Abstract
In recent years, technical and procedural advances have brought functional magnetic resonance imaging (fMRI) to the field of murine neuroscience. Due to its unique capacity to measure functional activity non-invasively, across the entire brain, fMRI allows for the direct comparison of large-scale murine and human brain functions. This opens an avenue for bidirectional translational strategies to address fundamental questions ranging from neurological disorders to the nature of consciousness. The key challenges of murine fMRI are: (1) to generate and maintain functional brain states that approximate those of calm and relaxed human volunteers, while (2) preserving neurovascular coupling and physiological baseline conditions. Low-dose anesthetic protocols are commonly applied in murine functional brain studies to prevent stress and facilitate a calm and relaxed condition among animals. Yet, current mono-anesthesia has been shown to impair neural transmission and hemodynamic integrity. By linking the current state of murine electrophysiology, Ca2+ imaging and fMRI of anesthetic effects to findings from human studies, this systematic review proposes general principles to design, apply and monitor anesthetic protocols in a more sophisticated way. The further development of balanced multimodal anesthesia, combining two or more drugs with complementary modes of action helps to shape and maintain specific brain states and relevant aspects of murine physiology. Functional connectivity and its dynamic repertoire as assessed by fMRI can be used to make inferences about cortical states and provide additional information about whole-brain functional dynamics. Based on this, a simple and comprehensive functional neurosignature pattern can be determined for use in defining brain states and anesthetic depth in rest and in response to stimuli. Such a signature can be evaluated and shared between labs to indicate the brain state of a mouse during experiments, an important step toward translating findings across species.
Keywords: mouse, fMRI–functional magnetic resonance imaging, anesthesia, translational mouse-human, brain functional connectivity, EEG, Ca2+ imaging, MIND signature
Introduction
Much of our understanding of human brain functions comes from murine studies. The ease of genetic modification and other practical and financial issues have made the mouse the best-explored mammalian model organism in neuroscience. A multitude of murine protocols and repositories provide reliable benchmarks in today’s brain research (including the Allen Brain Project1, the Blue Brain Project2, and the Mouse ENCODE Project3; Lein et al., 2007; Sunkin et al., 2013; Erö et al., 2018; Keller et al., 2018; Frankish et al., 2019). Still, it remains an open question to what extent, and under what conditions, findings from the mouse can be translated into an understanding of human brain functions. Although the mouse brain is not merely a miniaturized version of the human brain, its comparably small and flat neocortex exhibits striking functional and structural similarities, and the subcortical architecture is evolutionarily largely preserved (Ventura-Antunes et al., 2013; Hofman, 2014; Glickfeld and Olsen, 2017; Halley and Krubitzer, 2019).
In recent years, technical and procedural advances have brought functional magnetic resonance imaging (fMRI) to the field of murine neuroscience. The unique capacity of fMRI to measure functional brain activity non-invasively and across the entire brain relies on tight neurovascular coupling, in which increased neural activity triggers local elevations in cerebral blood flow (CBF), cerebral blood volume (CBV), and blood oxygenation (Hamilton et al., 2010; Hall et al., 2014). Each of these hemodynamic parameters can be assessed by fMRI as a surrogate for neural activity. Blood oxygenation level-dependent (BOLD) fMRI is the most popular approach due to its high sensitivity and comparably fast acquisition times (Ogawa et al., 1990; Kim, 2018). Modern MR scanners operating at ultrahigh magnetic field strengths (≥7 T) have been tailored for use with small rodents, and can achieve a relative spatial resolution analogous to that commonly used in human fMRI (voxels per anatomical region; voxel size of ~200 μm3). This permits direct comparisons of large-scale murine and human brain functions, and opens up opportunities to use a plethora of genetically engineered models to clarify a wide range of clinical and basic neuroscience issues—from the pathogenesis of neurological disorders to fundamental questions about consciousness.
Although the first report on murine fMRI was published more than two decades ago (Huang et al., 1996), its application to mice continues to require extensive refinements (Mandino et al., 2020). Aside from technical issues including signal amplification from the small mouse brain or its vulnerability to physiological perturbations, a fundamental problem of interspecies translation involves the non-voluntary task conundrum. In comparison to (adult) humans, a mouse has no interest in participating in an fMRI study. Head fixation, body restraint, and habituation to the acoustic noise produced by the MR gradients (up to 115 dB sound pressure) cause enormous stress to this remarkably rousable creature, which is far less amenable to training than rats (Jonckers et al., 2014, 2015; Low et al., 2016b; Dopfel and Zhang, 2018). Studying unbiased nociception in mice is almost impossible; even the application of aversive stimuli, like mild cooling of the paw, causes immediate withdrawal, and functional patterns across the brain reflect not only stress, fear, and anticipation, but also unrelated motor and sensory responses. This introduces tremendous complexity to fMRI of the awake mouse.
Low-dose anesthetic protocols are commonly applied in mouse fMRI to address these problems and to alleviate potential suffering due to stress, fear, and pain. The goal is to achieve high-quality translational data from sedated, i.e., calm, relaxed, and undistracted subjects. However, anesthetics have been shown to impair neural transmission (Baumgart et al., 2015; Hemmings et al., 2019) and also affect other aspects of murine physiology including hemodynamics (Franceschini et al., 2010; Masamoto and Kanno, 2012), thermoregulation (Reimann et al., 2016), respiration (van Alst et al., 2019), and cardiovascular control (Sinclair, 2003; Low et al., 2016a) in a dose-dependent manner. All these parameters can affect neurovascular coupling, which links the BOLD effect to the activity of neural populations. Therefore, anesthetic protocols in fMRI face two major challenges: first, to preserve functional brain states based on neural oscillation and transmission characteristics (see “Brain States, Anesthetic Depth, and Murine Consciousness” section), and second, to maintain hemodynamic integrity (see “Murine fMRI and Hemodynamic Integrity” section).
Recognizing the progress, opportunities, and challenges of murine functional brain mapping, this work provides a review of the literature on current anesthetic protocols and their meaning for murine functional neuroimaging. The aim is to introduce the basic principles of anesthesia to better understand and interpret the outcome of murine fMRI studies, and to develop novel anesthetic protocols and monitoring strategies dedicated to promoting reproducible and translational neuroimaging.
Brain States, Anesthetic Depth, and Murine Consciousness
In the murine as in the human brain, neural information is processed against a background of spontaneous, ongoing activity generated by the promiscuous firing of neurons or structured patterns of neural populations. These oscillatory dynamics can be assessed via invasive and non-invasive neural readout techniques, such as electroencephalography (EEG), local field potentials (LFP), or calcium (Ca2+) imaging, yielding distinct spatiotemporal profiles that can be considered as signatures of distinct brain states.
Cortical States of Wakefulness
Awake brain states undergo constant alterations in response to changing contexts of arousal, attention, and behavior (Steriade, 2000; Pfaff, 2005; Olcese et al., 2018; Poulet and Crochet, 2019). The spectrum of EEG patterns during these states ranges from synchronous low-frequency oscillations (<8 Hz) during quiet wakefulness to structured higher-frequency oscillations (~8–100 Hz) during attentional tasks and highly desynchronized high-frequency oscillations during aroused states (Petersen et al., 2003; Palva et al., 2005; Fries et al., 2007; Sachidhanandam et al., 2013; McGinley et al., 2015a; Olcese et al., 2018; Poulet and Crochet, 2019). The level of cortical activation (i.e., higher-frequency oscillations) is positively correlated to the level of general arousal, which is mediated by modulatory input from subcortical cholinergic and monoaminergic (dopaminergic, noradrenergic, serotonergic, and histaminergic) nuclei (Figure 1A; Aston-Jones et al., 1999; Pfaff, 2005; Harris and Thiele, 2011; Lee and Dan, 2012; McGinley et al., 2015b; Lecrux and Hamel, 2016; Ma et al., 2018). These arousal nuclei project into the cortex either directly or via the medial thalamus, and govern cortical states at both the global and local levels. Defined cortical regions have been shown to be remotely modulated, for instance, by inhibitory projections of the thalamic reticular nucleus (Lewis et al., 2015; Herrera et al., 2016; Fernandez et al., 2017) or excitatory projections of cholinergic neurons in a modality-selective manner (Kim et al., 2016; Záborszky et al., 2018).
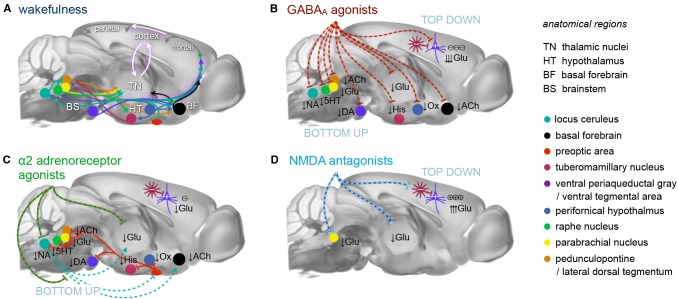
Figure 1.
Principal mechanisms of anesthesia. (A) Brain states are governed by a highly interconnected assembly of subcortical (arousal) nuclei. These nuclei employ distinct transmitter systems, including glutamate (Glu), noradrenaline (NA), serotonin (5HT), dopamine (DA), acetylcholine (ACh), histamine (His), and orexin (Ox). They project to the cortex directly and via higher-order nuclei of the thalamus. Thalamus and cortex are densely interconnected and heavily exchange information. Synchronous rhythmic thalamocortical activity can set the phase relations of distant cortical areas. Similar phase relations facilitate information transfer across the cortex and from frontal to parietal regions. All anesthetics generate distinct thalamocortical rhythms and alter the phase relationship of transmitting and receiving areas, leading to a successive breakdown of cortico-(thalamo-)cortical communication and eventually loss of consciousness. (B) GABAA agonists, including volatile ethers, affect brain states both top-down and bottom-up by inhibiting excitatory neurons in subcortical nuclei and directly in the cortex. (C) α2AR agonists exert their effects bottom-up mainly by inhibiting the locus coeruleus, which leads to a disinhibition of sleep promoting neurons in the preoptic area of the hypothalamus. (D) N-methyl-d-aspartate (NMDA) antagonists act primarily top-down in a dual mode: at low doses by inhibiting inhibitory interneurons leading to cortical excitation, and at higher dosages by also inhibiting excitatory cortical pyramidal neurons. Further suppression of nociception and arousal is mediated by blocking the parabrachial nucleus. These three routes present the major principles of anesthesia. All anesthetics in current use in preclinical fMRI act in one of these ways. Inspired by Franks (2008), Lee and Dan (2012) and Akeju and Brown (2017), plotted on an MR reference template of the Allen mouse brain atlas (Bakker et al., 2015).
Sensory stimuli are processed differently against the background of diverse cortical states (Shimaoka et al., 2018; Poulet and Crochet, 2019). Ensembles of neighboring neurons depend on a certain level of desynchronization to encode complex features of stimuli by concerting the firing rate, spike timing, and the temporal order at which they fire (Hopfield, 1995; Montemurro et al., 2008; Kayser et al., 2009; Mohajerani et al., 2013; Luczak et al., 2015; Montijn et al., 2016). Increasing synchronization may lend structure to features, but can also obscure them (Fries et al., 2007; Pachitariu et al., 2015; Olcese et al., 2018). An intermediate level of arousal has been found to enhance the consistency and signal strength of encoded stimuli and is associated with optimal sensory processing (Polack et al., 2013; Schneider and Logan, 2014; Schölvinck et al., 2015; McGinley et al., 2015a; Olcese et al., 2018; Shimaoka et al., 2018). Cortical information processing is substantially shaped by the activity of diverse inhibitory interneurons and relies on a balanced interplay of excitatory and inhibitory inputs (Isaacson and Scanziani, 2011; Rubin et al., 2017). Cortical states can become altered regionally and globally upon “attention” to a stimulus (Olcese et al., 2018; Poulet and Crochet, 2019). This flexibility permits the mode of sensory processing to adapt to situational demands.
Such modes of attention, sensory processing, and integration have been described in terms of cortical oscillations of distinct wavelengths (delta, theta, alpha, beta, and gamma, as reviewed in Fries et al., 2007; Schroeder and Lakatos, 2009; Saalmann et al., 2012; Fries, 2015; McVea et al., 2016; Jensen et al., 2019; Sikkens et al., 2019). The underlying rhythmic synchronization of neural assemblies is largely produced by a synchronized spiking of inhibitory interneurons at higher frequencies (reviewed in Fries et al., 2007; Jensen et al., 2019). The high magnitudes of slower rhythms are generated by other mechanisms (see “Brain States Under Anesthesia” section; reviewed in Neske, 2015; Sanchez-Vives et al., 2017). Neurons that act in phase are more likely to fire together, since the active and refractory periods of presynaptic and postsynaptic neurons are aligned (Saalmann, 2014). This temporal coding scheme can facilitate or inhibit information transfer between local and distant cell groups by varying their phase relations.
Cortical oscillations can propagate as traveling waves of neural depolarization and spiking activity across the cortical surface (reviewed in McVea et al., 2016; Kuroki et al., 2018; Muller et al., 2018). Higher frequencies are thereby nested within slower waves, leading to rich patterns of background information onto which local events are processed. The distinct and complex spatiotemporal dynamics of these traveling waves (e.g., radial, planar, spiraling, or rotating) are coordinated by recurrent cortical networks and distant projections from thalamic and subthalamic nuclei (Bhattacharya et al., 2019). Anesthesia can affect neural information processing at both the local level (by setting an imbalance of inhibitory and excitatory inputs) and globally (by spoiling phase relations of cell ensembles and disrupting information transfer across brain areas).
Brain States Under Anesthesia
Selecting an appropriate anesthetic protocol is in first place a question of choosing the right anesthetic class and dosage. Anesthetics currently used for murine fMRI (Table 1) can be divided into three classes based on their main molecular target receptors: γ-aminobutyric acid subtype A (GABAA) receptor agonists including volatile ethers, α2 adrenoreceptor (α2AR) agonists, and N-methyl-d-aspartate (NMDA) receptor antagonists. Each class exerts its sedating effects based on one of three key principles: (1) top-down by inhibiting cortical neurons directly (NMDA receptor antagonists); (2) bottom-up by suppressing subcortical arousal nuclei that affect cortical states (α2AR agonists); or (3) both, by inhibiting neurons across the entire brain (GABAA agonists and volatile ethers; Figure 1).
Table 1.
Anesthetic compounds used in mouse functional magnetic resonance imaging (fMRI).
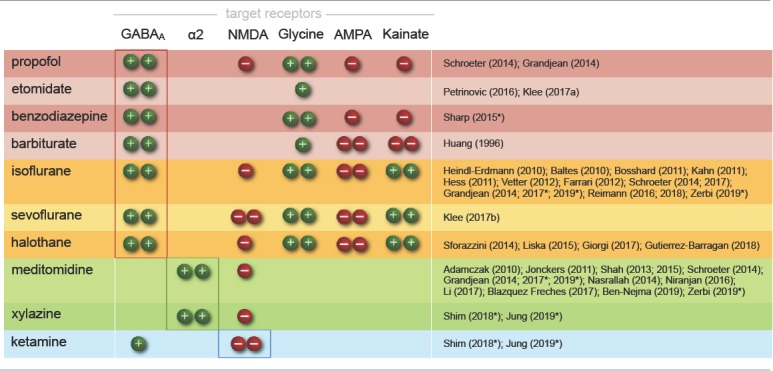 |
Each drug addresses distinct receptor types by either agonistic (+) or antagonistic (−) modulation. Single or double symbols are used to indicate whether potentiation or inhibition is mild or strong. Anesthetics are grouped into three classes based on their main target receptors: GABAA agonists, including injectable drugs and volatile ethers (red and yellow, respectively), α2AR agonists (green), and NMDA antagonists (blue). Alongside these key target receptors, the listed drugs modulate a host of further receptor types and may contribute to specific anesthetic effects (see, e.g., Alkire et al., 2008; Franks, 2008; McKinstry-Wu and Kelz, 2019). Halothane is chemically not an ether, yet it belongs to the group of halogenated vapors, like isoflurane and sevoflurane, which share largely overlapping target sites. Note that only drugs suitable for longitudinal studies are listed; the GABAA agonists α-chloralose and urethane have also been applied in murine fMRI (Ahrens and Dubowitz, 2001; Xu et al., 2003, 2005; Grandjean et al., 2014; Schroeter et al., 2014) and will be discussed in “Sensory Processing and the Key Challenges in Murine fMRI” and “Functional Connectivity and Murine Resting-State fMRI” sections. References are shortened to first author (and year) to save space and allow for more comprehensive referencing. Asterisks indicate use in multimodal protocols.
Because their target sites differ, each anesthetic class produces distinct cortical oscillatory dynamics (Figures 2A,B; Ching and Brown, 2014; Purdon et al., 2015; Flores et al., 2017), which vary with the anesthetic dosage (Figures 2B,C). Most anesthetics within one class further exhibit variations in their cortical signatures as they act at multiple receptor types (Table 1), interfering with partially overlapping pathways. Scientific advances over the last decades have revealed an increasingly comprehensive, yet still incomplete picture of the underlying mechanisms (Steriade, 2000; Campagna et al., 2003; Alkire et al., 2008; Franks, 2008; Brown et al., 2010, 2018a; Akeju and Brown, 2017; Flores et al., 2017; Hemmings et al., 2019). Here, we briefly introduce current models on how these rhythms are generated by the effects of different anesthetics, and how the brain states that are indicated by distinct rhythms affect neural transmission and processing.
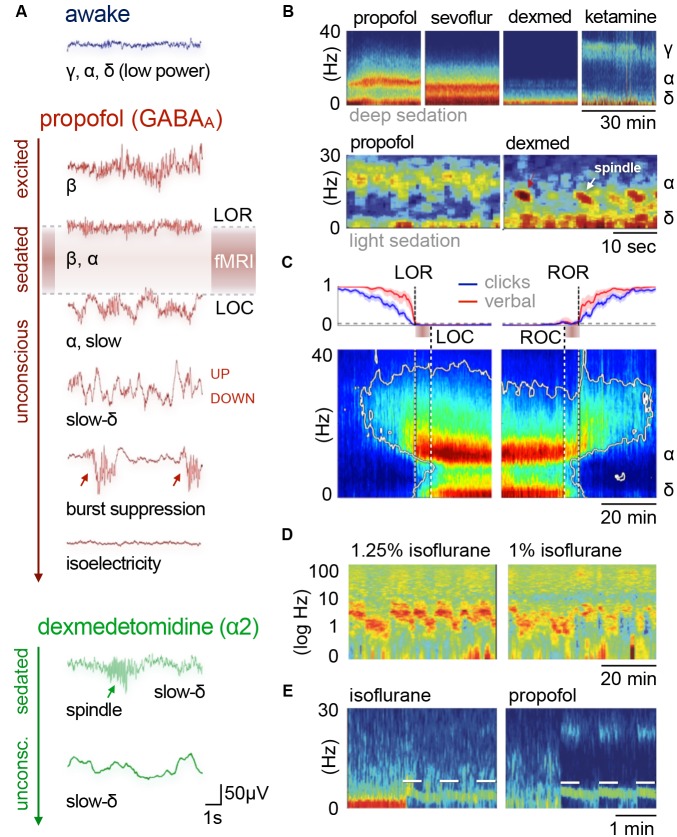
Figure 2.
Cortical oscillatory signatures of brain states under anesthesia. (A) Changes in oscillatory signatures for increasing doses of propofol and dexmedetomidine measured from the human anterior cortex. With increasing anesthetic depth, oscillation frequencies decrease and synchronize. (B) Distinct oscillatory signatures induced by different anesthetics (upper panel) and dosages (lower panel). The spectrogram represents the spectrum of frequencies in a time and frequency domain, which facilitates the identification of structured frequency bands. (C) Loss of responsiveness (LOR) and connected consciousness (LOC) induced by increasing doses of propofol. LOR is defined by suppression of responses to click and verbal commands (upper panel). Note the occurrence of alpha bands and the absence of slow-delta bands in the phase between LOR and LOC in the spectrogram. The same is true for return of consciousness (ROC) and responsiveness (ROR). In rodents, alpha waves coincide with the loss of righting reflex (LORR), which corresponds to LOR, and slow-delta waves are indicated by a complete loss of movement (LOM), which corresponds to LOC. (D) Brain states may not be fully stable when maintained via anesthesia. Transitions between two or more intermediate states occurred over longer periods of constant isoflurane concentration in rats. This feature has been defined as metastability. Note the logarithmic scale used to highlight the transitions that occur primarily in the lower frequency band (0–10 Hz). Local field potentials (LFP) recording was conducted in the anterior cingulate cortex (ACC). (E) Stimulation (white bars) of dopaminergic neurons of the ventral tegmental area in the rat shifts cortical states under sedation from slow delta (<4 Hz) towards θ power (isoflurane), or towards θ and β power (propofol). Oscillations: slow (<1 Hz), δ (1–4 Hz), θ (4–8 Hz), α (8–15 Hz), β (15–30 Hz), awake-γ (30–80 Hz), ketamine-γ (25–35 Hz), spindle (9–16 Hz). Adapted with permission from (A,B) Purdon et al. (2015); (B, lower panel) Akeju and Brown (2017); (C) Purdon et al. (2013); (D) Hudson (2017), and (E) Solt et al. (2014).
Thalamocortical Rhythmogenesis
The generation of cortical rhythms by anesthesia follows a common pattern for all anesthetics (as described in more detail below). All anesthetics exert their sedating effects by acting on specific target receptor types (Table 1), leading to inhibition and thus hyperpolarization of the affected neurons. Many types of excitatory and inhibitory neurons comprise specific membrane channels that open at distinct levels of hyperpolarization and lead to inward cation currents that cause the cells to quickly depolarize. Hence, the affected cells respond to strong inhibition with excitation, leading to post-inhibitory rebound spiking. These bursts of neural activity recur periodically, paced by the sum of inhibitory (hyperpolarizing) and excitatory (depolarizing) inputs that are required to exceed the threshold for rebound spiking. These inputs can be differently modulated by distinct anesthetic types and dosages. The resulting spiking patterns reverberate in recurrent neuronal circuits and can synchronize large networks of cortical neurons by reciprocal coupling, which manifests as cortical oscillations on EEG. These oscillations are generated either within the cortex or the thalamus, or between these two densely interconnected regions (Figure 3A; reviewed in McCormick and Bal, 1997; Steriade, 2001; Fuentealba and Steriade, 2005; Franks, 2008; Ching and Brown, 2014; Akeju and Brown, 2017).
Figure 3.
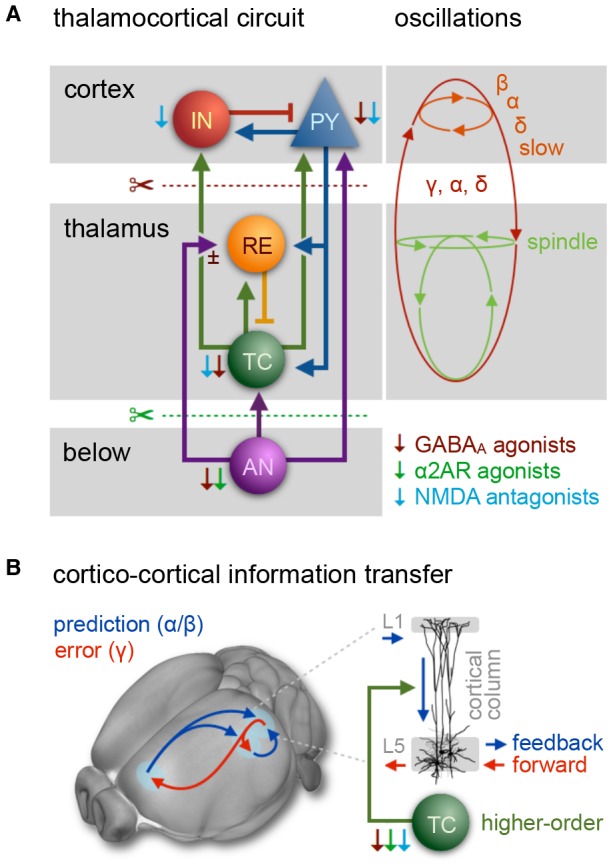
The thalamocortical circuit as wave generating unit, and cortical information transfer. (A) Scheme of thalamocortical circuit displays major interactions between GABAergic inhibitory interneurons (IN) and excitatory pyramidal cells (PY) in the cortex and thalamic GABAergic inhibitory reticular cells (RE) and excitatory thalamocortical neurons (TC). Arousal nuclei (AN) drive cortical desynchronization via cortical and thalamocortical circuits, including biphasic excitatory–inhibitory modulation (Sun et al., 2013). Anesthetic classes reduce the membrane potential of their respective target neurons (indicated by thin color-coded arrows), leading to inhibition and rebound spiking. Cortical oscillations are produced by synchronized periodic activity between two or more cell types. Transection below cortex (red scissors) leads to slow wave generation in the cortex; transection below thalamus (cerveau isolé, green scissors) leads to generation of sleep-like spindles and slow-delta waves. Anesthesia-induced oscillations: slow (<1 Hz), δ (1–4 Hz), α (8–15 Hz), β (15–30 Hz), γ (25–35 Hz), spindle (9–16 Hz). (B) Fronto-parietal information transfer along the cortical hierarchy. Feedback information (blue) is conveyed from higher-order frontal areas to primary sensory areas in the α/β band, feedforward information flow (red) from primary to higher-order areas in the γ band. In the predictive coding scheme feedback signals are considered predictive models of upcoming sensory states, whereas feedforward information represents the error resulting from a mismatch of a predictive model with an actual state. Feedback transmitted via long-range projections enters cortical columns at the dendritic trees of pyramidal cells in layer 1 (L1). The signal is transmitted to the soma of the same cells in layer 5, which requires driving input of higher-order TC nuclei. Feedback information transfer breaks down with the inhibition of higher-order TC nuclei at increasing depth of anesthesia. (A) Inspired by Ching and Brown (2014) and others. (B) Left panel: adapted from Sikkens et al. (2019). Note that visual higher-order areas in mice differ from those of primates, as reviewed in Glickfeld and Olsen (2017); Pennartz et al. (2019). Right panel: based on Suzuki and Larkum (2020).
Thalamic key players of rhythm generation are excitatory thalamocortical (TC) cells in intralaminar and medial thalamic nuclei (Baker et al., 2014; Flores et al., 2017). These higher-order TC nuclei receive and process input from cortical areas and serve as crucial relays of information transfer across the cortex (Theyel et al., 2010; Sherman, 2017; Mo and Sherman, 2019). Effective information transfer is thereby facilitated by frequency and phase synchronization of projection, relay, and target areas (Saalmann, 2014). In contrast, first-order TC nuclei primarily relay incoming sensory information to the cortex. First and higher-order TC nuclei undergo a major inhibitory impact of GABAergic thalamic reticular (RE) neurons, which project from the thin, outer shell of the thalamus—the thalamic reticular nucleus (as reviewed in Varela, 2014). RE neurons control TC traffic, synchronicity of firing patterns, and the level of arousal by targeted inhibition of TC cells (Sun et al., 2012). The level of depolarization in both TC and RE neurons is further regulated by afferents from cholinergic and monoaminergic arousal nuclei (Saper et al., 2010), which also modulate the membrane potential of cortical neurons: GABAergic inhibitory interneurons and glutamatergic excitatory pyramidal cells (Figure 3A).
GABAA Agonists Suppress Neural Activity Across the Central Nervous System
Volatile ethers and GABAA agonists such as isoflurane and propofol predominantly act on postsynaptic GABAA receptors of excitatory neurons by increasing the preference of those ligand-gated chloride channels for the open state (Bai et al., 1999; Lee and Maguire, 2014). This enhances the inward chloride current and facilitates hyperpolarization and thus inhibition of the postsynaptic cell. Presynaptic actions that impair neurotransmitter release (Hemmings et al., 2005, 2019; Baumgart et al., 2015) further contribute to reducing neural activity across the central nervous system (CNS), including the cortex, thalamus, striatum, brainstem, and even the spinal cord (Figure 1B; Bowery et al., 1987; Hemmings et al., 2005; Brown et al., 2010; Phillips et al., 2018). This exerts hypnotic effects and gradually renders the animal unconscious in a dose-dependent manner (Akeju and Brown, 2017; Flores et al., 2017).
An initial excitement is often observed at the induction of anesthesia, accompanied by a disinhibition of motor activity and the emergence of relatively fast cortical oscillatory activity in the beta band (~13–30 Hz; Figure 2A; Ching and Brown, 2014; Le Van Quyen et al., 2016). The precise mechanisms responsible for this paradoxical excitation have yet to be fully explained. Network models indicate the involvement of hyperpolarization-triggered inward currents that cause cortical circuits of excitatory and inhibitory neurons to produce periodic firing in the beta range (McCarthy et al., 2008). With increasing inhibitory tone and sedation, beta rhythms slow down to alpha frequencies (Figures 2A, 3A; McCarthy et al., 2008; Baker et al., 2014; Flores et al., 2017). This is the onset of stable sedation marked by the loss of the righting reflex (LORR; Baker et al., 2014; Flores et al., 2017).
Although alpha oscillations (8–15 Hz) are produced within the cortex (Mukamel et al., 2014), the generation of temporally and spatially coherent alpha rhythms across larger areas requires the participation of the thalamus (Ching et al., 2010; Flores et al., 2017). The GABAergic inhibition of TC cells is thought to trigger enhanced post-inhibitory rebound spiking that is paced by the decay rate of inhibition, and thus depends on the anesthetic dosage (Figure 3A; Ching and Brown, 2014). Thalamic and cortical alpha activities reinforce each other by reciprocal coupling, resulting in strong TC synchronicity (Figure 3A; Ching et al., 2010; Ching and Brown, 2014; Crunelli et al., 2015; Bhattacharya et al., 2019).
Dense recurrent projections from the thalamus convey broad spatial coherence of alpha oscillations in the frontal cortex (Flores et al., 2017). In contrast, occipital thalamic projections of high-threshold TC neurons, which produce coherent alpha oscillations in the awake state are suppressed by a reduction of hyperpolarization-activated cation currents, presumably by secondary drug effects (Ching et al., 2010; Cimenser et al., 2011; Vijayan et al., 2013). This causes a characteristic spatial shift in EEG alpha power from the posterior to the anterior part of the brain, a phenomenon known as anteriorization (Akeju et al., 2014b).
LORR is likely caused by impaired information transfer and integration across cortical areas due to changing oscillatory modes. When sending and receiving cells are tuned in phase, postsynaptic neurons receive input during their active periods and are more likely to fire. Phase alignment facilitates neural transmission across distant cortical areas, which also involves higher-order TC nuclei (Saalmann, 2014; Sherman, 2017; Mo and Sherman, 2019). Higher-order TC nuclei are engaged with GABAergic drug-induced frontal alpha waves in humans and rodents (Ching et al., 2010; Liu et al., 2013a; Baker et al., 2014; Flores et al., 2017), which might affect phase–frequency relationships between sending and receiving areas and thus contribute to the disruption of cross-cortical communication.
However, the loss of awake-alpha (and higher frequency) waves has been shown to be a more reliable marker for stable sedation (Blain-Moraes et al., 2015; Pavone et al., 2017). A loss of awake-alpha is typical for the first stage of non-rapid eye movement (N1) sleep (Prerau et al., 2017) and was correlated with a lack of behavioral response to stimuli in N1 sleep (Prerau et al., 2014) and under light sedation via sevoflurane (Pavone et al., 2017).
Awake-alpha waves play an essential role in information transfer and integration across the cortex (Jensen et al., 2019; Senzai et al., 2019). For instance, neural activity in the alpha band in contralateral sensorimotor areas has been shown to phase lock robustly to somatosensory stimuli. If these stimuli are perceived consciously, the stimulus locking spreads rapidly to the frontal, parietal, and ipsilateral sensorimotor regions (Palva et al., 2005). For unperceived stimuli, the phase locking is weak and restricted to the initial sensorimotor area.
Synchronous alpha activity in frontal, parietal, and sensorimotor regions facilitates recurrent information transfer between these areas due to a phase alignment of neural activity in transmitting and receiving areas. Such alpha-phase synchrony between primary sensory and higher-order cortical areas is considered a neural basis of attention, and is strongly mediated by frontal and parietal regions. Fronto-parietal information transfer is involved in many higher-order functions, including the formation of higher-order functional networks in the awake, resting state (see “Functional Connectivity and Murine Resting-State fMRI” section; Coull, 1998; Kastner and Ungerleider, 2000; Rees et al., 2002; Zeman, 2004; Klimesch et al., 2007; Palva and Palva, 2007; Sadaghiani et al., 2012; Fries, 2015; Han et al., 2019; Sikkens et al., 2019).
Recurrent processing along the cortical hierarchy accounts for the contextual modulation of stimuli, including perceptual and semantic interpretation, endowing them with spatiotemporal context and behavioral significance (Pennartz et al., 2019). One function of recurrent processing is expressed in the predictive coding framework, in which the brain is constantly generating and updating sensory models of the world (as reviewed in Pennartz et al., 2019; Sikkens et al., 2019). Feedback projections from higher-order (e.g., prefrontal) to early sensory areas are thought to convey or facilitate predictive models of upcoming sensory states via alpha/beta oscillations. A mismatch of a predictive model with actual sensory input results in error signals that are presumably fed forward from early sensory to higher-order areas via gamma oscillations—through different, partially overlapping cortical layers—to update the model in a perpetual feedback/feedforward loop (Figure 3B, left panel; van Kerkoerle et al., 2014; Zhang et al., 2014; Bastos et al., 2015; D’Souza et al., 2016; Chao et al., 2018; Michalareas et al., 2016; Kissinger et al., 2018; Zhang et al., 2019).
Feedback and feedforward information transfer are considered cornerstones of effective signal processing in the conscious brain (as reviewed in Lamme, 2018; Pennartz et al., 2019). LORR can probably be attributed to impaired feedback or feedforward information transfer (Pavone et al., 2017; Sanders et al., 2018; Redinbaugh et al., 2020). Long-range feedback modulation, which in primary sensory areas promotes both alpha waves (Jensen et al., 2019; Sikkens et al., 2019) and desynchronized activity (Harris and Thiele, 2011; Ecker et al., 2016), undergoes a breakdown with increasing anesthetic depth (Imas et al., 2005b; Raz et al., 2014; Hentschke et al., 2017; Sanders et al., 2018; Murphy et al., 2019).
A recent cellular study revealed that cortico-cortical feedback flow through the cortical layers (mediated by L5 pyramidal cells) requires the driving input of higher-order TC nuclei (Figure 3B, right panel; Suzuki and Larkum, 2020). Since these higher-order TC nuclei in turn depend on the driving input of cholinergic projections from the brainstem (Figure 3A; Trageser et al., 2006; Masri et al., 2006, 2008), they appear to be particularly susceptible to the effects of various anesthetics. At already 1% isoflurane the inhibition of higher-order TC nuclei causes a disruption of cortico-cortical feedback loops.
This causal relationship unifies two central hypotheses of consciousness, which had previously assumed a dependence on either cortical feedback or higher-order TC loops (Alkire et al., 2008; Boly et al., 2011; Mashour, 2014). Also, feedforward signal propagation toward higher-order cortical regions has been reported to be increasingly suppressed with thalamocortical inhibition (Massimini et al., 2005; Sellers et al., 2015; Casarotto et al., 2016; Hentschke et al., 2017; Sanders et al., 2018; Redinbaugh et al., 2020). Therefore, studying sensory pathways within the cortex of anesthetized mice is often limited to brain areas that receive direct sensory thalamic or subcortical input.
Neural responses to sensory stimuli in primary cortices are largely preserved across sensory modalities (Lamme et al., 1998; Detsch et al., 1999; Pack et al., 2001; Imas et al., 2005a; Greenberg et al., 2008; Schumacher et al., 2011; Haider et al., 2013; Milenkovic et al., 2014; Raz et al., 2014; Sellers et al., 2015). Yet, even low anesthetic dosages alter the spatial and temporal structure of neural firing patterns, thereby disrupting information processing, which relies on precise timing of ensemble activity (Luczak et al., 2015; Yuste, 2015). For example, neural responses to visual stimuli in mice anesthetized with 0.25–1% isoflurane extend into larger V1 areas and are temporally prolonged, as they are less shaped by directed inhibition compared to the awake state (Haider et al., 2013; Sellers et al., 2015). This has also been observed for other drugs and sensory modalities (Devonshire et al., 2010).
