Abstract
Background
As part of a randomised controlled trial of treatment with placebo versus 3 days of amoxicillin for nonsevere fast-breathing pneumonia among Malawian children aged 2–59 months, a subset of children was hospitalised for observation. We sought to characterise the progression of fast-breathing pneumonia among children undergoing repeat assessments to better understand which children do and do not deteriorate.
Methods
Vital signs and physical examination findings, including respiratory rate, arterial oxygen saturation measured by pulse oximetry (SpO2), chest indrawing and temperature were assessed every 3 h for the duration of hospitalisation. Children were assessed for treatment failure during study visits on days 1, 2, 3 and 4.
Results
Hospital monitoring data from 436 children were included. While no children had SpO2 90–93% at baseline, 7.4% (16 of 215) of children receiving amoxicillin and 9.5% (21 of 221) receiving placebo developed SpO2 90–93% during monitoring. Similarly, no children had chest indrawing at enrolment, but 6.6% (14 of 215) in the amoxicillin group and 7.2% (16 of 221) in the placebo group went on to develop chest indrawing during hospitalisation.
Conclusion
Repeat monitoring of children with fast-breathing pneumonia identified vital and physical examination signs not present at baseline, including SpO2 90–93% and chest indrawing. This information may support providers and policymakers in developing guidance for care of children with nonsevere pneumonia.
Short abstract
This study characterised the progression of fast-breathing pneumonia among children in Malawi. Repeat monitoring of children identified vital and physical exam signs not present at baseline, including oxygen saturation of 90–93% and chest indrawing. http://bit.ly/2vUlckS
Introduction
Nearly one million children worldwide die of pneumonia each year [1]. Despite its prevalence, pneumonia remains difficult to diagnose, particularly in low-resource settings (LRS), where diagnostic imaging is often unavailable [2]. A challenge in identifying pneumonia in the absence of diagnostics lies in differentiating those children with pneumonia and more severe illness from those with upper respiratory infections or other less severe conditions [3]. In LRS, pneumonia typically is diagnosed using the World Health Organization (WHO) Integrated Management of Childhood Illnesses (IMCI) requirements, which identify and treat pneumonia based on clinical signs and symptoms such as fast breathing and chest indrawing in children under 5 years with cough and/or difficulty breathing [4]. Previous research suggests that the diagnosis of pneumonia based on these subjective signs and symptoms is challenging, can be nonspecific, and may be less reliable than imaging methods [2, 5].
Given this low specificity, it is challenging to differentiate which children will deteriorate and which will improve. Several studies have sought to develop risk-score models to identify clinical signs that predict progression [2, 3, 6, 7]. These studies have identified factors, including very fast breathing and low arterial oxygen saturation measured by pulse oximetry (SpO2). However, most of this work uses measurements from a single time point, or daily measurements. Using inpatient monitoring data collected every 3 h as part of a randomised controlled trial of treatment with placebo versus 3 days of amoxicillin for fast-breathing pneumonia, we sought to analyse the progression of vital signs and physical examination findings among children aged 2–59 months diagnosed with fast-breathing pneumonia. Characterising the progression of pneumonia among children undergoing frequent serial assessments could make strides in better understanding which children deteriorate and which children do not, and the potential value of repeated evaluations. With this analysis, we seek to build on this body of work with longitudinal data collected every 3 h among children in Malawi diagnosed with fast-breathing pneumonia.
Methods
Participants and study design
Data for this analysis were obtained during a prospective, double-blind, randomised controlled two-arm, noninferiority trial that aimed to determine whether treatment with placebo in children uninfected with HIV, 2–59 months of age with nonsevere fast-breathing pneumonia was as effective as 3 days of treatment with amoxicillin [8, 9]. Children aged 2–59 months presenting to the outpatient departments of Kamuzu Central Hospital and Bwaila District Hospital in Lilongwe, Malawi with cough <14 days or difficulty breathing and fast breathing for age were enrolled in the study. A full list of inclusion and exclusion criteria is included in table 1. Children enrolled in the study were randomised to receive 3 days of either placebo (intervention) or amoxicillin (control) [10]. Study visits took place at days 1 (enrolment), 2, 3, 4 and 14.
TABLE 1.
Eligibility criteria
| Inclusion criteria |
|
| Exclusion criteria |
|
SpO2: arterial oxygen saturation measured by pulse oximetry; WHO: World Health Organization; IMCI: Integrated Management of Childhood Illnesses.
