Abstract
Glucose is the primary osmotic medium used in most peritoneal dialysis (PD) solutions, and long-term exposure to high glucose is a major contributor to peritoneal fibrosis. Our previous study revealed that M2 macrophages participate in the development of PD-related fibrosis in a rat model. In the present study, the effects of high glucose on peritoneal macrophage polarization in vivo and in vitro were further evaluated. Continuous ambulatory PD (CAPD) patients with an overnight dwell of 1.5 or 2.5% glucose dialysate were recruited for this study. Overnight effluent samples from patients with CAPD (2,000 ml) were centrifuged to collect cells from the peritoneal cavity. J774A.1 cells (murine macrophages from ascites) were cultured in different concentrations of glucose. Macrophage phenotype markers were detected by flow cytometry. The levels of cytokines in PD effluent and the supernatant of murine macrophages were detected by enzyme-linked immunosorbent assays. The activity of arginase was determined by quantitative colorimetric analysis. In total, 107 CAPD subjects (92 patients using 1.5% glucose dialysate and 15 patients using 2.5% glucose dialysate) were recruited. The percentage of M1 macrophages (CD14- and CCr7-positive cells) in the 1.5 and 2.5% glucose dialysate groups was 23.0±13.3 and 24.9±12.0%, respectively. The difference was not significant (P>0.05). The percentage of M2 macrophages (CD14- and CD206-positive cells) in the 1.5% glucose dialysate group (36.2±11.4%) was significantly decreased compared to the 2.5% glucose dialysate group (43.2±7.4%) (P<0.05). Murine macrophages were cultured in a high-glucose in vitro environment, and the percentage of M1 macrophages in 138.8 mmol/l glucose medium significantly increased over time. The percentage of M2 macrophages increased in a glucose concentration-dependent and time-dependent manner. Arginase 1 in murine macrophages and the level of transforming growth factor β1 in the supernatant increased in a glucose concentration-dependent manner. In conclusion, high glucose contributed to the polarization of peritoneal macrophages to the M2 phenotype, which may play an important role in the pathogenesis of PD-related fibrosis.
Keywords: high glucose, M1 macrophages, M2 macrophages, peritoneal fibrosis, transforming growth factor-β1
Introduction
Peritoneal dialysis (PD) is widely used for the treatment of end-stage renal disease (ESRD) (1). Glucose is the primary osmotic medium used in most PD solutions, and most PD patients undergo ultrafiltration through the use of high concentrations of glucose. Long-term exposure to a high concentration of glucose alters the structure and function of the peritoneal membrane, which may result in the development of peritoneal fibrosis and eventually cause peritoneal failure in PD patients (2,3). Certain studies have revealed that the immune function of the peritoneal cavity may be crucial for the development of PD-related perineal fibrosis (4,5).
Macrophages are ubiquitous tissue-resident components under homeostatic physiological conditions and are thought to serve as the first line of defence against infection (6). However, an increasing number of studies have revealed that they are a diverse set of cells polarized by different T-cell responses and cytokine environments. Generally, macrophages can be divided into classically activated macrophages (M1) and alternatively activated macrophages (M2) (7). M1 macrophages can be induced by Th1 cytokines, including interferon (IFN)-γ and lipopolysaccharide (LPS), which can phagocytose and destroy microbes, kill tumour cells and present antigens to T cells to evoke an adaptive immune response (8,9).
In contrast, M2 macrophages are induced in response to stimulation with IL-4, IL-10, and IL-13. Macrophages of this phenotype upregulate the surface levels of mannose (CD206), scavenger (CD204) and galactose-type receptors involved in debris clearance. Furthermore, M2 macrophages constitutively produce functional cytokines [including transforming growth factor β (TGF-β)] and express the enzyme Arginase 1 (Arg1) (10,11). Through the cytokines and enzyme pathways mentioned aforementioned, M2 macrophages may be involved in the downregulation of inflammation, the promotion of tumour growth, long-term tissue repair and tissue fibrosis. Our previous study also revealed that M2 macrophages contributed to the development of PD-related peritoneal fibrosis in a rat model (12).
However, the differentiation of macrophages in high glucose is not well understood. It is hypothesized that macrophages will polarize to the M2 phenotype in a high glucose environment. To test this hypothesis, the percentage of M1 and M2 macrophages in the 1.5 or 2.5% glucose dialysates of patients with CAPD was detected and the effect of high glucose on the phenotypic polarization of cultured murine macrophages from ascites was investigated. The functions of the cytokine TGF-β1 and the enzyme Arg1 were also detected.
