Abstract
We wanted to find the intensity of exercise that could increase brain- derived neurotrophic factor (BDNF) expression and improve spatial learning and memory without dietary control. C57BL/6 mice were fed a 60% high-fat diet (HFD) for 6 weeks to induce obesity. Obesity-induced mice were exercised on a treadmill for 8 weeks at various exercise in-tensities: HFD-control (n=7), HFD-low-intensity exercise (HFD-LIE, n= 7, 12 m/min for 75 min), HFD-middle intensity exercise (HFD-MIE, n=7, 15 m/min for 60 min) and HFD-high-intensity exercise (HFD-HIE, n=7, 18 m/min for 50 min). One week before sacrificing mice, the Morris wa-ter maze test was performed, and the hippocampus was immediately removed after sacrifice. The expression levels of BDNF (encoded by the gene Bdnf) and tropomyosin receptor kinase B (TrkB) in the hippo-campus were analyzed by quantitative real-time reverse transcription- polymerase chain reaction and western blot. In the last probe test of the Morris water maze test, occupancy in the target quadrant was sig-nificantly higher in the HFD-HIE group (P<0.05) than in the other groups. In addition, mRNA expression from the Bdnf promoter region was found to be significantly higher in the HFD-HIE group than in the other groups (P<0.001). Although there were some differences in the levels of signifi-cance, the expression levels of both BDNF and TrkB were significantly higher in the HFD-HIE group than in the other groups. Therefore, rela-tively high-intensity aerobic exercise can resist the adverse effects of a high-fat diet on the brain without dietary control.
Keywords: Obesity, High-fat diet, Exercise, Hippocampus, Brain-derived neurotrophic factor, Spatial learning
INTRODUCTION
Obesity is a global epidemic and is a risk factor that increases the risk of chronic diseases such as type II diabetes mellitus (Mokdad et al., 2003), nonalcoholic fatty liver disease (Loomba and Sanyal, 2013; Younossi et al., 2011), hypertension (Younossi et al., 2011), and stroke (Greenlund et al., 2004). Obesity was also found to be closely related to a decline in cognitive function. Animal studies have shown that obese mice have impaired learning and memory abilities (Farr et al., 2008; Winocur et al., 2005), and have higher cognitive impairments than wild type mice, especially in obese rats induced by high-fat diets (HFDs) (Farr et al., 2008; Greenwood and Winocur, 1996; Molteni et al., 2002; Stranahan et al., 2008; Winocur and Greenwood, 2005; Winocur et al., 2005). HFD-induced obese mice have also been shown to have brain insulin resistance and cognitive impairment (Kothari et al., 2017).
Increasing insulin resistance, oxidative stress, and inflammation caused by HFDs may decrease cognitive function (Freeman et al., 2014). Brain-derived neurotrophic factor (BDNF), which promotes the hippocampal neurogenesis responsible for memory and learning, decreases in expression by insulin resistance (Stranahan et al., 2008), oxidative stress (Wu et al., 2004), and inflammation (Barrientos et al., 2004; Murray and Lynch, 1998), and eventually decreases neurogenesis and brain plasticity. This can lead to a decrease in cognitive function. Therefore, the regulation of hippocampal BDNF expression in the brain may be an important factor to prevent cognitive decline by HFD.
Exercise is known to increase hippocampal BDNF expression, thereby increasing brain health and plasticity and protecting brain function (Cotman and Berchtold, 2002; Cotman and Engesser-Cesar, 2002). In addition, exercise has been reported to offset the detrimental effects of HFDs in rats by inducing hippocampal BDNF expression (Molteni et al., 2004). In another study, treadmill exercise was performed on rats fed an HFD, and the exercise was found to counteract the decrease in brain health caused by the HFD (Woo et al., 2013). This evidence supports the exercise’s ability to prevent degenerative changes in the brain resulting from obesity.
