Abstract
Circulating microRNAs (miRNAs) are potential biomarkers in various diseases. However, whether they could serve as biomarkers for human adult fulminant myocarditis (FM) is unknown. Circulating miRNA expression profiles were detected by microarray analysis and validated by quantitative real-time PCR arrays. Meanwhile, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis was used to determine the critical roles of these circulating miRNAs in FM. Moreover, correlation analysis was employed between miRNAs and the parameters of cardiac functions in FM. Finally, the sensitivity and specificity of circulating long non-coding RNA (lncRNA) expression in FM diagnosis were evaluated using receiver operating characteristic curve analysis. Both microarray and quantitative real-time PCR analysis showed that the expression of miR-4763-3p and miR-4281 were upregulated in the plasma of FM at the onset, and their levels were restored as the clinical symptom recovered. The predicted target genes of miR-4763-3p and miR-4281 are involved in several pathways, mainly inflammatory and cardiac injury response. Moreover, the miRNAs enrichment was negatively correlated with the severity of FM. In addition, the expression levels of circulating miR-4763-3p were unchanged in myocardial infarction (MI) patients but showed high sensitivity and specificity for FM diagnosis. This study provides a global profile of circulating miRNAs in patients with FM, among which miR-4763-3p could serve as a potential biomarker.
Keywords: circulating miRNA, biomarker, human, fulminant myocarditis
Graphical Abstract
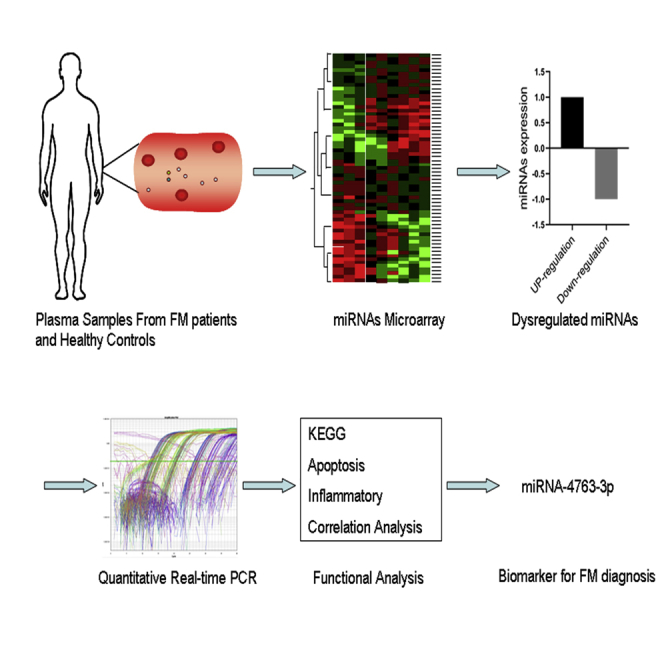
Fulminant myocarditis (FM) is the most severe form of acute myocarditis. However, the diagnostic biomarkers for suspected myocarditis are limited. Here, Chen and colleagues detected the global profile of circulating miRNAs in patients with FM, among which miR-4763-3p showed high sensitivity and specificity for FM diagnosis.
Introduction
Fulminant myocarditis (FM) is the most severe form of acute myocarditis,1 which may lead to serious hemodynamic dysfunction, acute heart failure, and even sudden death with a mortality rate up to 40%–70%.2,3 There are various etiologies of FM, including virus infection, immune and allergy.4, 5, 6 Diagnosis for FM is complicated, and it is more likely to be a clinical diagnosis rather than a histological or pathological diagnosis.7 An early and correct diagnosis may be helpful for the treatment and potentially reduce the mortality rate. Current laboratory examinations, such as troponin I or T, creatine kinase, lactic dehydrogenase, and brain natriuretic peptide (BNP) were applied in clinical diagnosis of cardiovascular diseases. However, the levels of these biomarkers are widely changed during myocardial injury8 and cardiac dysfunction9 and may not be specific for fulminant myocarditis diagnosis. Therefore, novel biomarkers with higher sensitivity and specificity in diagnosis of fulminant myocarditis are urgently needed.
MicroRNAs (miRNAs) are small non-coding RNAs that usually induce gene silencing by binding to the 3′ UTR of target mRNAs, which play significant roles in multiple diseases.10,11 Previous study showed that miRNAs might exist in plasma or serum in a stable form for more than 10 years.12 Certain circulating miRNAs, such as miR-665, miR-320a, and miR-22, contribute significant roles in heart failure (HF) and could serve as biomarkers for HF diagnosis.13, 14, 15 Cardiac-specific miRNA, miR-208a, is a novel biomarker for early detection of myocardial injury in human plasma.16 Circulating miR-122-5p was an independent predictor of neurological outcome and survival after cardiac arrest, improving the prediction of outcome after out-of-hospital cardiac arrest.17 However, whether they could serve as biomarkers for human adult FM is unknown.
The present study aims to characterize the expression profile of circulating miRNAs in FM patients and investigate potential biomarkers for FM diagnosis.