Crossing the Borders: GABAergic Slow Waves and Burst Suppression
Increasing doses of anesthetics bring a complete loss of movement (LOM), which coincides with a sudden rise in slow-delta power; i.e., delta oscillations (1–4 Hz) primarily in the frontal areas, and slow waves (<1 Hz) across the entire cortex (Figure 2A; Steriade et al., 1993b, c, d; Flores et al., 2017; Chamadia et al., 2019). Slow-delta waves are characterized by alternations of persistent desynchronized network activity (depolarized “UP” states) and generalized neural silence (hyperpolarized “DOWN” states) of varying duration (Steriade et al., 1993b, c, d, 2001; Luczak et al., 2007). Slow-delta rhythms impose strong oscillatory dominance on the cortical firing patterns by nesting oscillations of higher frequencies in distinctive sequences (Steriade, 2001; Fuentealba and Steriade, 2005). Delta oscillations are grouped themselves by slow waves into larger sequences, although both waveforms share a very similar structure.
The exact phase-amplitude coupling of slower and faster waves is a function of the anesthetic depth (Chamadia et al., 2019). With light sedation, alpha waves lie in the trough of slow-delta oscillations. With increasing anesthetic depth, they experience a shift in phase until they ride on top of the slow waves’ peaks. This phase-amplitude syntax causes a functional decoupling of neural activity, and thus cortical communication to collapse in crucial networks, leading to deep anesthesia (see “Functional Connectivity and Murine Resting-State fMRI” section).
Cortical delta and slow waves persist following removal of the thalamus, although under such conditions, slow waves (at ~0.3 Hz) clearly dominate the EEG (Figure 3A, red scissors; Steriade et al., 1993c; Steriade, 2001). Slow waves even occur in small cortical pieces in vitro (e.g., in the primary visual cortex) and are robust to various experimental perturbations (as reviewed in Sanchez-Vives et al., 2017). Therefore, they have been proposed to represent the default activity pattern of cortical networks. The UP states bear striking resemblance to the desynchronized firing patterns of the awake state (Destexhe et al., 2007). The transition between the two micro-states originates from activity-dependent adaptation, which accumulates during UP states, attenuates neural transmission, and eventually switches to the DOWN state. In this state of neural silence (or strongly reduced activity), the network recovers its excitability until it elicits the next sudden transition to the UP state, forced up and amplified by the firing rates of neighboring neurons (Compte et al., 2003; Destexhe and Contreras, 2006; Braun and Mattia, 2010; Mattia and Sanchez-Vives, 2012; Neske, 2015; Sanchez-Vives et al., 2017).
Delta oscillations consist of cortical and thalamic components that are generated in overlapping higher-order TC nuclei along with alpha oscillations at increasing inhibitory tone (Steriade et al., 1993c, 1994; Amzica and Steriade, 1997; Steriade, 2001; Flores et al., 2017). Thalamic delta rhythmogenesis results from the interplay between two distinct hyperpolarization-activated currents within TC neurons, whereas GABAergic RE cells provide the hyperpolarizing input under natural conditions (Figure 3A; McCormick and Pape, 1990; Soltesz et al., 1991; Steriade et al., 1994). Since TC cells are not synaptically coupled, they fire in frequency, but not necessarily in phase (Amzica and Steriade, 1997; Neske, 2015). Coupling of the clock-like thalamic delta patterns is mediated through interconnected cortical pyramidal cells, which generate bursts at similar intrinsic delta frequencies, thereby synchronizing cortical areas via recurrent TC projections (Amzica and Steriade, 1997; Neske, 2015). While delta power rises coherently in anterior areas, slow waves occur all over the cortex but not necessarily in phase, leading to cortical fragmentation at moderate doses and thus impaired information transmission (Lewis et al., 2012).
An essential component in generating slow and delta oscillations is the inhibition of subcortical arousal nuclei, which causes cortical and TC neurons to lose their depolarizing driving input (Figure 3A; Steriade et al., 1991, 1993a). An inhibition of key arousal nuclei is indeed sufficient to explain delta and slow wave generation in sleep (reviewed in Weber and Dan, 2016) and quiet wakefulness (Neske, 2015). However, the pattern characteristics of slow waves under natural conditions are distinct from those elicited by GABAergic anesthesia, which are produced under additional direct thalamic and cortical inhibition (Chauvette et al., 2011; Kenny et al., 2014; Busche et al., 2015; Akeju and Brown, 2017; Arena et al., 2017; Aggarwal et al., 2019). The full inhibitory package results in enhanced suppression of spontaneous desynchronized cortical background activity, which causes successively quiescent networks in the DOWN state with increasing anesthetic depth.
Such quiescent cortical networks of synchronous activity have been proposed to be ideal substrates for the propagation of dense traveling waves (Figure 4; Muller et al., 2018). Given that all neurons act in phase, neurotransmission reaches all post-synaptic cells during episodes of increased excitability. Triggering UP states in this low complexity environment instantly recruits neighboring neurons via massive recurrent excitation (Sanchez-Vives et al., 2017), so that even local events (like sensory inputs) can elicit dense traveling waves that entrain nearly all cells as they pass (Muller et al., 2018).
Figure 4.
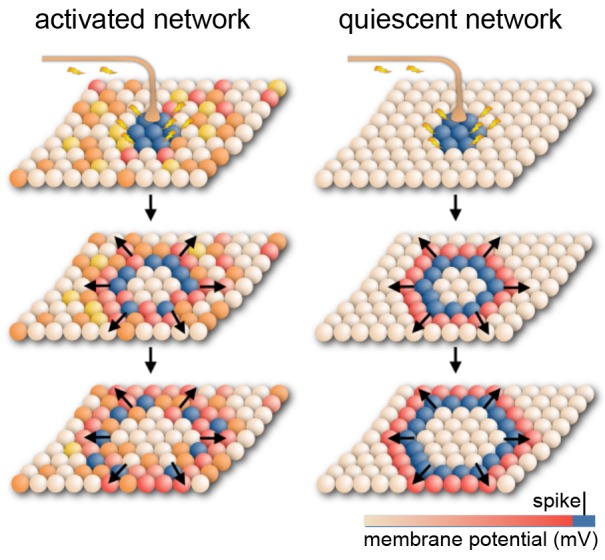
Model for cortical signal propagation by nearest neighbor recruitment. In an activated cortical network with desynchronized background activity (as observed in normal waking states), local stimulation elicits waves that weakly entrain neuronal spiking as they travel across the network. In a quiescent cortical network with almost no background activity (as in deeply anesthetized states) local stimulation elicits dense traveling waves, which recruit nearly all cells as they pass. Spheres represent neurons whose membrane potential is indicated by color. Based on Muller et al. (2018).
Such dense, traveling slow waves have been found to spread across the cortical surface, irrespective of anatomical boundaries, like a set of waves created by a drop in an oil bath (Figure 4; Massimini et al., 2004, 2007; Luczak et al., 2007; Stroh et al., 2013; Sanchez-Vives et al., 2017; Muller et al., 2018). Whether these waves remain local or propagate depends on the (local) brain state or desynchronization (Massimini et al., 2004, 2007; Nir et al., 2011; Vyazovskiy et al., 2011; Schwalm et al., 2017; Muller et al., 2018) and hence on anesthetic depth.
Aside from functional deafferentation and cortical inhibition, which provides a powerful substrate for long-range spread by nearest-neighbor recruitment, the synchronizing drive from the thalamus may add to the manifestation of slow waves (Crunelli and Hughes, 2010; Sheroziya and Timofeev, 2014; Crunelli et al., 2015; Neske, 2015). For the sake of translatability, functional brain studies should be generally performed at lower dosages than those leading to the dominance of slow wave and delta power, which affects neuronal excitability and stimulus–response properties (see “Anesthetic Depth and Consciousness—the Virtue of Translational Neuroimaging” section; Steriade et al., 1993b, c; Petersen et al., 2003; McGinley et al., 2015b).
Higher anesthetic dosages lead to burst suppression-characterized by increasingly prolonged “DOWN” micro-states alternating with periods of brief bursts of spikes and waves (Figure 2A; Steriade et al., 1994; Lewis et al., 2013; Amzica, 2015; Purdon et al., 2015). This is an increasingly hyperexcitable state in which large networks of cortical neurons suddenly discharge in tight synchrony (Steriade et al., 1994; Kroeger and Amzica, 2007; Ferron et al., 2009). Subsequently they fall into a post-burst refractory period, due to metabolic exhaustion (Hirsch and Taylor, 2010; Ching et al., 2012; Ching and Brown, 2014). This period of complete cortical silence is induced by adenosine triphosphate (ATP)-sensitive cation channels, which open when ATP decreases below critical levels (Cunningham et al., 2006). The resulting cation influx renders neurons unable to produce action potentials. Upon metabolic regeneration, the channels close again, and cortical networks gradually regain hyperexcitability by depolarizing, until they exhibit the next discharge (Kroeger and Amzica, 2007). Hyperexcitability is generated within the cortex as a result of the functional impairment of cortical afferents (Echlin et al., 1952; Henry and Scoville, 1952; Hughes, 1986; Niedermeyer et al., 1999; Kenny et al., 2014; Amzica, 2015).
Thalamic sensory transmission is preserved during isoelectric and burst episodes, although cortical discharge rates are substantially reduced during isoelectric periods (Detsch et al., 2002). During periods of cortical hyperexcitability, even subliminal stimuli are sufficient to trigger bursting activity (Yli-Hankala et al., 1993; Hartikainen et al., 1995; Hudetz and Imas, 2007; Kroeger and Amzica, 2007; Amzica, 2009). Spontaneous and evoked bursts can spread across large cortical areas (Steriade et al., 1993b; Timofeev et al., 2000; Land et al., 2012) and may further engage remote hippocampal (Sirota et al., 2003; Hahn et al., 2006; Ji and Wilson, 2007; Busche et al., 2015) and thalamic structures (Steriade et al., 1993c; Stroh et al., 2013; Sheroziya and Timofeev, 2015), presumably by excitatory projections (Leong et al., 2016). In this regard, burst suppression events can be considered as non- or quasi-periodic slow waves with a long refractory time and high discharge.
Further increasing the anesthetic dosage leads to longer suppression, shorter burst periods, and less reactivity to stimuli (Hartikainen et al., 1995), which culminates in complete neural inexcitability and finally isoelectricity due to increasing (thalamo)cortical and subcortical suppression (Figure 2A; Kroeger and Amzica, 2007).
α2AR Agonists Induce a Sleep-Like State by Suppressing General Arousal
Sympatholytics or α2AR agonists such as xylazine, medetomidine, or its potent dextro enantiomer dexmedetomidine exert their sedating and antinociceptive effects primarily by acting on presynaptic α2A adrenergic receptors of noradrenergic cells that project from the locus coeruleus. This results in a hyperpolarization of the affected neurons and a reduction in the release of noradrenaline to their target sites (Correa-Sales et al., 1992; Jorm and Stamford, 1993; Chiu et al., 1995; Van Bockstaele et al., 1999). The locus coeruleus is a major arousal nucleus that projects to the basal forebrain (a subcortical arousal structure, which regulates cortical states by cholinergic efferents; Nelson et al., 2005; Hoover and Vertes, 2007; Pal et al., 2018), intralaminar nucleus of the thalamus, thalamic reticular nucleus, preoptic area of the hypothalamus, and diffusely into the cortex (Figure 1C; Asanuma, 1992; Nelson et al., 2003; Samuels and Szabadi, 2008a; Saper et al., 2010; Zhang et al., 2015; Fu et al., 2017; Brown et al., 2018a). Nociceptive pathways are further affected by the direct activation of α2 receptors in the spinal cord (Andrieu et al., 2009).
Decreased noradrenaline release in the preoptic area of the hypothalamus causes disinhibition (and thus excitation) of local endogenous sleep-promoting cells that send inhibitory projections to other key arousal nuclei in the midbrain and pons (Figure 1C; Sherin et al., 1998; Saper et al., 2005; reviewed in Saper et al., 2010; Scammell et al., 2017). This causes an inhibition of widely-projecting neurons in these arousal nuclei, which in turn decreases the depolarizing input to thalamic and cortical areas, leading to sleep-like, spindle (9–16 Hz), and slow-delta (0.1–4 Hz) oscillations. This pattern is distinct from that induced by GABAA agonists due to the lack of direct cortical and thalamic inhibition (Figures 2A–C, 3A; Noreika et al., 2011; Baker et al., 2014; Nasrallah et al., 2014a; Akeju and Brown, 2017; Banks et al., 2017).
Similar sleep-like states were observed following a transection below the thalamus in cats, the so-called cerveau isolé (the isolated cerebrum; Figure 3A, green scissors; Bremer, 1935; Steriade et al., 1993c). This dramatically demonstrates that the disconnection of thalamic and cortical circuits from the input of subcortical arousal nuclei suffices to produce oscillatory and spiking patterns, similar to non-REM sleep at the N2 stage and sedation elicited by α2AR agonists (Akeju and Brown, 2017).
Under light sedation, spindle rhythms occur in brief bursts of ~0.5–3 s (Figures 2A,C; Baker et al., 2014; Nasrallah et al., 2014a; Purdon et al., 2015). Spindles are generated within the thalamic RE nucleus, which is considered the spindle pacemaker (Steriade et al., 1985; Halassa et al., 2011; Kim et al., 2012). Evidence for this includes the deafferentation of this structure from the cortex and the remaining thalamus, upon which the nucleus continues to generate spindles (Steriade et al., 1987). Rhythmogenesis thereby strongly depends on the level of hyperpolarization of thalamic RE neurons and the intactness of their long and thin dendrites, which are richly endowed with low-threshold hyperpolarization-triggered Ca2+ channels (as reviewed in Fuentealba and Steriade, 2005; Crandall et al., 2010; Astori et al., 2011; Zaman et al., 2011).
Suppression of arousal nuclei leads to decreased depolarizing input to RE cells (McCormick, 1992; Saper et al., 2010; Sun et al., 2013), which can cause hyperpolarization below the threshold, leading to strong dendritic Ca2+ spikes. This results in depolarization and rhythmic bursts of action potentials that are sustained by additional voltage-gated cation channels (reviewed in Fuentealba and Steriade, 2005; Lüthi, 2014). GABAergic transmission and electrical coupling in recurrent networks of RE neurons are sufficient to synchronize oscillations in the range of spindles (Bazhenov et al., 1999; Fuentealba and Steriade, 2005).
In the intact brain, the initiation and synchronization of spindles is supported by TC and cortical circuits. Glutamatergic stimuli can easily trigger low-threshold Ca2+ spikes in RE cells (Crandall et al., 2010). Therefore, spindles can be initiated by diverse inputs, including spontaneous oscillating TC cells or cortical volleys that impinge on RE networks (Figure 3A; Destexhe et al., 1996). For example, the cortical transitions to the UP state of slow waves may be quickly followed by a resulting spindle wave, which is a common sequence in slow wave sleep, known as the K-complex (Steriade et al., 1993b; Amzica and Steriade, 1997). Notably, K-complexes have not been described for sedation through α2AR agonists (Huupponen et al., 2008; Nasrallah et al., 2014a; Akeju et al., 2016a).
A burst of action potentials in an RE neuron causes hyperpolarization, rebound spiking and thus neural firing in multiple TC neurons; this reverberates in large thalamic networks by recurrent synapsing (Figure 3A; Bazhenov et al., 1999; Beenhakker and Huguenard, 2009). The strong synchronized rhythms of TC neurons can further entrain cortical pyramidal cells and interneurons in both prefrontal and sensory cortices (Peyrache et al., 2011). Cortico-cortical recruitment may cause further synchronization (Kandel and Buzsáki, 1997). The short spindle episodes are terminated via intrinsic ionic mechanisms in both thalamic RE and TC cells that are triggered by high concentrations of accumulated intracellular Ca2+ (alongside other strategies reviewed in Lüthi, 2014).
Delta waves resemble spindles in that they do not appear continuously in sleep or for light α2AR-induced sedation (Figure 2B; Steriade et al., 1993a, c; Baker et al., 2014). This changes at higher anesthetic dosages through a further suppression of depolarizing arousal inputs to thalamic RE neurons; they further hyperpolarize, and their firing patterns subsequently change from spindle to delta waveforms (Figures 2A,B; Nuñez et al., 1992; Destexhe et al., 1994).
The transition to delta rhythms is mirrored in TC circuits and entrains cortical networks. The result is continuous delta oscillation in thalamic and cortical areas, whose onset has been reported to coincide with a discrete drop in frequency at the instant of dexmedetomidine-induced LORR (Baker et al., 2014). This is accompanied by a significant phase shift of delta waves in the central medial thalamus as compared to cortical areas. Such phase shifts in higher-order TC nuclei can disrupt cortico-thalamo-cortical communication, causing a breakdown of information transfer between cortical areas (Slézia et al., 2011; Saalmann, 2014; Mo and Sherman, 2019; Suzuki and Larkum, 2020) and thus LORR.
First-order thalamic relay nuclei may not participate in producing continuous delta oscillations (Baker et al., 2014). Instead, they intensify spindle generation following LORR. The transient inhibition of TC neurons during spindle periods has been reported to prevent them from transferring sensory information to the cortex (Steriade and Contreras, 1995; Fuentealba and Steriade, 2005). Accordingly, spindle density correlates with the gating of sensory inputs in sleep (Dang-Vu et al., 2010; Wimmer et al., 2012; Chen et al., 2016).
However, in α2AR-induced sedation, primary sensory routes remain largely intact. Medetomidine has been shown to preserve cortical responsiveness to acoustic stimuli in primary sensory areas in rats (Banks et al., 2017). Subcutaneous electrostimulation of the paw has been reported to evoke slightly reduced potentials in the primary somatosensory cortex (S1) compared to GABAergic drugs (Hayton et al., 1999; Li et al., 2003). Yet, the amplitudes of somatosensory evoked potentials in S1 are not affected with increasing concentrations of medetomidine (Li et al., 2003; Nasrallah et al., 2014a). Spindle activity in the paw region of S1 occurs at medetomidine concentrations that are commonly used for fMRI (Nasrallah et al., 2014a).
The induction of LORR at continuous delta activity has been demonstrated in a study that kept rats in a rotating tube in which they had to constantly adapt their position until they rolled onto their sides and remained supine (Baker et al., 2014). Such constant active behavior increases the activity of arousal nuclei (Marlinski et al., 2012; Furth et al., 2017) and interferes with the “sleep-like” sedative state elicited by α2AR agonists (Kamibayashi and Maze, 2000; Venn and Grounds, 2001). In fact, sedation induced by α2AR agonists is far more arousable than for GABAergic drugs (see “Sensory Processing and the Key Challenges in Murine fMRI” and “Functional Connectivity and Murine Resting-State fMRI” sections; Noreika et al., 2011; Sanders et al., 2012; Akeju and Brown, 2017; Banks et al., 2017). Thus, higher anesthetic dosages were required to induce LORR in actively moving rats (Baker et al., 2014) than to induce the sedation sufficient for an fMRI experiment (Nasrallah et al., 2014a). For the GABAergic drug propofol, LORR occurred with the onset of alpha waves both in rotating tubes (Baker et al., 2014) and in the freely resting rodent (Flores et al., 2017).
NMDA Receptor Antagonists Primarily Affect Cortical Neural Activity
Ketamine is an NMDA receptor antagonist that provides dissociating, quasi-hypnotic effects by selectively binding and blocking NMDA receptors that are primarily expressed in cortical inhibitory and excitatory neurons, but also in thalamic TC and RE cells (Deleuze and Huguenard, 2016), and to a lesser extent in subcortical arousal nuclei and peripheral nerves (Gunduz-Bruce, 2009). Ketamine generates opposing effects in a dose-dependent manner. At lower concentrations, it binds preferentially to NMDA receptors on cortical GABAergic inhibitory interneurons, which show about 10-fold higher sensitivity to NMDA blockade than pyramidal neurons (Grunze et al., 1996).
NMDA receptor blockage prevents cation influx and depolarization, resulting in decreased GABAergic transmission to downstream excitatory neurons (Homayoun and Moghaddam, 2007; Seamans, 2008). Thus, excitatory neurons are disinhibited and become depolarized (Figure 1D, Brown et al., 2011; Phillips et al., 2018; Picard et al., 2019). An appropriate depolarization of cortical pyramidal neurons can elicit fast oscillations in the lower gamma band (Steriade et al., 1991; Nuñez et al., 1992; Gray and McCormick, 1996), synchronized by rhythmic inhibition (Buzsáki and Chrobak, 1995; Olufsen et al., 2003; Börgers et al., 2005).
The fast rhythm generation is promoted by ketamine actions in thalamic circuits, which switches the firing patterns of TC and RE neurons from the burst mode to the tonic generation of single action potentials (Anderson et al., 2017; Mahdavi et al., 2019). Such synchronous fast oscillations in the gamma band (25–80 Hz) typically emerge with cortical processing during higher-level mental activity, and in REM sleep, which is associated with dreaming mentation (Llinás and Ribary, 1993).
Low-dose ketamine elicits gamma waves in a narrow frequency band of ~25–35 Hz and diffuse excitatory cortical activity (Akeju et al., 2016b). At this stage, hallucinations, dissociated states, euphoria, and dysphoria have been reported in clinical use: they have been attributed to preserved communication across brain areas at low inhibitory modulation and control, as well as a disruption of dopaminergic neurotransmission in the prefrontal cortex (Moghaddam et al., 1997; Purdon et al., 2015).
At higher doses, ketamine increasingly begins to block NMDA receptors at excitatory pyramidal neurons, causing cortical inhibition to predominate. It further suppresses arousal pathways by blocking excitatory projections from the parabrachial nucleus and from the medial pontine reticular formation in the brainstem to the thalamus and to the basal forebrain (Boon and Milsom, 2008; Fuller et al., 2011; Brown et al., 2018a). In turn, gamma waves become interspersed with slow-delta oscillations (Figure 2B; Ruiz-Mejias et al., 2011; Akeju et al., 2016b), which are augmented by direct drug action in the thalamus (Kiss et al., 2011; Zhang et al., 2012). The suppression of higher-order TC loops (Suzuki and Larkum, 2020) and a breakdown of cortical coherence is considered likely to be the mechanism that induces LORR (Pal et al., 2015; de la Salle et al., 2016; Schroeder et al., 2016; Brown et al., 2018a).
Ketamine can cause regional hypo- and hyperactivation across the cortex (Porro et al., 2004) and introduces further complexity to the processing of external stimuli (Oye et al., 1992; Zandieh et al., 2003; Schwertner et al., 2018). Besides antinociception mediated by direct inhibition of peripheral nociceptive afferents expressing NMDA receptors (Sinner and Graf, 2008), ketamine has also been shown to persistently reduce aversive responses to noxious stimuli in a top-down manner by prolonged suppression of hyperactive neurons in the anterior cingulate cortex (ACC) in rodent chronic pain models (Zhou et al., 2018). Low dosages increase SEP in rats and mice, even compared to the awake state (Franceschini et al., 2010; Michelson and Kozai, 2018) although the degree depends on the mouse strain (Maxwell et al., 2006). SEP from the cortex to higher-order TC neurons are decreased, illustrating a disturbed functional state of cortico-thalamo-cortical and thus cortico-cortical circuits (Anderson et al., 2017). Low-dose ketamine increases power in the gamma band, but also delta power can increase more significantly than for low-dose isoflurane (Michelson and Kozai, 2018).
Metastability of Brain States, Hysteresis, and Behavioral Monitoring
The comparison of brain states induced by various anesthetic compounds and dosages illustrates the diversity of mechanisms that lead to sedation. Whether an adjusted brain state can be stably maintained throughout the entire duration of an fMRI session remains to be clarified. Volatile ethers and intravenously injectable anesthetics appear to permit relatively stable maintenance of cortical oscillation patterns (Figures 2B,C; Purdon et al., 2013, 2015; Flores et al., 2017). However, transitions between two or more brain states have been observed over longer periods (1 h) at fixed concentrations of isoflurane in rats (Hudson et al., 2014). The transitions occurred predominantly in the lower-frequency band and could be well observed when the data were expressed on a logarithmic scale (Figure 2D).
The authors referred to this condition as “metastable” or potentially “multistable,” given that intermediate brain states shifted between two or more attractors distributed in phase space (for further discussion, see Breakspear, 2017; Hudson, 2017). There is evidence that such meta- or multistability is a general feature of brain states under anesthesia for various compounds and across species, although studies dedicated to detail such transitions over long periods are sparse (reviewed in Hudson, 2017). To what extent the transition of brain states may jeopardize the concordance of results across experiments (e.g., by exerting significantly different effects on signal processing) remains an open question, and has to be established for specific anesthetic protocols applied.
The reported state transitions were observed during recovery of consciousness; after an initial concentration of 1.75% isoflurane for 1 h, which reliably produced burst suppression, the concentration was reduced by 0.5%, maintained at that level for 1 h, and the process was repeated over a total of 6 h. Given this design, the dynamics of metastability were likely swayed by neural inertia—an intrinsic feature of neural circuits to resist swift transitions between consciousness and unconsciousness (Friedman et al., 2010; Proekt and Hudson, 2018; Proekt and Kelz, 2018). During recovery of consciousness neural inertia tends to trap the brain in an unconscious state. Due to this “stickiness,” a lower anesthetic dosage is required to maintain a similar anesthetic depth for the emergence from unconsciousness, compared to the induction of anesthesia (Hudson et al., 2014; Hudson, 2017). This dependence of the brain state on its history (hysteresis effect) cannot be explained by pharmacokinetic actions (see “Functional Connectivity and Murine Resting-State fMRI” section; Kelz et al., 2008; Friedman et al., 2010), and should be considered in preclinical studies for which an initial bolus induction of anesthesia is common practice.
Another issue in maintaining an intermediate brain state throughout an fMRI experiment might arise from nociceptive or stressful stimuli. Clinical experience and preclinical studies have shown that noxious stimuli, as well as direct stimulation of key arousal nuclei, can shift cortical states from slow synchronized towards highly desynchronized oscillations (Figure 2E; Hudetz et al., 2003; Solt et al., 2014; Vazey and Aston-Jones, 2014; Akeju and Brown, 2017; Sanders, 2017; Pal et al., 2018; Hayat et al., 2019). When the activity of the arousal-related nuclei exceeds a certain level, the animal wakes up from light anesthesia, and falls back into sedation when the activity subsides (Figure 2E; Solt et al., 2014). Consequently, an anesthetic protocol should be tailored, in terms of class and dosage, to the experimental task at hand. If the aim of a study is to assess functional connectivity (FC) at rest, there is no need to adjust the anesthetic depth so that the mouse remains unresponsive to nociceptive stimuli.
This calls into question the idea of the minimum alveolar concentration (MAC) in rodent fMRI—a well-established behavioral measure of anesthetic depth for volatile ethers (Steffey, 2017). Briefly, a MAC of 1.0 is defined as the average of the lowest anesthetic concentration that prevents a behavioral response upon a standardized pain stimulus, and the highest concentration that still permits a nocifensive response in 50% of tested subjects (Eger et al., 1965; Quasha et al., 1980). A MAC of 0.7 in rats has been reported to suppress the righting reflex and active attempts to withdraw; at a MAC of 0.3 (“MAC-awake”), frequent movements of the snout including sniffing, chewing, licking, and gross limb movements still occurred (Hudetz, 2002). However, these behavioral markers are not dependent on the application of any nociceptive stimulus that is not applied in the actual experiment.
Anesthetic depth can be monitored by behavioral markers and pupil dilation diameter to assess the level of arousal (Erisken et al., 2014; McGinley et al., 2015a; Joshi et al., 2016; Reimer et al., 2016; Binda and Gamlin, 2017; Shimaoka et al., 2018). Behavioral observation is indispensable to determine the level of sedation and immobilization of an animal, although the capacity to infer the actual brain state from these responses is limited (Pal et al., 2018). Hence, it is highly encouraged to adjust and compare anesthetic protocols based on oscillatory signatures and neural response properties outside the MR environment. Behavioral monitoring becomes increasingly crucial as different anesthetic classes and combinations of drugs are employed to sedate the animal (see “Multimodal Anesthesia in Translational fMRI” section).
Anesthetic Depth and Consciousness—the Virtue of Translational Neuroimaging
In general, anesthetic mechanisms are highly conserved across species (Achermann and Borbély, 1997; Steriade et al., 2001; Mölle et al., 2002; Eschenko et al., 2006; Destexhe et al., 2007; Buzsáki and Moser, 2013; Shein-Idelson et al., 2016) and the oscillatory signatures of humans and mice are virtually similar upon applications of specific anesthesia and stages of anesthetic depth (Seth et al., 2005; Flores et al., 2017; Guidera et al., 2017; Hudson, 2017; Storm et al., 2017; Olcese et al., 2018). This relation provides a rough means with which to interpret brain states in terms of perceived anesthetic depth, stress, or pain. In humans, specific brain states can be linked to the introspective experience, as revealed by subjective reports. Using oscillatory signatures, a third-person observer can precisely specify the moment at which the “lights switch off,” from the first-person perspective (Purdon et al., 2015; Brown et al., 2018b). For GABAergic drugs, this is the emergence of slow-delta oscillations (Figure 2C; Purdon et al., 2013, 2015).
Traditionally, this transition point is considered to be the “loss of connected consciousness” (LOC; Sanders et al., 2012) and is defined as a loss of sensory perception and interoception—the perception of internal processes, including pain, anxiety, stress, discomfort, or sense of time (Purdon et al., 2013, 2015; Warnaby et al., 2016; Sleigh et al., 2018; Chamadia et al., 2019). This is distinguished from “disconnected” phenomenal consciousness, like the awareness of pure darkness (Sanders et al., 2012; Sleigh et al., 2018) or dreaming, although dreams have often been reported for medetomidine-induced LOC (Akeju and Brown, 2017; Mashour and Hudetz, 2017, 2018).
In the state of propofol-induced LOC, innocuous sensory stimuli such as words or tones could no longer be perceived, and no hemodynamic responses to these stimuli were detected using fMRI (Ní Mhuircheartaigh et al., 2013; Warnaby et al., 2016; Lichtner et al., 2018). However, BOLD patterns in response to nociceptive stimuli were partially preserved (Lichtner et al., 2018). These findings imply that the oscillatory signatures associated with LOC may be useful as a marker delineating the lower limit of anesthetic depth in sensory fMRI (Figures 2A,C).
Another transition point can be determined based on behavioral observation—the “loss of behavioral responsiveness” (LOR), which occurs earlier, approximately with the appearance of frontal alpha oscillations and before slow waves dominate the EEG (Figure 2C; Purdon et al., 2013; Warnaby et al., 2016). At this point, subjects fail to exhibit volitional responses to sensory or even noxious stimuli (Sanders et al., 2012; Ní Mhuircheartaigh et al., 2013; Purdon et al., 2013; Warnaby et al., 2016; Sleigh et al., 2018). Stimuli are still perceived and processed when they are applied, as indicated by fMRI data and subjective reports (Warnaby et al., 2016). Volunteers described this as a state of detachment from the stimuli and from “themselves”—i.e., they were not fully unconscious, but also not aware that the stimuli were related to them. Tones that were presented rather subtly and very briefly (1 kHz, 60 ms) were not sufficient to elicit significant BOLD responses beyond the thalamic relay nuclei. The BOLD patterns evoked by words and nociception, on the other hand, were reported to reflect closely those of the conscious state, with substantially lower activity in only the right dorsal anterior insular cortex (Warnaby et al., 2016). This area is reportedly associated with body ownership and self-agency (Warnaby et al., 2016; Lichtner et al., 2018; Sleigh et al., 2018). The anesthetic inhibition of the anterior insula and related interoceptive networks led the authors to reformulate their understanding of the hypnotic effects of anesthesia as a gradual disruption of “selfhood,” which occurs with an increase of anesthetic depth, finally leading to oblivion (Sleigh et al., 2018).
This offers not only a direct link between introspective reports, behavioral and neuroimaging markers, but also a vivid example of how the examination of large-scale networks using fMRI can complement models of LOC that are based on the suppression of feedback or feedforward information transfer along the cortical hierarchy (see “GABAA Agonists Suppress Neural Activity Across the Central Nervous System” section; reviewed in Pennartz et al., 2019; Sikkens et al., 2019). Because similar transitions in oscillatory signatures can be observed in humans and rodents, maintaining and stabilizing a brain state between LOR (corresponding to LORR in animals; Baker et al., 2014; Banks et al., 2017; Flores et al., 2017) and LOC (corresponding to LOM in animals, and the occurrence of slow-delta waves; Flores et al., 2017) could be an attractive target state in which to perform fMRI in mice, at least for sensory perception tasks (Figures 2A,C). Hemodynamic coupling does not appear to be directly affected by propofol (Veselis et al., 2005). However, potential vasomodulatory effects of anesthesia have to be taken into account when inferring dose-dependent suppression of neural activity based on fMRI (Table 2; Aksenov et al., 2015).
Table 2.
Effects on hemodynamic integrity of anesthetics used in mouse fMRI.
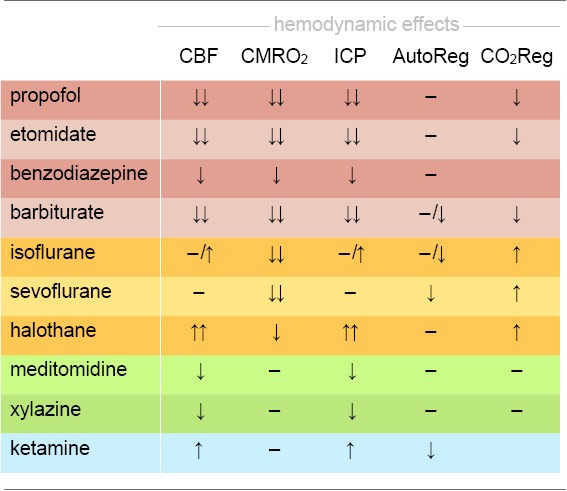 |
Anesthetics are listed according to their classes: GABAA agonists, including injectable drugs (red) and volatile ethers (yellow), α2AR agonists (green), and NMDA antagonists (blue). Vascular effects including vasodilation and vasoconstriction may impact on cerebral blood flow (CBF), intracranial pressure (ICP), or cerebral autoregulation (AutoReg). Drugs may further affect the cerebral metabolic rate of oxygen consumption (CMRO2) and the regulation of the CO2 metabolism (CO2Reg). Effect: ↓ reduction; ↓↓ strong reduction; ↑ increase; ↑↑ strong increase;–no change. Based on Bittner et al. (2019).
Murine fMRI and Hemodynamic Integrity
Research on murine fMRI has grown exponentially from only a handful of publications in the first decade (Huang et al., 1996; Ahrens and Dubowitz, 2001; Mueggler et al., 2003; Xu et al., 2003, 2005) to more than 50 in the following. Nevertheless, anesthesia in murine fMRI presents a multitude of challenges beyond the determination of functional brain states and neural response properties, and anesthetic protocols are still far from being effectively tailored to meet murine physiological and hemodynamic demands. Preserving hemodynamic integrity that approximates a physiological state is essential to exploit the two key advantages of murine fMRI: non-invasively investigating whole-brain functional dynamics, and the opportunity to directly compare these dynamics across species, including humans. The experiments that have been performed can be classified into at least four categories: (1) sensory perception; (2) nociception and pain; (3) FC based on the resting state; and (4) within-brain stimulation involving the use of opto- and chemogenetics. Each category reveals particular aspects of anesthetic effects in fMRI and deserves consideration in terms of the requirements for anesthetic protocols that are appropriate to obtaining neuroimaging data of translational value.
Sensory Processing and the Key Challenges in Murine fMRI
Sensory studies were among the earliest and most elementary applications of murine fMRI. Nevertheless, only a handful of publications report on innocuous sensory stimulation tasks that address the “natural” sensory perception routes of primary organs—like eyes, ears, nose, skin, or whiskers—using various anesthetic protocols. The very first mouse fMRI study (Huang et al., 1996) concerned visual perception and applied the GABAA-positive allosteric modulator and agonist pentobarbital; subsequent olfactory studies used urethane (Xu et al., 2003, 2005). Later visual (Niranjan et al., 2016) and auditory (Blazquez Freches et al., 2018) studies applied the α2AR agonist medetomidine, and deflection of the vibrissae was conducted under low-dose (0.5–1%) isoflurane (Kahn et al., 2011). All these studies identified modality-relevant sensory pathways, including primary sensory and partially preserved secondary cortical and thalamic structures. Higher-order or association areas have not been reported.
Technical Detection Limit
While this is in principle an encouraging situation, the reality of implementing mouse fMRI in the laboratory is fraught with peril. Sensory fMRI requires meticulous fine-tuning of murine physiology to compensate for anesthetic side effects, in conjunction with advanced technical equipment to boost the BOLD effect, which increases linearly with the magnetic field strength and venous blood volume (Kim, 2018). A recent study vividly illustrated how the field strength determines whether or not activation of entire brain areas may be detected. Subcutaneous electrostimulation of the murine paw is expected to elicit activity in the contralateral ventral thalamic nuclei, which relay the signal along the spinothalamic tract to the primary (S1) and secondary (S2) somatosensory cortices. Nevertheless, BOLD responses to electrostimulation were found to be limited to the contralateral S1 in mice under ketamine–xylazine at 9.4 T (Figure 5A; Shim et al., 2018), and under medetomidine at 9.4 (Nasrallah et al., 2014a) and 11.7 T (Adamczak et al., 2010). However, the same ketamine–xylazine protocol at 15 T revealed the expected BOLD patterns in all three somatosensory key areas (Figure 5A; Jung et al., 2019).
