Abstract
Purpose
We describe a modified injection technique that adheres a sustained-release dexamethasone intravitreal implant (Ozurdex®) to the vitreous base.
Observations
This modified technique was applied after removal of a prior dislocated Ozurdex® that migrated into the anterior chamber in one patient, and also on another patient bothered by perception of a large floater induced by a free-floating Ozurdex® in the vitreous cavity previously inserted with the conventional technique. The main feature of this new technique consisted of altering the conventional “bevel-up” orientation of the insertion needle tip towards the vitreous cavity to the modified “bevel-down” orientation of the needle tip directed towards the pars plana and vitreous base, for the purpose of adhering a portion of or the entire dexamethasone implant to the vitreous base. Neither patient developed postoperative complications with this technique.
Conclusions and importance
This modified insertion technique allows adherence of Ozurdex® to the vitreous base and avoids adverse effects associated with a free-floating Ozurdex®, such as its migration into the anterior chamber, or visual disturbance associated with movement of the implant.
Keywords: Sustained-release dexamethasone, Intravitreal injection, Intravitreal implant, Dislocation dexamethasone implant into anterior chamber, Modified insertion technique, Cystoid macular edema
Highlights
-
•
For conventional injection of sustained-release dexamethasone implant (Ozurdex®), needle tip is maintained in bevel-up position (facing vitreous cavity) before its insertion and after subsequent insertion and intraocular implant release.
-
•
For modified technique, needle-tip is first directed in conventional bevel-up position before insertion and re-directed in bevel-down position towards eye-wall after insertion.
-
•
Alternatively, needle is already in bevel-down position before its insertion, and maintained in same position after insertion with subsequent release and adherence of implant at vitreous base.
1. Introduction
The dexamethasone intravitreal implant (Ozurdex®, Allergan Inc., Irvine, CA) is a sustained-release preparation of dexamethasone embedded in a bioerodible copolymer consisting of poly (lactic-co-glycolic acid) that has been approved by the Federal Drug Administration of the United States for treatment of macular edema secondary to retinal vein occlusion in 2009, non-infectious posterior uveitis in 2010, and diabetic macular edema in 2014.1 Ozurdex® has been reported to be effective in treating diabetic macular edema refractory to anti-vascular endothelial growth factor therapy.2 It consists of a solid rod-shaped polymer with a sustained-release delivery system that allows a gradual delivery of dexamethsaone into the vitreous cavity.1 Given the variability of the responses to Ozurdex® due to multiple unique factors associated with individual patients and the underlying differing disease processes, the frequency of its insertion may range from every 2–6 months. Despite the beneficial therapeutic effects of Ozurdex, there are reports of its migration into the anterior chamber in patients with an opening or a defect in the lens-iris diaphragm, causing vision loss primarily due to corneal toxicity. Published management of the anteriorly migrated implant includes ocular massage in the supine position to coax the dislocated implant back into the vitreous cavity, its surgical removal, and prophylactic scleral fixation of the implant with sutures.3, 4, 5
In our report, we describe migration of an inserted Ozurdex® into the anterior chamber in one patient (Fig. 1). We then discuss detailed management of the dislocated Ozurdex® as well as the insertion of a new Ozurdex® by modification of the standard injection technique, in order to reduce the tendency for anterior migration of the second Ozurdex®. We also describe the use of this modified technique in another patient, who could not tolerate the moving image induced by a free-floating Ozurdex® previously inserted with the conventional technique
Fig. 1.
A sustained-release dexamethasone intravitreal implant (Ozurdex®) migrated into the anterior chamber, left eye.
2. Case report
2.1. Case 1
A 70-year-old woman presented with a history of pars plana vitrectomy and scleral buckle procedure for a retinal detachment of the left eye two years ago followed by cataract extraction and insertion of an anterior chamber intraocular lens. There was persistent pseudophakic cystoid macular edema despite repeated periocular triamcinolone injections (Kenalog, Bristol-Myers Squibb Company, Princeton, NJ) and intravitreal triamcinolone administration (Triescence® Alcon, Fort Worth, TX), left eye (Fig. 2A). Her visual acuity was right eye: 20/25 and left eye: 20/60. Subsequent treatment with insertion of an Ozurdex® resolved the recalcitrant cystoid macular edema without intraocular pressure elevation. However, several weeks later, the Ozurdex® migrated into the anterior chamber (Fig. 1). The patient was taken to the operating room, and the dislocated implant was engaged with a pair of 23-gauge disposable end-grasping forceps (Alcon, Fort Worth, Tx), via a 23-gauge trocar at the temporal pars plana. There was a chalk-like consistency associated with the dislocated Ozurdex®. During the process of its manipulation, it broke into two fragments. Both fragments were then successfully removed with the same pair of intraocular forceps. There were no complications. After removal of the dislocated Ozurdex®, there was recurrent cystoid macular edema associated with deterioration of her visual acuity to 20/100 in the same eye. Given the worsening vision and prior initially improved vision after insertion of the original Ozurdex®, she requested insertion of a second Ozurdex® despite the risk of a similar dislocation. After an extensive discussion of the benefits and risks, a consensus was reached to insert a second Ozurdex® with the modified technique described in detail below, in order to reduce the risk of its anterior migration. Following insertion of the second Ozurdex® with the modified technique, one end of the second implant was successfully adhered to the vitreous base. There was no anterior migration of the second implant, and there was marked reduction of the recurrent cystoid macular edema with visual acuity improvement to 20/30 (Fig. 2B). Fig. 3, Fig. 4 illustrate the details of the modified technique employed in this patient.