Results
Determination of Circulating miRNA Profiles in FM Patients
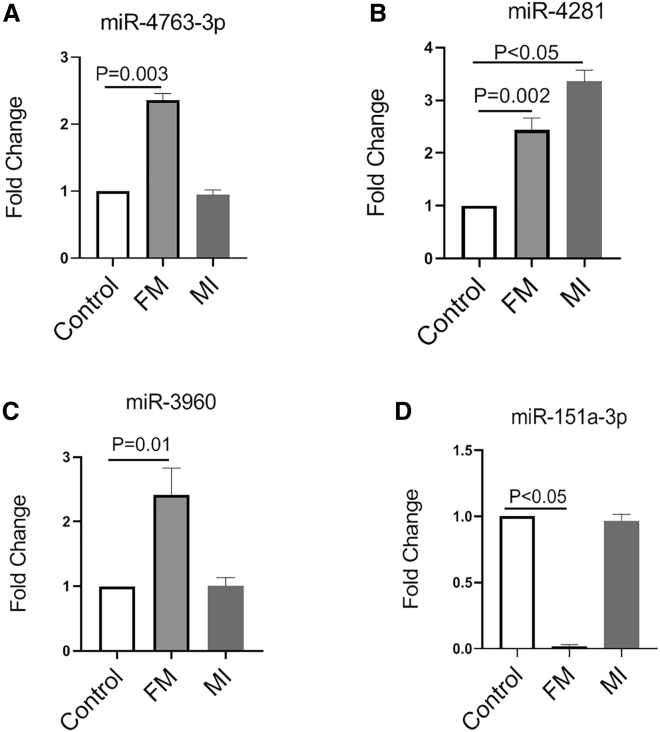
To identify the miRNAs profile in the plasma of FM patients, we subjected blood samples from FM and healthy controls to miRNA microarray analysis. The baseline characteristics of the FM patients and healthy controls were listed in Tables S1–S5. In total, 1,711 miRNAs were detected, among which three miRNAs were upregulated, including hsa-miR-4763-3p, hsa-miR-4281, and hsa-miR-3960, while one miRNA, hsa-miR-151a-3p, was downregulated (Figures 1A–1D; Table S6). Since miRNAs may be secreted into circulation during organ injury,16 we further compared the miRNAs profiles in plasma from acute myocardial infarction (MI) patients (Table S7) to evaluate whether these differently expressed miRNAs were specific responsible for the onset of fulminant myocarditis. As shown in Figure 1, the expression levels of hsa-miR-4763-3p, hsa-miR-3960, and hsa-miR-151a-3p were unchanged in MI patients, while hsa-miR-4281 was also increased in MI, compared to controls.
Figure 1.
Determination of Circulating miRNA Profiles in FM Patients
(A) The fold change of miR-4763-3p from microarray analysis (N = 8). (B) The fold change of miR-4281 from microarray analysis (N = 8). (C) The fold change of miR-3960 from microarray analysis (N = 8). (D) The fold change of miR-151-3p from microarray analysis (N = 8). FM, fulminant myocarditis; MI, myocardial infarction.
These results indicated that miR-4281 may be related with the cardiac injury induced by FM. And hsa-miR-4763-3p, hsa-miR-3960, and hsa-miR-151a-3p may be responsible for the initial pathological processes of FM, such as inflammatory reaction, but not the cardiomyocyte injury outcome of FM.
Validation of miRNA Expression by Quantitative Real-Time PCR
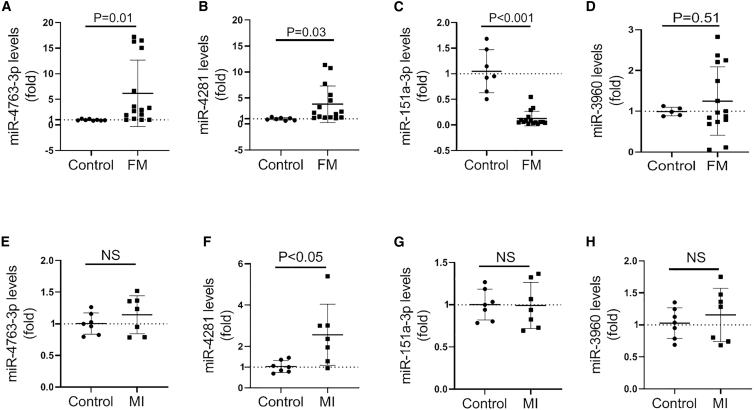
To further validate these microarray-identified miRNAs, we employed another fifteen plasma samples from a second cohort of FM patients for quantitative real-time PCR analysis. Consistently, comparing to the control group, the expression of miR-4763-3p and miR-4281 was significantly upregulated in FM patients, while miR-151a-3p level was significantly downregulated (Figures 2A–2C). Although the level of circulating miR-3960 was increased, there was a lack of significance (p = 0.51; Figure 2D). Moreover, another seven plasma samples from a second cohort of MI patients were also employed. Results of quantitative real-time PCR analysis showed that only miR-4281 expression level was increased in plasma from MI patients among the four dysregulated miRNAs, which was in line with the microarray analysis (Figures 2E–2H).
Figure 2.
Validation of miRNA Expression by Quantitative Real-Time PCR
(A) The expression of miR-4763-3p in plasma from FM patients detected by quantitative real-time PCR (N = 15). (B) The expression of miR-4281 in plasma from FM patients detected by quantitative real-time PCR (N = 15). (C) The expression of miR-151-3p in plasma from FM patients detected by quantitative real-time PCR (N = 15). (D) The expression of miR-3960 in plasma from FM patients detected by quantitative real-time PCR (N = 15). (E) The expression of miR-4763-3p in plasma from MI patients detected by quantitative real-time PCR (N = 7). (F) The expression of miR-4281 in plasma from MI patients detected by quantitative real-time PCR (N = 7). (G) The expression of miR-151-3p in plasma from MI patients detected by quantitative real-time PCR (N = 7). (H) The expression of miR-3960 in plasma from MI patients detected by quantitative real-time PCR (N = 7). FM, fulminant myocarditis; MI, myocardial infarction.
Together, both the microarray and quantitative real-time PCR assays suggested that circulating miR-4763-3p and miR-151a-3p were more specifically dysregulated in FM and may serve as potential biomarkers for FM.