Figure 5.
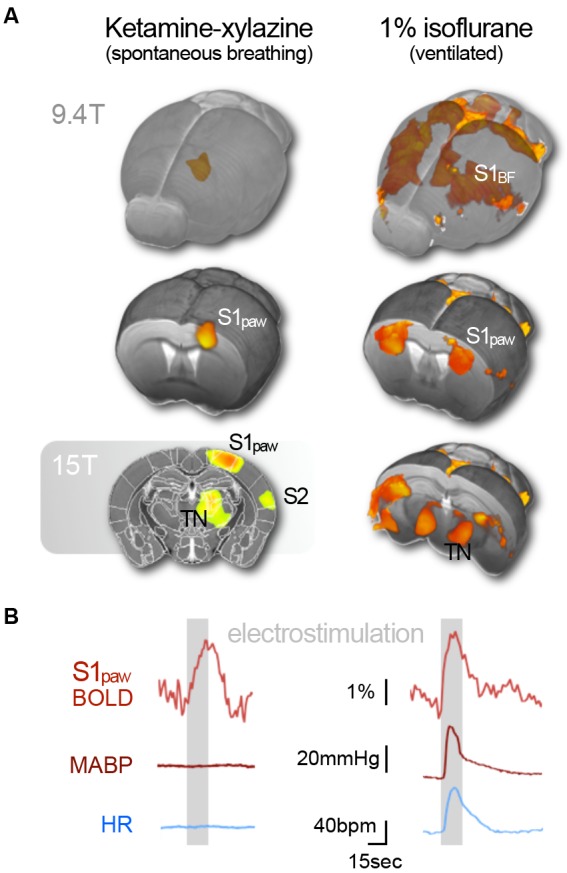
Subcutaneous electrostimulation of the paw in spontaneous breathing and mechanically ventilated mice under two different anesthetic regimens. (A) Blood oxygenation level-dependent (BOLD) patterns show unilateral responses in the contralateral paw region of S1 for spontaneous breathing mice under ketamine–xylazine. Increasing the field strength to 15 T reveals additional pattern in S2 and thalamic regions not visible at 9.4 T. In contrast large bilateral patterns were observed in ventilated mice under 1% isoflurane. (B) Signal time courses depict substantial surges in mean arterial blood pressure (MABP) and heart rate (HR) in ventilated, but not in spontaneous breathing animals. Data were acquired in-house reproducing findings from Reimann et al. (2018) and Shim et al. (2018). Functional map superimposed on gray shading (15 T) is adapted with permission from Jung et al. (2019).
To boost the BOLD signal, small-bore MR scanners can be equipped with a cryogenically cooled radiofrequency coil that amplifies the temporal SNR by a factor of up to 3 (reviewed in Niendorf et al., 2015). Increasing the statistical power by multiple repetitions of a task similarly facilitates the detection of weak BOLD effects. Both strategies reduce noise, but do not increase the sensitivity to T2*—the measure of relative changes in blood oxygenation, which determines the intrinsic technical detection limit of an MR system. To amplify a signal, it must be above the detection threshold. However, weak BOLD responses have been observed in the S2 and the thalamus at 9.4 T (Jung et al., 2019), strongly suggesting that these areas can be detected via signal amplification. The studies reported above employed MR surface coils that lose sensitivity for deeper brain areas. In addition to this technical issue, the low BOLD signal in deeper brain areas is likely physiological in nature, and may be caused by the sparser overall thalamic cell density, compared to the cortex (Meyer et al., 2013), by different densities of noradrenergic afferents in these areas (King et al., 1995; Wang et al., 1996) or otherwise asymmetric impact of reduced subthalamic drive, by unequal anesthetic effects on the vasculature in different brain regions (Lei et al., 2001), or by the experimental setup that can create a thermal gradient between the dorsal–ventral axis of the mouse brain (see below).
Vasomodulation, Body Temperature, and Respiratory Depression
The modulation of vascular properties across the brain is a key challenge for fMRI under anesthesia—even more so due to the temperature sensitivity of vessels. All anesthetics affect thermoregulation and render mice poikilothermic. Due to their high surface area-to-mass ratio, mice quickly adopt to their environmental temperature. Although blood circulation distributes heat energy across the body, heating pads below the trunk and cool surface MR units attached to the scalp may induce thermal gradients across the mouse, which means the brain temperature is neither homogeneous across all areas, nor accurately reflected by the temperature reading of a rectal probe (Reimann et al., 2016). Aside from a thermal dependency of neural activity that has been shown to alter brain states, and hence anesthetic depth (Volgushev et al., 2000; Reig et al., 2010; Sheroziya and Timofeev, 2015; Schwalm and Easton, 2016), vessels dilate with increasing temperature. Vasoconstriction induced by cooling the scalp can boost cortical BOLD responses to electrostimulation by about 30%, although this is not physiological (Baltes et al., 2011).
Many anesthetics directly affect vascular dilation and reactivity, which can substantially alter baseline CBF and hemodynamic responses (see Table 2, Franceschini et al., 2010; Masamoto and Kanno, 2012). In contrast to cooling, vasoconstrictive effects of α2AR such as medetomidine and xylazine (Sinclair et al., 2003; Fukuda et al., 2013) might hinder the thorough vasodilation of arterioles in response to neural activity and impair BOLD responses in mice (Nasrallah et al., 2014a; Schroeter et al., 2014). Anesthetic-induced vasodilation, on the other hand, increases the baseline blood flow and hence decreases the magnitude of relative changes in CBV, CBF, and BOLD in response to neural activity. Isoflurane is commonly reported to have strong vasodilatory effects (Sharp et al., 2015; Petrinovic et al., 2016; Cao et al., 2017), causing decreases of up to 75% in CBF responses to whisker stimulation at a concentration of only 1% in spontaneously breathing mice (Takuwa et al., 2012). Although isoflurane has been shown to directly dilate cerebral vessels (Iida et al., 1998; Leoni et al., 2011), its major vasodilatory potency is a result of its suppressive effects on respiration in a dose-dependent manner (van Alst et al., 2019).
Respiratory depression leads to hypercapnia—elevated blood CO2 levels—which causes strong cerebral vasodilation and increases the baseline CBF (Kety and Schmidt, 1948; Raper and Levasseur, 1971; Shimosegawa et al., 1995; Jones et al., 2005; Leoni et al., 2011; van Alst et al., 2019). Both rats and mice exhibit massively reduced or even fully ablated BOLD responses, when CO2 (5–10%) is added to the inspiratory gas (Schlegel, 2017; Munting et al., 2019; van Alst et al., 2019). Mechanically ventilated rats showed substantially higher BOLD responses to subcutaneous electrostimulation, and lower baseline CBF compared to spontaneously breathing rats at the same concentrations of isoflurane (van Alst et al., 2019). A comparison of cortical and thalamic baseline perfusion across anesthetic protocols in mechanically ventilated mice found isoflurane to be in a similar range as urethane, which is known to preserve hemodynamics and vascular diameters (Schroeter et al., 2014).
Hypercapnia has further effects on brain states. Since CO2 forms carbonic acid in water, elevated CO2 makes the blood more acidic. Both low pH and CO2 have been shown to impair neural transmission and excitability (Coulter et al., 1995; Tombaugh and Somjen, 1996; Sun et al., 1997; Meuth et al., 2003, 2006; Putnam et al., 2004; Williams et al., 2007; Sinning and Hübner, 2013). Hyperoxia, on the other hand, has been reported to cause vasoconstriction of arterioles (Duling and Berne, 1970; Pries et al., 1995) and to decrease baseline CBF (Matsuura et al., 2001; Matsuura and Kanno, 2002). This demonstrates that blood gas levels are crucial in fMRI and that respiration should be kept at physiological levels to preserve neural and especially hemodynamic responses.
Mechanical Ventilation and Stress Response
This has led to the widespread use of mechanical ventilation in murine fMRI, initially to detect highly reproducible BOLD responses to salient (“attention grabbing”) stimuli, like subcutaneous electrostimulation (Bosshard et al., 2010; Baltes et al., 2011), and later nociceptive heat stimuli (see “Nociception, Pain, and Physiological Confounds” section; Bosshard et al., 2015; Reimann et al., 2016). Mechanical ventilation was further introduced as part of a robust protocol for pharmacological fMRI in mice (Ferrari et al., 2012) and is utilized in resting-state fMRI (see “Functional Connectivity and Murine Resting-State fMRI” section; Grandjean et al., 2014). The commonly applied protocol features 1.0–1.3% isoflurane in combination with neuromuscular blockage to minimize motion-related noise and prevent stimulus-correlated movements. An application of classical electrostimulation block paradigms to the murine paw—stimulus trains of 15–30 s composed of rectangular pulses of 0.5–2 mA—revealed that this protocol leads to BOLD signal changes of substantial magnitudes, and a very specific pattern of distribution: BOLD effects occurred in bilateral clusters in S1, S2, the thalamus, and the insular cortex (Figure 5A; Bosshard et al., 2010; Baltes et al., 2011; Schroeter et al., 2017; Reimann et al., 2018; Schlegel et al., 2018; Shim et al., 2018), and were accompanied by strong, transient elevations in mean arterial blood pressure (MABP) and heart rate (HR; Figure 5B; Schroeter et al., 2014; Reimann et al., 2018). This was confirmed for various anesthetic protocols including low-dose isoflurane, medetomidine, propofol, and urethane in ventilated and paralyzed mice (Schroeter et al., 2014).
The same stimulation paradigm applied to spontaneously breathing mice evoked less prominent BOLD responses that were strictly limited to the paw region of the contralateral S1 as described above (Figure 5A; Adamczak et al., 2010; Nasrallah et al., 2014a; Shim et al., 2018). This was observed for medetomidine and ketamine–xylazine anesthesia. The additional patterns observed in contralateral thalamic relay nuclei and S2 at higher field strengths emphasize the somatosensory nature of the response along the spinothalamic tract (Jung et al., 2019). Weak responses in ipsilateral S1 have also been reported for some animals, likely due to inter-hemispheric projections (Schroeter et al., 2017; Shim et al., 2018; Jung et al., 2019), and no cardiovascular changes were observed during the stimulus periods in these studies (Figure 5B).
In contrast, the strong, transient cardiovascular surges in ventilated animals clearly indicate sympathetic activity, which is governed by medullary control areas and triggered by an autonomic stress response (Pfaff, 2005; Samuels and Szabadi, 2008b; Ulrich-Lai and Herman, 2009). Such a response involves activity of the locus coeruleus and other key nuclei of the brainstem and the forebrain arousal system, which innervate multiple cortical and subcortical areas (Figure 1A; Toussay et al., 2013; Lecrux and Hamel, 2016; Lecrux et al., 2019).
The specific reason for this strong sympathetic response in ventilated mice is not fully understood. It is known that endotracheal intubation and forced ventilation can induce excessive stress in humans and animals when the anesthetic depth is too low. We hypothesize that long stimulus trains in ventilated mice accumulate to engage the activity of subcortical arousal nuclei that drive the brain state gradually toward desynchronization (see “Metastability of Brain States, Hysteresis and Behavioral Monitoring” section; Hudetz et al., 2003; Solt et al., 2014; Vazey and Aston-Jones, 2014; Pal et al., 2018; Hayat et al., 2019). Elevated sympathetic activity causes a tightly coupled increase in cardiovascular and respiratory output (Pfaff, 2005; Ulrich-Lai and Herman, 2009). Yet, in ventilated mice, the respiration rate is fixed (to 80–90 bpm), which may lead to an allosteric mismatch (“prediction error”) between autonomic command and sensory feedback (Alheid and McCrimmon, 2008; Kleckner et al., 2017). It is unclear whether this drives the animal’s stress response. However, it appears that ongoing electrostimulation triggers an excitatory feed-forward loop that forces increased activity in reticulo-thalamo-cortical circuits, and causes bilateral patterns in the S1 barrel fields, and strong sympathetic outflow (Toussay et al., 2013; Lecrux and Hamel, 2016; Lecrux et al., 2019).
With isoflurane, this is a transient process, and mice usually fall back into stable sedation after a stimulation period, which is evident from HR and MABP traces (Schroeter et al., 2014; Reimann et al., 2018) as well as from behavioral observations in unparalyzed mice. Mice administered medetomidine while under ventilation have been shown to exhibit increases in their HRs progressively over the length of the fMRI scan following the first stimulation period (Schroeter et al., 2014). Medetomidine exerts its effects predominantly by inhibiting the locus coeruleus (Akeju and Brown, 2017). Once a competing mechanism engages this key arousal nucleus, the sedation is temporarily suppressed (Hayat et al., 2019), and the stimuli might be processed in the awakening, largely unaffected cortex (see “α2AR Agonists Induce a Sleep-Like State by Suppressing General Arousal” section). Again, medetomidine produces a state from which arousal is more likely to occur than through the use of GABAergic drugs, which suppress the activity of excitatory neurons across the entire brain (Figures 1B,C).
Mechanically ventilated mice anesthetized with medetomidine exhibited bilateral BOLD responses even to single electrical pulses applied to the paw (1 mA, 0.5 ms; Schlegel et al., 2015). The same single electrical pulses applied to ventilated mice under isoflurane, propofol, or urethane evoked BOLD responses contralaterally along the spinothalamic tract in S1, S2, and first-order TC nuclei at 9.4 T—very similar to the patterns observed for stimulus trains in spontaneously breathing mice under ketamine–xylazine anesthesia at 15.2 T (Figure 5; Jung et al., 2019), with the latter being more spatially defined. The greatest similarities have been reported for patterns elicited under propofol along with profound hemodynamic responses to electrostimulation. However, the sedation under propofol was found to be unstable for the adjusted dosage (Schroeter et al., 2014; Schlegel et al., 2015). In summary, for the tested regimens, single pulses of electrical stimulation in mechanically ventilated mice elicit patterns predominantly along the somatosensory axis, whereas long pulse trains evoke bilateral patterns that likely reflect a transient recruitment of arousal structures, which can drive the brain state towards consciousness.
Multiple publications have reported on subcutaneous electrostimulation of the paw in mechanically ventilated mice under isoflurane. Whether this protocol allows for the detection of non-salient, low-intensity sensory stimuli has not yet been investigated. There is evidence that stimulus trains in the somatosensory range (0.5 mA) lead either to bilateral patterns or to no response at all (Shim et al., 2018). For a free-breathing sedation adjusted at 0.5–1% isoflurane, significant BOLD responses to whisker deflection have been reported in a murine fMRI study (Kahn et al., 2011). In such lightly sedated mice, tracheal intubation and forced ventilation would act as heavy continuous stressors during an fMRI experiment. Accordingly, mechanical ventilation does not permit further decreases in isoflurane concentrations, and approximately 1–1.3% are advised to prevent panic and withdrawal from the endotracheal tube. Concentrations of 0.75–1.1% isoflurane were found necessary to avoid burst suppression and permit stable sedation in spontaneous breathing mice (Kozai et al., 2015; Michelson and Kozai, 2018). To create conditions as close as possible to those experienced by a calm human volunteer, alternative strategies to ventilation should be considered for sensory tasks.
Balancing Anesthesia in Spontaneously Breathing Mice
Increasing oxygen concentration in the carrier gas is a popular strategy to prevent hypocapnia in spontaneously breathing mice, although caution is advised: no differences in respiratory rates, pH, blood CO2 levels, or blood pressure have been found for mice breathing isoflurane in pure oxygen (100%) compared to medical air (21%). This has been explained by a decrease in lung volume for pure oxygen based on alveolar collapse, known as absorption atelectasis (Wilding et al., 2017). Hemodynamic responses to whisker stimulation were significantly reduced for mice breathing pure oxygen compared to medical air (Sharp et al., 2015). The exact concentration of oxygen in the breathing gas may be adjusted based on a bell-shaped stimulus–response curve with respect to the anesthetic protocol that is applied (Blazquez Freches et al., 2018).
A good strategy is to apply anesthesia that causes neither respiratory depression nor vasodilation. This makes α2AR agonists attractive to assess sensory processing in murine fMRI, preserving physiological breathing rates at about 120–190 bpm (Adamczak et al., 2010; Nasrallah et al., 2014a). However, α2AR agonists induce a sleep-like state and impair BOLD responses due to vasoconstriction (see above; Fukuda et al., 2013; Nasrallah et al., 2014a), which further initiates a transient increase in MABP followed by a reduction based on suppression of noradrenergic sympathetic ganglia (McCallum et al., 1998; Samuels and Szabadi, 2008a). A better choice might be the combination of two or more complementary anesthetics, to balance their respective actions and to tailor the desired effect (Fukuda et al., 2013). Combining the α2AR agonist xylazine together with ketamine preserves physiological breathing rates at about 180 bpm and leads to pronounced BOLD responses, at least in the contralateral S1 (Shim et al., 2018). Another promising alternative was introduced by balancing isoflurane anesthesia with fentanyl-fluanisone and midazolam, which allows the isoflurane level to be reduced to 0.5–0.8% (Sharp et al., 2015). This was reported to induce stable sedation and permit the detection of hemodynamic effects for whisker stimulation, with magnitudes and transition times similar to those observed in awake mice.
Balanced multimodal anesthesia holds great promise to circumvent the multiple challenges in murine fMRI, with the aim of preserving physiological conditions while sufficiently sedating the animal, and still ensuring a certain level of “connectedness” to sensory perception and interoception (see “Anesthetic Depth and Consciousness–the Virtue of Translational Neuroimaging” section; Sanders et al., 2012). Protocols for balanced multimodal anesthesia will be further discussed in “Multimodal Anesthesia in Translational fMRI” section.
Nociception, Pain, and Physiological Confounds
Nociception is “the neural process of encoding noxious stimuli.” Pain is defined as an unpleasant experience that may or may not arise from nociception (Davis et al., 2017). In contrast to fMRI tasks that probe the central processing of applied stimuli, fMRI of pain explicitly aims to identify neural correlates of a phenomenal quality (Mouraux and Iannetti, 2018). Its assessment is therefore hampered in the (sedated) rodent, which cannot report on the experience or level of pain during an fMRI task (Seth et al., 2005). This is an important domain for translational fMRI: in principle, it should be possible to first correlate nociceptive-evoked BOLD patterns with subjective ratings of pain to identify pain-specific functional neurosignatures in the human brain (Wager et al., 2013; Woo et al., 2017). In a second step, a corresponding signature could be sought in the sedated animal model (Tracey, 2017). This leads to an even more fundamental question in terms of anesthesia: to what extent is consciousness required to encode a pain-specific neurosignature?
Currently, we cannot answer this question, since a pain-specific neurosignature has not yet been found (Mouraux and Iannetti, 2018). Painful stimuli are processed within a widely distributed network that is often referred to as the “pain matrix” (Legrain et al., 2011)—a term derived from Melzack’s original neuromatrix theory of pain (see below; Melzack, 1989, 2005). It has been shown that salient and painful stimuli are processed by largely overlapping cortical areas in humans (Mouraux et al., 2011), and subcortical and arousal structures that are involved in nociception are also engaged by acute responses to stress (Reimann et al., 2016; Martins and Tavares, 2017). In other words, the neuromatrix is not pain-specific. This is a major obstacle for identifying pain-specific brain signatures, because painful stimuli are: (1) intrinsically salient (Legrain et al., 2011; Mouraux et al., 2011); and (2) often provoke autonomic stress responses (Reimann et al., 2016, 2018).
Such stress responses introduce severe complications in murine fMRI. They involve the activity of the nociceptive–medullary–autonomic circuit—a fundamental component of the “flight or fight” response, which triggers abrupt surges in HR and MABP (Figure 6A; Price, 2000; Pfaff, 2005; Brown et al., 2010). Since cerebral autoregulation has high-pass filter characteristics, slow changes in MABP are buffered to a certain extent, whereas more pronounced and rapid changes are reflected in the CBF (Hamner et al., 2019). The efficiency of dynamic autoregulation might be further impaired by vasomodulatory effects of anesthesia (Lee et al., 1994; Dagal and Lam, 2009; Sanders et al., 2011). Abrupt surges in MABP, well within the physiological range (80–120 mmHg in mice), can lead to stimulus-correlated increases in CBF causing widespread BOLD patterns across the brain (Figures 6B,C; Kalisch et al., 2001; Wang et al., 2006; Gozzi et al., 2007; Reimann et al., 2018). Veins are particularly prone to translate changes in CBF, into pronounced BOLD effects due to their low oxygen concentration (Reimann et al., 2018). In fact, significant clusters were observed along large draining veins (cluster threshold z = 3.1) that were highly correlated with transient MABP surges induced by methods of controlled pharmacological vasoconstriction (Figures 6A,D). Since large veins co-localize with key cortical regions of the neuromatrix (S1, S2, insula, and parietal cortex), this is a confounding factor in current murine fMRI studies of nociception. Aside from strategies to identify and correct the affected regions, fMRI protocols that track changes in CBV, such as vascular space occupancy (VASO) or iron-oxide-based CBV-fMRI, are less prone to such large-vein effects (for a review, see Huber et al., 2017) and can be used either as alternative techniques, or to normalize and correct BOLD data.
Figure 6.
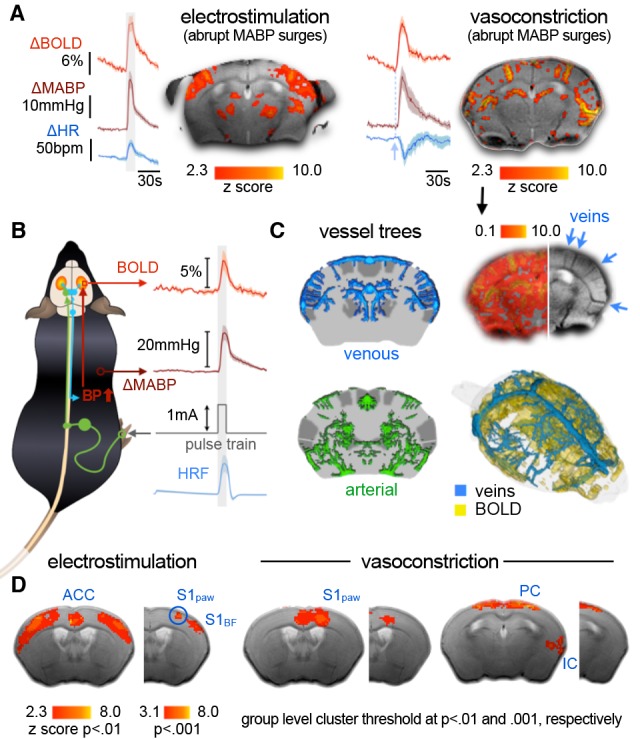
BOLD patterns evoked by abrupt surges in MABP. (A) Electro-stimulation evokes abrupt surges in MABP and HR. Mimicking these surges by intravenous injection of vasoconstricting drugs leads to BOLD patterns co-locating with large veins. Injection time point is indicated by dashed line. (B) Nociceptive stimuli or stress can elicit abrupt surges in MABP that increase the influx of oxygenated blood into the brain vasculature. MABP and BOLD patterns are highly correlated with each other and with the applied stimulus. This causes the hemodynamic response function (HRF)—that is modeled based on the stimulus paradigm—to reveal MABP-induced effects as significant patterns. (C) Significant patterns co-localize with large veins and can therefore be identified and corrected for, whereas widespread patterns remain below the statistical threshold. (D) At the group level, significant effects persist at more liberal thresholds, and few minor clusters even at more conservative standards. Adapted with permission from Reimann et al. (2018).
Further complexity is introduced by outreaching vasodilatory projections of specific arousal nuclei such as the locus coeruleus, or the nucleus basalis of Meynert in the basal forebrain that are thought to have a substantial impact on the cortical hemodynamic responses to noxious stimuli (Lecrux and Hamel, 2016; Uchida and Kagitani, 2018; Paquette et al., 2019). The extent to which these BOLD responses actually reflect neural activity remains unclear (Lecrux et al., 2019). Both abrupt elevations in MABP and remote vasodilatory projections of arousal nuclei can produce hemodynamic readouts that are indistinguishable from neurovascular coupling. This problem has hardly been explored and is currently considered a major hurdle for interpreting experimental data related to nociceptive fMRI in (ventilated) rodents (Paquette et al., 2018).
Despite these considerations, nociceptive pathways are largely preserved in murine BOLD fMRI using low-dose mono-anesthetic isoflurane anesthesia in mechanically ventilated and paralyzed mice (Reimann et al., 2016). Brief heat stimuli just above the Aδ nociceptor threshold (46°C) applied to the murine paw evoked BOLD effects in brain areas including the spinothalamic and spinoreticular tract, which have been well documented as having a functional involvement in murine and human nociception (Figure 7A). Employing a cryogenic transmit-receive unit (Niendorf et al., 2015) at 9.4 T has permitted resolving even the small nuclei of the habenular nociceptive pathway (Figure 7B, Reimann et al., 2016)—a pain modulating circuit known from human fMRI and from histological c-fos immunostaining in the mouse (Shelton et al., 2012a, b).
Figure 7.
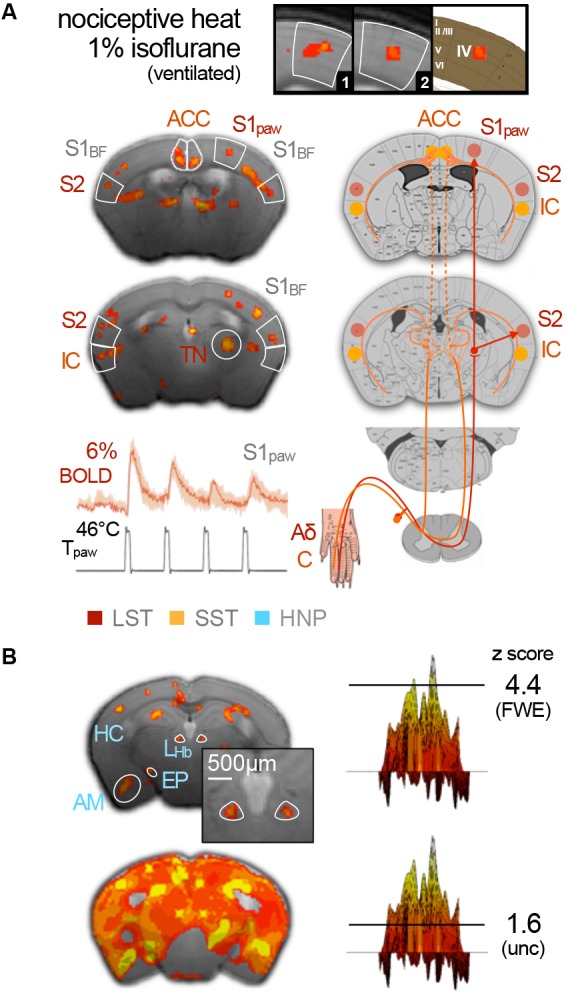
Murine nociception induced by heat stimuli applied to the paw. (A) BOLD patterns along the spinothalamic (LST) and spinoreticular tract (SST) depict well-known nociceptive routes in the mouse with high spatial accuracy, including thalamic nuclei (TN), primary (S1) and secondary (S2) somatosensory cortex, the barrel field (S1BF), and insular (IC) and ACC. (B) Involvement of the habenula nociceptive pathway (HNP) has also been observed, including amygdala (AM), hippocampal areas (HC), entopenduncular nucleus (EP), and the small nuclei of the lateral habenula (LHb). These highly precise patterns represented the tip of the iceberg of underlying BOLD effects, likely induced by MABP surges and vasodilative projections from subcortical arousal nuclei. Adapted from Reimann et al. (2016), http://creativecommons.org/licenses/by/4.0.
The detail of the BOLD patterns observed in this study was achieved by highly conservative thresholding, and has exposed peak values of extensive patterns spreading across large parts of the brain (Figure 7B). More liberal thresholding of heat-evoked BOLD effects in mice under comparable conditions revealed pattern distributions very similar to those observed for electrostimulation (Bosshard et al., 2015). In fact, nociceptive and arousal pathways are tightly interconnected (Craig, 2013). It is thus difficult to disentangle or exclude a potential contribution of ventilation-driven stress responses from those involved in nociception (as discussed in “Sensory Processing and the Key Challenges in Murine fMRI” section). Nociception drives arousal pathway activity, and hence cortical desynchronization, toward a transient emergence from anesthesia (Figure 2E; Hudetz et al., 2003; Solt et al., 2014; Vazey and Aston-Jones, 2014; Akeju and Brown, 2017; Sanders, 2017; Hayat et al., 2019). Therefore, it is important to develop protocols that permit stable sedation in receptive, spontaneously breathing animals. The continuous monitoring of MABP permits the tracking of this important confounder, but it is highly invasive and introduces postoperative nociception. Sympathetic activity is similarly reflected in HR and respiration. Both may rise with the stimulus, but should subsequently return to the baseline (Reimann et al., 2016, 2018). A progressively rising HR requires adjustment of the anesthetic dosage to avoid the animal waking (Nasrallah et al., 2014b; Schroeter et al., 2014; Shim et al., 2018).
The interconnection of nociceptive and arousal routes causes a transient lightening of anesthesia in response to nociceptive stimuli. This could explain why BOLD responses to noxious electrostimulation are partially preserved in humans even after propofol-induced LOC, whereas sensory evoked patterns vanish (see “Anesthetic Depth and Consciousness–the Virtue of Translational Neuroimaging” section; Lichtner et al., 2018). In relation to our initial question, of whether consciousness is required to evoke a pain-specific neurosignature, it could be said that nociception itself drives the brain state toward consciousness. The proposed architecture of the body-self neuromatrix is composed of sensory, affective, and cognitive subnetworks of brain areas. Their output patterns produce the multiple dimensions of the pain experience (sensory-discriminative, cognitive-evaluative, motivational-affective), which coincide with homeostatic and behavioral responses (Melzack, 2005; Craig, 2013; Seth and Tsakiris, 2018). Dynamic changes in the FC of these respective networks, triggered either by external nociceptive stimuli or by internal processes in animal models of chronic pain, may offer a more concrete representation of the neuromatrix, as was recently addressed as the dynamic pain connectome (Kucyi and Davis, 2017).
Functional Connectivity and Murine Resting-State fMRI
FC between two or more brain areas is defined by the correlation coefficient of their signal intensity courses over time: the higher the correlation, the higher the FC (Figure 8A; Petersen and Sporns, 2015). This simple relation is the key principle in defining functional networks across the entire brain—an opportunity that is unique to fMRI. FC is considered to be in large part the hemodynamic translation of simultaneous activity of neural populations, which is inherently limited by the physical connections between neurons (Mateo et al., 2017). This structural scaffold permits a repertoire of possible functional configurations that changes dynamically on a time scale depending on the level of organization: on the order of 1 ms for neurons, 10 ms for local circuits, 100 ms for EEG, and 1 s for fMRI (Mashour and Hudetz, 2018). This has two important implications: (1) the temporal changes in FC hold important information that is simply discarded when large fMRI time series are averaged assuming steady-state conditions (Preti et al., 2017); and (2) brain states can, in principle, be translated into FC and assessed via fMRI (Van de Ville et al., 2010).
Figure 8.
Functional connectivity (FC) in the mouse brain. (A) FC is defined by the correlation coefficient (cc) of remote brain areas. The left panel shows an assembly of important areas in murine FC, including the default mode network (DMN), cortical sensory-motor networks, and subcortical networks. (B) Murine FC correlation maps for five anesthetic protocols. Seed voxels in the anterior primary somatosensory cortex show anticorrelated time courses in the cingulate cortex. Anticorrelation is an essential criterion for anesthetic states in fMRI in which the FC is not restricted to its structural scaffold. (C) Correlation matrices in the rat for six different anesthetic protocols referenced against awake rats. Asterisks indicate statistically significant differences compared with the awake group (t-test, p < 0.05, false discovery rate corrected). (D) Optogenetic stimulation of the dorsal raphé nuclei that send serotinergic projections into large parts of the brain leading to wide patterns of negative BOLD effects. Adapted from (A) Grandjean et al. (2019a); (B) based on Grandjean et al. (2014), reproducible using original data from the open repository, https://central.xnat.org; (C) adapted with permission from Paasonen et al. (2018), (D) Grandjean et al. (2019b).
The integrity or disruption of FC is commonly used as an indicator of various types and stages of neurodegenerative diseases and neurophysiological malfunctions, or to study the effects of pharmacological interventions, genetically modified proteins or neural populations under optogenetic control (see “Deep Brain Stimulation, Opto- and Chemogenetics” section). Notably, the majority of murine resting-state studies have been conducted under anesthesia (Jonckers et al., 2011, 2014, 2015; Guilfoyle et al., 2013; Grandjean et al., 2014; Mechling et al., 2014; Nasrallah et al., 2014a; Sforazzini et al., 2014; Liska et al., 2015; Bukhari et al., 2017; Wu et al., 2017; Pan et al., 2018). The extent to which various anesthetic protocols intrinsically disrupt FC is therefore a critical issue that must be comprehensively addressed. A number of excellent reviews are already available on this issue for humans (Mashour and Hudetz, 2017, 2018) and mice (Hoyer et al., 2014; Jonckers et al., 2014, 2015; Pan et al., 2015; Gozzi and Schwarz, 2016; Chuang and Nasrallah, 2017; Sumiyoshi et al., 2019; Grandjean et al., 2020; Mandino et al., 2020). Here, we add to the existing literature by focusing on basic concepts of anesthesia-specific changes in FC with respect to the underlying mechanisms.
FC is remarkably similar across mammalian species. In 2014, 3 years after the first study on murine FC (Jonckers et al., 2011), it was shown that mice exhibit a medial fronto-parietal or “default mode” network (DMN) during the resting state (Stafford et al., 2014) similar to that of rats (Lu et al., 2012), monkeys (Vincent et al., 2007), and humans (Raichle et al., 2001). The DMN plays a fundamental role in baseline functions of the mammalian brain (Raichle, 2015). In humans, it is associated with mind-wandering and rumination. Whether the DMN entails comparable phenomenal qualities in mice remains speculative and may be interesting from an evolutionary or philosophical point of view (Seth et al., 2005; Havlik, 2017). In the presence of a salient stimulus, DMN connectivity becomes disrupted and shifts towards connectivity in the dorsal attention network (Raichle, 2015), in the same way that cortical states change with attention toward a stimulus (see “Cortical States of Wakefulness” and “Brain States Under Anesthesia” sections).
The DMN involves similar higher-order cortical regions along the medial fronto-parietal axis across species (Figure 8A; Raichle, 2015; Grandjean et al., 2020), although prefrontal and cingulate regions appear to perform the tasks of the precuneus, which is absent in rodents (as reviewed in Gozzi and Schwarz, 2016). Further functional networks have been described in the mouse, including a salience network and latero-cortical network, as well as various sensory, sensorimotor, cerebellar, limbic system, and basal ganglia networks (see Figure 8B for murine FC maps based on a seed voxel in S1 for different anesthetics; Jonckers et al., 2011, 2014; Grandjean et al., 2014, 2017b, 2020; Nasrallah et al., 2014b; Sforazzini et al., 2014; Zerbi et al., 2015; Shim et al., 2018; Gutierrez-Barragan et al., 2018).
Anesthesia-Induced Disruptions in FC
The network architecture is very similar between humans and mice (Bullmore and Sporns, 2012; Gămănuţ et al., 2018; Balsters et al., 2019; Fulcher et al., 2019), which has been utilized to pursue questions about human network configurations in the mouse (Fulcher and Fornito, 2016). Brain networks are organized around highly connected hubs. Substantial disruption of FC induced by anesthesia at these central nodes can cause widespread communication failure associated with LOC (Schröter et al., 2012; Lee H. et al., 2013; Moon et al., 2015; Bonhomme et al., 2016; Tononi et al., 2016; Mashour and Hudetz, 2018). Abnormal reorganization of hubs during recovery from anesthesia likely explains differences in FC strength and configuration observed prior to and following LOC (Långsjö et al., 2012; Liu et al., 2013b; Monti et al., 2013). This sheds light on the anesthetic hysteresis effect: namely, that different anesthetic dosages are required to induce a similar depth of anesthesia during the induction and recovery phases (see “Brain States Under Anesthesia” section). The translational utility of murine FC studies may depend on the capacity of the anesthetic protocol to preserve or restore the integrity of specific functional networks across the brain.