Materials and methods
Subjects
The study was approved by the Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University. Written informed consent was obtained from all participating subjects. A total of 107 patients with CAPD (age, ≥18 years; age range, 22–76 years; mean age, 50.94 years) were recruited from the First Affiliated Hospital of Sun Yat-sen University between January 2010 and March 2011, including 58 male patients and 49 female patients. The subjects were patients maintained on CAPD without peritonitis, another active infection or evident inflammation.
Sample collection
The night before the study visit, patients were instilled with 2,000 ml of PD solution (1.5% glucose dialysate or 2.5% glucose dialysate) for an overnight dwell (10 h). Effluent samples from patients with CAPD were centrifuged at 1,500 × g for 15 min at 4°C, and the cell sediment was washed twice with PBS. Subsequently, the cell concentration was adjusted to 1×106 cells/ml for flow cytometry. The supernatant of the dialysis effluent was stored at −80°C immediately to detect cytokines by ELISA.
Cell culture
J774A.1 cells, murine macrophages from ascites, were obtained from the American Type Culture Collection. Cells were maintained in RPMI-1640 media (Sigma-Aldrich; Merck KGaA) supplemented with 10% heat-inactivated foetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) at 37°C with 5% CO2 in 6-well cell culture plates (3 cm/well) until they formed a confluent monolayer.
Determination of viable cell number
A total of 2×106 cells/ml cells were incubated with 0.2 mg/ml MTT to determine cell viability. Following the MTT incubation, the purple formazan crystals were dissolved using DMSO and viability was assessed at a wavelength of 560 nm using an Infinite F50 R plate reader (Tecan Group, Ltd.).
Flow cytometry
To identify and measure the proportion of peritoneal cell types, markers on peritoneal macrophages from patients with CAPD were measured by triple colour flow cytometry using a FACSort flow cytometer (Beckman Coulter, Inc.). Anti-CD14 (clone M5E2; cat. no. 550787; 1:100; BD Biosciences), anti-CCr7 (clone 3D12; cat. no. 552176; 1:50; BD Biosciences) and anti-CD206 (clone 19.2; cat. no. 551135; 1:50; BD Biosciences) antibodies were labelled with PerCP, PE, and FITC, respectively. Class-matched isotype immunoglobulin PerCP, PE and FITC-conjugated negative control monoclonal antibodies were added to individual tubes for all samples to identify nonspecific binding. The broad macrophage gate was refined by plotting side scatter against CD14. In patients with CAPD, CD14- and CCr7-positive cells were considered M1 macrophages, and CD14- and CD206-positive cells were considered M2 macrophages. Murine macrophages (J774A.1 cells) cultured in different concentrations of glucose were considered M1 or M2 cells based on the expression of CCr7 or CD206, respectively. The flow cytometry data were analysing using WinMDI software (version 2.9; The Scripps Research Institute).
ELISA
The cytokine profiles of the dialysates of patients with CAPD were determined using ELISA kits, including IFN-γ (cat. no. BMS228), IL-2 (cat. no. BMS221-2), IL-4 (BMS225-2), and IL-13 (BMS231-3; eBioscience; Thermo Fisher Scientific, Inc.), according to the manufacturer's instructions. The concentrations of cytokines driving M2 in dialysate (including IL-4 and IL-13) could not be detected by ELISA, possibly because the concentration was too low in the dialysis effluent.
Arginase activity
The activity of murine macrophage Arg1 was determined in the aforementioned in vivo experiments using the QuantiChrom Arginase assay kit (cat. no. DARG-200; BioAssay Systems), according to the manufacturer's protocol, which measures the conversion of arginine into urea by arginase. Murine macrophages were lysed for 10 min at 4°C in 500 µl Tris-HCl (10 mM; pH 7.4) containing 1 µM pepstatin A, 1 µM leupeptin, and 0.4% (w/v) Triton X-100. Protein concentration was quantified using the bicinchoninic acid protein assay (Pierce Biotechnology; Thermo Fisher Scientific, Inc.), and the protein samples were standardized to the same concentration. Samples (40 µl) were incubated with 10 µl arginine buffer at 37°C for 2 h. Urea detection reagent containing anti-isonitrosopropiophenone was added and incubated at room temperature for 60 min. Subsequently, the optical density (OD) was measured at a wavelength of 430 nm. The readings were standardized to total protein against the OD of the control well of each sample (reaction without the incubation step).
