Abstract
Aims
The daily activity of osteoarthritis (OA) patients is limited by chronic pain and central sensitization. Although non-steroidal anti-inflammatory drugs (NSAIDs) and acetaminophen are the first-line drugs for the treatment of OA-related pain, their efficacy on central sensitization remains unclear. In the present study, we evaluated the effect of acetylsalicylic acid (ASA, Aspirin) using an OA model induced by monosodium iodoacetate (MIA), which has a similar disease progression to human OA.
Main methods
Secondary hyperalgesia was assessed at the plantar surface of the hind paw by Von Frey test. We evaluated the expression of acid-sensing ion channel 3 (ASIC3) in dorsal root ganglia and that of tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) in the spinal cord, which may cause secondary hyperalgesia in OA, by immunohistochemical analysis and real-time qPCR.
Key findings
The administration of ASA attenuated secondary hyperalgesia at 1–3 weeks after MIA, while celecoxib, a selective cyclooxygenase (COX)-2 inhibitor, failed to attenuate secondary hyperalgesia at week 2 after MIA injection, suggesting that ASA exerts its analgesic effect through a COX-2-independent pathway. Immunohistochemical analysis of the dorsal root ganglia indicated that ASA reduced the expression of ASIC3 during OA progression. Expression of TNF-α mRNA, but not IL-1β mRNA, in the spinal cord following MIA injection was suppressed by ASA administration.
Significance
These findings suggest that ASA may have the ability to attenuate secondary hyperalgesia through suppression of ASIC3 and/or TNF-α expression. ASA is therefore a clinically useful analgesic drug for treatment of secondary hyperalgesia in OA.
Keywords: Osteoarthritis, Acetylsalicylic acid, Secondary hyperalgesia, Monosodium iodoacetate, Rat model, Immunology, Pharmaceutical science, Pain research, Pharmacology
Osteoarthritis; Acetylsalicylic acid; Secondary hyperalgesia; Monosodium iodoacetate; Rat model; Immunology; Pharmaceutical science; Pain research; Pharmacology
1. Introduction
Osteoarthritis (OA) is one of the most common causes of chronic pain in aged people and is characterized by disruption of cartilage and hypertrophy of the synovium [1, 2]. OA patients have limited activity caused by chronic pain, heat hypersensitivity and central sensitization, impairing their quality of life [3, 4].
According to the National Institute for Health and Care Excellence (NICE) guidelines, if pain is not relieved by non-drug treatment including provision of information, instruction in self-management, exercise, reduction of weight, and electrotherapy, non-steroidal anti-inflammatory drugs (NSAIDs) and acetaminophen should be used as the first-line pain management drugs [5]. Although the efficacy of NSAIDs and acetaminophen on primary hyperalgesia has been evaluated previously, their efficacy on central sensitization is unknown. Central sensitization is a state of increased excitability in the central nervous system (CNS), including the spinal dorsal horn, which causes a facilitated response of spinal neurons to sensory inputs. It is important that the initial inputs reach the CNS to establish central sensitization. However, once central sensitization is established, peripheral inputs are no longer needed to maintain it [6]. Therefore, it is crucial to develop treatments that directly act on central sensitization before it can be established.
Acetylsalicylic acid (ASA, Aspirin) is one of the most widely used drugs. ASA has been used to treat symptoms of inflammation, including fever, swelling, and pain. Its mechanism of action is thought to be through inhibition of cyclooxygenase enzymes (COX-1 and COX-2), key enzymes in the biosynthesis of prostaglandin (PG)s [7], which sensitize nociceptors in the peripheral and dorsal horn neurons in the spinal cord [8]. In a monosodium iodoacetate (MIA) model, PG levels in the synovial fluid increase over time [9]. Beside COX inhibition, ASA may have an inhibitory action on acid-sensing ion channels (ASICs) [10]. These channels are permeable to cations and are activated by low extracellular pH [11]. ASICs contribute to pain processing and central sensitization in inflammatory states accompanied by severe tissue acidosis [12]. Of the four subtypes of ASICs, ASIC3 is predominantly detected in the peripheral nervous system. A previous study found that primary hyperalgesia was equally observed in both ASIC3 −/− and ASIC3 +/+ mice, but secondary hyperalgesia did not develop in ASIC3 −/− mice [13].
Different factors have been proposed as candidates for the development of central sensitization in OA. Proinflammatory cytokines play crucial roles in the development of hyperalgesia. Tumor necrosis factor-α (TNF-α) is upregulated in progressive joint arthritis [14, 15], and inhibition of TNF-α translation or activity reduced thermal hyperalgesia and mechanical allodynia [16]. Interleukin-1β (IL-1β) is also an important mediator of inflammatory pain [16].
The present study aimed to investigate the effect of ASA on central sensitization that was detected as secondary hyperalgesia in MIA-induced OA model rats. To clarify the mechanism of the analgesic activity of ASA, we assessed the effect of ASA on the expression of ASIC3, TNF-α, and IL-1β.
2. Materials and methods
2.1. Animals
Six-week-old male Sprague-Dawley (SD) rats were obtained from SLC Japan Inc. (Shizuoka, Japan). Rats were housed in groups of three per cage under standard conditions (23 °C ± 1 °C) with a 12 h dark/light cycle, with tap water and chow available ad libitum. Rats were acclimated for 4 days before use. All animal experiments were approved by the Animal Care and Use Committee of Lion Research Laboratories (Tokyo, Japan) and were conducted in accordance with the internal guidelines for animal experiments and the ethical policies of the Lion Corporation. These guidelines were created based on the Standards Relating to the Care and Keeping and Reducing Pain of Laboratory Animals under the jurisdiction of the Ministry of the Environment and Fundamental Guidelines for Proper Conduct of Animal Experiment and Related Activities in Research Institutions under the jurisdiction of Ministry of Health, Labor and Welfare, Japan.
2.2. Induction of osteoarthritis
An OA model was established in anesthetized rats (3% isoflurane) by intra-articular injection into the left knee of 25 μL saline (Sham group) or 3 mg MIA (Sigma) in 25 μL saline (MIA group) with a 27G needle and Hamilton syringe (Hamilton, Reno, NV) [17]. The right knees were similarly treated with 25 μL of saline as a control.
2.3. Animal behavior assessment
Secondary mechanical hyperalgesia was assessed by measuring the hind paw withdrawal threshold with a Dynamic Plantar Aesthesiometer (#37450, Ugo Basile, Varese, Italy). After accommodation for 10 min on a mesh grid cage, the sole of the hind paw was stimulated with a von Frey type filament until the rat withdrew it. The filament force was gradually increased up to 30 g in 40 s. Bias was minimized by random numbering of the animals of both groups and rapid measurements.
2.4. Histological analysis of knee joint
For reconfirmation of MIA model, histological changes and changes in the μCT were studied in the OA model, at 4 weeks post-MIA or saline injection. Rats were euthanized by inhalation of CO2. Thereafter, the left knee joint was removed and fixed in 3.7% formalin at 4 °C for 3 days for μCT imaging using an SMX-100CT (SHIMADZU Co., Ltd., Kyoto, Japan). For histochemical analysis, rats were euthanized by inhalation of CO2 at 1, 2, and 4 weeks post-MIA injection (n = 2 each) or at 4 weeks after saline injection (Sham, n = 5). The left knee joint was removed and fixed in 4% paraformaldehyde at 4 °C for 3 days. After thorough washing with saline three times, the knee joints were decalcified in 10% formic acid, which was changed every day, for 5 days, embedded in paraffin using ETP-150CH (Sakura Finetek Japan Co. Ltd., Tokyo, Japan), cut into 4 μm sections on a microtome (Leica SM2010 R, Leica Biosystems, Wetzlar, Germany), and stained with hematoxylin and eosin (H&E).