Of course, the technical equipment, animal preparation protocols, arousal states due to stress, murine susceptibility to vascular effects of anesthesia, and the applied statistical methods all have a crucial impact on the reliability of the data (Grandjean et al., 2020). It is therefore helpful to reference murine FC data against: (1) FC data derived from more stable systems like humans, monkeys, or rats under the same anesthesia; and (2) the molecular pathways that are known to be targeted by specific anesthetic protocols. Disruption of neural communication across brain areas occurs according to the principal target routes of an anesthetic regimen (see “Brain States Under Anesthesia” section) and may be translated into a discrete FC signature (Table 3). This relation makes the assessment of FC a highly relevant point of convergence for detailing and understanding brain states induced by anesthesia.
Table 3.
Functional connectivity (FC) disruptions reflect principal mechanisms of anesthesia.
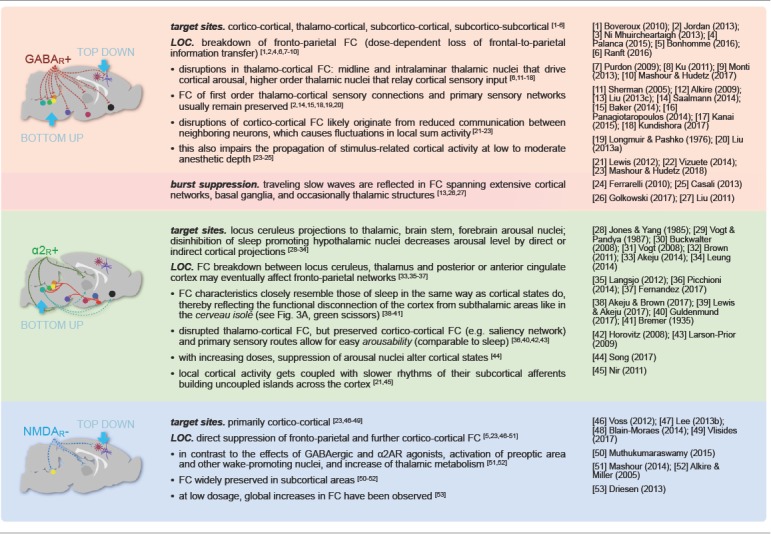 |
The table summarizes key effects on FC for the three major anesthetic classes: disruption of whole brain FC by GABAA receptor agonists (GABAR+, red), bottom-up disruption by α2 adrenoreceptor agonists (α2R+, green), and disruption of cortical FC by NMDA receptor antagonists (NMDAR-, blue). Loss of consciousness (LOC) is most likely mediated by suppression of frontal-to-parietal information transfer in the cortex and can be achieved by all three anesthetic classes either directly via disruption of cortico-cortical FC or indirectly via bottom-up pathways. Principal mechanisms of anesthesia are described in “Brain States Under Anesthesia” section and their reflection in FC patterns is discussed in “Functional Connectivity and Murine Resting-State fMRI” section. References are shortened to first author (and year) to save space and allow for comprehensive referencing.
Anticorrelation and Rich Dynamic Repertoires—Criteria for Appropriate FC
Discrete signatures of GABAergic drugs and volatile ethers, which suppress brain-wide neural activity include dose-dependent disruption of FC across the entire brain (for details, see Table 3; Boveroux et al., 2010; Schröter et al., 2012; Jordan et al., 2013; Ní Mhuircheartaigh et al., 2013; Palanca et al., 2015; Bonhomme et al., 2016; Ranft et al., 2016). This is reflected in a reduction in global connectedness, with stronger effects on higher-order cortical and thalamic FC compared to sensory areas, and fewer transitions between functional networks (Uhrig et al., 2018; Golkowski et al., 2019). This rigidity in the dynamics between networks correlates with a reduced ability to interact with external stimuli (see “GABAA Agonists Suppress Neural Activity Across the Central Nervous System” section). LOC has consistently been found to be correlated with a breakdown of fronto-parietal FC in humans and non-human primates induced by GABAergic drugs (Purdon et al., 2009; Boveroux et al., 2010; Jordan et al., 2013; Monti et al., 2013; Palanca et al., 2015; Ranft et al., 2016; Mashour and Hudetz, 2017; Uhrig et al., 2018), likely due to impaired frontal-to-parietal cortical information transfer (Ku et al., 2011; Jordan et al., 2013; Ranft et al., 2016). Although this relationship is also thought to exist in mice, further studies are required to assess the modes of murine fronto-parietal FC and their significance for wakefulness (Imas et al., 2005b; Mashour and Alkire, 2013; Sforazzini et al., 2014; Pal et al., 2018; Shofty et al., 2019; Grandjean et al., 2020).
On the other hand, burst suppression and slow waves that propagate unabated across the cortical surface (see “Crossing the Borders: GABAergic Slow Waves and Burst Suppression” section) promote FC that can span extensive cortical networks in humans and rats, occasionally reaching into the basal ganglia and thalamic structures (Liu et al., 2011, 2013b, c; Golkowski et al., 2017; Schwalm et al., 2017; Aedo-Jury et al., 2019). Isoflurane concentrations as low as 1.3% in ventilated rats were found sufficient to induce highly synchronized large-scale FC patterns in fronto-cortical and striatal regions, and to suppress most thalamo-cortical and intra-subcortical connections (Figure 8C; Paasonen et al., 2018).
Loss of information transfer and integration is further reflected in a reduced number of possible FC configurations (Hutchison et al., 2014; Barttfeld et al., 2015; Hudetz and Mashour, 2016; Ma et al., 2017; Uhrig et al., 2018). The repertoire of FC configurations can be evaluated via a sliding-window approach. In this approach, a window length of about 1 min is defined and shifted along the entire length of the resting-state time series, with a step size equal to the fMRI temporal resolution (typically 1–2 s). Each time-shifted segment then undergoes a correlation analysis, which gives access to dynamic aspects of FC, which have been reported to be well conserved across rats, monkeys, and humans (Majeed et al., 2011). Although blurred in temporal resolution, this technique permits the assessment of dynamic whole-brain signatures based on FC. Combining Ca2+ imaging or electrophysiology with fMRI (Thompson et al., 2013; Keilholz, 2014; Schmid et al., 2016; Schwalm et al., 2017; Lurie et al., 2018; Schlegel et al., 2018; Matsui et al., 2018; Aedo-Jury et al., 2019; van Alst et al., 2019), these signatures can be linked to the cortical states, and hence be employed to determine the nature and level of sedation (see “Brain States Under Anesthesia” and “Limitations in Maintaining Stable Brain States” sections).
The dynamical elaboration of a rich, flexible repertoire in the awake brain becomes increasingly constrained with increasing anesthetic dosage (Barttfeld et al., 2015; Uhrig et al., 2018). This is accompanied by a lack of anticorrelated areas—a further symptom of inflexible FC and confined information traffic pointing to LOC. A lack of anticorrelated areas was reported in mice ventilated at 1% isoflurane (Figure 8B; Grandjean et al., 2014). At 1.3% isoflurane broad cortical FC has been observed along with synchronized cortical slow wave activity in the Ca2+ signal assessed in the murine S1 (Schlegel et al., 2018). Further reducing the isoflurane concentration below 1–1.3% is not appropriate if the mice are ventilated and paralyzed (see “Sensory Processing and the Key Challenges in Murine fMRI” section). As head movements, including rhythmic, respiration-induced motion, account for a large part of the noise in FC (Kalthoff et al., 2011; Pais-Roldán et al., 2018), a trade-off has to be made between data quality and anesthetic depth. In ventilated rats, a reduction of isoflurane from 1.5% to 1% was sufficient to preserve distinct cortical networks and anticorrelated FC at a largely desynchronized frequency band in the EEG (Liu et al., 2013c). Due to the narrow dose range between stable sedation and burst suppression, careful adjustment of isoflurane concentrations is required to avoid unphysiological FC.
Compared to isoflurane and other volatile ethers, halothane exhibits a remarkably wide dose range between stable sedation and burst suppression (Murrell et al., 2008; Brown et al., 2018; McIlhone et al., 2018). A rich repertoire of dynamic FC patterns was observed for halothane-anesthetized mice, based on a varied set of recurring configurations of FC, with abundant anticorrelated areas (Sforazzini et al., 2014; Gutierrez-Barragan et al., 2018). At low dosages, other GABAergic drugs have been shown to preserve FC quite well with respect to the above criteria. The use of low-dose propofol and urethane was reported to achieve the best resemblance to FC in awake rats out of six different anesthetic regimens tested (Figure 8C; Paasonen et al., 2018). A similar observation was reported for mice, with propofol preserving spatially defined symmetrical cortico-cortical FC with anticorrelated cingulate areas (Figure 8B; Grandjean et al., 2014). Nevertheless, unstable sedation has been reported for this protocol in mice, which could be due to ventilation conditions, considering the general arousability of mice relative to rats. Comparing the capacity of anesthetic protocols to approximate the FC of the awake state in rats, propofol and urethane showed the best performance, followed by α-chloralose, while a protocol combining isoflurane and medetomidine (see below) was ranked 4th (Figure 8C).
“One-Dimensional” Disruption of FC
Medetomidine and other α2AR agonists, which suppress subcortical arousal nuclei (see “Brain States Under Anesthesia” section) disrupt FC in a bottom-up manner (Table 3). Disinhibition of sleep-promoting cells in the hypothalamus disrupts subcortico-subcortical and hence thalamo-cortical FC. This causes a discrete FC signature that reflects the functional disconnection of the cortex from subcortical areas, as anticipated on the basis of the cerveau isolé (“Brain States Under Anesthesia” section; Bremer, 1935; Guldenmund et al., 2017; Lewis and Akeju, 2017; Akeju and Brown, 2017). With increasing dosages, local cortical activity gets coupled with slower rhythms of subcortical afferents from the suppressed arousal nuclei, building uncoupled islands across the cortex (Nir et al., 2011; Lewis et al., 2012). This is reflected in disrupted interhemispheric cortico-cortical FC, which has been observed with increasing dosages of medetomidine in rats (Nasrallah et al., 2014a) and mice (Nasrallah et al., 2014b). The mechanism of bottom-up-induced LOC likely involves a breakdown of FC between the locus ceruleus, the thalamus, and the posterior or anterior cingulate cortex, which may eventually affect fronto-parietal FC (Långsjö et al., 2012; Fernandez et al., 2017). Medetomidine was found to preserve moderate intercortical connectivity in ventilated rats, although this was significantly reduced compared to that of awake rats, whereas thalamo-cortical activity was almost absent (Figure 8C; Pawela et al., 2008; Zhao et al., 2008; Paasonen et al., 2018).
Ketamine, on the other hand, which acts primarily in a top-down manner in the cortex, mediates LOC by direct suppression of fronto-parietal and further cortico-cortical FC (Table 3; Voss et al., 2012; Lee U. et al., 2013; Blain-Moraes et al., 2014; Muthukumaraswamy et al., 2015; Bonhomme et al., 2016; Vlisides et al., 2017; Mashour and Hudetz, 2018; Uhrig et al., 2018). In contrast to GABAergic and α2AR agonists, ketamine has been reported to largely preserve subthalamic FC, which is consistent with its activating effects on the preoptic area and other wake-promoting nuclei (Alkire and Miller, 2005; Mashour, 2014). The top-down and bottom-up actions of ketamine and medetomidine may be thought of as “one-dimensional” (Mashour and Hudetz, 2017). The dual effect of GABAergic drugs makes them more suitable for stable sedation by suppressing both subthalamo-cortical and cortico-cortical FC (Boveroux et al., 2010). To achieve a similar anesthetic stability by not overly stressing one pathway, drugs targeting either of these “one-dimensional” mechanisms are commonly combined (e.g., ketamine-xylazine or medetomidine-isoflurane) in order to target the complementary pathways.
Multimodal Anesthesia in Murine Resting-State fMRI
Combining isoflurane and medetomidine at levels roughly half their mono-anesthetic dosages (Lu et al., 2012; Paasonen et al., 2018; Sumiyoshi et al., 2019) has been shown to produce FC resembling that of awake rats substantially better than either mono-anesthetic protocol alone, yielding a correlation matrix that roughly corresponds to that of α-chloralose (Figure 8C; Paasonen et al., 2018). This medetomidine-isoflurane protocol was adapted for murine resting-state fMRI, where it was also described as providing the “best of both worlds”: reproducible, spatially defined networks, including symmetrical patterns of FC in bilateral sensory areas anticorrelated with cingulate regions, at a stable sedation in ventilated mice (Figure 8B; Grandjean et al., 2014).
Later studies revealed a high correspondence between FC and structural connectivity of cortico-cortical and cortico-striatal regions with the same medetomidine-isoflurane protocol in mice, whereas thalamo-frontal cortical FC was found suppressed (Grandjean et al., 2017b). Although a high correspondence between functional and structural connectivity has been associated with a constrained repertoire of FC configurations (Barttfeld et al., 2015; Uhrig et al., 2018), the sliding-window approach ultimately revealed rich patterns of dynamic FC under this protocol (Grandjean et al., 2017a). This incongruity could be due to methodological differences between the studies (e.g., the use of MR diffusion-based vs. neural tracer-based structural connectivity; Straathof et al., 2019). The exact relationship between the dynamics of FC and its correspondence to its scaffold as a function of anesthetic depth remains to be clarified across anesthetic protocols in mice.
Taken together, the preservation of a rich repertoire of FC configurations, along with spatially defined and anticorrelated FC in relevant networks, has made medetomidine-isoflurane the protocol of choice for studying murine FC. Low-dose halothane performs similarly well in mice, with the advantage of preserving thalamo-frontal cortical FC (Gutierrez-Barragan et al., 2018; Bertero et al., 2018; Mandino et al., 2020). Halothane is rarely used due to a risk of causing liver damage, although the risk is possibly no higher than that of other halogenated vapors (Mizobe, 2019).
A recent meta-analysis comparison across numerous labs, MR scanners, and animal preparation protocols (Grandjean et al., 2020) revealed greater specificity within elements of the DMN under medetomidine-isoflurane anesthesia in ventilated mice than in awake habituated mice, although the latter exhibited higher overall FC. This can be interpreted as an effect of environmental or internal distraction in awake mice, since DMN connectivity is known to be disrupted in the presence of salient stimuli (see above; Raichle, 2015). Contradictory findings have been reported for anesthetic effects on the DMN, although it appears that FC is principally supported at low anesthesia and disrupted with increasing anesthetic depth (Akeju et al., 2014a; Huang et al., 2014; Palanca et al., 2015; Ranft et al., 2016).
Resting-State fMRI in the Awake Mouse
Medetomidine-isoflurane and low-dose halothane are currently considered the most suitable anesthetic protocols available for the assessment of murine FC. Yet, carefully titrated multimodal protocols specifically designed to balance the complementary effects of their anesthetic compounds offer far more room for improvement (see “Multimodal Anesthesia in Translational fMRI” section). Comparing FC matrices for medetomidine-isoflurane against propofol with reference to the “ground truth” of an awake habituated rat gives an immediate impression of what we are heading for (Figure 8C). With respect to the non-voluntary task conundrum, resting-state applications have the best chance to produce reliable data from awake habituated mice. Nevertheless, training awake mice to tolerate the noisy scanner environment is far more challenging than for awake rats (Jonckers et al., 2014, 2015; Low et al., 2016b; Dopfel and Zhang, 2018), although further strategies are constantly being developed to facilitate this procedure (Yoshida et al., 2016; Madularu et al., 2017; Han et al., 2019; Chen et al., 2020).
Since brain states during wakefulness alter with the level of arousal, such a “ground truth” is still prone to reflect stress and restraint rather than the resting state, which becomes critical when comparing results with human volunteers (Bergmann et al., 2016). It might be useful to administer minimal anesthetic dosages (e.g., of anxiolytics) in the actual experiment to relax mice, which are already habituated. This would create a state of drowsy wakefulness that approximates the “ground truth” against which novel anesthetic protocols could be referenced. Ultimately, multimodal sedation may be instrumental to lock the mouse into a defined spectrum of brain states to minimize variations due to stress, arousal, and distraction, and to prevent effects due to habituation and reward.
Deep Brain Stimulation, Opto- and Chemogenetics
Functional reorganization and network properties can be further evaluated by using techniques that modulate neural activity from within the brain. The most popular methods are deep brain stimulation, opto- and chemogenetics. These techniques allow one, for example, to evaluate the reorganization of FC in response to locally targeted perturbations. This can be used as a measure for functional complexity of information processing in the anesthetized brain (perturbational complexity index; Casali et al., 2013; Mashour and Hudetz, 2018). Ketamine, for instance, has been shown to preserve cortical functional complexity, which is decreased by propofol with increasing dosage (Sarasso et al., 2015). The signal propagation of locally induced neural activity also provides useful information on FC, which has been shown to be impaired during anesthesia, sleep, and pathological unconsciousness (Massimini et al., 2005; Ferrarelli et al., 2010; Casali et al., 2013). In humans, such investigations are often limited to studying cortical FC using non-invasive transcranial magnetic stimulation. In preclinical fMRI deep brain stimulation can be more easily utilized to induce neural activity in subcortical areas (McIntyre and Anderson, 2016), although the electrodes are prone to cause imaging artifacts, distortions, and local signal loss (Lehto et al., 2018).
Optogenetics employs optical fiber probes that do not directly interfere with functional MR image acquisition. Excitation or inhibition of genetically modified neurons governed by light control can be combined with fMRI to detail spatial and temporal effects on evoked neural activity (Desai et al., 2011; Lai et al., 2015) or FC in remote brain regions (Ryali et al., 2016; Chan et al., 2017). Chemogenetics is a powerful minimally invasive complement to optogenetics, in which neurons are controlled by artificially designed ligands that are injected into the bloodstream (Roth, 2016). Both are important techniques to further investigate the mechanisms of FC (as recently summarized in Mandino et al., 2020), including the impact of distinct transmitter systems in mice. Exclusively selecting, for example, serotonergic neurons of the raphé nuclei (Figure 8D; Grandjean et al., 2019b) or noradrenergic neurons of the locus coeruleus (Zerbi et al., 2019) based on specific gene expression patterns can be used to detail the underpinnings of brain states in anesthesia and the circuits that drive awakening (Carter et al., 2010; Taylor et al., 2016).
Translational fMRI Across Brain States and Species
The key challenges of preclinical fMRI with a view to translation are: (1) to induce and maintain brain states that correspond with those of calm and relaxed human volunteers; and (2) to assure hemodynamic integrity—in other words, to preserve neurovascular coupling and physiological baseline conditions. Up to this point, we have discussed the general principles of anesthesia and have highlighted the need to recognize and overcome the shortcomings of current anesthetic protocols. This will require strategies to shape, maintain, and unambiguously identify distinct brain states in fMRI that meet the above criteria, to conduct reliable, reproducible studies and translate findings from mice to humans.
Multimodal Anesthesia in Translational fMRI
Preclinical fMRI aims for an animal that is sufficiently sedated, yet with a fully functional brain. This challenge puts high demands on anesthetic protocols to induce and maintain an intermediate brain state that closely approximates an awake, calm, relaxed, and undistracted state during the fMRI task. Current standard practices involving simple mono-anesthesia are outdated. Balanced multimodal anesthesia, in which two or more drugs are combined to balance their complementary effects, has come into use in the clinic (Brown et al., 2018a) and should be further developed for preclinical studies (see “Sensory Processing and the Key Challenges in Murine fMRI” and “Functional Connectivity and Murine Resting-State fMRI” section; Grandjean et al., 2014; Sharp et al., 2015; Shim et al., 2018).
By reducing the dose of individual compounds, a multimodal protocol reduces excessive stress on a single pathway, taking advantage of complementary modes of action to shape and maintain desired brain states. In principle, respiratory depression or hemodynamic side effects from specific drugs can be attenuated by balancing them with the effects of others. At the same time, combining anesthetic compounds through multimodal anesthesia introduces a higher level of complexity and potential cross-effects that require caution. A rigorous characterization of anesthetic actions will be necessary to understand and adjust the effects of individual drugs in multimodal approaches. This will require that brain states, hemodynamics, and physiology be monitored, evaluated, and reported to the community.
Defining Brain States in fMRI: Qualifiers and MIND Signature
To properly identify and communicate an anesthetic state in translational fMRI, we first need to define a set of functional characteristics that can identify an induced brain state with high specificity. Their purpose is to evaluate the anesthetic maintenance of an intermediate brain state, and qualify its approximation to the awake state. We will refer to these functional characteristics as qualifiers.
Among these qualifiers, we choose one identifier that allows the unambiguous assessment of whether a desired intermediate brain state has been maintained during the full length of the fMRI scan, based on a reliable neurophysiological signature pattern. It should permit: (1) identifying a brain state and the integrity of its qualifiers; and (2) comparing this state across subjects, trials, scanners, labs, and species. We will refer to this identifier of a specific brain state as a maintained, intermediate neurophysiologically-determined (MIND) signature.
Qualifiers
A number of qualifiers have the potential to illuminate characteristics of brain states and the functional implications that accompany them. The set of such qualifiers is growing rapidly through new findings and the development of novel analytical tools (as summarized in Bonhomme et al., 2019). Here, for the sake of brevity, we list only those that we consider most relevant and sufficient for defining brain states for fMRI in anesthetized animals.
Under these conditions, the set of functional characteristics needed to identify an anesthetically induced brain state should include measures of maintenance, arousability, neuronal stimulus–response features, and their hemodynamic translation, as well as FC and its dynamic repertoire (Figure 9). These qualifiers cover a range of informative features to identify and evaluate the degree to which an induced brain state approximates the calm, awake state.
Figure 9.
The functional characteristics (qualifiers) and MIND signature (identifier) of a specific brain state induced by an anesthetic protocol. The aim of anesthesia in fMRI is to induce stable sedation, while maintaining a brain state that approximates an awake, calm state. The protocol may be either mono-anesthetic (drug a; dose x) or multimodal (drug a, b, c; dose x, y, z). Qualifiers are defined as functional characteristics of an intermediate brain state that has been induced and maintained by the use of anesthesia: The cortical EEG signature permits to monitor anesthetic depth, to evaluate arousability by challenging the stability of a brain state via nociception or stress, and to probe stimulus-response features of sensory evoked potentials (SEP). Hemodynamic translation can be evaluated via fMRI, whereas shape and magnitude of the hemodynamic response function (HRF) provide information about anesthesia-induced vasomodulation. Anesthetic effects on functional connectivity (FC) across the brain can be assessed based on correlation matrices that depict interrelations of cortical and subcortical areas, including anticorrelations, and the dynamic repertoire of recurring sets of FC patterns over time. FC has great value as an identifier of a particular brain state and the integrity of its qualifiers. A fingerprint-like FC signature pattern can be used to unambiguously identify maintained intermediate brain states under anesthesia and communicate them across labs. This signature pattern is termed the maintained, intermediate neurophysiologically-determined (MIND) signature. Flanking tasks in fMRI with resting-state scans will permit a researcher to monitor the maintenance or variations in the brain state under anesthesia throughout the fMRI experiment.
Cortical EEG signatures provide valuable information about cortical synchronization at particular frequency bands (including correlates of phase-amplitude coupling, which can influence cortical information transfer and sensory processing; see “Brain States Under Anesthesia” section; Chamadia et al., 2019), the stability of a maintained intermediate brain state during rest and its response to nociception and stress (see “Metastability of Brain States, Hysteresis, and Behavioral Monitoring” section). Due to its real-time accessibility and high temporal resolution, EEG can be used to assess anesthetic depth and rousability, particularly when aligned with behavioral observations (outside the scanner).
The dynamics of neural stimulus–responses can be directly assessed via EEG (whose spatial resolution is limited due to the smoothing properties of the scalp and skull) or invasive electrophysiological recordings of SEP and Ca2+ signals in primary, secondary sensory, and higher-order cortical areas. Multichannel recordings (Muller et al., 2018) and large-scale assessment techniques of neural activity, including high-density EEG (Massimini et al., 2005, 2007), Ca2+- and voltage-sensitive dye imaging (McVea et al., 2016), can be used to assess the degree of complexity of signal processing and propagation across the cortex at different spatial and temporal scales (see also “Brain States Under Anesthesia” and “Deep Brain Stimulation, Opto- and Chemogenetics” sections). This provides insights into the effects of anesthesia on thalamo-cortical and cortico-cortical sensory transmission, as well as cortical information transfer (see “Brain States Under Anesthesia” section).
Hemodynamic integrity can be evaluated via the translation of neural responses into BOLD effects. Comparing SEP or evoked Ca2+ signals with BOLD effects reveals whether neural responses are triggered but become lost in hemodynamic translation (Aksenov et al., 2015). Neurovascular coupling varies across cortical areas and has been found to be more reliable in sensory vs. higher-order cortices (Ojemann et al., 2002, 2013; Conner et al., 2011). Therefore, an overall impairment of neurovascular coupling by anesthesia might lead to a selective loss of BOLD effects. Further information about vasoconstrictive or vasodilatory drug effects can be derived from the shape of the hemodynamic response function (HRF; see “Sensory Processing and the Key Challenges in Murine fMRI” section).
Since anesthesia primarily disrupts neural communication across the brain, its effects are most comprehensively reflected by measures of FC. An ideal sedation for fMRI would meet criteria of a close resemblance of the assessed correlation matrices with those of the awake references, including anticorrelated regions, and a rich repertoire of dynamic FC (see “Functional Connectivity and Murine Resting-State fMRI” section).
MIND Signature
Another advantage of FC in fMRI is that it can be acquired simply in the form of a resting-state scan without any additional equipment. This makes it a strong candidate measure for determining a MIND signature in translational fMRI. fMRI tasks can be flanked with brief resting-state scans that permit the brain state to be identified at regular points throughout an experiment. Such flanking approaches of FC have previously been shown to reveal valuable information on anesthetic effects on murine fMRI tasks (Nasrallah et al., 2014b; Schroeter et al., 2017; Shim et al., 2018). More advanced FC applications have been used to track spatial and temporal aspects of intermediate brain state transitions as a function of anesthesia in mice (Grandjean et al., 2017a; Gutierrez-Barragan et al., 2018) and rats (Schwalm et al., 2017; Aedo-Jury et al., 2019). Finally, the relations of FC and its structural scaffold have been already used to formulate resting-state dynamics as cortical signatures of anesthesia in monkeys (Barttfeld et al., 2015; Uhrig et al., 2018) and humans (Roberts et al., 2019).
Approaches like these have laid the groundwork for the development of a simple, yet comprehensive signature scheme for the unambiguous identification of anesthetically induced and maintained, intermediate brain states that (1) are adjusted to functionally approximate the awake state and (2) indicate the integrity of a defined set of functional qualifiers. This concept assumes a mechanistic connection between FC and the other qualifiers, so that specific functional properties can be inferred from the detection of a specific MIND signature (Figure 9). Such a mechanistic connection remains to be established—as well as the circumstances in which this assumption can be made with confidence.
Linking the MIND Signature to Other Qualifiers
Defining a MIND signature that could indicate the cortical state, stimulus–response properties, their hemodynamic translation, and dynamic FC in the form of one unique identifier would be extremely useful. The unambiguous identification of brain states during fMRI would also substantially improve the process of developing (and communicating) novel anesthetic protocols. A verifiable linkage between these qualifiers requires the simultaneous assessment of neural and hemodynamic readouts (see below). However, a large fraction of qualifiers can be assessed in separate measurements. It is reasonable to perform initial anesthetic adjustments outside the MR scanner, based on electrophysiological and behavioral responses to stimuli and cortical signatures during rest (see “Metastability of Brain States, Hysteresis, and Behavioral Monitoring” section), while under a tight control of physiological parameters. It is therefore important to select conditions under which the qualifiers measured outside the scanner can be linked to others obtained during fMRI.
Brain states, and thus stimulus response properties, are likely to change during fMRI scans due to the loud gradient noise, which can trigger activity in subcortical arousal nuclei (see “Metastability of Brain States, Hysteresis, and Behavioral Monitoring” section). In fact, fMRI at high levels of sound pressure substantially boosts BOLD responses to subcutaneous electrostimulation of the rat under α-chloralose, compared to silent fMRI protocols or after deafening by cochleotomy (Burke et al., 2000). Thus, hearing protection is recommended (Reimann et al., 2016). Sound pressure levels depend on the gradient coils, and can be reduced via sophisticated design of hardware and pulse sequences (Winkler et al., 2018).
Current efforts are underway to advance silent MR techniques using sweep imaging with Fourier transform (SWIFT; Idiyatullin et al., 2006, 2015). This approach renders obsolete the rapid and pulsed on/off switching of strong magnetic field gradients used for spatial encoding, and substantially lowers the acoustic noise level. SWIFT also permits zero echo time TE, which yields distortion- and artifact-free fMRI, even in the face of severe body motion and with electrodes implanted close to the region of interest (Lehto et al., 2018; Paasonen et al., 2020). This is a major advance for the development of sedation protocols to study FC in freely breathing animals and is further attractive for electrophysiological recordings or deep brain stimulation in fMRI. Until SWIFT is readily available for small-bore animal MR scanners, typical sound levels and vibrations produced by the gradients may be mimicked outside the scanner in the electrophysiological setup to test their effects on cortical states. Simultaneous fMRI and EEG (Sadaghiani et al., 2012; Thompson et al., 2013) or Ca2+ imaging (Schwalm et al., 2017; Schlegel et al., 2018) can permit a direct translation between the cortical state and whole-brain hemodynamic responses.
It remains to be clarified to what extent a signature based on FC is sufficient to indicate the integrity of the underlying functional properties of a brain state. Recent studies on the simultaneous imaging of murine cortex-wide FC of hemodynamic and neural calcium fluctuations in the resting state have found a strong relationship between both measurements (Murphy et al., 2017). Both measurements show sufficient resemblance in their intercortical FC for tracking similar brain states under anesthesia (Brier et al., 2019) and similar sets of dynamic FC configurations using a sliding-window approach (Matsui et al., 2018). Transitions in the dynamic FC configurations can be tracked via fMRI within a time scale of 1 or 2 s, and a sliding-window length below 1 min (Barttfeld et al., 2015, movie S1; Grandjean et al., 2017a; Lurie et al., 2018), which would be sufficient to resolve effects such as the metastable cortical state transitions that were observed via LFP recordings under constant isoflurane concentrations (see “Metastability of Brain States, Hysteresis, and Behavioral Monitoring” sections, Figure 2D; Hudson et al., 2014; Hudson, 2017). However, studies that investigate such brain state transitions under anesthesia linking electrophysiological or calcium recordings with dynamic FC are still pending.
Limitations in Maintaining Stable Brain States
Even when a brain state is stably maintained over time, it will exhibit a restricted set of recurrent FC configurations that vary within a defined dynamic range (Uhrig et al., 2018). Transitions between two intermediate brain states, as observed for metastable LFP cortical signatures (Hudson et al., 2014), should thus be classifiable as two distinct sets of recurrent FC configurations. Each of these sets varies within the internal dynamic range of its respective brain state. The MIND signature will comprise an indication of the variance of this range (narrow or broad) and the FC patterns that recur (Gutierrez-Barragan et al., 2018). A loss of recurrence and the appearance of new FC configurations would indicate a drift in brain states, and hence a change in anesthetic depth. Transitions in brain states become a confounding factor when they significantly alter the specific functional properties that are relevant for a given fMRI investigation.
The continuous anesthetic infusion via intraperitoneal or subcutaneous administration routes may present a practical experimental challenge because the uptake and distribution of agents depends on various biopharmaceutical and biological factors (comprehensively reviewed in Claassen, 1994). Whether and to what extent it is possible to maintain stable brain states under these conditions has to be established according to specific application procedures (Sirmpilatze et al., 2019). Future technical developments and novel methods should produce increasingly detailed functional information that will need to be integrated into single composite fMRI measurements (Breakspear, 2017; Belloy et al., 2018; Roberts et al., 2019; Song et al., 2019). This will produce a MIND signature that identifies brain states with increasing precision.
The MIND Signature in Translational fMRI
The MIND signature would be valuable as an indicator of specific brain states independent of minor changes in brain anatomy and thus hold even further value for translational fMRI. It would provide a framework for comparing the MIND signatures of humans and mice and facilitate the development of a scheme for systematic evaluation of functional similarity and divergence across species. This would allow for better translation of anesthetic effects from humans to mice and back, and will have three major benefits: (1) anesthetic effects could be referenced against an awake, but calm and relaxed volunteer—even within the same subject; (2) introspective reports could link specific MIND signatures to LOC and to the overall experience; and (3) determining precise translational limitations in general brain functions and specific anesthetic effects, to assess the scope for murine transgenic and optogenetic applications to explore human cognition and consciousness.
Future Perspectives
The need for effective anesthetic protocols and a better comprehension of their underpinnings is not exclusive to translational fMRI, but is equally important for preclinical researchers studying electrophysiology, Ca2+ imaging, and other modalities to probe brain functions. In clinical practice, balanced multimodal anesthesia is common and increasingly being used as a substitute for opioids, especially in light of the current opioid crisis in the US (Brown et al., 2018a). In basic research, cognitive scientists employ anesthesia to probe the neural correlates of consciousness. All these disparate fields produce valuable data that should be taken into account across research domains in pursuit of a deeper understanding of anesthetic effects on animal and human brain functions.
Yet accessing knowledge on anesthetic effects is not always straightforward. Much valuable information is buried in publications that focus on biological research questions and use anesthesia only as an experimental tool. Many anecdotal observations of crucial effects remain unpublished “lab lore” and are not available to the community. Having access to this information could substantially accelerate our progress in detailing anesthetic effects to develop multimodal protocols for a stable sedation with the best possible preservation of brain functions. A community-driven open database of key observations—collected from publications and lab notes—would make this information more accessible, promoting reproducibility and preventing research groups from running into the same “dead ends” already discovered by others. We are currently developing a web-based infrastructure for such an open initiative across neuroimaging communities, to facilitate the exchange of this information. We invite anyone interested in this initiative to contact the corresponding author.
Within the fMRI community, the additional assessments of resting-state fMRI at selected points throughout the experiment would help to specify the actual brain state and make comparisons of data across labs more reliable. The concept of the MIND signature could support this process by defining a set of qualifiers and one identifier readout that permits a reliable indication of a specific brain state and its related functional properties. Our future work is dedicated to further refining and establishing the MIND signature in a collaborative effort.
Conclusions
To make proper use of translational fMRI for a direct, non-invasive comparison between large-scale brain functions in humans and mice, it is essential to understand the impact of anesthesia on neural physiology. The animal is usually anesthetized to avoid variations due to stress and distraction, with the ultimate aim of achieving stable sedation while maintaining a functional brain state close to that of an awake, resting subject. Maintaining functional brain states and neurovascular coupling are the two major challenges of preclinical fMRI. Most anesthetics affect neural and hemodynamic integrity in different ways, based on their target receptors, which typically assigns them to one of the three main classes of anesthesia. Despite addressing different pathways, all classes exert their effects by spoiling the phase-frequency relationships of remote neuronal populations, which eventually leads to a breakdown of information transfer along the cortical hierarchy and thus LOC. The resulting disruption of higher-order circuits is reflected in FC, although the specifiability of markers for discrete functional stages remain to be clarified. Better knowledge of the mechanisms of action allows to balance different compounds in order to develop multimodal anesthetic protocols for translational fMRI, ideally revealing mainly species-specific variations and not the influence of anesthesia. The concept of the MIND signature has been proposed as an approach to determine and re-identify specific brain states and related functional properties induced by different anesthetic drugs and levels across studies, laboratories, and species.
Author Contributions
HR: conceiving and drafting the article, agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. TN: revising the article critically, final approval of the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Russ Hodge (Max Delbrück Center for Molecular Medicine, Berlin, Germany), Andreas Pohlmann, Jurjen Heij, Jason Millward (Berlin Ultrahigh Field Facility, Max Delbrück Center for Molecular Medicine, Berlin, Germany), and Nikoloz Sirmpilatze (Functional Imaging Laboratory, Deutsches Primatenzentrum, Göttingen, Germany) for valuable discussions, reading and commenting on the manuscript.
