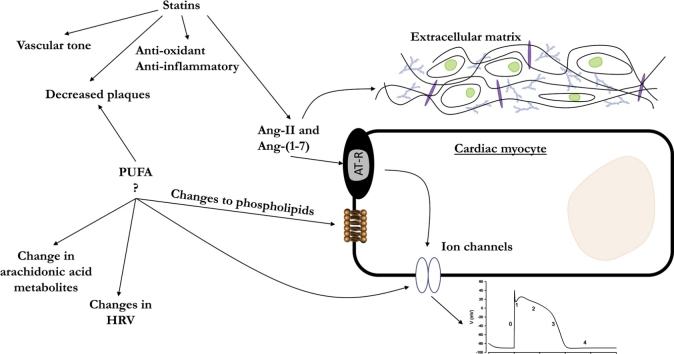
Graphical abstract
Keywords: Antiarrhythmic, Arrhythmia, Nti-arrhythmic, Statins, PUFA, ACE
Abstract
Traditional anti-arrhythmic drugs are classified by the Vaughan-Williams classification scheme based on their mechanisms of action, which includes effects on receptors and/or ion channels. Some known anti-arrhythmic drugs do not perfectly fit into this classification scheme. Other medications/molecules with established non-anti-arrhythmic indications have shown anti-arrhythmic properties worth exploring.
In this narrative review, we discuss the molecular mechanisms and evidence base for the anti-arrhythmic properties of traditional non-antiarrhythmic drugs such as inhibitors of the renin angiotensin system (RAS), statins and polyunsaturated fatty acids (PUFAs).
In summary, RAS antagonists, statins and PUFAs are ‘upstream target modulators’ that appear to have anti-arrhythmic roles. RAS blockers prevent the downstream arrhythmogenic effects of angiotensin II – the main effector peptide of RAS – and the angiotensin type 1 receptor. Statins have pleiotropic effects including anti-inflammatory, immunomodulatory, modulation of autonomic nervous system, anti-proliferative and anti-oxidant actions which appear to underlie their anti-arrhythmic properties. PUFAs have the ability to alter ion channel function and prevent excessive accumulation of calcium ions in cardiac myocytes, which might explain their benefits in certain arrhythmic conditions.
Clearly, whilst a number of anti-arrhythmic drugs exist, there is still a need for randomised trials to establish whether additional agents, including those already in clinical use, have significant anti-arrhythmic effects.
1. Introduction
Anti-arrhythmic drugs (AADs) modulate the activity of ion channels and receptors in the heart. The original Vaughan-Williams classification does not include drugs such as those belonging to the recently proposed class 0 (e.g. ivabradine) [which reduces the sino-atrial node (SAN) automaticity by blocking the pacemaker channel underlying the “funny current” (If, largely HCN4) [1]. Modification of the original classification scheme has become necessary as newer AADs (such as ivabradine, vernakalant, etc) and anti-arrhythmic properties of non-antiarrhythmic medications increasingly become apparent. In this review, we examine the evidence for anti-arrhythmic properties of drugs not traditionally thought of as antiarrhythmic.
1.1. Normal cardiac electrical activity
Cardiac activity relies on its action potential and excitation-contraction coupling. A normal cardiac impulse is generated by the pacemaker cells in the SAN, conducted through the atrioventricular node (AVN), and the His-Purkinje network (HPN) to facilitate activation of the ventricles. Cardiac arrhythmias occur from any abnormalities affecting electrical impulse generation, conduction or both. The SAN, AVN, HPN, coronary sinus (CS) and pulmonary veins (PVs) are capable of spontaneous electrical impulse generation (automaticity) underpinned by spontaneous diastolic depolarisations during phase 4 of the cardiac action potential; although the SAN sets the pace under normal physiological conditions with its pacemaker potential [1].
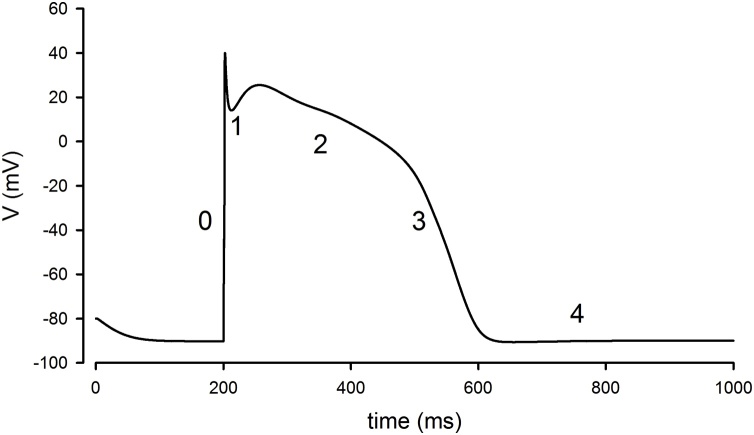
Ion fluxes at different phases of the cardiac potential determine whether there is resulting depolarisation or repolarisation (Fig. 1).
Fig. 1.
The ventricular action potential. Ventricular action potential simulated in python NEURON [150] using an adaptation of the DiFrancesco and Noble model [151] and stimulating with a 2 nA current injection at time 0.2 s. The four phases of the action potential are illustrated on the waveform. Phase 0 is the upstroke of the action potential resulting from the large rapid sodium (Na+) current, activated once the activation threshold is exceeded. Phase 1 occurs from the inactivation of the Na+ current while there is activation of a transient outward potassium (K+) current. Phase 2 is the plateau largely resulting from a balanced inward calcium (Ca2+) and outward delayed rectifier (K+) current. Phase 3, the downward stroke, occurs as the Ca2+ inactivates whilst the delayed rectifier current persists. In a ventricular myocyte, by phase 4 the cell has returned to the resting membrane potential and the voltage-gated currents will “reset” (recover from inactivation), ready for the next action potential. A key difference in nodal tissues (e.g. sinoatrial node) is that phase 4 of the nodal action potential (not shown) is a period of spontaneous depolarisation. Some established anti-arrhythmic drugs modulate specific phases of the action potential by their effects on specific ion currents e.g. Na+ (quinidine, lidocaine, mexiletine, flecainide) and K+ (amiodarone, sotalol, dofetilide). For instance, amiodarone modulates the hERG (human Ether-à-go-go-Related Gene) K+ channel that controls action potential duration [152].
There has been significant progress made in delineating the ion fluxes underlying the different phases of the human cardiac action potential since early attempts by electrophysiologists in the 1900′s using frog, sheep, calf and turtle myocardial models [2]. An initial depolarisation (phase 0) results from a large rapid flux of sodium current (INa) carried by the cell membrane’s fast activating sodium channels when the depolarisation threshold is exceeded. Phase 1, which follows, consists of minor repolarisation resulting from non-conducting inactivated sodium channels and the transient outward potassium current (Ito). The plateau (phase 2) results from a depolarising inward calcium flux carried by the L-type calcium (Ca) channels (which is induced by the initial inward sodium current) and a repolarising outward potassium current. Depolarisation of atrial myocytes activates a transient outward potassium current which rapidly inactivates, leading to a sustained outward current. The sustained current in atrial myocytes is due to activation of an ultra-rapid delayed rectifier potassium current (IKur) [3], [4]. The outward potassium conductance that persists throughout the plateau phase to effect repolarisation is referred to as the ‘delayed rectifier’ current. Phase 3 repolarisation is due to inactivation of the calcium current with persistence of the rapid and slow components of the delayed rectifier potassium current (IKr and IKs). Phase 4 is mediated by multiple potassium channels which carry the repolarising potassium current. These include the transient outward potassium current (Ito), and the delayed rectifier potassium current (IKr and IKs) [4]. The potassium current (IK1) establishes the resting membrane potential of the myocyte during this phase. Spontaneous diastolic (phase 4) depolarisation in cells capable of automaticity (such as nodal cells) is believed to be generated by activation of the inward If current during diastole [5]. Other currents including the potassium and calcium currents, potassium ATPase (IKATP) and the sodium-calcium exchanger (INCX), etc. may play roles in spontaneous diastolic depolarisation, but the extent of such roles need further clarification. More recent molecular characterisation has now largely, but not entirely led to identification of a number of ion channels important for each phase of the action potential (Fig. 2).
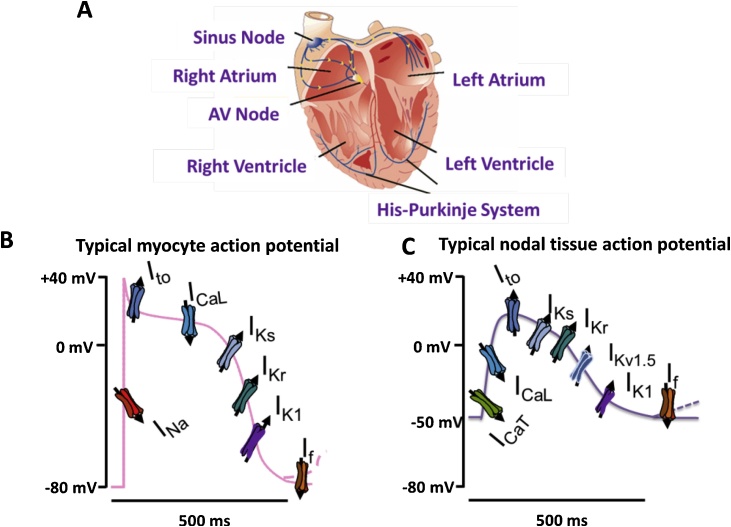
Fig. 2.
Illustration of cardiac action ion channel involvement in cardiac action potentials. (A) The basic cardiac structure with annotated conduction pathway from SAN to AVN via the His-Purkinje pathway. (B) The main ion channels involved with cardiac muscle action potentials, ventricular and atrial myocyte action potentials are similar; although typically the ventricular myocyte will have a broader plateau. (C) The nodal tissue, i.e., sinoatrial (SAN) and atrioventricular (AVN), are also similar with less pronounced spike and less negative resting membrane potential. Current (other name): Gene, Protein [additional genes/proteins]. INa: SCN5A, Nav1.5. Ito (fast): KCND2, Kv4.2 [KCND3, Kv4.3, KCNIP2, KChIP2]. Ito (slow): KCNA4, Kv1.4, ICa-L: CACNA1C, Cav1.2: IKs, KCNQ1, Kv7.1 [KCNE1, mink]. IKr: KCNH2, Kv11.1 [KCNE2, MiRP-1]. IK1: KCNJ2, Kir2.1 [KCNJ12, Kir2.2, KCNJ4, Kir2.3]. If : HCN4, HCN.
ICaT: CACNA1G Cav3.1 [CACNA1H, Cav3.2]. Kv1.5: KCNA5, Kv1.5. Figure from [153] With permission of Elsevier.
2. Angiotensin-Converting enzyme inhibitors/ angiotensin receptor blockers
The Renin-Angiotensin System (RAS) is an established key player in the regulation of fluid and electrolyte balance and blood pressure. Angiotensin II (Ang II) is the main effector peptide of RAS, acting primarily via angiotensin II type 1 (AT1R) and 2 receptors (AT2R) [6]. Angiotensin Converting Enzyme (ACE) is a central component of RAS (given its role in the generation of Ang II), and is a principal target in the management of cardiovascular (hypertension, heart failure, post myocardial infarction) and renal diseases. Ang II, acting on AT1R, ultimately leads to effects such as vasoconstriction, aldosterone and antidiuretic hormone (ADH) release, oxidative stress, hypertrophy, proliferation, and fibrosis [7]. In addition to the established effects, activation of RAS has been suggested to play a role in the generation and maintenance of arrhythmias such as atrial fibrillation (AF), as it modulates certain ion currents thought to be involved in the pathogenesis of the arrhythmia [8]. Blockade of RAS with Angiotensin Converting Enzyme Inhibitors (ACE-Is) and/or Angiotensin Receptor Blockers (ARBs), therefore, appears promising as a therapeutic target in the management of certain arrhythmic conditions.
