI) Gene Ontology Annotations and GenAge database were employed to investigate the genes responding to oxidative stress and aging associated genes in mouse.
2.5. Immunoblotting
Immunoblotting was performed as previously described [16,21]. Heart homogenate proteins were resolved by SDS-PAGE and transferred onto polyvinylidene difluoride membranes (Millipore, Bedford, MA). Rabbit antibodies against phospho-p38 MAPK (Thr180/Tyr182), p38 MAPK, phospho-SAPK/JNK (Thr183/Tyr185), SAPK/JNK, phopho-NF-κB (Ser536), NF-κB, and GAPDH from Cell signaling (Danvers, MA) were purchased and used according to protocols provided by the manufacturer. In addition, rabbit Sesn2 Ab was obtained from ProteinTech (Chicago, IL, USA).
2.6. Cytokine array
Expression of 111 circulating cytokines, chemokines and growth factors in mouse was examined by Proteome Profiler Mouse XL cytokine array (ARY028, R&D Systems). Serums from physiological and I/R challenged young-WT, aged-WT and Sesn2-KO mice were mixed with a ‘cocktail’ of biotinylated detection antibodies, followed by streptavidin-labeled horseradish peroxidase and then visualization by chemiluminescence-based detection. Density of the spots were quantified with the array-specifically designed software Quick spots HLImage++ from R&D Systems.
2.7. Superoxide analysis
Freshly isolated non-fixed left ventricle was sectioned in 5 μm thickness setting by cryostat at −20 °C. Sections were incubated with 10 μM MitoSOX Red (Thermo Fisher Scientific #M36008, Waltham, MA) for 10 min at 37 °C. MitoSOX was then removed with PBS. Subsequently, sections were incubated with 5 μg/mL wheat germ agglutinin (WGA), Alexa Fluor 488 conjugate (Thermo Fisher Scientific, #W11261, Waltham, MA) for 10 min at 37 °C. Labeled sections were then washed with Hank's balanced salt solution (HBSS) for twice. Images were taken with Keyence BZ-X710 All-in-One Fluorescence Microscope under 20X and 60X (oil immersion) objective magnification power with same exposure time for MitoSox and WGA, respectively. Fluorescence intensity was quantified with Image J software [35].
2.8. Myocardial histology
Left ventricular tissue from normal physiological or 45 min of ischemia and 24 h reperfusion was rapidly excised, cross-sectioned and fixed in 4% buffered paraformaldehyde. Fixed tissue was then paraffin embedded and sectioned and stained with H&E [36]. Slides were then assessed in a blinded fashion with Keyence BZ-X710 All-in-One Fluorescence Microscope under 20X and 60X (oil immersion) objective magnification power.
2.9. In vivo cardiac function evaluation by echocardiography
Trans-thoracic M-mode and Doppler mode echocardiography was performed using the VisualSonic Vevo 3100 system. The cardiac systolic and diastolic functions of five randomly selected animals from each group were assessed using the previously described protocol [37]. Simpson's measurements were performed to obtain the systolic function features, such as calculated averaged ejection fraction (EF), fractional shortening (FS), and stroke volume (SV) from various parameters and the diastolic function were assessed with E/A ratio [the early (E) to late (A) ventricular filling velocities ratio] [37,38].
2.10. Statistics analysis
The analysis results of cardiac function, superoxide accumulation, and immunoblotting were expressed as means ± standard error of the means (SEM). Two-tailed Student's t-test, one-way ANOVA with Tukey's test and Kruskal-Wallis test were used to perform the statistics comparison among a set of samples with Prism 8.0 (GraphPad Software). P < 0.05 was considered as significant difference.
2.11. Data availability
RNA-seq data were deposited in the NCBI Gene Expression Omnibus (GEO) data repository with the accession code as GSE130217.
3. Results
3.1. Sesn2 deficiency leads to an aging-like phenotype in mouse heart
Sesn2 is postulated to be an aging-related protein as it was reported that Sesn2 deletion results in age-related pathologies, such as lipid accumulation and cardiac dysfunction in Drosophila [16,19]. We confirmed that Sesn2 is an aging-related protein with decreased Sesn2 protein level in aged-WT heart compared to young-WT heart as well as the complete deletion of Sesn2 in Sesn2-KO heart (Fig. 1A). To determine the critical role of Sesn2 in heart, blinded histological H&E staining of heart sections was performed with young-WT, aged-WT, and Sesn2-KO heart tissue under physiological condition. Heart section images showed the increased degree of myocardial incompact, hemorrhage and spindle-shaped interstitial cells in the aged-WT and Sesn2-KO heart versus young-WT heart indicating the loss of Sesn2 results in aging-like myocardial structural phenotype (Fig. 1B).
Fig. 1.
Sesn2-KO heart exhibited aging-like phenotype. (A) Sesn2 protein (54 KDa) in the Young-WT, Aged-WT, and Sesn2-KO heart under physiological condition. (B) representative H&E stained histological images of Young-WT, Aged-WT, and Sesn2-KO heart in physiological condition. MIC: myocardial incompact; H: hemorrhage; S; spindle-shaped interstitial cells. (C) Venn diagram showing overlap of the significant differently expressed genes (DEGs) between Aged-WT and Sesn2-KO heart vs. Young-WT. (D) representative aging-associated genes expression level in Aged-WT and Sesn2-KO heart vsYoung-WT, alteration values are presented in log2 form according to the legend panel. (E) representative genes expression level in Aged-WT and Sesn2-KO heart vs.Young-WT which were clustered in Reactome signaling pathways from significant DEGs from the three categories inFig. 1C (36 referring to the unique DEGs in Aged-WT, 68 referring to the overlapped DEGs in Aged-WT and Sesn2-KO, 1004 referring to the unique DEGs in Sesn2-KO. Alteration ratios are presented in log2 form according to the legend panel. (F) DEGs distributions in critical pathways related to redox and immune system in Aged-WT and Sesn2-KO heart versus Young-WT. (G) representative DEGs' expression level in Aged-WT and Sesn2-KO heart versus Young-WT from the categories in Fig. 1F. Sample numbers were N = 3 per group for RNA-Seq analysis, alteration values are presented in log2 form according to the legend panel. Statistical comparison between each two groups was performed after normalization in DESeq and generated all DEGs with adjust p value < 0.05.
