Abstract
Lomentospora prolificans has caused outbreaks in immunocompromised patients. We performed whole genome sequencing (WGS) on 4 L. prolificans isolates from infections occurring during an 8-month period in the haematology unit at Hospital 1., and 2 isolates from unrelated infections at Hospital 2., showing a high number of mutational differences (>10,000 single nucleotide polymorphisms) between L. prolificans isolates from Hospital 1. Novel typing of isolates by WGS did not demonstrate a single causative strain.
Keywords: Lomentospora prolificans, Scedosporium prolificans, Fungal outbreak, Whole genome sequencing, Antifungal resistance
1. Introduction
Lomentospora prolificans is an environmental mold which is found particularly in Australia, Spain, Portugal, California and the southern United States [1]. It characteristically causes clinical infection in immunocompromised hosts and is notable for resistance to antifungals. Invasive disease occurs mainly in patients with haematological disease, stem cell transplantation and lung transplantation, with fewer cases in AIDS patients [2,3]. Overall mortality due to infection is high at 46.7%, with almost universal mortality with disseminated disease of 87.5% [2]. A cluster of clinical cases in our haematology unit prompted an examination for an environmental source and testing of the isolates to determine if a single strain caused the infections.
2. Clinical case presentation
We included four cases in the haematology unit at Hospital 1 which were all the probable or proven invasive L. prolificans infections in the hospital during an eight-month period according to established definitions [4]. Table 1. summarises the case demographics, underlying illness, clinical manifestations, antifungal therapy, and outcomes.
Case 1
(C1H1) was a 53 year old male with background of liver cirrhosis, type 2 diabetes mellitus and diffuse large B-cell lymphoma. He had a matched unrelated stem cell transplant 5th February 2015, complicated by acute graft-versus-host-disease treated with prednisolone and sirolimus, acute kidney injury requiring haemodialysis, and intensive care unit admission. He was on fluconazole prophylaxis. On the 19th May 2015 (Day 0) necrotic soft tissue was noticed at his heel. Swab D0, swab D+2 and tissue D+6 grew L. prolificans (fungal microscopy negative by Calcofluor White stain). MRI showed localized osteomyelitis of the calcaneum and overlying soft tissue inflammation. There were no abnormalities on CT chest and sinuses. On D+3 he commenced oral voriconazole with loading doses 400 mg b.d. on day one, followed by 200 mg b.d. (weight 88 kg), and oral terbinafine 250 mg once daily (creatinine clearance 54 ml/min). Serum voriconazole level was not obtained. D+15 he complained of new abdominal pain and was relatively hypotensive. The following day (D+16) he was found unresponsive, deceased.
Case 2
(C2H1) was a 71 year old female with chronic obstructive pulmonary disease who was diagnosed with chronic lymphocytic leukemia. This was treated with fludarabine, cyclophosphamide and rituximab in April 2015 without antifungal prophylaxis. She presented 26th May 2015 with fatigue, sweats, cough, and chest pain. CT chest showed nodules with surrounding halo sign suggestive of pulmonary mold infection. Expectorated sputum 27th May 2015 grew Klebsiella pneumoniae and a prior sputum taken 17th April 2015 had grown Aspergillus niger and Aspergillus terreus. She was treated with antibacterials and oral voriconazole 300 mg b.d., trough levels 0.6–1.3 mg/L, and it was ceased 2nd July. She had a rise in inflammatory markers and development of soft tissue nodules 7th July 2015 (D0). Disseminated infection was strongly suspected as hyphal elements were seen by Calcofluor White microscopy and L. prolificans was grown from urine and sputum, and hyphae and granulomatous inflammation were seen from biopsy of a soft tissue nodule (no growth, direct fungal PCR negative). CT chest and abdomen D+2 showed improved nodular lung lesions and no abnormalities in the kidneys. Blood culture was negative. Pending culture results, empirical liposomal amphotericin 400 mg once daily (patient weight ~80 kg) was commenced D0 to D+5, adding voriconazole (intravenous, 500 mg b.d. day one, then 300 mg b.d. ongoing) at D+2 with provisional Scedosporium spp from culture of urine and sputum. With identification of L. prolificans at D+7, we added oral terbinafine 250 mg once daily (creatinine clearance 20 ml/min) and IV anidulafungin (200 mg daily loading dose day one, then 100 mg daily ongoing) to ongoing voriconazole. Voriconazole trough level was 4.1 mg/L on D+9. On D+10 she developed sudden onset breathlessness, hypoxia with a bland chest X-ray, followed by reduced conscious state, and died D+12.