There is much evidence that exercise can rescue the harmful effects of HFD on the brain (Molteni et al., 2004; Woo et al., 2013). However, exercise uses different energy substrates according to exercise intensity (Gollnick, 1985), and physiological and inflammatory responses to the whole body after exercise (Bessa et al., 2016; Borsheim and Bahr, 2003). Indeed, it is known that the amount of exercise, type of exercise, and exercise intensity are related to BDNF and TrkB expression in the hippocampus (Berchtold et al., 2005). Most studies in nonobese subjects have reported higher BDNF and TrkB expression during low-intensity exercise than high-intensity exercise (Ghodrati-Jaldbakhan et al., 2017; Shih et al., 2013). Some studies have reported that high-intensity interval training is more effective for BDNF expression in the hippocampus than continuous low-intensity exercise (Freitas et al., 2018; Naghibzadeh et al., 2018). However, no studies have been conducted to determine which exercise intensity was most effective in improving BDNF and TrkB expression and cognitive function by performing tests with various exercise intensities with HFDs. Therefore, we wanted to identify what intensity of exercise would be effective to counteract the adverse effects of HFDs without dietary restrictions.
MATERIALS AND METHODS
Animals
Six-weeks-old male C57BL/6J mice (n=28) were purchased from Samtako (Daejeon, Korea). The mice were kept in a room maintained at 24°C with 50%–60% relative humidity and a 12-hr light/12-hr dark cycle. All animals were fed an HFD containing 60% fat (D12492, Research Diets, New Brunswick, NJ, USA) for 6 weeks. After 6 weeks of HFD intake, the animals were divided into HFD-control (n=7), HFD-low-intensity exercise (HFD-LIE, n=7), HFD-middle intensity exercise (HFD-MIE, n=7), and HFD-high-intensity exercise (HFD-HIE, n=7) groups. All animals maintained an HFD intake during the exercise intervention period. The duration of exercise intervention was 8 weeks. After the intervention period, brains were immediately extracted after inhalation anesthesia with CO2 gas. The hippocampus was separated with a spatula on a chilled stainless-steel block. All experimental protocols were approved by the Institutional Animal Care and Use and the Committee of Pusan National University (approval number, PNU-2018-1886).
Exercise protocol
All exercise groups were subjected to exercise on an animal treadmill (DJ-344, Daejong Instrument Industry, Daejeon, Korea). In week 1 of intervention, exercise was conducted at the same intensity for all mice (10 m/min for 30 min). Different intensities were applied to each group from week 2–8, and the running time was adjusted so that the total amount of exercise was equal for all exercise groups (total distance=900 m): HFD-LIE, 12 m/min for 75 min; HFD-MIE, 15 m/min for 60 min; HFD-HIE, 18 m/min for 50 min; average 15 m/min for 60 min. The exercise intensities mentioned in our study are all relative intensities in this study. Exercise training was conducted 5 times per week between the hours of 6:00 and 7:00 p.m. As control, nonexercising animals were placed on an idle treadmill for the same time period. Before each exercise training session, all running mice were allowed a 5-min warm-up phase with a slow increase in speed.
Morris water maze test
A mice water maze pool (90-cm diameter; sidewalls 45-cm height) was filled with warm water (26°C±1°C) in which a submerged platform was located (1 cm below water surface; 6-cm diameter in a fixed position). For each trial, the mice were placed in the water at one of four equidistant locations. The platform was recognized in clear water before testing. From day 1 of the test to day 3, white nontoxic water paints were dissolved in water 3 times a day to make the platform invisible, and then the time to visit the platform was measured for 60 sec. If the animal failed to find the platform, it was guided there and allowed to stay at the platform for 15 sec. After the 3-day trial, the probe test was performed the next day. The probe test was carried out for 1 min, and the time spent in the quadrant where the original platform located after removing the platform was measured. All data were derived by analyzing the video tracking system (SMART 3.0, Panlab, Barcelona, Spain).