In addition, through a comparative bioinformatics analysis of the aged-WT and Sesn2-KO myocardial transcriptome versus young-WT group, 104 and 1, 072 genes’ expression were significantly altered in aged-WT and Sesn2-KO heart, respectively (Fig. 1C). Interestingly, 68 out of 104 (65.4%) differentially expressed genes (DEGs) in aged-WT heart are identical with Sesn2-KO group. These identical DEGs from aged-WT and Sesn2-KO heart were clustered into the cardiac conduction related pathways further indicated that Sesn2 is age-associated protein in heart and it plays critical roles in preserving the performance of cardiac conduction and maintaining myocardial transcriptomic homeostasis (Fig. 1C and E).
Furthermore, by mapping the DEGs from aged-WT and Sesn2-KO vs. young-WT group against the mouse age-associated genes in GenAge database, there were six pro-longevity genes were down-regulated and four anti-longevity genes were up-regulated in aged-WT versus young-WT group, and this shift trend was aggravated in Sesn2-KO group indicating Sesn2 plays important roles in the aging process in the heart through regulating multiple longevity related genes, such as nitric oxide synthase 3 (Nos3), superoxide dismutase2 (Sod2), and cyclin-dependent kinase inhibitor 1 (Cdkn1A) (Fig. 1D). Intriguingly, majority of them are involved in response to reactive oxygen species (ROS) and mitogen-activated protein kinase (MAPK) signaling activation indicating the potential roles of age-related Sesn2 in regulating redox and immune system related signaling.
Of interest, the DEGs uniquely from Sesn2-KO group were clustered into the immune system related pathways through blasting against Reactome database suggested that Sesn2 is crucial to regulate immune response in heart (Fig. 1E). We also found that DEGs responding to ROS, MAPK and programmed cell death signaling activation from aged-WT and Sesn2-KO heart versus young-WT heart demonstrated that age-related Sesn2 pay roles in regulating ROS, MAPK signaling, immune system, as well as programmed cell death in heart (Fig. 1F and G).
3.2. Loss of Sesn2 aggravates pro-inflammatory signaling in the heart
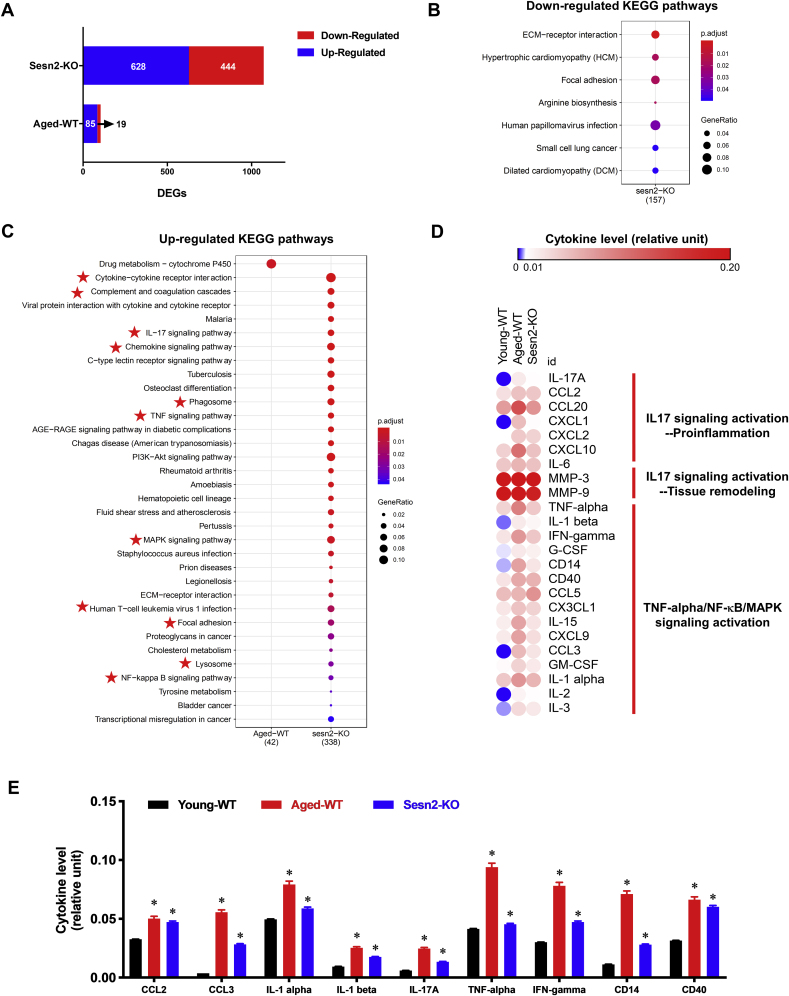
Bioinformatics analysis reported that 85 and 628 genes were significantly up-regulated, but 19 and 444 significantly down-regulated in aged-WT and Sesn2-KO myocardial transcriptome versus young-WT group (Fig. 2A). It was found that zero and seven KEGG pathways were significantly down-regulated, but one and 33 KEGG pathways significantly up-regulated in aged-WT and Sesn2-KO heart, respectively (p. adjust. value < 0.05) (Fig. 2B and C). Out of all up-regulated pathways in Sesn2-KO group, 11 of them were correlated with immune system (highlighted with star symbol), such as, Cytokine-cytokine receptor interaction, Complement and coagulation cascades, Chemokine signaling pathway (Fig. 2C). In the 11 immune system related pathways, IL-17 signaling pathway, TNF signaling pathway, MAPK signaling pathway, and Lysosome were pro-inflammation pathways suggested that Sesn2 depletion prompts cardiac chronic inflammation under physiological condition (Fig. 2C).
Fig. 2.