Procedures
Informed consent was obtained from caregivers of children prior to screening and again prior to enrolment. Study staff described the study purpose, procedures, risks and benefits. Caregivers were encouraged to ask questions and a comprehension checklist was administered prior to obtaining consent to ensure that caregivers fully comprehended the nature of the study.
Once enrolled, children were observed in the hospital for 2–8 h prior to being assessed for discharge. Children without fever or very fast breathing were discharged after 2 h, whereas those with fever and/or very fast breathing remained under observation. Children aged <6 months, those with moderate malnutrition (11.5–13.5 cm mid-upper arm circumference), and those with fever and a negative malaria test were hospitalised overnight and assessed for discharge on day 2 [11]. Observations were conducted by study nurses with immediate referral to a study clinician if further evaluation was needed. Children could remain under hospital observation until the morning of day 3. Those whose condition necessitated hospitalisation past the morning of day 3 were considered to have prolonged hospitalisation and met criteria to be classified as treatment failure (TF). Additional TF criteria are described in table 2.
TABLE 2.
Treatment failure criteria and hospital discharge criteria
| Treatment failure criteria |
|
| Hospital discharge criteria |
|
WHO: World Health Organization; IMCI: Integrated Management of Childhood Illnesses.
In addition to study assessments, vital signs (respiratory rate (RR), SpO2, and temperature), other respiratory signs (wheeze or stridor when calm), signs of severe respiratory distress (head nodding, nasal flaring, grunting, chest indrawing), and WHO IMCI general danger signs (lethargy or unconsciousness, convulsions, vomiting everything, and inability to drink or breastfeed) were assessed by study nurses every 3 h.
Children were assessed for TF by a study clinician during the observation period following enrolment. During observation, if a child developed a WHO IMCI general danger sign or sign of severe respiratory distress, hypoxaemia (SpO2 <90%), or RR 10 breaths higher than RR at enrolment, the child was hospitalised and considered a TF. Children were also assessed for TF during the study visits on days 1, 2, 3 and 4. TF was defined as any of the following on or before day 4: WHO IMCI general danger sign, severe respiratory distress, hypoxaemia, missing ≥2 study drug doses due to vomiting, change in antibiotics prescribed by a study clinician, hospitalisation due to pneumonia (if not initially admitted), prolonged hospitalisation or re-admission due to pneumonia, and death (table 2). Children determined to have TF were hospitalised and received second-line therapy based on standard of care at Kamuzu Central Hospital.
Statistical analysis
Measurements collected every 3 h during the hospital monitoring assessments described above were used for analysis. Data from the observation period and the hospitalisation immediately following enrolment (for those children admitted) were included. For children classified as having TF, hospital monitoring data from checks that occurred after a TF designation were excluded from the analysis.
Descriptive statistics were calculated regarding vital signs, signs of severe respiratory distress, and WHO IMCI general danger signs during the duration of this observation/hospitalisation. Progression of these signs over the first 24 h following enrolment was also described. In addition to the standard definition of hypoxaemia of SpO2 <90%, an SpO2 range of 90–93% was also assessed [2, 12–16].
Mean values at baseline were compared using two-sided t-tests assuming unequal variances. Differences in categorical variables were assessed using a chi-squared test, except for in analyses where the expected number in any cell was less than five, in which case Fisher's exact tests were used. The ability of SpO2, fever and fast breathing (measured during the first 24 h) to predict TF was calculated using receiver operating characteristic curves. All analyses were stratified by whether children were in the amoxicillin or placebo group, and further divided by whether children did or did not experience TF. No adjustments were made for multiple comparisons. Analyses were performed using Stata (Stata Corporation, College Station, TX, USA).
Ethical approvals
This study was conducted in accordance with the International Conference on Harmonisation, Good Clinical Practice and the Declaration of Helsinki 2008, and was approved by the Western Institutional Review Board in the state of Washington, USA; the College of Medicine Research and Ethics Committee, Blantyre, Malawi; and the Malawi Pharmacy, Medicines and Poisons Board. The study was registered at ClinicalTrials.gov under identifier NCT02760420.
Results
Enrolment in this study took place from June 2016 to June 2017. Overall, 1126 children were enrolled and 436 (38.7%) were hospitalised for observation. The duration of monitoring ranged from 2 h to 7 days (median=23.8 h; IQR=22.6–25.9). TF was documented for 48 children (11.0% of hospitalised children), comprising 16 children in the amoxicillin group and 32 children in the placebo group.