Quantitative real-time reverse transcription-polymerase chain reaction (qPCR)
The hippocampus was homogenized using the TissueLyser LT magnetic bead homogenizer (Qiagen, Hilden, Germany) with 50-mm stainless-steel beads for 5 min at 50 Hz. Total RNA was isolated using TRIzol reagent (Life Technologies, Carlsbad, CA, USA), and cDNA was synthesized from RNA using the M-MLV RT reaction buffer (Promega, Madison, WI, USA) according to the manufacturer’s instructions. The cDNA was mixed with forward and reverse primers for Bdnf coding (forward: GCGGCA-GATAAAAAGACTGC; reverse: GCAGCCTTCCTTGGTGTAAC), and Bdnf promoter (forward: GCCTTCATGCAACCGAA-GTA; reverse: CAGGACAGCAAAGCCACAAT) (Sleiman et al., 2016) in a 96-well plate containing AMPIGENE qPCR Green Mix Lo-ROX (Enzo Life Science, Farmingdale, NY, USA). To normalize mRNA levels among samples, Actb (forward: CCAACC-GTGAAAAGATGACC; reverse: CCATCACAATGCCTGTGGTA) was used as a housekeeping gene. Each sample was examined in triplicate. Sequences of all primers were entered 5′ to 3′ order. The reaction was performed using a LightCycler 96 real-time PCR system (Roche Diagnostics, Basel, Switzerland). The cycle threshold (Ct) values measured after the experiment were analyzed by the relative quantification 2−ΔΔC(T) method.
Western blot
Western blotting for TrkB and BDNF protein was performed. The hippocampal tissues were homogenized on ice and lysed in a lysis buffer containing 50 mM HEPES (pH, 7.5), 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 1 mM EGTA, 1.5 mM MgCl2·6H2O, 1 mM sodium orthovanadate, and 100 mM sodium fluoride. Protein content was measured using a Bio-Rad colorimetric protein assay kit (Bio-Rad, Hercules, CA, USA). Protein samples (30 μg) were separated on sodium dodecyl sulfate-polyacrylamide gel and transferred onto a nitrocellulose membrane. The membranes were incubated with 5% skim milk in Tris-buffered saline containing 0.1% Tween-20 and then incubated overnight at 4°C with the following primary antibodies: rabbit anti-TrkB antibody (#SC-8316, 1:1,000; Santa Cruz Biotechnology, Dallas, TX, USA), rabbit anti-BDNF antibody (#SC-546, 1:1,000; Santa Cruz Biotechnology), and mouse anti-β-actin (#SC-47778, 1:1,000, Santa Cruz Biotechnology). Subsequently, membranes were incubated for 1 hr with appropriate secondary antibodies (1:2,000; Vector Laboratories, Burlingame, CA, USA), and band detection was performed using the enhanced chemiluminescence detection kit (Santa Cruz Biotechnology) (Ko et al., 2018).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5.0 software (GraphPad Software, San Diego, CA, USA). The qPCR results were compared using the independent samples t-test with the other groups relative to the HFD-control determined by the 2−ΔΔC(T) method. Western blot and Morris water maze results were analyzed using one-way analysis of variance and Bonferroni post hoc test. All values are expressed as mean±standard deviation. Statistically significant differences were established at P<0.05.
RESULTS
Relatively high-intensity exercise improved spatial learning and memory in obese mice despite sustained HFD
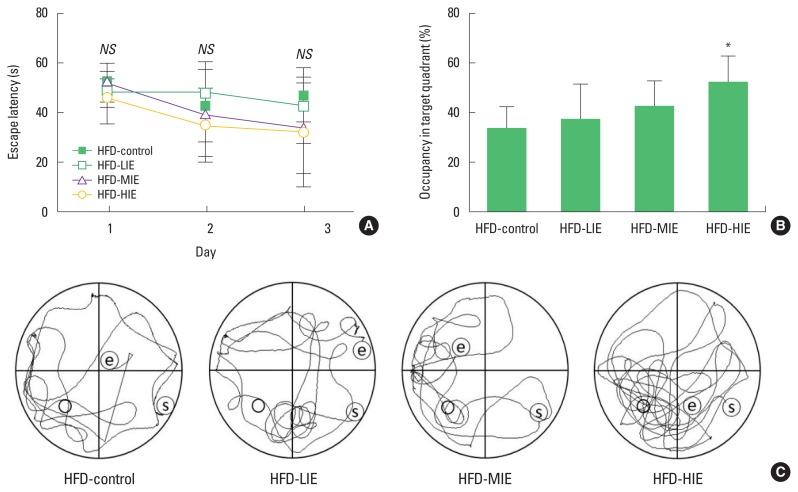
The platform arrival time (escape latency) of the mice was reduced during three platform cognitive training sessions per day, 3 days before the last probe test. However, the arrival time was reduced in all groups and no significant difference was observed between groups (Fig. 1A). In the probe test, a significant difference between the time spent on the target quadrant and the time spent in the remaining quadrants was identified only in the HFD-HIE group (P<0.05) (Fig. 1B, C). We visualized mouse movement paths over 1 min obtained in the video tracking system (data closest to the mean of each group) (Fig. 1C).