Sesn2 depletion provoked pro-inflammatory signaling. (A) differently expressed genes (DEGs) in Sesn2-KO-Sham vs. Young-WT-Sham and Aged-WT-Sham vs. Young-WT-Sham. Down-regulated DEGs are highlighted in blue and up-regulated DEGs are highlighted in red. (B) significantly down-regulated KEGG signaling and disease pathways of Sesn2-KO-Sham vs. Young-WT-Sham. Color represent p-value from lowest (red) to highest (blue) and the bubble size represent the significantly shifted gene ratio in the individual pathway. (C) significantly up-regulated KEGG signaling and disease pathways of Sesn2-KO-Sham and Aged-WT-Sham vs. Young-WT-Sham. Color represent p-value from lowest (red) to highest (blue) and the bubble size represent the significantly shifted gene ratio in the individual pathway. (D) heatmap visualization of serum cytokines level in Young-WT-Sham, Aged-WT-Sham, Sesn2-KO-Sham. Color was labeled with the intensity ratio of the individual cytokine vs. the reference according to the legend panel. (E) representative expression levels of 9 pro-inflammatory cytokines from cytokine array. Y axis is the intensity ratio of the individual cytokine vs. the reference. Values are mean ± SEM, N = 6 (*stands for p < 0.05 while comparing to young-WT-Sham). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Given the pro-inflammatory signaling activation in Sesn2-KO heart, and it was reported that chronic systemic inflammation is one of the characteristics in aging [39]. To determine whether Sesn2 deficiency contribute to the provoked inflammation, the Mouse XL Cytokine Array were applied to compare the serum inflammation related cytokine and chemokines in young-WT, aged-WT and Sesn2-KO mice. Heatmap analysis illustrated that 24 cytokines related to IL-17, TNF-α, NF-κB, MAPK pro-inflammatory signaling pathway were augmented in aged-WT and Sesn2-KO versus young-WT mice (Fig. 2D). Specifically, it was found that pro-inflammatory cytokine IL-17 were up-regulated in aged-WT and Sesn2-KO mice (Fig. 2E). Activation of IL-17 signaling pathway is responsible for the activation of downstream signaling cascades, resulting in the release of cytokines, such as, IL-1β, IL-6, IFN-γ and TNF-α which contributing to activate NF-κB and MAPK signaling and initiate of chronic inflammation and autoimmunity. Besides, it was noted that chemokines CCL2 and CCL3, the typical pro-inflammatory factors responsible for mediating the inflammatory leukocytes migration were significantly elevated in aged-WT and Sesn2-KO mice versus young-WT mice (Fig. 2E). The results demonstrated that Sesn2 plays roles in repressing inflammation and inhibiting pro-inflammatory signaling pathway in heart through regulating cytokine IL-17 signaling cascades in the circulation system.
3.3. Age-related Sesn2 impairment sensitizes the heart to I/R stress with exacerbated inflammation
To determine the effects of Sesn2 in the tolerance of heart, especially aged heart, to ischemia reperfusion injury, young-WT, aged-WT, Sesn2-KO mice were subjected to ligation of left anterior descending coronary artery (LAD) by suture to induce an in vivo regional ischemia for 45 min and then release of the suture to reperfuse for 24 h. Cardiac functions of the heart with sham or I/R operations were measured by using echocardiography. The results demonstrated there were not significantly different among young-WT, aged-WT, and Sesn2-KO hearts under normal physiological conditions in terms of cardiac systolic functions (Fig. 3A). However, the impaired cardiac diastolic function was observed in aged-WT heart under physiological condition with significantly decreased E/A ratio (Fig. 3A and Supplemental Table 1). Of interest, after in vivo regional ischemia for 45 min by LAD ligation and reperfusion for 24 h with release of the suture, cardiac systolic dysfunction was observed in young-WT group in terms of significantly decreased ejection fraction (EF) and fractional shortening (FS), and these effects were exacerbated in aged-WT and Sens2-KO groups (Fig. 3A and Supplemental Table 1).
Fig. 3.
Cardiac function assessment and myocardium histological analysis ofYoung-WT,Aged-WT and Sesn2-KO mice in responding to I/R. (A) Echocardiography showed that both Aged-WT and Sesn2-KO mice were intolerant to I/R injury as shown by ejection fraction (EF) and fractional shortening (FS). N = 5, Values are means ± SEM *p < 0.05. Diastolic dysfunction was demonstrated in aged-WT heart in both physiological and I/R stressed condition. Reprehensive images of M-mode and Doppler echocardiography for all six groups. The blue lines were the analysis performed on Vevo Image software to obtain the parameters for systolic and diastolic function analysis. (B) Representative H&E stained histological images of Young-WT, Aged-WT, and Sesn2-KO heart under physiological and I/R stressed condition. IF: Inflammation Filtration; MIC: myocardial incompact; H: hemorrhage; S; spindle-shaped interstitial cells. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Furthermore, to determine the roles of Sesn2 in heart to adapt to ischemia reperfusion injury, histological analysis was performed with young-WT, aged-WT, and Sesn2-KO heart tissue under physiological or I/R conditions. Heart section images showed that there was increased degree of disorganized myocardium, hemorrhage and inflammation filtration in young-WT mice after I/R stress and this phenomenon were exacerbated in the aged-WT and Sesn2-KO heart versus young-WT heart after I/R insults indicating the Sesn2 plays important roles in preserving myocardium structure and repressing inflammation (Fig. 3B).
Sesn2 is a stress-induced protein functioning in repressing ROS under oxidative stress conditions and regulating the mTOR signaling pathway, which is critical in proliferation, translation, metabolism, and inflammation [15,40]. To determine the cardioprotective mechanism of Sesn2 against I/R injury, RNA-Seq was performed to reveal the gene expression alteration profiles in myocardium of young-WT, aged-WT and Sesn2-KO mice in response to I/R stress. The results showed that there were neither protein nor transcriptional signal for Sesn2 in Sesn2-KO heart under physiological and I/R injured condition (Fig. 4A and B). Of interest, in response to I/R stress, the transcription of Sesn2 in young-WT mice was up-regulated in approximate 1.5-fold (Fig. 4B). However, there was no significant increase of Sesn2 transcript in aged-WT heart in response to I/R stress suggesting aged-WT heart were impaired to promote Sesn2 transactivation to adapt to I/R injury (Fig. 4B).
Fig. 4.