Footnotes
References
- Achermann P., Borbély A. A. (1997). Low-frequency (<1 Hz) oscillations in the human sleep electroencephalogram. Neuroscience 81, 213–222. 10.1016/s0306-4522(97)00186-3 [DOI] [PubMed] [Google Scholar]
- Adamczak J. M., Farr T. D., Seehafer J. U., Kalthoff D., Hoehn M. (2010). High field BOLD response to forepaw stimulation in the mouse. NeuroImage 51, 704–712. 10.1016/j.neuroimage.2010.02.083 [DOI] [PubMed] [Google Scholar]
- Aedo-Jury F., Schwalm M., Hamzehpour L., Stroh A. (2019). Brain states govern the spatio-temporal dynamics of resting state functional connectivity. bioRxiv [Preprint]. 10.1101/832998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal A., Brennan C., Shortal B., Contreras D., Kelz M. B., Proekt A. (2019). Coherence of visual-evoked γ oscillations is disrupted by propofol but preserved under equipotent doses of isoflurane. Front. Syst. Neurosci. 13:19. 10.3389/fnsys.2019.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrens E. T., Dubowitz D. J. (2001). Peripheral somatosensory fMRI in mouse at 11.7 T. NMR Biomed. 14, 318–324. 10.1002/nbm.709 [DOI] [PubMed] [Google Scholar]
- Akeju O., Brown E. N. (2017). Neural oscillations demonstrate that general anesthesia and sedative states are neurophysiologically distinct from sleep. Curr. Opin. Neurobiol. 44, 178–185. 10.1016/j.conb.2017.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akeju O., Kim S. E., Vazquez R., Rhee J., Pavone K. J., Hobbs L. E., et al. (2016a). Spatiotemporal dynamics of dexmedetomidine-induced electroencephalogram oscillations. PLoS One 11:e0163431. 10.1371/journal.pone.0163431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akeju O., Song A. H., Hamilos A. E., Pavone K. J., Flores F. J., Brown E. N., et al. (2016b). Electroencephalogram signatures of ketamine anesthesia-induced unconsciousness. Clin. Neurophysiol. 127, 2414–2422. 10.1016/j.clinph.2016.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akeju O., Loggia M. L., Catana C., Pavone K. J., Vazquez R., Rhee J., et al. (2014a). Disruption of thalamic functional connectivity is a neural correlate of dexmedetomidine-induced unconsciousness. Elife 3:e04499. 10.7554/eLife.04499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akeju O., Westover B., Pavone K. J., Sampson A. L., Hartnack K. E., Brown E. N., et al. (2014b). Effects of sevoflurane and propofol on frontal electroencephalogram power and coherence. Anesthesiology 121, 990–998. 10.1097/aln.0000000000000436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksenov D. P., Li L., Miller M. J., Iordanescu G., Wyrwicz A. M. (2015). Effects of anesthesia on BOLD signal and neuronal activity in the somatosensory cortex. J. Cereb. Blood Flow Metab. 35, 1819–1826. 10.1038/jcbfm.2015.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alheid G. F., McCrimmon D. R. (2008). The chemical neuroanatomy of breathing. Respir. Physiol. Neurobiol. 164, 3–11. 10.1016/j.resp.2008.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkire M. T., Asher C. D., Franciscus A. M., Hahn E. L. (2009). Thalamic microinfusion of antibody to a voltage-gated potassium channel restores consciousness during anesthesia. Anesthesiology 110, 766–773. 10.1097/aln.0b013e31819c461c [DOI] [PubMed] [Google Scholar]
- Alkire M. T., Hudetz A. G., Tononi G. (2008). Consciousness and anesthesia. Science 322, 876–880. 10.1126/science.1149213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkire M. T., Miller J. (2005). General anesthesia and the neural correlates of consciousness. Prog. Brain Res. 150, 229–244. 10.1016/s0079-6123(05)50017-7 [DOI] [PubMed] [Google Scholar]
- Amzica F. (2009). Basic physiology of burst-suppression. Epilepsia 50, 38–39. 10.1111/j.1528-1167.2009.02345.x [DOI] [PubMed] [Google Scholar]
- Amzica F. (2015). What does burst suppression really mean? Epilepsy Behav. 49, 234–237. 10.1016/j.yebeh.2015.06.012 [DOI] [PubMed] [Google Scholar]
- Amzica F., Steriade M. (1997). The K-complex: its slow (<1 Hz) rhythmicity and relation to delta waves. Neurology 49, 952–959. 10.1212/wnl.49.4.952 [DOI] [PubMed] [Google Scholar]
- Anderson P. M., Jones N., O’Brien T. J., Pinault D. (2017). The N-Methyl d-Aspartate glutamate receptor antagonist ketamine disrupts the functional state of the corticothalamic pathway. Cereb. Cortex 27, 3172–3185. 10.1093/cercor/bhw168 [DOI] [PubMed] [Google Scholar]
- Andrieu G., Roth B., Ousmane L., Castaner M., Petillot P., Vallet B., et al. (2009). The efficacy of intrathecal morphine with or without clonidine for postoperative analgesia after radical prostatectomy. Anesth. Analg. 108, 1954–1957. 10.1213/ane.0b013e3181a30182 [DOI] [PubMed] [Google Scholar]
- Arena A., Lamanna J., Gemma M., Ripamonti M., Ravasio G., Zimarino V., et al. (2017). Linear transformation of the encoding mechanism for light intensity underlies the paradoxical enhancement of cortical visual responses by sevoflurane. J. Physiol. 595, 321–339. 10.1113/jp272215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma C. (1992). Noradrenergic innervation of the thalamic reticular nucleus: a light and electron microscopic immunohistochemical study in rats. J. Comp. Neurol. 319, 299–311. 10.1002/cne.903190209 [DOI] [PubMed] [Google Scholar]
- Aston-Jones G., Rajkowski J., Cohen J. (1999). Role of locus coeruleus in attention and behavioral flexibility. Biol. Psychiatry 46, 1309–1320. 10.1016/s0006-3223(99)00140-7 [DOI] [PubMed] [Google Scholar]
- Astori S., Wimmer R. D., Prosser H. M., Corti C., Corsi M., Liaudet N., et al. (2011). The CaV3.3 calcium channel is the major sleep spindle pacemaker in thalamus. Proc. Natl. Acad. Sci. U S A 108, 13823–13828. 10.1073/pnas.1105115108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai D., Pennefather P. S., MacDonald J. F., Orser B. A. (1999). The general anesthetic propofol slows deactivation and desensitization of GABAA receptors. J. Neurosci. 19, 10635–10646. 10.1523/jneurosci.19-24-10635.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsters J. H., Zerbi V., Sallet J., Wenderoth N., Mars R. B. (2019). Primate homologs of mouse cortico- striatal circuits. bioRxiv [Preprint]. 10.1101/834481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R., Gent T. C., Yang Q., Parker S., Vyssotski A. L., Wisden W., et al. (2014). Altered activity in the central medial thalamus precedes changes in the neocortex during transitions into both sleep and propofol anesthesia. J. Neurosci. 34, 13326–13335. 10.1523/JNEUROSCI.1519-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker R., Tiesinga P., Kötter R. (2015). The scalable brain atlas: instant web-based access to public brain atlases and related content. Neuroinformatics 13, 353–366. 10.1007/s12021-014-9258-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltes C., Bosshard S., Mueggler T., Ratering D., Rudin M. (2011). Increased blood oxygen level-dependent (BOLD) sensitivity in the mouse somatosensory cortex during electrical forepaw stimulation using a cryogenic radiofrequency probe. NMR Biomed. 24, 439–446. 10.1002/nbm.1613 [DOI] [PubMed] [Google Scholar]
- Banks M. I., Moran N. S., Krause B. M., Grady S. M., Uhlrich D. J., Manning K. A. (2017). Evidence that loss of consciousness under anesthesia is not associated with impaired stimulus representation in auditory cortex. bioRxiv [Preprint]. 10.1101/21335529156030 [DOI] [Google Scholar]
- Barttfeld P., Uhrig L., Sitt J. D., Sigman M., Jarraya B., Dehaene S. (2015). Signature of consciousness in the dynamics of resting-state brain activity. Proc. Natl. Acad. Sci. U S A 112, 887–892. 10.1073/pnas.1418031112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos A. M., Vezoli J., Bosman C. A., Schoffelen J. M., Oostenveld R., Dowdall J. R., et al. (2015). Visual areas exert feedforward and feedback influences through distinct frequency channels. Neuron 85, 390–401. 10.1016/j.neuron.2014.12.018 [DOI] [PubMed] [Google Scholar]
- Baumgart J. P., Zhou Z.-Y., Hara M., Cook D. C., Hoppa M. B., Ryan T. A., et al. (2015). Isoflurane inhibits synaptic vesicle exocytosis through reduced Ca2+ influx, not Ca2+-exocytosis coupling. Proc. Natl. Acad. Sci. U S A 112, 11959–11964. 10.1073/pnas.1500525112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazhenov M., Timofeev I., Steriade M., Sejnowski T. J. (1999). Self-sustained rhythmic activity in the thalamic reticular nucleus mediated by depolarizing GABAA receptor potentials. Nat. Neurosci. 2, 168–174. 10.1038/5729 [DOI] [PubMed] [Google Scholar]
- Beenhakker M. P., Huguenard J. R. (2009). Neurons that fire together also conspire together: is normal sleep circuitry hijacked to generate epilepsy? Neuron 62, 612–632. 10.1016/j.neuron.2009.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belloy M. E., Naeyaert M., Abbas A., Shah D., Vanreusel V., Van Audekerke J., et al. (2018). Dynamic resting state fMRI analysis in mice reveals a set of Quasi-Periodic Patterns and illustrates their relationship with the global signal. NeuroImage 180, 463–484. 10.1016/j.neuroimage.2018.01.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann E., Zur G., Bershadsky G., Kahn I. (2016). The organization of mouse and human cortico-hippocampal networks estimated by intrinsic functional connectivity. Cereb. Cortex 26, 4497–4512. 10.1093/cercor/bhw327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertero A., Liska A., Pagani M., Parolisi R., Masferrer M. E., Gritti M., et al. (2018). Autism-associated 16p11.2 microdeletion impairs prefrontal functional connectivity in mouse and human. Brain 141, 2055–2065. 10.1093/brain/awy111 [DOI] [PubMed] [Google Scholar]
- Bhattacharya S., Le Cauchois M. B., Iglesias P. A., Chen Z. S. (2019). Thalamocortical network connectivity controls spatiotemporal dynamics of cortical and thalamic traveling waves. bioRxiv 6:7169 10.1101/780239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binda P., Gamlin P. D. (2017). Renewed attention on the pupil light reflex. Trends Neurosci. 40, 455–457. 10.1016/j.tins.2017.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner S., Gobel K., Meuth S. G. (2019). Cerebral Physiology. Oxford Textbook of Neuroscience and Anaesthesiology. Ann Arbor, MI: Oxford University Press. [Google Scholar]
- Blain-Moraes S., Lee U., Ku S., Noh G., Mashour G. A. (2014). Electroencephalographic effects of ketamine on power, cross-frequency coupling and connectivity in the α bandwidth. Front. Syst. Neurosci. 8:114. 10.3389/fnsys.2014.00114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blain-Moraes S., Tarnal V., Vanini G., Alexander A., Rosen D., Shortal B., et al. (2015). Neurophysiological correlates of sevoflurane-induced unconsciousness. Anesthesiology 122, 307–316. 10.1097/ALN.0000000000000482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazquez Freches G., Chavarrias C., Shemesh N. (2018). BOLD-fMRI in the mouse auditory pathway. NeuroImage 165, 265–277. 10.1016/j.neuroimage.2017.10.027 [DOI] [PubMed] [Google Scholar]
- Boly M., Garrido M. I., Gosseries O., Bruno M. A., Boveroux P., Schnakers C., et al. (2011). Preserved feedforward but impaired top-down processes in the vegetative state. Science 332, 858–862. 10.1126/science.1202043 [DOI] [PubMed] [Google Scholar]
- Bonhomme V., Staquet C., Montupil J., Defresne A., Kirsch M., Martial C., et al. (2019). General anesthesia: a probe to explore consciousness. Front. Syst. Neurosci. 13:36. 10.3389/fnsys.2019.00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonhomme V., Vanhaudenhuyse A., Demertzi A., Bruno M. A., Jaquet O., Bahri M. A., et al. (2016). Resting-state network-specific breakdown of functional connectivity during ketamine alteration of consciousness in volunteers. Anesthesiology 125, 873–888. 10.1097/aln.0000000000001275 [DOI] [PubMed] [Google Scholar]
- Boon J. A., Milsom W. K. (2008). NMDA receptor-mediated processes in the Parabrachial/Kolliker fuse complex influence respiratory responses directly and indirectly via changes in cortical activation state. Respir. Physiol. Neurobiol. 162, 63–72. 10.1016/j.resp.2008.04.002 [DOI] [PubMed] [Google Scholar]
- Börgers C., Epstein S., Kopell N. J. (2005). Background gamma rhythmicity and attention in cortical local circuits: a computational study. Proc. Natl. Acad. Sci. U S A 102, 7002–7007. 10.1073/pnas.0502366102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosshard S. C., Baltes C., Wyss M. T., Mueggler T., Weber B., Rudin M. (2010). Assessment of brain responses to innocuous and noxious electrical forepaw stimulation in mice using BOLD fMRI. Pain 151, 655–663. 10.1016/j.pain.2010.08.025 [DOI] [PubMed] [Google Scholar]
- Bosshard S. C., Stuker F., Von Deuster C., Schroeter A., Rudin M. (2015). BOLD fMRI of C-fiber mediated nociceptive processing in mouse brain in response to thermal stimulation of the forepaws. PLoS One 10:e0126513. 10.1371/journal.pone.0126513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveroux P., Vanhaudenhuyse A., Bruno M. A., Noirhomme Q., Lauwick S., Luxen A., et al. (2010). Breakdown of within- and between-network resting state functional magnetic resonance imaging connectivity during propofol-induced loss of consciousness. Anesthesiology 113, 1038–1053. 10.1097/ALN.0b013e3181f697f5 [DOI] [PubMed] [Google Scholar]
- Bowery N. G., Hudson A. L., Price G. W. (1987). GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience 20, 365–383. 10.1016/0306-4522(87)90098-4 [DOI] [PubMed] [Google Scholar]
- Braun J., Mattia M. (2010). Attractors and noise: twin drivers of decisions and multistability. NeuroImage 52, 740–751. 10.1016/j.neuroimage.2009.12.126 [DOI] [PubMed] [Google Scholar]
- Breakspear M. (2017). Dynamic models of large-scale brain activity. Nat. Neurosci. 20, 340–352. 10.1038/nn.4497 [DOI] [PubMed] [Google Scholar]
- Bremer F. (1935). Cerveau isole et Physiologie du sommeil. C. R. Soc. Biol. 118, 1235–1241. [Google Scholar]
- Brier L. M., Landsness E. C., Snyder A. Z., Wright P. W., Baxter G. A., Bauer A. Q., et al. (2019). Separability of calcium slow waves and functional connectivity during wake, sleep and anesthesia. Neurophotonics 6:035002. 10.1117/1.nph.6.3.035002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. N., Lydic R., Schiff N. D. (2010). General anesthesia, sleep and coma. N. Engl. J. Med. 363, 2638–2650. 10.1056/NEJMra0808281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. L., Zanos P., Wang L., Elmer G. I., Gould T. D., Shepard P. D. (2018). Isoflurane but not halothane prevents and reverses helpless behavior: a role for eeg burst suppression?. Int. J. Neuropsychopharmacol. 21, 777–785. 10.1093/ijnp/pyy029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. N., Pavone K. J., Naranjo M. (2018a). Multimodal general anesthesia: theory and practice. Anesth. Analg. 127, 1246–1258. 10.1213/ane.0000000000003668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. N., Purdon P. L., Akeju O., An J. (2018b). Using EEG markers to make inferences about anaesthetic-induced altered states of arousal. Br. J. Anaesth. 121, 325–327. 10.1016/j.bja.2017.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. N., Purdon P. L., Van Dort C. J. (2011). General anesthesia and altered states of arousal: a systems neuroscience analysis. Annu. Rev. Neurosci. 34, 601–628. 10.1146/annurev-neuro-060909-153200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckwalter J. A., Parvizi J., Morecraft R. J., Van Hoesen G. W. (2008). Thalamic projections to the posteromedial cortex in the macaque. J. Comp. Neurol. 507, 1709–1733. 10.1002/cne.21647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhari Q., Schroeter A., Cole D. M., Rudin M. (2017). Resting state fMRI in mice reveals anesthesia specific signatures of brain functional networks and their interactions. Front. Neural Circuits 11:5. 10.3389/fncir.2017.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E., Sporns O. (2012). The economy of brain network organization. Nat. Rev. Neurosci. 13, 336–349. 10.1038/nrn3214 [DOI] [PubMed] [Google Scholar]
- Burke M., Schwindt W., Ludwig U., Hennig J., Hoehn M. (2000). Facilitation of electric forepaw stimulation-induced somatosensory activation in rats by additional acoustic stimulation: an fMRI investigation. Magn. Reson. Med. 44, 317–321. [DOI] [PubMed] [Google Scholar]
- Busche M. A., Kekuš M., Adelsberger H., Noda T., Förstl H., Nelken I., et al. (2015). Rescue of long-range circuit dysfunction in Alzheimer’s disease models. Nat. Neurosci. 18, 1623–1630. 10.1038/nn.4137 [DOI] [PubMed] [Google Scholar]
- Buzsáki G., Chrobak J. J. (1995). Temporal structure in spa- tially organized neuronal ensembles: a role for interneuronal networks. Curr. Opin. Neurobiol. 5, 504–510. 10.1016/0959-4388(95)80012-3 [DOI] [PubMed] [Google Scholar]
- Buzsáki G., Moser E. I. (2013). Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat. Neurosci. 16, 130–138. 10.1038/nn.3304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagna J. A., Miller K. W., Forman S. A. (2003). Mechanisms of actions of inhaled anesthetics. N. Engl. J. Med. 348, 2110–2124. 10.1056/nejmra021261 [DOI] [PubMed] [Google Scholar]
- Cao R., Li J., Ning B., Sun N., Wang T., Zuo Z., et al. (2017). Functional and oxygen-metabolic photoacoustic microscopy of the awake mouse brain. NeuroImage 150, 77–87. 10.1016/j.neuroimage.2017.01.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter M. E., Yizhar O., Chikahisa S., Nguyen H., Adamantidis A., Nishino S., et al. (2010). Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat. Neurosci. 13, 1526–1533. 10.1038/nn.2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casali A. G., Gosseries O., Rosanova M., Boly M., Sarasso S., Casali K. R., et al. (2013). A theoretically based index of consciousness independent of sensory processing and behavior. Sci. Transl. Med. 5:198ra105. 10.1126/scitranslmed.3006294 [DOI] [PubMed] [Google Scholar]
- Casarotto S., Rosanova M., Gosseries O., Boly M., Massimini M., Sarasso S. (2016). “Exploring the neurophysiological correlates of loss and recovery of consciousness: perturbational complexity,” in Brain Function and Responsiveness in Disorders of Consciousness, eds Monti M. M., Sannita W. G. (Cham: Springer International Publishing; ), 93–104. [Google Scholar]
- Chamadia S., Pedemonte J. C., Hahm E. Y., Mekonnen J., Ibala R., Gitlin J., et al. (2019). Delta oscillations phase limit neural activity during sevoflurane anesthesia. Commun. Biol. 2:415. 10.1038/s42003-019-0664-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R. W., Leong A. T. L., Ho L. C., Gao P. P., Wong E. C., Dong C. M., et al. (2017). Low-frequency hippocampal-cortical activity drives brain-wide resting-state functional MRI connectivity. Proc. Natl. Acad. Sci. U S A 114, E6972–E6981. 10.1073/pnas.1703309114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Tong C., Han Z., Zhang K., Bo B., Feng Y., et al. (2020). Sensory evoked fMRI paradigms in awake mice. NeuroImage 204:116242. 10.1016/j.neuroimage.2019.116242 [DOI] [PubMed] [Google Scholar]
- Chao Z. C., Takaura K., Wang L., Fujii N., Dehaene S. (2018). Large-scale cortical networks for hierarchical prediction and prediction error in the primate brain. Neuron 100, 1252.e3–1266.e3. 10.1016/j.neuron.2018.10.004 [DOI] [PubMed] [Google Scholar]
- Chauvette S., Crochet S., Volgushev M., Timofeev I. (2011). Properties of slow oscillation during slow-wave sleep and anesthesia in cats. J. Neurosci. 31, 14998–15008. 10.1523/JNEUROSCI.2339-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Wimmer R. D., Wilson M. A., Halassa M. M. (2016). Thalamic circuit mechanisms link sensory processing in sleep and attention. Front. Neural Circuits 9:83. 10.3389/fncir.2015.00083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching S., Brown E. N. (2014). Modeling the dynamical effects of anesthesia on brain circuits. Curr. Opin. Neurobiol. 25, 116–122. 10.1016/j.conb.2013.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching S., Cimenser A., Purdon P. L., Brown E. N., Kopell N. J. (2010). Thalamocortical model for a propofol-induced α-rhythm associated with loss of consciousness. Proc. Natl. Acad. Sci. U S A 107, 22665–22670. 10.1073/pnas.1017069108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching S., Purdon P. L., Vijayan S., Kopell N. J., Brown E. N. (2012). A neurophysiological-metabolic model for burst suppression. Proc. Natl. Acad. Sci. U S A 109, 3095–3100. 10.1073/pnas.1121461109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu T. H., Chen M. J., Yang Y. R., Yang J. J., Tang F. I. (1995). Action of dexmedetomidine on rat locus coeruleus neurones: intracellular recording in vitro. Eur. J. Pharmacol. 285, 261–268. 10.1016/0014-2999(95)00417-j [DOI] [PubMed] [Google Scholar]
- Chuang K. H., Nasrallah F. A. (2017). Functional networks and network perturbations in rodents. NeuroImage 163, 419–436. 10.1016/j.neuroimage.2017.09.038 [DOI] [PubMed] [Google Scholar]
- Cimenser A., Purdon P. L., Pierce E. T., Walsh J. L., Salazar-Gomez A. F., Harrell P. G., et al. (2011). Tracking brain states under general anesthesia by using global coherence analysis. Proc. Natl. Acad. Sci. U S A 108, 8832–8837. 10.1073/pnas.1017041108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claassen V. (1994). “Neglected factors in pharmacology and neuroscience research: biopharmaceutics, animal characteristics, maintenance, testing conditions,” in Techniques in the Behavioral and Neural Sciences, Vol. 12, ed. Huston J. P. (New York, NY: Elsevier; ). [Google Scholar]
- Compte A., Sanchez-Vives M. V., McCormick D. A., Wang X. J. (2003). Cellular and network mechanisms of slow oscillatory activity (<1 Hz) and wave propagations in a cortical network model. J. Neurophysiol. 89, 2707–2725. 10.1152/jn.00845.2002 [DOI] [PubMed] [Google Scholar]
- Conner C. R., Ellmore T. M., Peters T. A., DiSano M. A., Tandon N. (2011). Variability of the relationship between electrophysiology and BOLD-fMRI across cortical regions in humans. J. Neurosci. 31, 12855–12865. 10.1523/JNEUROSCI.1457-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa-Sales C., Rabin B. C., Maze M. (1992). A hypnotic response to dexmedetomidine, an α 2 agonist, is mediated in the locus coeruleus in rats. Anesthesiology 76, 948–952. 10.1097/00000542-199206000-00013 [DOI] [PubMed] [Google Scholar]
- Coull J. T. (1998). Neural correlates of attention and arousal: insights from electrophysiology, functional neuroimaging and psychopharmacology. Prog. Neurobiol. 55, 343–361. 10.1016/s0301-0082(98)00011-2 [DOI] [PubMed] [Google Scholar]
- Coulter K. L., Perier F., Radeke C. M., Vandenberg C. A. (1995). Identification and molecular localization of a pH-sensing domain for the inward rectifier potassium channel HIR. Neuron 15, 1157–1168. 10.1016/0896-6273(95)90103-5 [DOI] [PubMed] [Google Scholar]
- Craig A. D. (2013). Cooling, pain and other feelings from the body in relation to the autonomic nervous system. Handb. Clin. Neurol. 117, 103–109. 10.1016/b978-0-444-53491-0.00009-2 [DOI] [PubMed] [Google Scholar]
- Crandall S. R., Govindaiah G., Cox C. L. (2010). Low-threshold Ca2+ current amplifies distal dendritic signaling in thalamic reticular neurons. J. Neurosci. 30, 15419–15429. 10.1523/JNEUROSCI.3636-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V., David F., Lörincz M. L., Hughes S. W. (2015). The thalamocortical network as a single slow wave-generating unit. Curr. Opin. Neurobiol. 31, 72–80. 10.1016/j.conb.2014.09.001 [DOI] [PubMed] [Google Scholar]
- Crunelli V., Hughes S. W. (2010). The slow (<1 Hz) rhythm of non-REM sleep: a dialogue between three cardinal oscillators. Nat. Neurosci. 13, 9–17. 10.1038/nn.2445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham M. O., Pervouchine D. D., Racca C., Kopell N. J., Davies C. H., Jones R. S., et al. (2006). Neuronal metabolism governs cortical network response state. Proc. Natl. Acad. Sci. U S A 103, 5597–5601. 10.1073/pnas.0600604103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagal A., Lam A. M. (2009). Cerebral autoregulation and anesthesia. Curr. Opin. Anaesthesiol. 22, 547–552. 10.1097/ACO.0b013e32833020be [DOI] [PubMed] [Google Scholar]
- Dang-Vu T. T., McKinney S. M., Buxton O. M., Solet J. M., Ellenbogen J. M. (2010). Spontaneous brain rhythms predict sleep stability in the face of noise. Curr. Biol. 20, R626–R627. 10.1016/j.cub.2010.06.032 [DOI] [PubMed] [Google Scholar]
- Davis K. D., Flor H., Greely H. T., Iannetti G. D., Mackey S., Ploner M., et al. (2017). Brain imaging tests for chronic pain: medical, legal and ethical issues and recommendations. Nat Rev Neurol 13, 624–638. 10.1038/nrneurol.2017.122 [DOI] [PubMed] [Google Scholar]
- de la Salle S., Choueiry J., Shah D., Bowers H., McIntosh J., Ilivitsky V., et al. (2016). Effects of ketamine on resting-state EEG activity and their relationship to perceptual/dissociative symptoms in healthy humans. Front. Pharmacol. 7, 348–348. 10.3389/fphar.2016.00348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleuze C., Huguenard J. R. (2016). Two classes of excitatory synaptic responses in rat thalamic reticular neurons. J. Neurophysiol. 116, 995–1011. 10.1152/jn.01121.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai M., Kahn I., Knoblich U., Bernstein J., Atallah H., Yang A., et al. (2011). Mapping brain networks in awake mice using combined optical neural control and fMRI. J. Neurophysiol. 105, 1393–1405. 10.1152/jn.00828.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destexhe A., Contreras D. (2006). Neuronal computations with stochastic network states. Science 314, 85–90. 10.1126/science.1127241 [DOI] [PubMed] [Google Scholar]
- Destexhe A., Contreras D., Sejnowski T. J., Steriade M. (1994). A model of spindle rhythmicity in the isolated thalamic reticular nucleus. J. Neurophysiol. 72, 803–818. 10.1152/jn.1994.72.2.803 [DOI] [PubMed] [Google Scholar]
- Destexhe A., Contreras D., Steriade M., Sejnowski T. J., Huguenard J. R. (1996). In vivo, in vitro, and computational analysis of dendritic calcium currents in thalamic reticular neurons. J. Neurosci. 16, 169–185. 10.1523/JNEUROSCI.16-01-00169.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destexhe A., Hughes S. W., Rudolph M., Crunelli V. (2007). Are corticothalamic ‘up’ states fragments of wakefulness? Trends Neurosci. 30, 334–342. 10.1016/j.tins.2007.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detsch O., Kochs E., Siemers M., Bromm B., Vahle-Hinz C. (2002). Increased responsiveness of cortical neurons in contrast to thalamic neurons during isoflurane-induced EEG bursts in rats. Neurosci. Lett. 317, 9–12. 10.1016/s0304-3940(01)02419-3 [DOI] [PubMed] [Google Scholar]
- Detsch O., Vahle-Hinz C., Kochs E., Siemers M., Bromm B. (1999). Isoflurane induces dose-dependent changes of thalamic somatosensory information transfer. Brain Res. 829, 77–89. 10.1016/s0006-8993(99)01341-4 [DOI] [PubMed] [Google Scholar]
- Devonshire I. M., Grandy T. H., Dommett E. J., Greenfield S. A. (2010). Effects of urethane anaesthesia on sensory processing in the rat barrel cortex revealed by combined optical imaging and electrophysiology. Eur. J. Neurosci. 32, 786–797. 10.1111/j.1460-9568.2010.07322.x [DOI] [PubMed] [Google Scholar]
- Dopfel D., Zhang N. (2018). Mapping stress networks using functional magnetic resonance imaging in awake animals. Neurobiol. Stress 9, 251–263. 10.1016/j.ynstr.2018.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza R. D., Meier A. M., Bista P., Wang Q., Burkhalter A. (2016). Recruitment of inhibition and excitation across mouse visual cortex depends on the hierarchy of interconnecting areas. Elife 5:e19332. 10.7554/eLife.19332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duling B. R., Berne R. M. (1970). Longitudinal gradients in periarteriolar oxygen tension. A possible mechanism for the participation of oxygen in local regulation of blood flow. Circ. Res. 27, 669–678. 10.1161/01.res.27.5.669 [DOI] [PubMed] [Google Scholar]
- Echlin F. A., Arnett V., Zoll J. (1952). Paroxysmal high voltage discharges from isolated and partially isolated human and animal cerebral cortex. Electroencephalogr. Clin. Neurophysiol. 4, 147–164. 10.1016/0013-4694(52)90004-7 [DOI] [PubMed] [Google Scholar]
- Ecker A. S., Denfield G. H., Bethge M., Tolias A. S. (2016). On the structure of neuronal population activity under fluctuations in attentional state. J. Neurosci. 36, 1775–1789. 10.1523/JNEUROSCI.2044-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eger E. I., II., Saidman L. J., Brandstater B. (1965). Minimum alveolar anesthetic concentration: a standard of anesthetic potency. Anesthesiology 26, 756–763. 10.1097/00000542-196511000-00010 [DOI] [PubMed] [Google Scholar]
- Erisken S., Vaiceliunaite A., Jurjut O., Fiorini M., Katzner S., Busse L. (2014). Effects of locomotion extend throughout the mouse early visual system. Curr. Biol. 24, 2899–2907. 10.1016/j.cub.2014.10.045 [DOI] [PubMed] [Google Scholar]
- Erö C., Gewaltig M.-O., Keller D., Markram H. (2018). A cell atlas for the mouse brain. Front. Neuroinform. 12:84. 10.3389/fninf.2018.00084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenko O., Mölle M., Born J., Sara S. J. (2006). Elevated sleep spindle density after learning or after retrieval in rats. J. Neurosci. 26, 12914–12920. 10.1523/JNEUROSCI.3175-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez L. M. J., Pellegrini C., Vantomme G., Béard E., Lüthi A., Astori S. (2017). Cortical afferents onto the nucleus Reticularis thalami promote plasticity of low-threshold excitability through GluN2C-NMDARs. Sci. Rep. 7:12271. 10.1038/s41598-017-12552-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarelli F., Massimini M., Sarasso S., Casali A., Riedner B. A., Angelini G., et al. (2010). Breakdown in cortical effective connectivity during midazolam-induced loss of consciousness. Proc. Natl. Acad. Sci. U S A 107, 2681–2686. 10.1073/pnas.0913008107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari L., Turrini G., Crestan V., Bertani S., Cristofori P., Bifone A., et al. (2012). A robust experimental protocol for pharmacological fMRI in rats and mice. J. Neurosci. Methods 204, 9–18. 10.1016/j.jneumeth.2011.10.020 [DOI] [PubMed] [Google Scholar]
- Ferron J.-F., Kroeger D., Chever O., Amzica F. (2009). Cortical inhibition during burst suppression induced with isoflurane anesthesia. J. Neurosci. 29, 9850–9860. 10.1523/JNEUROSCI.5176-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores F. J., Hartnack K. E., Fath A. B., Kim S.-E., Wilson M. A., Brown E. N., et al. (2017). Thalamocortical synchronization during induction and emergence from propofol-induced unconsciousness. Proc. Natl. Acad. Sci. U S A 114, E6660–E6668. 10.1073/pnas.1700148114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschini M. A., Radhakrishnan H., Thakur K., Wu W., Ruvinskaya S., Carp S., et al. (2010). The effect of different anesthetics on neurovascular coupling. NeuroImage 51, 1367–1377. 10.1016/j.neuroimage.2010.03.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankish A., Diekhans M., Ferreira A. M., Johnson R., Jungreis I., Loveland J., et al. (2019). GENCODE reference annotation for the human and mouse genomes. Nucleic Acids Res. 47, D766–D773. 10.1093/nar/gky955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks N. P. (2008). General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat. Rev. Neurosci. 9, 370–386. 10.1038/nrn2372 [DOI] [PubMed] [Google Scholar]
- Friedman E. B., Sun Y., Moore J. T., Hung H. T., Meng Q. C., Perera P., et al. (2010). A conserved behavioral state barrier impedes transitions between anesthetic-induced unconsciousness and wakefulness: evidence for neural inertia. PLoS One 5:e11903. 10.1371/journal.pone.0011903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P. (2015). Rhythms for cognition: communication through coherence. Neuron 88, 220–235. 10.1016/j.neuron.2015.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P., Nikolić D., Singer W. (2007). The γ cycle. Trends Neurosci. 30, 309–316. 10.1016/j.tins.2007.05.005 [DOI] [PubMed] [Google Scholar]
- Fu B., Yu T., Yuan J., Gong X., Zhang M. (2017). Noradrenergic transmission in the central medial thalamic nucleus modulates the electroencephalographic activity and emergence from propofol anesthesia in rats. J. Neurochem. 140, 862–873. 10.1111/jnc.13939 [DOI] [PubMed] [Google Scholar]
- Fuentealba P., Steriade M. (2005). The reticular nucleus revisited: intrinsic and network properties of a thalamic pacemaker. Prog. Neurobiol. 75, 125–141. 10.1016/j.pneurobio.2005.01.002 [DOI] [PubMed] [Google Scholar]
- Fukuda M., Vazquez A. L., Zong X., Kim S. G. (2013). Effects of the α2-adrenergic receptor agonist dexmedetomidine on neural, vascular and BOLD fMRI responses in the somatosensory cortex. Eur. J. Neurosci. 37, 80–95. 10.1111/ejn.12024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulcher B. D., Fornito A. (2016). A transcriptional signature of hub connectivity in the mouse connectome. Proc. Natl. Acad. Sci. U S A 113, 1435–1440. 10.1073/pnas.1513302113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulcher B. D., Murray J. D., Zerbi V., Wang X.-J. (2019). Multimodal gradients across mouse cortex. Proc. Natl. Acad. Sci. U S A 116, 4689–4695. 10.1073/pnas.1814144116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller P. M., Sherman D., Pedersen N. P., Saper C. B., Lu J. (2011). Reassessment of the structural basis of the ascending arousal system. J. Comp. Neurol. 519, 933–956. 10.1002/cne.22559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furth K. E., McCoy A. J., Dodge C., Walters J. R., Buonanno A., Delaville C. (2017). Neuronal correlates of ketamine and walking induced γ oscillations in the medial prefrontal cortex and mediodorsal thalamus. PLoS One 12:e0186732. 10.1371/journal.pone.0186732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gămănuţ R., Kennedy H., Toroczkai Z., Ercsey-Ravasz M., Van Essen D. C., Knoblauch K., et al. (2018). The mouse cortical connectome, characterized by an ultra-dense cortical graph, maintains specificity by distinct connectivity profiles. Neuron 97, 698.e10–715.e10. 10.1016/j.neuron.2017.12.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi A., Migliarini S., Galbusera A., Maddaloni G., Mereu M., Margiani G., et al. (2017). Brainwide mapping of endogenous serotonergic transmission via chemogenetic-fMRI. Cell Rep. 21, 910–918. 10.1016/j.celrep.2017.09.087 [DOI] [PubMed] [Google Scholar]
- Glickfeld L. L., Olsen S. R. (2017). Higher-order areas of the mouse visual cortex. Annu. Rev. Vis. Sci. 3, 251–273. 10.1146/annurev-vision-102016-061331 [DOI] [PubMed] [Google Scholar]
- Golkowski D., Ranft A., Kiel T., Riedl V., Kohl P., Rohrer G., et al. (2017). Coherence of BOLD signal and electrical activity in the human brain during deep sevoflurane anesthesia. Brain Behav. 7:e00679. 10.1002/brb3.679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golkowski D., Larroque S. K., Vanhaudenhuyse A., Plenevaux A., Boly M., Di Perri C., et al. (2019). Changes in whole brain dynamics and connectivity patterns during sevoflurane-and propofol-induced unconsciousness identified by functional magnetic resonance imaging. Anesthesiology 130, 898–911. 10.1097/aln.0000000000002704 [DOI] [PubMed] [Google Scholar]
- Gozzi A., Ceolin L., Schwarz A., Reese T., Bertani S., Crestan V., et al. (2007). A multimodality investigation of cerebral hemodynamics and autoregulation in pharmacological MRI. Magn. Reson. Imaging 25, 826–833. 10.1016/j.mri.2007.03.003 [DOI] [PubMed] [Google Scholar]
- Gozzi A., Schwarz A. J. (2016). Large-scale functional connectivity networks in the rodent brain. NeuroImage 127, 496–509. 10.1016/j.neuroimage.2015.12.017 [DOI] [PubMed] [Google Scholar]
- Grandjean J., Canella C., Anckaerts C., Ayranci G., Bougacha S., Bienert T., et al. (2020). Common functional networks in the mouse brain revealed by multi-centre resting-state fMRI analysis. NeuroImage 116278. 10.1016/j.neuroimage.2019.116278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean J., Canella C., Anckaerts C., Ayranci G., Bougacha S., Bienert T., et al. (2019a). Common functional networks in the mouse brain revealed by multi-centre resting-state fMRI analysis. bioRxiv [Preprint]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean J., Corcoba A., Kahn M. C., Upton A. L., Deneris E. S., Seifritz E., et al. (2019b). A brain-wide functional map of the serotonergic responses to acute stress and fluoxetine. Nat. Commun. 10:350. 10.1038/s41467-018-08256-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean J., Preti M. G., Bolton T. A. W., Buerge M., Seifritz E., Pryce C. R., et al. (2017a). Dynamic reorganization of intrinsic functional networks inthe mouse brain. NeuroImage 152, 497–508. 10.1016/j.neuroimage.2017.03.026 [DOI] [PubMed] [Google Scholar]
- Grandjean J., Zerbi V., Balsters J. H., Wenderoth N., Rudin M. (2017b). Structural basis of large-scale functional connectivity in the mouse. J. Neurosci. 37, 8092–8101. 10.1523/JNEUROSCI.0438-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean J., Schroeter A., Batata I., Rudin M. (2014). Optimization of anesthesia protocol for resting-state fMRI in mice based on differential effects of anesthetics on functional connectivity patterns. NeuroImage 102, 838–847. 10.1016/j.neuroimage.2014.08.043 [DOI] [PubMed] [Google Scholar]
- Gray C. M., McCormick D. A. (1996). Chattering cells: superficial pyramidal neurons contributing to the generation of synchronous oscillations in the visual cortex. Science 274, 109–113. 10.1126/science.274.5284.109 [DOI] [PubMed] [Google Scholar]
- Greenberg D. S., Houweling A. R., Kerr J. N. (2008). Population imaging of ongoing neuronal activity in the visual cortex of awake rats. Nat. Neurosci. 11, 749–751. 10.1038/nn.2140 [DOI] [PubMed] [Google Scholar]
- Grunze H. C. R., Rainnie D. G., Hasselmo M. E., Barkai E., Hearn E. F., McCarley R. W., et al. (1996). NMDA-dependent modulation of CA1 local circuit inhibition. J. Neurosci. 16, 2034–2043. 10.1523/JNEUROSCI.16-06-02034.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidera J. A., Taylor N. E., Lee J. T., Vlasov K. Y., Pei J., Stephen E. P., et al. (2017). Sevoflurane induces coherent slow-delta oscillations in rats. Front. Neural Circuits 11:36. 10.3389/fncir.2017.00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilfoyle D. N., Gerum S. V., Sanchez J. L., Balla A., Sershen H., Javitt D. C., et al. (2013). Functional connectivity fMRI in mouse brain at 7T using isoflurane. J. Neurosci. Methods 214, 144–148. 10.1016/j.jneumeth.2013.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guldenmund P., Vanhaudenhuyse A., Sanders R., Sleigh J., Bruno M. A., Demertzi A., et al. (2017). Brainfunctional connectivity differentiates dexmedetomidinefrom propofol and natural sleep. Br. J. Anaesth. 119, 674–684. 10.1093/bja/aex257 [DOI] [PubMed] [Google Scholar]
- Gunduz-Bruce H. (2009). The acute effects of NMDA antagonism: from the rodent to the human brain. Brain Res. Rev. 60, 279–286. 10.1016/j.brainresrev.2008.07.006 [DOI] [PubMed] [Google Scholar]
- Gutierrez-Barragan D., Basson M. A., Panzeri S., Gozzi A. (2018). Oscillatory brain states govern spontaneous fMRI network dynamics. bioRxiv [Preprint]. 10.1101/393389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn T. T. G., Sakmann B., Mehta M. R. (2006). Phase-locking of hippocampal interneurons’ membrane potential to neocortical up-down states. Nat. Neurosci. 9, 1359–1361. 10.1038/nn1788 [DOI] [PubMed] [Google Scholar]
- Haider B., Häusser M., Carandini M. (2013). Inhibition dominates sensory responses in the awake cortex. Nature 493, 97–100. 10.1038/nature11665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa M. M., Siegle J. H., Ritt J. T., Ting J. T., Feng G., Moore C. I. (2011). Selective optical drive of thalamic reticular nucleus generates thalamic bursts and cortical spindles. Nat. Neurosci. 14, 1118–1120. 10.1038/nn.2880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C. N., Reynell C., Gesslein B., Hamilton N. B., Mishra A., Sutherland B. A., et al. (2014). Capillary pericytes regulate cerebral blood flow in health and disease. Nature 508, 55–60. 10.1038/nature13165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halley A. C., Krubitzer L. (2019). Not all cortical expansions are the same: the coevolution of the neocortex and the dorsal thalamus in mammals. Curr. Opin. Neurobiol. 56, 78–86. 10.1016/j.conb.2018.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton N. B., Attwell D., Hall C. N. (2010). Pericyte-mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Front. Neuroenergetics 2:5. 10.3389/fnene.2010.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamner J. W., Ishibashi K., Tan C. O. (2019). Revisiting human cerebral blood flow responses to augmented blood pressure oscillations. J. Physiol. 597, 1553–1564. 10.1113/jp277321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z., Chen W., Chen X., Zhang K., Tong C., Zhang X., et al. (2019). Awake and behaving mouse fMRI during Go/No-Go task. NeuroImage 188, 733–742. 10.1016/j.neuroimage.2019.01.002 [DOI] [PubMed] [Google Scholar]
- Han H.-B., Lee K. E., Choi J. H. (2019). Functional dissociation of θ oscillations in the frontal and visual cortices and their long-range network during sustained attention. eNeuro 6:ENEURO.0248-19.2019. 10.1523/ENEURO.0248-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris K. D., Thiele A. (2011). Cortical state and attention. Nat. Rev. Neurosci. 12, 509–523. 10.1038/nrn3084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartikainen K. M., Rorarius M., Peräkylä J. J., Laippala P. J., Jäntti V. (1995). Cortical reactivity during isoflurane burst-suppression anesthesia. Anesth. Analg. 81, 1223–1228. 10.1213/00000539-199512000-00018 [DOI] [PubMed] [Google Scholar]
- Havlik M. (2017). Missing piece of the puzzle in the science of consciousness: resting state and endogenous correlates of consciousness. Conscious. Cogn. 49, 70–85. 10.1016/j.concog.2017.01.006 [DOI] [PubMed] [Google Scholar]
- Hayat H., Regev N., Matosevich N., Sales A., Paredes-Rodriguez E., Krom A. J., et al. (2019). Locus-coeruleus norepinephrine activity gates sensory-evoked awakenings from sleep. bioRxiv [Preprint]. 10.1101/539502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayton S. M., Kriss A., Muller D. P. (1999). Comparison of the effects of four anaesthetic agents on somatosensory evoked potentials in the rat. Lab. Anim. 33, 243–251. 10.1258/002367799780578219 [DOI] [PubMed] [Google Scholar]
- Heindl-Erdmann C., Axmann R., Kreitz S., Zwerina J., Penninger J., Schett G., et al. (2010). Combining functional magnetic resonance imaging with mouse genomics: new options in pain research. Neuroreport 21, 29–33. 10.1097/wnr.0b013e3283324faf [DOI] [PubMed] [Google Scholar]
- Hemmings H. C., Jr., Akabas M. H., Goldstein P. A., Trudell J. R., Orser B. A., Harrison N. L. (2005). Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol. Sci. 26, 503–510. 10.1016/j.tips.2005.08.006 [DOI] [PubMed] [Google Scholar]
- Hemmings H. C., Jr., Riegelhaupt P. M., Kelz M. B., Solt K., Eckenhoff R. G., Orser B. A., et al. (2019). Towards a comprehensive understanding of anesthetic mechanisms of action: a decade of discovery. Trends Pharmacol. Sci. 40, 464–481. 10.1016/j.tips.2019.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry C. E., Scoville W. B. (1952). Suppression-burst activity from isolated cerebral cortex in man. Electroencephalogr. Clin. Neurophysiol. 4, 1–22. 10.1016/0013-4694(52)90027-8 [DOI] [PubMed] [Google Scholar]
- Hentschke H., Raz A., Krause B. M., Murphy C. A., Banks M. I. (2017). Disruption of cortical network activity by the general anaesthetic isoflurane. Br. J. Anaesth. 119, 685–696. 10.1093/bja/aex199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera C. G., Cadavieco M. C., Jego S., Ponomarenko A., Korotkova T., Adamantidis A. (2016). Hypothalamic feedforward inhibition of thalamocortical network controls arousal and consciousness. Nat. Neurosci. 19, 290–298. 10.1038/nn.4209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess A., Axmann R., Rech J., Finzel S., Heindl C., Kreitz S., et al. (2011). From the cover: blockade of TNF-α rapidly inhibits pain responses in the central nervous system. Proc. Natl. Acad. Sci. U S A 108, 3731–3736. 10.1073/pnas.1011774108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch N., Taylor C. (2010). Pharmacological and pathological modulation of cerebral physiology. Anaesth. Intens. Care Med. 11, 349–354. 10.1016/j.mpaic.2010.05.011 [DOI] [Google Scholar]
- Hofman M. A. (2014). Evolution of the human brain: when bigger is better. Front. Neuroanat. 8:15. 10.3389/fnana.2014.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H., Moghaddam B. (2007). NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J. Neurosci. 27, 11496–11500. 10.1523/JNEUROSCI.2213-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover W. B., Vertes R. P. (2007). Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct. Funct. 212, 149–179. 10.1007/s00429-007-0150-4 [DOI] [PubMed] [Google Scholar]
- Hopfield J. J. (1995). Pattern recognition computation using action potential timing for stimulus representation. Nature 376, 33–36. 10.1038/376033a0 [DOI] [PubMed] [Google Scholar]
- Horovitz S. G., Fukunaga M., de Zwart J. A., van Gelderen P., Fulton S. C., Balkin T. J., et al. (2008). Low frequency BOLD fluctuations during resting wakefulness and light sleep: a simultaneous EEG-fMRI study. Hum. Brain Mapp. 29, 671–682. 10.1002/hbm.20428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer C., Gass N., Weber-Fahr W., Sartorius A. (2014). Advantages and challenges of small animal magnetic resonance imaging as a translational tool. Neuropsychobiology 69, 187–201. 10.1159/000360859 [DOI] [PubMed] [Google Scholar]
- Huang W., Plyka I., Li H., Eisenstein E. M., Volkow N. D., Springer C. S., Jr. (1996). Magnetic resonance imaging (MRI) detection of the murine brain response to light: temporal differentiation and negative functional MRI changes. Proc. Natl. Acad. Sci. U S A 93, 6037–6042. 10.1073/pnas.93.12.6037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z., Wang Z., Zhang J., Dai R., Wu J., Li Y., et al. (2014). Altered temporal variance and neural synchronization of spontaneous brain activity in anesthesia. Hum. Brain Mapp. 35, 5368–5378. 10.1002/hbm.22556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber L., Handwerker D. A., Jangraw D. C., Chen G., Hall A., Stüber C., et al. (2017). High-resolution CBV-fMRI allows mapping of laminar activity and connectivity of cortical input and output in human M1. Neuron 96, 1253.e7–1263.e7. 10.1016/j.neuron.2017.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudetz A. G. (2002). Effect of volatile anesthetics on interhemispheric EEG cross-approximate entropy in the rat. Brain Res. 954, 123–131. 10.1016/s0006-8993(02)03358-9 [DOI] [PubMed] [Google Scholar]
- Hudetz A. G., Imas O. A. (2007). Burst activation of the cerebral cortex by flash stimuli during isoflurane anesthesia in rats. Anesthesiology 107, 983–991. 10.1097/01.anes.0000291471.80659.55 [DOI] [PubMed] [Google Scholar]
- Hudetz A. G., Mashour G. A. (2016). Disconnecting consciousness: is there a common anesthetic end point? Anesth. Analg. 123, 1228–1240. 10.1213/ane.0000000000001353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudetz A. G., Wood J. D., Kampine J. P. (2003). Cholinergic reversal of isoflurane anesthesia in rats as measured by cross-approximate entropy of the electroencephalogram. Anesthesiology 99, 1125–1131. 10.1097/00000542-200311000-00019 [DOI] [PubMed] [Google Scholar]
- Hudson A. E. (2017). Metastability of neuronal dynamics during general anesthesia: time for a change in our assumptions? Front. Neural Circuits 11:58. 10.3389/fncir.2017.00058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson A. E., Calderon D. P., Pfaff D. W., Proekt A. (2014). Recovery of consciousness is mediated by a network of discrete metastable activity states. Proc. Natl. Acad. Sci. U S A 111, 9283–9288. 10.1073/pnas.1408296111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R. J. (1986). Extreme stereotypy in the burst suppression pattern. Clin. Electroencephalogr. 17, 162–168. [PubMed] [Google Scholar]
- Hutchison R. M., Hutchison M., Manning K. Y., Menon R. S., Everling S. (2014). Isoflurane induces dose-dependent alterations in the cortical connectivity profiles and dynamic properties of the brain’s functional architecture. Hum. Brain Mapp. 35, 5754–5775. 10.1002/hbm.22583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huupponen E., Maksimow A., Lapinlampi P., Särkelä M., Saastamoinen A., Snapir A., et al. (2008). Electroencephalogram spindle activity during dexmedetomidine sedation and physiological sleep. Acta Anaesthesiol. Scand. 52, 289–294. 10.1111/j.1399-6576.2007.01537.x [DOI] [PubMed] [Google Scholar]
- Idiyatullin D., Corum C. A., Garwood M. (2015). Multi-band-SWIFT. J. Magn. Reson. 251, 19–25. 10.1016/j.jmr.2014.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idiyatullin D., Corum C., Park J. Y., Garwood M. (2006). Fast and quiet MRI using a swept radiofrequency. J. Magn. Reson. 181, 342–349. 10.1016/j.jmr.2006.05.014 [DOI] [PubMed] [Google Scholar]
- Iida H., Ohata H., Iida M., Watanabe Y., Dohi S. (1998). Isoflurane and sevoflurane induce vasodilation of cerebral vessels via ATP-sensitive K+ channel activation. Anesthesiology 89, 954–960. 10.1097/00000542-199810000-00020 [DOI] [PubMed] [Google Scholar]
- Imas O. A., Ropella K. M., Ward B. D., Wood J. D., Hudetz A. G. (2005a). Volatile anesthetics enhance flash-induced γ oscillations in rat visual cortex. Anesthesiology 102, 937–947. 10.1097/00000542-200505000-00012 [DOI] [PubMed] [Google Scholar]
- Imas O. A., Ropella K. M., Ward B. D., Wood J. D., Hudetz A. G. (2005b). Volatile anesthetics disrupt frontal-posterior recurrent information transfer at gamma frequencies in rat. Neurosci. Lett. 387, 145–150. 10.1016/j.neulet.2005.06.018 [DOI] [PubMed] [Google Scholar]
- Isaacson J. S., Scanziani M. (2011). How inhibition shapes cortical activity. Neuron 72, 231–243. 10.1016/j.neuron.2011.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O., Spaak E., Zumer J. M. (2019). “Human brain oscillations: from physiological mechanisms to analysis and cognition,” in Magnetoencephalography, eds Supek S., Aine C. (Cham: Springer; ), 1–46. [Google Scholar]
- Ji D., Wilson M. A. (2007). Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat. Neurosci. 10, 100–107. 10.1038/nn1825 [DOI] [PubMed] [Google Scholar]
- Jonckers E., Delgado Y Palacios R., Shah D., Guglielmetti C., Verhoye M., Van der Linden A. (2014). Different anesthesia regimes modulate the functional connectivity outcome in mice. Magn. Reson. Med. 72, 1103–1112. 10.1002/mrm.24990 [DOI] [PubMed] [Google Scholar]
- Jonckers E., Shah D., Hamaide J., Verhoye M., Van der Linden A. (2015). The power of using functional fMRI on small rodents to study brain pharmacology and disease. Front. Pharmacol. 6:231. 10.3389/fphar.2015.00231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonckers E., Van Audekerke J., De Visscher G., Van der Linden A., Verhoye M. (2011). Functional connectivity fMRI of the rodent brain: comparison of functional connectivity networks in rat and mouse. PLoS One 6:e18876. 10.1371/journal.pone.0018876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M., Berwick J., Hewson-Stoate N., Gias C., Mayhew J. (2005). The effect of hypercapnia on the neural and hemodynamic responses to somatosensory stimulation. NeuroImage 27, 609–623. 10.1016/j.neuroimage.2005.04.036 [DOI] [PubMed] [Google Scholar]
- Jones B. E., Yang T. Z. (1985). The efferent projections from the reticular formation and the locus coeruleus studied by anterograde and retrograde axonal transport in the rat. J. Comp. Neurol. 242, 56–92. 10.1002/cne.902420105 [DOI] [PubMed] [Google Scholar]
- Jordan D., Ilg R., Riedl V., Schorer A., Grimberg S., Neufang S., et al. (2013). Simultaneous electroencephalographic and functional magnetic resonance imaging indicate impaired cortical top-down processing in association with anesthetic-induced unconsciousness. Anesthesiology 119, 1031–1042. 10.3410/f.718082755.793486169 [DOI] [PubMed] [Google Scholar]
- Jorm C. M., Stamford J. A. (1993). Actions of the hypnotic anaesthetic, dexmedetomidine, on noradrenaline release and cell firing in rat locus coeruleus slices. Br. J. Anaesth. 71, 447–449. 10.1093/bja/71.3.447 [DOI] [PubMed] [Google Scholar]
- Joshi S., Li Y., Kalwani R. M., Gold J. I. (2016). Relationships between pupil diameter and neuronal activity in the locus coeruleus, colliculi, and cingulate cortex. Neuron 89, 221–234. 10.1016/j.neuron.2015.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung W. B., Shim H. J., Kim S. G. (2019). Mouse BOLD fMRI at ultrahigh field detects somatosensory networks including thalamic nuclei. NeuroImage 195, 203–214. 10.1016/j.neuroimage.2019.03.063 [DOI] [PubMed] [Google Scholar]
- Kahn I., Desai M., Knoblich U., Bernstein J., Henninger M., Graybiel A. M., et al. (2011). Characterization of the functional MRI response temporal linearity via optical control of neocortical pyramidal neurons. J. Neurosci. 31, 15086–15091. 10.1523/JNEUROSCI.0007-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R., Elbel G.-K., Gössl C., Czisch M., Auer D. P. (2001). Blood pressure changes induced by arterial blood withdrawal influence bold signal in anesthesized rats at 7 tesla: implications for pharmacologic MRI. NeuroImage 14, 891–898. 10.1006/nimg.2001.0890 [DOI] [PubMed] [Google Scholar]
- Kalthoff D., Seehafer J. U., Po C., Wiedermann D., Hoehn M. (2011). Functional connectivity in the rat at 11.7T: impact of physiological noise in resting state fMRI. NeuroImage 54, 2828–2839. 10.1016/j.neuroimage.2010.10.053 [DOI] [PubMed] [Google Scholar]
- Kamibayashi T., Maze M. (2000). Clinical uses of α-2 adrenergic agonists. Anesthesiology 93, 1345–1349. 10.1097/00000542-200011000-00030 [DOI] [PubMed] [Google Scholar]
- Kanai R., Komura Y., Shipp S., Friston K. (2015). Cerebral hierarchies: predictive processing, precision and the pulvinar. Philos. Trans. R. Soc. Lond. B Biol. Sci. 370:20140169. 10.1098/rstb.2014.0169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel A., Buzsáki G. (1997). Cellular-synaptic generation of sleep spindles, spike-and-wave discharges, and evoked thalamocortical responses in the neocortex of the rat. J. Neurosci. 17, 6783–6797. 10.1523/JNEUROSCI.17-17-06783.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S., Ungerleider L. G. (2000). Mechanisms of visual attention in the human cortex. Annu. Rev. Neurosci. 23, 315–341. 10.1146/annurev.neuro.23.1.315 [DOI] [PubMed] [Google Scholar]
- Kayser C., Montemurro M. A., Logothetis N. K., Panzeri S. (2009). Spike-phase coding boosts and stabilizes information carried by spatial and temporal spike patterns. Neuron 61, 597–608. 10.1016/j.neuron.2009.01.008 [DOI] [PubMed] [Google Scholar]
- Keilholz S. D. (2014). The neural basis of time-varying resting-state functional connectivity. Brain Connect. 4, 769–779. 10.1089/brain.2014.0250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller D., Erö C., Markram H. (2018). Cell densities in the mouse brain: a systematic review. Front Neuroanat 12:83. 10.3389/fnana.2018.00083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelz M. B., Sun Y., Chen J., Cheng Meng Q., Moore J. T., Veasey S. C., et al. (2008). An essential role for orexins in emergence from general anesthesia. Proc. Natl. Acad. Sci. U S A 105, 1309–1314. 10.1073/pnas.0707146105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny J. D., Westover M. B., Ching S., Brown E. N., Solt K. (2014). Propofol and sevoflurane induce distinct burst suppression patterns in rats. Front. Syst. Neurosci. 8:237. 10.3389/fnsys.2014.00237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kety S. S., Schmidt C. F. (1948). The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men. J. Clin. Invest. 27, 484–492. 10.1172/jci101995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.-G. (2018). Biophysics of BOLD fMRI investigated with animal models. J. Magn. Reson. 292, 82–89. 10.1016/j.jmr.2018.04.006 [DOI] [PubMed] [Google Scholar]
- Kim J.-H., Jung A.-H., Jeong D., Choi I., Kim K., Shin S., et al. (2016). Selectivity of neuromodulatory projections from the basal forebrain and locus ceruleus to primary sensory cortices. J. Neurosci. 36, 5314–5327. 10.1523/JNEUROSCI.4333-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A., Latchoumane C., Lee S., Kim G. B., Cheong E., Augustine G. J., et al. (2012). Optogenetically induced sleep spindle rhythms alter sleep architectures in mice. Proc. Natl. Acad. Sci. U S A 109, 20673–20678. 10.1073/pnas.1217897109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King P. R., Gundlach A. L., Louis W. J. (1995). Quantitative autoradiographic localization in rat brain of α2-adrenergic and non-adrenergic I-receptor binding sites labelled by [3H]rilmenidine. Brain Res. 675, 264–278. 10.1016/0006-8993(95)00083-3 [DOI] [PubMed] [Google Scholar]
- Kiss T., Hoffmann W. E., Scott L., Kawabe T. T., Milici A. J., Nilsen E. A., et al. (2011). Role of thalamic projection in NMDA receptor-induced disruption of cortical slow oscillation and short-term plasticity. Front. Psychiatry 2:14. 10.3389/fpsyt.2011.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissinger S. T., Pak A., Tang Y., Masmanidis S. C., Chubykin A. A. (2018). Oscillatory encoding of visual stimulus familiarity. J. Neurosci. 38, 6223–6240. 10.1523/jneurosci.3646-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner I. R., Zhang J., Touroutoglou A., Chanes L., Xia C., Simmons W. K., et al. (2017). Evidence for a large-scale brain system supporting allostasis and interoception in humans. Nat. Hum. Behav. 1:0069. 10.1038/s41562-017-0069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee R., Mueggler T., Bruns A., Kienlin M., Künnecke B. (2017a). “Is sevoflurane a viable alternative anaesthetic for functional MRI in mice?” in Conference Paper: Annual Meeting and Exhibition of the International Society for Magnetic Resonance in Medicine at Volume: 25th (Honolulu, HI). [Google Scholar]
- Klee R., Mueggler T., Bruns A., Wyttenbach N., Ricci A., Gasser R., et al. (2017b). “Etomidate anaesthesia for fmri in mice revisited: subcutaneous administration facilitates experimental procedures,” in Conference Paper: Annual Meeting and Exhibition of the International Society for Magnetic Resonance in Medicine at Volume: 25th (Honolulu, HI). [Google Scholar]
- Klimesch W., Sauseng P., Hanslmayr S. (2007). EEG α oscillations: the inhibition-timing hypothesis. Brain Res. Rev. 53, 63–88. 10.1016/j.brainresrev.2006.06.003 [DOI] [PubMed] [Google Scholar]
- Kozai T. D., Du Z., Gugel Z. V., Smith M. A., Chase S. M., Bodily L. M., et al. (2015). Comprehensive chronic laminar single-unit, multi-unit, and local field potential recording performance with planar single shank electrode arrays. J. Neurosci. Methods 242, 15–40. 10.1016/j.jneumeth.2014.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeger D., Amzica F. (2007). Hypersensitivity of the anesthesia-induced comatose brain. J. Neurosci. 27, 10597–10607. 10.1523/JNEUROSCI.3440-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku S. W., Lee U., Noh G. J., Jun I. G., Mashour G. A. (2011). Preferential inhibition of frontal-to-parietal feedback connectivity is a neurophysiologic correlate of general anesthesia in surgical patients. PLoS One 6:e25155. 10.1371/journal.pone.0025155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucyi A., Davis K. D. (2017). The neural code for pain: from single-cell electrophysiology to the dynamic pain connectome. Neuroscientist 23, 397–414. 10.1177/1073858416667716 [DOI] [PubMed] [Google Scholar]
- Kundishora A. J., Gummadavelli A., Ma C., Liu M., Mccafferty C., Schiff N. D., et al. (2017). Restoring conscious arousal during focal limbic seizures with deep brain stimulation. Cereb. Cortex 27, 1964–1975. 10.1093/cercor/bhw035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki S., Yoshida T., Tsutsui H., Iwama M., Ando R., Michikawa T., et al. (2018). Excitatory neuronal hubs configure multisensory integration of slow waves in association cortex. Cell Rep. 22, 2873–2885. 10.1016/j.celrep.2018.02.056 [DOI] [PubMed] [Google Scholar]
- Lai H.-Y., Albaugh D. L., Kao Y.-C. J., Younce J. R., Shih Y.-Y. I. (2015). Robust deep brain stimulation functional MRI procedures in rats and mice using an MR-compatible tungsten microwire electrode. Magn. Reson. Med. 73, 1246–1251. 10.1002/mrm.25239 [DOI] [PubMed] [Google Scholar]
- Lamme V. A. F. (2018). Challenges for theories of consciousness: seeing or knowing, the missing ingredient and how to deal with panpsychism. Philos. Trans. R. Soc. Lond. B Biol. Sci. 373:20170344. 10.1098/rstb.2017.0344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamme V. A. F., Zipser K., Spekreijse H. (1998). Figure-ground activity in primary visual cortex is suppressed by anesthesia. Proc. Natl. Acad. Sci. U S A 95, 3263–3268. 10.1073/pnas.95.6.3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land R., Engler G., Kral A., Engel A. K. (2012). Auditory evoked bursts in mouse visual cortex during isoflurane anesthesia. PLoS One 7:e49855. 10.1371/journal.pone.0049855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Långsjö J. W., Alkire M. T., Kaskinoro K., Hayama H., Maksimow A., Kaisti K. K., et al. (2012). Returning from oblivion: imaging the neural core of consciousness. J. Neurosci. 32, 4935–4943. 10.1523/JNEUROSCI.4962-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson-Prior L. J., Zempel J. M., Nolan T. S., Prior F. W., Snyder A. Z., Raichle M. E. (2009). Cortical network functional connectivity in the descent to sleep. Proc. Natl. Acad. Sci. U S A 106, 4489–4494. 10.1073/pnas.0900924106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Van Quyen M., Muller L. E., II., Telenczuk B., Halgren E., Cash S., Hatsopoulos N. G., et al. (2016). High-frequency oscillations in human and monkey neocortex during the wake-sleep cycle. Proc. Natl. Acad. Sci. U S A 113, 9363–9368. 10.1073/pnas.1523583113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecrux C., Hamel E. (2016). Neuronal networks and mediators of cortical neurovascular coupling responses in normal and altered brain states. Philos. Trans. R. Soc. Lond. B Biol. Sci. 371:20150350. 10.1098/rstb.2015.0350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecrux C., Bourourou M., Hamel E. (2019). How reliable is cerebral blood flow to map changes in neuronal activity? Auton. Neurosci. 217, 71–79. 10.1016/j.autneu.2019.01.005 [DOI] [PubMed] [Google Scholar]
- Lee S.-H., Dan Y. (2012). Neuromodulation of brain states. Neuron 76, 209–222. 10.1016/j.neuron.2012.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. G., Hudetz A. G., Smith J. J., Hillard C. J., Bosnjak Z. J., Kampine J. P. (1994). The effects of halothane and isoflurane on cerebrocortical microcirculation and autoregulation as assessed by laser-Doppler flowmetry. Anesth. Analg. 79, 58–65. 10.1213/00000539-199407000-00012 [DOI] [PubMed] [Google Scholar]
- Lee U., Ku S., Noh G., Baek S., Choi B., Mashour G. A. (2013). Disruption of frontal-parietal communication by ketamine, propofol, and sevoflurane. Anesthesiology 118, 1264–1275. 10.1097/ALN.0b013e31829103f5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V., Maguire J. (2014). The impact of tonic GABAA receptor-mediated inhibition on neuronal excitability varies across brain region and cell type. Front. Neural Circuits 8:3. 10.3389/fncir.2014.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Mashour G. A., Noh G. J., Kim S., Lee U. (2013). Reconfiguration of network hub structure after propofol-induced unconsciousness. Anesthesiology 119, 1347–1359. 10.1097/aln.0b013e3182a8ec8c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrain V., Iannetti G. D., Plaghki L., Mouraux A. (2011). The pain matrix reloaded: a salience detection system for the body. Prog. Neurobiol. 93, 111–124. 10.1016/j.pneurobio.2010.10.005 [DOI] [PubMed] [Google Scholar]
- Lehto L. J., Filip P., Laakso H., Sierra A., Slopsema J. P., Johnson M. D., et al. (2018). Tuning neuromodulation effects by orientation selective deep brain stimulation in the rat medial frontal cortex. Front. Neurosci. 12:899. 10.3389/fnins.2018.00899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei H., Grinberg O., Nwaigwe C. I., Hou H. G., Williams H., Swartz H. M., et al. (2001). The effects of ketamine-xylazine anesthesia on cerebral blood flow and oxygenation observed using nuclear magnetic resonance perfusion imaging and electron paramagnetic resonance oximetry. Brain Res. 913, 174–179. 10.1016/s0006-8993(01)02786-x [DOI] [PubMed] [Google Scholar]
- Lein E. S., Hawrylycz M. J., Ao N., Ayres M., Bensinger A., Bernard A., et al. (2007). Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176. 10.1038/nature05453 [DOI] [PubMed] [Google Scholar]
- Leong A. T. L., Chan R. W., Gao P. P., Chan Y.-S., Tsia K. K., Yung W.-H., et al. (2016). Long-range projections coordinate distributed brain-wide neural activity with a specific spatiotemporal profile. Proc. Natl. Acad. Sci. U S A 113, E8306–E8315. 10.1073/pnas.1616361113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoni R. F., Paiva F. F., Henning E. C., Nascimento G. C., Tannus A., de Araujo D. B., et al. (2011). Magnetic resonance imaging quantification of regional cerebral blood flow and cerebrovascular reactivity to carbon dioxide in normotensive and hypertensive rats. NeuroImage 58, 75–81. 10.1016/j.neuroimage.2011.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung L. S., Luo T., Ma J., Herrick I. (2014). Brain areas that influence general anesthesia. Prog. Neurobiol. 122, 24–44. 10.1016/j.pneurobio.2014.08.001 [DOI] [PubMed] [Google Scholar]
- Lewis L. D., Akeju O. (2017). Hierarchy in disruption of large-scale networks across altered arousal states. Br. J. Anaesth. 119, 566–568. 10.1093/bja/aex297 [DOI] [PubMed] [Google Scholar]
- Lewis L. D., Ching S., Weiner V. S., Peterfreund R. A., Eskandar E. N., Cash S. S., et al. (2013). Local cortical dynamics of burst suppression in the anaesthetized brain. Brain 136, 2727–2737. 10.1093/brain/awt174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis L. D., Voigts J., Flores F. J., Schmitt L. I., Wilson M. A., Halassa M. M., et al. (2015). Thalamic reticular nucleus induces fast and local modulation of arousal state. Elife 4:e08760. 10.7554/eLife.08760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis L. D., Weiner V. S., Mukamel E. A., Donoghue J. A., Eskandar E. N., Madsen J. R., et al. (2012). Rapid fragmentation of neuronal networks at the onset of propofol-induced unconsciousness. Proc. Natl. Acad. Sci. U S A 109, E3377–E3386. 10.1073/pnas.1210907109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Li G., Wu D., Lu H., Hou Z., Ross C. A., et al. (2017). Resting-state functional MRI reveals altered brain connectivity and its correlation with motor dysfunction in a mouse model of Huntington’s disease. Sci. Rep. 7:16742. 10.1038/s41598-017-17026-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B. H., Lohmann J. S., Schuler H. G., Cronin A. J. (2003). Preservation of the cortical somatosensory-evoked potential during dexmedetomidine infusion in rats. Anesth. Analg. 96, 1155–1160. 10.1213/01.ane.0000053239.62623.32 [DOI] [PubMed] [Google Scholar]
- Lichtner G., Auksztulewicz R., Kirilina E., Velten H., Mavrodis D., Scheel M., et al. (2018). Effects of propofol anesthesia on the processing of noxious stimuli in the spinal cord and the brain. NeuroImage 172, 642–653. 10.1016/j.neuroimage.2018.02.003 [DOI] [PubMed] [Google Scholar]
- Liska A., Galbusera A., Schwarz A. J., Gozzi A. (2015). Functional connectivity hubs of the mouse brain. NeuroImage 115, 281–291. 10.1016/j.neuroimage.2015.04.033 [DOI] [PubMed] [Google Scholar]
- Liu X., Lauer K. K., Ward B. D., Li S. J., Hudetz A. G. (2013a). Differential effects of deep sedation with propofol on the specific and nonspecific thalamocortical systems: a functional magnetic resonance imaging study. Anesthesiology 118, 59–69. 10.1097/ALN.0b013e318277a801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Pillay S., Li R., Vizuete J. A., Pechman K. R., Schmainda K. M., et al. (2013b). Multiphasic modification of intrinsic functional connectivity of the rat brain during increasing levels of propofol. NeuroImage 83, 581–592. 10.1016/j.neuroimage.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Zhu X. H., Zhang Y., Chen W. (2013c). The change of functional connectivity specificity in rats under various anesthesia levels and its neural origin. Brain Topogr. 26, 363–377. 10.1007/s10548-012-0267-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Zhu X. H., Zhang Y., Chen W. (2011). Neural origin of spontaneous hemodynamic fluctuations in rats under burst-suppression anesthesia condition. Cereb. Cortex 21, 374–384. 10.1093/cercor/bhq105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Ribary U. (1993). Coherent 40-Hz oscillation characterizes dream state in humans. Proc. Natl. Acad. Sci. U S A 90, 2078–2081. 10.1073/pnas.90.5.2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longmuir I. S., Pashko L. (1976). The induction of cytochrome P-450 by hypoxia. Adv. Exp. Med. Biol. 75, 171–175. 10.1007/978-1-4684-3273-2_22 [DOI] [PubMed] [Google Scholar]
- Low L. A., Bauer L. C., Klaunberg B. A. (2016a). Comparing the effects of isoflurane and α chloralose upon mouse physiology. PLoS One 11:e0154936. 10.1371/journal.pone.0154936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low L. A., Bauer L. C., Pitcher M. H., Bushnell M. C. (2016b). Restraint training for awake functional brain scanning of rodents can cause long-lasting changes in pain and stress responses. Pain 157, 1761–1772. 10.1097/j.pain.0000000000000579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H., Zou Q., Gu H., Raichle M. E., Stein E. A., Yang Y. (2012). Rat brains also have a default mode network. Proc. Natl. Acad. Sci. U S A 109, 3979–3984. 10.1073/pnas.1200506109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczak A., Barthó P., Marguet S. L., Buzsáki G., Harris K. D. (2007). Sequential structure of neocortical spontaneous activity in vivo. Proc. Natl. Acad. Sci. U S A 104, 347–352. 10.1073/pnas.0605643104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczak A., McNaughton B. L., Harris K. D. (2015). Packet-based communication in the cortex. Nat. Rev. Neurosci. 16, 745–755. 10.1038/nrn4026 [DOI] [PubMed] [Google Scholar]
- Lurie J. D., Kessler D., Bassett D., Betzel R., Breakspear M., Keilholz S., et al. (2018). On the nature of resting fMRI and time-varying functional connectivity. PsyArXiv [Preprint]. [Google Scholar]