2.1. Molecular mechanisms of anti-arrhythmogenicity
Anti-arrhythmic roles for ACE-Is and AT1R blockers appear plausible on consideration of the molecular effects of some components of RAS. Chronic AF is associated with the down-regulation and up-regulation of AT1 and AT2 in human atrial tissue, respectively [9]. Similarly, interstitial fibrosis increases the levels of the AT2 receptor - mainly expressed on cardiac fibroblasts - where it opposes Ang II-induced hypertrophic signals [10]. Ang II and Ang-(1−7) themselves have a number of effects on cardiac ion channels critical to maintenance of cardiac rhythm (Table 1) together with potential indirect effects via modulation of the extracellular matrix [11].
Table 1.
Ang-II and Ang-(1-7) action actions on ion channels.
| Target | Action | References |
|---|---|---|
| IKs | Chronic in vivo Ang II exposure increases IKs in atrial myocytes, while decreasing them in ventricular myocytes. | [12] |
| Kv4.3 / Ito | Ang II can alter the current density of Ito in myocyte membranes. (1) Downregulation by internalisation, where angiotensin II receptor type 1 (AT1R) colocalises with Kv4.3, to form a molecular complex that is internalised via the well-established phenomenon of AT1 endocytosis. (2) Modulation of gating properties of Kv4.3; such that the Kv4.3 activation voltage threshold is increased/decreased. | [13,14,15] |
| ICaL | The L-type Ca channel current (ICaL) is increased in atrial myocytes after chronic exposure to Ang II, which contributes to plateau elevation of the action potential and prolongation of the APD. | [12] |
| Iti, IK | Ang II also increases the delayed rectifier potassium (IK), transient inward (Iti), pacemaker, and sodium-calcium exchanger (INCX) currents in pulmonary vein cardiomyocytes, whilst AT1 antagonists, such as losartan, decrease the Ito, Ik, Iti, and INCX currents [8]. | |
| INa | Ang-(1−7) significantly increases the cardiac sodium current (INa) densities, contributing to improved intra-atrial conduction, which reduces the likelihood of re-entry (and therefore decreases likelihood of arrhythmia induction and maintenance). | [16,17] |
RAS could also influence arrhythmogenicity via modulation of extracellular matrix protein expression and cardiac remodelling. Ang II leads to proliferation, while Ang-(1−7) leads to anti-proliferation. Progressive accumulation of fibrotic tissue in the myocardium is a major contributor to structural cardiac remodelling, along with dilatation and myocardial hypertrophy. Structural remodelling includes changes in both the cellular components (myofibroblasts, fibroblasts) and the extracellular matrix. Ang II has direct proliferative effects on atrial and ventricular fibroblasts and smooth muscle cells [11]. Ang II is also a potent stimulator of collagen synthesis by cardiac fibroblasts [18]. It promotes cellular growth and hypertrophy through the activation of mitogen-activated protein kinases (MAPKs). Ang II also promotes the expression of other profibrotic factors such as endothelin 1, ET-1 [18], while the downstream generation of aldosterone is also pro-fibrotic by direct or indirect stimulatory effects on fibroblasts or macrophages, respectively [19]. Chronic persistent and paroxysmal atrial fibrillation are associated with increased ACE activity, along with increased activated ERK-1/ERK-2 and the ERK activating kinases (MEK 1/MEK2) in the interstitial cells associated with marked atrial fibrosis.
2.2. Evidence from pre-clinical studies
AF is the commonest type of arrhythmia; and it is associated with remodelling (electrical and structural), which facilitate the occurrence of the arrhythmia. In many cases, the electrical remodelling is thought to be mediated by rate-induced intracellular calcium overload in the short term [20,21], and include reductions in: 1) the APD [22], 2) the atrial effective refractory period (ERP) [20,[22], [23], [24], [25]], 3) the ERP accommodation to heart rate [17,22], and 4) atrial conduction velocity (CV) [[22], [23], [24], [25]]; and prolonged 5) inter-atrial conduction time and 6) AF duration [26]. Reduced APD and ERP are generally regarded as important factors in re-entry-based AF [27]. Ang II/AT1 may play important roles in electrical remodelling, and their inhibition by ACE-Is/AT1R blockers may be preventive of such remodelling [25,28], as shown with candesartan [28], captopril [28], and irbesartan [27], [29,30]. Structural remodelling includes tissue fibroses [26], dilatation and hypertrophy. Mechanical stretch in response to haemodynamic load causes the release of Ang II which essentially feeds-forward to produce a hypertrophic response [31]. Ang II enhances synthesis of extracellular matrix collagen via both AT1R and AT2R [32]. Electrical heterogeneity from tissue fibrosis is thought to be a critical factor leading to the induction and promotion of arrhythmia [21], [33].
Chronic rapid atrial activation (by pacing) promotes the induction of sustained AF. In a canine model, this was shown to occur from a reduction in both the sodium current (INa) density and the atrial conduction velocity [17]. Similarly, Ang II leads to a reduction in transcription of the sodium channel (SCN5A) and the INa by promoting the formation of hydrogen peroxide (H2O2), which instigates the binding of nuclear factor kappa B (NF-κB) to the SCN5A promoter [34]. RAS inhibition with ACE-I or AT1R blocker prevents the down-regulation of the SCN5A and the INa [17], which can reduce the pro-arrhythmic risk in conditions with increased Ang II activity such as AF and heart failure (HF) [25].
Ang II plays a critical role in the induction of ventricular arrhythmias during ischaemia-reperfusion injury. This was demonstrated with the observation that the number of premature ventricular complexes (PVCs) in AT1R knock-out mice was much less compared to the number in wild-type controls in ischaemia-reperfusion injury; and that selective treatment with an AT1R blocker in wild-type mice prior to ischaemia ameliorated the occurrence of the PVCs [35].
Reduction in atrial fibrosis may also contribute to reduction in structural remodelling, and hence modulation of the pro-arrhythmic substrate. By virtue of their antifibrotic properties, RAS inhibitors are believed to play a role here by reducing the synthesis of collagen I molecules as well as promoting the degradation of collagen I fibres already formed [30], and reducing the expression of TGF-β [26].
2.3. Evidence from randomised trials
Potential beneficial effect of RAS inhibition on arrhythmias (e.g. AF) could be direct or secondary to neurohumoral modulation and antihypertensive effects. RAS inhibition has also been associated with indirect anti-arrhythmic effects – as identified for some clinical trials set-up for non-arrhythmic primary outcomes (i.e. observational effects or secondary outcome measures). Randomised controlled trials are weightier measures on the evidence scale; hence, a few trials have sought to prove a direct anti-arrhythmic effect for RAS inhibitors with rather mixed results (Table 2).
Table 2.
Randomised Trial data for RAS inhibition on arrhythmias.
| Study | Cohort size | Headline | Refs |
|---|---|---|---|
| TRAndolapril Cardiac Evaluation (TRACE) | 1577 | The study showed a significant difference in the development of AF in favour of the ACE-I. | [36] |
| In-hospital AF or flutter in the GISSI-3 trial | 17944 | Reduction in AF post-AMI. | [37] |
| Congestive Heart Failure (CHF) arrhythmias | 374 | Significant reduction in the frequency of ventricular arrhythmias such as PVCs, ventricular couplets and VT. | [38] |
| Studies Of Left Ventricular Dysfunction (SOLVD) | 55 | Significantly fewer patients with AF in enalapril group. | [39] |
| Valsartan Heart Failure Trial (Val-HEFT) | 4395 | Significantly lower AF incidence in patients with HF randomised to either valsartan or placebo on top of HF treatment. | [40] |
| Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) | 392 | Candesartan reduced incidence of AF in patients with symptomatic HF. | [41] |
| Heart Outcomes Prevention Evaluation (HOPE) | 8335 | Over 4.5 years of follow-up ramipril (compared to placebo) did not significantly reduce the incidence of AF in patients without known HF or left ventricular systolic dysfunction. | [42] |
| Valsartan Antihypertensive Long-term Use Evaluation (VALUE) | 15245 | Valsartan-based treatment of hypertension reduced the incidence of new-onset/sustained AF compared with an amlodipine-based treatment in hypertensive patients. | [43] |
| Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico–Atrial Fibrillation GISSI-AF trial | 1442 | Valsartan had no effect on the recurrence rate of AF in patients with a history of AF (including post successful cardioversion). | [44] |
| Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET) | 25577 | No changes in new onset AF in combining telmisartan and ramipril, but an increased risk of adverse effects. | [45] |
| Atrial Fibrillation Clopidogrel Trial with Irbesartan for Prevention of Vascular Events (ACTIVE 1) |
9016 | Irbesartan did not significantly reduce the risk of hospitalisation of patients AF. | [46] |
| Japanese Rhythm Management Trial II for Atrial Fibrillation (J-RHYTHM II) |
326 | Candesartan, combined with amplodipine, gave no advantage of amplodipine alone in terms of paroxysmal AF frequency. | [47] |
The TRAndolapril Cardiac Evaluation (TRACE) study generated very promising results. Patients with reduced left ventricular function (LVF) secondary to acute myocardial infarction (AMI) in sinus rhythm (SR) received either trandolapril or placebo and were followed up for 2–4 years for the primary outcome measure of the development and time to occurrence of AF in one 12-lead ECG recorded at an outpatient visit. The study showed a significant difference in the development of AF in favour of the ACE-I with a relative risk of approximately 50 % albeit with a broad confidence interval) in patients with left ventricular dysfunction (LVD) secondary to AMI (95 % CI: 0.26−0.76 [36]. In larger trial (GISSI-3) there was a 24 % reduction in AF incidence (OR 0.76; 95 % CI: 0.65−0.89) in patients with both an ACE-I (lisinopril) and nitrates compared to controls [37]. Further positive data showed that enalapril, given alongside maintenance therapy with digoxin and furosemide in Congestive Heart Failure (CHF) (New York Heart Association functional class II-III) significantly reduced the frequency of ventricular arrhythmias such as PVCs, ventricular couplets and VT [38]. A further retrospective study (SOLVD) involving patients with LVD in SR revealed a significant difference in the incidence of AF in patients who had received enalapril compared to those who received placebo after mean follow up of nearly 3 years. On multivariate analysis, enalapril was the most powerful predictor for risk reduction of AF (hazard ratio, HR, 0.22; 95 % CI: 0.11−0.44) [39]. Similarly, results from sub-analyses of the Valsartan Heart Failure Trial (Val-HEFT) showed a significant difference in AF incidence in patients with HF randomised to either valsartan or placebo on top of HF treatment. [40]. A meta-analysis of three ARB studies found a significant beneficial effect of RAS inhibition on the occurrence of AF in the setting of HF (OR 0.52; 95 % CI: 0.31−0.87) [48]. In contrast the HOPE trial, however, found that over 4.5 years of follow-up ramipril (compared to placebo) did not significantly reduce the incidence of AF in patients without known HF or left ventricular systolic dysfunction (LVSD) [42]. Studies suggests the beneficial effects of RAS inhibitors, such that they are, in preventing AF are not just secondary benefits from improved haemodynamics such as controlled BP [49,43].
However, a number of studies are less promising; in the GISSI-AF trial, valsartan was not associated with a reduction in the incidence of recurrent AF [44] and treatment with candesartan before and after electrical cardioversion also had no effect on the recurrence rate of AF [50]. Furthermore, other trials including ONTARGET, ACTIVE I, and J-RHYTHM II have found little additional beneficial effect against AF recurrence using RAS blockade along with an AAD (e.g. amiodarone, sotalol, propafenone) [51,[45], [46], [47]].
3. Statins
Statins are important medications with established indications in primary and secondary prevention of coronary heart disease. They decrease the cellular cholesterol content by selectively inhibiting the rate-limiting enzyme of cholesterol synthesis pathway (also known as ‘mevalonate pathway’): 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase. As well as limiting cholesterol biosynthesis, they lower serum and hepatic cholesterol and serum triglyceride concentrations.