Sesn2 deficiency sensitized the heart to I/R stress with exacerbated inflammation. (A) Sesn2 protein (54 KDa) in the Young-WT, Aged-WT, and Sesn2-KO heart under physiological and I/R stress conditions. (B) Sesn2 transcript level of Young-WT-Sham, Aged-WT-Sham, Sesn2-KO-Sham, Young-WT-I/R, Aged-WT-I/R and Sesn2-KO-I/R from RNA-Seq. Values are means ± SEM *p < 0.05 (N = 3). (C) the Venn diagram illustration of the DEGs similarities and differences among three groups, standing for Young-WT-I/R (black), Aged-WT-I/R (red) and Sesn2-KO-I/R (blue) myocardium vs. Young-WT-Sham. (D) the numbers of differently expressed genes (DEGs) in Young-WT-IR, Aged-WT-IR, and Sesn2-KO-IR normalized by Young-WT-Sham. Down-regulated DEGs are highlighted in blue and up-regulated DEGs are highlighted in red. (E) significantly up-regulated KEGG signaling and disease pathways of Young-WT-I/R, Aged-WT-I/R and Sesn2-KO-I/R myocardium vs. Young-WT-Sham. Color represent p-value from lowest (red) to highest (blue) and the bubble size represent the significantly shifted gene ratio in the individual pathway. (F) heatmap visualization of 111 serum cytokines level in Young-WT-Sham, Aged-WT-Sham, Sesn2-KO-Sham, Young-WT-I/R, Aged-WT-I/R and Sesn2-KO-I/R vs. Young-WT-Sham. Color represent the increase (red) and decrease (blue) of each component, alteration ratios are presented in log2 form according to the legend panel. (G) representative expression levels of 9 pro-inflammatory cytokines from cytokine array. Y axis is the intensity ratio of the individual cytokine vs. the reference. Values are mean ± SEM (*stands for p < 0.05 while comparing to Young-WT-Sham. #stands for p < 0.05 while comparing to Young-WT-I/R). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
In response to I/R stress, there were 819 DEGs in the young-WT, 1, 749 DEGs in aged-WT, and 2, 845 DEGs in Sesn2-KO myocardium suggesting that Sesn2 deficiency increased the cardiac transcriptomic sensitivity to I/R stress (Fig. 4C and D). Out of the 1, 749 DEGs, 1, 471 (84.1%) DEGs in aged-WT group were overlapping with Sesn2-KO group demonstrated the Sesn2 is substantially associated with aging in response to the I/R stress (Fig. 4C). In addition, in response to I/R stress, 42 (79.2%) of the 53 significantly up-regulated KEGG pathways were identical in aged-WT and Sesn2-KO hearts compared to the 26 (49.1%) ones with young-WT hearts (Fig. 4E). The results suggest that Sesn2 deficiency sensitized genes expression in response to I/R stress in the aged heart.
In young-WT hearts, pro-inflammation related signaling KEGG pathways, such as, IL-17, TNF-α, and NF-κB signaling were significantly up-regulated in response to I/R stress demonstrating the presence of inflammation after I/R stress (Fig. 4E). Thus, increased number of significantly up-regulated inflammation related pathways in Sesn2-KO and aged-WT group after I/R stress indicating excessive inflammation in Sesn2 defected hearts (Fig. 4E). In addition, cytokine array analysis results confirmed that provoked inflammation in young-WT mice after I/R stress with up-regulated CCL2, CCL3, TNF-α, CD14, and CD40 in serum and these effects were aggravated in aged-WT and Sesn2-KO mice (Fig. 4F and G). Interestingly, heatmap analysis of 111 cytokines in Fig. 4F illustrated that cytokinome profile of Sesn2-KO mice resemble the aged-WT versus young-WT mice after I/R stress indicating that Sesn2 is aging-associated stress inducible protein in the aspect of mediating inflammation response under I/R stress (Fig. 4F and Supplementary Fig. 1).
3.4. Sesn2 deficiency leads to intensified oxidative stress through disruption of redox homeostasis during I/R insults
Ischemia reperfusion injury is associated with the releasing of extensive ROS which initiates the inflammatory response including cell migration and up-regulation of pro-inflammatory cytokines [2,4,[7], [8], [9], [10], [11], [12], [13]]. Budanov et al. reported the redox-active domain homolog of Sestrins to AhpD, a component of alkyl-hydroperoxidereductase leading to the discovery of Sesn2 functioning in repressing ROS [41]. Sesn2 is expressed in skeletal muscle and myocardium suggesting the roles of Sesn2 in regulating ROS levels in these high-energy demanding and thus high mitochondrial contents tissues [41]. To characterize the effect of Sesn2 in regulating the redox homeostasis in myocardium, the intracellular superoxide (O2•−) levels in the physiological and I/R challenged fresh heart tissue were examined using MitoSox oxidation [35]. Compared to the young-WT heart, intensive intracellular O2•− levels were detected in aged-WT hearts and Sesn2-KO hearts under physiological conditions (Fig. 5A and B). Furthermore, I/R stress significantly up-regulated the intracellular O2•− levels in young-WT heart, this effect was further exacerbated in the aged-WT and Sesn2-KO hearts. The results suggest that Sesn2 is critical to repress the ROS accumulation in myocardium under both physiological and IR stress conditions (Fig. 5A and B).
Fig. 5.
Sesn2 deficiency agitated the intracellular redox homeostasis in hearts. (A) representative microscopy images (200X) showing MitoSOX and wheat germ agglutinin (WGA) fluorescence in the myocardium from Young-WT, aged-WT or Sesn2-KO hearts under physiological and I/R stressed conditions (upper panel). Quantification of MitoSOX oxidation from images. Values are means ± SEM (N = 5, *p < 0.05) with NIH Image J (Lower panel). (B) representative microscopy images (600X) showing MitoSOX and WGA fluorescence in the myocardium from Young-WT, aged-WT or Sesn2-KO hearts under physiological and I/R stressed conditions. (C) representative ROS related genes expression level in Aged-WT-Sham, Sesn2-KO-Sham, Young-WT-I/R, Aged-WT-I/R and Sesn2-KO-I/R heart vs. Young-WT-Sham, alteration ratios are presented in log2 form according to the legend panel.