Fig. 2.
There was persistent cystoid macular edema (CME) despite repeated periocular and intravitreal triamcinolone injections, with VA of 20/60, left eye. B. Following insertion of second Ozurdex® with the modified technique, one end of the second implant was successfully adhered to the vitreous base without its anterior migration. There was marked reduction of recurrent CME with visual acuity improvement to 20/30, left eye.
Fig. 3.
Conventional technique for insertion of an Ozurdex® with the needle tip first creating a beveled scleral tunnel before entering the vitreous cavity in an oblique angle, and its subsequent redirection towards the center of the globe in the “bevel-up” position.
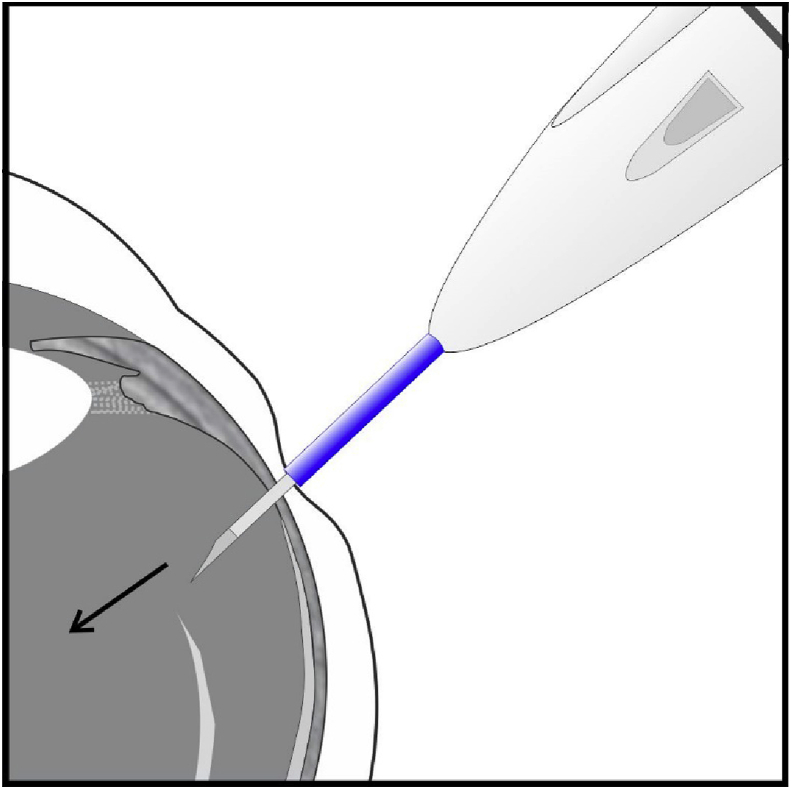
Fig. 4.
In the modified technique, the same beveled scleral tunnel is created, but during the redirection of the injection needle after its penetration through the sclera, the needle tip of the injection applicator is oriented instead in the opposite bevel-down position (the bevel of the needle facing the eye-wall). Alternatively, the tip of the needle is already placed in the bevel-down position before its insertion. Subsequent gentle compression of the injection button after needle insertion induces partial incarceration of one end of the implant at the inferior vitreous base and its opposite end dangled in the inferior vitreous cavity, or the entire injected Ozurdex® may adhere to the vitreous base in a horizontal orientation.
2.2. Case 2
A 69-year-old woman with a history of prior cataract extraction with a posterior chamber intraocular lens and a pars plana vitrectomy for endophthalmitis presented with marked retinal hemorrhage and edema due to a perfused central retinal vein occlusion, right eye. Visual acuity was 5/200. Repeated administration of anti-vascular endothelial growth factor medications, starting with multiple 1.25 mG of bevacizumab injections followed by multiple 2.0 mG of aflibercept injections, did not reduce the retinal hemorrhage and edema. Instead, injection of 4.0 mG of triamcinolone acetonide induced a reduction in the retinal hemorrhage and edema, but its therapeutic effects only lasted for a month, likely due to the status of pseudophakia and prior pars plana vitrectomy. Next, insertion of a dexamethasone implant with the conventional technique attained sustained therapeutic effects, and the visual acuity was improved to 20/200. However, she was greatly disturbed by the perception of a large moving floater induced by the free-floating dexamethasone implant. Subsequent repeat insertion of the dexamethasone implant with the modified technique achieved its adherence at the inferior vitreous base with extended therapeutic effects and avoided an annoying large floater for this patient. Fig. 5 of her right eye shows an Optos wide-field image of an entire dexamethasone implant at 2 weeks after its successful fixation in a horizontal orientation with the modified insertion technique at the vitreous base within the inferior pars plana.
Fig. 5.
Optos wide-field image of this 69-year-old woman's right eye shows the successful adherence of an entire Ozurdex® implant in a horizontal orientation at the vitreous base of the inferior pars plana at 2 weeks after its insertion with the modified technique for treatment of a perfused central retinal vein occlusion. Previously, she was bothered by the large floater induced by a free-floating dexamethasone implant inserted with the conventional technique.
3. Materials and methods
3.1. Modified insertion technique
According to the conventional technique for insertion of an Ozurdex® (see Ozurdex insertion video distributed by Allergan Inc.), the needle tip of the injection applicator is used to create a beveled scleral tunnel at the pars plana for the purpose of achieving a self-sealed wound.6 The needle that enters the vitreous cavity in an oblique angle is then redirected towards the center of the globe in the “bevel-up” position (the bevel of the needle facing the center of the vitreous cavity [Fig. 3]). The subsequent compression of the injection button on the insertion applicator results in release of a free-floating Ozurdex® into the vitreous cavity. In the modified technique, the same beveled scleral tunnel is created to achieve a self-sealed wound. But during the redirection of the injection needle after its penetration in an oblique angle through the sclera, the needle tip of the injection applicator is oriented instead in the opposite bevel-down position (the bevel of the needle facing the eye-wall). Alternately, the tip of the needle is initially placed in the bevel-down position before its penetration of the eye-wall in an oblique angle through a beveled scleral tunnel, so that it is already directed towards the eye-wall at the vitreous base after its entrance into the vitreous cavity. The subsequent gentle compression of the injection button on the insertion applicator allows a soft instead of forceful release of the Ozurdex® into the vitreous cavity, with the intent of causing one end of the implant partially incarcerated at the inferior vitreous base, while its opposite end is suspended in the inferior vitreous cavity, or the entire injected Ozurdex® may adhere to the vitreous base in a horizontal orientation (Fig. 4, Fig. 5).
For the purpose of proof of concept, both options of this modified technique were performed in a series of pig eyes. For this experiment, adherence of the dexamethasone implant at the vitreous base was achieved in 4 of 5 attempts utilizing option 1 (tip of needle first directed in bevel-up position before its redirection towards eye-wall after insertion), and adherence of the dexamethsaone implant to the vitreous base was successful in 4 of 4 attempts utilizing option 2 (tip of needle already in bevel-down position directed towards eye-wall before its insertion).
4. Discussion
In the literature, Khurana et al., and Rahimy et al. have reported case series of anterior migration of a free-floating Ozurdex® into the anterior chamber, particularly in certain vitrectomized patients, pseudophakic patients with a large iridectomy, and patients with an anterior chamber intraocular lens.4,7 They further described the risk of progressive corneal decompensation and associated vision loss, when the dislocated Ozurdex® was allowed to linger in the anterior chamber for a prolonged period.
Our first patient was forewarned regarding the possibility of repeat dislocation of the Ozurdex® into the anterior chamber despite the modified, “off-label” insertion technique. However, for this patient, the modified insertion technique did prevent the free-floating of the second Ozurdex® in the vitreous cavity, and therefore, avoidance of its anterior migration with the desired visual recovery. Subsequently, this patient received four more consecutive dexamethasone implants with the modified insertion technique with their successful partial incarceration at the vitreous base without their anterior migration. Despite the more anterior location of the partially incarcerated Ozurdex® in comparison to a posterior free-floating Ozurdex® injected with the conventional technique, the intended therapeutic target of resolving the cystoid macular edema was achieved after re-insertion of consecutive dexamethasone implants with the modified technique.