Statistical analysis
Statistical analyses were performed using SPSS (version 20.0; IBM Corp.). Data are presented as the mean ± SE. The Kolmogorov-Smirnov (K-S) test was used to analyse normality, and variance analysis was used to test variance equality before analysis by t-test. Comparisons between groups were performed using the independent sample Student's t-test and paired sample Student's t-test. One-way ANOVA followed by the LSD post hoc test was used for multiple groups. The χ2 was used to analyse nominal variables. P<0.05 was considered to indicate a statistically significant difference.
Results
Percentage of M1 and M2 macrophages in 1.5 and 2.5% glucose dialysates in an overnight dwell
To evaluate the difference in macrophage phenotypes between patients using 1.5% glucose solution and those using 2.5% glucose solution, the expression of surface markers on macrophages in overnight dialysates of PD patients was examined. In total, 107 CAPD subjects (92 patients using 1.5% glucose and 15 patients using 2.5% glucose) were recruited. Patient characteristics, including age, sex, and biological and haematological data, are listed in Table I. Compared to patients using 1.5% glucose dialysate, patients using 2.5% glucose dialysate exhibited a longer follow-up duration, a higher ratio of diabetes, and lower levels of haemoglobin, haemotocrit, and albumin. However, there were no differences in these clinical characteristics between the two groups (P>0.05).
Table I.
Clinical characteristics of patients using 1.5 and 2.5% glucose dialysates.
| Characteristics | Patients using 1.5% glucose dialysate (n=92) | Patients using 2.5% glucose dialysate (n=15) | P-value |
|---|---|---|---|
| Age (years) | 50.9±14.8 | 51.2±12.4 | 0.95 |
| Sex (male:female) | 50:42 (54.3:45.7%) | 8:7 (53.3:46.7%) | 0.69 |
| Follow-up duration (months) | 18.7±22.5 | 21.5±24.7 | 0.62 |
| Diabetes mellitus | 17 (20.1%) | 4 (28.6%) | 0.83 |
| White blood cell count (109) | 7.5±2.5 | 7.1±2.1 | 0.86 |
| Percentage of neutrophils (%) | 65.8±8.0 | 65.2±10.2 | 0.52 |
| Haemoglobin (g/l) | 104.3±19.4 | 101.4±12.3 | 0.65 |
| Haemotocrit (%) | 31.7±5.7 | 30.7±4.2 | 0.62 |
| Albumin (g/l) | 38.1±4.8 | 35.5±4.3 | 0.09 |
| Blood urine nitrogen (mmol/l) | 18.6±6.7 | 19.1±5.4 | 0.80 |
| Creatinine (µmol/l) | 986.9±334.5 | 1,039.5±367.8 | 0.65 |
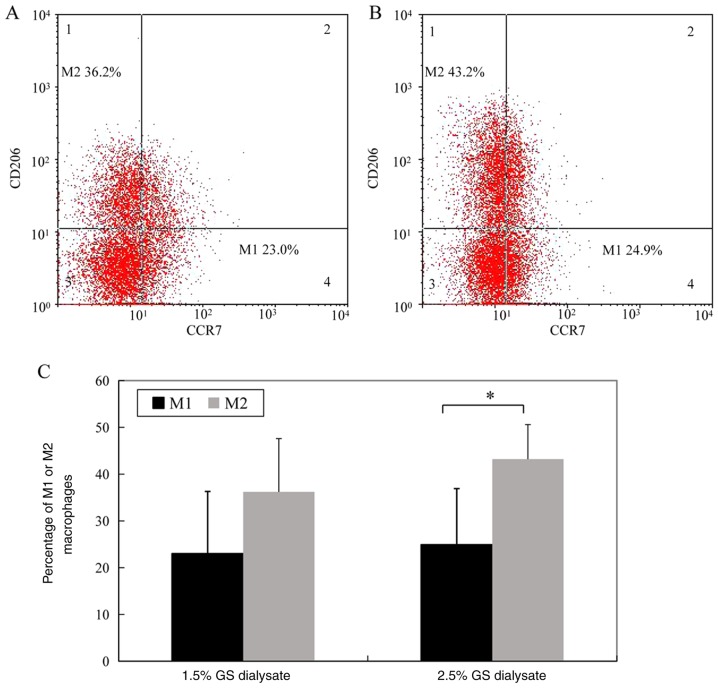
The percentage of M1 macrophages (CD14- and CCr7-positive cells) in the 1.5% glucose solution group and the 2.5% glucose dialysis solution group was 23.0±13.3 and 24.9±12.0%, respectively. The difference between the two groups was not significant. However, the percentage of M2 macrophages (CD14- and CD206-positive cells) in the 1.5% glucose solution group was significantly decreased compared 2.5% glucose solution group (36.2±11.4 vs. 43.2±7.4%, P<0.05) (Fig. 1; the cells in Fig. 1 were gated by CD14 as total macrophages).