To determine the mechanism of Sesn2 in modulating myocardium ROS, the expression profiles of genes related with cellular response to reactive oxygen species were extracted from MGI database [42]. Interestingly, there were 3 and 12 genes expression response to ROS significantly up-regulated in aged-WT and Sesn2-KO heart under physiological conditions implying the Sesn2 deficiency leading to excessive ROS accumulation resulting in the activation of transcriptional regulation in heart. This transcriptional modulation effect was also observed in young-WT, aged-WT, and Sesn2-KO heart after I/R insults which is consistent with the up-regulated the intracellular O2•− levels in all three groups. However, the expression alteration of regulatory genes of responding to ROS, such as, Sestrin 1 (Sesn1), Foxo3 (Forkhead box O-3) and Sirtuin 3 (Sirt3) were significantly down-regulated in Sesn2-KO heart compared to young-WT heart after I/R insults indicating Sesn2 plays roles in mediating the genes which are responsible to regulate the intracellular response to ROS (Fig. 5C). Furthermore, Superoxide dismutase 2 (Sod2) and Sphingomyelin phosphodiesterase 3 (Smpd3) which are responsible for removal of superoxide radicals were significantly down-regulated in Sesn2-KO heart compared to young-WT heart after I/R insults suggesting Sesn2 is crucial for the transactivation of the antioxidant genes under the I/R stress (Fig. 5C).
3.5. Sesn2 defected heart coaxes MAPK pro-inflammatory and apoptosis signaling in response to I/R stress
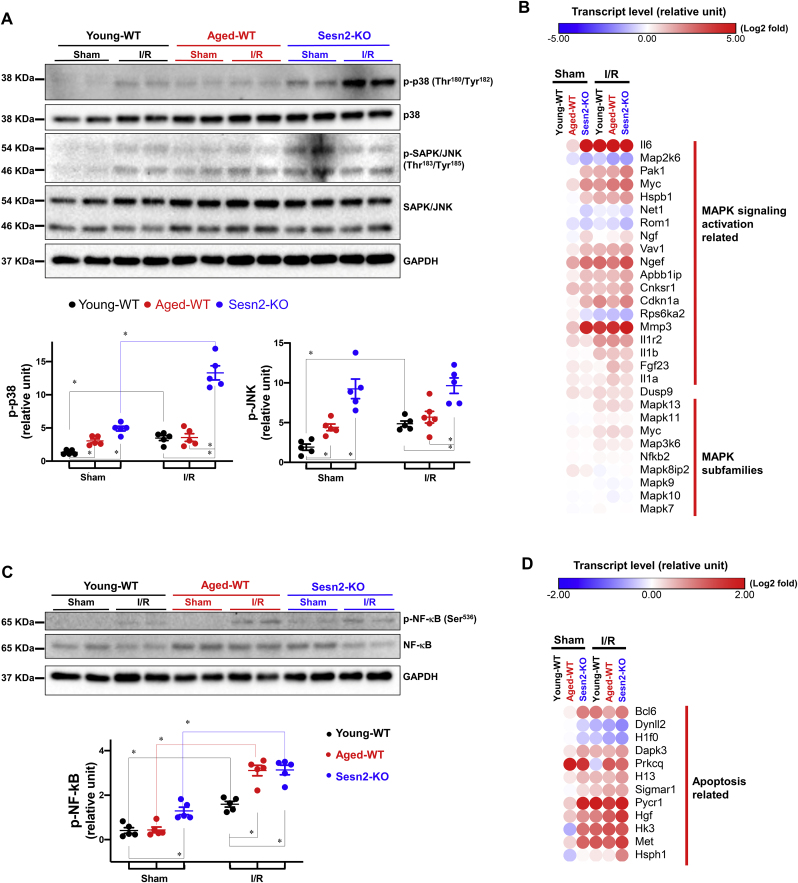
Reactive oxygen species (ROS), such as hydrogen peroxide was reported to active MAPK signaling pathway and induce programmed cell death [43]. The transcriptional up-regulation of MAPK signaling pathway in Sesn2-KO heart under physiological and I/R injured heart indicates the potential link between Sesn2 and MAPK signaling pathway in heart (Fig. 2, Fig. 4E). Transcriptome analysis showed that genes involved in the activation of the MAPK signaling pathway were significantly altered in the I/R group as shown in Fig. 6B suggesting the activation of MAPK signaling by I/R stress. Consistently, immunoblotting results showed the phosphorylation of p38 and JNK was significantly increased in the I/R versus the sham groups indicating the activation of MAPK in response to I/R stress (Fig. 6A). Furthermore, the activation of MAPK was found in both aged-WT and Sesn2-KO sham operations compared with the young-WT as shown by up-regulated p38 and JNK phosphorylation as well as MAPK activation genes expression (Fig. 6A and B). Of interest, the transcripts of MAPK subfamilies genes showed no significant differences in all six groups indicating the post-translational regulation of Sesn2 in mediating MAPK signaling pathway (Fig. 6B).
Fig. 6.
Sesn2 deficiency activated MAPK, NF-κB and apoptosis signaling pathway in responseto I/R. (A) representative Immunoblotting images of the phosphorylation of MAPK p38 and SAPK/JNK and total levels of p38 and SAPK/JNK, in Young-WT-Sham, Aged-WT-Sham, Sesn2-KO-Sham, Young-WT-I/R, Aged-WT-I/R and Sesn2-KO-I/R heart. Values are means ± SEM. *p < 0.05. (B) representative MAPK signaling activation related gene expression levels in Aged-WT-Sham, Sesn2-KO-Sham, Young-WT-I/R, Aged-WT-I/R and Sesn2-KO-I/R heart vs. Young-WT-Sham, alteration ratios are presented in log2 form according to the legend panel. (C) representative Immunoblotting images of the phosphorylation of NF-κB and total levels of NF-κB in Young-WT-Sham, Aged-WT-Sham, Sesn2-KO-Sham, Young-WT-I/R, Aged-WT-I/R and Sesn2-KO-I/R heart. Values are means ± SEM. *p < 0.05. (D) representative apoptosis signaling related gene expression levels in Aged-WT-Sham, Sesn2-KO-Sham, Young-WT-I/R, Aged-WT-I/R and Sesn2-KO-I/R heart vs. Young-WT-Sham, alteration ratios are presented in log2 form according to the legend panel.