Circulating miRNAs Profile in FM Patients with Follow-Up Treatment
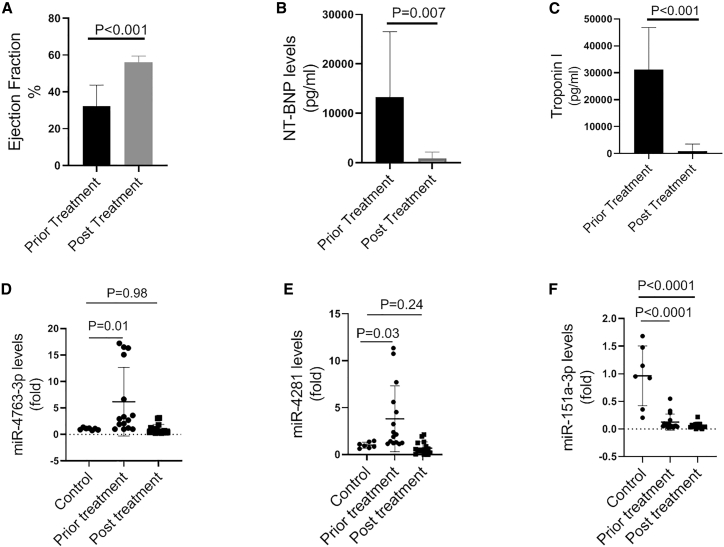
To further explore whether the levels of above miRNAs were associated with the initiation and progression of FM, we also detected the miRNAs profile in FM patients with follow-up treatment. After a systematic and comprehensive treatment, including temporary intra-aortic balloon pump (IABP) and/or extracorporeal membrane oxygenation (ECMO), cortisone and intravenous immunoglobulin (IVIg) treatment, continuous renal replacement therapy (CRRT), and anti-virus treatment (Table S8), the clinical symptom of FM was significantly improved, as evidenced by the increased left ventricular ejection fraction (LVEF) and decreased levels of NT-proBNP and cTnI (Figures 3A–3C). In line with this, the levels of miR-4763-3p and miR-4281 were restored to the normal level after proper treatments, while miR-151a-3p expression was still downregulated (Table S9). The levels of miR-4763-3p, miR-4281, and miR-151a-3p were also confirmed by quantitative real-time PCR in plasma from FM patients with follow-up treatment (Figures 3D–3F).
Figure 3.
Circulating miRNAs Profile in FM Patients with Follow-Up Treatment
(A) Left ventricular ejection fraction in FM patients before and after treatments (N = 15). (B) The concentration of NT-proBNP in FM patients before and after treatments (N = 15). (C) The concentration of cTnI in FM patients before and after treatments (N = 15). (D) The level of circulating miR-4763-3p in control or FM patients before and after treatments detected by quantitative real-time PCR (N = 15). (E) The level of circulating miR-4281 in control or FM patients before and after treatments detected by quantitative real-time PCR (N = 15). (F) The level of circulating miR-151-3p in control or FM patients before and after treatments detected by quantitative real-time PCR (N = 15).
Considering the above results, the expression of miR-4763-3p was much more specifically consistent with the progress of FM, which could potentially be biomarkers for FM diagnosis.
KEGG Pathway Analysis of Potential Biomarker miRNAs
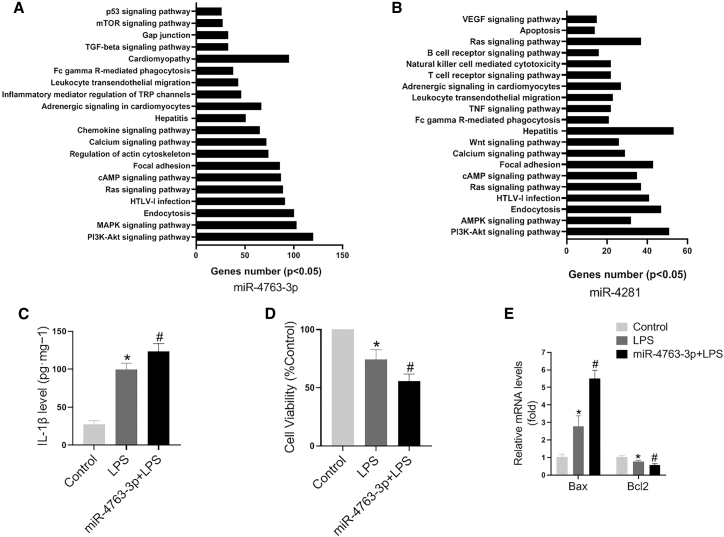
In order to gain insights into the functions of these circulating miRNAs, KEGG pathway analysis was applied to their target pool using DIANA tools. As shown in Figure 4A, the targets of miR-4763-3p were involved in many pathways, especially inflammatory reaction, such as T cells or B cell receptor, tumor necrosis factor (TNF), leukocyte transendothelial migration, and chemokine signaling pathway. Cell-survival-related pathways, such as apoptosis, vascular endothelial growth factor (VEGF), and PI3K-AKT, were also involved in the targets of miR-4281 (Figure 4B).
Figure 4.
KEGG Pathway Analysis of Potential Biomarker miRNAs
(A) The KEGG pathway analysis for miR-4763-3p target genes. (B) The KEGG pathway analysis for miR-4281 target genes. (C) IL-1β levels were detected in AC16 cells with various treatments (N = 5, ∗p < 0.05 versus control, #p < 0.05 versus LPS). (D) Cell viability was detected in AC16 cells with various treatments (N = 5, ∗p < 0.05 versus control, #p < 0.05 versus LPS). (E) Bax and Bcl2 expression levels were detected by quantitative real-time PCR in AC16 cells with various treatments (N = 5, ∗p < 0.05 versus control, #p < 0.05 versus LPS).
Moreover, the inflammation and apoptosis related functions of miR-4763-3p in cardiomyocytes were explored. Interleukin-1β (IL-1β) expression level was increased in AC16 cells after lipopolysaccharide (LPS) treatment, and miR-4763-3p promoted the increase of IL-1β expression induced by LPS (Figure 4C). Meanwhile, cell counting kit-8 (CCK-8) assays showed that miR-4763-3p aggravated the LPS-induced decrease of cell viability (Figure 4D). Furthermore, LPS treatment increased the expression levels of Bax and reduced those of Bcl2, while miR-4763-3p aggravated these effects (Figure 4E).
These indicated that miR-4763-3p could be predictable for inflammatory and cardiomyocyte injury responses, which were main characteristics of FM.