Baseline characteristics
In the amoxicillin group, most baseline characteristics were similar between children with and without TF, (table 3). However, while the sample was approximately equally split between males and females, 13 male children (80%) went on to have TF compared to 3 female children (20%) (p=0.017). Occurrence of fever also differed between the TF and non-TF groups, where 111 children (55.5%) in the TF group had fever at enrolment compared with 4 (26.7%) in the non-TF group (p=0.03). In the placebo group, there were no significant differences at baseline between the TF and non-TF groups.
TABLE 3.
Characteristics of enrolled children undergoing hospital monitoring
| Characteristic | Amoxicillin | Placebo | ||||||
| Overall (n=215) | No treatment failure (n=199) | Treatment failure (n=16) | p-value | Overall (n=221) | No treatment failure (n=189) | Treatment failure (n=32) | p-value | |
| Age months | 15.4±14.8 | 15.2±14.7 | 17.4±16.4 | 0.57 | 15.8±13.6 | 15.4±13.7 | 18.3±12.8 | 0.25 |
| 2–11 | 120 (55.8%) | 111 (55.8%) | 9 (56.3%) | 1.00 | 114 (51.6%) | 103 (54.5%) | 11 (34.4%) | 0.11 |
| 12–35 | 67 (31.2%) | 62 (31.1%) | 5 (31.3%) | 80 (36.2%) | 64 (33.9%) | 16 (50.0%) | ||
| 36–59 | 28 (13.0%) | 26 (13.1%) | 2 (12.4%) | 27 (12.2%) | 22 (11.6%) | 5 (15.6%) | ||
| Female | 108 (50.2%) | 105 (52.5%) | 3 (20.0%) | 0.017 | 123 (55.7%) | 104 (55.0%) | 19 (59.4%) | 0.65 |
| MUAC cm | 14.8±1.2 | 15.0±2.2 | 14.8±1.2 | 0.54 | 14.6±1.2 | 14.6±1.2 | 14.6±1.0 | 0.77 |
| <11.5 | 0 | 0 | 0 | 0.14 | 0 | 0 | 0 | 1.00 |
| 11.5–13.5 | 30 (14.0%) | 30 (15.1%) | 0 | 45 (30.4%) | 39 (20.6%) | 6 (18.8%) | ||
| >13.5 | 185 (86.0%) | 169 (84.9%) | 16 (100%) | 176 (79.6%) | 150 (79.4%) | 26 (81.2%) | ||
| Respiratory rate# breaths·min−1 | ||||||||
| Age 2–11 months¶ | 57.3±6.0 | 57.1±6.0 | 59.3±5.8 | 0.30 | 56.8±5.7 | 56.9±5.9 | 55.9±3.6 | 0.58 |
| <50 | 0 | 0 | 0 | 0.21 | 0 | 0 | 0 | 0.64 |
| 50–59 | 77 (64.2%) | 73 (65.8%) | 4 (44.4%) | 79 (69.3%) | 70 (68.0%) | 9 (81.8%) | ||
| 60–69 | 38 (31.7%) | 34 (30.6%) | 4 (44.4%) | 32 (28.1%) | 30 (29.1%) | 2 (18.2%) | ||
| ≥70 | 5 (4.2%) | 4 (3.6%) | 1 (1.1%) | 3 (2.6%) | 3 (2.9%) | 0 | ||
| Age 12–59 months | 48.9 (7.1) | 49.1 (7.0) | 46.3 (8.4) | 0.32 | 48.5 (7.1%) | 48.2 (7.0%) | 49.7 (7.4) | 0.37 |
| <40 | 0 | 0 | 0 | 0.10 | 0 | 0 | 0 | 0.52 |
| 40–49 | 55 (57.9%) | 49 (55.7%) | 6 (85.7%) | 64 (59.8%) | 53 (61.6%) | 11 (52.4%) | ||
| 50–59 | 32 (33.7%) | 32 (36.4%) | 0 | 37 (34.6%) | 29 (33.7%) | 8 (38.1%) | ||
| ≥60 | 8 (8.4%) | 7 (7.9%) | 1 (14.3%) | 6 (5.6%) | 4 (4.7%) | 2 (9.5%) | ||
| Oxygen saturation¶ | 98 (97–99) | 98.5 (97–99) | 98 (97–99) | 0.21 | 98 (97–99) | 98 (97–99) | 98 (97–99) | 0.89 |
| <90% | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 90–93% | 0 | 0 | 0 | 0 | 0 | 0 | ||
| ≥94% | 215 (100%) | 199 (100%) | 16 (100%) | 221 (100%) | 189 (100%) | 32 (100%) | ||
| Axillary temperature# °C | 37.9±1.0 | 38.0±1.0 | 37.7±0.9 | 0.27 | 37.8±1.0 | 37.8±1.0 | 37.8±0.9 | 0.91 |
| <38 | 100 (46.5%) | 89 (44.5%) | 11 (73.3%) | 0.03 | 112 (50.7%) | 92 (48.7%) | 20 (62.5%) | 0.15 |
| ≥38 | 115 (53.5%) | 111 (55.5%) | 4 (26.7%) | 109 (49.3%) | 97 (51.3%) | 12 (37.5%) | ||
| Heart rate# beats·min−1 | 151.3±14.3 | 151.1±14.1 | 153.9±17.0 | 0.45 | 150.2±13.7 | 150.5±14.1 | 148.7±11.2 | 0.51 |
Data are mean±sd, n (%) or median (interquartile range), unless otherwise stated. MUAC: mid-upper arm circumference. #: higher value between screening and enrolment visits; ¶: lower value between screening and enrolment visits.
The mean age at enrolment was 15.4 months among children in the amoxicillin group, and 15.8 months among children in the placebo group. All children had fast breathing at screening, as this was an inclusion criterion. Median SpO2 was 98% in both the amoxicillin and placebo groups, and was similar among those children who did and did not go on to experience TF. No child was hypoxaemic at baseline, as hypoxaemia was an exclusion criterion.
Hospital monitoring
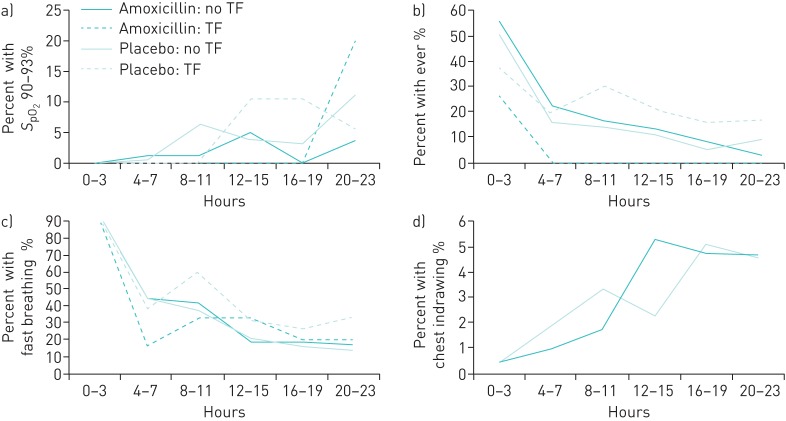
SpO2 90–93%, fast breathing, very fast breathing, chest indrawing and fever were documented in both the amoxicillin and placebo groups, as described below and in table 4. Vomiting everything was documented in one child, who was in the amoxicillin group; the remaining general and respiratory danger signs were not documented in any children. Temporal trends for SpO2 90–93%, fast breathing, chest indrawing and fever over the first 24 h of hospitalisation are shown in figure 1.
TABLE 4.
Clinical signs identified at any time during hospital monitoring
| Characteristic | Amoxicillin (n=215) | Placebo (n=221) | ||||||
| Overall (n=215) | No treatment failure (n=199) | Treatment failure (n=16) | p-value | Overall (n=221) | No treatment failure (n=189) | Treatment failure (n=32) | p-value | |
| Oxygen saturation 90–93% | 16 (7.4%) | 14 (7.0%) | 2 (12.5%) | 0.338 | 21 (9.5%) | 17 (9.0%) | 4 (12.5%) | 0.518 |
| Fast breathing | 214 (99.5%) | 198 (99.5%) | 16 (100%) | 0.776 | 221 (100%) | 189 (100%) | 32 (100%) | |
| Chest indrawing# | 14 (6.6%) | 16 (7.2%) | ||||||
| Fever | 122 (56.7%) | 117 (58.8%) | 6 (33.3%) | 0.032 | 117 (52.9%) | 100 (52.9%) | 17 (53.1%) | 0.98 |
A description of hospital monitoring checks among children prior to any treatment failure. Data collected during hospital monitoring assessments after children were classified as having treatment failure are excluded. This includes all hospital monitoring data from children classified as having treatment failure within 2 h following enrolment (n=7). Sample sizes in this table have been adjusted to remove these children. #: chest indrawing was a treatment failure criterion.
FIGURE 1.