Myocardial apoptosis is primarily triggered or accelerated during reperfusion after ischemia due to the intensive release of ROS and chemotactic cytokines which are regulated by MAPK and downstream NF-κB signaling pathway [43]. Immunoblotting results demonstrated the phosphorylation of NF-κB was augmented in the young-WT-I/R versus the sham group indicating the activation of NF-κB in myocardium after I/R insults (Fig. 6C). Transcriptomics analysis showed the up-regulation of multiple apoptosis related genes in the young-WT-I/R group versus the sham group indicating the initiation of myocardium apoptosis progress after I/R insults (Fig. 6D). These effects were found significantly exacerbated in aged-WT and Sesn2-KO hearts after I/R stress indicated the critical roles of Sesn2 in modulating myocardial apoptosis under I/R stress.
4. Discussion
To determine the correlation of Sesn2 and aging in heart as well as explore the cardioprotective roles and mechanisms of Sesn2 in adapting to cardiac I/R stress, various physiological, biochemical, histological and big data tools were applied to achieve the goal in this study. Our lab has demonstrated that Sesn2 is an age-related protein in heart with declined Sesn2 protein with aging which contributes to the susceptibility of aged heart towards ischemic reperfusion injury [16]. This study elucidates that Sesn2 is substantially associated with aging in heart with similar disorganized myocardium structure and resembled transcriptomics profile in physiological heart. The transcriptomics alteration in aged heart versus young heart was observed with regulation of aging-associated genes and immune system related genes under physiological condition. However, this effect was dramatically exacerbated in Sesn2 KO heart. It indicates that loss of Sesn2 resulting in the transcriptomics sensitivity in the heart. We further found that Sesn2 is required to repress inflammation showing as the provoke pro-inflammation signaling pathways and the systemic chronic inflammation in Sesn2 knockout physiological heart. Moreover, Sesn2 depletion induced the excessive intracellular ROS accumulation in physiological hearts. Combined the evidence of aging accompanying with impaired Sesn2, intensive oxidative stress, and chronic inflammation, Sesn2 is determined as a critical age-associated protein in modulating redox and immune system in heart. In response to I/R stress, the transcriptomic analysis showed resembled gene expression alteration profiles between Sesn2-KO and aged-WT heart and echocardiography cardiac function assessment demonstrated the likewise systolic dysfunction in both aged-WT and Sesn2-KO mice compared to young-WT group. In addition, the similarity in systemic cytokinome profile and cardiac intracellular ROS level of Sesn2-KO and aged-WT mice demonstrated that Sesn2 is a substantial age-related gene in responding to ischemia reperfusion stress through modulating redox homeostasis and regulating inflammation response.
As the homolog of AhpD, Sesn2 was reported to function in repressing ROS in various tissues [44]. It is clear now that Sesn2 could be stimulated by oxidative stress, Endoplasmic Reticulum (ER) stress, and hypoxia in cell [17]. Intriguingly, Sesn2 expressional activation was found impaired in aged-WT heart under ischemia reperfusion stress resulting in the intensive superoxide accumulation compared to young-WT heart. These effects were significantly exacerbated in Sesn2 KO heart accompanied with the maladaptive expression response to redox system under ischemia reperfusion stress. Superoxide was reported to induce Ca2+ overload in myocardial mitochondrial leading to activation of MAPK cascades through MAP3K, MAP2K, as well as JNK and p38 [43]. Activation of p38 and JNK signaling is participating in eliciting the activation of downstream NF-κB and promotes subsequent synthesis of TNF-α resulting in the initiation of cell death cascade [[45], [46], [47]]. NF-κB is a major transcriptional regulator that could transactivating immune system related genes in nuclear [48]. Combined with the activate MAPK signaling pathway, NF-κB signaling pathway, increased release of TNF-α, and increased degree of myocardium injury in aged-WT and Sesn2-KO hearts, Sesn2 is demonstrated to play critical roles in modulating inflammation and apoptosis through maintaining redox homeostasis in mouse hearts in responding to ischemia reperfusion injury.
Our previous studies reported that Sesn2 is critical for AMPK signaling activation in the heart during ischemic stress [15]. AMPK signaling is well-characterized in modulation nutrient metabolism and reported critical for regulating inflammation through inhibiting NF-κB activation, JNK signaling, and IL-1β expression [49,50]. In this study, we revealed that the antioxidant property of Sesn2 is critical to maintain redox homeostasis which is ascribed for the modulation of inflammatory response to I/R stress in heart. Besides, Sesn2 is found to regulate age-related genes expression and IL-17 signaling and its downstream pro-inflammatory cytokines as well as MAPK-NF-κB signaling. In aged heart, the impaired Sesn2 contributes to the intensified oxidative stress along with up-regulated pro-inflammatory signaling resulting to the greater myocardial damage after I/R stress.
In the past few decades, it has motivated intensive studies of the mechanisms of cardioprotection in I/R stress, especially for aging circumstance, which is a phenomenon with progressive declining in the functional maintenance of tissue homeostasis physiology, leading to higher sensitivity to a variety of stresses and diseases [16]. In this study, we demonstrated that Sesn2 is a critical factor in aged-related adaptive response to I/R stress. Through maintaining the cellular redox homeostasis, thus modulating inflammation response, Sesn2 facilitates the heart to adapt to the I/R injury. The implication of this study might encourage the researchers to pay attention in Sesn2 for developing the new therapeutic strategies of the cardiac diseases, especially for the aging population.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgements
These studies were supported by American Diabetes Association 1-17-IBS-296, NIH R01AG049835, R01GM124108, P20GM104357, P20GM103476 and P20GM121334.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101556.
