Abstract
Ginkgolide B (GB) is a diterpene lactone found in the leaves of the traditional Chinese medicinal plant Ginkgo that has been shown to have various pharmacological effects. However, the anti-apoptotic properties of GB in cardiovascular disease remain poorly understood. The present study aimed to investigate the effect of GB on hydrogen peroxide-induced cell injury in cardiac H9c2 cells, and to further clarify its protective mechanism of action. An in vitro hydrogen peroxide-treated H9c2 cell model was used in order to mimic myocardial ischemia-reperfusion (I/R) injury. Cell viability was assessed by the Cell Counting Kit-8 assay. The induction of apoptosis was determined by flow cytometry and staining was performed using Hoechst 33342. In addition, the effect of GB on the expression levels of apoptosis-associated proteins was evaluated by western blot analysis. The present study demonstrated that GB protected against hydrogen peroxide-induced cytotoxicity and cell apoptosis in H9c2 cardiac cells. GB upregulated the expression level of the anti-apoptotic protein Bcl-2 and downregulated the expression levels of the pro-apoptotic proteins cleaved caspase-3 and Bax in hydrogen peroxide-treated H9c2 cells. The molecular mechanism underlying the anti-apoptotic effects of GB was subsequently detected. GB pretreatment activated the PI3K/Akt/mTOR signaling pathway and caused an increase in the phosphorylation levels of Akt and mTOR in hydrogen peroxide-treated H9c2 cells. These results revealed that GB inhibited hydrogen peroxide-induced apoptosis in H9c2 cells via activation of the PI3K/Akt/mTOR signaling pathway. These findings indicate the potential therapeutic benefits of GB in the treatment of myocardial I/R injury.
Keywords: Ginkgolide B, myocardial ischemia/reperfusion injury, oxidative stress, cell apoptosis, phosphatidyl inositol 3 kinase/protein kinase B/mammalian target of rapamycin
Introduction
Acute myocardial infarction (AMI), resulting from sudden interruption of blood flow in the main coronary arteries remains a leading cause of morbidity and mortality worldwide (1). Currently, percutaneous coronary intervention (PCI) is the most effective minimally invasive treatment for AMI. PCI is performed in order to reopen the affected coronary artery and restore adequate blood flow to the ischemic myocardium. It aims to reduce the myocardial infarct size and to maintain heart function (2,3). However, following PCI treatment, ~4-7% of the patients experience recurrent chest pain, elevated troponin I/T levels and onset of hemodynamic disorders that are attributed to myocardial ischemia-reperfusion (I/R) injury (4). Cardiac I/R is characterized by restricted blood flow to the myocardium followed by reoxygenation associated with blood reperfusion when the coronary artery reopens (5). I/R injury is caused by mitochondrial dysfunction, calcium overload and excessive generation of mitochondrial reactive oxygen species (ROS), which eventually contribute to cardiomyocyte apoptosis and necrosis (6). Therefore, the prevention of myocardial I/R injury is essential for improving prognosis following PCI treatment.
Ginkgolide B (GB) is extracted from the leaves of an ancient Chinese medicinal plant Ginkgo. This compound is a diterpene lactone, which has been documented as a strong antagonist of platelet-activating factor receptor (7). Previous studies have shown that the anti-inflammatory, antioxidant and anti-apoptotic properties of GB have rendered it beneficial in ischemic and hemorrhagic stroke (8–11). Furthermore, it has been shown to improve cognitive function and the disease outcome of different types of cancer. Moreover, previous studies have demonstrated that GB plays a therapeutic role in cardiovascular diseases (12,13). For instance, GB can ameliorate oxidized low-density lipoprotein-induced endothelial cell dysfunction in human umbilical vein endothelial cells and inhibit inflammatory cascades in murine RAW264.7 macrophage-like cells (12). In addition, GB inhibits the production of ROS in doxorubicin-induced cardiotoxicity in H9c2 cells (13). However, the effect of GB on myocardial I/R injury remains unclear.
PI3Ks are a family of lipid kinases involved in the regulation of cellular activation, inflammatory responses and apoptosis (14). PI3K activation induces the formation of the second messenger phosphatidylinositol-3,4,5-triphosphate (PIP3) (15). PIP3 provides an Akt docking site for Akt phosphorylation and activation (16). As the central mediator of the PI3K signaling pathway, Akt promotes cell survival, apoptosis and proliferation by inducing the downstream mTOR complex (17). The mTOR signaling pathway is also involved in regulating cell growth, cell survival and protein synthesis (18). Therefore, the regulation of the PI3K/Akt/mTOR signaling pathway could affect cell viability and the induction of apoptosis on different cell types.