Proportion of children experiencing clinical signs during the first 24 h of hospital monitoring. a) Oxygen saturation measured by pulse oximetry (SpO2) 90–93%; b) fever; c) fast breathing; d) chest indrawing. Denominators (total children monitored during the designated time window) were as follows. Hours 0–3: amoxicillin, no treatment failure (TF): n=200; amoxicillin, TF: n=15; placebo, no TF: n=189; placebo, TF: n=32. Hours 4–7: amoxicillin, no TF: n=190; amoxicillin, TF: n=12; placebo, no TF: n=186; placebo, TF: n=31. Hours 8–11: amoxicillin, no TF: n=165; amoxicillin, TF: n=6; placebo, no TF: n=159; placebo, TF: n=20. Hours 12–15: amoxicillin, no TF: n=164; amoxicillin, TF: n=6; placebo, no TF: n=158; placebo, TF: n=19. Hours 16–19: amoxicillin, no TF: n=163; amoxicillin, TF: n=5; placebo, no TF: n=158; placebo, TF: n=19. Hours 20–23: amoxicillin, no TF: n=165; amoxicillin, TF: n=5; placebo, no TF: n=156; placebo, TF: n=18.
SpO2 measurements ranged from 90 to 100%. Sixteen children in the amoxicillin group (7.4%) and 21 children in the placebo group (9.5%) had SpO2 90–93%. In both the amoxicillin and placebo groups, a larger proportion of children with TF had SpO2 90–93% (7.0% versus 12.5%, p=0.338 in the amoxicillin group and 9.0% versus 12.5%, p=0.518 in the placebo group), but these differences were not statistically significant.
Fast breathing was identified in 214 children in the amoxicillin group (99.5%) and 222 children in the placebo group (100%). Approximately half (48.4%) of the children monitored had fast breathing documented one to two times during monitoring, whereas the other half (51.6%) had fast breathing documented three or more times. This followed a similar pattern between those children who went on to experience TF and those who did not.
Fourteen children in the amoxicillin group (6.6%) and 16 in the placebo group (7.2%) had chest indrawing identified; 10 had chest indrawing recorded at one time point, whereas 20 had chest indrawing recorded at multiple time points. The proportion of children with chest indrawing at a single time point versus multiple time points was similar in the amoxicillin and placebo groups. The proportion of children with chest indrawing in both groups increased over the first 24 h of monitoring (figure 1).
Fever was documented in 122 children in the amoxicillin group (56.7%) and 117 children in the placebo group (52.7%). In the amoxicillin group, a larger proportion of children without TF had fever documented compared to those children with TF (58.8% versus 33.3%, p=0.032). This pattern was not noted in the placebo group, where 52.9% of children with TF and 53.1% of children without TF had fever (p=0.98).
In both the amoxicillin and placebo groups, approximately 50% of those children without TF had fever at the initiation of hospital monitoring (figure 1). This dropped steadily through approximately hour 20. Among children with TF, 25–40% had fever at the initiation of hospital monitoring. In the TF groups, the proportions did not follow a consistent pattern through the remainder of monitoring; this may be due to the small number of children with TF.
SpO2 90–93%, fast breathing and fever all had very low sensitivity and high specificity to identify children who would experience TF when measured during the first 24 h from initiation of monitoring. Sensitivity tended to be higher in the placebo group, which may have been due to the higher number of children with TF in that group. For SpO2 90–93%, sensitivity was 10.0% and specificity was 95.4% in the amoxicillin group, versus 18.8% sensitivity and 87.7% specificity in the placebo group. Fast breathing had a sensitivity of 5.6% and specificity of 95.9% in the amoxicillin group, compared with 14.7% sensitivity and 89.1% specificity in the placebo group. For fever, sensitivity was 1.6% and specificity was 94.7% in the amoxicillin group; sensitivity was 15.9% and specificity was 88.2% in the placebo group.
Discussion
The value of repeat monitoring of vital signs among hospitalised children has been previously described [17, 18]. A multicountry trial found that models including both baseline data and vital sign information obtained after 12 or 24 h of patient monitoring more accurately predicted which children experienced TF when compared with models that included baseline data alone [17]. The value of repeat monitoring extends beyond pneumonia; continuous monitoring, including pulse oximetry, has also demonstrated potential for optimising outcomes among ill neonates [18].
Previous studies have characterised vital sign progression among children with pneumonia. A study enrolling children in the United States found a median time to clinical stability of 38.6 h for RR, 39.5 h for SpO2, and 14.5 h for temperature among children <2 years [19]. Additionally, a study in Taiwan assessed risk factors for pneumonia progression among hospitalised children and found persistent fever that did not respond to therapy within 72 h to be a risk factor for progression [20].
