Abstract
Blastomycosis is a systemic fungal infection which primarily involves the lungs but can disseminate to involve extrapulmonary sites. Current testing that exists includes sputum, urine, serum, and pathological tissue analysis. Radiological testing is often non-specific and highly variable. Here we present five cases of pulmonary blastomycosis with challenging radiographic presentations.
Keywords: Blastomycosis, Radiological findings in pulmonary blastomycosis, Pneumonia, Dimorphic fungi, Acute respiratory distress system
1. Introduction
Blastomyces, a group of thermally dimorphic fungi, grow as filamentous mold in the environment and as yeast in humans. The primary mode of infection is by inhalation of the conidia of Blastomyces dermatitidis (B. dermatitidis) or Blastomyces gilchristii (B. gilchristii) which results in a pyogranulomatous pulmonary infection. Infection occurs in both healthy and immunocompromised individuals and ranges from asymptomatic presentation, acute and/or chronic pneumonia, acute respiratory distress syndrome and even death. Dissemination occurs hematogenously; manifestations of extrapulmonary disease commonly occur in the skin, bones, genitourinary and central nervous systems.
Blastomyces is endemic to North America, particularly the states bordering the Ohio, Mississippi, and St. Lawrence Rivers, as well as the Great Lakes [1]. Rare cases of blastomycosis have been reported outside of North America; approximately 100 cases have been reported in continental Africa and less than 10 cases have been reported in India [2,3].
Delays in diagnosis of greater than 4 weeks have been observed in greater than 40% of patients, often due to the fact that blastomycosis is not considered as a primary agent of infection [4]. Here, we present five cases of pulmonary blastomycosis that have varying degrees of radiological findings in order to bring awareness to fellow clinicians.
2. Case presentation
2.1. Case 1
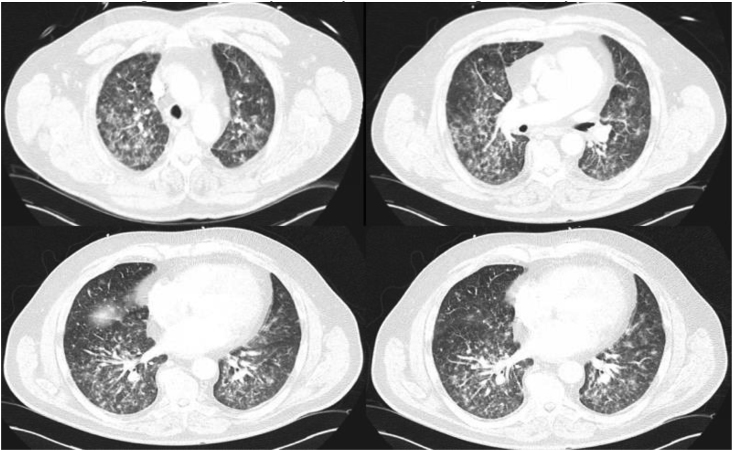
A 64-year-old male with a history of hypertension, hyperlipidemia and recent history of right lower extremity tibial pain who underwent a bone biopsy a week prior presented to the emergency department (ED) for acute hypoxic respiratory failure with chest x-ray (CXR) showing diffuse bilateral infiltrates. Antibacterial therapy was started with intravenous (IV) cefepime 2 gm every 8 h, IV vancomycin 1.5 gm every 24 h, and IV azithromycin 500 mg every 24 h. The patient had increasing oxygen requirements and became progressively lethargic requiring intubation with initiation of mechanical ventilation. Blood chemistries and hematological tests were unremarkable at the time. Computed tomography (CT) of the chest was performed which revealed persistence of bilateral interstitial and nodular opacities (Fig. 1). Work up for bacterial pneumonia was negative, including sputum cultures, urinary Streptococcal antigen, and urine Legionella antigen. Work up for fungal pneumonia was thus undertaken on day +5 of admission. Results from the patient's tibial biopsy returned positive for a dimorphic fungus the same day. Serum blastomyces antibody was positive along with urine blastomyces antigen. Cultures from respiratory samples obtained from bronchoscopy with bronchial washings revealed B. dermatitidis. IV amphotericin B was added to his antibiotic regimen at a dosage of 5mg/kg/day . He was then successfully extubated on day +14 and completed therapy with IV amphotericin B for 4 weeks. After completion of therapy, he was transitioned to oral itraconazole 200 mg three times daily for 3 days and then 200 mg twice a day for 12 months.
Fig. 1.
Bilateral reticulonodular alveolar and interstitial opacities of pulmonary blastomycosis.
2.2. Case 2
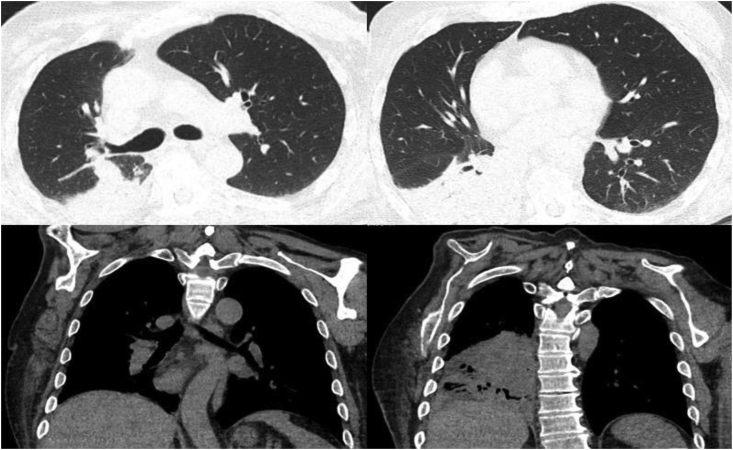
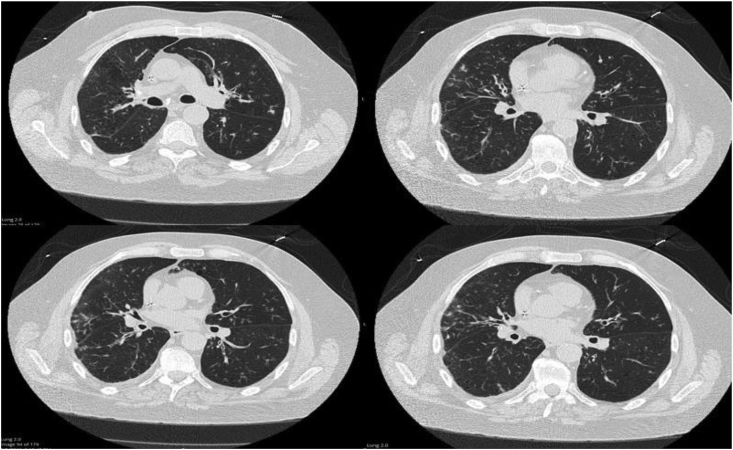
A 61-year-old male with a history of right-sided hemiparesis presented to the hospital for complaints of lethargy and chronic productive cough for a month. He denied fevers, chills, night sweats, or changes in weight. His initial biochemical and hematological tests were within normal limits. CT Chest revealed a 9 mm endobronchial lesion at the entry of the right lower lobe bronchus and right lower lobe opacification (Fig. 2). Patient was started on ceftriaxone and doxycycline to cover empirically for community acquired pneumonia. Sputum cultures returned negative, blastomyces antibody returned equivocal for blastomycosis, whereas histoplasma antibody returned positive with a titer of 1:32. The patient underwent bronchoscopy on day +5, and a globular appearing mass was visualized at the beginning of the right bronchus intermedius that appeared to be necrotic and mucinous and completely obstructed the bronchus. Bronchial washing, endobronchial biopsies and endobronchial brushings of the mass were taken. Preliminary pathology of the endobronchial mass revealed a budding yeast and anti-microbial treatment was switched to IV amphotericin B 5 mg/kg/day and IV piperacillin-tazobactam 4.5 g every 8 h. Both respiratory cultures from bronchial washings and pathology from the bronchial mass confirmed B. dermatitidis. Patient completed 2 weeks of IV amphotericin B followed by oral itraconazole 200mg twice a day for 12 months.
Fig. 2.
Right lower lobe atelectasis with endobronchial lesion.
2.3. Case 3
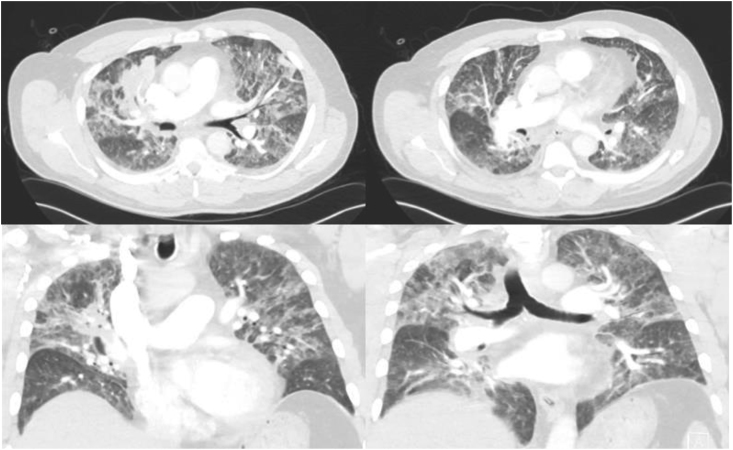
A 68-year-old male with a history of benign prostatic hypertrophy, chronic prostatitis and prostate cancer presented to the hospital with acute urinary retention due to prostatitis. On day +2 of admission the patient became febrile with leukocytosis (WBC 19.2 10^9 u/L ), acute kidney injury (Cr 1.4 mg/dL) and acute hypoxic respiratory failure; CT chest showed diffuse mixed interstitial, ground-glass and alveolar infiltrates in the upper and lower lobes bilaterally and mediastinal lymphadenopathy (Fig. 3). The patient was diagnosed with multilobar pneumonia, transferred to the intensive care unit on day +3 and started on high-flow nasal oxygen therapy and broad-spectrum antibiotics (IV vancomycin 1.25 gm every 24 h, IV piperacillin-tazobactam 4.5 gm every 8 h, IV levofloxacin 750 mg every 48 h). His respiratory status continued to deteriorate and the patient was intubated and managed for acute respiratory distress syndrome. A bronchoscopy performed on the same day removed purulent fluid and bronchoalveolar lavage (BAL) was performed. Repeat CXR demonstrated worsening infiltrates and decision was made to start IV amphotericin B 5mg/kg/day. The patient continued to worsen and unfortunately succumbed to his respiratory failure requiring transfer to a higher-level center for extracorporeal membrane oxygenation. On day +7 after transfer, fungal cultures of the BAL were positive for very few yeasts; final results via nuclear ribosomal internal transcribed spacer demonstrated B. dermatitidis.
Fig. 3.
Diffuse bilateral mixed alveolar and interstitial opacities with hilar and mediastinal lymphadenopathy.
2.4. Case 4
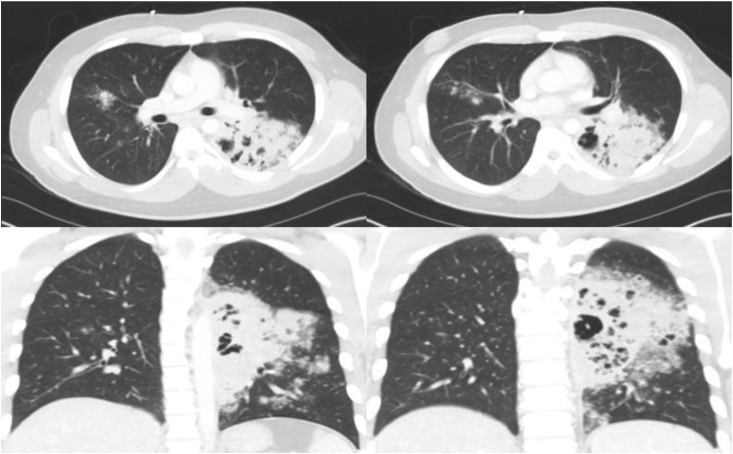
A 29-year-old male with a history of seizures initially presented to an outside hospital ED for pleuritic chest pain. CT chest demonstrated a small rounded area of airspace opacification within the posterior medial left lower lobe and surrounding ground glass opacification suggestive of a small pneumonia. The patient was subsequently discharged from the ED with oral azithromycin 250 mg daily for 5 days. The patient presented to the ED on 2 additional occasions, being discharged with cyclobenzaprine and ibuprofen first, with a subsequent ED visit resulting in hospitalization and discharge with a 7-day course of oral amoxicillin-clavulanic acid 875-125 mg twice a day. He again re-presented to our ED, 17 days later, with complaints of fever, chills and an approximately 22 pounds weight loss. Labs were significant for a leukocytosis of 33.2 10^9 u/L , AST/ALT elevation of 66/87 IU/L and procalcitonin of 0.61 ng/mL. CT chest showed a confluent consolidative process with tree in bud pattern opacification, predominantly at the left lower lobe (Fig. 4). He was started on broad-spectrum antibiotics (IV vancomycin 1.25 gm every 24 h, piperacillin-tazobactam 4.5 gm every 8 h, IV levofloxacin 750 mg every 24 h). Bronchoscopy performed on day +2 of admission showed a significant amount of purulent secretions at the superior segment of the left lower lobe with erythematous and inflamed mucosa. The blastomycosis immunodiffusion was positive, and BAL fungal culture was positive for B. dermatitidis. The patient was initially treated with IV amphotericin B at a dose of 5 mg/kg/day for a 14-day course and was discharged on a 6-month course of oral itraconazole 200 mg twice daily.
Fig. 4.
Left upper lobe consolidation with multiple cavitary lesions suspicious for necrotizing pneumonia. Right upper and left lower lobe tree-in-bud opacities.
2.5. Case 5
A 65-year-old male with a 150 pack per year smoking history and history of chronic hypoxic respiratory failure secondary to severe chronic obstructive pulmonary disease presented to the ED with a one-week history of productive cough of whitish-green sputum and bilateral lower extremity weakness. Labs were significant for creatinine elevation (1.75 mg/dL), transaminitis (AST/ALT 85/111 IU/L), leukocytosis (11.8 10^9 u/L ), B natriuretic peptide elevation (1264 pg/mL). CT chest revealed tree-in-bud opacities in bibasilar lung fields and a cavitary nodule in the right lower lobe, concerning for endobronchial fungal infection versus infectious bronchitis. He was admitted to the hospital and treated for presumed infectious bronchitis with a 5-day course of IV azithromycin 500 mg daily and IV ceftriaxone 1 gm daily. A repeat CT chest performed on day +3 later continued to show findings consistent with a calcified granuloma in the right upper lobe, diffuse airway thickening and tree-in-bud opacities (Fig. 5). Bronchoscopy revealed grossly normal airways and BAL fungal culture revealed budding yeast consistent with B. dermatitidis. Serum immunodiffusion was also positive for blastomycosis. The patient was treated with a 10-day course of oral amoxicillin-clavulanic acid 875-125 mg and a 6-month course of oral itraconazole 200 mg twice daily.
Fig. 5.
Bilateral tree-in-bud opacities, worse on the right.
3. Discussion
The epidemiology of Blastomycosis in North America is largely based on retrospective studies, clinical reports and analyses of outbreaks due to a lack of standardized antigen test. The annual incidence of blastomycosis ranges from 0.2 to 1.94 cases per 100,000 persons and approximately half of infected persons have subclinical or asymptomatic illness and are unreported, likely resulting in a decreased incidence [5,6].
Phylogenetic and population genetic analyses have identified two genetically isolated monophyletic clades within the pathogenic fungus B. dermatitidis and B. gilchristii [7]. Most cases of blastomycosis have been reported in North America, predominantly in the states bordering the Ohio and Mississippi river basins and midwestern states and Canadian provinces located around the Great Lakes. Outside of North America, blastomycosis has been reported occasionally in Africa, Central and South America and India.
In the endemic areas, Blastomyces inhabits ecological niches characterized by decaying organic material, forested sandy soils with low pH, and rotting wood located near water sources, and outbreaks have often been associated with waterways [8]. Exposure to soil appears to be the common factor associated with both endemic and epidemic disease.
The primary site of infection of B. dermatitidis is known to be lungs and the pathogenesis begins with inhalation of aerosolized conidia. Conidial forms of B. dermatitidis are very small in size (2–10 μm) and are easily inhaled into the lungs. Some reach the periphery of the lung while others stay central. Once they reach the lungs via inhalation, they initiate a granulomatous inflammatory reaction, which is mediated by neutrophils, monocytes, and macrophages. Generally, the immune response from the host cell can destroy the conidia before converting them into yeast. However, at times, the conidia become resistant to the host response and convert to the yeast form, resulting in an infection. Once they convert to yeast form in the lungs, they can further disseminate and reach extrapulmonary sites such as skin, bone, and the central nervous system.
Historically, chemical blood tests, body fluid (urine/sputum), or tissue specimens have been used to help physicians diagnose blastomycosis. This process, however, is operator dependent and requires experience with slide analysis in order to differentiate between fungi [9]. The gold standard for diagnosis is growth of the fungus on culture. This process can take 2–4 weeks and anticipating final culture results potentially delays treatment [10]. More recently, antigen enzyme immunoassays (EIA) are used for detection of the fungal antigen in urine, serum, and bodily fluid [10,11]. This testing allows rapidly available results with high sensitivity (92.9%) with moderate specificity (79.3%). It can also be used in monitoring resolution of the disease in response to antifungal therapy [11].
Diagnosing pulmonary blastomycosis radiographically can be challenging. Radiographic findings in pulmonary blastomycosis can vary in location (airspace, lobar and interstitial) and symmetry [12]. Of these, the most common presentation is airspace consolidation, which often manifests as an ill-defined, highly-attenuated material compared to the pulmonary parenchyma. Rarely do we see lobar consolidation reported. Interstitial disease often manifests as consolidation as well and is frequently described as reticular changes that are bilateral and diffuse.
Mass-like lesions occur secondary to airspace opacities. Reportedly, they are paramediastinal and perihilar, roughly between 3 to 10 cm [12]. Unfortunately, these mass-like lesions are indistinguishable from malignant lesions and undergo resection. Its counterpart, nodules, are less commonly observed and are quite variable in size and number. Of the radiographic findings listed above, the least common manifestation of blastomycosis is cavitary lesions.
A retrospective study of 353 patients completed in Canada reviewed CT scan images of 318 patients during a 17.7-year period which revealed air bronchograms were the most common finding among patients, followed by consolidation, and nodules [13].
Our five patients (Table 1) from the state of Illinois had varying degrees of radiographic presentations. There does not appear to be an identified pattern when it comes to clinical presentation and radiological imaging. As seen in cases one-three and five, acute pulmonary manifestations tend to cause parenchymal disease with alveolar infiltrates, tree-in-bud opacities, and interstitial disease. However, this is not mutually exclusive as our second patient did have an endobronchial lesion complicated by osseous blastomycosis. Case four represents a chronic indolent infection where many cavitary lesions were formed.
Table 1.
Characteristics of reported cases.
| Case Number | Age, Y/Sex | Comorbidities | Geographic Location | Diagnostic Testing for Blastomyces | Diagnostic Image Findings |
|---|---|---|---|---|---|
| 1 | 64/M | HTN, HLD | Effingham, Illinois | Serum and urine blastomyces antigen positive. Bronchial washings with B. dermatitidis. Tibial biopsy with B. dermatitidis | Bilateral reticulonodular alveolar and interstitial opacities |
| 2 | 61/M | RS hemiparesis (gunshot) | Pana, Illinois | Serum blastomyces antigen positive. Bronchial washings and endobronchial biopsies revealed B. dermatitidis | Right lower lobe atelectasis with endobronchial lesion |
| 3 | 68/M | HTN, HLD, BPH, chronic prostatitis, prostate cancer | Decatur, Illinois | BAL positive for B. dermatitidis | Diffuse bilateral mixed alveolar and interstitial opacities with hilar and mediastinal lymphadenopathy |
| 4 | 29/M | Seizures, 1 PPD Smoke | Springfield, Illinois | Serum blastomyces immunodiffusion positive. BAL with B. dermatitidis | Left upper lobe consolidation with multiple cavitary lesions suspicious for necrotizing pneumonia. Right upper and left lower lobe tree-in-bud opacities |
| 5 | 65/M | HTN, CAD, CHF, COPD, DMII, 150 pack year smoker | Pana, Illinois | Serum blastomyces immunodiffusion positive. BAL fungal culture with B. dermatitidis | Bilateral tree-in-bud opacities, worse on the right |
Abbreviations: LLE, left lower extremity; RS, right-side; HTN, hypertension; HLD, hyperlipidemia; BPH, benign prostatic hyperplasia; CT, computed tomography; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; DMII, type 2 diabetes; BAL, bronchoalveolar lavage: PPD, pack per day.
Blastomycosis is a diagnostic dilemma that requires a high clinical suspicion. This case series highlights the importance of clinical acuity in the absence of clear radiological findings.
Declaration of competing interest
There are no conflicts of interest to disclose.
Acknowledgements
The authors are thankful to the microbiology department at Memorial Medical Center and St. John's Hospital in Springfield, Illinois for identification and speciation of these organisms.
References
- 1.Benedict K., Roy M., Chiller T., Davis J.P. Epidemiologic and ecologic features of blastomycosis: a review. Curr. Fungal Infect. Rep. 2012 Dec 1;6(4):327–335. [Google Scholar]
- 2.Baily G.G., Robertson V.J., Neill P., Garrido P., Levy L.F. Blastomycosis in Africa: clinical features, diagnosis, and treatment. Rev. Infect. Dis. 1991 Sep 1;13(5):1005–1008. doi: 10.1093/clinids/13.5.1005. [DOI] [PubMed] [Google Scholar]
- 3.Randhawa H.S., Chowdhary A., Kathuria S., Roy P., Misra D.S., Jain S., Chugh T.D. Blastomycosis in India: report of an imported case and current status. Med. Mycol. 2013 Feb 1;51(2):185–192. doi: 10.3109/13693786.2012.685960. [DOI] [PubMed] [Google Scholar]
- 4.McBride J.A., Gauthier G.M., Klein B.S. Clinical manifestations and treatment of blastomycosis. Clin. Chest Med. 2017 Sep 1;38(3):435–449. doi: 10.1016/j.ccm.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC Blastomycosis--Wisconsin, 1986-1995. MMWR. Morbidity and mortality weekly report. 1996 Jul 19;45(28):601. [PubMed] [Google Scholar]
- 6.Furcolow M.L., Chick E.W., Busey J.F., Menges R.W. Prevalence and incidence studies of human and canine blastomycosis: I. Cases in the United States, 1885–1968. Am. Rev. Respir. Dis. 1970 Jul;102(1):60–67. doi: 10.1164/arrd.1970.102.1.60. [DOI] [PubMed] [Google Scholar]
- 7.Brown EM, McTaggart LR, Zhang SX, Low DE, Stevens DA. Phylogenetic Analysis Reveals a Cryptic Species Blastomyces Gilchristii, sp. Nov..
- 8.Klein B.S., Vergeront J.M., Weeks R.J., Kumar U.N., Mathai G., Varkey B., Kaufman L., Bradsher R.W., Stoebig J.F., Davis J.P., Investigation Team Isolation of Blastomyces dermatitidis in soil associated with a large outbreak of blastomycosis in Wisconsin. N. Engl. J. Med. 1986 Feb 27;314(9):529–534. doi: 10.1056/NEJM198602273140901. [DOI] [PubMed] [Google Scholar]
- 9.Durkin M., Witt J., LeMonte A., Wheat B., Connolly P. Antigen assay with the potential to aid in diagnosis of blastomycosis. J. Clin. Microbiol. 2004 Oct 1;42(10):4873–4875. doi: 10.1128/JCM.42.10.4873-4875.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frost H.M., Novicki T.J. Blastomyces antigen detection for diagnosis and management of blastomycosis. J. Clin. Microbiol. 2015 Nov 1;53(11):3660–3662. doi: 10.1128/JCM.02352-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mongkolrattanothai K., Peev M., Wheat L.J., Marcinak J. Urine antigen detection of blastomycosis in pediatric patients. Pediatr. Infect. Dis. J. 2006 Nov 1;25(11):1076–1078. doi: 10.1097/01.inf.0000241144.89426.2a. [DOI] [PubMed] [Google Scholar]
- 12.Fang W., Washington L., Kumar N. Imaging manifestations of blastomycosis: a pulmonary infection with potential dissemination. Radiographics. 2007 May;27(3):641–655. doi: 10.1148/rg.273065122. [DOI] [PubMed] [Google Scholar]
- 13.Ronald S., Strzelczyk J., Moore S., Trepman E., Cheang M., Limerick B., Wiebe L., Sarsfield P., MacDonald K., Meyers M., Embil J.M. Computed tomographic scan evaluation of pulmonary blastomycosis. Can. J. Infect Dis. Med. Microbiol. 2009;20(4):112–116. doi: 10.1155/2009/763018. [DOI] [PMC free article] [PubMed] [Google Scholar]