Fig. 1.
The Morris water maze test confirmed that relatively high-intensity exercise improved learning and memory in obese mice. (A) Changes in platform arrival time (escape latency) for all groups during training session. (B) Probe test confirmed that relatively high-intensity exercise improved spatial memory on obese mice. (C) Mouse swimming path during probe testing confirmed by video tracking system. Data are presented as mean±standard deviation. HFD, high-fat diet; LIE, low-intensity exercise; MIE, middle intensity exercise; HIE, high-intensity exercise. *P<0.05.
Relatively high-intensity exercise increased mRNA expression of hippocampal Bdnf in obese mice despite sustained HFD
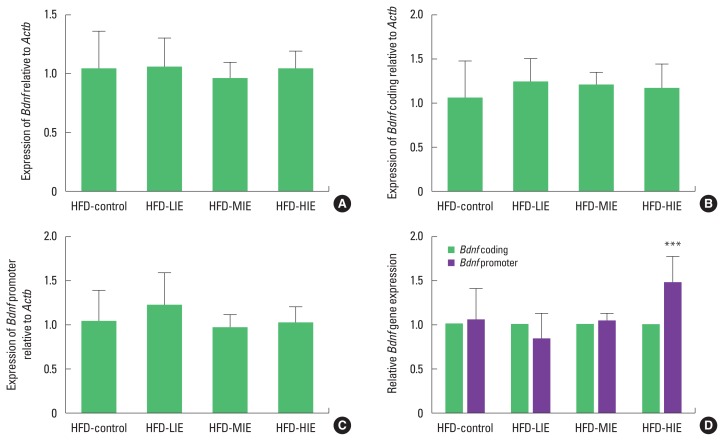
We tried to confirm expression with various primer combinations because Bdnf is regulated by alternative splicing (Aid et al., 2007). When Bdnf coding, and Bdnf promoter sequences were identified, there was no significant difference between the groups (Fig. 2A–C). However, we measured the relative expression level of the Bdnf promoter against Bdnf coding and found that the HFD-HIE group had higher Bdnf expression than the other groups (P< 0.001) (Fig. 2D).
Fig. 2.
Relatively high-intensity exercise increases mRNA expression of Bdnf in the hippocampus of obese mice. (A) Expression of Bdnf/Actb. (B) Expression of Bdnf coding gene/Actb. (C) Expression of Bdnf promoter gene/Actb. (D) Relatively high-intensity exercise increases Bdnf promoter expression compared to Bdnf coding gene. Data are presented as mean±standard deviation. HFD, high-fat diet; LIE, low-intensity exercise; MIE, middle intensity exercise; HIE, high-intensity exercise. ***P<0.001.
Relatively high-intensity exercise increased hippocampal BDNF protein expression despite sustained HFD
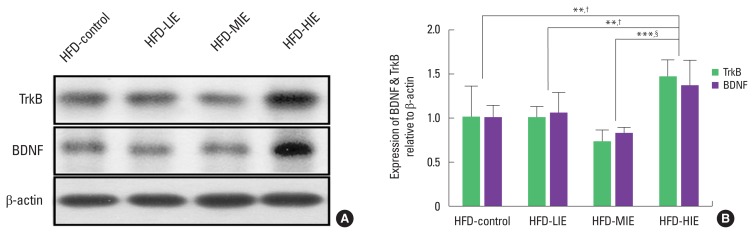
Both BDNF and TrkB expression levels were higher in the HFD-HIE group than in the other groups. In terms of TrkB, the HFD- control (P<0.01) and HFD-LIE groups (P<0.01) showed similar levels in comparison with HFD-HIE group, and the significance level of the HFD-MIE group (P<0.001) was larger than other exercise intensity groups (Fig. 3A, B). In the case of BDNF, the HFD- control and HFD-LIE groups showed similar levels to the HFD-HIE group (P<0.05), and the significance level of the HFD-MIE group was greater (P<0.001).