3.1. Molecular mechanisms
A counter-regulatory mechanism of reduced cholesterol biosynthesis is upregulation of low-density lipoprotein (LDL) receptor expression in the hepatocyte membrane, with accelerated hepatic clearance of circulating LDL cholesterol [52]. Furthermore, statins show some additional pharmacodynamic (‘pleiotropic’) effects that are inexplicable with the lipid-lowering mechanism alone [53]. A number of these pleiotropic effects are ultimately cardioprotective.
By reducing the production of mevalonate (a key intermediate compound during cholesterol biosynthesis) through the inhibition of HMG-CoA reductase, statins impair the downstream production of isoprenoid intermediates (e.g. farnesylpyrophosphate and gerany-geranylpyrophosphate). This implies inhibition of post-translational modifications (e.g. transferase-catalysed addition of farnesyl and geranylgeranyl isoprenoids to small hydrolase enzymes, such as Ras and Ras-like proteins (e.g. Rho and Rac), that hydrolyse guanosine triphosphate, i.e. GTPases [54]). Rho and its downstream target, Rho-associated coiled-coil-containing kinase (ROCK), are mediators of endothelial dysfunction [55], hence their inhibition can improve endothelial function, and decrease vascular inflammation and atherosclerosis [56]. Endothelial Peroxisome Proliferator-Activated Receptor-gamma (PPAR-γ) protects against oxidative stress and endothelial dysfunction by suppressing the activity of Rho and ROCK [57]. Statin inhibition of Rho and ROCK is a key underlying mechanism mediating some of the pleiotropic effects [56,58],59]. Furthermore, the Rho/ROCK pathway contributes to myocyte apoptosis and hypertrophy and interstitial fibrosis occurring as part of pathological cardiac hypertrophy secondary to Ang II [55,56]. The mechanisms underlying some pleiotropic effects and potential anti-arrhythmic effects of statins are listed in Table 3.
Table 3.
Mechanisms underlying pleiotropic and potential anti-arrhythmic effects of statins ROCK: Rho-associated coiled-coil-containing kinase; eNOS: endothelial Nitric Oxide Synthase; PI(3)K: Phosphatidylinositol-3-OH kinase; Akt: protein kinase B; mRNA: messenger ribonucleic acid; MMP: Matrix Metalloproteinase; GTPase: Guanosine Triphosphate Phosphohydrolase; NADPH: Nicotinamide Adenine Dinucleotide Phosphate Hydrogen; SMC: Smooth Muscle Cell; IL- Interleukin; TNF-α: Tumour Necrosis Factor-alpha; CRP: C-reactive protein; TGF-β: Transforming Growth Factor – beta; NF-κB: Nuclear Factor-kappa B; MAPK: Mitogen-Activated-Protein Kinase; HCM: Hypertrophic Cardiomyopathy.
| Effect | Mechanism | References |
|---|---|---|
| Improved vascular tone: | ||
| Up-regulation of endothelial nitric oxide synthase (eNOS) | Inhibition of ROCK (which downregulates eNOS) | [59,81] |
| Activation on PI(3)K/Akt pathway (which increases eNOS activity) | [68,82,83] | |
| Post-transcriptional eNOS mRNA stabilization | [67] | |
| Increase in endothelial progenitor cells | Activation on PI(3)K/Akt pathway | [84,85] |
| Inhibition of endothelin 1 | [86] | |
| Improved endothelial function | Inhibition of superoxide formation | [64] |
| Reduced plaque vulnerability | Reduction of arterial wall myocyte migration and proliferation | [87] |
| Inhibition of macrophage cholesterol esterification | [88] | |
| Inhibition of matrix metalloproteinase (e.g. MMP-2, MMP-9, MMP-12) secretion | [89,90] | |
| Anti-oxidant effects: | ||
| Inhibition of Ang II-induced superoxide formation in myocytes and vascular SMC | Inhibition of GTPase Rac1 required for NAD(P)H oxidase activity | [91] |
| Reduced mRNA expression of NADPH oxidase subunits (Nox1, p22phox) | [92] | |
| Anti-inflammatory effects | Reduced stimulation of pro-inflammatory cytokines (e.g. TNF-α, IL-1β, IL-6, IL-8, etc.) and CRP | [93] |
| Inhibition of RhoA-mediated TNF-α-induced NF-κB activation | [94] | |
| Attenuation of Ang II-mediated cardiovascular remodelling | Inhibition of Rac1-mediated NADPH oxidase activity in vascular SMCs and heart | [91] |
| Reduction of activated Ras and MAPK in a transgenic model of human HCM | [95] | |
| Inhibition of TGF-1β-Smad 2/3 signalling pathway | [96] | |
Briefly, the possible underlying mechanisms of anti-arrhythmic effects of statins include:
-
-
atherosclerotic plaque stabilisation and regression [60,61], (i.e. improved myocardial perfusion and less risk of ischaemia-reperfusion or scar-related arrhythmia, etc.),
-
-
inhibition of oxidised LDL (oxidised LDL upregulates AT1R possibly via a nuclear factor kappa B- NFκB-dependent pathway) [62,63],
-
-
anti-oxidant, anti-inflammatory and anti-proliferative effects [[64], [65], [66]],
-
-
regulation of nitric-oxide-dependent coronary arterial tone and endothelial function [67,68],
-
-
attenuation of arrhythmogenic structural remodelling via mechanisms including upregulation of transcription factor GATA-6 expression and inhibition of the Rho pathway [[69], [70], [71], [72]],
-
-
improvement in the heterogeneity of cardiac repolarisation [73] (i.e. QT dispersion, QTd – increased QTd is associated with the incidence of life-threatening ventricular arrhythmias [74,75]);
-
-
increase in heart rate variability, HRV (decreased HRV is believed to be associated with reduced parasympathetic tone and predicts arrhythmic events [76,77]) and
-
-
changes in transmembrane ion channel conductance [[78], [79], [80]].
3.2. Statins and ventricular arrhythmias
MADIT-CRT (Multicentre Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy) was a randomised trial that examined the potential anti-arrhythmic benefit of statin use in non-ischaemic cardiomyopathy (NICM). Statin use was associated with a significant (77 %) decrease in the risk of cardiac death or life-threatening ventricular arrhythmias (VT/VF) [97]. Similarly, MADIT-II trial also showed beneficial anti-arrhythmic effect of statin use in implantable cardioverter-defibrillator (ICD) patients by lowering the risk of VT/VF and cardiac death [98]. Statin use in patients with atherosclerotic heart disease (ASHD) treated with ICD, led to decreased probability (40 % decrease in relative hazard) in the recurrence of VT/VF in the Antiarrhythmics Versus Implantable Defibrillators (AVID) trial [99]; while in the Global Registry of Acute Coronary Events (GRACE), there was association with reduced VT, VF or cardiac arrest (as well as AF) [100]. Furthermore, the Thai Registry of Acute Coronary Syndrome (TRACS) – associated statins with decreased incidence of ventricular arrhythmias (VA) [101].
In summary, statins appear to show beneficial protective effect against life-threatening ventricular arrhythmias. The possible mechanisms underlying this protective effect may be related to effects including improved coronary artery tone, atherosclerotic plaque stabilisation, anti-inflammatory, anti-oxidative, and improved cardiac repolarisation heterogeneity [79].
3.2.1. Statins and atrial fibrillation
Inflammation and oxidative stress have been of key interest among the possible mechanisms that underlie AF pathogenesis. In acute inflammatory states, such as cardiac or non-cardiac surgery and myo-pericarditis, new-onset AF coincides with peak levels of inflammatory biomarkers [102]. Theoretically, statins can protect against AF by reducing the burden of vascular disease and by mitigating atrial remodelling via pleiotropic anti-inflammatory, anti-oxidative, anti-proliferative and antithrombotic effects; as well as by improvement of endothelial function and neurohormonal regulation. Statins may act through these pleiotropic effects in reducing AF morbidity and mortality [79].
Statins decreased the risk of new-onset AF by 19 % - more so with CHADS2 score ≥ 2 than CHADS2 = 1 (Taiwan’s National Health Insurance research database) [103]. On the contrary, Cabratosa-Alves et al., reported only minimal protective effect of statins against new-onset AF in lone hypertension in a Spanish registry [104].
A meta-analysis by Fang et al., showed an overall significant reduced risk of AF incidence/recurrence [105].
Inflammation and abnormal oxidative stress may be the main pathophysiological features related to atrial remodelling and enhanced myocardial tissue inflammation, resulting in AF onset, recurrence, and persistence [106]. Statins are well-known to reduce inflammation and oxidative stress - which could underlie their anti-arrhythmic effect [107].
4. Polyunsaturated fatty acids (PUFAs)
Polyunsaturated fatty acids (PUFAs) are best known as constituents of fish oils, but they are also found in soybean and certain rapeseed oils but not ordinary olive oil [108]. N-3 and n-6 PUFAs are involved in important cell homeostasis, contributing to plasma membrane structure, cell metabolism and response to oxidative stress and inflammation [109,110]. In an early study Kromhout et al. 1985 [111] reported that coronary heart disease of those eating substantial quantities of fish was half that of the control group. Since then, cardiovascular health benefits of n-3 PUFA have been widely reported in terms of coronary heart disease and sudden death in animal models of AF, but less so in terms of reduced human atrial fibrillation or ventricular arrythmia [112]. Broadly, there appears to be an inverse correlation between fish intake and incidence of AF [[113], [114], [115]], but a recent Cochrane systematic review of PUFA for prevention of cardiovascular disease reports that clinical evidence of beneficial effects of PUFA on atrial fibrillation is of very low quality [116].
4.1. Molecular mechanisms
Experimental studies show numerous potential molecular mechanisms by which PUFAs could potentially alter cardiac rhythmicity. At the level of the heart itself, PUFA exposure can alter myocardial membrane phospholipid composition and can, in turn, be anti-inflammatory or decrease potential Ca2+ overload [[117], [118], [119], [120], [121], [122]]. Either directly, or indirectly via such phospholipid changes, PUFA can decrease membrane excitability via alteration of ion channel activity (e.g., Na, K, L-type calcium) and currents (e.g., Ito, Ik, voltage-dependent sodium current) [[123], [124], [125], [126], [127]]. Ca2+ release through the ryanodine receptor is also decreased by PUFA [128] and there is an additional notable anti-VA potential of PUFA associated with connexin (cx43) mediate myocyte-myocyte coupling [128,129]. (Table 4).
Table 4.
Possible mechanisms underlying anti-arrhythmic effect of polyunsaturated fatty acids Ito: transient outward potassium current; Ik: delayed rectifier potassium current; PUFA Polyunsaturated Fatty Acids; SR: Sarcoplasmic Reticulum; TXA2: Thromboxane A2; AA: Arachidonic acid; HRV: Heart Rate Variability; NEFA: Non-esterified Fatty Acids; cGMP: cyclic guanosine monophosphate; TGFβ1: Transforming Growth Factor β1; Akt: protein kinase B; EGF: Epidermal Growth Factor.
| Effect | Mechanism | References |
|---|---|---|
| Reduced substrate vulnerability: | ||
| Altered myocyte electrophysiology | Modulation of ion channel (e.g. Na, K, L-type calcium) conductivity and currents (e.g. Ito, Ik, voltage-dependent sodium current) leading to reduced myocyte excitability. | [123,124,125,126,127] |
| Direct inhibition of SR calcium ion release channel/ryanodine receptor gating. | [128] | |
| Modulation of connexins. | [129,130] | |
| Changes in myocardial membrane phospholipids | Insertion of n3-PUFA into cell membranes alters protein function and signalling: e.g. anti-inflammatory; anti-thrombosis – reduced platelet aggregation and adhesion via reduced production of TXA2. Altered cardiac myocyte membrane phospholipid composition: reducing n6-PUFA while increasing n3-PUFA is antiarrhythmic by reducing calcium ion availability Lowering of pro-arrhythmic membrane NEFA concentration, preventing intracellular calcium overload. |
[117,118,119,120,121,122] |
| Altered balance of AA metabolites | Increased prostacyclin (anti-arrhythmic) to TXA2 (pro-arrhythmic) ratio. | [131,132] |
| Improved HRV | Calcium channel blocking effect on cardiac myocytes. | [133,134] |
| Modulation of sympathetic nervous system. | [135] | |
| Reduction of trigger events and remodelling | ||
| Reduced atherosclerosis | Decreased plaque inflammation and increased plaque stability; reduced neovascularisation. | [136,137,138] |
| Reduced thrombosis | Reduced platelet aggregation. | [136] |
| Reduced cardiac fibrosis | Increase cGMP levels which inhibit TGFβ1-induced cardiac fibrosis by blocking phosphorylation and nuclear translocation of Smad2/3 as well as inhibitory effects on some structural remodelling signalling molecules (e.g. Akt, EGF). | [139,140], |
4.2. PUFA and ventricular arrhythmias (VA)
Beginning in the 1980s, these fatty acids were documented to confer protection against arrhythmias in Wistar rats after coronary artery ligation-induced ischaemic injury [141]. Albert et al., also reported significant inverse correlation of n-3 PUFA levels with the risk of sudden death among men with no prior history of cardiovascular disease (i.e. high blood n3-PUFA associated with low risk of sudden death) [121]. The clinical data on potential benefit for VA are mixed, two trials in the same year (2005) reported apparently opposite results. Leaf et al., a marginally statistically significant reduction of life-threatening VA (relative risk reduction of 38 %) in an ICD population [142], but in similar study Raitt et al., found the opposite with increased risk of VT/VF in the n3-PUFA group [143]. This echoed the early work of Burr et al. 1989 challenging patients with increased consumption of fatty fish [144]. The differences in conclusions were striking since in both studies there were similar patient condition, cohort size, PUFA dose and change in the benchmark red blood cell membrane PUFA concentrations. The lack of anti-arrhythmic effects seen clinically in the latter study was very much supported by a more recent canine model where again n-3 PUFA were actually pro-arrhythmic under some conditions [145].
4.3. PUFA and atrial fibrillation (AF)
Despite the positive correlative data (above) linking reduced AF with high fish consumption, the clinical trials investigating whether n-3 PUFA protect against AF are again disappointing. In the large OPERA multicentre trial, fish oil failed to reduce post-operative AF [146] and a similar lack of efficacy of prescription n3-PUFA was reported for paroxysmal AF over a 6 month period [147]. AF is a common complication of coronary artery bypass graft surgery, but n3-PUFA supplementation also failed to reduce AF in this scenario too [148].
In summary there is still no consensus to what the anti-arrhythmic potential of PUFA really are, if any, despite approaching 40 years of investigation [149].
5. Future directions and conclusions
In summary, RAS blockers, statins and PUFAs appear to have beneficial roles in decreasing the incidence and recurrence of arrhythmias. The foregoing discussions illustrate that while some insight has been gained in the potential molecular pathways underlying their plausible anti-arrhythmic roles, further clarification of these mechanisms and additional direct evidence of antiarrhythmic effects through randomised controlled trials are necessary.
Only a few randomised trials to date have examined the potential anti-arrhythmic role of RAS blockers with no clear significant beneficial effects. However, there might still be some justification for future attempts aiming to identify direct anti-arrhythmic evidence through larger randomised controlled trials in patients with or without underlying cardiac disease. Future studies with statins could attempt to elucidate further the underlying molecular pathways explaining their pleiotropic effects. Furthermore, the identification of direct evidence for their anti-arrhythmic beneficial effects and the specific underlying pleiotropic effect at play are necessary. Further research into the differing effects of the major components of PUFAs (DHA and EPA), as well as clarification of the molecular mechanisms underlying their potential anti-arrhythmic roles are also required.
Based on the evidence examined above, it may not be possible to propose RAS blockers, statins or PUFA (EPA and DHA) are primary AADs. However, there appears to be strong molecular plausibility and evidence base supporting their use for obtaining adjunctive anti-arrhythmic benefits alone or in combination with already established AADs.
Declaration of Competing Interest
None.
Acknowledgement
This work was funded by BBSRC grant award to RBJ; Grant number BB/S008136/1.
References
- 1.Lei M., Wu L., Terrar D.A., Huang C.L.H. Modernized classification of cardiac antiarrhythmic drugs. Circulation. 2018;138(October 17):1879–1896. doi: 10.1161/CIRCULATIONAHA.118.035455. [DOI] [PubMed] [Google Scholar]
- 2.WEIDMANN S. Ion movements underlying the cardiac action potential. Am. Heart J. 1961;vol. 61(August 3):298–302. doi: 10.1016/0002-8703(61)90598-1. [DOI] [PubMed] [Google Scholar]
- 3.Wang Z., Fermini B., Nattel S. Sustained depolarization-induced outward current in human atrial myocytes. Evidence for a novel delayed rectifier K+ current similar to Kv1.5 cloned channel currents. Circ. Res. 1993;vol. 73(December 6):1061–1076. doi: 10.1161/01.res.73.6.1061. [DOI] [PubMed] [Google Scholar]
- 4.Chen L., Sampson K.J., Kass R.S. Cardiac Delayed Rectifier Potassium Channels in Health and Disease. Card. Electrophysiol. Clin. 2016;vol. 8(no. 2):307–322. doi: 10.1016/j.ccep.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DiFrancesco D. The role of the funny current in pacemaker activity. Circ. Res. 2010;vol. 106(February 3):434–446. doi: 10.1161/CIRCRESAHA.109.208041. [DOI] [PubMed] [Google Scholar]
- 6.K. D. Singh and S. S. Karnik, Angiotensin Receptors: Structure, Function, Signaling and Clinical Applications. [DOI] [PMC free article] [PubMed]
- 7.Fyhrquist F., Saijonmaa O. Renin-angiotensin system revisited. J. Intern. Med. 2008;264(September 3):224–236. doi: 10.1111/j.1365-2796.2008.01981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y.J., Chen Y.C., Tai C.T., Yeh H.I., Lin C.I., Chen S.A. Angiotensin II and angiotensin II receptor blocker modulate the arrhythmogenic activity of pulmonary veins. Br. J. Pharmacol. 2006;vol. 147(no. 1):12–22. doi: 10.1038/sj.bjp.0706445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goette A., Arndt M., Rocken C., Spiess A., Staack T., Geller J.C., Huth C., Ansorge S., Klein H.U., Lendeckel U. Regulation of angiotensin II receptor subtypes during atrial fibrillation in humans. Circulation. 2000;vol. 101(June 23):2678–2681. doi: 10.1161/01.cir.101.23.2678. [DOI] [PubMed] [Google Scholar]
- 10.Tsutsumi Y., Matsubara H., Ohkubo N., Mori Y., Nozawa Y., Murasawa S., Kijima K., Maruyama K., Masaki H., Moriguchi Y., Shibasaki Y., Kamihata H., Inada M., Iwasaka T. Angiotensin II type 2 receptor is upregulated in human heart with interstitial fibrosis, and cardiac fibroblasts are the major cell type for its expression. Circ. Res. 1998;vol. 83(November 10):1035–1046. doi: 10.1161/01.res.83.10.1035. [DOI] [PubMed] [Google Scholar]
- 11.McEwan P.E., Gray G.A., Sherry L., Webb D.J., Kenyon C.J. Differential effects of angiotensin II on cardiac cell proliferation and intramyocardial perivascular fibrosis in vivo. Circulation. 1998;vol. 98(December 24):2765–2773. doi: 10.1161/01.cir.98.24.2765. [DOI] [PubMed] [Google Scholar]
- 12.Zankov D.P., Salloum F.N., Jiang M., Tseng G.-N. Chronic in vivo angiotensin II administration differentially modulates the slow delayed rectifier channels in atrial and ventricular myocytes. Hear. Rhythm. 2019;vol. 16(no. 1):108–116. doi: 10.1016/j.hrthm.2018.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu H., Gao J., Wang H., Wymore R., Steinberg S., McKinnon D., Rosen M.R., Cohen I.S. Effects of the renin-angiotensin system on the current I(to) in epicardial and endocardial ventricular myocytes from the canine heart. Circ. Res. 2000;vol. 86(May. 10):1062–1068. doi: 10.1161/01.res.86.10.1062. [DOI] [PubMed] [Google Scholar]
- 14.Akar F.G., Wu R.C., Deschenes I., Armoundas A.A., Piacentino V., 3rd, Houser S.R., Tomaselli G.F. Phenotypic differences in transient outward K+ current of human and canine ventricular myocytes: insights into molecular composition of ventricular Ito. Am. J. Physiol. Heart Circ. Physiol. 2004;vol. 286(February. 2):H602–H609. doi: 10.1152/ajpheart.00673.2003. [DOI] [PubMed] [Google Scholar]
- 15.Doronin S.V., Potapova I.A., Lu Z., Cohen I.S. Angiotensin receptor type 1 forms a complex with the transient outward potassium channel Kv4.3 and regulates its gating properties and intracellular localization. J. Biol. Chem. 2004;279(November. 46):48231–48237. doi: 10.1074/jbc.M405789200. [DOI] [PubMed] [Google Scholar]
- 16.Wang X., Li G. Angiotensin-(1-7) prevent atrial tachycardia induced sodium channel remodeling. Pacing Clin. Electrophysiol. 2014;vol. 37(October.10):1349–1356. doi: 10.1111/pace.12427. [DOI] [PubMed] [Google Scholar]
- 17.Gaspo R., Bosch R.F., Bou-Abboud E., Nattel S. Tachycardia-induced changes in Na+ current in a chronic dog model of atrial fibrillation. Circ. Res. 1997;vol. 81(December 6):1045–1052. doi: 10.1161/01.res.81.6.1045. [DOI] [PubMed] [Google Scholar]
- 18.Dzeshka M.S., Lip G.Y.H., Snezhitskiy V., Shantsila E. Cardiac Fibrosis in Patients With Atrial Fibrillation. J. Am. Coll. Cardiol. 2015;vol. 66(August 8):943–959. doi: 10.1016/j.jacc.2015.06.1313. [DOI] [PubMed] [Google Scholar]
- 19.Kong P., Christia P., Frangogiannis N.G. The pathogenesis of cardiac fibrosis. Cell. Mol. Life Sci. 2014;71(February 4):549–574. doi: 10.1007/s00018-013-1349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goette A., Honeycutt C., Langberg J.J. Electrical remodeling in atrial fibrillation. Time course and mechanisms. Circulation. 1996;vol. 94(December 11):2968–2974. doi: 10.1161/01.cir.94.11.2968. [DOI] [PubMed] [Google Scholar]
- 21.Kumagai K., Nakashima H., Urata H., Gondo N., Arakawa K., Saku K. Effects of angiotensin II type 1 receptor antagonist on electrical and structural remodeling in atrial fibrillation. J. Am. Coll. Cardiol. 2003;vol. 41(June. 12):2197–2204. doi: 10.1016/s0735-1097(03)00464-9. [DOI] [PubMed] [Google Scholar]
- 22.Yue L., Feng J., Gaspo R., Li G.R., Wang Z., Nattel S. Ionic remodeling underlying action potential changes in a canine model of atrial fibrillation. Circ. Res. 1997;vol. 81(October 4):512–525. doi: 10.1161/01.res.81.4.512. [DOI] [PubMed] [Google Scholar]
- 23.Gaspo R., Bosch R.F., Talajic M., Nattel S. Functional mechanisms underlying tachycardia-induced sustained atrial fibrillation in a chronic dog model. Circulation. 1997;vol. 96(December 11):4027–4035. doi: 10.1161/01.cir.96.11.4027. [DOI] [PubMed] [Google Scholar]
- 24.Daoud E.G., Bogun F., Goyal R., Harvey M., Man K.C., Strickberger S.A., Morady F. Effect of atrial fibrillation on atrial refractoriness in humans. Circulation. 1996;vol. 94(October 7):1600–1606. doi: 10.1161/01.cir.94.7.1600. [DOI] [PubMed] [Google Scholar]
- 25.Wang X., Li G. Irbesartan prevents sodium channel remodeling in a canine model of atrial fibrillation. J. Renin. Syst. 2018;19(January 1) doi: 10.1177/1470320318755269. 1470320318755269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kataoka N., Nishida K., Kinoshita K., Sakamoto T., Nakatani Y., Tsujino Y., Mizumaki K., Inoue H., Kinugawa K. Effect of irbesartan on development of atrial fibrosis and atrial fibrillation in a canine atrial tachycardia model with left ventricular dysfunction, association with p53. Heart Vessels. 2016;vol. 31(December 12):2053–2060. doi: 10.1007/s00380-016-0853-7. [DOI] [PubMed] [Google Scholar]
- 27.Wu J., Ding W.-G., Zhao J., Zang W.-J., Matsuura H., Horie M. Irbesartan-mediated AT1 receptor blockade attenuates hyposmotic-induced enhancement of I Ks current and prevents shortening of action potential duration in atrial myocytes. J. Renin. Syst. 2014;15(December 4):341–347. doi: 10.1177/1470320312474855. [DOI] [PubMed] [Google Scholar]
- 28.Nakashima H., Kumagai K., Urata H., Gondo N., Ideishi M., Arakawa K. Angiotensin II antagonist prevents electrical remodeling in atrial fibrillation. Circulation. 2000;vol. 101(June 22):2612–2617. doi: 10.1161/01.cir.101.22.2612. [DOI] [PubMed] [Google Scholar]
- 29.Madrid A.H., Bueno M.G., Rebollo J.M.G., Marin I., Pena G., Bernal E., Rodriguez A., Cano L., Cano J.M., Cabeza P., Moro C. Use of irbesartan to maintain sinus rhythm in patients with long-lasting persistent atrial fibrillation: a prospective and randomized study. Circulation. 2002;vol. 106(July 3):331–336. doi: 10.1161/01.cir.0000022665.18619.83. [DOI] [PubMed] [Google Scholar]
- 30.Madrid A.H., Escobar C., Rebollo J.M.G., Marin I., Bernal E., Nannini S., Limon L., Peng J., Moro C. Angiotensin receptor blocker as adjunctive therapy for rhythm control in atrial fibrillation: results of the irbesartan-amiodarone trial. Card. Electrophysiol. Rev. 2003;vol. 7(September 3):243–246. doi: 10.1023/B:CEPR.0000012391.95928.d2. [DOI] [PubMed] [Google Scholar]
- 31.Sadoshima J., Xu Y., Slayter H.S., Izumo S. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell. 1993;vol. 75(December 5):977–984. doi: 10.1016/0092-8674(93)90541-w. [DOI] [PubMed] [Google Scholar]
- 32.Mifune M., Sasamura H., Shimizu-Hirota R., Miyazaki H., Saruta T. Angiotensin II type 2 receptors stimulate collagen synthesis in cultured vascular smooth muscle cells. Hypertens. (Dallas, Tex. 1979) 2000;vol. 36(November 5):845–850. doi: 10.1161/01.hyp.36.5.845. [DOI] [PubMed] [Google Scholar]
- 33.Li D., Fareh S., Leung T.K., Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999;vol. 100(July 1):87–95. doi: 10.1161/01.cir.100.1.87. [DOI] [PubMed] [Google Scholar]
- 34.Shang L.L., Sanyal S., Pfahnl A.E., Jiao Z., Allen J., Liu H., Dudley S.C.J. NF-kappaB-dependent transcriptional regulation of the cardiac scn5a sodium channel by angiotensin II. Am. J. Physiol., Cell Physiol. 2008;294(January 1):C372–C379. doi: 10.1152/ajpcell.00186.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harada K., Komuro I., Hayashi D., Sugaya T., Murakami K., Yazaki Y. Angiotensin II type 1a receptor is involved in the occurrence of reperfusion arrhythmias. Circulation. 1998;vol. 97(February 4):315–317. doi: 10.1161/01.cir.97.4.315. [DOI] [PubMed] [Google Scholar]
- 36.Pedersen O.D., Bagger H., Køber L., Torp-Pedersen C. Trandolapril reduces the incidence of atrial fibrillation after acute myocardial infarction in patients with left ventricular dysfunction. Circulation. 1999 doi: 10.1161/01.cir.100.4.376. [DOI] [PubMed] [Google Scholar]
- 37.Pizzetti F., Turazza F.M., Franzosi M.G., Barlera S., Ledda A., Maggioni A.P., Santoro L., Tognoni G. Incidence and prognostic significance of atrial fibrillation in acute myocardial infarction: the GISSI-3 data. Heart. 2001;vol. 86(November 5):527–532. doi: 10.1136/heart.86.5.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Webster M.W., Fitzpatrick M.A., Nicholls M.G., Ikram H., Wells J.E. Effect of enalapril on ventricular arrhythmias in congestive heart failure. Am. J. Cardiol. 1985;vol. 56(September 8):566–569. doi: 10.1016/0002-9149(85)91186-5. [DOI] [PubMed] [Google Scholar]
- 39.Vermes E., Tardif J.-C., Bourassa M.G., Racine N., Levesque S., White M., Guerra P.G., Ducharme A. Enalapril decreases the incidence of atrial fibrillation in patients with left ventricular dysfunction: insight from the Studies of left Ventricular Dysfunction (SOLVD) trials. Circulation. 2003;107(June 23):2926–2931. doi: 10.1161/01.CIR.0000072793.81076.D4. [DOI] [PubMed] [Google Scholar]
- 40.Maggioni A.P., Latini R., Carson P.E., Singh S.N., Barlera S., Glazer R., Masson S., Cere E., Tognoni G., Cohn J.N. Valsartan reduces the incidence of atrial fibrillation in patients with heart failure: results from the Valsartan Heart Failure Trial (Val-HeFT) Am. Heart J. 2005;vol. 149(March 3):548–557. doi: 10.1016/j.ahj.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 41.Ducharme A., Swedberg K., Pfeffer M.A., Cohen-Solal A., Granger C.B., Maggioni A.P., Michelson E.L., McMurray J.J.V., Olsson L., Rouleau J.L., Young J.B., Olofsson B., Puu M., Yusuf S. Prevention of atrial fibrillation in patients with symptomatic chronic heart failure by candesartan in the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Am. Heart J. 2006;152(1):86–92. [PubMed] [Google Scholar]
- 42.Salehian O., Healey J., Stambler B., Alnemer K., Almerri K., Grover J., Bata I., Mann J., Matthew J., Pogue J., Yusuf S., Dagenais G., Lonn E. Impact of ramipril on the incidence of atrial fibrillation: results of the Heart Outcomes Prevention Evaluation study. Am. Heart J. 2007;vol. 154(September 3):448–453. doi: 10.1016/j.ahj.2007.04.062. [DOI] [PubMed] [Google Scholar]
- 43.Schmieder R.E., Kjeldsen S.E., Julius S., McInnes G.T., Zanchetti A., Hua T.A. Reduced incidence of new-onset atrial fibrillation with angiotensin II receptor blockade: the VALUE trial. J. Hypertens. 2008;vol. 26(March 3):403–411. doi: 10.1097/HJH.0b013e3282f35c67. [DOI] [PubMed] [Google Scholar]
- 44.Disertori M., Latini R., Barlera S., Franzosi M.G., Staszewsky L., Pietro Maggioni A., Lucci D., Di Pasquale G., Tognoni G. Valsartan for prevention of recurrent atrial fibrillation. N. Engl. J. Med. 2009;vol. 360(April 16):1606–1617. doi: 10.1056/NEJMoa0805710. [DOI] [PubMed] [Google Scholar]
- 45.The ONTARGET Investigators Telmisartan, Ramipril, or Both in Patients at High Risk for Vascular Events. N. Engl. J. Med. 2008;vol. 358(April 15):1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 46.Yusuf S., Healey J.S., Pogue J., Chrolavicius S., Flather M., Hart R.G., Hohnloser S.H., Joyner C.D., Pfeffer M.A., Connolly S.J. Irbesartan in patients with atrial fibrillation. N. Engl. J. Med. 2011;vol. 364(March 10):928–938. doi: 10.1056/NEJMoa1008816. [DOI] [PubMed] [Google Scholar]
- 47.Yamashita T., Inoue H., Okumura K., Kodama I., Aizawa Y., Atarashi H., Ohe T., Ohtsu H., Kato T., Kamakura S., Kumagai K., Kurachi Y., Koretsune Y., Saikawa T., Sakurai M., Sato T., Sugi K., Nakaya H., Hirai M., Hirayama A., Fukatani M., Mitamura H., Yamazaki T., Watanabe E., Ogawa S. Randomized trial of angiotensin II-receptor blocker vs. Dihydropiridine calcium channel blocker in the treatment of paroxysmal atrial fibrillation with hypertension (J-RHYTHM II study) Europace. 2011;13(April 4):473–479. doi: 10.1093/europace/euq439. [DOI] [PubMed] [Google Scholar]
- 48.Schneider M.P., Hua T.A., Böhm M., Wachtell K., Kjeldsen S.E., Schmieder R.E. Prevention of atrial fibrillation by renin-angiotensin system inhibition. JAC. 2010;55(21):2299–2307. doi: 10.1016/j.jacc.2010.01.043. [DOI] [PubMed] [Google Scholar]
- 49.Wachtell K., Lehto M., Gerdts E., Olsen M.H., Hornestam B., Dahlöf B., Ibsen H., Julius S., Kjeldsen S.E., Lindholm L.H., Nieminen M.S., Devereux R.B. Angiotensin II receptor blockade reduces new-onset atrial fibrillation and subsequent stroke compared to atenolol: the Losartan Intervention for End point reduction in hypertension (LIFE) study. J. Am. Coll. Cardiol. 2005;45(5):712–719. doi: 10.1016/j.jacc.2004.10.068. [DOI] [PubMed] [Google Scholar]
- 50.Tveit A., Grundvold I., Olufsen M., Seljeflot I., Abdelnoor M., Arnesen H., Smith P. Candesartan in the prevention of relapsing atrial fibrillation ⋆. Int. J. Cardiol. 2007;vol. 120:85–91. doi: 10.1016/j.ijcard.2006.08.086. [DOI] [PubMed] [Google Scholar]
- 51.M. Palardy, A. Ducharme, S. Nattel, J.-C. Tardif, M. White, N. Racine, K. Tetreault, F. Dabouz, M. Talajic, and D. Roy, Absence of protective effect of renin-angiotensin system inhibitors on atrial fibrillation development: insights from the Canadian Trial of Atrial Fibrillation (CTAF), Can. J. Cardiol., vol. 24, September 9, pp. 709–713. [DOI] [PMC free article] [PubMed]
- 52.C. R. Sirtori, The pharmacology of statins, Pharmacol. Res., vol. 88, pp. 3–11, October 4. [DOI] [PubMed]
- 53.Kavalipati N., Shah J., Ramakrishan A., Vasnawala H. Pleiotropic effects of statins. Indian J. Endocrinol. Metab. 2015;vol. 19(no. 5):554–562. doi: 10.4103/2230-8210.163106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stubbs E.B.J. Isoprenylation of monomeric GTPases in human trabecular meshwork cells. Methods Mol. Biol. 2017;1609:217–229. doi: 10.1007/978-1-4939-6996-8_18. [DOI] [PubMed] [Google Scholar]
- 55.T. M. De Silva, D. A. Kinzenbaw, M. L. Modrick, L. D. Reinhardt, and F. M. Faraci, Heterogeneous Impact of ROCK2 on Carotid and Cerebrovascular Function, Hypertens. (Dallas, Tex. 1979), vol. 68, September 3, 809–817. [DOI] [PMC free article] [PubMed]
- 56.Sawada N., Liao J.K. Rho/Rho-associated coiled-coil forming kinase pathway as therapeutic targets for statins in atherosclerosis. Antioxid. Redox Signal. 2014;vol. 20(March 8):1251–1267. doi: 10.1089/ars.2013.5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Silva T.M., Li Y., Kinzenbaw D.A., Sigmund C.D., Faraci F.M. Endothelial PPARgamma (Peroxisome proliferator-activated receptor-gamma) is essential for preventing endothelial dysfunction with aging. Hypertens. (Dallas, Tex. 1979) 2018;72(July 1):227–234. doi: 10.1161/HYPERTENSIONAHA.117.10799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Laufs U., Endres M., Stagliano N., Amin-Hanjani S., Chui D.S., Yang S.X., Simoncini T., Yamada M., Rabkin E., Allen P.G., Huang P.L., Bohm M., Schoen F.J., Moskowitz M.A., Liao J.K. Neuroprotection mediated by changes in the endothelial actin cytoskeleton. J. Clin. Invest. 2000;vol. 106(July 1):15–24. doi: 10.1172/JCI9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takemoto M., Sun J., Hiroki J., Shimokawa H., Liao J.K. Rho-kinase mediates hypoxia-induced downregulation of endothelial nitric oxide synthase. Circulation. 2002;vol. 106(July 1):57–62. doi: 10.1161/01.cir.0000020682.73694.ab. [DOI] [PubMed] [Google Scholar]
- 60.Rosenson R.S., Tangney C.C. Antiatherothrombotic properties of statins: implications for cardiovascular event reduction. JAMA. 1998;vol. 279(May 20):1643–1650. doi: 10.1001/jama.279.20.1643. [DOI] [PubMed] [Google Scholar]
- 61.Nissen S.E. Halting the progression of atherosclerosis with intensive lipid lowering: results from the Reversal of Atherosclerosis with Aggressive Lipid Lowering (REVERSAL) trial. Alami J. 2005;118(Suppl December):22–27. doi: 10.1016/j.amjmed.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 62.Li D.Y., Zhang Y.C., Philips M.I., Sawamura T., Mehta J.L. Upregulation of endothelial receptor for oxidized low-density lipoprotein (LOX-1) in cultured human coronary artery endothelial cells by angiotensin II type 1 receptor activation. Circ. Res. 1999;vol. 84(May 9):1043–1049. doi: 10.1161/01.res.84.9.1043. [DOI] [PubMed] [Google Scholar]
- 63.Li D., Saldeen T., Romeo F., Mehta J.L. Oxidized LDL upregulates angiotensin II type 1 receptor expression in cultured human coronary artery endothelial cells: the potential role of transcription factor NF-kappaB. Circulation. 2000;vol. 102(October 16):1970–1976. doi: 10.1161/01.cir.102.16.1970. [DOI] [PubMed] [Google Scholar]
- 64.Giroux L.M., Davignon J., Naruszewicz M. Simvastatin inhibits the oxidation of low-density lipoproteins by activated human monocyte-derived macrophages. Biochim. Biophys. Acta - Lipids Lipid Metab. 1993;vol. 1165(no. 3):335–338. doi: 10.1016/0005-2760(93)90145-y. [DOI] [PubMed] [Google Scholar]
- 65.Kwak B., Mulhaupt F., Myit S., Mach F. Statins as a newly recognized type of immunomodulator. Nat. Med. 2000;vol. 6(December 12):1399–1402. doi: 10.1038/82219. [DOI] [PubMed] [Google Scholar]
- 66.Laufs U., Marra D., Node K., Liao J.K. 3-Hydroxy-3-methylglutaryl-CoA reductase inhibitors attenuate vascular smooth muscle proliferation by preventing rho GTPase-induced down-regulation of p27(Kip1) J. Biol. Chem. 1999;vol. 274(July 31):21926–21931. doi: 10.1074/jbc.274.31.21926. [DOI] [PubMed] [Google Scholar]
- 67.Laufs U., Fata V.L., Liao J.K. Inhibition of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase blocks hypoxia-mediated down-regulation of endothelial nitric oxide synthase. J. Biol. Chem. 1997;vol. 272(December 50):31725–31729. doi: 10.1074/jbc.272.50.31725. [DOI] [PubMed] [Google Scholar]
- 68.Kureishi Y., Luo Z., Shiojima I., Bialik A., Fulton D., Lefer D.J., Sessa W.C., Walsh K. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat. Med. 2000;vol. 6(September 9):1004–1010. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Q., Guo Y.-Z., Zhang Y.-T., Xue J.-J., Chen Z.-C., Cheng S.-Y., Ou M.-D., Cheng K.-L., Zeng W.-J. The effects and mechanism of atorvastatin on pulmonary hypertension due to left heart disease. PLoS One. 2016;11(7):e0157171. doi: 10.1371/journal.pone.0157171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nishimura T., Faul J.L., Berry G.J., Vaszar L.T., Qiu D., Pearl R.G., Kao P.N. Simvastatin attenuates smooth muscle neointimal proliferation and pulmonary hypertension in rats. Am. J. Respir. Crit. Care Med. 2002;vol. 166(November 10):1403–1408. doi: 10.1164/rccm.200203-268OC. [DOI] [PubMed] [Google Scholar]
- 71.Liu B., Wang X., Yu L., Zhou T., Wang X., Liu H. Simvastatin restores down-regulated GATA-6 expression in pulmonary hypertensive rats. Exp. Lung Res. 2009;vol. 35(June 5):411–426. doi: 10.1080/01902140902736819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Akahori H., Tsujino T., Naito Y., Matsumoto M., Sasaki N., Iwasaku T., Eguchi A., Sawada H., Hirotani S., Masuyama T. Atorvastatin ameliorates cardiac fibrosis and improves left ventricular diastolic function in hypertensive diastolic heart failure model rats. J. Hypertens. 2014;vol. 32(July 7):1534–1541. doi: 10.1097/HJH.0000000000000184. discussion 1541. [DOI] [PubMed] [Google Scholar]
- 73.Tekin A., Tekin G., Sezgin A.T., Muderrisoglu H. Short- and long-term effect of simvastatin therapy on the heterogeneity of cardiac repolarization in diabetic patients. Pharmacol. Res. 2008;vol. 57(May 5):393–397. doi: 10.1016/j.phrs.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 74.Yu G.L., Cheng I.R., Zhao S.P., Zhuang H.P., Cai X.Y. Clinical significance of QT dispersion after exercise in patients with previous myocardial infarction. Int. J. Cardiol. 1998;vol. 65(August 3):255–260. doi: 10.1016/s0167-5273(98)00120-x. [DOI] [PubMed] [Google Scholar]
- 75.Vrtovec B., Okrajsek R., Golicnik A., Ferjan M., Starc V., Radovancevic B. Atorvastatin therapy increases heart rate variability, decreases QT variability, and shortens QTc interval duration in patients with advanced chronic heart failure. J. Card. Fail. 2005;vol. 11(December 9):684–690. doi: 10.1016/j.cardfail.2005.06.439. [DOI] [PubMed] [Google Scholar]
- 76.Cripps T.R., Malik M., Farrell T.G., Camm A.J. Prognostic value of reduced heart rate variability after myocardial infarction: clinical evaluation of a new analysis method. Br. Heart J. 1991;vol. 65(January 1):14–19. doi: 10.1136/hrt.65.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Odemuyiwa O., Malik M., Farrell T., Bashir Y., Poloniecki J., Camm J. Comparison of the predictive characteristics of heart rate variability index and left ventricular ejection fraction for all-cause mortality, arrhythmic events and sudden death after acute myocardial infarction. Am. J. Cardiol. 1991;vol. 68(no. 5):434–439. doi: 10.1016/0002-9149(91)90774-f. [DOI] [PubMed] [Google Scholar]
- 78.Laszlo R., Menzel K.A., Bentz K., Schreiner B., Kettering K., Eick C., Schreieck J. Atorvastatin treatment affects atrial ion currents and their tachycardia-induced remodeling in rabbits. Life Sci. 2010;vol. 87(October 15–16):507–513. doi: 10.1016/j.lfs.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 79.Kostapanos M.S., Liberopoulos E.N., Goudevenos J.A., Mikhailidis D.P., Elisaf M.S. Do statins have an antiarrhythmic activity? Cardiovasc. Res. 2007;vol. 75(July 1):10–20. doi: 10.1016/j.cardiores.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 80.Ali N., Begum R., Faisal M.S., Khan A., Nabi M., Shehzadi G., Ullah S., Ali W. Current statins show calcium channel blocking activity through voltage gated channels. BMC Pharmacol. Toxicol. 2016;vol. 17(September 1):43. doi: 10.1186/s40360-016-0086-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shin H.K., Salomone S., Potts E.M., Lee S.-W., Millican E., Noma K., Huang P.L., Boas D.A., Liao J.K., Moskowitz M.A., Ayata C. Rho-kinase inhibition acutely augments blood flow in focal cerebral ischemia via endothelial mechanisms. J. Cereb. Blood Flow Metab. 2007;vol. 27(May 5):998–1009. doi: 10.1038/sj.jcbfm.9600406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dimmeler S., Fleming I., Fisslthaler B., Hermann C., Busse R., Zeiher A.M. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;vol. 399(June 6736):601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 83.Michell B.J., Griffiths J.E., Mitchelhill K.I., Rodriguez-Crespo I., Tiganis T., Bozinovski S., de Montellano P.R., Kemp B.E., Pearson R.B. The Akt kinase signals directly to endothelial nitric oxide synthase. Curr. Biol. 1999;vol. 9(July 15):845–848. doi: 10.1016/s0960-9822(99)80371-6. [DOI] [PubMed] [Google Scholar]
- 84.Dimmeler S., Aicher A., Vasa M., Mildner-Rihm C., Adler K., Tiemann M., Rütten H., Fichtlscherer S., Martin H., Zeiher A.M. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J. Clin. Invest. 2001;vol. 108(August 3):391–397. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kusuyama T., Omura T., Nishiya D., Enomoto S., Matsumoto R., Murata T., Takeuchi K., Yoshikawa J., Yoshiyama M. The effects of HMG-CoA reductase inhibitor on vascular progenitor cells. J. Pharmacol. Sci. 2006;vol. 101(August 4):344–349. doi: 10.1254/jphs.fp0060102. [DOI] [PubMed] [Google Scholar]
- 86.Hernandez-Perera O., Perez-Sala D., Navarro-Antolin J., Sanchez-Pascuala R., Hernandez G., Diaz C., Lamas S. Effects of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors, atorvastatin and simvastatin, on the expression of endothelin-1 and endothelial nitric oxide synthase in vascular endothelial cells. J. Clin. Invest. 1998;vol. 101(June 12):2711–2719. doi: 10.1172/JCI1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bellosta S., Bernini F., Ferri N., Quarato P., Canavesi M., Arnaboldi L., Fumagalli R., Paoletti R., Corsini A. Direct vascular effects of HMG-CoA reductase inhibitors. Atherosclerosis. 1998;vol. 137(Suppl April):S101–S109. doi: 10.1016/s0021-9150(97)00319-5. [DOI] [PubMed] [Google Scholar]
- 88.Tanaka K., Yasuhara M., Suzumura K., Narita H., Suzuki T. Effects of fluvastatin and its major metabolites on low-density lipoprotein oxidation and cholesterol esterification in macrophages. Jpn. J. Pharmacol. 2001;vol. 86(July 3):289–296. doi: 10.1254/jjp.86.289. [DOI] [PubMed] [Google Scholar]
- 89.Massaro M., Zampolli A., Scoditti E., Carluccio M.A., Storelli C., Distante A., De Caterina R. Statins inhibit cyclooxygenase-2 and matrix metalloproteinase-9 in human endothelial cells: anti-angiogenic actions possibly contributing to plaque stability. Cardiovasc. Res. 2009;vol. 86(November 2):311–320. doi: 10.1093/cvr/cvp375. [DOI] [PubMed] [Google Scholar]
- 90.Roth L., Rombouts M., Schrijvers D.M., Martinet W., De Meyer G.R.Y. Cholesterol-independent effects of atorvastatin prevent cardiovascular morbidity and mortality in a mouse model of atherosclerotic plaque rupture. Vascul. Pharmacol. 2016;vol. 80:50–58. doi: 10.1016/j.vph.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 91.Wassmann S., Laufs U., Baumer A.T., Muller K., Konkol C., Sauer H., Bohm M., Nickenig G. Inhibition of geranylgeranylation reduces angiotensin II-mediated free radical production in vascular smooth muscle cells: involvement of angiotensin AT1 receptor expression and Rac1 GTPase. Mol. Pharmacol. 2001;vol. 59(March 3):646–654. doi: 10.1124/mol.59.3.646. [DOI] [PubMed] [Google Scholar]
- 92.Wassmann S., Laufs U., Muller K., Konkol C., Ahlbory K., Baumer A.T., Linz W., Bohm M., Nickenig G. Cellular antioxidant effects of atorvastatin in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol. 2002;vol. 22(February 2):300–305. doi: 10.1161/hq0202.104081. [DOI] [PubMed] [Google Scholar]
- 93.Bedi O., Dhawan V., Sharma P.L., Kumar P. Pleiotropic effects of statins: new therapeutic targets in drug design. Naunyn Schmiedebergs Arch. Pharmacol. 2016 doi: 10.1007/s00210-016-1252-4. [DOI] [PubMed] [Google Scholar]
- 94.Xu H., Liu P., Liang L., Danesh F.R., Yang X., Ye Y., Zhan Z., Yu X., Peng H., Sun L. RhoA-mediated, tumor necrosis factor alpha-induced activation of NF-kappaB in rheumatoid synoviocytes: inhibitory effect of simvastatin. Arthritis Rheum. 2006;vol. 54(November 11):3441–3451. doi: 10.1002/art.22169. [DOI] [PubMed] [Google Scholar]
- 95.Senthil V., Chen S.N., Tsybouleva N., Halder T., Nagueh S.F., Willerson J.T., Roberts R., Marian A.J. Prevention of cardiac hypertrophy by atorvastatin in a transgenic rabbit model of human hypertrophic cardiomyopathy. Circ. Res. 2005;vol. 97(August 3):285–292. doi: 10.1161/01.RES.0000177090.07296.ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yagi S., Aihara K., Ikeda Y., Sumitomo Y., Yoshida S., Ise T., Iwase T., Ishikawa K., Azuma H., Akaike M., Matsumoto T. Pitavastatin, an HMG-CoA reductase inhibitor, exerts eNOS-independent protective actions against angiotensin II induced cardiovascular remodeling and renal insufficiency. Circ. Res. 2008;vol. 102(January 1):68–76. doi: 10.1161/CIRCRESAHA.107.163493. [DOI] [PubMed] [Google Scholar]
- 97.Buber J., Goldenberg I., Moss A.J., Wang P.J., McNitt S., Hall W.J., Eldar M., Barsheshet A., Shechter M. Reduction in Life-Threatening Ventricular Tachyarrhythmias in Statin-Treated Patients With Nonischemic Cardiomyopathy Enrolled in the MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy) J. Am. Coll. Cardiol. 2012;vol. 60(August 8):749–755. doi: 10.1016/j.jacc.2012.03.041. [DOI] [PubMed] [Google Scholar]
- 98.Vyas A.K., Guo H., Moss A.J., Olshansky B., McNitt S.A., Hall W.J., Zareba W., Steinberg J.S., Fischer A., Ruskin J., Andrews M.L. Reduction in ventricular tachyarrhythmias with statins in the Multicenter Automatic Defibrillator Implantation Trial (MADIT)-II. J. Am. Coll. Cardiol. 2006;vol. 47(February 4):769–773. doi: 10.1016/j.jacc.2005.09.053. [DOI] [PubMed] [Google Scholar]
- 99.Mitchell L.B., Powell J.L., Gillis A.M., Kehl V., Hallstrom A.P., Investigators A. Are Lipid-Lowering Drugs Also Antiarrhythmic Drugs? An Analysis of the Antiarrhythmics Versus Implantable Defibrillators (AVID) Trial. J. Am. Coll. Cardiol. 2003;vol. 42(no. 1):81–87. doi: 10.1016/s0735-1097(03)00498-4. [DOI] [PubMed] [Google Scholar]
- 100.Vedre A., Gurm H.S., Froehlich J.B., Kline-Rogers E., Montalescot G., Gore J.M., Brieger D., Quill A.L., Eagle K.A. Impact of prior statin therapy on arrhythmic events in patients with acute coronary syndromes (from the Global Registry of Acute Coronary Events [GRACE]) Am. J. Cardiol. 2009;vol. 104(December 12):1613–1617. doi: 10.1016/j.amjcard.2009.07.045. [DOI] [PubMed] [Google Scholar]
- 101.Apiyasawat S., Sritara P., Ngarmukos T., Sriratanasathavorn C., Kasemsuwan P. Association of statin therapy with ventricular arrhythmias among patients with acute coronary syndrome. Heart Asia. 2013;vol. 5(no. 1):39–41. doi: 10.1136/heartasia-2012-010225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bruins P., te Velthuis H., Yazdanbakhsh A.P., Jansen P.G., van Hardevelt F.W., de Beaumont E.M., Wildevuur C.R., Eijsman L., Trouwborst A., Hack C.E. Activation of the complement system during and after cardiopulmonary bypass surgery: postsurgery activation involves C-reactive protein and is associated with postoperative arrhythmia. Circulation. 1997;vol. 96(November 10):3542–3548. doi: 10.1161/01.cir.96.10.3542. [DOI] [PubMed] [Google Scholar]
- 103.Hung C.-Y., Lin C.-H., Loh E.-W., Ting C.-T., Wu T.-J. CHADS2 score, statin therapy, and risks of atrial fibrillation. Am. J. Med. 2013;126(February 2):133–140. doi: 10.1016/j.amjmed.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 104.Alves-Cabratosa L., Garcia-Gil M., Comas-Cufi M., Ponjoan A., Marti-Lluch R., Parramon D., Blanch J., Elosua-Bayes M., Ramos R. Statins and new-onset atrial fibrillation in a cohort of patients with hypertension. Analysis of electronic health records, 2006-2015. PLoS One. 2017;vol. 12(no. 10):e0186972. doi: 10.1371/journal.pone.0186972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fang W., Li H.-J., Zhang H., Jiang S. The role of statin therapy in the prevention of atrial fibrillation: a meta-analysis of randomized controlled trials. Br. J. Clin. Pharmacol. 2012;vol. 74(November 5):744–756. doi: 10.1111/j.1365-2125.2012.04258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Maggioni A.P., Fabbri G., Lucci D., Marchioli R., Franzosi M.G., Latini R., Nicolosi G.L., Porcu M., Cosmi F., Stefanelli S., Tognoni G., Tavazzi L. Effects of rosuvastatin on atrial fibrillation occurrence: ancillary results of the GISSI-HF trial. Eur. Heart J. 2009;vol. 30(October 19):2327–2336. doi: 10.1093/eurheartj/ehp357. [DOI] [PubMed] [Google Scholar]
- 107.Plenge J.K., Hernandez T.L., Weil K.M., Poirier P., Grunwald G.K., Marcovina S.M., Eckel R.H. Simvastatin lowers C-reactive protein within 14 days: an effect independent of low-density lipoprotein cholesterol reduction. Circulation. 2002;vol. 106(September 12):1447–1452. doi: 10.1161/01.cir.0000029743.68247.31. [DOI] [PubMed] [Google Scholar]
- 108.Trautwein E.A., Rieckhoff D., Kunath-Rau A., Erbersdobler H.F. Replacing saturated fat with PUFA-rich (sunflower oil) or MUFA-rich (rapeseed, olive and high-oleic sunflower oil) fats resulted in comparable hypocholesterolemic effects in cholesterol-fed hamsters. Ann. Nutr. Metab. 1999;vol. 43(no. 3):159–172. doi: 10.1159/000012782. [DOI] [PubMed] [Google Scholar]
- 109.Awada M., Soulage C.O., Meynier A., Debard C., Plaisancie P., Benoit B., Picard G., Loizon E., Chauvin M.-A., Estienne M., Peretti N., Guichardant M., Lagarde M., Genot C., Michalski M.-C. Dietary oxidized n-3 PUFA induce oxidative stress and inflammation: role of intestinal absorption of 4-HHE and reactivity in intestinal cells. J. Lipid Res. 2012;vol. 53(October 10):2069–2080. doi: 10.1194/jlr.M026179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Laye S., Nadjar A., Joffre C., Bazinet R.P. Anti-Inflammatory Effects of Omega-3 Fatty Acids in the Brain: Physiological Mechanisms and Relevance to Pharmacology. Pharmacol. Rev. 2018;vol. 70(January 1):12–38. doi: 10.1124/pr.117.014092. [DOI] [PubMed] [Google Scholar]
- 111.Kromhout D., Bosschieter E.B., Cor de Lezenne C. The inverse relation between fish consumption and 20-year mortality from coronary heart disease. N. Engl. J. Med. 1985;312(19):1205–1209. doi: 10.1056/NEJM198505093121901. [DOI] [PubMed] [Google Scholar]
- 112.Mozaffarian D., Wu J.H.Y. Omega-3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011;58(20):2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 113.Mozaffarian D., Psaty B.M., Rimm E.B., Lemaitre R.N., Burke G.L., Lyles M.F., Lefkowitz D., Siscovick D.S. Fish intake and risk of incident atrial fibrillation. Circulation. 2004;vol. 110(no. 4):368–373. doi: 10.1161/01.CIR.0000138154.00779.A5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Virtanen J.K., Mursu J., Voutilainen S., Tuomainen T.P. Serum long-chain n-3 polyunsaturated fatty acids and risk of hospital diagnosis of atrial fibrillation in men. Circulation. 2009;vol. 120(no. 23):2315–2321. doi: 10.1161/CIRCULATIONAHA.109.852657. [DOI] [PubMed] [Google Scholar]
- 115.Berry J.D., Prineas R.J., van Horn L., Passman R., Larson J., Goldberger J., Snetselaar L., Tinker L., Liu K., Lloyd-Jones D.M. Dietary Fish Intake and Incident Atrial Fibrillation (from the Women’s Health Initiative) Am. J. Cardiol. 2010;vol. 105(no. 6):844–848. doi: 10.1016/j.amjcard.2009.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Abdelhamid A.S., Martin N., Bridges C., Brainard J.S., Wang X., Brown T.J., Hanson S., Jimoh O.F., Ajabnoor S.M., Deane K.H., Song F., Hooper L. Polyunsaturated fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2018 doi: 10.1002/14651858.CD012345.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fischer S., Weber P.C. Thromboxane A3 (TXA3) is formed in human platelets after dietary eicosapentaenoic acid (C20:5 omega 3) Biochem. Biophys. Res. Commun. 1983;116(November 3):1091–1099. doi: 10.1016/s0006-291x(83)80254-x. [DOI] [PubMed] [Google Scholar]
- 118.Siess W., Roth P., Scherer B., Kurzmann I., Bohlig B., Weber P.C. Platelet-membrane fatty acids, platelet aggregation, and thromboxane formation during a mackerel diet. Lancet (London, England) 1980;vol. 1(March 8166):441–444. doi: 10.1016/s0140-6736(80)90995-2. [DOI] [PubMed] [Google Scholar]
- 119.Nair S.S., Leitch J.W., Falconer J., Garg M.L. Prevention of cardiac arrhythmia by dietary (n-3) polyunsaturated fatty acids and their mechanism of action. J. Nutr. 1997;vol. 127(March 3):383–393. doi: 10.1093/jn/127.3.383. [DOI] [PubMed] [Google Scholar]
- 120.McLennan P.L., Abeywardena M.Y., Charnock J.S. Reversal of the arrhythmogenic effects of long-term saturated fatty acid intake by dietary n-3 and n-6 polyunsaturated fatty acids. Am. J. Clin. Nutr. 1990;51(January 1):53–58. doi: 10.1093/ajcn/51.1.53. [DOI] [PubMed] [Google Scholar]
- 121.Albert C.M., Campos H., Stampfer M.J., Ridker P.M., Manson J.E., Willett W.C., Ma J. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N. Engl. J. Med. 2002;vol. 346(April 15):1113–1118. doi: 10.1056/NEJMoa012918. [DOI] [PubMed] [Google Scholar]
- 122.Jouven X., Charles M.A., Desnos M., Ducimetiere P. Circulating nonesterified fatty acid level as a predictive risk factor for sudden death in the population. Circulation. 2001;vol. 104(August 7):756–761. doi: 10.1161/hc3201.094151. [DOI] [PubMed] [Google Scholar]
- 123.Xiao Y.F., Gomez A.M., Morgan J.P., Lederer W.J., Leaf A. Suppression of voltage-gated L-type Ca2+ currents by polyunsaturated fatty acids in adult and neonatal rat ventricular myocytes. Proc. Natl. Acad. Sci. U. S. A. 1997;vol. 94(April. 8):4182–4187. doi: 10.1073/pnas.94.8.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xiao Y.F., Wright S.N., Wang G.K., Morgan J.P., Leaf A. Fatty acids suppress voltage-gated Na+ currents in HEK293t cells transfected with the alpha-subunit of the human cardiac Na+ channel. Proc. Natl. Acad. Sci. U. S. A. 1998;vol. 95(March 5):2680–2685. doi: 10.1073/pnas.95.5.2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Leifert W.R., Jahangiri A., Saint D.A., McMurchie E.J. Effects of dietary n-3 fatty acids on contractility, Na+ and K+ currents in a rat cardiomyocyte model of arrhythmia. J. Nutr. Biochem. 2000;vol.11(7–8):382–392. doi: 10.1016/s0955-2863(00)00094-2. [DOI] [PubMed] [Google Scholar]
- 126.Kang J.X., Xiao Y.F., Leaf A. Free, long-chain, polyunsaturated fatty acids reduce membrane electrical excitability in neonatal rat cardiac myocytes. Proc. Natl. Acad. Sci. U. S. A. 1995;vol. 92(April 9):3997–4001. doi: 10.1073/pnas.92.9.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Leaf A., Xiao Y.F. The modulation of ionic currents in excitable tissues by n-3 polyunsaturated fatty acids. J. Membr. Biol. 2001;vol. 184(December 3):263–271. doi: 10.1007/s00232-001-0095-0. [DOI] [PubMed] [Google Scholar]
- 128.Swan J.S., Dibb K., Negretti N., O’Neill S.C., Sitsapesan R. Effects of eicosapentaenoic acid on cardiac SR Ca(2+)-release and ryanodine receptor function. Cardiovasc. Res. 2003;vol. 60(November 2):337–346. doi: 10.1016/s0008-6363(03)00545-5. [DOI] [PubMed] [Google Scholar]
- 129.Bacova B., Radosinska J., Knezl V., Kolenova L., Weismann P., Navarova J., Barancik M., Mitasikova M., Tribulova N. Omega-3 fatty acids and atorvastatin suppress ventricular fibrillation inducibility in hypertriglyceridemic rat hearts: implication of intracellular coupling protein, connexin-43. J. Physiol. Pharmacol. 2010;vol. 61(December 6):717–723. [PubMed] [Google Scholar]
- 130.Benova T., Knezl V., Viczenczova C., Bacova B.S., Radosinska J., Tribulova N. Acute anti-fibrillating and defibrillating potential of atorvastatin, melatonin, eicosapentaenoic acid and docosahexaenoic acid demonstrated in isolated heart model. J. Physiol. Pharmacol. 2015;vol. 66(February 1):83–89. [PubMed] [Google Scholar]
- 131.Parratt J.R., Coker S.J., Wainwright C.L. Eicosanoids and susceptibility to ventricular arrhythmias during myocardial ischaemia and reperfusion. J. Mol. Cell. Cardiol. 1987;vol.19(Suppl 5 October):55–66. doi: 10.1016/s0022-2828(87)80610-7. [DOI] [PubMed] [Google Scholar]
- 132.Abeywardena M.Y., McLennan P.L., Charnock J.S. Differential effects of dietary fish oil on myocardial prostaglandin I2 and thromboxane A2 production. Am. J. Physiol. 1991;vol. 260(February Pt 2):H379–H385. doi: 10.1152/ajpheart.1991.260.2.H379. [DOI] [PubMed] [Google Scholar]
- 133.Hallaq H., Smith T.W., Leaf A. Modulation of dihydropyridine-sensitive calcium channels in heart cells by fish oil fatty acids. Proc. Natl. Acad. Sci. U. S. A. 1992;vol. 89(March 5):1760–1764. doi: 10.1073/pnas.89.5.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pepe S., Bogdanov K., Hallaq H., Spurgeon H., Leaf A., Lakatta E. Omega 3 polyunsaturated fatty acid modulates dihydropyridine effects on L-type Ca2+ channels, cytosolic Ca2+, and contraction in adult rat cardiac myocytes. Proc. Natl. Acad. Sci. U. S. A. 1994;vol. 91(September 19):8832–8836. doi: 10.1073/pnas.91.19.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Christensen J.H., Korup E., Aaroe J., Toft E., Moller J., Rasmussen K., Dyerberg J., Schmidt E.B. Fish consumption, n-3 fatty acids in cell membranes, and heart rate variability in survivors of myocardial infarction with left ventricular dysfunction. Am. J. Cardiol. 1997;vol. 79(June 12):1670–1673. doi: 10.1016/s0002-9149(97)00220-8. [DOI] [PubMed] [Google Scholar]
- 136.Borow K.M., Nelson J.R., Mason R.P. Biologic plausibility, cellular effects, and molecular mechanisms of eicosapentaenoic acid (EPA) in atherosclerosis. Atherosclerosis. 2015;vol. 242(no. 1):357–366. doi: 10.1016/j.atherosclerosis.2015.07.035. [DOI] [PubMed] [Google Scholar]
- 137.Cawood A.L., Ding R., Napper F.L., Young R.H., Williams J.A., Ward M.J.A., Gudmundsen O., Vige R., Payne S.P.K., Ye S., Shearman C.P., Gallagher P.J., Grimble R.F., Calder P.C. Eicosapentaenoic acid (EPA) from highly concentrated n−3 fatty acid ethyl esters is incorporated into advanced atherosclerotic plaques and higher plaque EPA is associated with decreased plaque inflammation and increased stability. Atherosclerosis. 2010;vol. 212(no. 1):252–259. doi: 10.1016/j.atherosclerosis.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 138.Nishio R., Shinke T., Otake H., Nakagawa M., Nagoshi R., Inoue T., Kozuki A., Hariki H., Osue T., Taniguchi Y., Iwasaki M., Hiranuma N., Konishi A., Kinutani H., Shite J., Hirata K. Stabilizing effect of combined eicosapentaenoic acid and statin therapy on coronary thin-cap fibroatheroma. Atherosclerosis. 2014;vol. 234(no. 1):114–119. doi: 10.1016/j.atherosclerosis.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 139.Chen J., Shearer G.C., Chen Q., Healy C.L., Beyer A.J., Nareddy V.B., Gerdes A.M., Harris W.S., O’Connell T.D., Wang D. Omega-3 fatty acids prevent pressure overload-induced cardiac fibrosis through activation of cyclic GMP/protein kinase G signaling in cardiac fibroblasts. Circulation. 2011;vol. 123(February 6):584–593. doi: 10.1161/CIRCULATIONAHA.110.971853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ramadeen A., Laurent G., dos Santos C.C., Hu X., Connelly K.A., Holub B.J., Mangat I., Dorian P. n-3 Polyunsaturated fatty acids alter expression of fibrotic and hypertrophic genes in a dog model of atrial cardiomyopathy. Hear. Rhythm. 2010;vol. 7(April 4):520–528. doi: 10.1016/j.hrthm.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 141.McLennan P.L., Abeywardena M.Y., Charnock J.S. Influence of dietary lipids on arrhythmias and infarction after coronary artery ligation in rats. Can. J. Physiol. Pharmacol. 1985;vol. 63(November 11):1411–1417. doi: 10.1139/y85-232. [DOI] [PubMed] [Google Scholar]
- 142.Leaf A., Albert C.M., Josephson M., Steinhaus D., Kluger J., Kang J.X., Cox B., Zhang H., Schoenfeld D. Prevention of fatal arrhythmias in high-risk subjects by fish oil n-3 fatty acid intake. Circulation. 2005;vol. 112(November 18):2762–2768. doi: 10.1161/CIRCULATIONAHA.105.549527. [DOI] [PubMed] [Google Scholar]
- 143.Raitt M.H., Connor W.E., Morris C., Kron J., Halperin B., Chugh S.S., McClelland J., Cook J., MacMurdy K., Swenson R., Connor S.L., Gerhard G., Kraemer D.F., Oseran D., Marchant C., Calhoun D., Shnider R., McAnulty J. Fish oil supplementation and risk of ventricular tachycardia and ventricular fibrillation in patients with implantable defibrillators: a randomized controlled trial. JAMA. 2005;vol. 293(June 23):2884–2891. doi: 10.1001/jama.293.23.2884. [DOI] [PubMed] [Google Scholar]
- 144.Burr M.L., Gilbert J.F., Holliday R.M., Elwood P.C., Fehily A.M., Rogers S., Sweetnam P.M., Deadman N.M. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART) Lancet. 1989;334(8666):757–761. doi: 10.1016/s0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]
- 145.Billman G.E., Carnes C.A., Adamson P.B., Vanoli E., Schwartz P.J. Dietary Omega-3 Fatty Acids and Susceptibility to Ventricular Fibrillation. Circ. Arrhythmia Electrophysiol. 2012;vol. 5(no. 3):553–560. doi: 10.1161/CIRCEP.111.966739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.M. R, M. D, S. G, M. A, F. P, G. T, L. R, L. P, L. F, O. P, P. R, S. M, T. L Fish oil for the prevention of post-operative atrial fibrillation - the omega-3 fatty acids for prevention of post-operative atrial fibrillation (OPERA) trial. Circulation. 2012;vol. 126(no. 23):2781. [Google Scholar]
- 147.Kowey P.R., Reiffel J.A., Ellenbogen K.A., Naccarelli G.V., Pratt C.M. Efficacy and safety of prescription omega-3 fatty acids for the prevention of recurrent symptomatic atrial fibrillation: A randomized controlled trial. JAMA J. Am. Med. Assoc. 2010;304(21):2363–2372. doi: 10.1001/jama.2010.1735. [DOI] [PubMed] [Google Scholar]
- 148.Sandesara C.M., Chung M.K., Van Wagoner D.R., Barringer T.A., Allen K., Ismail H.M., Zimmerman B., Olshansky B. A randomized, placebo‐controlled trial of omega‐3 fatty acids for inhibition of supraventricular arrhythmias after cardiac surgery: the FISH trial. J. Am. Heart Assoc. 2012;1(3) doi: 10.1161/JAHA.111.000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Billman G.E. Omega-3 polyunsaturated fatty acids and cardiac rhythm: an introduction. Front. Physiol. 2012;3(December) doi: 10.3389/fphys.2012.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hines M.L., Davison A.P., Muller E. NEURON and Python. Front. Neuroinform. 2009;vol. 3:1. doi: 10.3389/neuro.11.001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.DiFrancesco D., Noble D. A model of cardiac electrical activity incorporating ionic pumps and concentration changes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1985;307(January 1133):353–398. doi: 10.1098/rstb.1985.0001. [DOI] [PubMed] [Google Scholar]
- 152.Lo Y.-C., Kuo C.-C. Temperature Dependence of the Biophysical Mechanisms Underlying the Inhibition and Enhancement Effect of Amiodarone on hERG Channels. Mol. Pharmacol. 2019;vol. 96(September 3):330–344. doi: 10.1124/mol.119.116400. [DOI] [PubMed] [Google Scholar]
- 153.Huang H., Pugsley M.K., Fermini B., Curtis M.J., Koerner J., Accardi M., Authier S. Cardiac voltage-gated ion channels in safety pharmacology: review of the landscape leading to the CiPA initiative. J. Pharmacol. Toxicol. Methods. 2017;87:11–23. doi: 10.1016/j.vascn.2017.04.002. [DOI] [PubMed] [Google Scholar]