Association between Circulating miRNA Expression and the Severity of FM
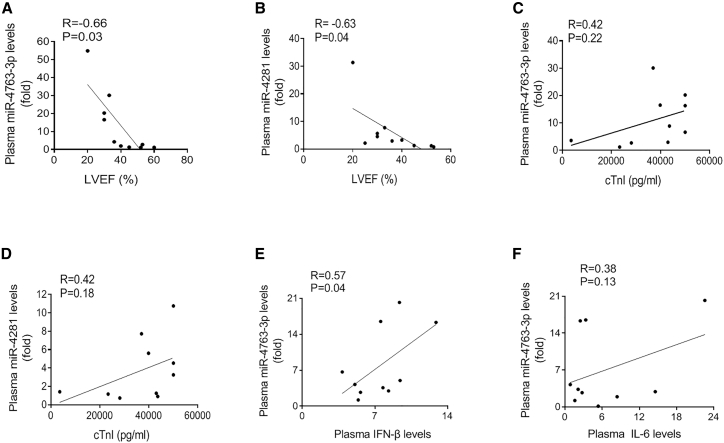
To determine whether the expression levels of circulating miR-4763-3p and miR-4281 are related with the severity of FM, we performed linear correlation analyses between miRNAs and the LVEF or cTnI levels. We found that miR-4763-3p or miR-4281 enrichment was negatively correlated with LVEF (R = −0.66, p = 0.03; R = −0.63, p = 0.04; respectively; Figures 5A and 5B). However, neither miR-4763-3p nor miR-4281 expression level was correlated with cTnI concentration (R = 0.42, p = 0.22; R = 0.42, p = 0.18; respectively; Figures 5C and 5D). Furthermore, the levels of inflammatory cytokines were also detected. Results showed that the concentration of IFN-β was positively correlated with the miR-4763-3p expression level (Figures 5E and 5F).
Figure 5.
Association between Circulating miRNA Expression and the Severity of FM
(A) The correlation between circulating miR-4763-3p level and LVEF (N = 10). (B) The correlation between circulating miR-4281 level and LVEF (N = 10). (C) The correlation between circulating miR-4763-3p level and cTnI concentration (N = 10). (D) The correlation between circulating miR-4281 level and cTnI concentration (N = 10). (E) The correlation between circulating miR-4763-3p level and interferon-β (IFN-β) concentration (N = 10). (F) The correlation between circulating miR-4763-3p level and IL-6 concentration (N = 10).
These results suggested that the abundances of circulating miR-4763-3p and miR-4281 might be a cardiomyocyte injury-independent predictive factor for FM diagnosis.
Evaluation of miRNAs in Plasma as Novel Biomarkers for FM
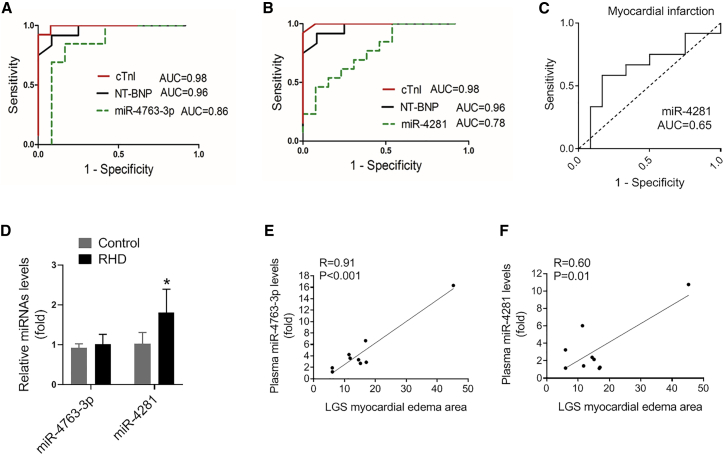
To further investigate the power of these miRNAs as potential biomarkers for FM, we performed ROC analysis in the same FM patients before and after treatment to assess the value of miR-4763-3p and miR-4281 for FM progress diagnosis.18,19 The receiver operating characteristic (ROC) curves of miR-4763-3p and miR-4281 reflected strong values, with an under curve (AUC) of 0.85 (95% confidence interval 0.68 to 1.00) and 0.78 (95% confidence interval 0.60 to 0.95), respectively (Figures 6A and 6B). As miR-4281 was also increased in the plasma of MI patients, the ROC analysis showed that the AUC of miR-4281 in MI was 0.65. These results suggested that miR-4763-3p could potentially serve as a biomarker for FM diagnosis and treatment with high sensitivity and specificity. In addition, the expression of miR-4763-3p was unchanged in the plasma from rheumatic heart disease patients with acute heart failure, while miR-4281 was slightly increased (Figure 6D). Cardiovascular magnetic resonance (CMR) was also employed according to the Lake Louise Criteria. The correlation between the expression level of miR-4763 and miR-4281 and severity of FM was reflected by edema or necrosis detected by MRI. As shown in Figures 6E and 6F, the expression level of miR-4763 was highly positively correlated with the severity of FM (R = 0.91, p < 0.001), while the expression level of miR-4281 was moderately positively correlated with the severity of FM (R = 0.60, p = 0.01).
Figure 6.
Evaluation of microRNAs in Plasma as Novel Biomarkers for FM
(A) The ROC curve for miR-4763-3p in FM patients. (B) The ROC curve for miR-4281 in FM patients. (C) The ROC curve for miR-4281 in MI patients. (D) The expression of miR-4763-3p and miR-4281 was detected by quantitative real-time PCR in the plasma of rheumatic heart disease patients with acute heart failure (∗p < 0.05 versus control). (E) The correlation between circulating miR-4763-3p level and myocardial edema area (N = 9). (F) The correlation between circulating miR-4281 level and myocardial edema area (N = 9).