Fig. 3.
Relatively high-intensity exercise increases protein expression of tropomyosin receptor kinase B (TrkB) and brain-derived neurotrophic factor (BDNF). (A) Detected image of Western blot. (B) The protein expression of TrkB and BDNF was significantly higher than that of all other groups. Data are presented as mean±standard deviation. HFD, high-fat diet; LIE, low-intensity exercise; MIE, middle intensity exercise; HIE, high-intensity exercise. **P<0.01 and ***P<0.001 for TrkB. †P<0.05 and §P<0.001 for BDNF.
DISCUSSION
Obesity is widely known to have harmful effects on brain health. For example, epidemiologic evidence reported that Western diets were associated with a smaller hippocampus size, both intake and overweight (Cherbuin et al., 2015; Jacka et al., 2015). Animal studies have shown that HFD-induced obesity reduces BDNF expression (Liu et al., 2014; Molteni et al., 2002; Park et al., 2010) and neurogenesis (Park et al., 2010) and increases inflammation in the hippocampus (Liu et al., 2014).
Generally, the most effective treatment for obesity is the combination of proper exercise and caloric restriction (Reed et al., 2010; Swift et al., 2014). The effect of exercise that resists obesity is not only due to negative energy balance, but also due to various beneficial effects. Regardless of dietary control, exercise helps to repair defects in the AMPK (AMP-activated protein kinase) and SIRT-1 (sirtuin-1) pathways caused by obesity (de Las Heras et al., 2018), and it has additional effects such as improved insulin sensitivity (Goodyear and Kahn, 1998) and vascular health (Watts et al., 2004). However, opinions on good exercise intensity to treat obesity are not clear, due to the inflammatory response caused by exercise. From an immunological point of view, exercise depends on the intensity of the inflammatory response (Mackinnon and Hooper, 1994). Several recent studies have suggested that exercise may improve insulin sensitivity by increasing the polarization of M2 macrophages (M1 macrophages have the opposite effect) associated with anti-inflammatory cytokines in adipose tissue (Kawanishi et al., 2010; Oliveira et al., 2013). However, these studies also overlook differences in inflammatory responses according to exercise intensity. In general, high-intensity exercise acutely increases the inflammatory response, mainly due to inflammatory cytokines associated with the differentiation of M1 macrophages (Pedersen et al., 1998). Taken together, there is no confirmed difference in the inflammatory response in the hippocampus according to the difference in exercise intensity, as in adipose tissue.
In our study, although obese mice were sustained on an HFD, we found that regular high-intensity exercise improved spatial learning and memory as well as BDNF expression. Thus, the temporary increase in inflammatory response due to high-intensity aerobic exercise does not appear to additionally increase the inflammatory response in the hippocampus, but rather appears to reduce it. These results are expected to be similar in adipose tissue and should be confirmed in further studies. It is essential to clearly understand the meaning of “high-intensity exercise” mentioned in this study. The high-intensity exercise mentioned in this study is a word relative to the other exercise intensity groups set in this study: 50 min of exercise performed on a treadmill at a speed of 18 m/min. This is an intensity of exercise that can be performed regularly daily even in obese mice and does not mean high-resistance or exhaustive exercise. In our opinion, the high-intensity exercise protocol in this study may be close to a regular moderate exercise protocol in other many previous studies (Baek et al., 2018a; Baek et al., 2018b; Baek et al., 2019; Dos Santos et al., 2019). Therefore, in actual clinical applications, these facts must be reflected to set exercise intensity.
