Abstract
Mucormycosis isolated to the mandible is a rare presentation occurring generally after dental procedures. The case we report presented with discharging sinuses over facial region with radiological appearance of isolated osteomyelitis of the mandible. The patient used to apply an addictive dental powder over his teeth leading to caries. Following this, he pulled out all his teeth, which probably led to his condition. Invasive sampling revealed mucormycosis. An extensive search for an underlying immunodeficiency revealed that the patient had chronic granulomatous disease (CGD). Despite a prolonged course of L-Amphotericin B, the patient continued to have intermittent pus discharge and surgical debridement and curettage was eventually required. The patient had a chronic course with minimal soft tissue involvement which initially did not raise the suspicion of mucormycosis. The main learning point is that an unusual invasive fungal infection in an otherwise healthy host can be the first symptom of an underlying primary immunodeficiency, like CGD. Invasive fungal infections in patients with CGD often have an indolent course.
Keywords: Mucormycosis, Mandible, Osteomyelitis, Liposomal amphotericin B, Chronic granulomatous disease
Highlights
-
•
Mucormycosis isolated to the mandible is a rare presentation, occurring generally after dental procedures.
-
•
Self-extraction of teeth as in our patient can result in isolated mucormycosis of the mandible.
-
•
Isolated mucormycosis of the mandible can be a presenting symptom of chronic granulomatous disease.
-
•
Combination of medical and surgical treatment is usually needed in mandibular mucormycosis.
1. Introduction
Mucormycosis (zygomycosis) has high prevalence in India due to high endemicity of diabetes and humid climate, which are conducive to the growth of the fungi. It is known to cause life-threatening infections, both in immunocompromised individuals, like in uncontrolled diabetes mellitus (especially in keto-acidosis), hematological malignancies, hematopoietic stem cell/solid organ transplant settings, long term glucocorticoid therapy, persistent neutropenia, and immunocompetent individuals, in whom the incidence is on the rise [[1], [2], [3], [4]]. Additional risk factors include iron overload or chelation with deferoxamine therapy, injection drug use, prophylaxis with voriconazole or echinocandins, breach of skin or mucosa due to trauma, burns or surgical wounds [[1], [2], [3], [4]]. Among the known presentations, mucormycosis isolated to the mandible is a rare presentation and only few case reports exist of the same, occurring generally after dental procedures [[5], [6], [7], [8]]. In patients with chronic granulomatous disease, Aspergillus is the most commonly reported fungal infection while mucormycosis infection is rare and mainly in patients receiving immunosuppression for autoinflammatory manifestations like Systemic Lupus erythematosus (SLE)/Juvenile Idiopathic Arthritis (JIA) [9,10]. Surgical treatment coupled with Liposomal Amphotericin B (L-AMB), as the drug of choice, are the mainstay of treatment of mucor, which is a highly resilient organism. We present the case of an individual with mucormycosis of the mandible, later detected to have chronic granulomatous disease.
2. Case report
We report a case of a 37-year-old male, presented to the out-patient department of our tertiary care centre (Day 0), after having visited several hospitals, with the complaints of loosening of teeth in lower jaw for 10 months, pus discharge (foul-smelling) from within oral cavity for 9 months, swelling over bilateral pre-auricular region (right followed by left), a discharging sinus in bilateral pre-auricular region and insidious onset hearing loss for 6 months. He had a history of tension-type headache for 5 years with history of Ibuprofen and Paracetamol overuse. He also reported depressive symptoms along with insomnia, for which he was abusing Alprazolam.
Further history revealed a history of use of a locally prepared dental powder with addictive potential, commonly used in rural India. This led to poor dental hygiene and periodontal health, possibly leading to loosening of his teeth. There is also history of abuse of an unknown oral medication for weight gain, probably a steroid.
He visited a local dentist who advised extraction of teeth because of caries. Not compliant with the advice he pulled out the incisors in his lower jaw himself. This led to pus discharge from within the oral cavity and loosening of all teeth in lower jaw, about a month later, and he pulled all the teeth out one after another. Over the next 3 months, he developed swelling over the right pre-auricular region followed by left, discharging sinus in bilateral pre-auricular region followed by angle of jaw (right). The pus discharge was sero-sanguineous, with no passage of grains.
There was history of weight loss, loss of appetite and easy fatiguability for 6 months. He had no past or family history of similar complaints. There was no history of recurrent infections in the past. No history of tuberculosis in self or family or contact with a case of tuberculosis. No history of any radiation to face through radiotherapy. No history of use of injectable bisphosphonates. Personal history revealed that he was a non-smoker/non-alcohol user. He used to chew betel nut and used a locally prepared dental powder. He was born out of a consanguineous marriage.
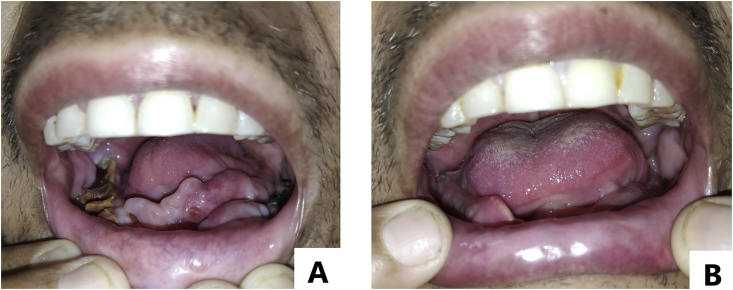
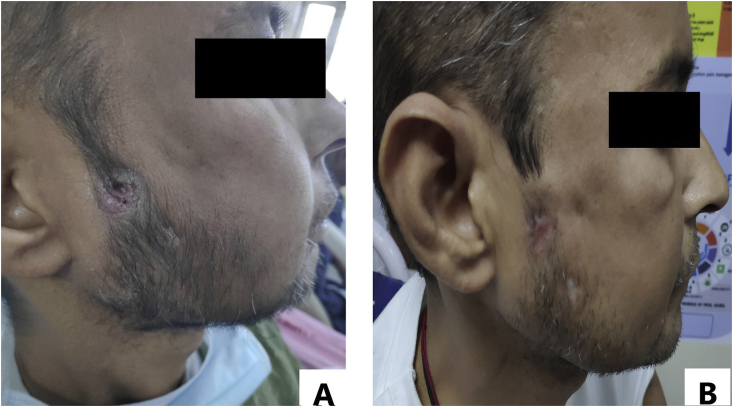
On examination, the patient was vitally stable and afebrile, thinly built with a Body Mass Index (BMI) of 17.2 kg/m2. He had pallor and diffuse alopecia with thinning of hair. Oral cavity showed no teeth in lower alveolus. There was a central smooth proliferative growth over lower gingiva. Posteriorly alveolar bone was exposed and appeared brown-black but no visible pus point (Fig. 1A). Upper teeth were nicotine-stained without loosening or pus discharge. Buccal mucosa, palate and tongue appeared healthy. Mouth opening was normal. Sinus tracts opened ~2 cm anterior to right tragus (Fig. 2A), 4 cm anterior to left tragus and near right angle of mandible. A pus point was present over right pre-auricular sinus while the openings of other two sinuses showed signs of healing with scar tissue. Systemic examination was within normal limits except for profound bilateral hearing loss.
Fig. 1.
[A, B]: Smooth proliferative growth in oral cavity, with alveolar bone exposed posteriorly appearing brown-black. [A] Pre-treatment [B] At discharge. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 2.
[A, B]: Sinus tract [A] At admission [B] At hospital discharge.
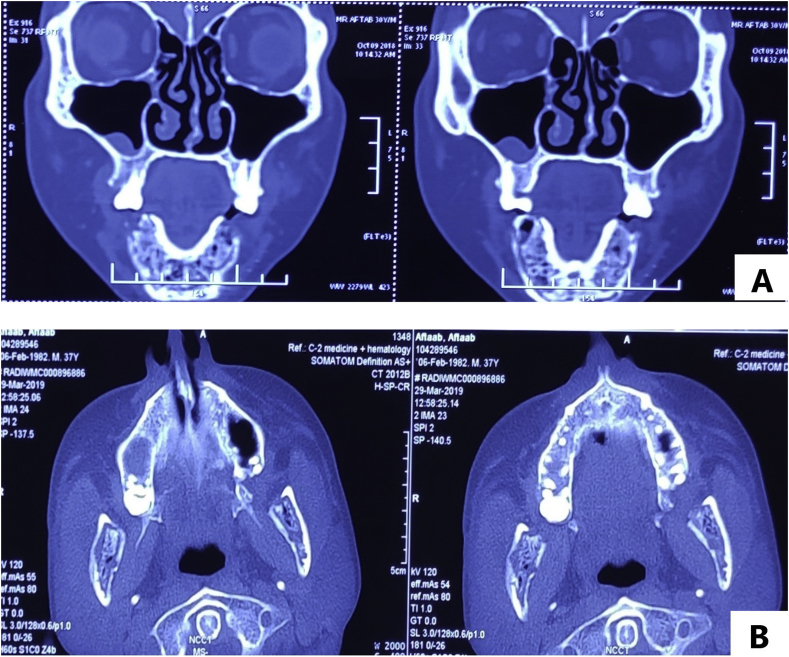
Ortho-pantomogram (OPG) done at initial presentation to the dentist showed all teeth intact. Non-contrast Computed Tomography (NCCT) face, done in an outside hospital (Fig. 3A), showed moth-eaten pattern of bone destruction involving entire mandible, reaching condylar processes with cortical erosion and multiple intra-medullary air spaces (suggestive of chronic osteomyelitis). Previous pus cultures showed no growth with negative Acid Fast Bacilli (AFB) staining and GeneXpert. However, Potassium hydroxide preparation (KOH test) showed a doubtful possibility of a-septated hyphae.
Fig. 3.
[A, B]: CT face coronal [A] At onset [B] At admission.
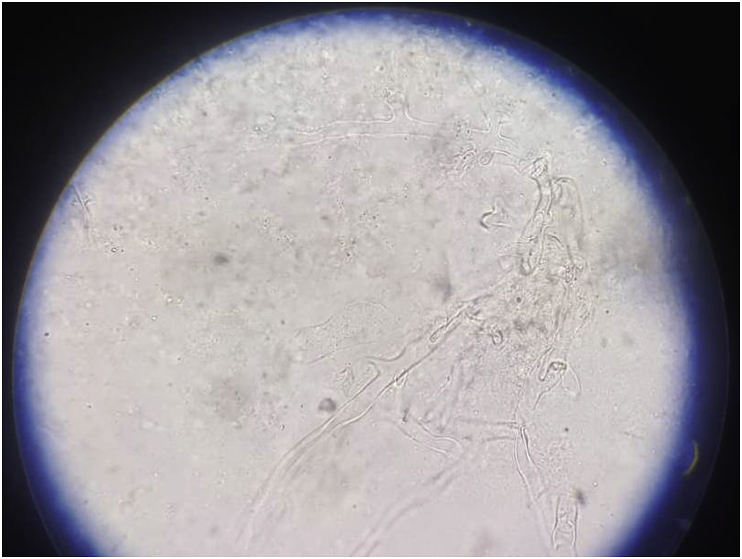
After admission on day 1, blood investigations showed low Hemoglobin (6.7g/dL), elevated ESR (63mm/h) and low albumin (1.9g/dL). Urea and creatinine were normal at presentation (28mg/dL, 0.9mg/dL). Intravenous (IV) Augmentin was started empirically to cover for cervico-facial actinomycosis after sending cultures. Pus discharge showed no growth and fungal stains, AFB stain and GeneXpert were negative. Blood sent for bacterial, anaerobic and fungal culture was sterile. Contrast-Enhanced Computed Tomography (CECT) done on day 2 showed progression of disease (Fig. 3B). A biopsy was taken from the central proliferative growth within the oral cavity, on day 3, KOH mount did not show any fungal elements. While the histopathological examination (HPE) report was awaited, IV L-AMB was started on day 3, at 3mg/kg with a high suspicion of Mucor spp infection. Simultaneously, workup was sent to look for evidence of immunosuppression, which included investigations to look for Diabetes mellitus (blood glucose-Fasting/PostPrandial, Hemoglobin A1c), Chronic kidney disease (Ultrasound KUB, intact Parathyroid hormone/vitamin D), autoimmune workup (Anti Nuclear Antibody, urine protein/active sediments), HIV, HBsAg, anti HCV which were all normal/negative and CD4 level (658) which was normal. Pure tone audiometry revealed bilateral sensorineural hearing loss and records showed that he had received Amikacin outside for about a month. Workup was also sent to look for primary T cell/NK cell immunodeficiency and Chronic Granulomatous Disease. Meanwhile, the HPE report showed benign fibroepithelial polypoid tissue (chronic inflammatory reaction). Bone biopsy was done on day 10, from the site of maximum uptake on Positron Emission Tomography (PET) and the KOH mount revealed broad a-septated hyphae (Fig. 4) which confirmed the diagnosis, further testing with Polymerase Chain reaction (PCR) was not performed as it would not have led to change in the provided treatment. Culture was negative. IV L-AMB dose was increased to 5mg/kg (day 10). There was a response to increased dose of L-AMB with cessation of pus discharge from the right pre-auricular sinus within 1.5 days (day 12). The biopsy was also sent for culture which grew Klebsiella pneumoniae, and IV Imipenem was started (day 12) based on sensitivity pattern for 2 weeks (till day 25). PET did not show evidence of any systemic infection. The patient subjectively felt better after increasing the dose in terms of increased appetite, oral intake, mobility, and general well-being.
Fig. 4.
KOH mount from biopsy.
The patient developed acute kidney injury on day 22 (AKI) but L-AMB was continued. An NCCT face repeated 4 weeks after L-AMB therapy (day 32) did not show any resolution in the chronic osteomyelitis. A decision was then taken for surgical debridement and curettage (done on day 34) to remove the necrotic tissue and decrease burden of the disease. Hemi/total mandibulectomy was not considered due to the morbidity associated with the procedure. The debrided tissue was sent for cultures, AFB staining, fungal stain, and GeneXpert. KOH mount showed tissue studded with a-septated hyphae with HPE and fungal stains confirming the same (Fig. 5[A-D]). L-AMB was continued and regular intra-oral necrotic slough removal and packing was done. The immunodeficiency workup revealed low neutrophil oxidative index (NOI) 26.6 (168 in control), suggestive of CGD (day 45). Genetic confirmation was not performed due to financial constraints. Cotrimoxazole prophylaxis was started. L-AMB was combined with oral posaconazole for 10 days (days 51–60) and the patient was then discharged in a stable ambulatory state (day 61). (Fig. 1, Fig. 2B). He was discharged on oral Posaconazole suspension (4 × 200mg), to be taken with fatty meal and carbonated drinks after giving a total of 13.45 g of L-AMB.
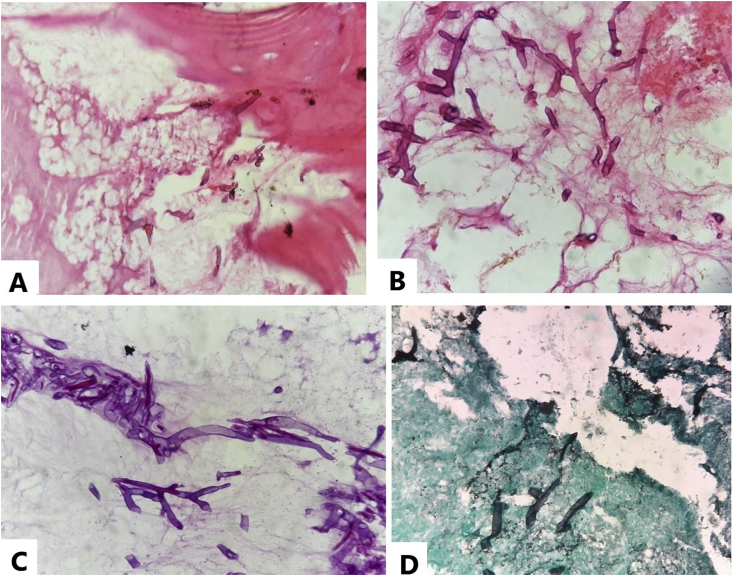
Fig. 5.
[A, B, C, D]: Histopathological images of debrided necrotic bone. Hematoxylin and Eosin images 200x [A] and 400x [B] show fragments of dead bony trabeculae with necrosis and aseptate, wide-angle (≥90°) branching hyphae with non-parallel walls. [C] Periodic Acid Schiff-Diastase, 400x and [D] Gomori Methenamine stain, 400x highlighting hyphael forms of mucor.
3. Discussion
Mucormycosis, an infective disease caused by Mucor genera of the order Mucorales fungi is primarily a disease of immunocompromised and diabetics. The most common involvement is sinus (39%) followed by pulmonary (24%) and cutaneous (19%) as found in a review of 929 cases of mucormycosis [11]. Depending upon the involvement, the mode of transmission is either spore inhalation or direct inoculation of spores. Currently, there are only a few case reports of mandibular Mucormycosis, stating that it is a very rare entity.
We searched for case reports reporting mucormycosis of mandible using the PubMed interface of MEDLINE (National Library of Medicine). The terms used were mandibular mucormycosis, periodontal mucormycosis, and oral mucormycosis (summarized in Table 1).
Table 1.
Reported cases of isolated mucormycosis of mandible.
| Study type/Author | Age/Sex | Risk factor | Management | Outcome |
|---|---|---|---|---|
| Report/McSpadden et al. | 63/M | CML, post bone marrow transplant with Graft vs Host disease | Surgical | Death |
| Report/Oswal et al. | 68/F | Uncontrolled Diabetes mellitus (DM)/dental extraction | Surgical + L-AMB 50mg OD (1 day) | Death |
| Series/AB Urs et al. | 26/M | Dental extraction | Surgical + L-AMB | Satisfactory resolution |
| Report/Ojeda-Uribe M. | 55/F | AML/uncontrolled DM | Surgical + ABLC (Amphotericin B Lipid Complex) (5mg/kg)+ Caspofungin ABLC: 19.6 g (56 days) with Caspofungin followed by 12.2 g (35 days) |
Satisfactory resolution |
| Reports (2)/Bakathir | 14/M 49/M |
1.AML/dental extraction 2. DM with Diabetic ketoacidosis/ALL |
Surgical + L-AMB (Ambisome) for both | Resolution in both |
| Report/Salisbury et al. | 60/M | AML/oral tobacco use/dental extraction | Surgical + AMB | Satisfactory resolution at 1y |
| Report/Jones et al. | 43/M | AML with renal dysfunction | Surgical + AMB (80mg/d) | Satisfactory resolution at 1y |
| Report/Brown and Finn | 57/M | Uncontrolled DM with Chronic Kidney Disease | Total mandibulectomy + AMB | Death |
| Reports (2)/Mutan Hamdi Aras | 15/M 6/M |
1.AML 2.Neuroblastoma |
1. Surgical + AMB (80mg/d x 2months) 2. Surgical + AMB (50mg/d x 2months) |
Death (leukemia) Death (neuroblastoma) |
| Report/Dogan et al. | 7/M | AML | Surgical + L-AMB 80mg/d | Death |
| Reports (3)/Cohen A et al. | 21/F 14/M 41/M |
1.B-ALL 2. ALL 3. AML, voriconazole therapy for 14d |
Surgical (marginal mandibulectomy)+ L-AMB in all cases |
Satisfactory resolution followed by reconstruction of mandible (all cases) |
| Our case | 37/M | Self-extraction of teeth, renal dysfunction, chronic granulomatous disease | Surgical debridement + L-AMB (Fungisome-13.45g) + Posaconazole 800mg/d | Satisfactory resolution (clinically at 4 weeks) |
An extensive search revealed only 15 reported cases to date, of isolated mucormycosis of mandible with satisfactory resolution in 9 cases and death in 6, with most patients dying of the underlying condition. Most common underlying risk factor for mucor in this review was leukemia, (11/15 cases). Four cases had uncontrolled diabetes as a risk factor with only 2 having diabetes as the sole factor. Dental extraction was present in 4 cases.
There were many points against mucormycosis at initial presentation in our case, making it an atypical presentation. Historically and clinically, 8-9 month-long history, with patient not becoming toxic and severely ill was against mucor. Necrosis was only limited to bone with no skin changes. Mucormycosis causing isolated involvement of the mandible is a rare presentation and it is not a common etiology for cervico-facial discharging sinuses. CT image with no soft tissue component and no invasion beyond the confines of the bone also made mucormycosis less likely.
The spores of Mucor are ubiquitous, so the chance of infection and disease progression depends upon the host's immune system status. An active search for such states is warranted if the diagnosis of Mucormycosis is kept. As in our case, the immunodeficiency evaluation revealed Chronic Granulomatous Disease. Although Aspergillosis is the most common fungal disease in CGD patients, Mucormycosis is also seen in these patients, mainly in the setting of treatment of inflammatory complications with immunosuppressive drugs, like steroids or methotrexate [9,10]. The use of local dental powder and manual extraction of teeth by the patient himself appear to be the crucial modes of spore inoculation to gums and mandible. Apart from CGD, chronic abuse of NSAIDs, alprazolam, the dental powder and development of acute kidney injury need more study to turn up as recognizable risk factors. It is vital that we understand the patient's background mental state and health, which led him to behave in such a way so as to pull out his teeth himself. Comparing mucormycosis in patients of CGD, with common risk factors like Diabetes, neutropenia or iron overload, suggests that presentation of invasive fungal infections in CGD is mostly indolent with onset in most patients being preceded by an immunosuppressive regimen for 3 or more weeks, in the absence of traditional risk factors like neutropenia and Diabetes [9].
PET confirmed, locally progressive disease with no dissemination is probably the cause of uncomplicated benign course and early therapy response. This report also recognizes that being aseptate, mucor hyphae are very fragile making their detection difficult, as seen in our first attempt of diagnosis. Our patient was initially treated with L-AMB and later on shifted to Posaconazole. Posaconazole has been studied as a salvage therapy in zygomycosis and has proved efficient in 80% of Amphotericin B resistant disease or when facing adverse drug events [12].
Currently, there are no guidelines on dosage and duration of antifungal therapy in cases of Mucormycosis, with treatment duration completely based on clinical recovery.
4. Conclusion
Though the most common form of Mucormycosis is rhino-cerebral, clinicians do encounter other rare forms like locally invading mandibular Mucormysosis without any dissemination as in our case. Invasive fungal infections in patients with CGD often have an indolent course and can be a presenting symptom of underlying primary immunodeficiency, e.g. CGD [10]. Currently, there is a need for more evidence for setting a definite dose and duration of antifungal therapy to alleviate the financial burden on patient and drug-related adverse events.
Declaration of competing interest
There are no conflicts of interest.
Acknowledgement
There are no acknowledgements to be made.
References
- 1.Petrikkos G., Skiada A., Lortholary O., Roilides E., Walsh T.J., Kontoyiannis D.P. Epidemiology and clinical manifestations of mucormycosis. Clin. Infect. Dis. 2012;54(Suppl. 1):S23–S34. doi: 10.1093/cid/cir866. [DOI] [PubMed] [Google Scholar]
- 2.Tansir G., Rastogi N., Ramteke P., Kumar P., Soneja M., Biswas A. Disseminated mucormycosis: a sinister cause of neutropenic fever syndrome. Intractable Rare Dis Res. 2017;6:310–313. doi: 10.5582/irdr.2017.01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chakrabarti A., Singh R. Mucormycosis in India: unique features. Mycoses. 2014;57(Suppl. 3):85–90. doi: 10.1111/myc.12243. [DOI] [PubMed] [Google Scholar]
- 4.Prakash H., Ghosh A.K., Rudramurthy S.M., Singh P., Xess I., Savio J. A prospective multicenter study on mucormycosis in India: epidemiology, diagnosis, and treatment. Med. Mycol. 2018;57(4):395–402. doi: 10.1093/mmy/myy060. [DOI] [PubMed] [Google Scholar]
- 5.Gupta N., Kumar R., Soneja M., Singh G., Khot W., Malla S. Mucor menace in an immunocompetent young male after dental manipulation. J. Fam. Med. Prim. Care. 2019;8:757–759. doi: 10.4103/jfmpc.jfmpc_412_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown Orval E., Finn Richard. Mucormycosis of the mandible. J. Oral Maxillofac. Surg. 1986;44(2):132–136. doi: 10.1016/0278-2391(86)90196-5. [DOI] [PubMed] [Google Scholar]
- 7.Aras Mutan Hamdi, Kara Muhammed Isa, Erkiliç Suna, Ay Sinan. Mandibular mucormycosis in immunocompromised patients: report of 2 cases and review of the literature. J. Oral Maxillofac. Surg. 2012;70(6) doi: 10.1016/j.joms.2011.05.012. 1362-8, 0278–2391. [DOI] [PubMed] [Google Scholar]
- 8.Prakash Oswal Nitin, Pushkar Kiran Gadre, Prachee Sathe, Kiran Shrikrishna Gadre . vol. 2012. 2012. (“Mucormycosis of Mandible with Unfavorable Outcome,” Case Reports in Dentistry). Article ID 257940, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vinh D.C., Freeman A.F., Shea Y.R., Malech H.L., Abinun M., Weinberg G.A. Mucormycosis in chronic granulomatous disease: association with iatrogenic immunosuppression. J. Allergy Clin. Immunol. 2009;123:1411–1413. doi: 10.1016/j.jaci.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warris A., Henriet S.S.V. Invasive fungal infections in the child with chronic granulomatous disease. Curr Fungal Infect Rep. 2014;8:37–44. doi: 10.1007/s12281-013-0168-4. [DOI] [Google Scholar]
- 11.Roden M., Zaoutis T., Buchanan W., Knudsen T. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin. Infect. Dis. 2005;41(5):634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 12.Greenberg R., Mullane K., van Burik J., Raad I., Abzug M., Anstead G. Posaconazole as salvage therapy for zygomycosis. Antimicrob. Agents Chemother. 2005;50(1):126–133. doi: 10.1128/AAC.50.1.126-133.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]