Discussion
FM onsets urgently, progresses rapidly and may lead to sever heart failure or even sudden death. Timely and accurate diagnosis is in urgent need for saving life. Currently, diagnostic biomarkers for suspected myocarditis are limited. A comprehensive strategy including the history, clinical symptoms, and noninvasive test results is helpful to distinguish the disease. Previously, our group reported a Chinese society of cardiology expert consensus statement on the diagnosis and treatment of adult fulminant myocarditis based on the clinical diagnosis on FM patients from 2017 to 2018. When patients have following signs: sudden onset of disease, obvious initial symptoms of viral infection (such as fever, cough, stomachache, etc.), rapid emerging severe hemodynamic dysfunction, serious myocardial injury, and diffuse ventricular wall motion decrease, the clinical diagnosis of fulminant myocarditis can be made.2 As expected for these criteria, CMR was also employed according to the Lake Louise Criteria, evidenced by the signs of intracellular and interstitial edema, hyperemia, and capillary leakage, as well as cellular necrosis and fibrosis. If two or more of the three tissue-based criteria are positive, myocardial inflammation can be predicted with a diagnostic accuracy of 78%.20,21 Therefore, in the current study, the diagnosis of FM was mainly based on the clinical syndrome and CMR analysis according to the 2013 European Society of Cardiology position statement, 2017 Chinese Society of Cardiology expert consensus statement, and 2009 International Consensus Group on Cardiovascular Magnetic Resonance in Myocarditis statement.2,20,22
FM is identified as an inflammatory process that occurs in the myocardium and causes hemodynamic abnormality and heart failure. The pathogenesis of FM involves direct myocardial infection and indirect injuries mainly induced by immune response.23 A deep exploration of its pathogenesis is needed to accomplish early diagnosis and identify new treatment targets. Viral infections, such as enteroviruses and adenovirus, are thought to be responsible for the majority of FM.3 Less frequent causes include autoimmune disease,5,6 bacterial infection, and drugs. Endomyocardial biopsy (EMB) is widely accepted for diagnosing myocarditis, based upon histopathology, immunohistology and molecular techniques to identify exact viral genomes.24 However, the limitations of EMB also have to be considered, such as the samples error, which might produce false-negative results due to the missing of inflammation area.25,26 Meanwhile, as a few lymphocytes reside in interstitium under normal condition, the number of interstitial inflammatory cells to confirm the diagnosis of lymphocytic myocarditis is uncertain,27 whereas the overall complication rate is 6%; for example, perforation and tamponade.24,28 Due to various reasons, endomyocardial biopsy was only performed in limited patients, but not all the patients in the current study (data not shown). Additionally, virus serological test was detected in some FM patients in the current study. The detailed patient information was list in Tables S1–S7. In addition, a recent study showed that circular RNAs (circRNAs) might have substantial roles in pediatric FM and could serve as promising biomarkers.29 In general, diagnostic biomarkers for suspected myocarditis are limited.
miRNAs play significant roles in cardiovascular diseases and could serve as diagnosis biomarkers. FM is a kind of rapidly progressive disease that needs rapid diagnosis. Traditional quantitative real-time PCR analysis needs at least 1.5 h to detect the miRNA expression. However, adaptions of the standard qRT-PCR techniques have been established recently. With the advances of technology, new quantitative real-time PCR method and instrumentation was produced by multiple biotechnology companies, such as Cephid GeneXpert, BioFire FilmArray, and Roche Cobas Liat, and the detection time could be shortened to 30 min. Nevertheless, the expression pattern and functions of miRNAs in FM remain elusive. In order to find more specific and sensitive biomarkers for FM diagnosis, miRNA profiles were performed in plasma samples from FM patients in the current study. Here, we found that circulating miR-4763-3p and miR-4281 were upregulated in FM patients and restored to the normal level when the clinical symptom was recovery. Recent studies have shown that IL-2 upregulates miR-4281 expression, and miR-4281 facilitates iTreg development by enhancing FOXP3 expression.30 Global miRNA expression analysis shows that miR-4281 is specifically expressed in both naive and memory Tregs.31 Meanwhile, KEGG pathway analysis for miR-4763-3p and miR-4281 target genes mainly contributed to inflammation and immune response. These findings preliminarily suggested that miR-4763-3p and miR-4281 might contribute to FM via these pathways. Moreover, the expression of miR-4763-3p and miR-4281 was correlated with the severity of FM, characterized by LVEF. In addition, the expression of miR-4763-3p was unchanged in rheumatic heart disease patients with acute heart failure, while miR-4281 was slightly increased and ROC analysis showed that miR-4763-3p were specific and sensitive for FM diagnosis.
In the current study, we found that miR-4281 expression was elevated in the plasma of FM and MI patients, and it was negatively correlated with LVEF value, which indicated that miR-4281 was related to myocardial injury. However, no correlation was observed between miR-4281 and cTnI. The reasons might be as follows: (1) the source of miR-4281 and cTnI may be different. miR-4281 could be derived from cardiomyocytes, endothelial cells, or immune cells. cTnI is mainly derived from cardiomyocytes, which is usually considered as a specific biomarker for cardiomyocyte injury. (2) The elevated expression of cTnI in the plasma is usually released by cardiomyocytes due to cell death such as apoptosis and necrosis in myocardial infarction.32,33 The cardiomyocytes prefer dysfunction rather than death in the process of FM, and heart function could return to normal after proper treatment.2,34 The underlying mechanisms of miR-4281 expression regulation in blood are unclear, except for passive release after cell injury, and various stresses such as inflammatory response may induce active release of miR-4281 via exosomes or other ways. (3) The half-time of miR-4281 and cTnI in plasma might be different, which may lead to the different expression patterns during the disease process. We collected the samples at their admission, but the disease process might be various among the patients.
Moreover, the source of these miRNAs needs further investigation, which is important to reveal the molecular mechanism of FM. miR-4763-3p and miR-4281 are not well conservative among species (mice, rat, and human), so animal models may not truly reflect their distribution in human organs. According to the UCSC database and miRBase database, human miR-4763-3p is widely distributed in immune system, nervous system, muscle, and heart in humans. However, there are no distribution data of miR-4281 and miR-151a-3p in the database. To explore the source of these dysregulated miRNAs in plasma, the tissues such as heart, muscle, spleen, or lymph node from FM patients should be collected and detected. Moreover, heart tissue contains cardiomyocytes, endothelial cells, fibroblasts, immune cells, etc., all of which may be involved in the process of FM.
In conclusion, our data suggested that miR-4763-3p might be potential biomarkers for FM.
Materials and Methods
Patient and Public Involvement Statement
Patients with FM admitted to Tongji Hospital between 2017 and 2018 were recruited in this study. The inclusion criteria for patients with FM were according to the 2013 European Society of Cardiology position statement, 2017 Chinese Society of Cardiology expert consensus statement, and 2009 International Consensus Group on Cardiovascular Magnetic Resonance in Myocarditis statement.2,20,22 These patients were clinically diagnosed by sudden onset of disease, obvious initial symptoms of viral infection, rapid emerging severe hemodynamic dysfunction, myocardial injury, and diffuse ventricular wall motion decrease. This study was approved by the Ethics Review Board of Tongji Hospital and Tongji Medical College and conformed to the principles of the Declaration of Helsinki (IRB ID: TJ-C20160202). Informed consents were signed by the subjects recruited in the study or by family members in case of incapacity.