Hydrogen peroxide (H2O2) is a key metabolite in oxidative stress that is widely used to simulate myocardial I/R injury (19). The H9c2 cell line, derived from a rat embryonic heart ventricle, is considered a close surrogate for cardiomyocytes and has been proven to be ideal for cardiomyocyte signal transduction studies (20). Therefore, in the present study, H2O2 treatment in H9c2 cardiac cells was used to simulate myocardial I/R. In addition, the present study investigated the cardioprotective effect of GB on H2O2-induced apoptosis in H9c2 cells and its interaction with the PI3K/Akt/mTOR signaling pathway.
Materials and methods
Cell culture
H9c2 rat cardiomyocyte cells were purchased from The Cell Bank of Type Culture Collection of the Chinese Academy of Sciences and cultured in high-glucose DMEM (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 U/ml streptomycin at 37°C in a humidified atmosphere containing 5% CO2. The cells were passaged every 4 days and the culture medium was replaced every 2 days.
Establishment of H9c2 cell oxidative stress model
H9c2 cells (5×103 cells per well) were seeded in 96-well plates and incubated at 37°C in a 5% CO2 humidified incubator overnight. Subsequently, the cells were exposed to different H2O2 concentrations (200, 400, 600 and 800 µM) and harvested at different time points (4, 8 and 12 h). The appropriate concentration and exposure time of H2O2 used for the establishment of the oxidative stress model in H9c2 cells was determined by the Cell Counting Κit-8 (CCK-8) assay.
Drug preparation and cell treatment
GB was purchased from Sigma-Aldrich; Merck KGaA. The stock solution was prepared by dissolving GB in DMSO at 100 mM. The working solution of GB was obtained by diluting the stock solution in DMEM to the desired concentrations. To avoid the DMSO-induced cytotoxicity, the final concentration of DMSO was retained to <1%. H9c2 cells were pretreated with GB for 20 h prior to co-incubation with H2O2. The total time of GB-pretreatment group is the same as single GB-treated group. Prior to cell pretreatment with GB at 0.01, 0.1, 1, 10 and 100 µM for 1 h, the cells were treated with PI3K inhibitor LY294002 (Selleck Chemicals) at concentrations of 5, 10, 20 and 40 µM.
Cell viability assay
Cell viability was determined by the CCK-8 assay (Dojindo Molecular Technologies, Inc.), according to the manufacturer's protocol. H9c2 cells (5×103 cells per well) were seeded in 96-well plates overnight and were pretreated with different concentrations of LY294002, GB and H2O2. To measure cell viability, 10 µl CCK-8 assay solution was added into each well, containing 90 µl medium, and the cells were then incubated in the dark at 37°C for additional 2 h. Absorbance was measured at 450 nm in a microplate reader (BioTek Instruments, Inc.).
Hoechst 33342 staining
Typical morphological features of apoptotic cells were observed by Hoechst 33342 staining (Nanjing KeyGen Biotech Co., Ltd.). Briefly, the cells were washed twice with PBS following H2O2 incubation with or without GB pretreatment and stained with Hoechst 33342 for 15 min in a 5% CO2 incubator at 37°C in the dark. Finally, the cells were washed once with PBS and the images were captured by fluorescence microscopy (magnification, ×200; Zeiss Axiovert A1). The apoptotic cells with nuclear chromatin condensation and fragmentation were stained bright blue. A total of three fluorescence images per well were captured randomly, and the percentage of apoptotic cells was calculated using the following equation: (The number of apoptotic cells/the total number of cells) ×100.
Annexin V-FITC/propidium iodide (PI) assay
The induction of apoptosis was examined using the Annexin V-FITC/PI double staining assay (BD Biosciences; Becton-Dickinson and Company). Briefly, H9c2 cells were gently washed with ice-cold PBS, digested with 0.25% Trypsin without ethylenediaminetetraacetic acid (Gibco; Thermo Fisher Scientific, Inc.) and resuspended in complete culture medium. The cells were then centrifuged at 120 × g for 5 min at 4°C and the cell pellet was resuspended in ice-cold PBS and centrifuged at the same conditions. Finally, the cells were resuspended in 500 µl 1X binding buffer supplemented with 5 µl Annexin V-FITC and 5 µl PI. The cells were incubated at room temperature for 15 min in the dark and were analyzed by flow cytometry (BD FACSCalibur™; BD Biosciences; Becton-Dickinson and Company). Data was analyzed using FlowJo version 7.6.1 software (FlowJo LLC).