Figure 1.
The percentage of M1 and M2 macrophages in 1.5 and 2.5% glucose dialysates of an overnight dwell. M1 and M2 phenotypes were detected by flow cytometry for patients using (A) 1.5% glucose, and (B) 2.5% glucose. (C) Statistical analysis of the percentage of M1 and M2 macrophages were compared between patients using 1.5 and 2.5% glucose dialysates. Each bar represents the mean ± SEM. *P<0.05 vs. M2. GS, glucose.
Determination of the number of viable cells
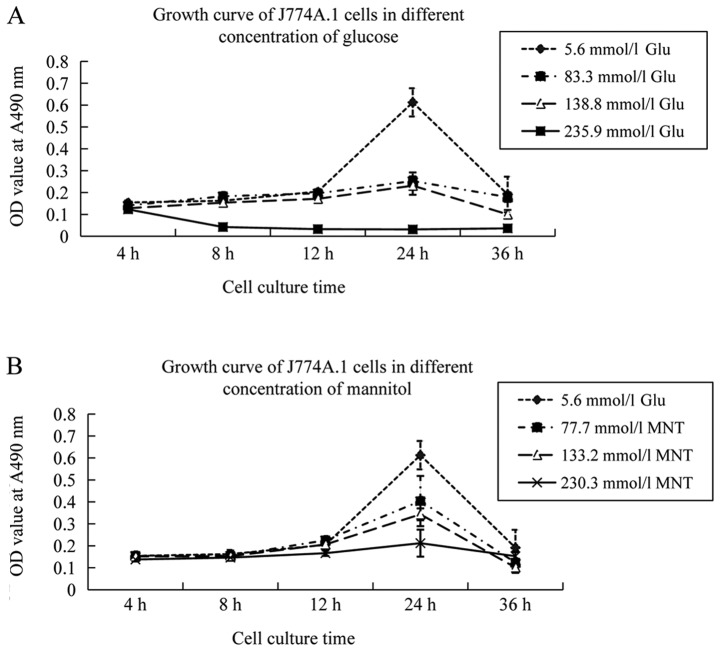
Four types of culture media were prepared: 5.6 mmol/l glucose was equivalent to the physiological glucose concentration, 83.3 mmol/l glucose was equivalent to 1.5% glucose, 138.8 mmol/l glucose was equivalent to 2.5% glucose, and 235.9 mmol/l glucose was equivalent to 4.25% glucose. A concentration of 5.6 mmol/l glucose was selected as the control concentration because 5.6 mmol/l glucose is the physiological glucose concentration in a healthy body. The latter three glucose concentrations were in accord with the glucose levels in the PD solution.
The growth of murine peritoneal macrophages incubated in 83.3 and 138.8 mmol/l glucose medium for 24 h was inhibited compared to that of macrophages incubated in 5.6 mmol/l glucose medium. Cell death was common when murine peritoneal macrophages were incubated in 235.9 mmol/l glucose medium for 4 h. To investigate the effect of osmotic pressure on the cell growth of murine peritoneal macrophages, these cells were incubated in the same osmotic media with 5.6 mmol/l glucose, 77.7 mmol/l mannitol, 133.2 mmol/l mannitol or 235.9 mmol/l mannitol. The data revealed that cell viability was not decreased at 4, 8 or 12 h (P>0.05). The aforementioned results indicated that osmotic pressure had a weaker effect on the growth of murine peritoneal macrophages within 12 h. Therefore, 5.6, 83.3 and 138.8 mmol/l concentrations were selected to study the differentiation of macrophages (Fig. 2).
Figure 2.
Growth curve of the J774A.1 cell line. The growth curve of the J774A.1 cell line at various concentrations of (A) glucose and (B) MNT with the same osmotic pressure. OD, optical density; glu, glucose; MNT, mannitol.
Differentiation of M1 and M2 macrophages in a high-glucose environment
J774A.1 cells, a murine macrophage line from ascites, were cultured in 5.6, 83.3 or 138.8 mmol/l glucose medium for 4, 8 or 12 h. The percentage of M1 macrophages (CCr7-positive cells) in 138.8 mmol/l glucose medium increased as time progressed (P<0.05). The percentage of M2 macrophages (CD206-positive cells) increased in a glucose concentration-dependent and time-dependent manner. The differences were significant (P<0.05) (Fig. 3A and B).
Figure 3.