To be specific, the diagnostic criteria for FM were as follows: (1) rapid onset of symptoms of acute HF within less than 2 weeks; (2) severe hemodynamic compromise requiring high doses of vasopressors (≥5 μg/kg/min of dopamine or dobutamine); (3) use of mechanical circulatory supports, such as intra-aortic balloon pumps and/or venoarterial extracorporeal membrane oxygenation, in the early phase (day 1 or 2 of admission); and (4) presence of myocarditis confirmed by cardiovascular magnetic resonance (CMR) performed before discharge. Only CMR-confirmed patients were enrolled. Coronary angiography was performed in patients older than 25 years to rule out AMI. The exclusion criteria were: (1) patients younger than 11 years; (2) patients with possible acute coronary syndrome, but unable to undergo coronary angiography to distinguish the two conditions; (3) patients with myocardial injury caused by sepsis, chemotherapeutical agents, or poison; (4) patients with unstable hemodynamics or shock caused by hypovolemia.
RNA Extraction
Plasma samples for miRNA microarray analysis were collected from patients in Division of Cardiology, Tongji Hospital, and stored at −80°C until use. Total RNA was isolated using TRIzol-LS (Invitrogen, Carlsbad, CA) and purified with RNeasy mini kit (QIAGEN, Shanghai, China) according to the manufacturer’s instructions. RNA quantity and quality were measured by NanoDrop ND-1000. The ratio of the absorbance at 260 and 280 nm (optical density [OD] 260/280) of isolated RNA was between 1.8 and 1.9.
miRNA Microarray
Sample labeling and array hybridization were performed by Kangcheng Biotechnology (Shanghai, China) according to the Agilent miRNA Microarray System with miRNA Complete Labeling and Hyb Kit protocol (Agilent Technology). Briefly, total miRNA from each sample was labeled with cyanine 3-pCp under the action of T4 RNA ligase. 1 μg of each labeled RNA was fragmented by adding 11 μL 10 × blocking agent and 2.2 μL of 25× fragmentation buffer, then heated at 60°C for 30 min, and finally 55 μL 2 × GE hybridization buffer was added to dilute the labeled RNA. 100 μL of hybridization solution was dispensed into the gasket slide and assembled to the gene expression microarray slide. The slides were incubated for 17 h at 65°C in an Agilent hybridization oven. The hybridized arrays were washed, fixed, and scanned using the Agilent microarray scanner (part number G2505C). Scanned images were then imported into GenePix Pro 6.0 software (Axon) for grid alignment and data extraction. The content source of this human miRNA microarray slide was from miRBase database (Release 21.0) by targeting 2,549 human miRNAs, of which 1,711 miRNAs were detected in our samples. The detection rate of miRNAs is about 67%. The microarray data was submitted to GEO database and the access number was GEO: GSE148153.
Validation by Quantitative Real-Time PCR
Total RNA from each sample was converted into cDNA using Bulge-Loop miRNA qRT-PCR Starter Kit (Ribobio, Guangzhou, China). The expression of miRNAs was evaluated by quantitative real-time PCR analysis according to the manufacturer’s protocol. Cel-miR-39-3p was used as the exogenous control. The expression of miRNAs was quantified by quantitative real-time PCR using Power SYBR Green PCR Master Mix (Invitrogen, Carlsbad, CA) on a 7900HT FAST real-time PCR system (Life Technologies, Carlsbad, CA). Quantitative PCR was performed using standard settings: 95°C (10 min), 40 cycles of 95°C (10 s), 60°C (10 s), and 72°C (15 s). The primers of miRNAs were designed and synthesized by Ribobio (Guangzhou, China). PCR reactions were performed in a total volume of 10 μL containing of 1 μL of 100 ng/μL sample cDNA, 5 μL of 2× SYBR Green Mix, 0.5 μL of 5 μM of miRNA forward primer, 0.5 μL of 5 μM reverse primer, and 3 μL of RNase/DNase free water. Relative expression levels were calculated with the 2-ΔΔct relative quantification method as previously described.35
Bioinformatic Analysis of miRNAs Targets and Pathway
The target genes of miRNAs were predicted by TargetScan (http://www.targetscan.org/vert_72/). DAVID Bioinformatics Resources was employed for KEGG pathway analysis of selected genes. p < 0.05 was considered statistically significant.
Culture of AC16 Cells
The cell line of human cardiomyocytes (AC16) was cultured in DMEM supplemented with 10% fetal bovine serum at 37°C in humidified air containing 5% of CO2. Cells were seeded into a 24-well or 96-well plates. The cells were transfected with miRNA mimics for 12 h followed by LPS treatment for another 12 h. Then AC16 cells were collected and analyzed.
IL-1β Assay
The levels of IL-1β were detected by the human IL-1β ELISA kit (Boster, Shanghai, China) in AC16 cells. The cells were transfected with miRNA mimics for 12 h followed by LPS treatment for another 12 h. The levels of IL-1β were determined by specific enzyme immunoassay kits in accordance with the manufacturer’s instructions.
Cell Viability Assay
AC16 cells were seeded into 96-well plates. Then cells were transfected with miRNA mimics for 12 h followed by LPS treatment for another 12 h. Then, cell viability was assessed by CCK-8 assay (Beyotime, Shanghai, China) according to the manufacturer’s protocol.
Statistical Analysis
Data are described as mean ± SD. All of these analyses were performed on GraphPad Prism 8.0 (GraphPad software, CA). A paired t test was used for analyzing the paired data or by unpaired t test around the premise that the data was normal distribution and with equal variance. For variables without normal distribution, Mann-Whitney U test was performed. Person correlation analyses were conducted to evaluate the relationships between candidate miRNAs and clinical parameters. The ROC curves were used to assess miRNAs as diagnostic tools for distinguishing FM. p < 0.05 was considered statistically significant.
Author Contributions
X.N. and M.H. designed the study, analyzed and interpreted the data, and drafted the paper. J.W., P.C., F.W., J.L., C.L., T.Y., H.Z., G.C., K.M., and J.J. contributed to data acquisition. D.W.W. and C.C. designed the study and drafted the paper.
Conflicts of interest
The authors declare no competing interests.