In our study, the monitoring of vital and physical exam signs every 3 h among hospitalised enrolled children allowed for an in-depth characterisation of illness progression that would not have been possible using vital sign data collected through study visits alone. The value of this periodic monitoring is demonstrated by the SpO2 findings. While no children had SpO2 90–93% documented during screening/enrolment, 7.4% of children in the amoxicillin group and 9.5% of children in the placebo group went on to have SpO2 90–93% measured during the monitoring period. Identifying these dips in SpO2 can be critically important, as described by previous literature. A study describing hypoxemia among children with acute lower respiratory infections found that for children with SpO2 below 95%, the OR of death was 3.56 [21]. Similarly, while no children had chest indrawing at enrolment, approximately 7% of children developed chest indrawing during monitoring, showing that a proportion of children diagnosed with fast-breathing pneumonia may subsequently develop chest-indrawing pneumonia, or that chest indrawing may be missed in the initial assessment. These proportions were similar in the amoxicillin and placebo groups.
Conversely, the proportion of children with fever was higher at enrolment than at 24 h, most likely due to the administration of paracetamol, a fever-reducing medication, during hospitalisation. The amoxicillin group had a larger proportion of children with fever in the non-TF group (55.5% versus 26.7%, p=0.03), which may also be due to the administration of paracetamol: 75.9% of children without TF and 93.8% of children with TF were administered paracetamol. Another explanation for this may be the protocol-specified hospitalisation of all children with fever but with a negative malaria test. If these children were otherwise not as ill as the other hospitalised children, this may have introduced bias into the relationship between fever and TF. This difference in fever between the TF and non-TF groups was not noted in the placebo group, although the administration of paracetamol was similarly differential.
While fast breathing was present among all children at enrolment, it was transient during monitoring. Repeat measurements of RR are important to improve the reliability and validity of this measure [22–24]. However, fast breathing is known to be a nonspecific assessment tool for pneumonia, as children may exhibit fast breathing for a variety of reasons, including malaria, asthma, and metabolic acidosis, as well as nonpathological reasons [2, 25, 26].
The progression of vital signs and physical examination findings over the first 24 h of hospital monitoring followed a similar pattern among children receiving amoxicillin and those receiving placebo. However, more children receiving placebo went on to experience TF, and the parent study identified a day 4 TF rate of 4% among children receiving amoxicillin and 7% among children receiving placebo (adjusted relative risk, 1.78) [6]. This analysis, therefore, may indicate that the first 24 h of treatment are not sufficient to determine the child's response. Alternatively, considering the overall low TF rate and the 3% absolute difference in the TF rate between children in the amoxicillin and placebo groups of the parent study, this may also indicate that a substantial proportion of children in this analysis did not have bacterial pneumonia. The difference in the relationships between fever and TF as well as sex and TF between the amoxicillin and placebo groups may suggest that a different infectious process is occurring in children with TF between the two groups. Indeed, sex differences in paediatric infectious diseases may vary across pathogens [27].
There are several limitations to this analysis. Statistical analyses were not adjusted for multiple comparisons and observed statistically significant results may be spurious. The association of vital and physical exam signs experienced during hospital monitoring with subsequent TF is complicated by the design of the parent study (i.e. the association of chest indrawing and TF could not be assessed because chest indrawing itself was a TF criterion). Aside from the treatment group assignments, these children were receiving standard supportive care in a hospital setting, so their vital and physical exam signs may have been affected by the administration of fever-reducing medication, which impacted the ability of this analysis to assess the progression of fever. Children with severe disease or comorbidities such as HIV were excluded, which may limit the generalizability of these findings. Finally, in order to be admitted for monitoring, children had to meet certain criteria (aged <6 months, moderate malnutrition, or fever with a negative malaria rapid diagnostic test), which may have introduced artefacts into the analysis.
Conclusions
This secondary analysis describes the progression of pneumonia over the first 24 h of hospitalisation among children aged 2–59 months enrolled in a fast-breathing pneumonia treatment study, some receiving antibiotic treatment and some receiving placebo. Children in the amoxicillin and placebo treatment groups experienced similar trends across RR, SpO2, chest indrawing and fever. Overall, regular monitoring of vital and physical exam signs among children with fast-breathing pneumonia led to the identification of clinical signs that were not apparent at baseline. This information may support providers and policymakers in developing guidance for care of children with nonsevere pneumonia, particularly with regard to establishing appropriate guidelines for determining when to admit or discharge children presenting with nonsevere pneumonia at the outpatient level.