Author contributions
D. Ren and J. Li designed and conducted the study; D. Ren, N. Quan, J. Fedorova, J. Zhang, Z. He performed data collection and analysis; D. Ren, N. Quan, J. Fedorova, J. Zhang, Z. He and J. Li interpreted data; and D. Ren and J. Li wrote the manuscript.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Benjamin E.J., Muntner P., Alonso A., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Das S.R., Delling F.N., Djousse L., Elkind M.S.V., Ferguson J.F., Fornage M., Jordan L.C., Khan S.S., Kissela B.M., Knutson K.L., Kwan T.W., Lackland D.T., Lewis T.T., Lichtman J.H., Longenecker C.T., Loop M.S., Lutsey P.L., Martin S.S., Matsushita K., Moran A.E., Mussolino M.E., O'Flaherty M., Pandey A., Perak A.M., Rosamond W.D., Roth G.A., Sampson U.K.A., Satou G.M., Schroeder E.B., Shah S.H., Spartano N.L., Stokes A., Tirschwell D.L., Tsao C.W., Turakhia M.P., VanWagner L.B., Wilkins J.T., Wong S.S., Virani S.S., American Heart Association Council on E., Prevention Statistics C., Stroke Statistics S. Heart disease and stroke statistics-2019 update: a report from the American heart association. Circulation. 2019;139(10):e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2.Buja L.M. Myocardial ischemia and reperfusion injury. Cardiovasc. Pathol. 2005;14(4):170–175. doi: 10.1016/j.carpath.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Hausenloy D.J., Yellon D.M. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J. Clin. Invest. 2013;123(1):92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Groot H., Rauen U. Ischemia-reperfusion injury: processes in pathogenetic networks: a review. Transplant. Proc. 2007;39(2):481–484. doi: 10.1016/j.transproceed.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Wu X.J., Sun X.H., Wang S.W., Chen J.L., Bi Y.H., Jiang D.X. Mifepristone alleviates cerebral ischemia-reperfusion injury in rats by stimulating PPAR γ. Eur. Rev. Med. Pharmacol. Sci. 2018;22(17):5688–5696. doi: 10.26355/eurrev_201809_15836. [DOI] [PubMed] [Google Scholar]
- 6.Ames W.R., 3rd, A, Kowada M., Thurston J.M., Majno G. Cerebral ischemia. II. The no-reflow phenomenon. Am. J. Pathol. 1968;52(2):437. [PMC free article] [PubMed] [Google Scholar]
- 7.Hsieh Y.H., Huang S.S., Wei F.C., Hung L.M. Resveratrol attenuates ischemia-reperfusion-induced leukocyte-endothelial cell adhesive interactions and prolongs allograft survival across the MHC barrier. Circ. J. 2007;71(3):423–428. doi: 10.1253/circj.71.423. [DOI] [PubMed] [Google Scholar]
- 8.Chang N.J., Weng W.H., Chang K.H., Liu E.K., Chuang C.K., Luo C.C., Lin C.H., Wei F.C., Pang S.T. Genome-wide gene expression profiling of ischemia-reperfusion injury in rat kidney, intestine and skeletal muscle implicate a common involvement of MAPK signaling pathway. Mol. Med. Rep. 2015;11(5):3786–3793. doi: 10.3892/mmr.2015.3235. [DOI] [PubMed] [Google Scholar]
- 9.Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;284(1):G15–G26. doi: 10.1152/ajpgi.00342.2002. [DOI] [PubMed] [Google Scholar]
- 10.Gilbert T.B., Hoffman J.W., Jr., Poston R.S., Silldorff E.P. Myocardial reperfusion injury: etiology, mechanisms, and therapies. J. Extra Corpor. Technol. 2004;36(4):391–411. [PubMed] [Google Scholar]
- 11.Hossmann KA. Pathophysiology and therapy of experimental stroke. Cell. Mol. Neurobiol. 2006;26(7–8):1057–1083. doi: 10.1007/s10571-006-9008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy E., Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol. Rev. 2008;88(2):581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Land W.G. The role of postischemic reperfusion injury and other nonantigen-dependent inflammatory pathways in transplantation. Transplantation. 2005;79(5):505–514. doi: 10.1097/01.tp.0000153160.82975.86. [DOI] [PubMed] [Google Scholar]
- 14.Lesnefsky E.J., Hoppel C.L. Ischemia-reperfusion injury in the aged heart: role of mitochondria. Arch. Biochem. Biophys. 2003;420(2):287–297. doi: 10.1016/j.abb.2003.09.046. [DOI] [PubMed] [Google Scholar]
- 15.Morrison A., Chen L., Wang J., Zhang M., Yang H., Ma Y., Budanov A., Lee J.H., Karin M., Li J. Sestrin2 promotes LKB1-mediated AMPK activation in the ischemic heart. Faseb. J. 2015;29(2):408–417. doi: 10.1096/fj.14-258814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quan N., Sun W., Wang L., Chen X., Bogan J.S., Zhou X., Cates C., Liu Q., Zheng Y., Li J. Sestrin2 prevents age-related intolerance to ischemia and reperfusion injury by modulating substrate metabolism. Faseb. J. 2017;31(9):4153–4167. doi: 10.1096/fj.201700063R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pasha M., Eid A.H., Eid A.A., Gorin Y., Munusamy S. Sestrin2 as a novel biomarker and therapeutic target for various diseases. Oxid. Med. Cell. Longev. 2017:3296294. doi: 10.1155/2017/3296294. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J.H., Budanov A.V., Talukdar S., Park E.J., Park H.L., Park H.W., Bandyopadhyay G., Li N., Aghajan M., Jang I., Wolfe A.M., Perkins G.A., Ellisman M.H., Bier E., Scadeng M., Foretz M., Viollet B., Olefsky J., Karin M. Maintenance of metabolic homeostasis by Sestrin2 and Sestrin3. Cell Metabol. 2012;16(3):311–321. doi: 10.1016/j.cmet.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J.H., Budanov A.V., Park E.J., Birse R., Kim T.E., Perkins G.A., Ocorr K., Ellisman M.H., Bodmer R., Bier E., Karin M. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science. 2010;327(5970):1223–1228. doi: 10.1126/science.1182228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Budanov A.V., Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134(3):451–460. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quan N., Wang L., Chen X., Luckett C., Cates C., Rousselle T., Zheng Y., Li J. Sestrin2 prevents age-related intolerance to post myocardial infarction via AMPK/PGC-1alpha pathway. J. Mol. Cell. Cardiol. 2018;115:170–178. doi: 10.1016/j.yjmcc.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J., Yang L., Rezaie A.R., Li J. Activated protein C protects against myocardial ischemic/reperfusion injury through AMP-activated protein kinase signaling. J. Thromb. Haemostasis. 2011;9(7):1308–1317. doi: 10.1111/j.1538-7836.2011.04331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown J., Pirrung M., McCue L.A. FQC dashboard: integrates FastQC results into a web-based, interactive, and extensible FASTQ quality control tool. Bioinformatics. 2017;33(19):3137–3139. doi: 10.1093/bioinformatics/btx373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.S. Ghosh, C.-K.K. Chan, Analysis of RNA-seq data using TopHat and Cufflinks, Plant Bioinformatics, Springer2016, pp. 339-361. [DOI] [PubMed]