2.5. Administration of drugs
ASA (Yoshida Pharmaceutical Co., Ltd., Tokyo, Japan) or celecoxib (Tokyo Chemical Industry Co., Ltd., Tokyo, Japan) was suspended in 5% gum arabic (Wako, Japan). After fasting for 10–16 h, rats were orally administrated by gavage ASA solution (75 mg/kg B.W.), or celecoxib solution (30 mg/kg B.W.), or vehicle solution (5% gum Arabic, 5 mL/kg), and paw withdrawal threshold was evaluated 1 h after administration of the drug.
2.6. Reagents for UPLC-MS/MS experiments
ASA, Salicylic acid (SA), Gentisic acid (GA), 2,3-dihydroxybenzoic acid (2,3-DHBA), Salicyluric acid (SUA) and o-Toluic acid (o-TA) were purchased from Tokyo Chemical Industry Co., Ltd. (Chuo-ku, Tokyo, Japan). Acetylsalicylic acid-d4 (ASA-d4) and Gentisic acid-d3 (GA-d3) were purchased from Toronto Research Chemicals (North York, ON, Canada). Salicylic acid-d4 (SA-d4) was purchased from Sigma-Aldrich Co. LLC (St. Louis, MO, USA). Acetonitrile (LC-MS grade) was purchased from Kanto chemical (Chuo-ku, Tokyo, Japan), Ammonium Acetate Solution (HPLC grade) was purchased from FUJIFILM Wako Pure Chemical Corporation (Chuo-ku, Osaka, Japan) and Formic acid (LC-MS grade) was purchased from Honeywell Fluka™ (Morris Plains, NJ, USA).
2.7. UPLC-MS/MS analysis
UPLC-MS/MS analysis was performed on an Acquity UPLC H-class system (Waters, USA) equipped with a binary solvent delivery system, a solvent delivery compartment with high pressure mixing, and an autosampler. MS/MS detection was achieved using a Xevo™ TQ mass spectrometer (Waters, USA) in the negative modes (ESI-). The analytes were separated with a Waters ACQUITY UPLC BEH C18 column (1.7 μm, 2.1 × 50 mm) and a gradient elution using acetonitrile and 0.1% (v/v) formic acid-water containing 0.5 mM ammonium acetate as the mobile phases. The capillary voltage and the extractor voltage were kept respectively at 2.5 kV and 3.0 V, with a source temperature of 120 °C and desolvation temperature of 350 °C. The cone gas and desolvation gas were set respectively at 30 and 600 L/h (nitrogen), and argon was used as collision gas. Measured metabolites were ASA, SA, GA, 2,3-DHBA, SUA, surrogate (deuterium-labeled) compounds as ASA-d4, SA-d4, GA-d3, and o-TA spiked for alternative internal standard of SUA and 2,3-DHBA.
MS/MS experiments were carried out in multiple reaction monitoring (MRM) mode with precursor-to-product ion transitions monitored for each analyte: MRM transitions were as follows; GA (m/z 153.2→108.1), GA-d3 (m/z 155.2→110.1), SUA (m/z 194.2→93.1), 2,3-DHBA (m/z 153.2→109.1), o-TA (m/z 135.2→91.1), SA (m/z 137.2→93.1), ASA (m/z 137.2→93.1), SA-d4 (m/z 141.2→97.1), ASA-d4 (m/z 141.2→97.1). The data acquisition and data processing were performed using Masslynx 4.1 software.
2.8. Pretreatment for UPLC-MS/MS experiments
Blood samples were obtained from the abdominal aorta in 4 inflamed rats under anesthesia with 3% isoflurane. The brain, spinal cord, and both knees were removed from inflamed rats after euthanasia with CO2 and frozen immediately. A suspension was made by pulverizing the frozen tissue and adding ultrapure water according to tissue weight. Centrifugal separation was carried out at 13000 × g at 4 °C for 5 min, and the supernatant was collected. Initially, 400 μL of a surrogate mixture solution (in acetonitrile) including 0.02 ppm GA-d3, 1.56 ppm SA-d4 and 0.02 ppm ASA-d4 was added to 100 μL of the supernatant. Centrifugal separation was carried out at 13000 × g at 4 °C for 5 min, and then 400 μL of the supernatant was collected. The collected supernatant was evaporated to dryness with nitrogen gas. Extracts were added to 50 μL of 0.1 % formic acid and acetonitrile (9:1) including 5.1 ppm o-Toluic acid (o-TA) and injected into the UPLC-MS/MS.
2.9. Quantitative analysis of ASA metabolite
The limit of quantification (LOQ) was determined at signal-to-noise (S/N) ratios of about 10. The LOQ was determined to be 50 ppb for ASA, 10 ppb for SA, 50 ppb for GA, 103 ppb for SUA, and 10 ppb for 2, 3-DHBA. The surrogate (deuterium-labeled) compounds were the optimal internal standard because it has a physical characteristic and dissolution time equal to the detection metabolites.
2.10. Immunohistochemistry of dorsal root ganglia (DRGs)
The expression of ASIC3 was measured by immunohistochemical analysis of the DRGs of rats to clarify the mechanism of ASA activity. One week before tissue collection at each time point, 4–6 rats each (total of 43 rats) were anesthetized with 3% isoflurane, and 0.05 mg Fast Blue (POLYSCIENCES, inc., Warrington, PA) in 10 μL saline was intra-articularly injected to the left knee [18]. Animals recovered from anesthesia and survived for a week. On the day of tissue collection, vehicle (5% gum Arabic, 5 mL/kg) or ASA solution (75 mg/kg B.W.) was orally administered by gavege, and 1 h later, rats were anesthetized with 3% isoflurane and perfused with cold 1.5% paraformaldehyde in saline. The abdominal cavity was opened by midline incision, and the intestine was moved to one side, then muscles and fascia over the lumbar vertebrae were removed to expose them. Vertebrae were counted from the caudal end (next to the sacral bone) and L3-L5 vertebrae were marked. Spinous, transverse and articular processes, and vertebral arch were removed with the bone trimmer to expose DRGs. DRGs with short stretch of ventral and dorsal roots, and spinal nerve attached (without cleaning), were collected. L3 - L5 DRGs were removed and fixed in 4% paraformaldehyde overnight, and then cryoprotected in 30% sucrose solution at 4 °C. After embedding in OCT compound on dry ice, 20 μm sections were cut at -20 °C using a cryostat microtome (Leica CM3050 S, Leica Biosystems, Wetzlar, Germany), with every other section collected, dried, and stored at -80 °C. Nonspecific protein binding was blocked with 1% bovine serum albumin in TBS-T (Tris Buffered Saline with Tween 20) for 1 h at room temperature. After washing with 0.1 M PBS (pH 7.4), sections were incubated at 4 °C overnight with rabbit anti-ASIC3 antibody (1:500, Alomone Labs, Jerusalem, Israel). The next day, sections were washed with PBS three times and incubated for 1 h at room temperature with donkey anti-rabbit IgG (H + L) (conjugated with Alexa Fluor 555, 1:500, Invitrogen, Carlsbad, CA). After thorough washing with PBS three times, sections were mounted with SlowFade[™] Diamond Antifade Mountant (Invitrogen, Carlsbad, CA) under a coverslip for observation on a microscope (BX53, OLYMPUS, Tokyo, Japan). Rats with more than 50 FB-labeled neurons in L3-L5 DRGs were analyzed and others were discarded. As a result samples from 4‒6 rats were analyzed further in each group. FB-labeled neurons were mainly observed in L3 and L4 DRGs, and ASIC3 positive neurons were therefore only counted in these DRGs.
2.11. Real-time PCR
The expression of cytokines that might be responsible for secondary hyperalgesia was assessed using real-time PCR. On the day of tissue collection, vehicle (5% gum Arabic, 5 mL/kg) or ASA solution (75 mg/kg B.W.) was orally administered by gavage to MIA rats except rats for 0 w (neither vehicle nor ASA), and 1 h later rats were killed by decapitation under anesthesia with 3 % isoflurane. L3-L5 spinal cord was removed from VEH group and ASA group 1–4 weeks post-MIA injection, snap frozen in liquid nitrogen and stored at −80 °C. Tissue samples were then homogenized and total RNA was extracted using AllPrep DNA/RNA/Protein Mini Kit (QIAGEN, Germany) following the manufacturer's instructions. RNA (500 ng) was transcribed to cDNA using ReverTra Ace(R) qPCR RT Master Mix (TOYOBO, Japan). Real-time PCR was carried out with KOD SYBR(R) qPCR Mix (TOYOBO, Japan), using the following primer pairs: (forward) 5′-AAATGGGCTCCCTCTCATCAGTTC-3′, (reverse) 5′-TCTGCTTGGTGGTTTGCTAC GAC-3′ for TNFα; (forward) 5′-TCATCTTTGAAGAAGAGCCCGTCC -3′, (reverse) 5′-TGCAGTGCAGCTGTCTAATGGGAA -3′ for IL-1β; (forward) 5′-CCCCCAATGTATCCGTTGTG-3′, (reverse) 5′-TAGCCCAGGATGCCCTTTAGT-3′ for GAPDH. GAPDH expression was used as the endogenous control.
2.12. Statistical analysis
Animals were randomly assigned to each group. Data are represented as means ± standard error of the mean (S.E.M). Statistical analysis was carried out using SPSS, version 22 (SPSS Inc., Chicago, Illinois, USA). Behavioral testing was analyzed with two-way ANOVA followed by Dunnett's test. One-way ANOVA followed by Dunnett's test was used for comparing ASIC3, TNFα, and IL-1β expression. The effect of the drugs was evaluated using Student's t-test. A p-value < 0.05 was considered statistically significant.
3. Results
3.1. MIA induced cartilage damage and secondary hyperalgesia
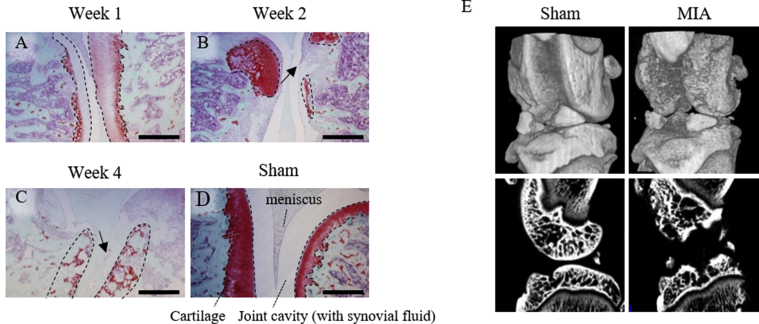
To confirm that our method inducing knee joint OA by MIA works the knee joints of MIA injected rats were evaluated with μCT and histopathological images. The sham group showed smooth cartilage surfaces and normal cartilage thickness for this age (Figure 1D). The MIA group showed loss of cartilage matrix, presented as thin or almost no area of red staining of safranin, 1 week after MIA injection and continually thereafter (Figure 1A-C). At 4 weeks when OA was most advanced, the MIA group had an irregular cartilage surface(Figure 1E), and the cartilage was lacy appearance and almost lost (Figure 1C).
Figure 1.
Histology of the knee joint. (A–D) To confirm the OA progression, histological sections were stained with Safranin O at 1, 2, and 4 weeks after injection of MIA. Anatomical labels of knee joint are shown in D. Scale bar: 1 mm. Loss of cartilage matrix is recognized as smaller or almost no area or lacy appearance (C) of red staining of Safranin, as shown with dotted line. This change occurred from 1 week after injection of MIA. The arrows in B and C show osteophytes. (E) μCT images of the knees of rat of the sham group (left) and MIA group (right) 4 weeks after injection. The upper panels are three-dimensional reconstruction images and the lower panels are the sagittal plane images. MIA group showed irregular surface and destroyed cartilage.
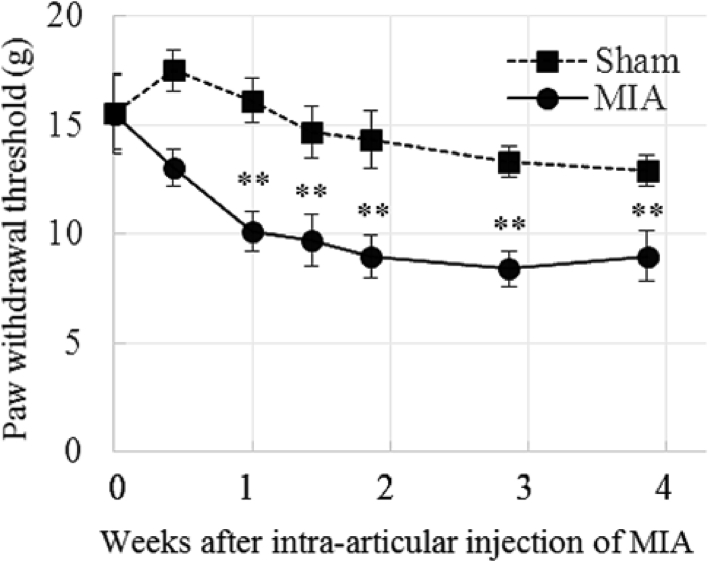
The time course of secondary hyperalgesia in the MIA model was established using the von Frey test. The paw withdrawal threshold (PWT) of the sham group remained almost constant during the entire observation period, while that of the ipsilateral limb of the MIA group gradually decreased after MIA injection. Statistical analysis revealed that there were significant differences in ‘time’ (F (6, 98) = 5.73, p < 0.001) and ‘treatment’ (MIA and sham) (F (1, 98) = 47.90, p < 0.001), and no interaction between the two factors (F (6, 98) = 1.44, p > 0.1). Post hoc analysis (Dunnett's test) revealed that the PWT of the MIA group decreased from day 7 to day 27 compared to that of day 0 (F (6, 49) = 6.38) (Figure 2), while no difference (F (6, 49) = 1.99, p > 0.05) was observed in the sham group compared with that of day 0. This result demonstrated that secondary hyperalgesia appeared 1 week after MIA injection, and continued thereafter.
Figure 2.
Time course of secondary hyperalgesia in the MIA model. Paw withdrawal threshold (PWT) (g) evaluated with the von Frey test. Means ± S.E.M, N = 8 (Sham group), N = 8 (MIA group), ∗∗: p < 0.01, vs Day 0 (ANOVA followed by Dunnett's test) The PWT of the sham group remained almost constant, while that of the MIA group gradually decreased showing progression of secondary hyperalgesia.
3.2. ASA ameliorated secondary hyperalgesia
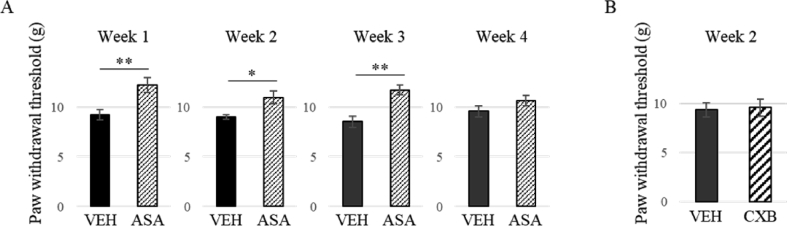
To investigate the effect of ASA on secondary hyperalgesia, the PWT of the ipsilateral hind paw was measured from 1 to 4 weeks after MIA injection. At weeks 1, 2, and 3, nociceptive response was improved 1 h after administration of ASA (p < 0.05 or 0.01, t-test), while the effect of ASA was not significant at week 4 (Figure 3A). Celecoxib, a selective COX-2 inhibitor, was not effective in reversing the reduced PWT at week 2 (Figure 3B).
Figure 3.
Effect of aspirin (A, ASA) and celecoxib (B) on secondary hyperalgesia. (A) Change in the withdrawal threshold (g) after administration of VEH or ASA 1, 2, 3, and 4 weeks after MIA injection. Secondary hyperalgesia at 1–3 weeks after MIA injection was reversed by ASA but not that at 4 weeks after MIA injection. N = 7–11. (B) Effect of celecoxib (CXB). CXB failed to reverse the secondary hyperalgesia 2 weeks after MIA injection. N = 6–7, Means ± S.E.M, ∗∗: p < 0.01, ∗: p < 0.05, Student's t test.
3.3. Tissue distribution of ASA and its metabolites
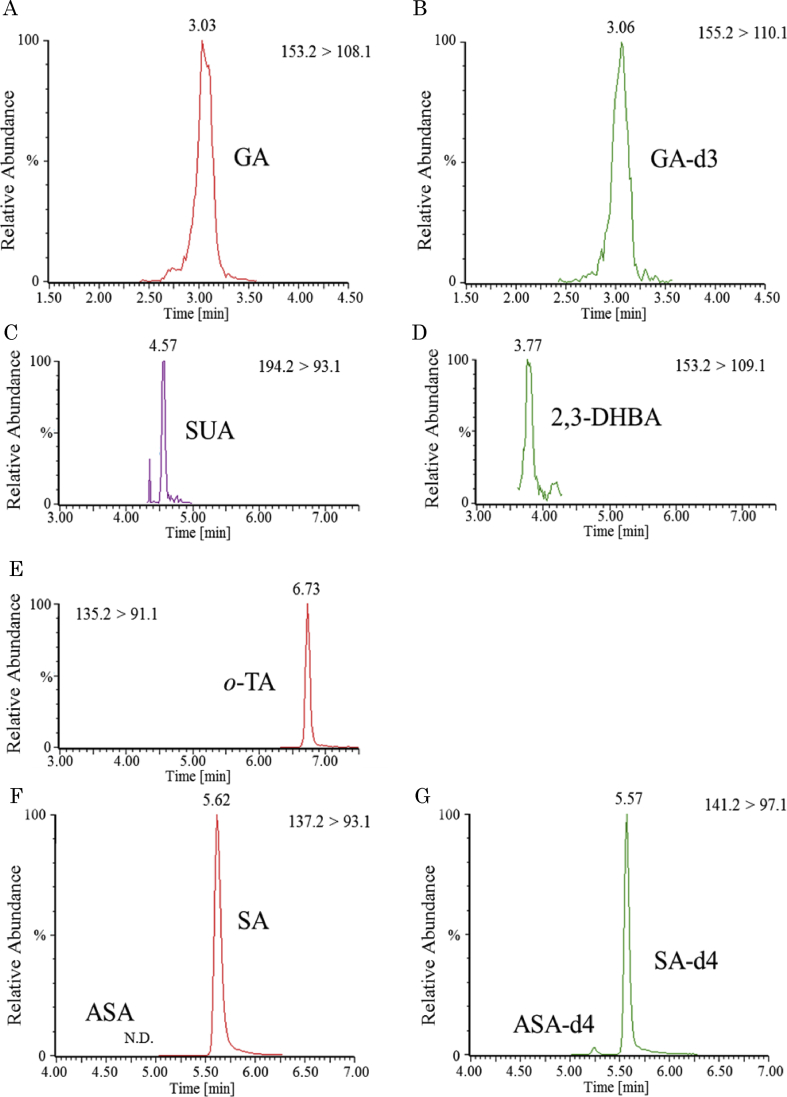
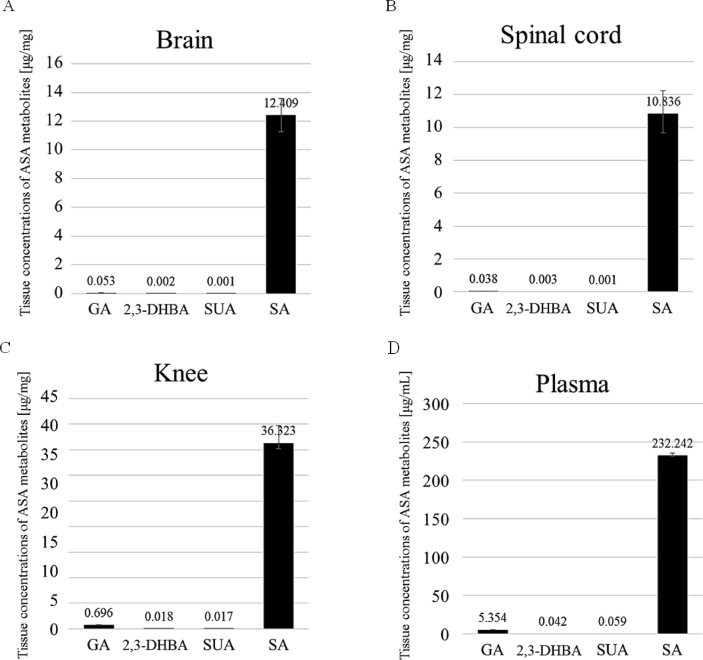
For confirmation of the tissue distribution of ASA and its metabolites at 1 h after administration, their concentration was measured in the brain, spinal cord, knee, and plasma. Sample chromatograms from tissues of a rat are shown in Figure 4. ASA was not detected (detection limit is 50 ppb, Figure 4F, G), but 4 metabolites (SA, GA, 2, 3-DHBA and SUA) were detected in all tissues (Figure 5). SA was the most abundant metabolite in all tissues (Figure 5).
Figure 4.
Sample LC-MS/MS chromatograms of a knee sample obtained with MRM after oral administration of ASA at a dose of 75 mg/kg b.w. GA (A), GA-d3 for the internal standard of GA (B), SUA (C), 2,3-DHBA (D), o-TA for the internal standard of SUA and 2,3-DHBA (E), ASA (left peak, not detected), SA (right peak) (F), ASA-d4 (left peak), SA-d4 (right peak) for the internal standard of ASA and SA m respectively (G). ASA was not detected (limit of quantification 50 ppb). GA: Gentisic acid, SUA: Salicyluric acid, 2,3-DHBA: 2,3-dihydroxybenzoic acid, o-TA: o-Toluic acid, SA: Salicylic acid.
Figure 5.
Tissue concentrations of ASA and its metabolites after oral administration of ASA at a dose of 75 mg/kg b.w. [presented in μg/mg or mL (plasma)]. Four metabolites (GA, 2, 3-DHBA, SA, SUA) were detected in all tissues (Brain (A), Spinal cord (B), Knee (C), Plasma (D)). SA was the most abundant metabolite in all tissues. Each value represents the mean ± S.E.M. of 4 rats. Note that the plasma concentration is presented in μg/mL different from that for the rest of the tissues (μg/mg). GA: Gentisic acid, SUA: Salicyluric acid, 2,3-DHBA: 2,3-dihydroxybenzoic acid, o-TA: o-Toluic acid, SA: Salicylic acid.
3.4. ASA decreased ASIC3 expression in knee joint afferents
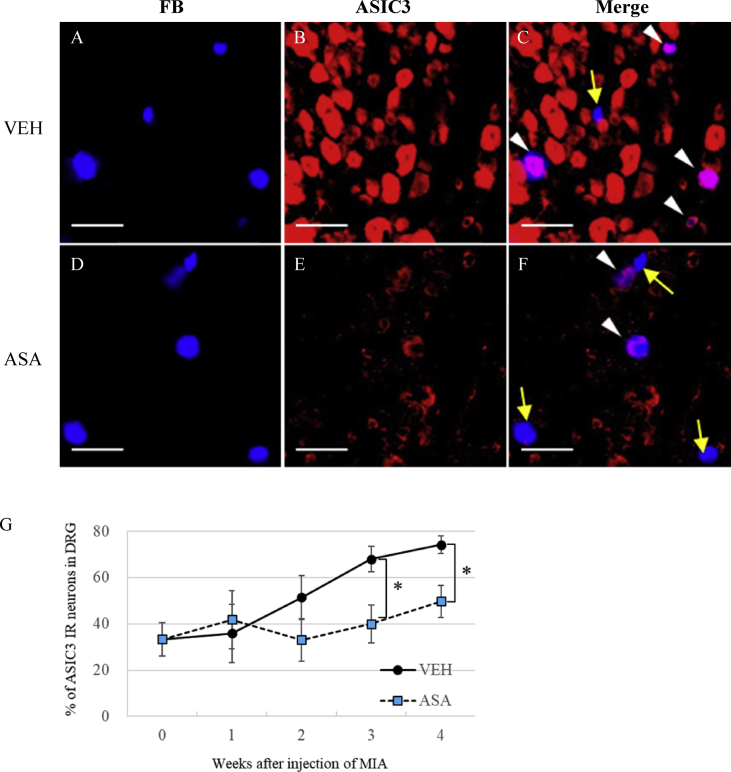
Because celecoxib had no effect on reduced PWT, we hypothesized that factors other than COX-2 may induce secondary hyperalgesia, and that ASA inhibits non-COX-2 factors as the mechanism of its analgesic action. We first quantified the levels of ASIC3 in the primary afferent neurons innervating the knee joint in OA rats. A total of 310–678 DRG neurons were labeled with FB in the vehicle group of MIA rats (sample photo in Figure 6A) (Table 1). Many DRG neurons were ASIC3 positive in the vehicle group of MIA rats (Figure 6B), and many of FB labeled neurons were double-labeled with ASIC3 (Figure 6C). The percentage of ASIC3-positive DRG neurons in the knee joint afferent neurons (FB positive) of the ipsilateral limb gradually increased after MIA injection. Compared with that at week 0 (33.3 ± 7.1%), the ASIC3 level significantly increased at week 3 (68.0 ± 5.4%) and week 4 (74.2 ± 3.8%) (Figure 6G).
Figure 6.
Effect of ASA on ASIC3 expression in DRG. (A–F) Fluorescence microscope image of FB- (A, D), ASIC3- (B, E) labeled DRG neurons and their overlay (C, F) 3 weeks after MIA injection (1 week after FB injection). Arrowheads (white) indicate ASIC3 positive cells in FB-labeled neurons and yellow arrows indicate ASIC3 negative cells in FB-labeled neurons innervating the knee joint. Scale bar: 100 μm. (G) Effect of ASA on ASIC3 expression in DRG. ASIC3 expression was counted in FB-labeled neurons in L3-4 DRGs and quantified as the percentage of total FB-labeled neurons. Means ± S.E.M, N = 4–6, ∗: p < 0.05, Student's t test compared with Vehicle at the same time point.
Table 1.
The number of DRG neurons labeled with FB after MIA injection.
| Rat No. |
0w | 1w |
2w |
3w |
4w |
||||
|---|---|---|---|---|---|---|---|---|---|
| VEH | ASA | VEH | ASA | VEH | ASA | VEH | ASA | ||
| 1 | 80 | 73 | 101 | 82 | 118 | 95 | 76 | 75 | 55 |
| 2 | 95 | 118 | 99 | 56 | 61 | 61 | 80 | 63 | 56 |
| 3 | 113 | 111 | 113 | 58 | 60 | 89 | 107 | 117 | 89 |
| 4 | 88 | 228 | 151 | 113 | 103 | 89 | 77 | 55 | 54 |
| 5 | 205 | 58 | 126 | 122 | 70 | ||||
| 6 |
97 |
||||||||
| average | 113 | 133 | 116 | 73 | 94 | 91 | 85 | 78 | 65 |
Next, we checked the effect of ASA administration on ASIC3 expression in the knee joint afferent neurons of MIA rats. The percentage of ASIC3 positive neurons in the ASA group was less than that of the VEH group (Figure 6D-F). The proportion of neurons labeled with ASIC3 in the knee joint afferent neurons did not change in the ASA group (40.0 ± 8.3% at week 3, 49.7 ± 6.9% at week 4) (Figure 6G). Statistical analysis with two-way ANOVA revealed that there were significant differences in both ‘time’ (F (4, 39) = 3.90, p < 0.01) and ‘treatment’ (vehicle or ASA) (F (1, 39) = 5.93, p < 0.05), but that the interaction between ‘treatment’ and ‘time’ (F (4, 39) = 1.56, p > 0.05) was not significant. ASIC3 expression in the ASA group was significantly lower at weeks 3 and 4 than that in the VEH group (p < 0.05, student t test). We conclude that ASIC3 expression was suppressed by ASA at weeks 3 and 4.
3.5. ASA decreased TNF-α mRNA, but not IL-1β mRNA in the spinal cord
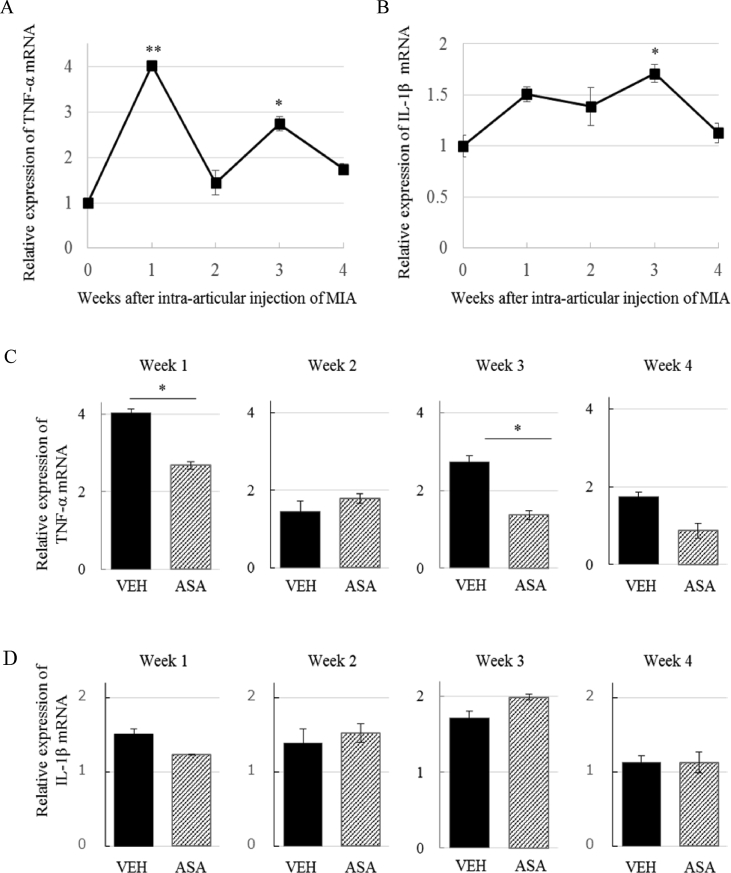
We assessed the expression of TNF-α and IL-1β in the L3-5 spinal cord after injection of MIA over time. TNF-α mRNA expression was upregulated more than 4-fold at week 1, and fluctuated at a lower level up to week 4, with a significant increase at week 3 (Figure 7A). The expression of IL-1β mRNA tended to increase between week 1 and week 3, then returned to basal level at week 4 (Figure 7B). One and 3 weeks after MIA injection, administration of ASA resulted in a significant reduction in TNF-α expression in the spinal cord (Figure 7C). However, IL-1β mRNA levels were unaffected by ASA treatment at week 3 (Figure 7D).
Figure 7.
Effects of ASA on cytokine (TNF-α, IL-1β) expression in the spinal cord. (A) Time course of change in TNF-α mRNA expression in the spinal cord of the VEH group. Significant increase was observed 1 and 3 weeks after MIA injection. Means ± S.E.M, N = 3–6, ∗∗: p < 0.01, ∗: p < 0.05 vs Week 0 (One-way ANOVA with post hoc Dunnett's test) (B) Time course of change in IL-1β mRNA expression in the spinal cord. Significant increase was observed 3 weeks after MIA injection. N = 4–6, Means ± S.E.M, ∗: p < 0.05 vs week 0 (One-way ANOVA with post hoc Dunnett's test) (C) Expression of TNF-αmRNA after administration of VEH or ASA 1, 2, 3, and 4 weeks after MIA injection. ASA suppressed TNF-α expression 1 and 3 weeks after MIA injection. Means ± S.E.M, N = 4–6, ∗: p < 0.05, Student's t test. (D) Expression of IL-1β mRNA after administration of VEH or ASA 1, 2, 3, and 4 weeks after MIA injection. IL-1β mRNA levels were not significantly changed by ASA at week 3 compared with VEH. Means ± S.E.M, N = 3–6, Student's t test.
4. Discussion
In the present study, we found that ASA, which is a widely used NSAID, diminished central sensitization in a rat OA model induced by MIA that displays multiple components of symptoms and disease progression that are similar to those of human OA [19, 20]. We also confirmed that the state of the cartilage and secondary hyperalgesia gradually worsened after MIA injection.
Although ASA was effective at treating secondary hyperalgesia, celecoxib at a dose of 30 mg/kg did not affect the PWT at week 2. A previous study on an animal OA model reported a significant improvement in PWT following celecoxib administration (30 mg/kg) on day 3, but no effect on day 21 and 35 [21]. This suggests that the inhibition of COX-2 is analgesic only if administered in the early-phase of OA. We hypothesize that ASA suppresses secondary hyperalgesia via a COX-2 independent pathway.
The PWT score is a measure of secondary hyperalgesia and improved following ASA administration at 1, 2, and 3 weeks after MIA injection, but not at 4 weeks after MIA injection. The MIA group at 4 weeks had the osteophyte, almost no cartilage and narrow joint cavity. These damages might have resulted in stronger mechanical impact to nociceptors in the bones of the knee. A recent study also showed that bone marrow damage, synovitis and cartilage loss were severe in MIA rats at 4 weeks, in addition, that activated macrophages expressing nerve growth factor (NGF) accumulates in the synovium [22]. These authors reported synovial NGF increased in the late phase (4W) of MIA model, and it contributed to mechanical hyperalgesia in this phase. Moreover, neuropathic component may also be responsible to pain in this phase [23, 24]. These factors might have made late-phase secondary hyperalgesia in MIA model resistant to ASA.
ASIC3 plays a crucial role in secondary hyperalgesia, but not in primary hyperalgesia [13]. In the present study, ASIC3 levels in the primary afferent neurons innervating the knee joint in OA rats gradually increased until week 4 with OA progression by 2-fold that of week 0, while ASA treatment reduced ASIC3 expression in DRGs. Because ASA metabolites were found in the knee joint and spinal cord, including the spinal fluid, ASA may act directly or indirectly on DRG neurons. ASIC3 (pH50 6.4–6.6) is predominantly localized in the peripheral nervous system [11]. The pH of the synovial fluid of OA patients is about 6; therefore, it is possible that ASIC3 is activated in nerve fibers in the knee joint. ASA has the ability to inhibit the sustained current component of ASIC3 [10]. We demonstrated that ASA suppressed the protein expression of ASIC3 in DRGs. There are 3 possible mechanisms for the observed suppression of ASIC3 expression in DRGs: First, ASA could suppress NGF pathway, which is required for ASIC3 synthesis. Some studies have reported that NGF is abundant in the knee of OA models and patients [22, 25, 26]. Antagonism of NGF was associated with a reduction in joint pain and improvement in function [27]. NGF tightly controls the expression of the ASIC3 encoding gene in sensory neurons [28]. During in vitro experiments, ASA or SA inhibited this NGF-dependent transcription activity in the presence of high NGF expression [28]. Therefore, the observed reduction in ASIC3 expression may be due to suppression of axonal transport of NGF from locally inflamed areas to DRGs. Second, ASA could promote the degradation of ASIC3. The fragmentation mechanism of ASIC3 is not well understood; however, in the present study, the reduction of ASIC3 occurred at 1 h after oral administration of ASA. It is possible that ASIC3 was degraded by ASA in this time frame. Third, ASA could promote axonal transport from DRGs to peripheral neuron terminals. Since ASA directly suppresses ASIC3 currents [10], ASIC3 transported to the periphery can no longer play a role in hyperalgesia.
ASIC3 inhibition is only one of possibilities for analgesic mechanism of ASA on secondary hyperalgesia. In previous report, TRPV1 antagonist inhibited mechanically evoked responses of knee joint afferents in MIA rats [29]. Furthermore ASA reduced TRPV1 activity in HEK293 cells [30]. Therefore, we think that there is also a possibility that ASA inhibits secondary hyperalgesia through inhibition of TRPV1. Monocyte chemoattractant protein-1 (MCP-1) can be an another factor involved in analgesic mechanism of ASA. MCP-1 is a 14-kDa glycoprotein of the CC chemokine family and a potent chemoattractant for monocyte recruitment. MCP-1 has been found as one of the key factors to start the inflammatory process through the binding to chemokine (C–C motif) receptor (CCR) 2. Some studies reported that ASA inhibits MCP-1 expression in TNF-α stimulated Human vascular endothelial cells [31], which are most commonly used cells for experiment, signaling of which is indispensable for the persistence of allodynia but not for its development [32]. It would be necessary to study the involvement of not only ASIC3 but also TRPV1, MCP-1, and CCR2 or other factors.
TNF-α, and IL-1β are candidate mediators involved in central sensitization [13, 33, 34, 35]. In the present study, the expression of TNF-α significantly increased at 1 and 3 weeks after MIA injection. Although IL-1β and TNF-α expression both increased at week 3, ASA inhibited the mRNA expression of TNF-α only. A transient increase in TNF-α expression was observed in exercised muscle in a lengthening contraction model [36]. There is a possibility that TNF-α has a different role at early or late phase OA. In bortezomib-induced painful peripheral neuropathy model, TNF-α in the neurons and IL-1β in astrocytes played a crucial role in the development of allodynia [37]. In an MIA model, proinflammatory cytokines might be derived from different cell types to induce pain. If ASA acted on the specific cell type producing TNF-α, then the effect of ASA on TNF-α may be specific, explaining why IL-1β was unaffected. The presence of activated microglia and astrocytes in the spinal cord in an MIA model has been previously reported [38]. Furthermore the administration of minocycline, an inhibitor of glial cell activation, significantly attenuated hyperalgesia [38]. Thus, the immune cells in neural tissue were key factors involved in the development of hyperalgesia. In the present study, ASA affect the expression of cytokines. Therefore, it is possible that ASA attenuated secondary hyperalgesia, possibly through the control of the activity of immune cells or immune recruiting. Further investigation into the protein expression of proinflammatory cytokines and the activity of glial cells, which produce cytokines, would be illuminating in this regard.
The cartilage of OA patients is more permeable to ASA than healthy cartilage [39]. ASIC3 may serve as a pH sensor in synoviocytes and as an inhibitor for the synthesis of hyaluronic acid, which plays a crucial role in the normal function of joint tissues [40]. Therefore, it is possible that ASA suppresses the change of the joint cartilage, as reported by a previous human study showing that continuous treatment with low dose ASA reduced the progression of knee OA [41]. In the present experiment we studied only short time (1h) effects of single administration of ASA, therefore, we did not check the effects on structural changes. It would be necessary to check the change in the joint structure by histology and/or μCT in future study when effects of continuous administration are studied.
ASA and indomethacin had different effects on inflammation and nociception according to a formalin test in mice [42]. ASA suppressed both the early and the late phases of nociception, but indomethacin suppressed the late phase only. This suggests that inhibition of cyclooxygenase activity has no major effect on the early phase in the formalin test, and that ASA may have a direct antinociceptive action in addition to its anti-inflammatory effects. Furthermore, ASA and diclofenac inhibited the activity of ASIC3, but many other analgesic agents (indomethacin, nimesulide, rofecoxib, piroxicam, tolmetin, acetaminophen, etodolac, and naprozen) were ineffective [10]. Thus, ASA seems to have multiple suppressive actions on nociception. To provide effective treatment, we need better understanding of the different analgesic abilities of NSAIDs.
5. Conclusion
The present study evaluated the effects of ASA on secondary hyperalgesia in a rat OA model induced by MIA. The levels of ASIC3 expression in the primary afferent neurons innervating the knee joint in OA rats gradually increased with OA progression, while ASA treatment suppressed ASIC3 up-regulation. ASA also decreased the expression of TNF-α mRNA induced by MIA, but did not affect the expression of IL-1β mRNA in the spinal cord. In conclusion, ASA attenuated secondary hyperalgesia, possibly through the ASIC3 and/or TNF-α pathways.
Declarations
Author contribution statement
M. Niibori: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Y. Kudo, T. Hayakawa, K. Ikoma-Seki, R. Kawamata and A. Sato: Performed the experiments; Analyzed and interpreted the data.
K. Mizumura: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was fully funded by Lion Corporation, Japan (https://www.lion.co.jp/en/). The Lion Corporation provided support in the form of salaries for authors M. Niibori, Y. Kudo, T. Hayakawa, K. Ikoma-Seki, R. Kawamata and A. Sato. K. Mizumura received consulting fee from Lion Corporation.
Competing interest statement
The authors declare the following conflict of interests: M. Niibori, Y. Kudo, T. Hayakawa, K. Ikoma-Seki, R. Kawamata and A. Sato are employees of Lion Corporation. This does not alter our adherence to policies of Heliyon on sharing data and materials. The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional information
No additional information is available for this paper.
References
- 1.Arden N., Nevitt M.C. Osteoarthritis : Epidemiology. Best Pract. Res. Clin. Rheumatol. 2006;20:3–25. doi: 10.1016/j.berh.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Goldring S.R., Goldring M.B. Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage bone crosstalk. Nat. Rev. Rheumatol. 2016;12:632–644. doi: 10.1038/nrrheum.2016.148. [DOI] [PubMed] [Google Scholar]
- 3.Imamura M., Imamura S.T., Kaziyama H.H.S., Targino R.A., Hsing W.T., De Souza L.P.M., Cutait Ma.M., Fregni F., Camanho G.L. Impact of nervous system hyperalgesia on pain, disability, and quality of life in patients with knee osteoarthritis: a controlled analysis. Arthritis Rheum. 2008;59:1424–1431. doi: 10.1002/art.24120. [DOI] [PubMed] [Google Scholar]
- 4.Lee Y.C., Lu B., Bathon J.M., Haythornthwaite J.A., Smith M.T., Page G.G., Edwards R.R. Pain sensitivity and pain reactivity in osteoarthritis. Arthritis Care Res. (Hoboken) 2010;63:320–327. doi: 10.1002/acr.20373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The National Institute for Health and Care Excellence . 2014. Osteoarthritis: Care and Management Osteoarthritis: Care and Management Clinical Guideline.https://www.nice.org.uk/guidance/cg177/resources/osteoarthritis-care-and-management-pdf-35109757272517 [Google Scholar]
- 6.Coderre T.J., Katz J. Peripheral and central hyperexcitability: differential signs and symptoms in persistent pain. Behav. Brain Sci. 1997;20:404–419. doi: 10.1017/s0140525x97251484. [DOI] [PubMed] [Google Scholar]
- 7.Brunton L.L., Hilal-Dandan R., Knollmann B.C. Lipid-derived autacoids: eicosanoids and platelet-activating factor. In: Emer G.A.F., Smyth M., Grosser Tilo, editors. thirteenth ed. Vol. 675. McGraw-Hill Educationn, United States of America; 2017. pp. 691–696. (Goodman Gilman’s Pharmacol. Basis Ther. 13th Ed.). [Google Scholar]
- 8.Schaible H.-G., Ebersberger A., Von Banchet G.S. Mechanisms of pain in arthritis. Ann. N. Y. Acad. Sci. 2002;966:343–354. doi: 10.1111/j.1749-6632.2002.tb04234.x. [DOI] [PubMed] [Google Scholar]
- 9.Hu H., Yang B., Li Y., Zhang S., Li Z. Blocking of the P2X7 receptor inhibits the activation of the MMP-13 and NF-κB pathways in the cartilage tissue of rats with osteoarthritis. Int. J. Mol. Med. 2016;38:1922–1932. doi: 10.3892/ijmm.2016.2770. [DOI] [PubMed] [Google Scholar]
- 10.Voilley N., De Weille J., Mamet J., Lazdunski M. Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. J. Neurosci. 2001;21:8026–8033. doi: 10.1523/JNEUROSCI.21-20-08026.2001. 21/20/8026 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wemmie J.A., Taugher R.J., Kreple C.J. Acid-sensing ion channels in pain and disease. Nat. Rev. Neurosci. 2013;14:461–471. doi: 10.1038/nrn3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deval E., Gasull X., Noël J., Salinas M., Baron A., Diochot S., Lingueglia E. Acid-sensing ion channels (ASICs): pharmacology and implication in pain. Pharmacol. Ther. 2010;128:549–558. doi: 10.1016/j.pharmthera.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Ikeuchi M., Kolker S.J., Burnes L.A., Walder R.Y., Sluka K.A. Role of ASIC3 in the primary and secondary hyperalgesia produced by joint inflammation in mice. Pain. 2008;137:662–669. doi: 10.1016/j.pain.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeong J.W., Kim J., Choi E.O., Kwon D.H., Kong G.M., Choi I.W., Kim B.H., Kim G.Y., Lee K.W., Kim K.Y., Kim S.G., Choi Y.W., Hong S.H., Park C., Choi Y.H. Schisandrae fructus ethanol extract ameliorates inflammatory responses and articular cartilage damage in monosodium iodoacetate-induced osteoarthritis in rats. EXCLI J. 2017;16:265–277. doi: 10.17179/excli2017-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choudhary D., Kothari P., Tripathi A.K., Singh S., Adhikary S., Ahmad N., Kumar S., Dev K., Mishra V.K., Shukla S., Maurya R., Mishra P.R., Trivedi R. Spinacia oleracea extract attenuates disease progression and sub-chondral bone changes in monosodium iodoacetate-induced osteoarthritis in rats. BMC Compl. Alternative Med. 2018;18:1–16. doi: 10.1186/s12906-018-2117-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sachs D., Coelho F.M., Costa V.V., Lopes F., Pinho V., Amaral F.A., Silva T.A., Teixeira A.L., Souza D.G., Teixeira M.M. Cooperative role of tumour necrosis factor-α, interleukin-1β and neutrophils in a novel behavioural model that concomitantly demonstrates articular inflammation and hypernociception in mice. Br. J. Pharmacol. 2011;162:72–83. doi: 10.1111/j.1476-5381.2010.00895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalbhen D.A. Chemical model of osteoarthritis--a pharmacological evaluation. J. Rheumatol. 1987;14 Spec:130–131. [PubMed] [Google Scholar]
- 18.Hanesch U., Heppelmann B. A simple method for a specific retrograde labelling of dorsal root and sympathetic ganglion cells innervating the knee joint of the cat. J. Neurosci. Methods. 1995;63:55–59. doi: 10.1016/0165-0270(95)00086-0. [DOI] [PubMed] [Google Scholar]
- 19.Combe R., Bramwell S., Field M.J. The monosodium iodoacetate model of osteoarthritis: a model of chronic nociceptive pain in rats? Neurosci. Lett. 2004;370:236–240. doi: 10.1016/j.neulet.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi K., Imaizumi R., Sumichika H., Tanaka H., Goda M., Fukunari A., Komatsu H. Sodium iodoacetate-induced experimental osteoarthritis and associated pain model in rats. J. Vet. Med. Sci. 2003;65:1195–1199. doi: 10.1292/jvms.65.1195. [DOI] [PubMed] [Google Scholar]
- 21.Gong K., Shao W., Chen H., Wang Z., Luo Z.J. Rat model of lumbar facet joint osteoarthritis associated with facet-mediated mechanical hyperalgesia induced by intra-articular injection of monosodium iodoacetate. J. Formos. Med. Assoc. 2011;110:145–152. doi: 10.1016/S0929-6646(11)60024-7. [DOI] [PubMed] [Google Scholar]
- 22.Sakurai Y., Fujita M., Kawasaki S., Sanaki T., Yoshioka T., Higashino K., Tofukuji S., Yoneda S., Takahashi T., Koda K., Asaki T., Hasegawa M., Morioka Y. Contribution of synovial macrophages to rat advanced osteoarthritis pain resistant to cyclooxygenase inhibitors. Pain. 2019;160:895–907. doi: 10.1097/j.pain.0000000000001466. [DOI] [PubMed] [Google Scholar]
- 23.Ivanavicius S.P., Ball A.D., Heapy C.G., Westwood F.R., Murray F., Read S.J. Structural pathology in a rodent model of osteoarthritis is associated with neuropathic pain: increased expression of ATF-3 and pharmacological characterisation. Pain. 2007;128:272–282. doi: 10.1016/j.pain.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 24.Im H., Kim J., Li X., Kotwal N., Sumner D.R., Andre J., Wijnen V., Davis F.J., Yan D., Levine B., Henry J.L., Kroin J.S. Alteration of sensory neurons and spinal response to an experimental osteoarthritis pain model. Arthritis Rheum. 2011;62:2995–3005. doi: 10.1002/art.27608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orita S., Ishikawa T., Miyagi M., Ochiai N., Inoue G., Eguchi Y., Kamoda H., Arai G., Toyone T., Aoki Y., Kubo T., Takahashi K., Ohtori S. Pain-related sensory innervation in monoiodoacetate-induced osteoarthritis in rat knees that gradually develops neuronal injury in addition to inflammatory pain. BMC Muscoskel. Disord. 2011;12:134. doi: 10.1186/1471-2474-12-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halliday D.A., Zettler C., Rush R.A., Scicchitano R., McNeil J.D. Elevated nerve growth factor levels in the synovial fluid of patients with inflammatory joint disease. Neurochem. Res. 1998;23:919–922. doi: 10.1023/a:1022475432077. [DOI] [PubMed] [Google Scholar]
- 27.Lane N.E., Schnitzer T.J., Birbara C.A., Mokhtarani M., Shelton D.L., Smith M.D., Brown M.T. Tanezumab for the treatment of pain from osteoarthritis of the knee. N. Engl. J. Med. 2010;363:1521–1531. doi: 10.1056/NEJMoa0901510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mamet J., Lazdunski M., Voilley N. How nerve growth factor drives physiological and inflammatory expressions of acid-sensing ion channel 3 in sensory neurons. J. Biol. Chem. 2003;278:48907–48913. doi: 10.1074/jbc.M309468200. [DOI] [PubMed] [Google Scholar]
- 29.Kelly S., Chapman R.J., Woodhams S., Sagar D.R., Turner J., Burston J.J., Bullock C., Paton K., Huang J., Wong A., McWilliams D.F., Okine B.N., Barrett D.A., Hathway G.J., Walsh D.A., Chapman V. Increased function of pronociceptive TRPV1 at the level of the joint in a rat model of osteoarthritis pain. Ann. Rheum. Dis. 2015;74:252–259. doi: 10.1136/annrheumdis-2013-203413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maurer K., Binzen U., Mörz H., Bugert P., Schedel A., Treede R.D., Greffrath W. Acetylsalicylic acid enhances tachyphylaxis of repetitive capsaicin responses in TRPV1-GFP expressing HEK293 cells. Neurosci. Lett. 2014;563:101–106. doi: 10.1016/j.neulet.2014.01.050. [DOI] [PubMed] [Google Scholar]
- 31.Yang Y.Y., Hu C.J., Chang S.M., Tai T.Y., Leu S.J. Aspirin inhibits monocyte chemoattractant protein-1 and interleukin-8 expression in TNF-α stimulated human umbilical vein endothelial cells. Atherosclerosis. 2004;174:207–213. doi: 10.1016/j.atherosclerosis.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 32.Miller R.E., Tran P.B., Das R., Ghoreishi-Haack N., Ren D., Miller R.J., Malfait A.M. CCR2 chemokine receptor signaling mediates pain in experimental osteoarthritis. Proc. Natl. Acad. Sci. U. S. A. 2012;109:20602–20607. doi: 10.1073/pnas.1209294110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Izumi M., Ikeuchi M., Ji Q., Tani T. Local ASIC3 modulates pain and disease progression in a rat model of osteoarthritis. J. Biomed. Sci. 2012;19:1. doi: 10.1186/1423-0127-19-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calich A.L.G., Domiciano D.S., Fuller R. Osteoarthritis: can anti-cytokine therapy play a role in treatment? Clin. Rheumatol. 2010;29:451–455. doi: 10.1007/s10067-009-1352-3. [DOI] [PubMed] [Google Scholar]
- 35.Hakim A.W., Dong X.-D., Svensson P., Kumar U., Cairns B.E. TNFα mechanically sensitizes masseter muscle afferent fibers of male rats. J. Neurophysiol. 2009;102:1551–1559. doi: 10.1152/jn.00326.2009. [DOI] [PubMed] [Google Scholar]
- 36.Murase S., Terazawa E., Queme F., Ota H., Matsuda T., Hirate K., Kozaki Y., Katanosaka K., Taguchi T., Urai H., Mizumura K. Bradykinin and nerve growth factor play pivotal roles in muscular mechanical hyperalgesia after exercise (Delayed-Onset muscle soreness) J. Neurosci. 2010;30:3752–3761. doi: 10.1523/JNEUROSCI.3803-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Z.-Y., Zhang Y.-P., Zhang J., Zhang S.-B., Li D., Huang Z.-Z., Xin W.-J. The possible involvement of JNK activation in the spinal dorsal horn in bortezomib-induced allodynia: the role of TNF-α and IL-1β. J. Anesth. 2016;30:55–63. doi: 10.1007/s00540-015-2077-x. [DOI] [PubMed] [Google Scholar]
- 38.Sagar D.R., Burston J.J., Hathway G.J., Woodhams S.G., Pearson R.G., Bennett A.J., Kendall D.A., Scammell B.E., Chapman V. The contribution of spinal glial cells to chronic pain behaviour in the monosodium iodoacetate model of osteoarthritic pain. Mol. Pain. 2011;7:1. doi: 10.1186/1744-8069-7-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palmoski M.J., Colyer R.A., Brandt K.D. Marked suppression by salicylate of the augmented proteoglycan synthesis in osteoarthritic cartilage. Arthritis Rheum. 1980;23:83–91. doi: 10.1002/art.1780230114. [DOI] [PubMed] [Google Scholar]
- 40.Kolker S.J., Walder R.Y., Usachev Y., Hillman J., Boyle D.L., Firestein G.S., Sluka K.A. Acid-sensing ion channel 3 expressed in type B synoviocytes and chondrocytes modulates hyaluronan expression and release. Ann. Rheum. Dis. 2010;69:903–909. doi: 10.1136/ard.2009.117168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wluka A.E., Ding C., Wang Y., Jones G., Urquhart D.M., Cicuttini F.M. Aspirin is associated with reduced cartilage loss in knee osteoarthritis: data from a cohort study. Maturitas. 2015;81:394–397. doi: 10.1016/j.maturitas.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 42.Hunskaar S., Berge O.G., Hole K. Dissociation between antinociceptive and anti-inflammatory effects of acetylsalicylic acid and indomethacin in the formalin test. Pain. 1986;25:125–132. doi: 10.1016/0304-3959(86)90014-X. [DOI] [PubMed] [Google Scholar]