Acknowledgments
We thank our colleagues from the Division of Cardiology, Tongji Hospital, for technical assistance and stimulating discussions during this investigation. This work was supported by grants from the National Natural Science Foundation of China (81822002 and 31771264 to C.C. and 91839302, 81630010, and 81790624 to D.W.W.) and Tongji Hospital Clinical Research Flagship Program (2019CR207 to D.W.W.). No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtm.2020.05.005.
Contributor Information
Dao Wen Wang, Email: dwwang@tjh.tjmu.edu.cn.
Chen Chen, Email: chenchen@tjh.tjmu.edu.cn.
Supplemental Information
References
- 1.Ammirati E., Veronese G., Cipriani M., Moroni F., Garascia A., Brambatti M., Adler E.D., Frigerio M. Acute and Fulminant Myocarditis: a Pragmatic Clinical Approach to Diagnosis and Treatment. Curr. Cardiol. Rep. 2018;20:114. doi: 10.1007/s11886-018-1054-z. [DOI] [PubMed] [Google Scholar]
- 2.Wang D., Li S., Jiang J., Yan J., Zhao C., Wang Y., Ma Y., Zeng H., Guo X., Wang H., Section of Precision Medicine Group of Chinese Society of Cardiology. Editorial Board of Chinese Journal of Cardiology. Working Group of Adult Fulminant Myocarditis Chinese society of cardiology expert consensus statement on the diagnosis and treatment of adult fulminant myocarditis. Sci. China Life Sci. 2019;62:187–202. doi: 10.1007/s11427-018-9385-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta S., Markham D.W., Drazner M.H., Mammen P.P. Fulminant myocarditis. Nat. Clin. Pract. Cardiovasc. Med. 2008;5:693–706. doi: 10.1038/ncpcardio1331. [DOI] [PubMed] [Google Scholar]
- 4.Al-Amoodi M., Rao K., Rao S., Brewer J.H., Magalski A., Chhatriwalla A.K. Fulminant myocarditis due to H1N1 influenza. Circ. Heart Fail. 2010;3:e7–e9. doi: 10.1161/CIRCHEARTFAILURE.110.938506. [DOI] [PubMed] [Google Scholar]
- 5.Berg D.D., Vaduganathan M., Nohria A., Davids M.S., Alyea E.P., Torre M., Padera R.F., Jr. Immune-related fulminant myocarditis in a patient receiving ipilimumab therapy for relapsed chronic myelomonocytic leukaemia. Eur. J. Heart Fail. 2017;19:682–685. doi: 10.1002/ejhf.806. [DOI] [PubMed] [Google Scholar]
- 6.Liu W., Nakamura H., Shioji K., Tanito M., Oka S., Ahsan M.K., Son A., Ishii Y., Kishimoto C., Yodoi J. Thioredoxin-1 ameliorates myosin-induced autoimmune myocarditis by suppressing chemokine expressions and leukocyte chemotaxis in mice. Circulation. 2004;110:1276–1283. doi: 10.1161/01.CIR.0000141803.41217.B6. [DOI] [PubMed] [Google Scholar]
- 7.Altesha M.A., Ni T., Khan A., Liu K., Zheng X. Circular RNA in cardiovascular disease. J. Cell. Physiol. 2019;234:5588–5600. doi: 10.1002/jcp.27384. [DOI] [PubMed] [Google Scholar]
- 8.Hamm C.W., Goldmann B.U., Heeschen C., Kreymann G., Berger J., Meinertz T. Emergency room triage of patients with acute chest pain by means of rapid testing for cardiac troponin T or troponin I. N. Engl. J. Med. 1997;337:1648–1653. doi: 10.1056/NEJM199712043372302. [DOI] [PubMed] [Google Scholar]
- 9.Mukoyama M., Nakao K., Hosoda K., Suga S., Saito Y., Ogawa Y., Shirakami G., Jougasaki M., Obata K., Yasue H. Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J. Clin. Invest. 1991;87:1402–1412. doi: 10.1172/JCI115146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai E.C. Micro RNAs are complementary to 3′ UTR sequence motifs that mediate negative post-transcriptional regulation. Nat. Genet. 2002;30:363–364. doi: 10.1038/ng865. [DOI] [PubMed] [Google Scholar]
- 11.Yuan W., Tang C., Zhu W., Zhu J., Lin Q., Fu Y., Deng C., Xue Y., Yang M., Wu S., Shan Z. CDK6 mediates the effect of attenuation of miR-1 on provoking cardiomyocyte hypertrophy. Mol. Cell. Biochem. 2016;412:289–296. doi: 10.1007/s11010-015-2635-4. [DOI] [PubMed] [Google Scholar]
- 12.Kim J., Kwon J., Kim M., Do J., Lee D., Han H. Low-dielectric-constant polyimide aerogel composite films with low water uptake. Polym. J. 2016;48:829–834. [Google Scholar]
- 13.Goren Y., Kushnir M., Zafrir B., Tabak S., Lewis B.S., Amir O. Serum levels of microRNAs in patients with heart failure. Eur. J. Heart Fail. 2012;14:147–154. doi: 10.1093/eurjhf/hfr155. [DOI] [PubMed] [Google Scholar]
- 14.Li H., Fan J., Yin Z., Wang F., Chen C., Wang D.W. Identification of cardiac-related circulating microRNA profile in human chronic heart failure. Oncotarget. 2016;7:33–45. doi: 10.18632/oncotarget.6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan J., Li H., Nie X., Yin Z., Zhao Y., Zhang X., Yuan S., Li Y., Chen C., Wang D.W. MiR-665 aggravates heart failure via suppressing CD34-mediated coronary microvessel angiogenesis. Aging (Albany NY) 2018;10:2459–2479. doi: 10.18632/aging.101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang G.K., Zhu J.Q., Zhang J.T., Li Q., Li Y., He J., Qin Y.W., Jing Q. Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur. Heart J. 2010;31:659–666. doi: 10.1093/eurheartj/ehq013. [DOI] [PubMed] [Google Scholar]