- 25.Langmead B., Salzberg S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9(4):357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langmead B., Trapnell C., Pop M., Salzberg S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10(3):R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trapnell C., Roberts A., Goff L., Pertea G., Kim D., Kelley D.R., Pimentel H., Salzberg S.L., Rinn J.L., Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012;7(3):562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trapnell C., Williams B.A., Pertea G., Mortazavi A., Kwan G., van Baren M.J., Salzberg S.L., Wold B.J., Pachter L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010;28(5):511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H., Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., Marth G., Abecasis G., Durbin R., Genome S. project data processing, the sequence alignment/map format and SAMtools. Bioinformatics. 2009;25(16):2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anders S., Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11(10):R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu G., Wang L.G., Han Y., He Q.Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16(5):284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Croft D M.A., Haw R., Milacic M., Weiser J., Wu G., Caudy M., Garapati P., Gillespie M., Kamdar M.R., Jassal B. The Reactome pathway knowledgebase. Nucleic Acids Res. 2013;42(D1):D472–D477. doi: 10.1093/nar/gkt1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Case A.J., Tian J., Zimmerman M.C. Increased mitochondrial superoxide in the brain, but not periphery, sensitizes mice to angiotensin II-mediated hypertension. Redox Biol. 2017;11:82–90. doi: 10.1016/j.redox.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elrod J.W., Calvert J.W., Morrison J., Doeller J.E., Kraus D.W., Tao L., Jiao X., Scalia R., Kiss L., Szabo C., Kimura H., Chow C.W., Lefer D.J. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc. Natl. Acad. Sci. U. S. A. 2007;104(39):15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li X., Liu J., Hu H., Lu S., Lu Q., Quan N., Rousselle T., Patel M.S., Li J. Dichloroacetate ameliorates cardiac dysfunction caused by ischemic insults through AMPK signal pathway-not only shifts metabolism. Toxicol. Sci. 2019;167(2):604–617. doi: 10.1093/toxsci/kfy272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao S., Ho D., Vatner D.E., Vatner S.F. Echocardiography in mice. Curr. Protoc. Mouse Biol. 2011;1:71–83. doi: 10.1002/9780470942390.mo100130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ovadya Y., Landsberger T., Leins H., Vadai E., Gal H., Biran A., Yosef R., Sagiv A., Agrawal A., Shapira A., Windheim J., Tsoory M., Schirmbeck R., Amit I., Geiger H., Krizhanovsky V. Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat. Commun. 2018;9(1):5435. doi: 10.1038/s41467-018-07825-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bodmer D., Levano-Huaman S. Sesn2/AMPK/mTOR signaling mediates balance between survival and apoptosis in sensory hair cells under stress. Cell Death Dis. 2017;8(10) doi: 10.1038/cddis.2017.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Budanov A.V., Sablina A.A., Feinstein E., Koonin E.V., Chumakov P.M. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science. 2004;304(5670):596–600. doi: 10.1126/science.1095569. [DOI] [PubMed] [Google Scholar]
- 42.Eppig J.T., Smith C.L., Blake J.A., Ringwald M., Kadin J.A., Richardson J.E., Bult C.J. Mouse genome informatics (MGI): resources for mining mouse genetic, genomic, and biological data in support of primary and translational research. Methods Mol. Biol. 2017;1488:47–73. doi: 10.1007/978-1-4939-6427-7_3. [DOI] [PubMed] [Google Scholar]
- 43.Son Y., Kim S., Chung H.T., Pae H.O. Reactive oxygen species in the activation of MAP kinases. Methods Enzymol. 2013;528:27–48. doi: 10.1016/B978-0-12-405881-1.00002-1. [DOI] [PubMed] [Google Scholar]
- 44.Budanov A.V., Lee J.H., Karin M. Stressin' Sestrins take an aging fight. EMBO Mol. Med. 2010;2(10):388–400. doi: 10.1002/emmm.201000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shuto T., Xu H., Wang B., Han J., Kai H., Gu X.-X., Murphy T.F., Lim D.J., Li J.-D. Activation of NF-κB by nontypeable Hemophilus influenzae is mediated by toll-like receptor 2-TAK1-dependent NIK–IKKα/β–IκBα and MKK3/6–p38 MAP kinase signaling pathways in epithelial cells. Proc. Natl. Acad. Sci. U. S. A. 2001;98(15):8774–8779. doi: 10.1073/pnas.151236098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cabal-Hierro L., Rodriguez M., Artime N., Iglesias J., Ugarte L., Prado M.A., Lazo P.S. TRAF-mediated modulation of NF-kB AND JNK activation by TNFR2. Cell. Signal. 2014;26(12):2658–2666. doi: 10.1016/j.cellsig.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 47.Blaser H., Dostert C., Mak T.W., Brenner D. TNF and ROS crosstalk in inflammation. Trends Cell Biol. 2016;26(4):249–261. doi: 10.1016/j.tcb.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 48.Lim E.J., Lee S.H., Lee J.G., Kim J.R., Yun S.S., Baek S.H., Lee C. Toll-like receptor 9 dependent activation of MAPK and NF-kB is required for the CpG ODN-induced matrix metalloproteinase-9 expression. Exp. Mol. Med. 2007;39(2):239–245. doi: 10.1038/emm.2007.27. [DOI] [PubMed] [Google Scholar]
- 49.Xiang H.C., Lin L.X., Hu X.F., Zhu H., Li H.P., Zhang R.Y., Hu L., Liu W.T., Zhao Y.L., Shu Y., Pan H.L., Li M. AMPK activation attenuates inflammatory pain through inhibiting NF-kappaB activation and IL-1beta expression. J. Neuroinflammation. 2019;16(1):34. doi: 10.1186/s12974-019-1411-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mancini S.J., Salt I.P. Investigating the role of AMPK in inflammation. Methods Mol. Biol. 2018;1732:307–319. doi: 10.1007/978-1-4939-7598-3_20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA-seq data were deposited in the NCBI Gene Expression Omnibus (GEO) data repository with the accession code as GSE130217.