Case 3
(C3H1) was a 63 year old male admitted 5th July 2015 with a new diagnosis of acute myeloid leukemia, treated with idarubicin and cytarabine with oral posaconazole MR tablet 300 mg antifungal prophylaxis, trough level on the 15th July of 1.7 mg/L. Febrile neutropenia with abdominal pain developed 14th July, and CT abdomen showed right colon wall oedema and stranding consistent with typhlitis. Escherichia coli was grown from blood 14th July, and L. prolificans was grown from blood 19th July (D0), and also from blood cultures on D+2, D+3, and D+4. On D+2 he started IV voriconazole 400 mg b.d. (weight 72 kg, so 5.6 mg/kg/dose), oral terbinafine 500 mg b.d. (creatinine clearance 38 ml/min), and IV anidulafungin (200 mg day one, followed by 100 mg daily ongoing). On D+2 he complained of a painful eye however no vitreous or retinal pathology was evident by ophthalmologist examination and CT eye was unremarkable. On D+3 he developed hypotension, acute kidney injury, bilateral hilar infiltrates on chest X-ray, and coagulopathy. He died D+5.
Case 4
(C4H1) was a 25 year old female with acute myeloid leukemia who had a matched unrelated stem cell transplant 30th December 2015. L. prolificans grew from blood culture taken 7th January 2016 (D0), when she had febrile neutropenia, CT abdomen evidence of typhlitis, and sinus opacification without bony erosion on CT sinuses. CT chest was unremarkable. She was commenced D+4 on IV voriconazole (loading dose 400 mg b.d. day one, followed by 250 mg b.d., weight 64 kg), oral terbinafine 500 mg b.d. ongoing (creatinine clearance 100 ml/min), and IV anidulafungin (200 mg day one, 100 mg daily ongoing).Voriconazole dose was increased to 400 mg b.d. D+33. The first trough voriconazole level at D+13 was therapeutic at 3.2 mg/L, from D+32 to D+52 levels were subtherapeutic 0.2–0.4 mg/L (the majority of voriconazole was given orally in this period). Right sternoclavicular joint pain developed with inflammatory changes on MRI D+40 and Fluorodeoxyglucose (FDG) uptake on Positron emission tomography (PET) scan D+42. This was surgically debrided D+50 and tissue samples grew L. prolificans after 10 days incubation. New areas of kidney hypoattenuation were seen by CT abdomen D+57 (not present on PET scan), and urine taken D+61 grew L.prolificans. Miltefosine was added D+73 at 150 mg once daily, reduced due to nausea to 120 mg once daily D+98, which was continued. Voriconazole doses were increased stepwise at D+71 (250 mg b.d), D+73 (300 mg b.d.), D+93 (350 mg b.d.), D+99 (450 mg b.d.), and trough voriconazole levels in this period D+53 to D+126 were slightly higher than before at 0.5–1.6 mg/L. With new back pain, PET scan was repeated D+126 which showed new FDG uptake at the S1 vertebra and the right 4th costosternal junction. Biopsy of the 4th costosternal junction grew L. prolificans after 6 days incubation. From D+127 to D+147 trough voriconazole levels were 3.6–10 mg/L. She developed acute abdominal pain with acidosis D+146 thought to be secondary to gut graft-versus-host-disease, was palliated and died D+149. Of note, the majority of the time voriconazole was given intravenously, and she only had therapeutic levels on doses of 350 mg b.d. or higher suggesting she may have been a rapid or ultrarapid metabolizer of voriconazole, however we never tested cytochrome p450 2C19 alleles.