Several studies have shown that exercise can resist the deterioration of brain health caused by obesity, but independent effects of exercise and exercise intensity have not been identified. We report for the first time, in the case of obesity, that relatively high-intensity exercise can be effective in resisting the adverse effects of obesity on the brain, even without dietary control.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;85:525–535. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek KW, Cha HJ, Ock MS, Kim HS, Gim JA, Park JJ. Effects of regular-moderate exercise on high-fat diet-induced intramyocellular lipid accumulation in the soleus muscle of Sprague-Dawley rats. J Exerc Rehabil. 2018a;14:32–38. doi: 10.12965/jer.1835166.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek KW, Gim JA, Park JJ. Regular moderate aerobic exercise improves high-fat diet-induced nonalcoholic fatty liver disease via monoacylglycerol O-acyltransferase 1 pathway suppression. J Sport Health Sci. 2018b doi: 10.1016/j.jshs.2018.09.001. [Epub]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek KW, Jo JO, Kang YJ, Song KS, Yu HS, Park JJ, Choi YH, Cha HJ, Ock MS. Exercise training reduces the risk of opportunistic infections after acute exercise and improves cytokine antigen recognition. Pflugers Arch. 2020;472:235–244. doi: 10.1007/s00424-019-02281-4. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Sprunger DB, Campeau S, Watkins LR, Rudy JW, Maier SF. BDNF mRNA expression in rat hippocampus following contextual learning is blocked by intrahippocampal IL-1β administration. J Neuroimmunol. 2004;155:119–126. doi: 10.1016/j.jneuroim.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133:853–861. doi: 10.1016/j.neuroscience.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Bessa AL, Oliveira VN, Agostini GG, Oliveira RJ, Oliveira AC, White GE, Wells GD, Teixeira DN, Espindola FS. Exercise intensity and recovery: biomarkers of injury, inflammation, and oxidative stress. J Strength Cond Res. 2016;30:311–319. doi: 10.1519/JSC.0b013e31828f1ee9. [DOI] [PubMed] [Google Scholar]
- Borsheim E, Bahr R. Effect of exercise intensity, duration and mode on post-exercise oxygen consumption. Sports Med. 2003;33:1037–1060. doi: 10.2165/00007256-200333140-00002. [DOI] [PubMed] [Google Scholar]
- Cherbuin N, Sargent-Cox K, Fraser M, Sachdev P, Anstey KJ. Being overweight is associated with hippocampal atrophy: the PATH through life study. Int J Obes (Lond) 2015;39:1509–1514. doi: 10.1038/ijo.2015.106. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Engesser-Cesar C. Exercise enhances and protects brain function. Exerc Sport Sci Rev. 2002;30:75–79. doi: 10.1097/00003677-200204000-00006. [DOI] [PubMed] [Google Scholar]
- de Las Heras N, Klett-Mingo M, Ballesteros S, Martin-Fernandez B, Escribano O, Blanco-Rivero J, Balfagon G, Hribal ML, Benito M, Lahera V, Gomez-Hernandez A. Chronic exercise improves mitochondrial function and insulin sensitivity in brown adipose tissue. Front Physiol. 2018;9:1122. doi: 10.3389/fphys.2018.01122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos CMM, Diniz VLS, Bachi ALL, Dos Santos de Oliveira LC, Ghazal T, Passos MEP, de Oliveira HH, Murata G, Masi LN, Martins AR, Levada-Pires AC, Curi R, Hirabara SM, Sellitti DF, Pithon-Curi TC, Gorjao R. Moderate physical exercise improves lymphocyte function in melanoma-bearing mice on a high-fat diet. Nutr Metab (Lond) 2019;16:63. doi: 10.1186/s12986-019-0394-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr SA, Yamada KA, Butterfield DA, Abdul HM, Xu L, Miller NE, Banks WA, Morley JE. Obesity and hypertriglyceridemia produce cognitive impairment. Endocrinology. 2008;149:2628–2636. doi: 10.1210/en.2007-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman LR, Haley-Zitlin V, Rosenberger DS, Granholm AC. Damaging effects of a high-fat diet to the brain and cognition: a review of proposed mechanisms. Nutr Neurosci. 2014;17:241–251. doi: 10.1179/1476830513Y.0000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas DA, Rocha-Vieira E, Soares BA, Nonato LF, Fonseca SR, Martins JB, Mendonca VA, Lacerda AC, Massensini AR, Poortamns JR, Meeusen R, Leite HR. High intensity interval training modulates hippocampal oxidative stress, BDNF and inflammatory mediators in rats. Physiol Behav. 2018;184:6–11. doi: 10.1016/j.physbeh.2017.10.027. [DOI] [PubMed] [Google Scholar]