- 17.Devaux Y., Salgado-Somoza A., Dankiewicz J., Boileau A., Stammet P., Schritz A., Zhang L., Vausort M., Gilje P., Erlinge D., TTM-trial investigators Incremental Value of Circulating MiR-122-5p to Predict Outcome after Out of Hospital Cardiac Arrest. Theranostics. 2017;7:2555–2564. doi: 10.7150/thno.19851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim S.Y., Lee J.H., Gide T.N., Menzies A.M., Guminski A., Carlino M.S., Breen E.J., Yang J.Y.H., Ghazanfar S., Kefford R.F. Circulating Cytokines Predict Immune-Related Toxicity in Melanoma Patients Receiving Anti-PD-1-Based Immunotherapy. Clin. Cancer Res. 2019;25:1557–1563. doi: 10.1158/1078-0432.CCR-18-2795. [DOI] [PubMed] [Google Scholar]
- 19.Jiang L., Lee S.C., Ng T.C. Pharmacometabonomics Analysis Reveals Serum Formate and Acetate Potentially Associated with Varying Response to Gemcitabine-Carboplatin Chemotherapy in Metastatic Breast Cancer Patients. J. Proteome Res. 2018;17:1248–1257. doi: 10.1021/acs.jproteome.7b00859. [DOI] [PubMed] [Google Scholar]
- 20.Friedrich M.G., Sechtem U., Schulz-Menger J., Holmvang G., Alakija P., Cooper L.T., White J.A., Abdel-Aty H., Gutberlet M., Prasad S., International Consensus Group on Cardiovascular Magnetic Resonance in Myocarditis Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J. Am. Coll. Cardiol. 2009;53:1475–1487. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yilmaz A., Ferreira V., Klingel K., Kandolf R., Neubauer S., Sechtem U. Role of cardiovascular magnetic resonance imaging (CMR) in the diagnosis of acute and chronic myocarditis. Heart Fail. Rev. 2013;18:747–760. doi: 10.1007/s10741-012-9356-5. [DOI] [PubMed] [Google Scholar]
- 22.Caforio A.L., Pankuweit S., Arbustini E., Basso C., Gimeno-Blanes J., Felix S.B., Fu M., Helio T., Heymans S., Jahns R. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648. doi: 10.1093/eurheartj/eht210. [DOI] [PubMed] [Google Scholar]
- 23.Maisch B., Ruppert V., Pankuweit S. Management of fulminant myocarditis: a diagnosis in search of its etiology but with therapeutic options. Curr. Heart Fail. Rep. 2014;11:166–177. doi: 10.1007/s11897-014-0196-6. [DOI] [PubMed] [Google Scholar]
- 24.Anderson L., Pennell D. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Eur. Heart J. 2008;29 doi: 10.1093/eurheartj/ehn189. 1696–1696. [DOI] [PubMed] [Google Scholar]
- 25.Yan M., Chen C., Gong W., Yin Z., Zhou L., Chaugai S., Wang D.W. miR-21-3p regulates cardiac hypertrophic response by targeting histone deacetylase-8. Cardiovasc. Res. 2015;105:340–352. doi: 10.1093/cvr/cvu254. [DOI] [PubMed] [Google Scholar]
- 26.Chow L.H., Radio S.J., Sears T.D., McManus B.M. Insensitivity of right ventricular endomyocardial biopsy in the diagnosis of myocarditis. J. Am. Coll. Cardiol. 1989;14:915–920. doi: 10.1016/0735-1097(89)90465-8. [DOI] [PubMed] [Google Scholar]
- 27.Hauck A.J., Kearney D.L., Edwards W.D. Evaluation of postmortem endomyocardial biopsy specimens from 38 patients with lymphocytic myocarditis: implications for role of sampling error. Mayo Clin. Proc. 1989;64:1235–1245. doi: 10.1016/s0025-6196(12)61286-5. [DOI] [PubMed] [Google Scholar]
- 28.Feng B., Chen S., Gordon A., Chakrabarti S. Anti-inflammatory and Antifibrotic Effects of Endothelial miRNA-146 in the Heart in Diabetes. Diabetes. 2016;65 A125–A125. [Google Scholar]
- 29.Zhang L., Han B., Wang J., Liu Q., Kong Y., Jiang D., Jia H. Differential expression profiles and functional analysis of circular RNAs in children with fulminant myocarditis. Epigenomics. 2019;11:1129–1141. doi: 10.2217/epi-2019-0101. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y., Liu W., Chen Y., Liu J., Wu K., Su L., Zhang W., Jiang Y., Zhang X., Zhang Y. A Cellular MicroRNA Facilitates Regulatory T Lymphocyte Development by Targeting the FOXP3 Promoter TATA-Box Motif. J. Immunol. 2018;200:1053–1063. doi: 10.4049/jimmunol.1700196. [DOI] [PubMed] [Google Scholar]
- 31.Smigielska-Czepiel K., van den Berg A., Jellema P., van der Lei R.J., Bijzet J., Kluiver J., Boots A.M., Brouwer E., Kroesen B.J. Comprehensive analysis of miRNA expression in T-cell subsets of rheumatoid arthritis patients reveals defined signatures of naive and memory Tregs. Genes Immun. 2014;15:115–125. doi: 10.1038/gene.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sandoval Y., Nowak R., deFilippi C.R., Christenson R.H., Peacock W.F., McCord J., Limkakeng A.T., Sexter A., Apple F.S. Myocardial Infarction Risk Stratification With a Single Measurement of High-Sensitivity Troponin I. J. Am. Coll. Cardiol. 2019;74:271–282. doi: 10.1016/j.jacc.2019.05.058. [DOI] [PubMed] [Google Scholar]
- 33.Thygesen K., Alpert J.S., Jaffe A.S., Chaitman B.R., Bax J.J., Morrow D.A., White H.D., Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction Fourth Universal Definition of Myocardial Infarction (2018) J. Am. Coll. Cardiol. 2018;72:2231–2264. [Google Scholar]
- 34.Caforio A.L.P., Malipiero G., Marcolongo R., Iliceto S. Myocarditis: A Clinical Overview. Curr. Cardiol. Rep. 2017;19:63. doi: 10.1007/s11886-017-0870-x. [DOI] [PubMed] [Google Scholar]
- 35.Nie X., Fan J., Li H., Yin Z., Zhao Y., Dai B., Dong N., Chen C., Wang D.W. miR-217 Promotes Cardiac Hypertrophy and Dysfunction by Targeting PTEN. Mol. Ther. Nucleic Acids. 2018;12:254–266. doi: 10.1016/j.omtn.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.