M1 and M2 macrophage detection. (A) Detection of the percentage of M1 and M2 macrophages at various concentrations of glucose at 4, 8 and 12 h using flow cytometry. (B) Statistical analysis of the percentage of M1 and M2 macrophages at various concentrations of glucose at 4, 8 and 12 h. Each bar represents the mean ± SEM. *P<0.05, 138.8 mmol/l vs. 83.3 or 83.3 mmol/l vs. 5.6 mmol/l; #P<0.05, 8 vs. 4 or 12 h vs. 8 h. PE, phycoerythrin; glu, glucose.
Activity of Arg1 in macrophages under different glucose concentrations
To determine the effect of high glucose on functional enzymes in M2 macrophages, arginase activity in murine peritoneal macrophages exposed to different concentrations of glucose were examined. Quantitative colorimetric analysis revealed that the activity of Arg1 in macrophages incubated in different media for 12 h was 0.0008±0.0001 U/l (5.6 mmol/l), 0.1187±0.0287 U/l (83.3 mmol/l) and 0.2301±0.03013 U/l (138.8 mmol/l). The activity increased in a concentration-dependent manner (P<0.05) (Fig. 4).
Figure 4.
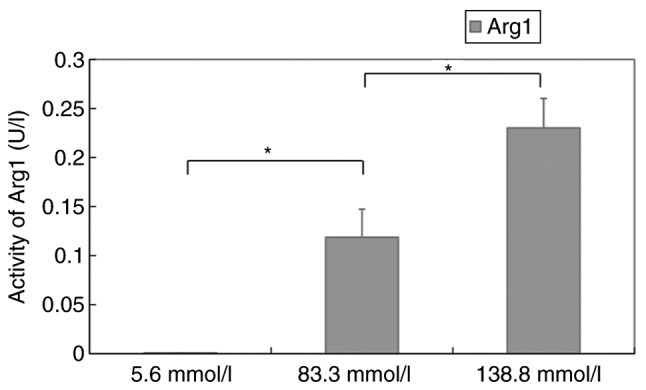
Activity of Arg1 in macrophages at various glucose concentrations. Each bar represents the mean ± SEM. *P<0.05, between groups. Arg1, arginase 1.
Level of TGF-β1 in murine macrophages cultured in different glucose concentrations
ELISA revealed that the levels of TGF-β1 in macrophages incubated in different media for 12 h were 32.6±3.1 pg/ml (5.6 mmol/l), 65.47±4.5 pg/ml (83.3 mmol/l) and 83.2±6.3 pg/ml (138.8 mmol/l). The levels also increased in a concentration-dependent manner (P<0.05) (Fig. 5).
Figure 5.

Expression level of TGF-β1 in murine macrophages cultured in various glucose concentrations. Each bar represents the mean ± SEM. *P<0.05 between groups. TGF-β1, transforming growth factor β1.
Discussion
In the present study, it was demonstrated that the percentage of M2 macrophages significantly increased in PD patients using 2.5% glucose dialysate compared with patients using 1.5% glucose dialysate. Moreover, murine peritoneal macrophages were cultured in high glucose, and the percentage of M2 macrophages increased in a glucose concentration-dependent and time-dependent manner. The percentage of M1 macrophages in 138.8 mmol/l glucose medium increased over time. In addition, the activity of Arg1, the functional enzyme of M2 macrophages, in murine macrophages exposed to different concentrations of glucose increased in a concentration-dependent manner. The level of TGF-β1 in the supernatant of murine macrophages also increased in a glucose concentration-dependent manner.
During PD, peritoneal membranes are continuously exposed to PD fluids. However, these solutions have glucose concentrations 10–50-fold higher than physiological concentrations, as well as lactate and an acidic pH (13). PD is limited by chronic fibrotic remodelling of the peritoneal wall after years of therapy (14). Local peritoneal inflammation is the driver of these chronic changes (15). Macrophages are a population of immune cells; they have been identified as key players in the fibrotic cascade and have the capacity to exert either injury-inducing or repair-promoting effects (16,17). Habib et al revealed that a characteristic mononuclear cell infiltrate consisting of CD4-positive T cells and M2 macrophages dominated the peritoneum of encapsulating peritoneal sclerosis (EPS) patients, which indicated that these mononuclear cells participated in the pathogenesis of EPS (18). Another study revealed that when a scavenger of macrophages was used to intervene in an animal model of peritoneal fibrosis, peritoneal thickness, collagen-I, fibronectin and CD206 were downregulated (19). Our previous data revealed that the number and ratio of peritoneal M2 macrophages were significantly increased in peritoneal fibrosis model rats (12,20). In the present study, the results also revealed that the percentage of M2 macrophages was much higher in the effluent of patients using a 2.5% glucose solution than in that of patients using a 1.5% glucose solution. These data indicated that macrophages were polarized to the M2 phenotype in high glucose in vivo.