Another advantage of a partial or complete incarceration of the Ozurdex® at the inferior vitreous base is the avoidance of large movements of a free-floating implant in the vitreous cavity particularly during positioning changes, which tend to be a nuisance for certain individuals, such as our second patient. Thus, in addition to reducing the risk of implant dislocation in those patients with a large opening in the lens-iris diaphragm, this modified technique may also benefit those patients who are annoyed by the movements associated with a free-floating dexamethasone implant. For both patients, there was a lack of intraocular pressure spikes, no evidence of any persistent scleral or conjunctival wound leak or fistula, or exposure of a portion of the injected Ozurdex® through the scleral insertion site. There was also absence of persistent inflammation or infection.
5. Conclusions
This modified insertion technique secures the dexamethasone implant in the vitreous base and decreases the disturbance associated with a free-floating implant in the vitreous cavity. No complications or disadvantages were observed with the use of the modified technique and the technique was successfully used for subsequent additional dexamethasone implants in our first patient. However, further studies are needed to establish the efficacy and safety of Ozurdex® when inserted with the described modified technique.
6. Patient consent
The report does not contain any personal information that could lead to the identification of the patients. Additionally, the photograph of a dexamethasone implant in the anterior chamber, and the photograph of a dexamethasone implant lodged at the inferior vitreous base do not contain any personal markings that could lead to the identification of the patients.
Acknowledgements and disclosures
No external funding or grant support for this case report.
The authors have no proprietary interests in any material or products mentioned in this manuscript.
The authors discuss an off-label insertion technique of a Food and Drug Administration-approved device.
Daniel H Lee does not have any financial disclosures.
Clement K Chan has received research support from the following entities: Roche-Genentech, Regeneron, Allergan, National Eye Institute, Chengdu Kanghong Biotechnology, and Regenerative Patch Technologies. Allergan provided the pig eyes for the proof-of-concept experiment, but no input or contributions to any aspect of this manuscript, such as funding, concept, design, write-up, conclusion, or recommendation.
All authors attest that they meet the current ICMJE criteria for Authorship.
The authors thank Aileen Arakaki Chan DDS for preparation of the figures for this manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Daniel H. Lee, Email: danielhjlee90@gmail.com.
Clement K. Chan, Email: cchan@desertretina.com.
References
- 1.Allergan U.S.A. June 2014. https://www.google.com/search?sxsrf=ACYBGNTEm-HXc5sSMNTAPpSOeNG7tkTNIQ%3A1571632664859&source=hp&ei=GDatXbrxMcfm-gTV_4KgAg&q=Allergan+USA.+Ozurdex+%28dexamethasone+intravitreal+implant%29+for+Intravitreal+Injection+-+Highlights+of+Prescribing+Information.+%282018%29.&oq=Allergan+USA.+Ozurdex+%28dexamethasone+intravitreal+implant%29+for+Intravitreal+Injection+-+Highlights+of+Prescribing+Information.+%282018%29.&gs_l=psy-ab.12 (Ozurdex (Dexamethasone Intravitreal Implant) for Intravitreal Injection - Full Prescribing Information, Page 5). 3353.3353..4889...0.0..0.0.0.......1....2j1..gws-wiz.&ved=0ahUKEwi63dD1w6zlAhVHs54KHdW_ACQQ4dUDCAs#spf=1571632678795) [Google Scholar]
- 2.Busch C., Zur D., Fraser-Bell S. Shall we stay, or shall we switch? Continued anti-VEGF therapy versus early switch to dexamethasone implant in refractory diabetic macular edema. Acta Diabetol. 2018;55:789–796. doi: 10.1007/s00592-018-1151-x. [DOI] [PubMed] [Google Scholar]
- 3.Ha J.Y., Jang J.Y., Ji Y.S. Management of anterior chamber migration of dexamethasone intravitreal implant. Kor J Ophthalmol. 2017;31:574–575. doi: 10.3341/kjo.2017.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahimy E., Khurana R.N. Anterior segment migration of dexamethasone implant: risk factors, complications, and management. Curr Opin Ophthalmol. 2017;28:246–251. doi: 10.1097/ICU.0000000000000365. [DOI] [PubMed] [Google Scholar]
- 5.Mateo C., Alkabes M., Burés-Jelstrup A. Scleral fixation of dexamethasone intravitreal implant (Ozurdex®) in a case of angle-supported lens implantation. Int Ophthalmol. 2014;34:661–665. doi: 10.1007/s10792-013-9841-4. [DOI] [PubMed] [Google Scholar]
- 6.Eyetube. Ozurdex Precision Program . 2010. Video Guide to Injection Technique.https://eyetube.net/video/crosce/ [Google Scholar]
- 7.Khurana R.N., Appa S.N., McCannel C.A. Dexamethasone implant anterior chamber migration: risk factors, complications, and management strategies. Ophthalmology. 2014;121:67–71. doi: 10.1016/j.ophtha.2013.06.033. [DOI] [PubMed] [Google Scholar]