Western blot analysis
H9c2 cells were washed with ice-cold PBS, pelleted by cell scraper and lysed in 100 µl lysis buffer containing 1 µl phosphatase inhibitor, 0.1 µl protease inhibitor and 0.5 µl PMSF. The extracted proteins were quantified with the bicinchoninic acid Protein assay kit (Thermo Fisher Scientific, Inc.). A total of 20 µg protein extracts per lane were separated by 6–15% SDS-PAGE. The proteins were transferred onto polyvinylidene difluoride membranes or nitrocellulose filter membrane. The membranes were blocked with 5% BSA (Gibco; Thermo Fisher Scientific, Inc.) at room temperature for 2 h and incubated overnight at 4°C with primary antibodies against rabbit anti-Bax (1:1,000; cat. no. 2772), rabbit anti-Bcl-2 (1:1,000; cat. no. 2870), rabbit anti-cleaved caspase-3 (1:1,000; cat. no. 9664), rabbit anti-caspase-3 (1:1,000; cat. no. 9662), rabbit anti-Akt (1:1,000; cat. no. 9272), rabbit anti-phosphorylated (p)-Akt (p-Akt; 1:1,000; cat. no. 4060), rabbit anti-mTOR (1:1,000; cat. no. 2983), rabbit anti-p-mTOR (p-mTOR; 1:1,000; cat. no. 5536), and rabbit anti-GAPDH (1:1,000; cat. no. 5174; all from Cell Signaling Technology, Inc.). The membranes were subsequently washed for 2 h with TBST (10 mM Tris, pH 7.5, 150 mM NaCl, and 0.05% Tween-20), and incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:500; cat. no. sc-2004; Santa Cruz Biotechnology, Inc.) for 1 h at room temperature. The antigen-antibody complexes on the membranes were detected using the SuperSignal™ West Femto Maximum Sensitivity substrate (Thermo Fisher Scientific, Inc.) and quantified on the ChemiDoc™ XRS Imaging system (Bio-Rad Laboratories, Inc.).
Statistical analysis
All experiments were repeated three times. The data are presented as the mean ± SD. Statistical differences among groups were analyzed using a one-way ANOVA, followed by a Tukey's post-hoc test. All statistical analyses were performed using the GraphPad Prism 5 software (GraphPad Software, Inc.). P<0.05 or P<0.001 were considered to indicate a statistically significant difference.
Results
GB decreases H2O2-induced cytotoxicity in H9c2 cells
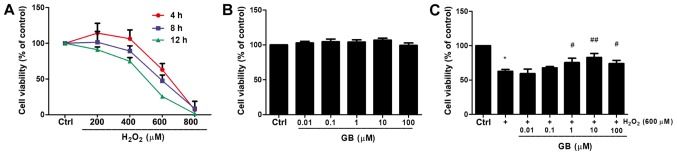
H2O2 reduced cell viability in a dose- and time- dependent manner (Fig. 1A). For instance, treatment of H9c2 cells with 600 µM H2O2 for 4 h reduced cell viability to 63.11% compared with that noted in untreated cells. The H9c2 cell state in this condition was not too poor to conduct subsequent experiments. Therefore, 600 µΜ H2O2 was selected as the optimal treatment concentration for subsequent experiments. The effect of GB alone on cell viability was also investigated. Cell viability was not significantly affected by GB treatment for 24 h even at concentrations up to 100 µM (Fig. 1B). Subsequently, the effects of H9c2 cell-GB pretreatment in the protection of the cells against H2O2-induced cytotoxicity were examined. GB pretreatment at 10 µM concentration for 20 h prior to co-incubation with 600 µM H2O2 for 4 h revealed the highest inhibitory effect on the induction of cell cytotoxicity caused by treatment of 600 µM H2O2 for 4 h (Fig. 1C). Therefore, 10 µM GB was used in subsequent experiments.
Figure 1.