To mimic the high-glucose microenvironment of peritoneal dialysis in vivo, murine peritoneal macrophages in a high-glucose in vitro environment were cultured. The present research revealed that the percentage of M2 macrophages increased in a glucose concentration-dependent and time-dependent manner, which was in accord with the observations of peritoneal macrophages in PD patients using high-glucose PD solution. The number of M1 and M2 macrophages was markedly decreased after treatment with 5.6 mmol/l glucose at 36 h since cell death was common when macrophages were incubated in glucose-containing medium, especially high-glucose-containing medium, for more than 24 h (21). Pavlou et al cultured bone marrow cells long-term in high-glucose and normal-glucose medium. They revealed that long-term exposure to high glucose increased the expression of M2 macrophage markers (Arg-1 and IL-10) (22). This result is in accordance with our data. The in vitro study and other previous research suggest that high glucose contributes to macrophage polarization to the M2 phenotype, which may be an important step in PD-related fibrosis.
Murine macrophages were cultured in a high-glucose in vitro environment to detect M1 macrophage markers. The present results revealed that the percentage of M1 macrophages in 138.8 mmol/l glucose medium increased over time. Hyperglycaemia has been reported to act directly on monocyte macrophage cell lines by driving the activation of these immunocytes towards an M1 phenotype (a proinflammatory state) (23). Qin et al also revealed that treating Ana-1 macrophages with advanced glycation end-products (AGEs) caused higher mRNA levels and increased production of IL-1β and TNF-α (proinflammatory cytokines) (24). These results suggest that high glucose and AGEs increase the secretion of inflammatory cytokines by macrophages to initiate an inflammatory response. Inflammation is another potential mechanism through which high glucose may damage peritoneal membranes and eventually result in peritoneal fibrosis.
The cytokine environment is the key factor for macrophage differentiation. In the presence of IFN-γ, macrophages may polarize to the M1 phenotype. In the presence of Th2 cytokines, macrophages are alternatively activated towards the M2 phenotype (9). Thus, the levels of Th1 cytokines and Th2 cytokines were detected to observe the microenvironment of the abdominal cavity for macrophage polarization. The present data revealed that cytokines driving the M1 phenotype, including IL-2 and INF-γ, were enhanced in the effluent of patients using 2.5% solution. The difference was not significant. This phenomenon was consistent with the trend of M1 macrophages. However, cytokines driving M2, including IL-4 and IL-13, were undetectable because their concentrations were too low.
Arginase is a marker enzyme of M2 macrophages, and its expression is strictly regulated by exogenous stimulation with IL-4, IL-10, and IL-13 (25). It is important in nitrogen elimination through its hydrolysis of L-arginine to L-ornithine. L-ornithine can be used to generate proline and hydroxyproline, the latter of which is an amino acid essential for the synthesis of collagen. Arg1 also competes with iNOS, an enzyme that controls the production of NO in Th1 cytokine-stimulated M1 macrophages (26). In the present study, it was demonstrated that the activity of arginase in murine macrophages was increased in a concentration-dependent manner when macrophages were exposed to different concentrations of glucose, which indicated that M2 macrophages may promote peritoneal tissue fibrosis through the Arg1 pathway.
M2 activation is provoked in response to stimulation with Th2 cytokines, and functional cytokines (including TGF-β1) are produced (9). Numerous studies have revealed that TGF-β1 is a central mediator in fibrogenic processes and that the activation of the TGF-β1 signalling pathway plays a detrimental role in the pathogenesis of progressive fibrosis (27–29). In the present study, it was demonstrated that the level of TGF-β1 also increased in the supernatant of murine macrophages in a glucose concentration-dependent manner. The present results indicated that the participation of M2 macrophages in PD-related fibrosis was partially resolved by the secretion of the functional cytokine TGF-β1.