- Lüthi A. (2014). Sleep spindles: where they come from, what they do. Neuroscientist 20, 243–256. 10.1177/1073858413500854 [DOI] [PubMed] [Google Scholar]
- Ma S., Allocca G., Ong-Palsson E. K., Singleton C. E., Hawkes D., McDougall S. J., et al. (2017). Nucleus incertus promotes cortical desynchronization and behavioral arousal. Brain Struct. Funct. 222, 515–537. 10.1007/s00429-016-1230-0 [DOI] [PubMed] [Google Scholar]
- Ma S., Hangya B., Leonard C. S., Wisden W., Gundlach A. L. (2018). Dual-transmitter systems regulating arousal, attention, learning and memory. Neurosci. Biobehav. Rev. 85, 21–33. 10.1016/j.neubiorev.2017.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madularu D., Mathieu A. P., Kumaragamage C., Reynolds L. M., Near J., Flores C., et al. (2017). A non-invasive restraining system for awake mouse imaging. J. Neurosci. Methods 287, 53–57. 10.1016/j.jneumeth.2017.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdavi A., Qin Y., Aubry A.-S., Cornec D., Kulikova S., Pinault D. (2019). Sleep-related thalamocortical spindles and delta oscillations are reduced during a ketamine-induced psychosis-relevant transition state. bioRxiv [Preprint]. 10.1101/833459 [DOI] [Google Scholar]
- Majeed W., Magnuson M., Hasenkamp W., Schwarb H., Schumacher E. H., Barsalou L., et al. (2011). Spatiotemporal dynamics of low frequency BOLD fluctuations in rats and humans. NeuroImage 54, 1140–1150. 10.1016/j.neuroimage.2010.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandino F., Cerri D. H., Garin C. M., Straathof M., van Tilborg G. A. F., Chakravarty M. M., et al. (2020). Animal functional magnetic resonance imaging: trends and path toward standardization. Front. Neuroinform. 13:78. 10.3389/fninf.2019.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlinski V., Sirota M. G., Beloozerova I. N. (2012). Differential gating of thalamocortical signals by reticular nucleus of thalamus during locomotion. J. Neurosci. 32, 15823–15836. 10.1523/JNEUROSCI.0782-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins I., Tavares I. (2017). Reticular formation and pain: the past and the future. Front. Neuroanat. 11:51. 10.3389/fnana.2017.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masamoto K., Kanno I. (2012). Anesthesia and the quantitative evaluation of neurovascular coupling. J. Cereb. Blood Flow Metab. 32, 1233–1247. 10.1038/jcbfm.2012.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashour G. A. (2014). Top-down mechanisms of anesthetic-induced unconsciousness. Front. Syst. Neurosci. 8:115. 10.3389/fnsys.2014.00115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashour G. A., Alkire M. T. (2013). Evolution of consciousness: phylogeny, ontogeny, and emergence from general anesthesia. Proc. Natl. Acad. Sci. U S A 110, 10357–10364. 10.1073/pnas.1301188110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashour G. A., Hudetz A. G. (2017). Bottom-up and top-down mechanisms of general anesthetics modulate different dimensions of consciousness. Front. Neural Circuits 11:44. 10.3389/fncir.2017.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashour G. A., Hudetz A. G. (2018). Neural correlates of unconsciousness in large-scale brain networks. Trends Neurosci. 41, 150–160. 10.1016/j.tins.2018.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masri R., Trageser J. C., Bezdudnaya T., Li Y., Keller A. (2006). Cholinergic regulation of the posterior medial thalamic nucleus. J. Neurophysiol. 96, 2265–2273. 10.1152/jn.00476.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masri R., Bezdudnaya T., Trageser J. C., Keller A. (2008). Encoding of stimulus frequency and sensor motion in the posterior medial thalamic nucleus. J. Neurophysiol. 100, 681–689. 10.1152/jn.01322.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimini M., Ferrarelli F., Esser S. K., Riedner B. A., Huber R., Murphy M., et al. (2007). Triggering sleep slow waves by transcranial magnetic stimulation. Proc. Natl. Acad. Sci. U S A 104, 8496–8501. 10.1073/pnas.0702495104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimini M., Ferrarelli F., Huber R., Esser S. K., Singh H., Tononi G. (2005). Breakdown of cortical effective connectivity during sleep. Science 309, 2228–2232. 10.1126/science.1117256 [DOI] [PubMed] [Google Scholar]
- Massimini M., Huber R., Ferrarelli F., Hill S., Tononi G. (2004). The sleep slow oscillation as a traveling wave. J. Neurosci. 24, 6862–6870. 10.1523/JNEUROSCI.1318-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo C., Knutsen P. M., Tsai P. S., Shih A. Y., Kleinfeld D. (2017). Entrainment of arteriole vasomotor fluctuations by neural activity is a basis of blood-oxygenation-level-dependent “resting-state” connectivity. Neuron 96, 936.e3–948.e3. 10.1016/j.neuron.2017.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T., Murakami T., Ohki K. (2018). Neuronal origin of the temporal dynamics of spontaneous BOLD activity correlation. Cereb. Cortex 29, 1496–1508. 10.1093/cercor/bhy045 [DOI] [PubMed] [Google Scholar]
- Matsuura T., Kanno I. (2002). Effect of nitric oxide synthase inhibitor on the local cerebral blood flow evoked by rat somatosensory stimulation under hyperoxia. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 131, 267–274. 10.1016/s1095-6433(01)00450-0 [DOI] [PubMed] [Google Scholar]
- Matsuura T., Kashikura K., Kanno I. (2001). Hemodynamics of local cerebral blood flow induced by somatosensory stimulation under normoxia and hyperoxia in rats. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 129, 363–372. 10.1016/s1095-6433(00)00354-8 [DOI] [PubMed] [Google Scholar]
- Mattia M., Sanchez-Vives M. V. (2012). Exploring the spectrum of dynamical regimes and timescales in spontaneous cortical activity. Cogn. Neurodyn. 6, 239–250. 10.1007/s11571-011-9179-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell C. R., Ehrlichman R. S., Liang Y., Trief D., Kanes S. J., Karp J., et al. (2006). Ketamine produces lasting disruptions in encoding of sensory stimuli. J. Pharmacol. Exp. Ther. 316, 315–324. 10.1124/jpet.105.091199 [DOI] [PubMed] [Google Scholar]
- McCallum J. B., Boban N., Hogan Q., Schmeling W. T., Kampine J. P., Bosnjak Z. J. (1998). The mechanism of α2-adrenergic inhibition of sympathetic ganglionic transmission. Anesth. Analg. 87, 503–510. 10.1097/00000539-199809000-00001 [DOI] [PubMed] [Google Scholar]
- McCarthy M. M., Brown E. N., Kopell N. (2008). Potential network mechanisms mediating electroencephalographic β rhythm changes during propofol-induced paradoxical excitation. J. Neurosci. 28, 13488–13504. 10.1523/JNEUROSCI.3536-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A. (1992). Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Prog. Neurobiol. 39, 337–388. 10.1016/0301-0082(92)90012-4 [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Bal T. (1997). Sleep and arousal: thalamocortical mechanisms. Ann. Rev. Neurosci. 20, 185–215. 10.1146/annurev.neuro.20.1.185 [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Pape H. C. (1990). Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamocortical neurones. J. Physiol. 431, 291–318. 10.1113/jphysiol.1990.sp018331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinley M. J., David S. V., McCormick D. A. (2015a). Cortical membrane potential signature of optimal states for sensory signal detection. Neuron 87, 179–192. 10.1016/j.neuron.2015.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinley M. J., Vinck M., Reimer J., Batista-Brito R., Zagha E., Cadwell C. R., et al. (2015b). Waking state: rapid variations modulate neural and behavioral responses. Neuron 87, 1143–1161. 10.1016/j.neuron.2015.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlhone A. E., Beausoleil N. J., Kells N. J., Johnson C. B., Mellor D. J. (2018). Effects of halothane on the electroencephalogram of the chicken. Vet. Med. Sci. 4, 98–105. 10.1002/vms3.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre C. C., Anderson R. W. (2016). Deep brain stimulation mechanisms: the control of network activity via neurochemistry modulation. J. Neurochem. 139, 338–345. 10.1111/jnc.13649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinstry-Wu A., Kelz M. B. (2019). Neural Mechanisms of Anaesthetics. Oxford Textbook of Neuroscience and Anaesthesiology. New York, NY: Oxford University Press. [Google Scholar]
- McVea D. A., Murphy T. H., Mohajerani M. H. (2016). Large scale cortical functional networks associated with slow-wave and spindle-burst-related spontaneous activity. Front. Neural Circuits 10:103. 10.3389/fncir.2016.00103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechling A. E., Hubner N. S., Lee H. L., Hennig J., von Elverfeldt D., Harsan L. A. (2014). Fine-grained mapping of mouse brain functional connectivity with resting-state fMRI. NeuroImage 96, 203–215. 10.1016/j.neuroimage.2014.03.078 [DOI] [PubMed] [Google Scholar]
- Melzack R. (1989). Labat lecture. Phantom limbs. Reg. Anesth. 14, 208–211. [PubMed] [Google Scholar]
- Melzack R. (2005). Evolution of the neuromatrix theory of pain. The Prithvi Raj Lecture: presented at the third World Congress of World Institute of Pain, Barcelona 2004. Pain Pract. 5, 85–94. 10.1111/j.1533-2500.2005.05203.x [DOI] [PubMed] [Google Scholar]
- Meuth S. G., Budde T., Kanyshkova T., Broicher T., Munsch T., Pape H. C. (2003). Contribution of TWIK-related acid-sensitive K+ channel 1 (TASK1) and TASK3 channels to the control of activity modes in thalamocortical neurons. J. Neurosci. 23, 6460–6469. 10.1523/JNEUROSCI.23-16-06460.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuth S. G., Kanyshkova T., Meuth P., Landgraf P., Munsch T., Ludwig A., et al. (2006). Membrane resting potential of thalamocortical relay neurons is shaped by the interaction among TASK3 and HCN2 channels. J. Neurophysiol. 96, 1517–1529. 10.1152/jn.01212.2005 [DOI] [PubMed] [Google Scholar]
- Meyer H. S., Egger R., Guest J. M., Foerster R., Reissl S., Oberlaender M. (2013). Cellular organization of cortical barrel columns is whisker-specific. Proc. Natl. Acad. Sci. U S A 110, 19113–19118. 10.1073/pnas.1312691110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalareas G., Vezoli J., van Pelt S., Schoffelen J. M., Kennedy H., Fries P. (2016). Alpha-beta and gamma rhythms subserve feedback and feedforward influences among human visual cortical areas. Neuron 89, 384–397. 10.1016/j.neuron.2015.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson N. J., Kozai T. D. Y. (2018). Isoflurane and ketamine differentially influence spontaneous and evoked laminar electrophysiology in mouse V1. J. Neurophysiol. 120, 2232–2245. 10.1152/jn.00299.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milenkovic N., Zhao W.-J., Walcher J., Albert T., Siemens J., Lewin G. R., et al. (2014). A somatosensory circuit for cooling perception in mice. Nat. Neurosci. 17, 1560–1566. 10.1038/nn.3828 [DOI] [PubMed] [Google Scholar]
- Mizobe T. (2019). The halothane hepatitis that was not. Br. J. Anaesth. 124, e2–e3. 10.1016/j.bja.2019.09.018 [DOI] [PubMed] [Google Scholar]
- Mo C., Sherman S. M. (2019). A sensorimotor pathway via higher-order thalamus. J. Neurosci. 39, 692–704. 10.1523/JNEUROSCI.1467-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B., Adams B., Verma A., Daly D. (1997). Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J. Neurosci. 17, 2921–2927. 10.1523/JNEUROSCI.17-08-02921.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohajerani M. H., Chan A. W., Mohsenvand M., LeDue J., Liu R., McVea D. A., et al. (2013). Spontaneous cortical activity alternates between motifs defined by regional axonal projections. Nat. Neurosci. 16, 1426–1435. 10.1038/nn.3499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mölle M., Marshall L., Gais S., Born J. (2002). Grouping of spindle activity during slow oscillations in human non-rapid eye movement sleep. J. Neurosci. 22, 10941–10947. 10.1523/JNEUROSCI.22-24-10941.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montemurro M. A., Rasch M. J., Murayama Y., Logothetis N. K., Panzeri S. (2008). Phase-of-firing coding of natural visual stimuli in primary visual cortex. Curr. Biol. 18, 375–380. 10.1016/j.cub.2008.02.023 [DOI] [PubMed] [Google Scholar]
- Monti M. M., Lutkenhoff E. S., Rubinov M., Boveroux P., Vanhaudenhuyse A., Gosseries O., et al. (2013). Dynamic change of global and local information processing in propofol-induced loss and recovery of consciousness. PLoS Comput. Biol. 9:e1003271. 10.1371/journal.pcbi.1003271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montijn J. S., Meijer G. T., Lansink C. S., Pennartz C. M. A. (2016). Population-level neural codes are robust to single-neuron variability from a multidimensional coding perspective. Cell Rep. 16, 2486–2498. 10.1016/j.celrep.2016.07.065 [DOI] [PubMed] [Google Scholar]
- Moon J. Y., Lee U., Blain-Moraes S., Mashour G. A. (2015). General relationship of global topology, local dynamics, and directionality in large-scale brain networks. PLoS Comput. Biol. 11:e1004225. 10.1371/journal.pcbi.1004225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouraux A., Diukova A., Lee M. C., Wise R. G., Iannetti G. D. (2011). A multisensory investigation of the functional significance of the “pain matrix”. NeuroImage 54, 2237–2249. 10.1016/j.neuroimage.2010.09.084 [DOI] [PubMed] [Google Scholar]
- Mouraux A., Iannetti G. D. (2018). The search for pain biomarkers in the human brain. Brain 141, 3290–3307. 10.1093/brain/awy281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueggler T., Baumann D., Rausch M., Staufenbiel M., Rudin M. (2003). Age-dependent impairment of somatosensory response in the amyloid precursor protein 23 transgenic mouse model of Alzheimer’s disease. J. Neurosci. 23, 8231–8236. 10.1523/JNEUROSCI.23-23-08231.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukamel E. A., Pirondini E., Babadi B., Wong K. F., Pierce E. T., Harrell P. G., et al. (2014). A transition in brain state during propofol-induced unconsciousness. J. Neurosci. 34, 839–845. 10.1523/JNEUROSCI.5813-12.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller L., Chavane F., Reynolds J., Sejnowski T. J. (2018). Cortical travelling waves: mechanisms and computational principles. Nat. Rev. Neurosci. 19, 255–268. 10.1038/nrn.2018.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munting L. P., Derieppe M. P. P., Suidgeest E., Denis de Senneville B., Wells J., van der Weerd L. (2019). Influence of different isoflurane anesthesia protocols on murine cerebral hemodynamics measured with pseudo-continuous arterial spin labeling. NMR Biomed. 32:e4105. 10.1002/nbm.4105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy S. D., Shaw A. D., Jackson L. E., Hall J., Moran R., Saxena N. (2015). Evidence that subanesthetic doses of ketamine cause sustained disruptions of NMDA and AMPA-mediated frontoparietal connectivity in humans. J. Neurosci. 35, 11694–11706. 10.1523/JNEUROSCI.0903-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M. C., Chan K. C., Kim S.-G., Vazquez A. L. (2017). Macroscale variation in resting-state neuronal activity and connectivity assessed by simultaneous calcium imaging, hemodynamic imaging and electrophysiology. NeuroImage 169, 352–362. 10.1016/j.neuroimage.2017.12.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C., Krause B., Banks M. (2019). Selective effects of isoflurane on cortico-cortical feedback afferent responses in murine non-primary neocortex. Br. J. Anaesth. 123, 488–496. 10.1016/j.bja.2019.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell J. C., Waters D., Johnson C. B. (2008). Comparative effects of halothane, isoflurane, sevoflurane and desflurane on the electroencephalogram of the rat. Lab. Anim. 42, 161–170. 10.1258/la.2007.06019e [DOI] [PubMed] [Google Scholar]
- Nasrallah F. A., Lew S. K., Low A. S., Chuang K. H. (2014a). Neural correlate of resting-state functional connectivity under α2 adrenergic receptor agonist, medetomidine. NeuroImage 84, 27–34. 10.1016/j.neuroimage.2013.08.004 [DOI] [PubMed] [Google Scholar]
- Nasrallah F. A., Tay H. C., Chuang K. H. (2014b). Detection of functional connectivity in the resting mouse brain. NeuroImage 86, 417–424. 10.1016/j.neuroimage.2013.10.025 [DOI] [PubMed] [Google Scholar]
- Nasrallah F. A., Tan J., Chuang K. H. (2012). Pharmacological modulation of functional connectivity: α2-adrenergic receptor agonist alters synchrony but not neural activation. NeuroImage 60, 436–446. 10.1016/j.neuroimage.2011.12.026 [DOI] [PubMed] [Google Scholar]
- Nelson L. E., Lu J., Guo T., Saper C. B., Franks N. P., Maze M. (2003). The α2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology 98, 428–436. 10.1097/00000542-200302000-00024 [DOI] [PubMed] [Google Scholar]
- Nelson C. L., Sarter M., Bruno J. P. (2005). Prefrontal cortical modulation of acetylcholine release in posterior parietal cortex. Neuroscience 132, 347–359. 10.1016/j.neuroscience.2004.12.007 [DOI] [PubMed] [Google Scholar]
- Neske G. T. (2015). The slow oscillation in cortical and thalamic networks: mechanisms and functions. Front. Neural Circuits 9:88. 10.3389/fncir.2015.00088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ní Mhuircheartaigh R., Warnaby C., Rogers R., Jbabdi S., Tracey I. (2013). Slow-wave activity saturation and thalamocortical isolation during propofol anesthesia in humans. Sci. Transl. Med. 5:208ra148. 10.1126/scitranslmed.3006007 [DOI] [PubMed] [Google Scholar]
- Niedermeyer E., Sherman D. L., Geocadin R. J., Hansen H. C., Hanley D. F. (1999). The burst-suppression electroencephalogram. Clin. Electroencephalogr. 30, 99–105. 10.1177/155005949903000305 [DOI] [PubMed] [Google Scholar]
- Niendorf T., Pohlmann A., Reimann H. M., Waiczies H., Peper E., Huelnhagen T., et al. (2015). Advancing cardiovascular, neurovascular and renal magnetic resonance imaging in small rodents using cryogenic radiofrequency coil technology. Front. Pharmacol. 6:255. 10.3389/fphar.2015.00255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir Y., Staba R. J., Andrillon T., Vyazovskiy V. V., Cirelli C., Fried I., et al. (2011). Regional slow waves and spindles in human sleep. Neuron 70, 153–169. 10.1016/j.neuron.2011.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niranjan A., Christie I. N., Solomon S. G., Wells J. A., Lythgoe M. F. (2016). fMRI mapping of the visual system in the mouse brain with interleaved snapshot GE-EPI. NeuroImage 139, 337–345. 10.1016/j.neuroimage.2016.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noreika V., Jylhänkangas L., Móró L., Valli K., Kaskinoro K., Aantaa R., et al. (2011). Consciousness lost and found: subjective experiences in an unresponsive state. Brain Cogn. 77, 327–334. 10.1016/j.bandc.2011.09.002 [DOI] [PubMed] [Google Scholar]
- Nuñez A., Amzica F., Steriade M. (1992). Voltage-dependent fast (20–40 Hz) oscillations in long-axoned neocortical neurons. Neuroscience 51, 7–10. 10.1016/0306-4522(92)90464-d [DOI] [PubMed] [Google Scholar]
- Ogawa S., Lee T. M., Kay A. R., Tank D. W. (1990). Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl. Acad. Sci. U S A 87, 9868–9872. 10.1073/pnas.87.24.9868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojemann G. A., Ramsey N. F., Ojemann J. (2013). Relation between functional magnetic resonance imaging (fMRI) and single neuron, local field potential (LFP) and electrocorticography (ECoG) activity in human cortex. Front. Hum. Neurosci. 7:34. 10.3389/fnhum.2013.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojemann G. A., Schoenfield-McNeill J., Corina D. (2002). Anatomic subdivisions in human temporal cortical neuronal activity related to recent verbal memory. Nat. Neurosci. 5, 64–71. 10.1038/nn785 [DOI] [PubMed] [Google Scholar]
- Olcese U., Oude Lohuis M. N., Pennartz C. M. A. (2018). Sensory processing across conscious and nonconscious brain states: from single neurons to distributed networks for inferential representation. Front. Syst. Neurosci. 12:49. 10.3389/fnsys.2018.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olufsen M., Whittington M. A., Camperi M., Kopell N. (2003). New functions for the gamma rhythm: population tuning and preprocessing for the beta rhythm. J. Comput. Neurosci. 14, 33–54. 10.1023/a:1021124317706 [DOI] [PubMed] [Google Scholar]
- Oye I., Paulsen O., Maurset A. (1992). Effects of ketamine on sensory perception: evidence for a role of N-methyl-D-aspartate receptors. J. Pharmacol. Exp. Ther. 260, 1209–1213. [PubMed] [Google Scholar]
- Paasonen J., Laakso H., Pirttimäki T., Stenroos P., Salo R. A., Zhurakovskaya E., et al. (2020). Multi-band SWIFT enables quiet and artefact-free EEG-fMRI and awake fMRI studies in rat. NeuroImage 206:116338. 10.1016/j.neuroimage.2019.116338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paasonen J., Stenroos P., Salo R. A., Kiviniemi V., Grohn O. (2018). Functional connectivity under six anesthesia protocols and the awake condition in rat brain. NeuroImage 172, 9–20. 10.1016/j.neuroimage.2018.01.014 [DOI] [PubMed] [Google Scholar]
- Pachitariu M., Lyamzin D. R., Sahani M., Lesica N. A. (2015). State-dependent population coding in primary auditory cortex. J. Neurosci. 35, 2058–2073. 10.1523/JNEUROSCI.3318-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pack C. C., Berezovskii V. K., Born R. T. (2001). Dynamic properties of neurons in cortical area MT in alert and anaesthetized macaque monkeys. Nature 414, 905–908. 10.1038/414905a [DOI] [PubMed] [Google Scholar]
- Pais-Roldán P., Biswal B., Scheffler K., Yu X. (2018). Identifying respiration-related aliasing artifacts in the rodent resting-state fMRI. Front. Neurosci. 12:788. 10.3389/fnins.2018.00788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal D., Dean J. G., Liu T., Li D., Watson C. J., Hudetz A. G., et al. (2018). Differential role of prefrontal and parietal cortices in controlling level of consciousness. Curr. Biol. 28, 2145.e5–2152.e5. 10.1016/j.cub.2018.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal D., Hambrecht-Wiedbusch V. S., Silverstein B. H., Mashour G. A. (2015). Electroencephalographic coherence and cortical acetylcholine during ketamine-induced unconsciousness. Br. J. Anaesth. 114, 979–989. 10.1093/bja/aev095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanca B. J., Mitra A., Larson-Prior L., Snyder A. Z., Avidan M. S., Raichle M. E. (2015). Resting-state functional magnetic resonance imaging correlates of sevoflurane-induced unconsciousness. Anesthesiology 123, 346–356. 10.1097/aln.0000000000000731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva S., Linkenkaer-Hansen K., Näätänen R., Palva J. M. (2005). Early neural correlates of conscious somatosensory perception. J. Neurosci. 25, 5248–5258. 10.1523/jneurosci.0141-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva S., Palva J. M. (2007). New vistas for α-frequency band oscillations. Trends Neurosci. 30, 150–158. 10.1016/j.tins.2007.02.001 [DOI] [PubMed] [Google Scholar]
- Pan W. J., Billings J. C., Grooms J. K., Shakil S., Keilholz S. D. (2015). Considerations for resting state functional MRI and functional connectivity studies in rodents. Front. Neurosci. 9:269. 10.3389/fnins.2015.00269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W. J., Billings J., Nezafati M., Abbas A., Keilholz S. (2018). Resting state fMRI in rodents. Curr. Protoc. Neurosci. 83:e45. 10.1002/cpns.45 [DOI] [PubMed] [Google Scholar]
- Paquette T., Jeffrey-Gauthier R., Leblond H., Pich E. M. (2018). Functional neuroimaging of nociceptive and pain-related activity in the spinal cord and brain: insights from neurovascular coupling studies. Anat. Rec. 301, 1585–1595. 10.1002/ar.23854 [DOI] [PubMed] [Google Scholar]
- Paquette T., Tokunaga R., Touj S., Leblond H., Piché M. (2019). Regulation of cortical blood flow responses by the nucleus basalis of Meynert during nociceptive processing. Neurosci. Res. 149, 22–28. 10.1016/j.neures.2019.01.008 [DOI] [PubMed] [Google Scholar]
- Pavone K. J., Su L., Gao L., Eromo E., Vazquez R., Rhee J., et al. (2017). Lack of responsiveness during the onset and offset of sevoflurane anesthesia is associated with decreased awake-α oscillation power. Front. Syst. Neurosci. 11:38. 10.3389/fnsys.2017.00038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawela C. P., Biswal B. B., Cho Y. R., Kao D. S., Li R., Jones S. R., et al. (2008). Resting-state functional connectivity of the rat brain. Magn. Reson. Med. 59, 1021–1029. 10.1002/mrm.21524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennartz C. M. A., Dora S., Muckli L., Lorteije J. A. M. (2019). Towards a Unified View on Pathways and Functions of Neural Recurrent Processing. Trends Neurosci. 42. 10.1016/j.tins.2019.07.005 [DOI] [PubMed] [Google Scholar]
- Petersen C. C. H., Hahn T. T. G., Mehta M., Grinvald A., Sakmann B. (2003). Interaction of sensory responses with spontaneous depolarization in layer 2/3 barrel cortex. Proc. Natl. Acad. Sci. U S A 100, 13638–13643. 10.1073/pnas.2235811100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen S. E., Sporns O. (2015). Brain networks and cognitive architectures. Neuron 88, 207–219. 10.1016/j.neuron.2015.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrinovic M. M., Hankov G., Schroeter A., Bruns A., Rudin M., Von Kienlin M., et al. (2016). A novel anesthesia regime enables neurofunctional studies and imaging genetics across mouse strains. Sci. Rep. 6:24523. 10.1038/srep24523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrache A., Battaglia F. P., Destexhe A. (2011). Inhibition recruitment in prefrontal cortex during sleep spindles and gating of hippocampal inputs. Proc. Natl. Acad. Sci. U S A 108, 17207–17212. 10.1073/pnas.1103612108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff D. (2005). Brain Arousal and Information Theory: Neural and Genetic Mechanisms. Cambridge, MA: Harvard University Press. page 71–72, 123. [Google Scholar]
- Phillips W. A., Bachmann T., Storm J. F. (2018). Apical function in neocortical pyramidal cells: a common pathway by which general anesthetics can affect mental state. Front. Neural Circuits 12:50. 10.3389/fncir.2018.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard N., Takesian A. E., Fagiolini M., Hensch T. K. (2019). NMDA 2A receptors in parvalbumin cells mediate sex-specific rapid ketamine response on cortical activity. Mol. Psychiatry 24, 828–838. 10.1038/s41380-018-0341-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picchioni D., Reith R. M., Nadel J. L., Smith C. B. (2014). Sleep, plasticity and the pathophysiology of neurodevelopmental disorders: the potential roles of protein synthesis and other cellular processes. Brain Sci. 4, 150–201. 10.3390/brainsci4010150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack P.-O., Friedman J., Golshani P. (2013). Cellular mechanisms of brain state-dependent gain modulation in visual cortex. Nat. Neurosci. 16, 1331–1339. 10.1038/nn.3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porro C. A., Cavazzuti M., Giuliani D., Vellani V., Lui F., Baraldi P. (2004). Effects of ketamine anesthesia on central nociceptive processing in the rat: a 2-deoxyglucose study. Neuroscience 125, 485–494. 10.1016/j.neuroscience.2004.01.039 [DOI] [PubMed] [Google Scholar]
- Poulet J. F. A., Crochet S. (2019). The cortical states of wakefulness. Front. Syst. Neurosci. 12:64. 10.3389/fnsys.2018.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prerau M. J., Brown R. E., Bianchi M. T., Ellenbogen J. M., Purdon P. L. (2017). Sleep neurophysiological dynamics through the lens of multitaper spectral analysis. Physiology 32, 60–92. 10.1152/physiol.00062.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prerau M. J., Hartnack K. E., Obregon-Henao G., Sampson A., Merlino M., Gannon K., et al. (2014). Tracking the sleep onset process: an empirical model of behavioral and physiological dynamics. PLoS Comput. Biol. 10:e1003866. 10.1371/journal.pcbi.1003866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preti M. G., Bolton T. A., Van De Ville D. (2017). The dynamic functional connectome: state-of-the-art and perspectives. NeuroImage 160, 41–54. 10.1016/j.neuroimage.2016.12.061 [DOI] [PubMed] [Google Scholar]
- Price D. D. (2000). Psychological and neural mechanisms of the affective dimension of pain. Science 288, 1769–1772. 10.1126/science.288.5472.1769 [DOI] [PubMed] [Google Scholar]
- Pries A. R., Secomb T. W., Gaehtgens P. (1995). Design principles of vascular beds. Circ. Res. 77, 1017–1023. 10.1161/01.res.77.5.1017 [DOI] [PubMed] [Google Scholar]
- Proekt A., Hudson A. E. (2018). A stochastic basis for neural inertia in emergence from general anaesthesia. Br. J. Anaesth. 121, 86–94. 10.1016/j.bja.2018.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proekt A., Kelz M. (2018). Schrödinger’s cat: anaesthetised and not! Br. J. Anaesth. 120, 424–428. 10.1016/j.bja.2017.11.068 [DOI] [PubMed] [Google Scholar]
- Purdon P. L., Pierce E. T., Bonmassar G., Walsh J., Harrell P. G., Kwo J., et al. (2009). Simultaneous electroencephalography and functional magnetic resonance imaging of general anesthesia. Ann. N Y Acad. Sci. 1157, 61–70. 10.1111/j.1749-6632.2008.04119.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdon P. L., Pierce E. T., Mukamel E. A., Prerau M. J., Walsh J. L., Wong K. F., et al. (2013). Electroencephalogram signatures of loss and recovery of consciousness from propofol. Proc. Natl. Acad. Sci. U S A 110, E1142–E1151. 10.1073/pnas.1221180110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdon P. L., Sampson A., Pavone K. J., Brown E. N. (2015). Clinical electroencephalography for anesthesiologists: part I: background and basic signatures. Anesthesiology 123, 937–960. 10.1097/ALN.0000000000000841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam R. W., Filosa J. A., Ritucci N. A. (2004). Cellular mechanisms involved in CO2 and acid signaling in chemosensitive neurons. Am. J. Physiol. Cell Physiol. 287, C1493–C1526. 10.1152/ajpcell.00282.2004 [DOI] [PubMed] [Google Scholar]
- Quasha A. L., Eger E. I., II., Tinker J. H. (1980). Determination and applications of MAC. Anesthesiology 53, 315–334. 10.1097/00000542-198010000-00008 [DOI] [PubMed] [Google Scholar]
- Raichle M. E. (2015). The brain’s default mode network. Annu. Rev. Neurosci. 38, 433–447. 10.1146/annurev-neuro-071013-014030 [DOI] [PubMed] [Google Scholar]
- Raichle M. E., Macleod A. M., Snyder A. Z., Powers W. J., Gusnard D. A., Shulman G. L. (2001). A default mode of brain function. Proc. Natl. Acad. Sci. U S A 98, 676–682. 10.1073/pnas.98.2.676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranft A., Golkowski D., Kiel T., Riedl V., Kohl P., Rohrer G., et al. (2016). Neural correlates of sevoflurane-induced unconsciousness identified by simultaneous functional magnetic resonance imaging and electroencephalography. Anesthesiology 125, 861–872. 10.1097/ALN.0000000000001322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper A. J., Levasseur J. E. (1971). Accurate sustained measurement of intra-luminal pressure from the microvasculature. Cardiovasc. Res. 5, 589–593. 10.1093/cvr/5.4.589 [DOI] [PubMed] [Google Scholar]
- Raz A., Grady S. M., Krause B. M., Uhlrich D. J., Manning K. A., Banks M. I. (2014). Preferential effect of isoflurane on top-down vs. bottom-up pathways in sensory cortex. Front. Syst. Neurosci. 8:191. 10.3389/fnsys.2014.00191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redinbaugh M. J., Phillips J. M., Kambi N. A., Mohanta S., Andryk S., Dooley G. L., et al. (2020). Thalamus modulates consciousness via layer-specific control of cortex. Neuron [Epub ahead of print]. 10.1016/j.neuron.2020.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees G., Kreiman G., Koch C. (2002). Neural correlates of consciousness in humans. Nat. Rev. Neurosci. 3, 261–270. 10.1038/nrn783 [DOI] [PubMed] [Google Scholar]
- Reig R., Mattia M., Compte A., Belmonte C., Sanchez-Vives M. V. (2010). Temperature modulation of slow and fast cortical rhythms. J. Neurophysiol. 103, 1253–1261. 10.1152/jn.00890.2009 [DOI] [PubMed] [Google Scholar]
- Reimann H. M., Hentschel J., Marek J., Huelnhagen T., Todiras M., Kox S., et al. (2016). Normothermic mouse functional MRI of acute focal thermostimulation for probing nociception. Sci. Rep. 6:17230. 10.1038/srep17230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann H. M., Todiras M., Hodge R., Huelnhagen T., Millward J. M., Turner R., et al. (2018). Somatosensory BOLD fMRI reveals close link between salient blood pressure changes and the murine neuromatrix. NeuroImage 172, 562–574. 10.1016/j.neuroimage.2018.02.002 [DOI] [PubMed] [Google Scholar]
- Reimer J., Mcginley M. J., Liu Y., Rodenkirch C., Wang Q., Mccormick D. A., et al. (2016). Pupil fluctuations track rapid changes in adrenergic and cholinergic activity in cortex. Nat. Commun. 7:13289. 10.1038/ncomms13289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. A., Gollo L. L., Abeysuriya R. G., Roberts G., Mitchell P. B., Woolrich M. W., et al. (2019). Metastable brain waves. Nat. Commun. 10:1056. 10.1038/s41467-019-08999-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth B. L. (2016). DREADDs for neuroscientists. Neuron 89, 683–694. 10.1016/j.neuron.2016.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R., Abbott L. F., Sompolinsky H. (2017). Balanced excitation and inhibition are required for high-capacity, noise-robust neuronal selectivity. Proc. Natl. Acad. Sci. U S A 114, E9366–E9375. 10.1073/pnas.1705841114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Mejias M., Ciria-Suarez L., Mattia M., Sanchez-Vives M. V. (2011). Slow and fast rhythms generated in the cerebral cortex of the anesthetized mouse. J. Neurophysiol. 106, 2910–2921. 10.1152/jn.00440.2011 [DOI] [PubMed] [Google Scholar]
- Ryali S., Shih Y.-Y. I., Chen T., Kochalka J., Albaugh D., Fang Z., et al. (2016). Combining optogenetic stimulation and fMRI to validate a multivariate dynamical systems model for estimating causal brain interactions. NeuroImage 132, 398–405. 10.1016/j.neuroimage.2016.02.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalmann Y. B. (2014). Intralaminar and medial thalamic influence on cortical synchrony, information transmission and cognition. Front. Syst. Neurosci. 8:83. 10.3389/fnsys.2014.00083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalmann Y. B., Pinsk M. A., Wang L., Li X., Kastner S. (2012). The pulvinar regulates information transmission between cortical areas based on attention demands. Science 337, 753–756. 10.1126/science.1223082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachidhanandam S., Sreenivasan V., Kyriakatos A., Kremer Y., Petersen C. C. H. (2013). Membrane potential correlates of sensory perception in mouse barrel cortex. Nat. Neurosci. 16, 1671–1677. 10.1038/nn.3532 [DOI] [PubMed] [Google Scholar]
- Sadaghiani S., Scheeringa R., Lehongre K., Morillon B., Giraud A. L., D’Esposito M., et al. (2012). α-band phase synchrony is related to activity in the fronto-parietal adaptive control network. J. Neurosci. 32, 14305–14310. 10.1523/jneurosci.1358-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels E. R., Szabadi E. (2008a). Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part I: principles of functional organisation. Curr. Neuropharmacol. 6, 235–253. 10.2174/157015908785777229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels E. R., Szabadi E. (2008b). Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part II: physiological and pharmacological manipulations and pathological alterations of locus coeruleus activity in humans. Curr. Neuropharmacol. 6, 254–285. 10.2174/157015908785777193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vives M. V., Massimini M., Mattia M. (2017). Shaping the default activity pattern of the cortical network. Neuron 94, 993–1001. 10.1016/j.neuron.2017.05.015 [DOI] [PubMed] [Google Scholar]
- Sanders T. H. (2017). Stimulation of cortico-subthalamic projections amplifies resting motor circuit activity and leads to increased locomotion in dopamine-depleted mice. Front. Integr. Neurosci. 11:24. 10.3389/fnint.2017.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders R. D., Banks M. I., Darracq M., Moran R., Sleigh J., Gosseries O., et al. (2018). Propofol-induced unresponsiveness is associated with impaired feedforward connectivity in cortical hierarchy. Br. J. Anaesth. 121, 1084–1096. 10.1016/j.bja.2018.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders R. D., Degos V., Young W. L. (2011). Cerebral perfusion under pressure: is the autoregulatory “plateau” a level playing field for all? Anaesthesia 66, 968–972. 10.1111/j.1365-2044.2011.06915.x [DOI] [PubMed] [Google Scholar]
- Sanders R., Tononi G., Laureys S., Sleigh J. (2012). Unresponsiveness ≢ unconsciousness. Anesthesiology 116, 946–959. 10.1097/ALN.0b013e318249d0a7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper C. B., Fuller P. M., Pedersen N. P., Lu J., Scammell T. E. (2010). Sleep state switching. Neuron 68, 1023–1042. 10.1016/j.neuron.2010.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper C. B., Scammell T. E., Lu J. (2005). Hypothalamic regulation of sleep and circadian rhythms. Nature 437, 1257–1263. 10.1038/nature04284 [DOI] [PubMed] [Google Scholar]
- Sarasso S., Boly M., Napolitani M., Gosseries O., Charland-Verville V., Casarotto S., et al. (2015). Consciousness and complexity during unresponsiveness induced by propofol, xenon, and ketamine. Curr. Biol. 25, 3099–3105. 10.1016/j.cub.2015.10.014 [DOI] [PubMed] [Google Scholar]
- Scammell T. E., Arrigoni E., Lipton J. O. (2017). Neural circuitry of wakefulness and sleep. Neuron 93, 747–765. 10.1016/j.neuron.2017.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel F. (2017). Improving Mouse FMRI and Our Understanding of Its Underpinnings. [Dissertation] Zürich, Switzerland: ETH Zurich. [Google Scholar]
- Schlegel F., Schroeter A., Rudin M. (2015). The hemodynamic response to somatosensory stimulation in mice depends on the anesthetic used: implications on analysis of mouse fMRI data. NeuroImage 116, 40–49. 10.1016/j.neuroimage.2015.05.013 [DOI] [PubMed] [Google Scholar]
- Schlegel F., Sych Y., Schroeter A., Stobart J., Weber B., Helmchen F., et al. (2018). Fiber-optic implant for simultaneous fluorescence-based calcium recordings and BOLD fMRI in mice. Nat. Protoc. 13, 840–855. 10.1038/nprot.2018.003 [DOI] [PubMed] [Google Scholar]
- Schmid F., Wachsmuth L., Schwalm M., Prouvot P. H., Jubal E. R., Fois C., et al. (2016). Assessing sensory versus optogenetic network activation by combining (o)fMRI with optical Ca2+ recordings. J. Cereb. Blood Flow Metab. 36, 1885–1900. 10.1177/0271678x15619428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider D. W., Logan G. D. (2014). Modelling response selection in task switching: testing the contingent encoding assumption. Q. J. Exp. Psychol. 67, 1074–1095. 10.1080/17470218.2013.843009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schölvinck M. L., Saleem A. B., Benucci A., Harris K. D., Carandini M. (2015). Cortical state determines global variability and correlations in visual cortex. J. Neurosci. 35, 170–178. 10.1523/jneurosci.4994-13.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder C. E., Lakatos P. (2009). Low-frequency neuronal oscillations as instruments of sensory selection. Trends in Neurosci. 32, 9–18. 10.1016/j.tins.2008.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder K. E., Irwin Z. T., Gaidica M., Nicole Bentley J., Patil P. G., Mashour G. A., et al. (2016). Disruption of corticocortical information transfer during ketamine anesthesia in the primate brain. NeuroImage 134, 459–465. 10.1016/j.neuroimage.2016.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter A., Grandjean J., Schlegel F., Saab B. J., Rudin M. (2017). Contributions of structural connectivity and cerebrovascular parameters to functional magnetic resonance imaging signals in mice at rest and during sensory paw stimulation. J. Cereb. Blood Flow Metab. 37, 2368–2382. 10.1177/0271678x16666292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter A., Schlegel F., Seuwen A., Grandjean J., Rudin M. (2014). Specificity of stimulus-evoked fMRI responses in the mouse: the influence of systemic physiological changes associated with innocuous stimulation under four different anesthetics. NeuroImage 94, 372–384. 10.1016/j.neuroimage.2014.01.046 [DOI] [PubMed] [Google Scholar]
- Schröter M. S., Spoormaker V. I., Schorer A., Wohlschlager A., Czisch M., Kochs E. F., et al. (2012). Spatiotemporal reconfiguration of large-scale brain functional networks during propofol-induced loss of consciousness. J. Neurosci. 32, 12832–12840. 10.1523/jneurosci.6046-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher J. W., Schneider D. M., Woolley S. M. N. (2011). Anesthetic state modulates excitability but not spectral tuning or neural discrimination in single auditory midbrain neurons. Neuroscience 106, 500–514. 10.1152/jn.01072.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwalm M., Easton C. (2016). Cortical temperature change: a tool for modulating brain states? eNeuro 3:ENEURO.0096-16.2016. 10.1523/eneuro.0096-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwalm M., Schmid F., Wachsmuth L., Backhaus H., Kronfeld A., Jury F. A., et al. (2017). Cortex-wide BOLD fMRI activity reflects locally-recorded slow oscillation-associated calcium waves. Elife 6:e27602. 10.7554/elife.27602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwertner A., Zortea M., Torres F. V., Caumo W. (2018). Effects of subanesthetic ketamine administration on visual and auditory event-related potentials (ERP) in humans: a systematic review. Front. Behav. Neurosci. 12:70. 10.3389/fnbeh.2018.00070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans J. (2008). Losing inhibition with ketamine. Nat. Chem. Biol. 4, 91–93. 10.1038/nchembio0208-91 [DOI] [PubMed] [Google Scholar]
- Sellers K. K., Bennett D. V., Hutt A., Williams J. H., Fröhlich F. (2015). Awake vs. anesthetized: layer-specific sensory processing in visual cortex and functional connectivity between cortical areas. J. Neurophysiol. 113, 3798–3815. 10.1152/jn.00923.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senzai Y., Fernandez-Ruiz A., Buzsáki G. (2019). Layer-specific physiological features and interlaminar interactions in the primary visual cortex of the mouse. Neuron 101, 500.e5–513.e5. 10.1016/j.neuron.2018.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth A. K., Baars B. J., Edelman D. B. (2005). Criteria for consciousness in humans and other mammals. Conscious. Cogn. 14, 119–139. 10.1016/j.concog.2004.08.006 [DOI] [PubMed] [Google Scholar]
- Seth A. K., Tsakiris M. (2018). Being a beast machine: the somatic basis of selfhood. Trends Cogn. Sci. 22, 969–981. 10.1016/j.tics.2018.08.008 [DOI] [PubMed] [Google Scholar]
- Sforazzini F., Schwarz A. J., Galbusera A., Bifone A., Gozzi A. (2014). Distributed BOLD and CBV-weighted resting-state networks in the mouse brain. NeuroImage 87, 403–415. 10.1016/j.neuroimage.2013.09.050 [DOI] [PubMed] [Google Scholar]
- Shah D., Blockx I., Guns P., Paul P., Deyn D., Van Dam D., et al. (2015). Acute modulation of the cholinergic system in the mouse brain detected by pharmacological resting-state functional MRI. NeuroImage 109, 151–159. 10.1016/j.neuroimage.2015.01.009 [DOI] [PubMed] [Google Scholar]
- Shah D., Jonckers E., Praet J., Vanhoutte G., Delgado Y. P., Bigot C., et al. (2013). Resting state FMRI reveals diminished functional connectivity in a mouse model of amyloidosis. PLoS One 8:e84241. 10.1371/journal.pone.0084241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. S., Shaw K., Boorman L., Harris S., Kennerley A. J., Azzouz M., et al. (2015). Comparison of stimulus-evoked cerebral hemodynamics in the awake mouse and under a novel anesthetic regime. Sci. Rep. 5:14890. 10.1038/srep14890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shein-Idelson M., Ondracek J. M., Liaw H. P., Reiter S., Laurent G. (2016). Slow waves, sharp waves, ripples, and REM in sleeping dragons. Science 352, 590–595. 10.1126/science.aaf3621 [DOI] [PubMed] [Google Scholar]
- Shelton L., Becerra L., Borsook D. (2012a). Unmasking the mysteries of the habenula in pain and analgesia. Prog. Neurobiol. 96, 208–219. 10.1016/j.pneurobio.2012.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton L., Pendse G., Maleki N., Moulton E. A., Lebel A., Becerra L., et al. (2012b). Mapping pain activation and connectivity of the human habenula. J. Neurophysiol. 107, 2633–2648. 10.1152/jn.00012.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherin J. E., Elmquist J. K., Torrealba F., Saper C. B. (1998). Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J. Neurosci. 18, 4705–4721. 10.1523/jneurosci.18-12-04705.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman S. M. (2017). Functioning of circuits connecting thalamus and cortex. Compr. Physiol. 7, 713–739. 10.1002/cphy.c160032 [DOI] [PubMed] [Google Scholar]
- Sheroziya M., Timofeev I. (2014). Global intracellular slow-wave dynamics of the thalamocortical system. J. Neurosci. 34, 8875–8893. 10.1523/jneurosci.4460-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheroziya M., Timofeev I. (2015). Moderate cortical cooling eliminates thalamocortical silent states during slow oscillation. J. Neurosci. 35, 13006–13019. 10.1523/jneurosci.1359-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim H.-J., Jung W. B., Schlegel F., Lee J., Kim S., Lee J., et al. (2018). Mouse fMRI under ketamine and xylazine anesthesia: robust contralateral somatosensory cortex activation in response to forepaw stimulation. NeuroImage 177, 30–44. 10.1016/j.neuroimage.2018.04.062 [DOI] [PubMed] [Google Scholar]
- Shimaoka D., Harris K. D., Carandini M. (2018). Effects of arousal on mouse sensory cortex depend on modality. Cell Rep. 22, 3160–3167. 10.1016/j.celrep.2018.02.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimosegawa E., Kanno I., Hatazawa J., Fujita H., Iida H., Miura S., et al. (1995). Photic stimulation study of changing the arterial partial pressure level of carbon dioxide. J. Cereb. Blood Flow Metab. 15, 111–114. 10.1038/jcbfm.1995.12 [DOI] [PubMed] [Google Scholar]
- Shofty B., Bergmann E., Zur G., Asleh J., Bosak N., Kavushansky A., et al. (2019). Autism-associated Nf1 deficiency disrupts corticocortical and corticostriatal functional connectivity in human and mouse. Neurobiol. Dis. 130:104479. 10.1016/j.nbd.2019.104479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikkens T., Bosman C. A., Olcese U. (2019). The role of top-down modulation in shaping sensory processing across brain states: implications for consciousness. Front. Syst. Neurosci. 13:31. 10.3389/fnsys.2019.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair M. D. (2003). A review of the physiological effects of α2-agonists related to the clinical use of medetomidine in small animal practice. Can. Vet. J. 44, 885–897. [PMC free article] [PubMed] [Google Scholar]
- Sinclair D. R., Chung F., Smiley A. (2003). General anesthesia does not impair simulator driving skills in volunteers in the immediate recovery period—a pilot study. Can. J. Anaesth. 50, 238–245. 10.1007/bf03017791 [DOI] [PubMed] [Google Scholar]
- Sinner B., Graf B. M. (2008). Ketamine. Handb. Exp. Pharmacol. 182, 313–333. 10.1007/978-3-540-74806-9_15 [DOI] [PubMed] [Google Scholar]
- Sinning A., Hübner C. A. (2013). Minireview: pH and synaptic transmission. FEBS Lett. 587, 1923–1928. 10.1016/j.febslet.2013.04.045 [DOI] [PubMed] [Google Scholar]
- Sirmpilatze N., Baudewig J., Boretius S. (2019). Temporal stability of fMRI in medetomidine-anesthetized rats. Sci. Rep. 9:16673. 10.1038/s41598-019-53144-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirota A., Csicsvari J., Buhl D., Buzsáki G. (2003). Communication between neocortex and hippocampus during sleep in rodents. Proc. Natl. Acad. Sci. U S A 100, 2065–2069. 10.1073/pnas.0437938100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh J., Warnaby C., Tracey I. (2018). General anaesthesia as fragmentation of selfhood: insights from electroencephalography and neuroimaging. Br. J. Anaesth. 121, 233–240. 10.1016/j.bja.2017.12.038 [DOI] [PubMed] [Google Scholar]
- Slézia A., Hangya B., Ulbert I., Acsády L. (2011). Phase advancement and nucleus-specific timing of thalamocortical activity during slow cortical oscillation. J. Neurosci. 31, 607–617. 10.1523/jneurosci.3375-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solt K., Van Dort C. J., Chemali J. J., Taylor N. E., Kenny J. D., Brown E. N. (2014). Electrical stimulation of the ventral tegmental area induces reanimation from general anesthesia. Anesthesiology 121, 311–319. 10.1097/ALN.0000000000000117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song A. H., Kucyi A., Napadow V., Brown E. N., Loggia M. L., Akeju O. (2017). Pharmacological modulation of noradrenergic arousal circuitry disrupts functional connectivity of the locus ceruleus in humans. J. Neurosci. 37, 6938–6945. 10.1523/jneurosci.0446-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B., Ma N., Liu G., Zhang H., Yu L., Liu L., et al. (2019). Maximal flexibility in dynamic functional connectivity with critical dynamics revealed by fMRI data analysis and brain network modelling. J. Neural Eng. 16:056002. 10.1088/1741-2552/ab20bc [DOI] [PubMed] [Google Scholar]
- Soltesz I., Lightowler S., Leresche N., Jassik-Gerschenfeld D., Pollard C. E., Crunelli V. (1991). Two inward currents and the transformation of low frequency oscillations of rat and cat thalamocortical cells. J. Physiol. 441, 175–197. 10.1113/jphysiol.1991.sp018745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford J. M., Jarrett B. R., Miranda-Dominguez O., Mills B. D., Cain N., Mihalas S., et al. (2014). Large-scale topology and the default mode network in the mouse connectome. Proc. Natl. Acad. Sci. U S A 111, 18745–18750. 10.1073/pnas.1404346111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffey E. P. (2017). Methodology for determining minimum alveolar concentration: a critical appraisal. Vet. Anaesth. Analg. 44, 2–6. 10.1016/j.vaa.2016.12.001 [DOI] [PubMed] [Google Scholar]
- Steriade M., Curró Dossi R., Nunez A. (1991). Network modulation of a slow intrinsic oscillation of cat thalamocortical neurons implicated in sleep delta waves: cortical potentiation and brainstem cholinergic suppression. J. Neurosci. 11, 3200–3217. 10.1523/jneurosci.11-10-03200.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M. (2000). Corticothalamic resonance, states of vigilance and mentation. Neuroscience 101, 243–276. 10.1016/s0306-4522(00)00353-5 [DOI] [PubMed] [Google Scholar]
- Steriade M. (2001). Impact of network activities on neuronal properties in corticothalamic systems. J. Neurophysiol. 86, 1–39. 10.1152/jn.2001.86.1.1 [DOI] [PubMed] [Google Scholar]
- Steriade M., Amzica F., Contreras D. (1994). Cortical and thalamic cellular correlates of electroencephalographic burst-suppression. Electroencephalogr. Clin. Neurophysiol. 90, 1–16. 10.1016/0013-4694(94)90108-2 [DOI] [PubMed] [Google Scholar]
- Steriade M., Amzica F., Nufiez A. (1993a). Cholinergic and noradrenergic modulation of the slow (approximately 0.3 Hz) oscillation in neocortical cells. J. Neurophysiol. 70, 1385–1400. 10.1152/jn.1993.70.4.1385 [DOI] [PubMed] [Google Scholar]
- Steriade M., Contreras D., Curró Dossi R., Nuñez A. (1993b). The slow (<1 Hz) oscillation in reticular thalamic and thalamocortical neurons: scenario of sleep rhythm generation in interacting thalamic and neocortical networks. J. Neurosci. 13, 3284–3299. 10.1523/jneurosci.13-08-03284.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M., Nuñez A., Amzica F. (1993c). Intracellular analysis of relations between the slow (<1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. J. Neurosci. 13, 3266–3283. 10.1523/jneurosci.13-08-03266.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M., Nuñez A., Amzica F. (1993d). A novel slow (<1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J. Neurosci. 13, 3252–3265. 10.1523/jneurosci.13-08-03252.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M., Contreras D. (1995). Relations between cortical and thalamic cellular events during transition from sleep pattern to paroxysmal activity. J. Neurosci. 15, 623–642. 10.1523/jneurosci.15-01-00623.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M., Deschenes M., Domich L., Mulle C. (1985). Abolition of spindle oscillations in thalamic neurons disconnected from nucleus reticularis thalami. J. Neurophysiol. 54, 1473–1497. 10.1152/jn.1985.54.6.1473 [DOI] [PubMed] [Google Scholar]
- Steriade M., Domich L., Oakson G., Deschênes M. (1987). The deafferented reticularis thalami nucleus generates spindle rhythmicity. J. Neurophysiol. 57, 260–273. 10.1152/jn.1987.57.1.260 [DOI] [PubMed] [Google Scholar]
- Steriade M., Timofeev I., Grenier F. (2001). Natural waking and sleep states: a view from inside neocortical neurons. J. Neurophysiol. 85, 1969–1985. 10.1152/jn.2001.85.5.1969 [DOI] [PubMed] [Google Scholar]
- Storm J. F., Boly M., Casali A. G., Massimini M., Olcese U., Pennartz C. M. A., et al. (2017). Consciousness regained: disentangling mechanisms, brain systems and behavioral responses. J. Neurosci. 37, 10882–10893. 10.1523/jneurosci.1838-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straathof M., Sinke M. R., Dijkhuizen R. M., Otte W. M. (2019). A systematic review on the quantitative relationship between structural and functional network connectivity strength in mammalian brains. J. Cereb. Blood Flow Metab. 39, 189–209. 10.1177/0271678x18809547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroh A., Adelsberger H., Groh A., Rühlmann C., Fischer S., Schierloh A., et al. (2013). Making waves: initiation and propagation of corticothalamic Ca2+ waves in vivo. Neuron 77, 1136–1150. 10.1016/j.neuron.2013.01.031 [DOI] [PubMed] [Google Scholar]
- Sumiyoshi A., Keeley R. J., Lu H. (2019). Physiological considerations of functional magnetic resonance imaging in animal models. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 4, 522–532. 10.1016/j.bpsc.2018.08.002 [DOI] [PubMed] [Google Scholar]
- Sun Y. M., Favre I., Schild L., Moczydlowski E. (1997). On the structural basis for size-selective permeation of organic cations through the voltage-gated sodium channel. Effect of alanine mutations at the DEKA locus on selectivity, inhibition by Ca2+ and H+ and molecular sieving. J. Gen. Physiol. 110, 693–715. 10.1085/jgp.110.6.693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W., McConnell E., Pare J. F., Xu Q., Chen M., Peng W., et al. (2013). Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science 339, 197–200. 10.1126/science.1226740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y. G., Wu C. S., Renger J. J., Uebele V. N., Lu H. C., Beierlein M. (2012). GABAergic synaptic transmission triggers action potentials in thalamic reticular nucleus neurons. J. Neurosci. 32, 7782–7790. 10.1523/jneurosci.0839-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkin S. M., Ng L., Lau C., Dolbeare T., Gilbert T. L., Thompson C. L., et al. (2013). Allen brain atlas: an integrated spatio-temporal portal for exploring the central nervous system. Nucleic Acids Res. 41, D996–D1008. 10.1093/nar/gks1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Larkum M. E. (2020). General anesthesia decouples cortical pyramidal neurons. Cell 180, 666.e13–676.e13. 10.1016/j.cell.2020.01.024 [DOI] [PubMed] [Google Scholar]
- Takuwa H., Matsuura T., Obata T., Kawaguchi H., Kanno I., Ito H. (2012). Hemodynamic changes during somatosensory stimulation in awake and isoflurane-anesthetized mice measured by laser-Doppler flowmetry. Brain Res. 1472, 107–112. 10.1016/j.brainres.2012.06.049 [DOI] [PubMed] [Google Scholar]
- Taylor N. E., Van Dort C. J., Kenny J. D., Pei J., Guidera J. A., Vlasov K. Y., et al. (2016). Optogenetic activation of dopamine neurons in the ventral tegmental area induces reanimation from general anesthesia. Proc. Natl. Acad. Sci. U S A 113, 12826–12831. 10.1073/pnas.1614340113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theyel B. B., Llano D. A., Sherman S. M. (2010). The corticothalamocortical circuit drives higher-order cortex in the mouse. Nat. Neurosci. 13, 84–88. 10.1038/nn.2449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson G. J., Merritt M. D., Pan W. J., Magnuson M. E., Grooms J. K., Jaeger D., et al. (2013). Neural correlates of time-varying functional connectivity in the rat. NeuroImage 83, 826–836. 10.1016/j.neuroimage.2013.07.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeev I., Grenier F., Bazhenov M., Sejnowski T. J., Steriade M. (2000). Origin of slow cortical oscillations in deafferented cortical slabs. Cereb. Cortex 10, 1185–1199. 10.1093/cercor/10.12.1185 [DOI] [PubMed] [Google Scholar]
- Tombaugh G. C., Somjen G. G. (1996). Effects of extracellular pH on voltage-gated Na+, K+ and Ca2+ currents in isolated rat CA1 neurons. J. Physiol. 493, 719–732. 10.1113/jphysiol.1996.sp021417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G., Boly M., Massimini M., Koch C. (2016). Integrated information theory: from consciousness to its physical substrate. Nat. Rev. Neurosci. 17, 450–461. 10.1038/nrn.2016.44 [DOI] [PubMed] [Google Scholar]
- Toussay X., Basu K., Lacoste B., Hamel E. (2013). Locus coeruleus stimulation recruits a broad cortical neuronal network and increases cortical perfusion. J. Neurosci. 33, 3390–3401. 10.1523/jneurosci.3346-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey I. (2017). Neuroimaging mechanisms in pain: from discovery to translation. Pain 158, S115–S122. 10.1097/j.pain.0000000000000863 [DOI] [PubMed] [Google Scholar]
- Trageser J. C., Burke K. A., Masri R., Li Y., Sellers L., Keller A. (2006). State-dependent gating of sensory inputs by zona incerta. J. Neurophysiol. 96, 1456–1463. 10.1152/jn.00423.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S., Kagitani F. (2018). Effect of basal forebrain stimulation on extracellular acetylcholine release and blood flow in the olfactory bulb. J. Physiol. Sci. 68, 415–423. 10.1007/s12576-017-0542-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhrig L., Sitt J. D., Jacob A., Tasserie J., Barttfeld P., Dupont M., et al. (2018). Resting-state dynamics as a cortical signature of anesthesia in monkeys. Anesthesiology 129, 942–958. 10.1097/aln.0000000000002336 [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai Y. M., Herman J. P. (2009). Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 10, 397–409. 10.1038/nrn2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Alst T. M., Wachsmuth L., Datunashvili M., Albers F., Just N., Budde T., et al. (2019). Anesthesia differentially modulates neuronal and vascular contributions to the BOLD signal. NeuroImage 195, 89–103. 10.1016/j.neuroimage.2019.03.057 [DOI] [PubMed] [Google Scholar]
- Van Bockstaele E. J., Saunders A., Telegan P., Page M. E. (1999). Localization of mu-opioid receptors to locus coeruleus-projecting neurons in the rostral medulla: morphological substrates and synaptic organization. Synapse 34, 154–167. [DOI] [PubMed] [Google Scholar]
- Van de Ville D., Britz J., Michel C. M. (2010). EEG microstate sequences in healthy humans at rest reveal scale-free dynamics. Proc. Natl. Acad. Sci. U S A 107, 18179–18184. 10.1073/pnas.1007841107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kerkoerle T., Self M. W., Dagnino B., Gariel-Mathis M. A., Poort J., van der Togt C., et al. (2014). Alpha and gamma oscillations char- acterize feedback and feedforward processing in monkey visual cortex. Proc. Natl. Acad. Sci. U S A 111, 14332–14341. 10.1073/pnas.1402773111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela C. (2014). Thalamic neuromodulation and its implications for executive networks. Front. Neural Circuits 8:69. 10.3389/fncir.2014.00069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazey E. M., Aston-Jones G. (2014). Designer receptor manipulations reveal a role of the locus coeruleus noradrenergic system in isoflurane general anesthesia. Proc. Natl. Acad. Sci. U S A 111, 3859–3864. 10.1073/pnas.1310025111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venn R. M., Grounds R. M. (2001). Comparison between dexmedetomidine and propofol for sedation in the intensive care unit: patient and clinician perceptions. Br. J. Anaesth. 87, 684–690. 10.1093/bja/87.5.684 [DOI] [PubMed] [Google Scholar]
- Ventura-Antunes L., Mota B., Herculano-Houzel S. (2013). Different scaling of white matter volume, cortical connectivity and gyrification across rodent and primate brains. Front. Neuroanat. 7:3. 10.3389/fnana.2013.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veselis R. A., Feshchenko V. A., Reinsel R. A., Beattie B., Akhurst T. J. (2005). Propofol and thiopental do not interfere with regional cerebral blood flow response at sedative concentrations. Anesthesiology 102, 26–34. 10.1097/00000542-200501000-00008 [DOI] [PubMed] [Google Scholar]
- Vetter I., Touska F., Hess A., Hinsbey R., Sattler S., Lampert A., et al. (2012). Ciguatoxins activate specific cold pain pathways to elicit burning pain from cooling. EMBO J. 3, 3795–3808. 10.1038/emboj.2012.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayan S., Ching S., Purdon P. L., Brown E. N., Kopell N. J. (2013). Thalamocortical mechanisms for the anteriorization of α rhythms during propofol-induced unconsciousness. J. Neurosci. 33, 11070–11075. 10.1523/jneurosci.5670-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent J. L., Patel G. H., Fox M. D., Snyder A. Z., Baker J. T., Van Essen D. C., et al. (2007). Intrinsic functional architecture in the anaesthetized monkey brain. Nature 447, 83–86. 10.1038/nature05758 [DOI] [PubMed] [Google Scholar]
- Vizuete J. A., Pillay S., Ropella K. M., Hudetz A. G. (2014). Graded defragmentation of cortical neuronal firing during recovery of consciousness in rats. Neuroscience 275, 340–351. 10.1016/j.neuroscience.2014.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlisides P. E., Bel-Bahar T., Lee U., Li D., Kim H., Janke E., et al. (2017). Neurophysiologic correlates of ketamine sedation and anesthesia: a high-density electroencephalography study in healthy volunteers. Anesthesiology 127, 58–69. 10.1097/aln.0000000000001671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt B. A., Hof P. R., Friedman D. P., Sikes R. W., Vogt L. J. (2008). Norepinephrinergic afferents and cytology of the macaque monkey midline, mediodorsal and intralaminar thalamic nuclei. Brain Struct. Funct. 212, 465–479. 10.1007/s00429-008-0178-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt B. A., Pandya D. N. (1987). Cingulate cortex of the rhesus monkey: II. Cortical afferents. J. Comp. Neurol. 262, 271–289. 10.1002/cne.902620208 [DOI] [PubMed] [Google Scholar]
- Volgushev M., Vidyasagar T. R., Chistiakova M., Yousef T., Eysel U. T. (2000). Membrane properties and spike generation in rat visual cortical cells during reversible cooling. J. Physiol. 522, 59–76. 10.1111/j.1469-7793.2000.0059m.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss B., Thienel R., Reske M., Kellermann T., Sheldrick A. J., Halfter S., et al. (2012). Cholinergic blockade under working memory demands encountered by increased rehearsal strategies: evidence from fMRI in healthy subjects. Eur. Arch. Psychiatry Clin. Neurosci. 262, 329–339. 10.1007/s00406-011-0267-6 [DOI] [PubMed] [Google Scholar]
- Vyazovskiy V. V., Olcese U., Hanlon E. C., Nir Y., Cirelli C., Tononi G. (2011). Local sleep in awake rats. Nature 472, 443–447. 10.1038/nature10009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T. D., Atlas L. Y., Lindquist M. A., Roy M., Woo C. W., Kross E. (2013). An fMRI-based neurologic signature of physical pain. N. Engl. J. Med. 368, 1388–1397. 10.1056/NEJMoa1204471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Foniok T., Wamsteeker J. I., Qiao M., Tomanek B., Vivanco R. A., et al. (2006). Transient blood pressure changes affect the functional magnetic resonance imaging detection of cerebral activation. NeuroImage 31, 1–11. 10.1016/j.neuroimage.2005.12.004 [DOI] [PubMed] [Google Scholar]
- Wang R., Macmillan L. B., Fremeau R. T., Jr., Magnuson M. A., Lindner J., Limbird L. E. (1996). Expression of α2-adrenergic receptor subtypes in the mouse brain: evaluation of spatial and temporal information imparted by 3 kb of 5′ regulatory sequence for the α 2A AR-receptor gene in transgenic animals. Neuroscience 74, 199–218. 10.1016/0306-4522(96)00116-9 [DOI] [PubMed] [Google Scholar]
- Warnaby C. E., Seretny M., Ni Mhuircheartaigh R., Rogers R., Jbabdi S., Sleigh J., et al. (2016). Anesthesia-induced suppression of human dorsal anterior insula responsivity at loss of volitional behavioral response. Anesthesiology 124, 766–778. 10.1097/aln.0000000000001027 [DOI] [PubMed] [Google Scholar]
- Weber F., Dan Y. (2016). Circuit-based interrogation of sleep control. Nature 538, 51–59. 10.1038/nature19773 [DOI] [PubMed] [Google Scholar]
- Wilding L. A., Hampel J. A., Khoury B. M., Kang S., Machado-Aranda D., Raghavendran K., et al. (2017). Benefits of 21% oxygen compared with 100% oxygen for delivery of isoflurane to mice (Mus musculus) and rats (Rattus norvegicus). J. Am. Assoc. Lab. Anim. Sci. 56, 148–154. [PMC free article] [PubMed] [Google Scholar]
- Williams R. H., Jensen L. T., Verkhratsky A., Fugger L., Burdakov D. (2007). Control of hypothalamic orexin neurons by acid and CO2. Proc. Natl. Acad. Sci. U S A 104, 10685–10690. 10.1073/pnas.0702676104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer R. D., Astori S., Bond C. T., Rovó Z., Chatton J. Y., Adelman J. P., et al. (2012). Sustaining sleep spindles through enhanced SK2-channel activity consolidates sleep and elevates arousal threshold. J. Neurosci. 32, 13917–13928. 10.1523/jneurosci.2313-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler S. A., Schmitt F., Landes H., de Bever J., Wade T., Alejski A., et al. (2018). Gradient and shim technologies for ultra high field MRI. NeuroImage 168, 59–70. 10.1016/j.neuroimage.2016.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo C. W., Schmidt L., Krishnan A., Jepma M., Roy M., Lindquist M. A., et al. (2017). Quantifying cerebral contributions to pain beyond nociception. Nat. Commun. 8:14211. 10.1038/ncomms14211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T., Grandjean J., Bosshard S. C., Rudin M., Reutens D., Jiang T. (2017). Altered regional connectivity reflecting effects of different anaesthesia protocols in the mouse brain. NeuroImage 149, 190–199. 10.1016/j.neuroimage.2017.01.074 [DOI] [PubMed] [Google Scholar]
- Xu F., Liu N., Kida I., Rothman D. L., Hyder F., Shepherd G. M. (2003). Odor maps of aldehydes and esters revealed by functional MRI in the glomerular layer of the mouse olfactory bulb. Proc. Natl. Acad. Sci. U S A 100, 11029–11034. 10.1073/pnas.1832864100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F., Schaefer M., Kida I., Schafer J., Liu N., Rothman D. L., et al. (2005). Simultaneous activation of mouse main and accessory olfactory bulbs by odors or pheromones. J. Comp. Neurol. 489, 491–500. 10.1002/cne.20652 [DOI] [PubMed] [Google Scholar]
- Yli-Hankala A., Lindgren L., Porkkala T., Jantti V. (1993). Nitrous oxide-mediated activation of the EEG during isoflurane anaesthesia in patients. Br. J. Anaesth. 70, 54–57. 10.1093/bja/70.1.54 [DOI] [PubMed] [Google Scholar]
- Yoshida K., Mimura Y., Ishihara R., Nishida H., Komaki Y., Minakuchi T., et al. (2016). Physiological effects of a habituation procedure for functional MRI in awake mice using a cryogenic radiofrequency probe. J. Neurosci. Methods 274, 38–48. 10.1016/j.jneumeth.2016.09.013 [DOI] [PubMed] [Google Scholar]
- Yuste R. (2015). From the neuron doctrine to neural networks. Nat. Rev. Neurosci. 16, 487–497. 10.1038/nrn3962 [DOI] [PubMed] [Google Scholar]
- Záborszky L., Gombkoto P., Varsanyi P., Gielow M. R., Poe G., Role L. W., et al. (2018). Specific basal forebrain-cortical cholinergic circuits coordinate cognitive operations. J. Neurosci. 38, 9446–9458. 10.1523/jneurosci.1676-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman T., Lee K., Park C., Paydar A., Choi J. H., Cheong E., et al. (2011). Cav2.3 channels are critical for oscillatory burst discharges in the reticular thalamus and absence epilepsy. Neuron 70, 95–108. 10.1016/j.neuron.2011.02.042 [DOI] [PubMed] [Google Scholar]
- Zandieh S., Hopf R., Redl H., Schlag M. G. (2003). The effect of ketamine/xylazine anesthesia on sensory and motor evoked potentials in the rat. Spinal Cord 41, 16–22. 10.1038/sj.sc.3101400 [DOI] [PubMed] [Google Scholar]
- Zeman A. (2004). Theories of visual awareness. Prog. Brain Res. 144, 321–329. 10.1016/s0079-6123(03)14422-6 [DOI] [PubMed] [Google Scholar]
- Zerbi V., Floriou-Servou A., Markicevic M., Vermeiren Y., Sturman O., Privitera M., et al. (2019). Rapid reconfiguration of the functional connectome after chemogenetic locus coeruleus activation. Neuron 103, 702.e5–718.e5. 10.1016/j.neuron.2019.05.034 [DOI] [PubMed] [Google Scholar]
- Zerbi V., Grandjean J., Rudin M., Wenderoth N. (2015). Mapping the mouse brain with rs-fMRI: an optimized pipeline for functional network identification. NeuroImage 123, 11–21. 10.1016/j.neuroimage.2015.07.090 [DOI] [PubMed] [Google Scholar]
- Zhang R. V., Featherstone R. E., Melynchenko O., Gifford R., Weger R., Liang Y., et al. (2019). High-beta/low-gamma frequency activity reflects top-down predictive coding during a spatial working memory test. Exp. Brain Res. 237, 1881–1888. 10.1007/s00221-019-05558-3 [DOI] [PubMed] [Google Scholar]
- Zhang S., Hu S., Hu J., Wu P. L., Chao H. H., Li C. S. (2015). Barratt impulsivity and neural regulation of physiological arousal. PLoS One 10:e0129139. 10.1371/journal.pone.0129139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Xu M., Kamigaki T., Hoang Do J. P., Chang W.-C., Jenvay S., et al. (2014). Long-range and local circuits for top-down modulation of visual cortex processing. Science 345, 660–665. 10.1126/science.1254126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Yoshida T., Katz D. B., Lisman J. E. (2012). NMDAR antagonist action in thalamus imposes delta oscillations on the hippocampus. J. Neurophysiol. 107, 3181–3189. 10.1152/jn.00072.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F., Zhao T., Zhou L., Wu Q., Hu X. (2008). BOLD study of stimulation-induced neural activity and resting-state connectivity in medetomidine-sedated rat. NeuroImage 39, 248–260. 10.1016/j.neuroimage.2007.07.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Zhang Q., Martinez E., Dale J., Hu S., Zhang E., et al. (2018). Ketamine reduces aversion in rodent pain models by suppressing hyperactivity of the anterior cingulate cortex. Nat. Commun. 9:3751. 10.1038/s41467-018-06295-x [DOI] [PMC free article] [PubMed] [Google Scholar]