Case 5
(C5H2) was a 69 year old female without past medical history who presented on 7th December 2016 with flexor tendon tenosynovitis one week after a thorn injury. Tissue samples grew L. prolificans and she was cured with several months of voriconazole with terbinafine. Trough voriconazole levels were 0.6–1.1 mg/L. Doses were not recorded.
Case 6
(C6H2) was a 45 year old indigenous male from a remote region of Western Australia. He had acute myeloid leukemia treated with idarubicin and cytarabine from the 4th May 2015. He presented on the 15th June 2015 with back pain, MRI demonstrated changes consistent with osteomyelitis and discitis at L1 and L2 vertebrae. Bone biopsy 20th June (D0) saw hyphal elements on Calcofluor White microscopy and grew L. prolificans. He was treated with voriconazole and terbinafine (doses not recorded). CT chest D+20 showed a few nodules less than 10 mm diameter. Trough voriconazole level D+22 was 0.2 mg/L, D+28 was 4.5 mg/L, D+40 was 0.4 mg/L, D+45 was 2.2 mg/L, and D+52 was 1.2 mg/L. CT chest repeated D+45 showed increasing infiltrates consistent with pulmonary mold infection. He deteriorated acutely with respiratory failure and was found unresponsive, deceased D+56.
Table 1.
Clinical characteristics of the cases with Lomentospora prolificans infection.
| Case number | Hospital | GenBank accession no. | Date of isolate | Site of infection | Age (yrs) | Sex | Disease | Antifungal treatment | Survival from positive isolate (days) |
|---|---|---|---|---|---|---|---|---|---|
| 1 (C1H1) | 1 | SAMN10849956 | 19/05/15 | Soft tissue | 53 | M | AlloHCTa Cirrhosis |
Voriconazole Terbinafine |
16 |
| 2 (C2H1) | 1 | SAMN10849957 | 07/07/15 | Disseminated | 71 | F | CLLb | Voriconazole Terbinafine Anidulafungin |
12 |
| 3 (C3H1) | 1 | SAMN10849958 | 19/07/15 | Disseminated | 63 | M | AMLc | Voriconazole Terbinafine Anidulafungin |
5 |
| 4 (C4H1) | 1 | SAMN10849959 | 07/01/16 | Disseminated | 25 | F | AlloHCTa | Voriconazole Terbinafine Anidulafungin Miltefosine |
149 |
| 5 (C5H2) | 2 | SAMN10849960 | 08/12/16 | Tendon sheath | 70 | F | Nil | Voriconazole Terbinafine |
Alive |
| 6 (C6H2) | 2 | SAMN10849961 | 20/06/15 | Vertebrae | 45 | M | AMLc | Voriconazole Terbinafine |
56 |
Allogeneic haematopoietic stem cell transplant.
Chronic lymphocytic leukemia.
Acute myeloid leukemia.
In Hospital 1., some cases were admitted to the haematology ward at the same time (Case 1. and Case 2.; and Case 2. and Case 3.), though none of the four cases of Hospital 1. were admitted to a common room on the ward at any time. The high-efficiency particulate air (HEPA) filters in the air conditioning system and positive pressure airflow systems in the rooms in which the patients were admitted had passed routine checks during the period. At the time of these cases, there were minor earthworks at a traffic light adjacent to the hospital and also water leakage from plumbing at a distant part of the hospital. One cubic metre of air for fungal culture was sampled in affected patient rooms, other areas of the haematology ward, the cancer outpatient centre, the main hospital concourse, car park and open grounds. Swabs for fungal culture were taken from patient sinks and various other surfaces of patient rooms in the haematology ward. However there was no growth of L. prolificans from any of the environmental samples.
3. Identification and minimum inhibitory concentration testing
L. prolificans was identified by characteristic macroscopic and microscopic features in addition to in-house Sanger sequencing of the internal transcribed spacer region 1 and 2 of the ribosomal DNA gene as previously described [5]. Antifungal susceptibility testing was performed by microbroth dilution with the Sensititre® YeastOne YO10 system (TREK Diagnostic Systems, West Sussex, UK). The study was approved with a waiver of informed patient consent by the Fiona Stanley Hospital Human Research and Ethics Committee, RGS number 191.