Acknowledgments
We thank the dedicated study staff at the University of North Carolina Project, Lilongwe Medical Relief Fund Trust for providing patient care and data collection; the University of North Carolina Project Lilongwe Community Advisory Board for their work as participant advocates and their assistance with community outreach; Triclinium Clinical Development for facilitating data review and management; and the Malawi Ministry of Health. We also thank the trial participants, their caregivers and the local community in Lilongwe, Malawi for their participation and support.
Footnotes
Conflict of interest: J. Lenahan reports grants from the Bill and Melinda Gates Foundation during the conduct of the study.
Conflict of interest: E. Nkwopara reports grants from the Bill and Melinda Gates Foundation during the conduct of the study.
Conflict of interest: M. Phiri has nothing to disclose.
Conflict of interest: T. Mvalo reports grants from the Save the Children Federation, Inc., during the conduct of the study.
Conflict of interest: M.T. Couasnon has nothing to disclose.
Conflict of interest: K. Turner has nothing to disclose.
Conflict of interest: C. Ndamala has nothing to disclose.
Conflict of interest: E.D. McCollum reports grants from the Bill and Melinda Gates Foundation outside the submitted work.
Conflict of interest: S. May reports grants from the Bill and Melinda Gates Foundation during the conduct of the study.
Conflict of interest: A.S. Ginsburg reports grants from the Bill and Melinda Gates Foundation during the conduct of the study.
Support Statement: This trial was funded by grant OPP1105080 from the Bill & Melinda Gates Foundation.
References
- 1.McAllister DA, Liu L, Shi T, et al. . Global, regional, and national estimates of pneumonia morbidity and mortality in children younger than 5 years between 2000 and 2015: a systematic analysis. Lancet Glob Health 2019; 7: e47–e57. doi: 10.1016/S2214-109X(18)30408-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Modi P, Mark Munyaneza RB, Goldberg E, et al. . Oxygen saturation can predict pediatric pneumonia in a resource-limited setting. J Emerg Med 2013; 45: 752–760. doi: 10.1016/j.jemermed.2013.04.041 [DOI] [PubMed] [Google Scholar]
- 3.Oostenbrink R, Thompson M, Lakhanpaul M, et al. . Children with fever and cough at emergency care. Eur J Emerg Med 2013; 20: 273–280. doi: 10.1097/MEJ.0b013e32835771fd [DOI] [PubMed] [Google Scholar]
- 4.WHO. Integrated Management of Childhood Illness: Chart Booklet. Geneva, World Health Organization, 2014. [Google Scholar]
- 5.Florin TA, Ambroggio L, Brokamp C, et al. . Reliability of examination findings in suspected community-acquired pneumonia. Pediatrics 2017; 140: e20170310. doi: 10.1542/peds.2017-0310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tuti T, Agweyu A, Mwaniki P, et al. . An exploration of mortality risk factors in non-severe pneumonia in children using clinical data from Kenya. BMC Med 2017; 15: 1–12. doi: 10.1186/s12916-016-0759-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deardorff K V, McCollum ED, Ginsburg AS. Pneumonia risk stratification scores for children in low-resource settings: a systematic literature review. Pediatr Infect Dis J 2018; 37: 743–748. doi: 10.1097/INF.0000000000001883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ginsburg AS, Mvalo T, Nkwopara E, et al. . Placebo vs amoxicillin for nonsevere fast-breathing pneumonia in Malawian children aged 2 to 59 months: a double-blind, randomized clinical noninferiority trial. JAMA Pediatr 2019; 173: 21–28. doi: 10.1001/jamapediatrics.2018.3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ginsburg AS, May SJ, Nkwopara E, et al. . Methods for conducting a double-blind randomized controlled clinical trial of three days versus five days of amoxicillin dispersible tablets for chest indrawing childhood pneumonia among children two to 59 months of age in Lilongwe, Malawi: a study protoc. BMC Infect Dis 2018; 18: 476. doi: 10.1186/s12879-018-3379-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO. Revised WHO classification and treatment of pneumonia in children at health facilities: evidence summaries. Geneva, World Health Organization, 2014. [PubMed] [Google Scholar]
- 11.Laillou A, Prak S, De Groot R, et al. . Optimal screening of children with acute malnutrition requires a change in current WHO guidelines as MUAC and WHZ identify different patient groups. PLoS ONE 2014; 9: 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanc J, Locatelli I, Rarau P, et al. . Retrospective study on the usefulness of pulse oximetry for the identification of young children with severe illnesses and severe pneumonia in a rural outpatient clinic of Papua New Guinea. PLoS ONE 2019; 14: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah SN, Bachur RG, Simel DL, et al. . Does this child have pneumonia? The rational clinical examination systematic review. JAMA 2017; 318: 462–471. doi: 10.1001/jama.2017.9039 [DOI] [PubMed] [Google Scholar]
- 14.Lynch T, Platt R, Gouin S, et al. . Can we predict which children with clinically suspected pneumonia will have the presence of focal infiltrates on chest radiographs? Pediatrics 2004; 113: 186–189. doi: 10.1542/peds.113.3.e186 [DOI] [PubMed] [Google Scholar]
- 15.Majumdar SR, Eurich DT, Gamble JM, et al. . Oxygen saturations less than 92% are associated with major adverse events in outpatients with pneumonia: a population-based cohort study. Clin Infect Dis 2011; 52: 325–331. doi: 10.1093/cid/ciq076 [DOI] [PubMed] [Google Scholar]
- 16.Neuman MI, Monuteaux MC, Scully KJ, et al. . Prediction of pneumonia in a pediatric emergency department. Pediatrics 2011; 128: 246–253. doi: 10.1542/peds.2010-3367 [DOI] [PubMed] [Google Scholar]
- 17.Fu LY, Ruthazer R, Wilson I, et al. . Brief hospitalization and pulse oximetry for predicting amoxicillin treatment failure in children with severe pneumonia. Pediatrics 2006; 118: e1822–e1830. doi: 10.1542/peds.2005-2673 [DOI] [PubMed] [Google Scholar]
- 18.Sahni R. Continuous noninvasive monitoring in the neonatal ICU. Curr Opin Pediatr 2017; 29: 141–148. doi: 10.1097/MOP.0000000000000459 [DOI] [PubMed] [Google Scholar]
- 19.Wolf RB, Edwards K, Grijalva CG, et al. . Time to clinical stability among children hospitalized with pneumonia. J Hosp Med 2015; 10: 380–383. doi: 10.1002/jhm.2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang CY, Chang L, Liu CC, et al. . Risk factors of progressive community-acquired pneumonia in hospitalized children: a prospective study. J Microbiol Immunol Infect 2015; 48: 36–42. doi: 10.1016/j.jmii.2013.06.009 [DOI] [PubMed] [Google Scholar]
- 21.Junge S, Palmer A, Greenwood BM, et al. . The spectrum of hypoxaemia in children admitted to hospital in The Gambia. West Africa Trop Med Int Heal 2006; 11: 367–372. doi: 10.1111/j.1365-3156.2006.01570.x [DOI] [PubMed] [Google Scholar]
- 22.Muro F, Mtove G, Mosha N, et al. . Effect of context on respiratory rate measurement in identifying non-severe pneumonia in African children. Trop Med Int Heal 2015; 20: 757–765. doi: 10.1111/tmi.12492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ginsburg AS, Lenahan JL, Izadnegahdar R, et al. . A systematic review of tools to measure respiratory rate in order to identify childhood pneumonia. Am J Respir Crit Care Med 2018; 197: 1116–1127. doi: 10.1164/rccm.201711-2233CI [DOI] [PubMed] [Google Scholar]
- 24.Lanaspa M, Valim C, Acacio S, et al. . High reliability in respiratory rate assessment in children with respiratory symptomatology in a rural area in Mozambique. J Trop Pediatr 2014; 60: 93–98. doi: 10.1093/tropej/fmt081 [DOI] [PubMed] [Google Scholar]
- 25.Chisti M, Salam M, Bardhan P, et al. . Influences of dehydration on clinical features of radiological pneumonia in children attending an urban diarrhoea treatment centre in Bangladesh. Ann Trop Paediatr 2010; 30: 311–316. doi: 10.1179/146532810X12858955921230 [DOI] [PubMed] [Google Scholar]
- 26.Saha D, Ronan A, Khan WA, et al. . Diagnosis of pneumonia in children with dehydrating diarrhoea. J Heal Popul Nutr 2014; 32: 14–18. [PMC free article] [PubMed] [Google Scholar]
- 27.Muenchhoff M, Goulder PJR. Sex differences in pediatric infectious diseases. J Infect Dis 2014; 209: S120–S126. doi: 10.1093/infdis/jiu232 [DOI] [PMC free article] [PubMed] [Google Scholar]