Detection of H9c2 cell viability. (A) Time- and dose-dependent H2O2-induced cytotoxicity was determined by a CCK-8 assay. (B) H9c2 cells were treated with GB (0–100 µM) for 24 h and cell viability was detected by the CCK-8 assay. (C) Indicated GB concentrations were administered 20 h prior to the 4 h incubation with 600 µM H2O2 and a CCK-8 assay was performed to measure cell viability. The data are presented as the mean ± SD (n=3). *P<0.05 vs. the Ctrl group; #P<0.05 vs. the H2O2-treated group; ##P<0.001 vs. the H2O2-treated group. GB, Ginkgolide B; H2O2, hydrogen peroxide; CCK-8, Cell Counting Kit-8; Ctrl, control.
GB reduces H2O2-induced cell apoptosis in H9c2 cells
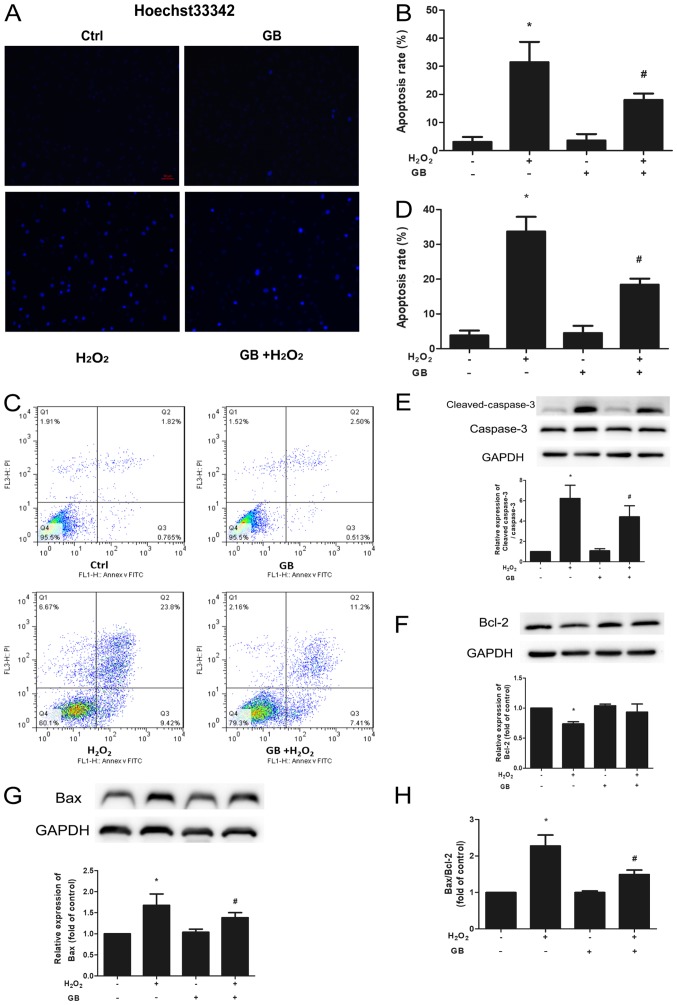
Chromatin condensation and fragmentation are the typical features of apoptotic cells so Hoechst 33342 staining was used to evaluate induction of cell apoptosis caused by H2O2. Pretreatment of the cells with 10 µM GB for 20 h significantly decreased the percentage of apoptotic cells, compared with that noted following treatment of the cells with 600 µM H2O2 for 4 h (Fig. 2A and B). Furthermore, the Annexin V-FITC/PI assay was further used to evaluate the induction of cell apoptosis. Treatment of the cells with 600 µM H2O2 for 4 h led to a significant increase in cell apoptosis compared with that noted in the control group, whereas this effect was inhibited by GB pretreatment (Fig. 2C and D). The expression levels of the apoptotic proteins Bcl-2, Bax, caspase-3 and cleaved caspase-3, were determined by western blot analysis. Both Bax and cleaved caspase-3 protein levels were significantly elevated in the H2O2-treated group, whereas Bcl-2 levels were downregulated (Fig. 2E-H). In addition, GB pretreatment significantly decreased the expression levels of Bax and cleaved caspase-3 and increased the expression levels of Bcl-2 compared with those of the H2O2-treated group. Both the Bax/Bcl-2 and the cleaved caspase-3/caspase-3 ratios were significantly decreased in the GB-pretreatment group compared with those of the H2O2-treated group.
Figure 2.