Minimum inhibitory concentrations (MICs) of the isolates from the six clinical cases are presented in Table 2., showing very high MICs for the triazoles, echinocandins and amphotericin B.
Table 2.
Antifungal minimum inhibitory concentrations of the Lomentospora prolificans isolates.
| Case number | Itraconazole | Posaconazole | Voriconazole | Anidulafungin | Caspofugin | Micafungin | Amphotericin | ||
|---|---|---|---|---|---|---|---|---|---|
| 1 (C1H1) | >16 | 8 | 2 | 2 | 1 | 1 | 8 | ||
| 2 (C2H1) | >16 | >8 | >8 | 2 | 1 | 2 | >8 | ||
| 3 (C3H1) | 16 | >8 | >8 | 2 | 8 | 4 | >8 | ||
| 4 (C4H1) | >16 | >8 | 8 | 2 | 4 | 2 | 4 | ||
| 5 (C5H2) | >16 | >8 | 4 | 2 | 2 | 2 | >8 | ||
| 6 (C6H2) | >16 | >8 | 8 | 2 | 1 | 2 | >8 | ||
4. Whole genome sequencing
Isolates from Hospital 1. were compared to two unrelated isolates from Hospital 2. Whole genome sequencing (WGS) using a Nextseq platform was performed on the four isolates from the case cluster, two unrelated isolates, and an ATCC isolate. A whole genome sequence of L. prolificans strain (NCBI accession number NLAX00000000) was used as the reference sequence [6]. The Illumina paired-end sequence data were analysed to identify single nucleotide polymorphisms (SNPs) in the core genome by comparison with the reference sequence with Snippy version 4.0 (http://www.vicbioinformatics.com/software.snippy.shtml). A maximum likelihood tree was constructed using MEGA v7.0 with 1000 bootstraps [7].
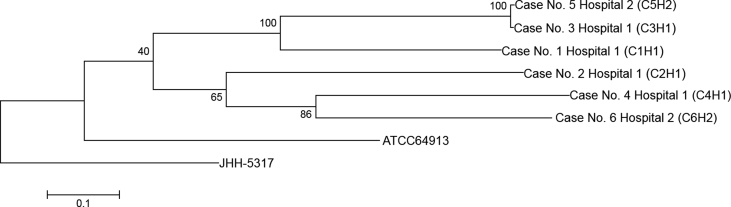
By phylogenetic analysis there appeared to be L. prolificans isolates circulating in both hospitals which diverged from a common ancestor linking to the ATCC64913 strain (see Fig. 1.). Other than the close genomic similarity shared between isolates from Case 5 and Case 3 (39 SNPs) for which there was no clinical connection, the other isolates contain a high number of mutational differences (>10,000 SNPs) between them. The results suggest no genetic relationship between the 4 clinically clustered cases in Hospital 1.
Fig. 1.
Phylogeny tree based on maximum likelihood of Lomentospora prolificans strains from four clinically clustered cases from Hospital 1, two unrelated cases from Hospital 2, an ATCC strain (ATCC 64913), and the reference sequence (JHH-5317). Numbers at the branch root indicate the bootstrap value supporting the replicates.
We decided not to change routine anti-mold prophylaxis of posaconazole to patients with acute myeloid leukemia and graft-versus-host-disease because of the resistance of L. prolificans to antifungal agents including high MICs for voriconazole and terbinafine, and we did not extend anti-mold prophylaxis to patients who would not normally require it. Without any change in practice, no further cases occurred.
5. Discussion
The occurrence of four cases in an eight-month period of invasive L. prolificans infection in our haematology unit at Hospital 1. was very concerning due to the rarity of this pathogen, its resistance to antifungal therapy and the high attributable mortality. WGS of the isolates suggested it was not the same strain causing infection. This does not rule out the possibility of a single common source involving multiple strains of L. prolificans, however a source was not identified by environmental sampling.