Overall, it was demonstrated that high glucose contributed to the polarization of macrophages to the M2 phenotype, which may be involved in the pathogenesis of peritoneal fibrosis induced by PD solutions with high concentrations of glucose. Moreover, M2 macrophage function in PD-related fibrosis may be associated with arginase production and the TGF-β1 signalling pathway. These results also indicated that the blockade of M2 macrophage polarization may be a favorable candidate for therapeutic intervention for PD-related fibrosis, however, the latter will require further studies.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science Foundation of China (grant no. 81700670) and the Medical Research Fund of Guangdong Province (grant no. 20161182070992).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JL performed the cell experiments and wrote the manuscript. QK collected the tissue samples and analyzed the data. WHa designed the study. WHu designed the study and revised the manuscript. All the authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University. Written informed consent was obtained from all participating subjects.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Wong B, Ravani P, Oliver MJ, Holroyd-Leduc J, Venturato L, Garg AX, Quinn RR. Comparison of patient survival between hemodialysis and peritoneal dialysis among patients eligible for both modalities. Am J Kidney Dis. 2018;71:344–351. doi: 10.1053/j.ajkd.2017.08.028. [DOI] [PubMed] [Google Scholar]
- 2.Cho Y, Badve S, Hawley C, Wiggins K, Johnson D. Biocompatible peritoneal dialysis fluids: Clinical outcomes. Int J Nephrol. 2012;2012:812609. doi: 10.1155/2012/812609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies S. Unraveling the mechanisms of progressive peritoneal membrane fibrosis. Kidney Int. 2016;89:1185–1187. doi: 10.1016/j.kint.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 4.Liappas G, González-Mateo GT, Sánchez-Díaz R, Lazcano JJ, Lasarte S, Matesanz-Marín A, Zur R, Ferrantelli E, Ramírez LG, Aguilera A, et al. Immune-regulatory molecule CD69 controls peritoneal fibrosis. J Am Soc Nephrol. 2016;27:3561–3576. doi: 10.1681/ASN.2015080909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liao CT, Andrews R, Wallace LE, Khan MW, Kift-Morgan A, Topley N, Fraser DJ, Taylor PR. Peritoneal macrophage heterogeneity is associated with different peritoneal dialysis outcomes. Kidney Int. 2017;91:1088–1103. doi: 10.1016/j.kint.2016.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Locati M, Curtale G, Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol. 2020;15:123–147. doi: 10.1146/annurev-pathmechdis-012418-012718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon S, Plüddemann A, Martinez Estrada F. Macrophage heterogeneity in tissues: Phenotypic diversity and functions. Immunol Rev. 2014;262:36–55. doi: 10.1111/imr.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Murray P, Allen J, Biswas S, Fisher E, Gilroy D, Goerdt S, Gordon S, Hamilton J, Ivashkiv L, Lawrence T, et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon S, Martinez F. Alternative activation of macrophages: Mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Xue J, Sharma V, Hsieh M, Chawla A, Murali R, Pandol S, Habtezion A. Alternatively activated macrophages promote pancreatic fibrosis in chronic pancreatitis. Nat Commun. 2015;6:7158. doi: 10.1038/ncomms8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu W, Jiang Z, Zhang Y, Liu Q, Fan J, Luo N, Dong X, Yu X. Characterization of infiltrating macrophages in high glucose-induced peritoneal fibrosis in rats. Mol Med Rep. 2012;6:93–99. doi: 10.3892/mmr.2012.890. [DOI] [PubMed] [Google Scholar]
- 13.Bartosova M, Schaefer B, Vondrak K, Sallay P, Taylan C, Cerkauskiene R, Dzierzega M, Milosevski-Lomic G, Büscher R, Zaloszyc A, et al. Peritoneal dialysis vintage and glucose exposure but not peritonitis episodes drive peritoneal membrane transformation during the first years of PD. Front Physiol. 2019;10:356. doi: 10.3389/fphys.2019.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehrotra R, Devuyst O, Davies S, Johnson D. The current state of peritoneal dialysis. J Am Soc Nephrol. 2016;27:3238–3252. doi: 10.1681/ASN.2016010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helmke A, Nordlohne J, Balzer MS, Dong L, Rong S, Hiss M, Shushakova N, Haller H, von Vietinghoff S. CX3CL1-CX3CR1 interaction mediates macrophage-mesothelial cross talk and promotes peritoneal fibrosis. Kidney Int. 2019;95:1405–1417. doi: 10.1016/j.kint.2018.12.030. [DOI] [PubMed] [Google Scholar]
- 16.Pellicoro A, Ramachandran P, Iredale J, Fallowfield J. Liver fibrosis and repair: Immune regulation of wound healing in a solid organ. Nat Rev Immunol. 2014;14:181–194. doi: 10.1038/nri3623. [DOI] [PubMed] [Google Scholar]
- 17.Palomar R, López-Hoyos M, Morales P, Marín MJ, Alvarez L, Ruiz-Soto M, Rodrigo E, Fernandez-Fresnedo G, Lm de Francisco A, Arias M. Analysis of peritoneal leukocyte population with different dialysis fluids. Clin Nephrol. 2009;72:137–142. doi: 10.5414/CNP72137. [DOI] [PubMed] [Google Scholar]
- 18.Habib SM, Abrahams AC, Korte MR, Zietse R, de Vogel LL, Boer WH, Dendooven A, Clahsen-van Groningen MC, Betjes MG. CD4-positive T Cells and M2 macrophages dominate the peritoneal infiltrate of patients with encapsulating peritoneal sclerosis. PLoS One. 2015;10:e0120174. doi: 10.1371/journal.pone.0120174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kushiyama T, Oda T, Yamada M, Higashi K, Yamamoto K, Oshima N, Sakurai Y, Miura S, Kumagai H. Effects of liposome-encapsulated clodronate on chlorhexidine gluconate-induced peritoneal fibrosis in rats. Nephrol Dial Transplant. 2011;26:3143–3154. doi: 10.1093/ndt/gfr068. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Jiang ZP, Su N, Fan JJ, Ruan YP, Peng WX, Li YF, Yu XQ. The role of peritoneal alternatively activated macrophages in the process of peritoneal fibrosis related to peritoneal dialysis. Int J Mol Sci. 2013;14:10369–10382. doi: 10.3390/ijms140510369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shang Y, Fang N, Wang F, Wang H, Wang Z, Tang M, Peng J, Zhang Y, Zhang W, Zhong M. MicroRNA-21, induced by high glucose, modulates macrophage apoptosis via programmed cell death 4. Mol Med Rep. 2015;12:463–469. doi: 10.3892/mmr.2015.3398. [DOI] [PubMed] [Google Scholar]
- 22.Pavlou S, Lindsay J, Ingram R, Xu H, Chen M. Sustained high glucose exposure sensitizes macrophage responses to cytokine stimuli but reduces their phagocytic activity. BMC Immunol. 2018;19:24. doi: 10.1186/s12865-018-0261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan Y, Wang Y, Cai L, Cai Y, Hu J, Yu C, Li J, Feng Z, Yang S, Li X, Liang G. Inhibition of high glucose-induced inflammatory response and macrophage infiltration by a novel curcumin derivative prevents renal injury in diabetic rats. Br J Pharmacol. 2012;166:1169–1182. doi: 10.1111/j.1476-5381.2012.01854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin Q, Niu J, Wang Z, Xu W, Qiao Z, Gu Y. Astragalus membranaceus inhibits inflammation via phospho-P38 mitogen-activated protein kinase (MAPK) and nuclear factor (NF)-κB pathways in advanced glycation end product-stimulated macrophages. Int J Mol Sci. 2012;13:8379–8387. doi: 10.3390/ijms13078379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pauleau AL, Rutschman R, Lang R, Pernis A, Watowich S, Murray P. Enhancer-mediated control of macrophage-specific arginase I expression. J Immunol. 2004;172:7565–7573. doi: 10.4049/jimmunol.172.12.7565. [DOI] [PubMed] [Google Scholar]
- 26.Gotoh T, Sonoki T, Nagasaki A, Terada K, Takiguchi M, Mori M. Molecular cloning of cDNA for nonhepatic mitochondrial arginase (arginase II) and comparison of its induction with nitric oxide synthase in a murine macrophage-like cell line. FEBS Lett. 1996;395:119–122. doi: 10.1016/0014-5793(96)01015-0. [DOI] [PubMed] [Google Scholar]
- 27.Strippoli R, Moreno-Vicente R, Battistelli C, Cicchini C, Noce V, Amicone L, Marchetti A, Del Pozo M, Tripodi M. Molecular mechanisms underlying peritoneal EMT and fibrosis. Stem Cells Int. 2016;2016:3543678. doi: 10.1155/2016/3543678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Q, Bajo MA, Del Peso G, Yu X, Selgas R. Preventing peritoneal membrane fibrosis in peritoneal dialysis patients. Kidney Int. 2016;90:515–524. doi: 10.1016/j.kint.2016.03.040. [DOI] [PubMed] [Google Scholar]
- 29.Kariya T, Nishimura H, Mizuno M, Suzuki Y, Matsukawa Y, Sakata F, Maruyama S, Takei Y, Ito Y. TGF-β1-VEGF-A pathway induces neoangiogenesis with peritoneal fibrosis in patients undergoing peritoneal dialysis. Am J Physiol Renal Physiol. 2018;314:F167–F180. doi: 10.1152/ajprenal.00052.2017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.