- Ghodrati-Jaldbakhan S, Ahmadalipour A, Rashidy-Pour A, Vafaei AA, Miladi-Gorji H, Alizadeh M. Low- and high-intensity treadmill exercise attenuates chronic morphine-induced anxiogenesis and memory impairment but not reductions in hippocampal BDNF in female rats. Brain Res. 2017;1663:20–28. doi: 10.1016/j.brainres.2017.02.024. [DOI] [PubMed] [Google Scholar]
- Gollnick PD. Metabolism of substrates: energy substrate metabolism during exercise and as modified by training. Fed Proc. 1985;44:353–357. [PubMed] [Google Scholar]
- Goodyear LJ, Kahn BB. Exercise, glucose transport, and insulin sensitivity. Annu Rev Med. 1998;49:235–261. doi: 10.1146/annurev.med.49.1.235. [DOI] [PubMed] [Google Scholar]
- Greenlund KJ, Croft JB, Mensah GA. Prevalence of heart disease and stroke risk factors in persons with prehypertension in the United States, 1999–2000. Arch Intern Med. 2004;164:2113–2118. doi: 10.1001/archinte.164.19.2113. [DOI] [PubMed] [Google Scholar]
- Greenwood CE, Winocur G. Cognitive impairment in rats fed high-fat diets: a specific effect of saturated fatty-acid intake. Behav Neurosci. 1996;110:451–459. doi: 10.1037//0735-7044.110.3.451. [DOI] [PubMed] [Google Scholar]
- Jacka FN, Cherbuin N, Anstey KJ, Sachdev P, Butterworth P. Western diet is associated with a smaller hippocampus: a longitudinal investigation. BMC Med. 2015;13:215. doi: 10.1186/s12916-015-0461-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanishi N, Yano H, Yokogawa Y, Suzuki K. Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat-diet-induced obese mice. Exerc Immunol Rev. 2010;16:105–118. [PubMed] [Google Scholar]
- Ko IG, Kim SE, Hwang L, Jin JJ, Kim CJ, Kim BK, Kim H. Late starting treadmill exercise improves spatial leaning ability through suppressing CREP/BDNF/TrkB signaling pathway following traumatic brain injury in rats. J Exerc Rehabil. 2018;14:327–334. doi: 10.12965/jer.1836248.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothari V, Luo Y, Tornabene T, O’Neill AM, Greene MW, Geetha T, Babu JR. High fat diet induces brain insulin resistance and cognitive impairment in mice. Biochim Biophys Acta Mol Basis Dis. 2017;1863:499–508. doi: 10.1016/j.bbadis.2016.10.006. [DOI] [PubMed] [Google Scholar]
- Liu Y, Fu X, Lan N, Li S, Zhang J, Wang S, Li C, Shang Y, Huang T, Zhang L. Luteolin protects against high fat diet-induced cognitive deficits in obesity mice. Behav Brain Res. 2014;267:178–188. doi: 10.1016/j.bbr.2014.02.040. [DOI] [PubMed] [Google Scholar]
- Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686–690. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- Mackinnon LT, Hooper S. Mucosal (secretory) immune system responses to exercise of varying intensity and during overtraining. Int J Sports Med. 1994;15( Suppl 3):S179–183. doi: 10.1055/s-2007-1021134. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- Molteni R, Barnard RJ, Ying Z, Roberts CK, Gomez-Pinilla F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience. 2002;112:803–814. doi: 10.1016/s0306-4522(02)00123-9. [DOI] [PubMed] [Google Scholar]
- Molteni R, Wu A, Vaynman S, Ying Z, Barnard RJ, Gomez-Pinilla F. Exercise reverses the harmful effects of consumption of a high-fat diet on synaptic and behavioral plasticity associated to the action of brain-derived neurotrophic factor. Neuroscience. 2004;123:429–440. doi: 10.1016/j.neuroscience.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Murray CA, Lynch MA. Evidence that increased hippocampal expression of the cytokine interleukin-1 beta is a common trigger for age- and stress-induced impairments in long-term potentiation. J Neurosci. 1998;18:2974–2981. doi: 10.1523/JNEUROSCI.18-08-02974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naghibzadeh M, Ranjbar R, Tabandeh MR, Habibi A. Effects of two training programs on transcriptional levels of neurotrophins and glial cells population in hippocampus of experimental multiple sclerosis. Int J Sports Med. 2018;39:604–612. doi: 10.1055/a-0608-4635. [DOI] [PubMed] [Google Scholar]
- Oliveira AG, Araujo TG, Carvalho BM, Guadagnini D, Rocha GZ, Bagarolli RA, Carvalheira JB, Saad MJ. Acute exercise induces a phenotypic switch in adipose tissue macrophage polarization in diet-induced obese rats. Obesity (Silver Spring) 2013;21:2545–2556. doi: 10.1002/oby.20402. [DOI] [PubMed] [Google Scholar]
- Park HR, Park M, Choi J, Park KY, Chung HY, Lee J. A high-fat diet impairs neurogenesis: involvement of lipid peroxidation and brain-derived neurotrophic factor. Neurosci Lett. 2010;482:235–239. doi: 10.1016/j.neulet.2010.07.046. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Ostrowski K, Rohde T, Bruunsgaard H. The cytokine response to strenuous exercise. Can J Physiol Pharmacol. 1998;76:505–511. doi: 10.1139/cjpp-76-5-505. [DOI] [PubMed] [Google Scholar]
- Reed JL, De Souza MJ, Williams NI. Effects of exercise combined with caloric restriction on inflammatory cytokines. Appl Physiol Nutr Metab. 2010;35:573–582. doi: 10.1139/H10-046. [DOI] [PubMed] [Google Scholar]
- Shih PC, Yang YR, Wang RY. Effects of exercise intensity on spatial memory performance and hippocampal synaptic plasticity in transient brain ischemic rats. PLoS One. 2013;8:e78163. doi: 10.1371/journal.pone.0078163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleiman SF, Henry J, Al-Haddad R, El Hayek L, Abou Haidar E, Stringer T, Ulja D, Karuppagounder SS, Holson EB, Ratan RR, Ninan I, Chao MV. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. Elife. 2016;5:e15092. doi: 10.7554/eLife.15092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Norman ED, Lee K, Cutler RG, Telljohann RS, Egan JM, Mattson MP. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus. 2008;18:1085–1088. doi: 10.1002/hipo.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift DL, Johannsen NM, Lavie CJ, Earnest CP, Church TS. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis. 2014;56:441–447. doi: 10.1016/j.pcad.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts K, Beye P, Siafarikas A, Davis EA, Jones TW, O’Driscoll G, Green DJ. Exercise training normalizes vascular dysfunction and improves central adiposity in obese adolescents. J Am Coll Cardiol. 2004;43:1823–1827. doi: 10.1016/j.jacc.2004.01.032. [DOI] [PubMed] [Google Scholar]
- Winocur G, Greenwood CE. Studies of the effects of high fat diets on cognitive function in a rat model. Neurobiol Aging. 2005;26( Suppl 1):46–49. doi: 10.1016/j.neurobiolaging.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Winocur G, Greenwood CE, Piroli GG, Grillo CA, Reznikov LR, Reagan LP, McEwen BS. Memory impairment in obese Zucker rats: an investigation of cognitive function in an animal model of insulin resistance and obesity. Behav Neurosci. 2005;119:1389–1395. doi: 10.1037/0735-7044.119.5.1389. [DOI] [PubMed] [Google Scholar]
- Woo J, Shin KO, Park SY, Jang KS, Kang S. Effects of exercise and diet change on cognition function and synaptic plasticity in high fat diet induced obese rats. Lipids Health Dis. 2013;12:144. doi: 10.1186/1476-511X-12-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A, Ying Z, Gomez-Pinilla F. The interplay between oxidative stress and brain-derived neurotrophic factor modulates the outcome of a saturated fat diet on synaptic plasticity and cognition. Eur J Neurosci. 2004;19:1699–1707. doi: 10.1111/j.1460-9568.2004.03246.x. [DOI] [PubMed] [Google Scholar]
- Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, Srishord M. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9:524–530. doi: 10.1016/j.cgh.2011.03.020. [DOI] [PubMed] [Google Scholar]