Other clusters or outbreaks with L. prolificans have been described. Guerrero et al. described six haematology patients with invasive infection with L. prolificans in a 14-month period. The organism was identified from air samples from two rooms and PCR fingerprinting suggested the two environmental samples and four clinical samples were identical. In response, HEPA filters and positive pressure airflow systems were installed into the rooms of the haematology ward, which aborted the outbreak [8]. Alvarez et al. reported four fatal cases in haematology patients over a one month period. At the time, the haematology ward was being refurbished and haematology patients were admitted to a standard ward with construction work occurring on the same floor. No additional cases were identified after haematology patients were moved to an alternative floor [9].
During nosocomial fungal outbreaks more generally, construction and disturbances to airflow systems are the commonest precipitants, and haematology or bone marrow transplant units are the most frequently affected patient groups. Suggested measures for prevention include communication between hospital engineering and infection prevention teams, risk assessments of construction work, measures to control the airborne dissemination of fungal spores such as barriers and portable HEPA filters, and surveillance for health-care associated mold infection [10].
L. prolificans is notorious for being resistant to azoles, echinocandins and polyenes. Current guidelines suggest treatment of voriconazole with terbinafine [11], however these antifungals are barely active [3] and consequently outcomes in immunocompromised patients are very poor [1], as was illustrated in our study. Miltefosine has in vitro activity with MICs of approximately 4 mg/L and in vitro led to a major reduction in amphotericin B and voriconazole MICs when combined with these antifungals [12]. Steady-state serum concentrations of 15–40 mg/L have been reported at the end of miltefosine treatment for visceral leishmaniasis, although it should be noted that the drug is highly protein bound (96%) in serum [13]. Miltefosine has been used with some success in combination with voriconazole and terbinafine [14], and may have prolonged survival in Case 4. in our study where it was utilized in combination with voriconazole, terbinafine and anidulafungin. Active antifungals are urgently required for L. prolificans. In vitro activity is evident for some novel antifungals such as ibrexafungerp, fosmanogepix, AR-12 and olorofim [15,16]. Results are eagerly awaited for olorofim as the agent most progressed along clinical development, with a global open-label phase 2b clinical study (FORMULA-OLS, NCT03583164) recruiting patients with difficult fungal infection including lomentosporiosis [17].
In summary, we have reported four cases of L. prolificans infection in haematology patients at a single institution in an eight-month period for which no common link was found. The infections were all fatal, generally within weeks of fungal diagnosis. WGS as a novel molecular epidemiological tool for this organism suggested the infections were not caused by a single strain. We could not find an environmental source by sampling, and without a change in practice, fortunately cases ceased to occur.
Declaration of competing interest
There are none
Acknowledgements
Nil.