Protective effect of GB against H2O2-induced apoptosis in H9c2 cells. (A) Percentage of apoptotic cells was determined by Hoechst 33342 staining. Blue fluorescence indicated apoptosis induction (magnification, ×200). (B) The apoptosis rate was significantly decreased in the GB-pretreated cells compared with that of the H2O2-treated cells. (C and D) Percentage of apoptotic rate in H9c2 cells was determined by flow cytometry using Annexin V-FITC/PI staining. Cell apoptosis was inhibited by 10 µM GB pretreatment for 20 h prior to 600 µM H2O2 treatment for 4 h. (E) cleaved caspase-3, (F) Bcl-2 and (G) Bax, caspase-3 protein expression levels were measured by western blot analysis. GAPDH was used as an internal control. (H) The Bax/Bcl-2 ratio was calculated. The data are presented as the mean ± SD (n=3). *P<0.05 vs. Ctrl group; #P<0.05 vs. the H2O2-treated group. H2O2, 600 µM H2O2-treated for 4 h group; GB, 10 µM GB-treated for 24 h group; GB + H2O2, 10 µM GB-pretreated for 20 h prior to co-incubation with 600 µM H2O2 for 4 h group. GB, Ginkgolide B; H2O2, hydrogen peroxide; Ctrl, control group.
GB enhances the phosphorylation levels of Akt and mTOR
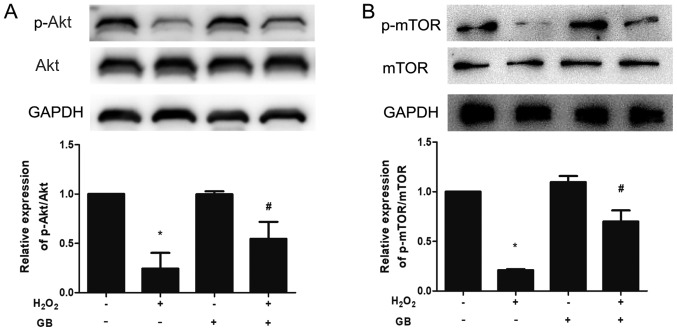
The expression levels of p-Akt and p-mTOR were downregulated in the H2O2-treated group, whereas GB pretreatment was able to reverse the effects of H2O2 (Fig. 3A and B).
Figure 3.
Akt and mTOR phosphorylation are involved in the protective effect of GB in H2O2-induced H9c2 cells. (A) Total Akt and p-Akt protein expression levels were measured by western blot analysis. (B) Total mTOR and p-mTOR protein expression levels were measured by western blot analysis. The data are presented as the mean ± SD (n=3). *P<0.05 vs. the control group; #P<0.05 vs. the H2O2-treated group. GB, Ginkgolide B; H2O2, hydrogen peroxide; p-, phosphorylated.
Effect of the PI3K inhibitor LY294002 on the H2O2-induced cytotoxicity in H9c2 cells
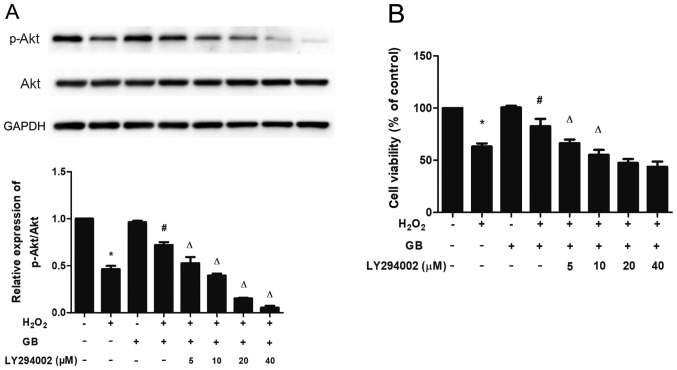
To determine whether the activation of Akt and mTOR was associated with the protective effects of GB, the LY294002 inhibitor was used to investigate the expression levels of p-Akt and to assess cell viability. Pretreatment of the cells with LY294002 and GB downregulated the expression levels of p-Akt in a dose-dependent manner, compared with those noted in the single GB-pretreatment group. In addition, LY294002 and GB pretreatment reduced cell viability compared with that of the single GB-pretreatment group. These results indicated that GB elicited its protective effects via activation of the PI3K/Akt/mTOR signaling pathway (Fig. 4A and B).
Figure 4.
PI3K inhibitor LY294002 inhibits the protective effect of GB in H2O2-induced H9c2 cells. (A) Total Akt and p-Akt protein expression levels were measured by western blot analysis. (B) Indicated concentrations of LY294002 were administered 1 h prior to GB and H2O2 treatment and Cell Counting Kit-8 assay was performed to measure cell viability. The data are presented as the mean ± SD (n=3). *P<0.05 vs. the control group; #P<0.05 vs. the H2O2-treated group; ΔP<0.05 vs. the GB-pretreated group. GB, Ginkgolide B; H2O2, hydrogen peroxide; p-, phosphorylated.
Discussion
The present study provides evidence regarding the protective effects of GB on H2O2-induced cytotoxicity in H9c2 cells. These effects were mediated by the inhibition of cell apoptosis. Furthermore, it was shown that the GB-induced protective effect was mediated via activation of the PI3K/Akt/mTOR signaling pathway.
The process of apoptosis that was initially described by Kerr et al (21) is a form of programmed cell death with certain morphological features, such as narrowed cell volume, chromatin condensation, nuclear fragmentation and apoptotic body formation (22). In the present study, GB inhibited the induction of cell apoptosis by H2O2. Two major pathways of apoptosis, namely the death receptor-mediated and the mitochondrial-mediated apoptotic pathways have been identified. Both pathways result in caspase-dependent cell death (23). The members of the Bcl-2 family of proteins, which is composed of anti- and pro-apoptotic factors, are involved in the mitochondrial-mediated apoptotic pathway (24). Bax is a pro-apoptotic protein of the Bcl-2 family that is negatively regulated by Bcl-2 (anti-apoptotic protein). Consequently, the Bax/Bcl-2 ratio can act as an indicator that determines the cell susceptibility to apoptosis and the balance between anti- and pro-apoptotic factors (25). Caspase-3 is one of the most important members of the caspase family and is considered the central effector of apoptosis activated by upstream initiator caspases. Caspase-3 is cleaved to produce the final cleaved caspase-3 protein form (26). In the present study, GB pretreatment significantly decreased cleaved caspase-3 and Bax expression levels, whereas it upregulated Bcl-2 expression levels in H2O2-treated H9c2 cells, resulting in a declined Bax/Bcl-2 ratio and increased cell viability. These results indicated that GB exhibited protective effects against the H2O2-induced cytotoxicity in H9c2 cells partly through its anti-apoptotic properties.
Previous studies have shown that the activation of the PI3K/Akt/mTOR signaling pathway promotes cell proliferation and inhibits cell apoptosis (27,28). In the current study, GB pretreatment inhibited cell apoptosis by inducing Akt and mTOR phosphorylation. To further confirm this observation, H9c2 cells were treated with the PI3K inhibitor LY294002 and it was shown that the GB-induced Akt phosphorylation was partially blocked by the LY294002 inhibitor. In addition, LY294002 treatment partially reversed the protective effect of GB in maintaining cell viability. The aforementioned results suggested that GB exerted protective effects against cell apoptosis via the activation of the PI3K/Akt/mTOR signaling pathway.
All in vitro experiments were repeated three times in this study, this is a limitation of the study so in future experiments there should be a higher number of repeats. Additional in vivo and clinical studies are also required to support the in vitro results reported in the current study. Previous studies demonstrated that PCI treatment followed by remote ischemic preconditioning (RIPC) exhibited protective effects on myocardial I/R injury (29) and contrast-induced nephropathy (CIN) (30,31). Furthermore, the activation of Akt may mediate the target organ protection by RIPC (32). The present study suggested that GB pretreatment could trigger the activation of Akt during oxidative stress. In conclusion, GB pretreatment could be used to alleviate myocardial I/R injury and CIN following PCI treatment. However, additional clinical trials need to be conducted in the future in order to confirm this hypothesis.
Acknowledgements
Not applicable.
Funding
The present study was funded by the grant from Jiangsu Province Nature Science Youth Foundation (grant no. BK20141020).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
JL and PW performed the majority of the experiments and drafted the manuscript; ZX, JZ and JL performed some of the experiments and collected the data; and JL and ZY designed the study. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Anderson JL, Morrow DA. Acute myocardial infarction. N Engl J Med. 2017;376:2053–2064. doi: 10.1056/NEJMra1606915. [DOI] [PubMed] [Google Scholar]
- 2.Canfield J, Totary-Jain H. 40 years of percutaneous coronary intervention: History and future directions. J Pers Med. 2018;8:E33. doi: 10.3390/jpm8040033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou QL, Teng F, Zhang YS, Sun Q, Cao YX, Meng GW. FPR1 gene silencing suppresses cardiomyocyte apoptosis and ventricular remodeling in rats with ischemia/reperfusion injury through the inhibition of MAPK signaling pathway. Exp Cell Res. 2018;370:506–518. doi: 10.1016/j.yexcr.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 4.Luo Y, Pan YZ, Zeng C, Li GL, Lei XM, Liu Z, Zhou SF. Altered serum creatine kinase level and cardiac function in ischemia-reperfusion injury during percutaneous coronary intervention. Med Sci Monit. 2011;17:Cr474–Cr479. doi: 10.12659/MSM.881932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J Clin Invest. 2013;123:92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basheer WA, Fu Y, Shimura D, Xiao S, Agvanian S, Hernandez DM, Hitzeman TC, Hong T, Shaw RM. Stress response protein GJA1-20k promotes mitochondrial biogenesis, metabolic quiescence, and cardioprotection against ischemia/reperfusion injury. JCI Insight. 2018;3:121900. doi: 10.1172/jci.insight.121900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nabavi SM, Habtemariam S, Daglia M, Braidy N, Loizzo MR, Tundis R, Nabavi SF. Neuroprotective effects of ginkgolide B against ischemic stroke: A review of current literature. Curr Top Med Chem. 2015;15:2222–2232. doi: 10.2174/1568026615666150610142647. [DOI] [PubMed] [Google Scholar]
- 8.Gill I, Kaur S, Kaur N, Dhiman M, Mantha AK. Phytochemical ginkgolide B attenuates amyloid-β1-42 induced oxidative damage and altered cellular responses in human neuroblastoma SH-SY5Y cells. J Alzheimers Dis. 2017;60(Suppl 1):S25–S40. doi: 10.3233/JAD-161086. [DOI] [PubMed] [Google Scholar]
- 9.Zhi Y, Pan J, Shen W, He P, Zheng J, Zhou X, Lu G, Chen Z, Zhou Z. Ginkgolide B inhibits human bladder cancer cell migration and invasion through MicroRNA-223-3p. Cell Physiol Biochem. 2016;39:1787–1794. doi: 10.1159/000447878. [DOI] [PubMed] [Google Scholar]
- 10.Nash KM, Shah ZA. Current perspectives on the beneficial role of ginkgo biloba in neurological and cerebrovascular disorders. Integr Med Insights. 2015;10:1–9. doi: 10.4137/IMI.S25054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shu ZM, Shu XD, Li HQ, Sun Y, Shan H, Sun XY, Du RH, Lu M, Xiao M, Ding JH, Hu G. Ginkgolide B protects against ischemic stroke via modulating microglia polarization in mice. CNS Neurosci Ther. 2016;22:729–739. doi: 10.1111/cns.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng Z, Yang X, Zhang L, Ansari IA, Khan MS, Han S, Feng Y. Ginkgolide B ameliorates oxidized low-density lipoprotein-induced endothelial dysfunction via modulating Lectin-like ox-LDL-receptor-1 and NADPH oxidase 4 expression and inflammatory cascades. Phytother Res. 2018;32:2417–2427. doi: 10.1002/ptr.6177. [DOI] [PubMed] [Google Scholar]
- 13.Gao J, Chen T, Zhao D, Zheng J, Liu Z. Ginkgolide B exerts cardioprotective properties against doxorubicin-induced cardiotoxicity by regulating reactive oxygen species, akt and calcium signaling pathways in vitro and in vivo. PLoS One. 2016;11:e0168219. doi: 10.1371/journal.pone.0168219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu X, Xu C, Zhou X, Cui B, Lu Z, Jiang H. PI3K/Akt signaling pathway involved in cardioprotection of preconditioning with high mobility group box 1 protein during myocardial ischemia and reperfusion. Int J Cardiol. 2011;150:222–223. doi: 10.1016/j.ijcard.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 15.Latronico MV, Costinean S, Lavitrano ML, Peschle C, Condorelli G. Regulation of cell size and contractile function by AKT in cardiomyocytes. Ann NY Acad Sci. 2004;1015:250–260. doi: 10.1196/annals.1302.021. [DOI] [PubMed] [Google Scholar]
- 16.Yao H, Han X, Han X. The cardioprotection of the insulin-mediated PI3K/Akt/mTOR signaling pathway. Am J Cardiovasc Drugs. 2014;14:433–442. doi: 10.1007/s40256-014-0089-9. [DOI] [PubMed] [Google Scholar]
- 17.Aoyagi T, Matsui T. Phosphoinositide-3 kinase signaling in cardiac hypertrophy and heart failure. Curr Pharm Des. 2011;17:1818–1824. doi: 10.2174/138161211796390976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sciarretta S, Forte M, Frati G, Sadoshima J. New insights into the role of mTOR signaling in the cardiovascular system. Circ Res. 2018;122:489–505. doi: 10.1161/CIRCRESAHA.117.311147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang X, Jiang H, Shi Y. Upregulation of heme oxygenase-1 expression by curcumin conferring protection from hydrogen peroxide-induced apoptosis in H9c2 cardiomyoblasts. Cell Biosci. 2017;7:20. doi: 10.1186/s13578-017-0146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watkins SJ, Borthwick GM, Arthur HM. The H9C2 cell line and primary neonatal cardiomyocyte cells show similar hypertrophic responses in vitro. In Vitro Cell Dev Biol Anim. 2011;47:125–131. doi: 10.1007/s11626-010-9368-1. [DOI] [PubMed] [Google Scholar]
- 21.Kerr JF, Wyllie AH, Currie AR. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takemura G, Kanoh M, Minatoguchi S, Fujiwara H. Cardiomyocyte apoptosis in the failing heart-a critical review from definition and classification of cell death. Int J Cardiol. 2013;167:2373–2386. doi: 10.1016/j.ijcard.2013.01.163. [DOI] [PubMed] [Google Scholar]
- 23.Mughal W, Dhingra R, Kirshenbaum LA. Striking a balance: Autophagy, apoptosis, and necrosis in a normal and failing heart. Curr Hypertens Rep. 2012;14:540–547. doi: 10.1007/s11906-012-0304-5. [DOI] [PubMed] [Google Scholar]
- 24.Watson EC, Grant ZL, Coultas L. Endothelial cell apoptosis in angiogenesis and vessel regression. Cell Mol Life Sci. 2017;74:4387–4403. doi: 10.1007/s00018-017-2577-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lomonosova E, Chinnadurai G. BH3-only proteins in apoptosis and beyond: An overview. Oncogene. 2008;27(Suppl 1):S2–S19. doi: 10.1038/onc.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edlich F. BCL-2 proteins and apoptosis: Recent insights and unknowns. Biochem Biophys Res Commun. 2018;500:26–34. doi: 10.1016/j.bbrc.2017.06.190. [DOI] [PubMed] [Google Scholar]
- 27.Chang J, Xue X, Song C, Liu B, Gao L. Ginkgolide B promotes cell growth in endothelial progenitor cells through miR-126 and the Akt signaling pathway. Mol Med Rep. 2017;16:5627–5632. doi: 10.3892/mmr.2017.7254. [DOI] [PubMed] [Google Scholar]
- 28.Fulda S. Synthetic lethality by co-targeting mitochondrial apoptosis and PI3K/Akt/mTOR signaling. Mitochondrion. 2014;19:85–87. doi: 10.1016/j.mito.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 29.Slagsvold KH, Moreira JB, Rognmo O, Hoydal M, Bye A, Wisloff U, Wahba A. Remote ischemic preconditioning preserves mitochondrial function and activates pro-survival protein kinase Akt in the left ventricle during cardiac surgery: A randomized trial. Int J Cardiol. 2014;177:409–417. doi: 10.1016/j.ijcard.2014.09.206. [DOI] [PubMed] [Google Scholar]
- 30.Moretti C, Cerrato E, Cavallero E, Lin S, Rossi ML, Picchi A, Sanguineti F, Ugo F, Palazzuoli A, Bertaina M, et al. The EUROpean and Chinese cardiac and renal remote ischemic preconditioning study (EURO-CRIPS CardioGroup I): A randomized controlled trial. Int J Cardiol. 2018;257:1–6. doi: 10.1016/j.ijcard.2017.12.033. [DOI] [PubMed] [Google Scholar]
- 31.Pistolesi V, Regolisti G, Morabito S, Gandolfini I, Corrado S, Piotti G, Fiaccadori E. Contrast medium induced acute kidney injury: A narrative review. J Nephrol. 2018;31:797–812. doi: 10.1007/s40620-018-0498-y. [DOI] [PubMed] [Google Scholar]
- 32.Zhao Y, Zheng ZN, Pi YN, Liang X, Jin SQ. Cardioprotective effects of transfusion of late-phase preconditioned plasma may be induced by activating the reperfusion injury salvage kinase pathway but not the survivor activating factor enhancement pathway in rats. Oxid Med Cell Longev. 2017;2017:8526561. doi: 10.1155/2017/8526561. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.