References
- 1.Cortez K.J., Roilides E., Quiroz-Telles F., Meletiadis J., Antachopolous C., Knudsen T. Infections caused by Scedosporium spp. Clin. Microbiol. Rev. 2008;21(1):157–197. doi: 10.1128/CMR.00039-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodriguez-Tudela J.L., Berenguer J., Guarro J., Serda Kantarcioglu A., Horre R., Sybren de Hoog G. Epidemiology and outcome of Scedosporium prolificans infection, a review of 162 cases. Med. Mycol. 2009;47(Special issue):359–370. doi: 10.1080/13693780802524506. [DOI] [PubMed] [Google Scholar]
- 3.Cooley L., Spelman D., Thursky K., Slavin M. Infection with Scedosporium apiospermum and S. prolificans, Australia. Emerg. Infect. Dis. 2007;13:1170–1177. doi: 10.3201/eid1308.060576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Pauw B., Walsh T.J., Donnelly J.P., Stevens D.A., Edwards J.E., Calandra T. Revised definitions of invasive fungal disease from the European organization for research and treatment of cancer/invasive fungal infections cooperative group and the national institute of allergy and infectious diseases mycoses study group (EORTC/MSG) consensus group. Clin. Infect. Dis. 2008;46(12):1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pryce T.M., Palladino S., Kay I.D., Coombs G.W. Rapid identification of fungi by sequencing the ITS1 and ITS2 regions using an automated capillary electrophoresis system. Med. Mycol. 2003;41(5):369–381. doi: 10.1080/13693780310001600435. [DOI] [PubMed] [Google Scholar]
- 6.Luo R., Zimin A., Workman R., Fan Y., Pertea G., Grossman N. First draft genome sequence of the pathogenic fungus Lomentospora prolificans (formerly Scedosporium prolificans) G3. 2017;7:3831–3836. doi: 10.1534/g3.117.300107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar S., Stecher G., Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33(7):1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guerrero A., Torres P., Teresa Duran M., Ruiz-Díez B., Rosales M., Rodirguez-Tudela J.L. Airborne outbreak of nosocomial Scedosporium prolificans infection. Lancet. 2001;357:1267–1268. doi: 10.1016/S0140-6736(00)04423-8. [DOI] [PubMed] [Google Scholar]
- 9.Alvarez M., Ponga B.L., Rayon C., Gala J.G., Porto M.C.R., Gonzalez M. Nosocomial outbreak caused by Scedosporium prolificans (inflatum): four fatal cases in leukemic patients. J. Clin. Microbiol. 1995;33(12):3290–3295. doi: 10.1128/jcm.33.12.3290-3295.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanamori H., Rutala W.A., Sickbert-Bennett E.E., Weber D.J. Review of fungal outbreaks and infection prevention in healthcare settings during construction and renovation. Clin. Infect. Dis. 2015;61(3):433–444. doi: 10.1093/cid/civ297. [DOI] [PubMed] [Google Scholar]
- 11.Tortorano A.M., Richardson M., Roilides E., van Diepeningen A., Caira M., Munoz P. ESCMID and ECMM joint guidelines on diagnosis and management of hyalohyphomycosis: Fusarium spp., Scedosporium spp. and others. Clin. Microbiol. Infect. 2014;20(Suppl. 3):27–46. doi: 10.1111/1469-0691.12465. [DOI] [PubMed] [Google Scholar]
- 12.Compain F., Botterel F., Sitterlé E., Paugam A., Bougnoux M.-E., Dannaoui E. In vitro activity of miltefosine in combination with voriconazole or amphotericin B against clinical isolates of Scedosporium spp. J. Med. Microbiol. 2015;64:309–311. doi: 10.1099/jmm.0.000019. [DOI] [PubMed] [Google Scholar]
- 13.Dorlo T.P.C., Balasegaram M., Beijnen J.H., de Vries P.J. Miltefosine: a review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J. Antimicrob. Chemother. 2012;67:2576–2597. doi: 10.1093/jac/dks275. [DOI] [PubMed] [Google Scholar]
- 14.Kesson A.M., Bellemore M.C., O'Mara T.J., Ellis D.H., Sorrell T.C. Scedosporium prolificans osteomyelitis in an immunocompetent child treated with a novel agent, hexacylphospocholine (Miltefosine), in combination with terbinafine and voriconazole: a case report. Clin. Infect. Dis. 2009;48:1257–1261. doi: 10.1086/597772. [DOI] [PubMed] [Google Scholar]
- 15.Biswas C., Law D., Birch M., Halliday C., Sorrell T.C., Rex J. In vitro activity of the novel antifungal compound F901318 against Australian Scedosporium and Lomentospora fungi. Med. Mycol. 2018;56(8):1050–1054. doi: 10.1093/mmy/myx161. [DOI] [PubMed] [Google Scholar]
- 16.Rauseo A.M., Coler-Reilly A., Larson L., Spec A. Hope on the horizon: novel fungal treatments in development. Open Forum Infect. Dis. 2020;7(2):ofaa016. doi: 10.1093/ofid/ofaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.F2G F2G expands investor syndicate and progresses Phase IIb study for novel antifungal. F2G News & Publications. https://www.f2g.com/news Available at: