Graphical abstract
Keywords: Helicobacter pylori, Medicinal plants, Secondary metabolites, Combat antibiotic resistance
Highlights
-
•
The effectiveness of the eradication therapy of H. pylori is hampered by increasing resistance against antibiotics.
-
•
In the recent drug technology scenario, Medicinal plants are repositories for novel synthetic substances.
-
•
Medicinal plants is the ideal therapy to combat resistance.
Abstract
Worldwide, Helicobacter pylori (H. pylori) is regarded as the major etiological agent of peptic ulcer and gastric carcinoma. Claiming about 50 percent of the world population is infected with H. pylori while therapies for its eradication have failed because of many reasons including the acquired resistance against its antibiotics. Hence, the need to find new anti-H.pylori medications has become a hotspot with the urge of searching for alternative, more potent and safer inhibitors. In the recent drug technology scenario, medicinal plants are suggested as repositories for novel synthetic substances. Hitherto, is considered as ecofriendly, simple, more secure, easy, quick, and less toxic traditional treatment technique. This review is to highlight the anti-H. pylori medicinal plants, secondary metabolites and their mode of action with the aim of documenting such plants before they are effected by cultures and traditions that is expected as necessity.
1. Introduction
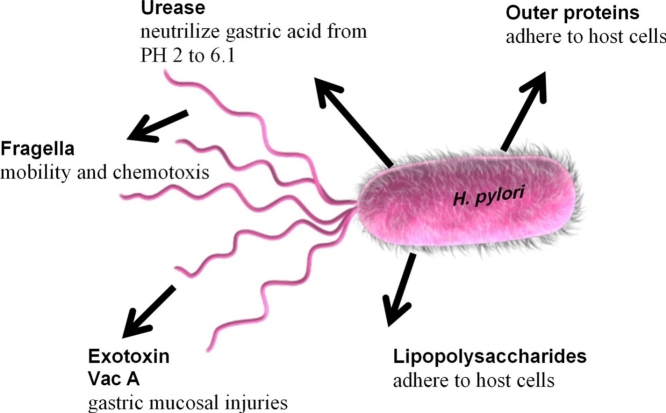
Helicobacter pylori (H. pylori) is a spiral-shaped Gram-negative bacteria colonized in the gastrointestinal tract. H. pylori infection leads to peptic ulceration, gastritis, and gastric carcinoma [1]. About 50 % of the world population is estimated to be infected by this bacterium [2]. The colonization of H. pylori is caused by its infectious agents as shown in Fig. 1 and Table 1.
Fig. 1.
Virulence agents of H. pylori. IL: Interleukin; TLR4: Toll-like receptor 4; NF-κB: Nuclear factor-kappaB; NIK: NF-κB-inducing kinase; VacA: Vacuolating cytotoxin A; CagA: Cytotoxin-associated gene antigen; PAK1: p21-activated kinase; IKKα/β: IκB kinase α/β; MAPK: Mitogen-activated protein kinase; MEK1/2: MAPK/ERK kinase 1/2; INF-γ: Interferon-γ; NOD1: Nucleotide-binding oligomerisation domain protein 1; ICAM-1: Intercellular adhesion molecule-1; iNOS: Inducible nitric oxide synthase, COX-2: Cyclooxygenase-2; MKK4: MAPK kinase 4; LPS: Lipopolysaccharide; TNF-α: Tumor necrosis factor-α.
Table 1.
Virulence agents of H. pylori.
| Vrulence agent | H. pylori Function |
|---|---|
| Vacuolating cytotoxin A (VacA) | Induce Cyto C release |
| Cytotoxicity | |
| Cag Pathogenicity Island (CagPAI) | Induce inflammation |
| Cag genes (Cag E,G,I,H, L and M) | Coding for 40-kb is a major virulence factor of H. pylori. |
| Urease | Causing epithelium cells toxicity |
| Disrupting cell tight junctions | |
| Buffers stomach acid | |
| Sheathing antigen | |
| Duodenal ulcer promoting A (DupA) | Induce inflammation |
| Outer inflammatory protein A (OipA) | Induce inflammation for IL-8 |
| H. pylori neutrophil activation protein (HP-NAP) | Activation of neutrophil |
| BabA | Adhesin |
| Flagella | Movements through mucin |
2. Pharmacological therapies
Numerous pharmacological studies have been reported for the eradication of H. pylori. Proton-pump inhibitors, antibiotics, bismuth saltsand H2-blockers (intragastric pH control drug) are recommended standard therapies [3]. A few issues may arise upon those eradication therapies, for example, the cost, the high global prevalence and the uprising resistance to available antibiotics. Consequently, some patients undergoing many of these drug regimens experience therapeutic failure [3]. Moreover, these therapies include getting too many medications which might cause side effects that, along with significant cost regarding the treatment, promote inadequate patient compliance. It is extremely desirable to explore for alternative strategies with agents to prevent or manage H. pylori-associated gastric tumor.
The quest regarding new anti-H. pylori therapies has driven exploration in the field of therapeutic plants. Many studies have been performed on a great number of plant varieties. Natural products exhibit their own anti-H. pylori actions via different mechanisms. While therapeutic agents have either antisecretory or healing effects, prophylactic compounds produce their effect via their antioxidant and anti-inflammatory mechanisms.
3. Mechanisms of medicinal plants as anti-H. pylori
Many natural products have anti-H. pylori potentials. The mechanisms of such potentials include urease inhibition, DNA damage, protein synthesis inhibition, and anti-inflammatory effects. In addition to the anti-H. pylori effects due to some enzymes like dihydrofolate reductase and myeloperoxidase N-acetyltransferase.
3.1. Urease inhibition
The potent effect of resveratrol as anti-H. pylori is mainly owing to ureaseinhibition [4]. The anti- H. pylori actions of Paeonia lactiflora roots is due to the hydrophobicity of 1,2,3,4,6-penta-O-galloyl-β-d-glucopyranose which facilitates thebinding to membranes leading to the loss of membrane integrity as well as urease inhibition [5]. Both the CHCl3 fraction and EtOH extract of Calophyllum brasiliense stem bark has been reported to decrease H. pylori and urease activity in Wistar rats as confirmed by histopathology [6]. The mode of action of mixed cranberry and oregano water extract may be due to inhibition of proline dehydrogenase and urease activvity [7]. BothCalotropis procera and Acacia nilotica extracts inhibit urease activity through competitive mechanisms [8].
3.2. Oxidative stress
2-Methoxy-1,4-naphthoquinoneexhibits strong anti H. pylori action. 2-methoxy-1,4-naphthoquinone is metabolized in H. pylori membrane by flavoenzymes and produces a high amount of free radicals that may damage cellular macromolecules and may lead to H. pylori death [9].
3.3. Anti-adhesion activity
Borage, parsley, and turmeric water extracts are found to be able to decrease adhesion of H. pylori [10]. The Liquoriceroot aqueous extract and polysaccharides exhibite strong anti-adhesive activity of human gastric mucosa aliquots with fluorescent-labeled H. pylori [11]. The Pelargonium sidoides root extract display antiadhesive activity [12]. The diterpene Plaunotol, isolated from the plau-noi leaves, is also found to inhibit adhesion of H. pylori as well as inhibition of IL-8 secretion [13].
4. Structure activity relationship
Plantswith anti H. pylori activityconsist of various phytocompounds, such as alkaloids, flavonoids, saponins, terpenes, and polysaccharides, which responsible for antimicrobial activity (Fig. 2) are discussed within this review in Table 2.
Fig. 2.
Mechanisms of action of phytocompounds against microorganisms.
Table 2.
Restorative herbs having anti-H. pylori action.
| Plant Names | Part and extract | Active ingredients responsible for the activity | Activity | Refs. |
|---|---|---|---|---|
| Aesculus hippocastanum | EtOH extract | Saponin (Aescine) | Antisecretory effect | [31] |
| Acacia nilotica | flower aceton extract | Not identified | Urease inhibitor | [8] |
| Achillea millefolium | MeOH extract of aerial parts | Not identified | Antioxidant | [45,46] |
| Ageratina pichinchensis | EtOH extract | 3,5-diprenyl-4-hydroxyacetophenone | Maintaenence NO, PG, SH release | [47] |
| Ageratum conyzoides | MeOH extract of the entire plant | Not identified | Not detected | [48] |
| Agrimonia pilosa Ledeb. | Aqueous extract of whole plant | Not identified | Not detected | [49] |
| Alchornea triplinervia | MeOH and EtOAc extracts | Not identified | Antisecretory | [50,51] |
| Increase PGE2 | ||||
| Decrease gastric injuries | ||||
| Increase mucus | ||||
| Promote epithelial cell | ||||
| Allium sativum | Oil and aqeous extract | Thiosulfinates | Interfere with cell wall | [52,53,54,55,56] |
| Diallyl disulfide | Causing cell lysis and Triggering autolysis | |||
| Aloe vera | Polysachharide fraction | Lectins | Increase mucus | [57] |
| Inhibit aminopyrin uptake | ||||
| Reduce TNF-α | ||||
| Alpinia speciosa | EtOH extract of root | Not identified | Inhibit H.pylori | [58] |
| Amphipterygium adstringens. | CH2Cl2 extract | 3a-hydroxymasticadienonic acid, b-sitosterol | Gastroprotective | [59] |
| 3-epi-oleanolic acid | ||||
| Angelica sinensis | EtOH extract | Polysaccharide indomethacin | Inhibition of MPO activity | [60] |
| Anisomeles indica | Stem and leaves EtOH extract | Not identified | Inhibit IL-12 and TNF-α, | [58] |
| Annona cherimola | Stem and leaves MeOH extract | Not identified | Not detected | [61] |
| Anthemis altissima | Isolated compounds from arial part | Sesquiterpene lactones | Not detected | [62] |
| Tatridin-A, sivasinolide, 1-epi-tatridin B, altissin, desacetyl-β-cyclopyrethrosin, | ||||
| Aralia elata | Root bark | Araloside A | Gastric lesion inhibitor ulcer formation inhibitor | [33] |
| Arrabidaea chica | HydroEtOHic extract of leaves | Flavones and flavonols | Inhibit H. pylori | [63] |
| Artemisia ludoviciana | Leaves and stem aqueous extract | Artemisin | Bactericidal kinetics | [61] |
| Morphological degeneration | ||||
| Atractylodes ovata | EtOH extract | Sesquiterpenoid | -Inhibition of MMP-2 | [64] |
| Atractylenolide III | -MMP-9 expression | |||
| Bixa orellana | EtOH extract of seeds | Not identified | Not detected | [65] |
| Boesenbergia rotunda | EtOH extract | Flavanone | Antioxidant | [66] |
| Pinostrobin | Decrease gastric motility | |||
| Bombax malabaricum | EtOH extract of root | Not identified | Not detected | [58] |
| Boronia pinnata | Whole shrub extract | Cinnamic acid derivative (boropinic acid) | Anti-ulcer agent | [67] |
| Brassica oleracea | Broccoli sprouts | Not identified | On human volunteers | [68] |
| Brazilian propolis | Propolis extract | 3-hydroxy-2,2dimethyl-8-prenylchromane-propenoic acid | Anti-H.pylori invitro | [69] |
| Bridelia micrantha | Acetone and EtOAc extracts of stem bark | Not identified | Anti-inflammatory | [70,71] |
| Byrsonima crassa | Leaves MeOH and CHCl3 extracts | Not identified | Immunostimulatory | [72] |
| Byrsonima fagifolia | Leaves MeOH extract | Not identified | Gastroprotective | [73] |
| Antidiarrheal | ||||
| Antibacterial Immunomodulatory | ||||
| Byrsonima intermedia | Leaves MeOH extract | Not identified | Antioxidant | [74] |
| Calophyllum b8rasiliense | Hexane, HydroEtOH extract and Ch2Cl2 fraction of stem bark | Mixture of chromanone | Decreased urease, | [6,75] |
| Reduce H. pylori in pathological analysis | ||||
| Calotropis procera | Acetone and MeOH extracts of leaves and flowers | Not identified | Urease inhibitor | [8] |
| Camellia sinensis | MeOH and water extracts of young shoots | Catechin | Urease inhibitor | [27,76,77] |
| Anti-inflammatory | ||||
| Carum carvi L. | Fruit MeOH | Not identified | Not detected | [78] |
| Casearia sylvestris | Leaves EtOH extract | Terpenoids | Decrease ulcerative size | [79] |
| Eradicate H. pylori | ||||
| Chamomilla recutita | Oil extract of flowers | Catechin | Urease inhibitor | [65,80,81,82] |
| 70 % aqueous | Decreasegastric mucosal injury | |||
| MeOH 96 % ethanol | ||||
| Cinnamomum cassia | Bark aqueous EtOH | Not identified | Suppression of IL-8 | [46] |
| Cinnamomum verum | Essential oils of dry bark | Cinnamaldehyde | Urease inhibitor | [83,84,85,86] |
| Cistus laurifolius | Flowers CHCl3 fraction | Isorhamnetin | Inhibit ulcer | [87,88] |
| Kaempferol 3,7-dimethyl ether, quercetin 3,7-dimethyl ether | Eradicate H.pylori | |||
| Citrus aurantium | EtOH extract | Monoterpene | indomethacin, ischemia reperfusion | [89] |
| b-Myrcene | ||||
| Citrus lemon | Essential oil | Monoterpene | Mucus production | [90] |
| Indomethacin | HSP-70 activation | |||
| Limonene | Vasoactive intestinal peptide and NO release | |||
| Maintenance of PGE2 and glutathione levels | ||||
| Cocculus hirsutus | EtOH extract of leaves | Alkaloids | Anti H. pylori | [91] |
| Cochlospermum tinctorium | Acidified EtOH | Polysaccharide | Antioxidant | [40] |
| Arabinogalactans II | Immunomodulatory | |||
| Combretum molle | Stem bark acetone extract was the best | Flavonoids | Gastroprotective | [92] |
| Coptis chinensis | Rhizome aqueous extract | Alkaloid | Inhibit ulcer | [93] |
| Eradicate H.pylori | ||||
| Croton reflexifolius | EtOH extract | Diterpenoid | Gastroprotective | [94] |
| Polyalthic acid | Block sulfhydryl groups | |||
| Inhibit NO synthase | ||||
| Croton sublyratus | Leaves extract | Terpenoid (Plaunotol) | Suppress IL-8 secretion | [95] |
| Cuminum cyminum | EtOH extracts of seeds | Phenolic compounds | Antioxidant | [96] |
| Cuphea aequipetala | Leaves aqueous extract | Phenolic compounds | Reduce gastric lesions | [61] |
| Inhibit ulcer | ||||
| Curcuma amada | Rhizome 70 % EtOH | Curcumin | Inhibit proton potassium ATPase | [97] |
| Cupressus sempervirens | Essential oil | Monoterpenes | Not detected | [98] |
| Curcuma longa | Polyphenolic rich extract of the root | Curcumin | Chemo-preventative | [99] |
| Cymbopogon citratus | Essential oil | Terpenes | Inhibit COX | [98] |
| Inhibit NO synthase Activate K+ATP channel and α2 receptors. | ||||
| Cyrtocarpa procera | Hexane extracts from stem bark | Not identified | Gastroprotective | [59,61,100] |
| Anti-inflammatory | ||||
| Davilla elliptica | Leaves MeOH extract | Not identified | Anti-inflammatory Gastroprotective | [101] |
| Davilla nítida | Leaves MeOH extract | Not identified | Anti-inflammatory Gastroprotective | [101] |
| Daucus carota | Essential oil of seed | Carvacrol and nerol | Decrease pH | [102] |
| Derris trifoliate | Petroleum ether and stemCHCl3 extracts | Not identified | Eradicate H. Pylori | [103] |
| Gastroprotective | ||||
| Desmostachya bipinnata | Wholeplant | Flavonoids (4-methoxy quercetin-7-O-glucoside) | Chemopreventive agent | [104,105] |
| Diethyl ether extract | ||||
| Dittrichia viscosa | Aerial parts essential oil (Oxygenated fractions) | 3-methoxy cuminyl isobutyrate | Antibacterial action | [81,106] |
| Eucalyptus torelliana | Hexane extract of leaves | Saponin and taninns | Decrease gastric acid | [107] |
| Increase pH gastric juice | ||||
| Eugenia caryophillus | EtOH extracts of flowers | Eugenol | Increase activity at acidic pH | [84,108] |
| Eugenia caryophyllata | Flowers aqueous extract | Essential oil | Anti-inflammatory | [49] |
| Eupatorium aschenbornianum | EtOH extract | Chromene | Antioxidant activity | [109] |
| Encecanescin | ||||
| Evodia rutaecarpa | Alkaloids rich extract | 1-Methyl-2-[(Z)-7-tridecenyl]-4-(1 H)-quinolone | Anti-inflammatory | [110] |
| Very strong Anti-H.pylori | ||||
| Feijoa sellowiana | Fruit Acetone Extract | Flavone | Inhibit H+/K+ATPase activity and Increase PGE2 | [111] |
| Ferulago campestris | Root extract | Coumarins (Aegelinol and Benzoyl aegelinol) | Not detected | [112,113,114,115] |
| Foeniculum vulgare | MeOH extract of the seeds | Not identified | Antioxidant | [45,46] |
| Garcinia achachairu | Acidified ethanol of the seeds | Polyisoprenylated benzophenone | Gastroprotective | [116] |
| Guttiferone A | ||||
| Geranium wilfordii | EtOH extracts and EtOAc fraction | 1,2,3,6-tetra-O-galloyl-β-d-glucose and corilagin | Not detected | [117] |
| Geum iranicum | Aqueous fraction of the roots | Tannins | Gastroprotective | [118] |
| Eugenol | ||||
| Glycyrrhiza glabra | Water extract of the root | Polysaccharide | Anti-adhesive activity | [11,29] |
| Flavonoids (glabridin) | Inhibit dihydrofolate reductase | |||
| Inhibit DNA gyrase | ||||
| Glycyrrhiza uralensis | MeOH extract of roots | licoricidin licoisoflavone B | Chemopreventive agents | [119,120] |
| licoric | ||||
| Guaiacum coulteri | Bark MeOH extract | Not identified | Antibacterial action | [61] |
| Hancornia speciose | Hydroalcoholic extract of the bark | Not identified | Antibacterial action | [121] |
| Hericium erinaceus | Hydroalcoholic extract of bark | Not identified | Antibacterial action | [122] |
| Hydrastis canadensis | MeOH extract of rhizome | Isoquinoline alkaloids | Inhibit bacterial efflux pumps, Inhibit of nucleic acid synthesis, Inhibite the enzyme dihydrofolate reductase | [123,124,125,126] |
| Berberine | ||||
| Hydrastine | ||||
| Hyptis suaveolens | EtOH extract | Diterpene, Indomethacin Suaveolol |
NO, PGE2, SH compounds | [127] |
| Impatiens balsamina | Pod acetone, EtoAc, terpenoid fraction | 2Methoxy1,4naphthoquinone | Produce ROS to damage H pylori cell membrane | [9] |
| Stigmasta7,22-diene3βol | ||||
| Ixeris chinensis | Boiling water,EtOH and CHCl3 extract was the active one | Not identified | Antibacterial | [128] |
| Antiadhesive | ||||
| Anti-inflammatory | ||||
| Inhibit IL-8, NO, TNF-α | ||||
| Jatropha isabelli | Acidified EtOH | Monoterpene | Gastroprotective | [129] |
| 1,4-Epoxy-ρ-menthan- 2-ol | ||||
| Sesquiterpene | ||||
| Cyperenoic acid | ||||
| Triterpene | ||||
| Acetyl aleuritolic acid | ||||
| 9b,13a- Dihydroxyisabellione | ||||
| Diterpene | ||||
| Jatropholone A | ||||
| Jatropholone B Jatrophone | ||||
| Juglans regia | Fruit MeOH extract | Xanthanolide | Not detected | [130] |
| Larrea divaricata | Branches and leaves aqueous extract | Nordihydroguaiaretic acid | Anti-inflammatory | [131] |
| Gastroprotective | ||||
| Anti-gastric cancer | ||||
| Lycopodium cernua | Whole plant hexane extract | The powerful compound was found in hexane fraction | Not detected | [48] |
| Magnoliae officinalis | Ether fraction of cortex | Magnolol | Antigastritic, antioxidant, neutralize acid, inhibit the secretion of gastric acid | [132] |
| Mallotus phillipinesis | 70 % EtOH extract of fruit | Isorottlerin, rottlerin | Not detected | [97] |
| 3′-prenylrubranine, 5,7-dihydroxy-8-methyl-6-prenylflavanone | ||||
| Malva sylvestris | Inflorescence and leaves EtOH Extract | Not identified | Not detected | [65] |
| Mangifera indica | Pet-ether and EtOH extracts of leaves | Mangiferin | Gastroprotective Antisecretory, antioxidant | [133,134] |
| Mentha piperita | Leaves andstem aqueous extract | Essential oil | antisecretory,antioxidant, anti-inflammatory, and antiapoptotic actions | [61] |
| Menthol | ||||
| Mentha sp. | EtOH extract | Monoterpene | Increase PGE2 | [38,39] |
| Indomethacin pyloric ligature | Antiapoptotic,Antioxidant | |||
| Menthol | Anti-inflammatory | |||
| Morus alba | leaves EtOH extract | Steroid, Albosteroid | Antisecretory | [135,136] |
| Pyloric ligature | Antioxidant | |||
| Mitrella kentii | EtOH extract | Chalcone | Antiapoptotic, antioxidant | [137] |
| Desmosdumotin C | Inhibit COX-2 | |||
| Musa acuminata | Crude flavonoids extract | Flavonoids | Increase mucus | [138,139] |
| Leucocyanidin | ||||
| Myristica fragrans | MeOH extracts of seeds and aerial parts | Not identified | Gastroprotective | [97,140] |
| Myroxylon peruiferum | Isolated compound | Isoflavone | Inhibit NADH oxidation | [141] |
| Cabreuvin | ||||
| Myrtus communis | Essential oil | Monoterpenes | Inhibit urease | [86,142] |
| Olea europaea | Leaves MeOH extract | Not identified | Increase gastric flora | [143] |
| Reduce H. pylori | ||||
| Ocimum sanctum | Fixed oil | Not identified | Inhibit lipoxygenase | [144] |
| Antisecretory | ||||
| Histamine antagonistic | ||||
| Origanum majorana L. | Aerial parts MeOH extract | Phenolic compounds | Enhance protective host defence | [45] |
| Oroxylum indicum | Crude Flavone glycosides | 7-O-methylchrysin, 5-hydroxy-749-dimethoxyflavone, oroxylin A, chrysin, and baicalein | Gastroprotective | [145, [146] |
| Paeonia lactiflora | Root lipid fraction | Lysophosphatidic acid Paeonol benzoic acid methyl gallate,1,2,3,4,6-penta- O-galloyl-β -D-glucopyranose |
Increase PG E2 Decrease membrane integrity Inhibit urease Inhibit UreB (an adhesin) |
[5,147] |
| Panax ginseng | Polysaccharides fraction | Galacturonic acid | Anti-adhesive | [148,149] |
| Papaver somniferum | Alkaloids | Porphine | Not detected | [150] |
| Pausinystalia yohimbe | Alkaloids | Yohimbine | Decrease ulcer | [44] |
| Peperomia pellucida | EtOH extract | Allylbenzene Dillapiole |
Gastroprotective | [151] |
| Persea americana | MeOH extracts of leaf | Procyanidins | Inhibit urease | [61] |
| Piper carpunya | Flavonoids rich extract of the leaves | Vitexin Isovitexin Rhamnopyranosylvitexin Isoembigenin |
Releasemyeloperoxidase Inhibite H+,K + ATPase activity N-Acetylation |
[154] |
| Piper multiplinervium | Hydroxybenzoic acid prenylated derivative | 3-farnesyl-2-hydroxybenzoic acid | Treat stomach aches | [155] |
| Pistacia lentiscus | Mastic gum | Triterpenic acids | Induce blebbing Cellular fragmentation Morphological abnormalities in H. pylori cells |
[156,157,158,159] |
| Plectranthus grandis | EtOH extract | Diterpenes 3b-Hydroxy-3- deoxibarbatusin Barbatusin |
K+ATP channel NO, TRPV1 channels | [160] |
| Plumbago zeylanica | EtOAc of rhizome | Naphthoquinone Plumbagin |
Bactericidal activity | [58,161] |
| Polygala cyparissias | EtOH extract | Xantone | Anti-ulcer Gastroprotective |
[162] |
| Polygonum tinctorium | Leaf juice | Tryptanthrin Kaempferol |
decrease numbers of colonies in gerbils stomachs | [163] |
| Polygala cyparissias | EtOH extract | Sterol a-Spinasterol |
Reduce percentage of lesion area Reduce ulcer index |
[162] |
| Potentilla fruticose | Aqueous extracts of aerial part | Not identified | Antibacterial action | [164] |
| Prunus dulcis | Polyphenol-rich extracts of skin | Protocatechuic acid | Post gastric plus duodenal digestion | [165] |
| Prumnopitys andina | Acidified EtOH | Diterpene, acetic acid Ferruginol |
PGE2 production Inhibit lipoperoxidation |
[37] |
| Psoralea corylifolia | Seeds extract | Psoracorylifols | Antibacterial | [166] |
| Pteleopsis suberosa | MeOH extract of stem bark | Oleanane saponine Arjunglucoside I | AntivacA/cagA positive and metronidazole-resistant strains | [167] |
| Punica granatum | EtOH, MeOH, BuOH and aqueous extracts from fruit peel | Phenolic compounds | Chang hydrophobicity of H. pylori cell surface | [130,168,169] |
| Phyllanthus niruri | Aqueous extracts of leaves | Ellagic acid Hydroxycinnamic acid |
Damage H.pylori cell membrane | [103,152] |
| Physalis alkekengi | EtOAc extract of the aerial parts | Quercetin Physalindicanols A kaempferol Blumenol A |
Antiinflammatory Antiulcer invivo Analgesic |
[153] |
| Qualea parviflora | MeOH extract of bark | Triterpenes Saponins |
Maintaine GSH levels Increase SH compounds Stimulate PGE2 synthesis |
[170] |
| Rabdosia trichocarpa | MeOH extract from entire plants | Diterpene Trichorabdal A |
Strong antibacterial action | [171] |
| Rhei Rhizoma | Rhizome | Emodin | Damage DNA H. Pylori | [30] |
| Rheum palmatum | Rhizome | Rhein | Inhibite N-acetyltransferase | [172] |
| Rheum rhaponticum L. | Root EtOH Extract | Not identified | Anti-inflammatory | [56] |
| Rosmarinus officinalis | Leaves MeOH extract | Not identified | Antiulcer, vasodilator Gastroprotective |
[45] |
| Rubus imperialis | EtOH extract | Triterpene 2b,3b-19a-Trihydroxy ursolic acid |
Not detected | [173] |
| Rubus ulmifolius | Leaves extract Flavonoids | Ellagic Kampferol |
Reduce gastric PH Participate No and SH |
[26] |
| Ruta graveolens | Aqueous EtOH extract of leaves | Polyphenols | Antioxidant Anti-inflammatory Inhibit IL-8 secretion |
[46] |
| Salvia mirzayanii | MeOH extract of leaves | Not identified | Not detected | [174] |
| Sanguinaria Canadensis | MeOH extracts of rhizome | Sanguinarine, chelerythrine, two benzophenanthridine alkaloids | Anti ulcer | [123,175] |
| Santalum album | hydro-alcoholic extract of stem | (Z)-R-santalol (7), (Z)-β-santalol, (Z)-lanceol | Strong antiulcer | [176] |
| Schinus molle | EtOH extract | Flavonol, Rutin | Antioxidant | [177] |
| Sclerocarya birrea | Essential oil | Terpinen- 4-ol | Decrease membrane integrity | [110,178] |
| Senecio brasiliensis | Inflorescences | Integerrimine, retrorsine, senecionine, usaramine, and seneciphylline | Increase mucus | [42,43] |
| Pyrrolizidine alkaloids | Increase PG | |||
| Simaba ferruginea | Rhizome fractions | Alkaloid | Antiulcerogenic | [41] |
| Canthin-6-one | Reduce myeloperoxidase malondialdehyde | |||
| Reduce plasma IL-8 | ||||
| Scleria striatinux | MeOH extract of roots | Okundoperoxide | Antibacterial | [48] |
| Solanum paniculatum L. | New isolated steroids saponins | diosgenin 3-O-b-d-glucopyranosyl(10 → 69)-O-b-d-glucopyranoiside. | Decrease gastric lesion | [179] |
| Decrease levels of MPO in the mucosa | ||||
| Sphacele chamaedryoide | EtOH extract Diterpene | Horminone, Carnosol | Gastroprotective | [180] |
| Taxoquinone | Inhibit gastric lesions | |||
| Stachys setifera | MeOH extracts of leaves | Not identified | Not detected | [181] |
| Strychnos pseudoquina | Leaves MeOH extract | Alkaloid enriched fraction | Increase cell proliferation in gastric mucosa | [182] |
| Syzygium aromaticum | Flower buds | Flavonoids | Antiulcerogenic | [183,184] |
| Tannins | Antisecretory | |||
| Increase PGE | ||||
| Tabebuia impetiginosa | Inner bark | (hydroxymethyl)anthraquin | Strong antibacterial | [185] |
| anthraquinone-2-carboxylic | ||||
| Lapachol, plumbagin | ||||
| Termitomyces eurhizus | Mushroom | Polysaccharides fraction | Stimulate mucosal regeneration and proliferation | [186] |
| Restoring gastric mucus | ||||
| Increase PG E2 | ||||
| Modulate COX-1 and COX-2 | ||||
| Reduce TNF-α and IL-1b | ||||
| Terminalia spinosa | Young branches crude extract | Not identified | Not detected | [187] |
| Terminalia chebula | Aqueous extracts of fruit | Chebulinic acid | Improve secretory of B runner gland | [188,189,190] |
| Ethyl gallate gallic acid | ||||
| Thymus vulgaris | Essential oils | Monoterpenes | Gastroprotective | [191] |
| Anti-inflammatory | ||||
| Tithonia diversifolia | EtOH extract | Sesquiterpene | Gastroprotective | [192] |
| Indomethacin, Tagitinin C | ||||
| Trachyspermum copticum | Mixture of petroleum / MeOH extract of fruit and leaves | Not identified | Antibacterial | [78,193] |
| Vaccinium macrocarpon | Cranberry juice | Polyphenols | Anti-adhesive | [194,195] |
| Vitis venefera | Grape seeds | Resveratrol | Chemopreventative | [4] |
| Flavonoids | ||||
| Antioxidant | ||||
| Xanthium brasilicum | Aerial parts MeOH, diethyl ether and benzene | Not identified | Antimicrobial | [78] |
| Zataria multiflora | Essential oils of aerial parts | Thymol, carvacrol | Enhance mucosa Cytoprotective | [83,196] |
| Zingiber officinalis | Root extract | 6-gingesulphonic acid | Inhibit thromboxane synthetase | [45,197,198,199,200,201,202] |
| 6-shogaol, Arcurcumene | ||||
| Gingerols |
Methanol: MeOH; Ethanol: EtOH; Butanol: BuOH; Dichloromethan: CH2Cl2; Chloroform:CHCl3; Prostaglandin: PG; Tumor necrosis factor: TNF; Interlokin: IL; Cyclooxiginase: COX; Nitric oxide: NO; sulfhydryl : SH.
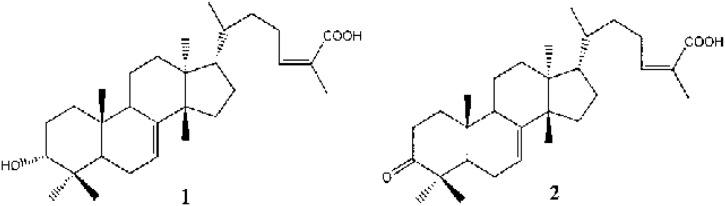
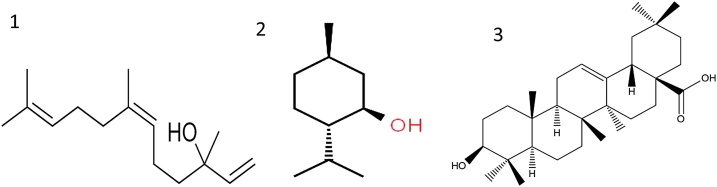
4.1. Sterol
The presence of a free OH group in C-3 is necessary for the antiulcer action of triterpenoids and sterols consistently, the only structural difference between the active 3a-hydroxymasticadienonic acid (Fig. 3, 1) and the inactive masticadienonic acid (Fig. 3, 2) is the presence of an OH group and a C O group in the C-3 [14,15].
Fig. 3.
Chemical structure of 3a-hydroxymasticadienonic acid (1) and masticadienonic acid (2).
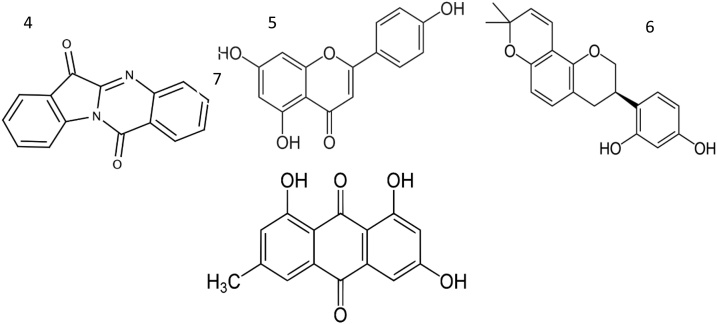
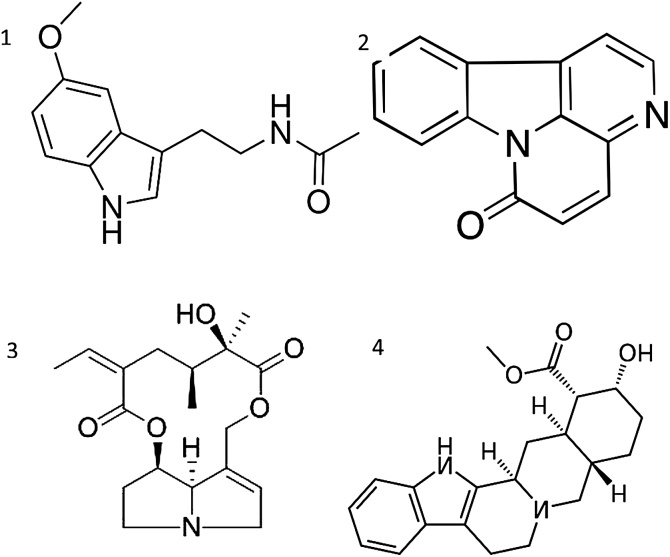
4.2. Flavonoids
Flavonoids have been used in the treatment of countless diseases [[16], [17], [18], [19], [20], [21]]. Flavonoids (Fig. 4) are found to display as antisecretory and cytoprotective agents by increasing PG levels, inhibiting H. pylori, decreasing histamine, and antioxidants [22]. The structure activity relationship shows that the presence of OCH3 group in the C-5 or C-7 positions, the double bonds at C-2 and C-3 and the presence pof an intact C-ring appear to increase gastroprotection potential. On the other hand, substitution with OH or OCH3 groups at C-3, C-6, or C-8 diminish the gastroprotective action.
Fig. 4.
Chemical structure of anti-H.Pylori flavonoids 1) Quercetin 2) Kampferol 3) Catchin 4) tryptanthrin 5) Apigenin 6) Glabridin 7) Emodin.
Flavonoids can kill microbs by 1) membrane disruption by apigenin, catechin, naringenin, quercetin, and rhamnetin and inhibition of nucleic acid synthesis 2) inhibit dihydrofolate reductase by epicatechin, 3) inhibithelicase by luteolin and myricetin, d) inhibitgyrase/topoisomerase by apigenin, kaempferol and quercetin, 4) inhibit bacterial virulence by quercetin and kaempferol 5) inhibit quorum sensing by epicatechin, naringenin, quercetin and kaempferol 6) inhibit fatty acid synthase and peptidoglycan synthesis by taxifolin, kaempferol, luteolin, myricetin and quercetin7) inhibit Ala–Ala dipeptide synthesis by gaiangin, kaempferol, and kaempferol-3-O-glucoside, 8) inhibitpeptidoglycan crosslinking by apigenin and quercetin. 9) inhibit refflux pumps by diadzein, genistein, epicatechin and quercetin10) inhibit NADH-cytochrome c reductase activity in the bacterial respiratory chain by chalcon11) inhibit ATP synthase by epicatchin, quercetin, quercetrin, and silymarin [23].
As shown in Fig. 4, quercetin decreases lipid peroxide and neutrophil leukocyte infiltration, in the H. pylori colonization [24]. The blend of kaempferol and tryptanthrin reduce the viability of H. pylori invivo [25,26]. Upon giving green tea product that is consisted of catechin to H. pylori-infected Mongolian gerbils, both of gastritis and the prevalence of H. pylori were significantly suppressed [27]. Besides, apigenin treatments effectively eradicated H. pylori, atrophic gastritis, and gastric cancer rates in H. pylori-infected Mongolian gerbils. Apigenin is reported to have excellent ability to inhibit H. pylori as well as possessing potent anti-gastric cancer [28]. As for Glabridin, it possesses a strong inhibitory effect on dihydrofolate reductase and DNA gyrase [29]. While emodin; a major phytocompound of Rhizoma Rhei induces H. pylori DNA damage [30].
4.3. Steroid saponin
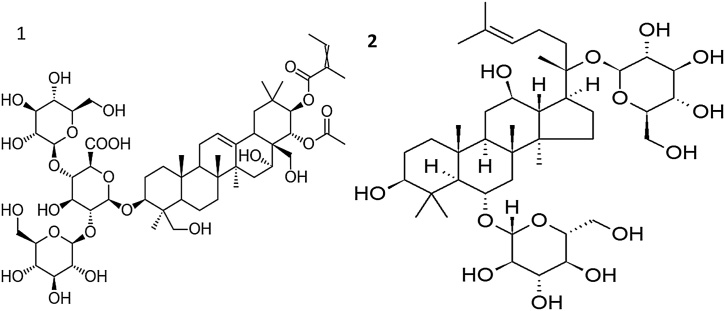
Aescine (Fig. 5) reduces the severity of ulcers by decreasing gastric secretion [31], while Ginsenoside increases the amount of mucus [32].
Fig. 5.
Chemical structure of Aescine (1) and Ginsenoside (2).
According to Lee et al. [33], the saponins display antisecretory action by inhibiting acid secretion, total acid output, and lowering the pH of gastric juice [34].
4.4. Terpenes
Nerolidol (Fig. 6) has an antiulcerogenic and cytoprotective effect by increasing mucus production via increasing the PG, improving the gastric blood flow, and increasing the secretion of gastric bicarbonate and mucus [35]. In addition, terpenoids act as antioxidants, reduce the lipid peroxidation levels, and increase the activity of antioxidant enzymes in the gastric mucosa [36,37]. Menthol is a monoterpene that increases the maintenance of SH compounds and the amount of mucus and PG production. It also possesses an antisecretory effect, in addition to antioxidant, anti-inflammatory, and antiapoptotic actions [38,39]. Oleanolic acid is a triterpene that improves healing in the ulcer model. The low toxicity and the widespread occurrence in various plants support the potential development of new antiulcer drug based on triterpenes or their derivatives [37].
Fig. 6.
Chemical structure of anti-H.pylori terpens 1) Nerolidol 2) Menthol 3) Oleanolic acid.
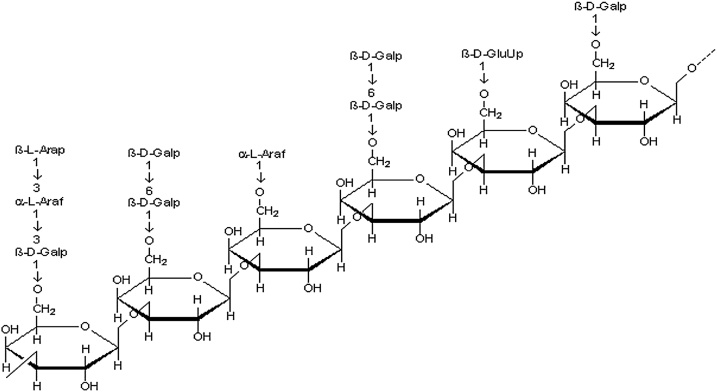
4.5. Polysaccharides
Arabinogalactan (Fig. 7) has the ability to bind on the gastric mucosa acting as a protective layer, in addition to its antisecretory activity towards gastric juice. The mucosal protective activity of Arabinogalactan is provided by an increased mucus synthesis and free radical scavenging activity. The particular mechanisms of polysaccharides are described by their potential to bind on the surface of the gastrointestinal mucosa, thereby acting as a protective layer, in addition to their antisecretory action. Their mucosal protective potentials are provided by an increased mucus synthesis and their antioxidant activity. Pectic polysaccharides obtained by aqueous extraction represent examples of the main polysaccharides displaying gastric antiulcer action [40].
Fig. 7.
chemical structure of Arabinogalactan.
4.6. Alkaloids
Canthin-6-one (Fig. 8), isolated from Simaba ferruginea rhizome has been shown to be antiulcerogenic [41], while integerrimine isolated from Senecio brasiliensis was found to increase mucus and PG levels [42,43]. Melatonin, as a hormone, has the ability to scavenge free radical and ameliorating gastric blood flow [43]. Yohimbine, isolated from Pausinystalia yohimbe, decreases ulcers [44].
Fig. 8.
Chemical structure of Melatonin (1), Canthin-6-one (2), Integerrimine (3), Yohimbine (4).
5. Conclusion
H. pylori inhibition with antibiotic therapies has a limitation mainly owing to antibiotic resistance. Medicinal herbs provide another opportunity to inhibit H. pylori. Medicinal herbs might also provide successful approach to decrease stomach cancer. However, potential cytotoxicity and side effects might present from those herbs. Therefore, further cytotoxicity investigation will be required.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Lawal T.O., Igbokwe C.O., Adeniyi B.A. Antimicrobial activities and the bactericidal kinetics of Allium ascalonicum Linn. whole plant) against standard and clinical strains of Helicobacter pylori: support for ethnomedical use. J. Nat. Sci. Res. 2014;4(8):48–56. [Google Scholar]
- 2.Conteduca V., Sansonno D., Lauletta G., Russi S., Ingravallo G., Dammacco F. H. pylori infection and gastric cancer: state of the art. Int. J. Oncol. 2013;42(1):5–18. doi: 10.3892/ijo.2012.1701. [DOI] [PubMed] [Google Scholar]
- 3.Wolle K., Malfertheiner P. Treatment of Helicobacter pylori. Best Pract. Res. Clin. Gastroenterol. 2007;21(2):315–324. doi: 10.1016/j.bpg.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Paulo L., Oleastro M., Gallardo E., Queiroz J.A., Domingues F. Anti-Helicobacter pylori and urease inhibitory activities of resveratrol and red wine. Food Res. Int. 2011;44(4):964–969. doi: 10.1016/j.foodres.2011.02.017. [DOI] [Google Scholar]
- 5.Ngan L.T.M., Moon J.K., Shibamoto T., Ahn Y.J. Growth-inhibiting, bactericidal, and urease inhibitory effects of Paeonia lactiflora root constituents and related compounds on antibiotic-susceptible and-resistant strains of Helicobacter pylori. J. Agric. Food Chem. 2012;60(36):9062–9073. doi: 10.1021/jf3035034. [DOI] [PubMed] [Google Scholar]
- 6.do Carmo Souza M., Beserra A.M.S., Martins D.C., Real V.V., dos Santos R.A.N., Rao V.S., da Silva R.M., de Oliveira Martins D.T. In vitro and in vivo anti-Helicobacter pylori activity of Calophyllum brasiliense Camb. J. Ethnopharmacol. 2009;123(3):452–458. doi: 10.1016/j.jep.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 7.Lin Y.T., Kwon Y.I., Labbe R.G., Shetty K. Inhibition of Helicobacter pylori and associated urease by oregano and cranberry phytochemical synergies. Appl. Environ. Microbiol. 2005;71(12):8558–8564. doi: 10.1128/AEM.71.12.8558-8564.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amin M., Anwar F., Naz F., Mehmood T., Saari N. Anti-Helicobacter pylori and urease inhibition activities of some traditional medicinal plants. Molecules. 2013;18(2):2135–2149. doi: 10.3390/molecules18022135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y.C., Li W.Y., Wu D.C., Wang J.J., Wu C.H., Liao J.J., Lin C.K. In vitro activity of 2-methoxy-1, 4-naphthoquinone and stigmasta-7, 22-diene-3β-ol from Impatiens balsamina L. against multiple antibiotic-resistant Helicobacter pylori. Evid. Based Complement. Altern. Med. 2011;2011 doi: 10.1093/ecam/nep147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Mahony R., Al-Khtheeri H., Weerasekera D., Fernando N., Vaira D., Holton J., Basset C. Bactericidal and anti-adhesive properties of culinary and medicinal plants against Helicobacter pylori. World J. Gastroenterol. 2005;11(47):7499. doi: 10.3748/wjg.v11.i47.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wittschier N., Faller G., Hensel A. Aqueous extracts and polysaccharides from liquorice roots (Glycyrrhiza glabra L.) inhibit adhesion of Helicobacter pylori to human gastric mucosa. J. Ethnopharmacol. 2009;125(2):218–223. doi: 10.1016/j.jep.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Wittschier N., Faller G., Hensel A. An extract of Pelargonium sidoides (EPs 7630) inhibits in situ adhesion of Helicobacter pylori to human stomach. Phytomedicine. 2007;14(4):285–288. doi: 10.1016/j.phymed.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Takagi A., Koga Y., Aiba Y., Kabir A.M., Watanabe S., Ohta‐Tada U., Osaki T., Kamiya S., Miwa T. Plaunotol suppresses interleukin‐8 secretion induced by Helicobacter pylori: therapeutic effect of plaunotol on H. pylori infection. J. Gastroenterol. Hepatol. 2000;15(4):374–380. doi: 10.1046/j.1440-1746.2000.02168.x. [DOI] [PubMed] [Google Scholar]
- 14.Giner-Larza E.M., Máñez S., Giner R.M., Recio M.C., Prieto J.M., Cerdá-Nicolás M., Ríos J. Anti-inflammatory triterpenes from Pistacia terebinthus galls. Planta Med. 2002;68(04):311–315. doi: 10.1055/s-2002-26749. [DOI] [PubMed] [Google Scholar]
- 15.Navarrete A., Trejo-Miranda J.L., Reyes-Trejo L. Principles of root bark of Hippocratea excelsa (Hippocrataceae) with gastroprotective activity. J. Ethnopharmacol. 2002;79(3):383–388. doi: 10.1016/S0378-8741(01)00414-7. [DOI] [PubMed] [Google Scholar]
- 16.El-Gengaihi S.E., Hamed M.A., Aboubaker D.H., Mossa A.T. Flavonoids from sugar beet leaves as hepatoprotective agent. Int. J. Pharm. Pharm. Sci. 2016;8:281–286. [Google Scholar]
- 17.El-Gengaihi S.E., Mossa A.T.H., Refaie A.A., Aboubaker D. Hepatoprotective efficacy of Cichorium intybus L. extract against carbon tetrachloride-induced liver damage in rats. J. Diet. Suppl. 2016;13:570–584. doi: 10.3109/19390211.2016.1144230. [DOI] [PubMed] [Google Scholar]
- 18.Baker D.A., Al-Moghazy M., ElSayed A.A.A. The in vitro cytotoxicity, antioxidant and antibacterial potential of Satureja hortensis L. essential oil cultivated in Egypt. Bioorg. Chem. 2020;95:103559. doi: 10.1016/j.bioorg.2019.103559. https://www.researchgate.net/publication/301490287 [DOI] [PubMed] [Google Scholar]
- 19.Abou Baker D.H., Rady Hanaa M. Bioassay-guided approach employed to isolate and identify anticancer compounds from Physalis peruviana calyces. Plant Arch. 2020;20(1):3285–3291. https://www.researchgate.net/publication/340132967 [Google Scholar]
- 20.Abou Baker D.H. Achillea millefolium L. ethyl acetate fraction induces apoptosis and cell cycle arrest in human cervical cancer (HeLa) cells. Ann. Agric. Sci. 2020 doi: 10.1016/j.aoas.2020.03.003. In press. [DOI] [Google Scholar]
- 21.Salam M.A., Ibrahim B.M., El-Batran S.E., El-Gengaihi S.E., Baker D.H. Study of the possible antihypertensive and hypolipidemic effects of an herbal mixture on l-name-induced hypertensive rats. Asian J. Pharm. Clin. Res. 2016;9:85–90. doi: 10.22159/ajpcr.2016.v9i5.12175. [DOI] [Google Scholar]
- 22.Coelho R.G., Batista L.M., Santos L.C.D., Brito A.R.M.D.S., Vilegas W. Phytochemical study and antiulcerogenic activity of Syngonanthus bisulcatus (Eriocaulaceae) Rev. Bras. Cinêc. Farm. 2006;42(3):413–417. doi: 10.1590/S1516-93322006000300010. [DOI] [Google Scholar]
- 23.Olaleye S.B., Farombi E.O. Attenuation of indomethacin‐and HCl/ethanol‐induced oxidative gastric mucosa damage in rats by kolaviron, a natural biflavonoid of Garcinia kola seed. Phytother. Res. 2006;20(1):14–20. doi: 10.1002/ptr.1793. [DOI] [PubMed] [Google Scholar]
- 24.González-Segovia R., Quintanar J.L., Salinas E., Ceballos-Salazar R., Aviles-Jiménez F., Torres-López J. Effect of the flavonoid quercetin on inflammation and lipid peroxidation induced by Helicobacter pylori in gastric mucosa of guinea pig. J. Gastroenterol. 2008;43(6):441. doi: 10.1007/s00535-008-2184-7. [DOI] [PubMed] [Google Scholar]
- 25.Kataoka M., Hirata K., Kunikata T., Ushio S., Iwaki K., Ohashi K., Ikeda M., Kurimoto M. Antibacterial action of tryptanthrin and kaempferol, isolated from the indigo plant (Polygonum tinctorium Lour.), against Helicobacter pylori-infected Mongolian gerbils. J. Gastroenterol. 2001;36(1):5–9. doi: 10.1007/s005350170147. [DOI] [PubMed] [Google Scholar]
- 26.Martini S., D’Addario C., Colacevich A., Focardi S., Borghini F., Santucci A., Figura N., Rossi C. Antimicrobial activity against Helicobacter pylori strains and antioxidant properties of blackberry leaves (Rubus ulmifolius) and isolated compounds. Int. J. Antimicrob. Agents. 2009;34(1):50–59. doi: 10.1016/j.ijantimicag.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Matsubara S., Shibata H., Ishikawa F., Yokokura T., Takahashi M., Sugimura T., Wakabayashi K. Suppression of Helicobacter pylori-induced gastritis by green tea extract in Mongolian gerbils. Biochem. Biophys. Res. Commun. 2003;310(3):715–719. doi: 10.1016/j.bbrc.2003.09.066. [DOI] [PubMed] [Google Scholar]
- 28.Kuo C.H., Weng B.C., Wu C.C., Yang S.F., Wu D.C., Wang Y.C. Apigenin has anti-atrophic gastritis and anti-gastric cancer progression effects in Helicobacter pylori-infected Mongolian gerbils. J. Ethnopharmacol. 2014;151(3):1031–1039. doi: 10.1016/j.jep.2013.11.040. [DOI] [PubMed] [Google Scholar]
- 29.Asha M.K., Debraj D., Edwin J.R., Srikanth H.S., Muruganantham N., Dethe S.M., Anirban B., Jaya B., Deepak M., Agarwal A. In vitro anti-Helicobacter pylori activity of a flavonoid rich extract of Glycyrrhiza glabra and its probable mechanisms of action. J. Ethnopharmacol. 2013;145(2):581–586. doi: 10.1016/j.jep.2012.11.033. [DOI] [PubMed] [Google Scholar]
- 30.Wang H.H., Chung J.G. Emodin-induced inhibition of growth and DNA damage in the Helicobacter pylori. Curr. Microbiol. 1997;35(5):262–266. doi: 10.1007/s002849900250. [DOI] [PubMed] [Google Scholar]
- 31.Marhuenda E., Martin M.J., Alarcon Lastra C.D.L. Antiulcerogenic activity of aescine in different experimental models. Phytother. Res. 1993;7(1):13–16. doi: 10.1002/ptr.2650070105. [DOI] [Google Scholar]
- 32.Jeong C.S., Hyun J.E., Kim Y.S., Lee E.S. Ginsenoside RB 1 the anti-ulcer constituent from the head of Panax ginseng. Arch. Pharm. Res. 2003;26(11):906. doi: 10.1007/BF02980198. [DOI] [PubMed] [Google Scholar]
- 33.Lee E.B., Kim O.J., Kang S.S., Jeong C. Araloside A, an antiulcer constituent from the root bark of Aralia elata. Biol. Pharm. Bull. 2005;28(3):523–526. doi: 10.1248/bpb.28.523. [DOI] [PubMed] [Google Scholar]
- 34.Klopell F.C., Lemos M., Sousa J.P.B., Comunello E., Maistro E.L., Bastos J.K., De Andrade S.F. Nerolidol, an antiulcer constituent from the essential oil of Baccharis dracunculifolia DC (Asteraceae) Z. Naturforschung C. 2007;62(7–8):537–542. doi: 10.1515/znc-2007-7-812. [DOI] [PubMed] [Google Scholar]
- 35.Ohta Y., Kamiya Y., Imai Y., Arisawa T., Nakano H. Plaunotol prevents the progression of acute gastric mucosal lesions induced by compound 48/80, a mast cell degranulator, in rats. Pharmacology. 2005;74(4):182–192. doi: 10.1159/000085388. [DOI] [PubMed] [Google Scholar]
- 36.Kim J.H., Kim Y.S., Song G.G., Park J.J., Chang H.I. Ulcers and gastrointestinal health. Eur. J. Pharmacol. 2005;514(1):53–59. doi: 10.1016/j.ejphar.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 37.Rodríguez J.A., Theoduloz C., Yáñez T., Becerra J., Schmeda-Hirschmann G. Gastroprotective and ulcer healing effect of ferruginol in mice and rats: assessment of its mechanism of action using in vitro models. Life Sci. 2006;78(21):2503–2509. doi: 10.1016/j.lfs.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 38.Rozza A.L., Hiruma-Lima C.A., Takahira R.K., Padovani C.R., Pellizzon C.H. Effect of menthol in experimentally induced ulcers: pathways of gastroprotection. Chem. Biol. Interact. 2013;206(2):272–278. doi: 10.1016/j.cbi.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Rozza A.L., de Faria F.M., Brito A.R.S., Pellizzon C.H. The gastroprotective effect of menthol: involvement of anti-apoptotic, antioxidant and anti-inflammatory activities. PLoS One. 2014;9(1):e86686. doi: 10.1371/journal.pone.0086686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nergard C.S., Diallo D., Inngjerdingen K., Michaelsen T.E., Matsumoto T., Kiyohara H., Yamada H., Paulsen B.S. Medicinal use of Cochlospermum tinctorium in Mali: anti-ulcer-, radical scavenging-and immunomodulating activities of polymers in the aqueous extract of the roots. J. Ethnopharmacol. 2005;96(1–2):255–269. doi: 10.1016/j.jep.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 41.Almeida E.S.S., Filho V.C., Niero R., Clasen B.K., Balogun S.O., Oliveira Martins D.T. Pharmacological mechanisms underlying the anti-ulcer activity of methanol extract and canthin-6-one of Simaba ferruginea A. St-Hil. in animal models. J. Ethnopharmacol. 2011;134:630–636. doi: 10.1016/j.jep.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 42.Toma W., Trigo J.R., Bensuaski de Paula A.C., Souza Brito. ARM Preventive activity of pyrrolizidine alkaloids from Senecio brasiliensis (Asteraceae) on gastric and duodenal induced ulcer on mice and rats. J. Ethnopharmacol. 2004;95:345–351. doi: 10.1016/j.jep.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 43.Konturek P.C., Konturek S.J., Majka J., Zembala M., Hahn E.G. Melatonin affords protection against gastric lesions induced by ischemia-reperfusion possibly due to its antioxidant and mucosal microcirculatory effects. Eur. J. Pharmacol. 1997;322(1):73–77. doi: 10.1016/S0014-2999(97)00051-4. [DOI] [PubMed] [Google Scholar]
- 44.Ozaki Y. Pharmacological studies of indole alkaloids obtained from domestic plants, Uncaria rhynchophylla Miq. and Amsonia elliptica Roem. Et Schult. Nihon yakurigaku zasshi. Folia Pharmacol. Jpn. 1989;94(1):17–26. doi: 10.1254/fpj.94.17. [DOI] [PubMed] [Google Scholar]
- 45.Mahady G.B., Pendland S.L., Stoia A., Hamill F.A., Fabricant D., Dietz B.M., Chadwick L.R. In vitro susceptibility of Helicobacter pylori to botanical extracts used traditionally for the treatment of gastrointestinal disorders. Phytother. Res. 2005;19(11):988–991. doi: 10.1002/ptr.1776. [DOI] [PubMed] [Google Scholar]
- 46.Zaidi S.F., Muhammad J.S., Shahryar S., Usmanghani K., Gilani A.H., Jafri W., Sugiyama T. Anti-inflammatory and cytoprotective effects of selected Pakistani medicinal plants in Helicobacter pylori-infected gastric epithelial cells. J. Ethnopharmacol. 2012;141(1):403–410. doi: 10.1016/j.jep.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 47.Sánchez-Mendoza M.E., Rodríguez-Silverio J., Rivero-Cruz J.F., Rocha-González H.I., Pineda-Farías J.B., Arrieta J. Antinociceptive effect and gastroprotective mechanisms of 3, 5-diprenyl-4-hydroxyacetophenone from Ageratina pichinchensis. Fitoterapia. 2013;87:11–19. doi: 10.1016/j.fitote.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 48.Ndip R.N., Tarkang A.E.M., Mbullah S.M., Luma H.N., Malongue A., Ndip L.M., Nyongbela K., Wirmum C., Efange S.M. In vitro anti-Helicobacter pylori activity of extracts of selected medicinal plants from North West Cameroon. J. Ethnopharmacol. 2007;114(3):452–457. doi: 10.1016/j.jep.2007.08.037. [DOI] [PubMed] [Google Scholar]
- 49.Li H., Meng L., Liu F., Wei J.F., Wang Y.Q. H+/K+-ATPase inhibitors: a patent review. Expert Opin. Ther. Pat. 2013;23(1):99–111. doi: 10.1517/13543776.2013.741121. [DOI] [PubMed] [Google Scholar]
- 50.Lima Z.P., Calvo T.R., Silva E.F., Pellizzon C.H., Vilegas W., Brito A.R.M.S., Bauab T.M., Hiruma-Lima C.A. Brazilian medicinal plant acts on prostaglandin level and Helicobacter pylori. J. Med. Food. 2008;11(4):701–708. doi: 10.1089/jmf.2007.0676. [DOI] [PubMed] [Google Scholar]
- 51.Lima Z.P., Bonamin F., Calvo T.R., Vilegas W., Santos L.C., Rozza A.L., Pellizzon C.H., Rocha L.R., Hiruma-Lima C.A. Effects of the ethyl acetate fraction of Alchornea triplinervia on healing gastric ulcer in rats. Pharmaceuticals. 2011;4(11):1423–1433. doi: 10.3390/ph4111423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Gara E.A., Hill D.J., Maslin D.J. Activities of garlic oil, garlic powder, and their diallyl constituents against Helicobacter pylori. Appl. Environ. Microbiol. 2000;66(5):2269–2273. doi: 10.1128/AEM.66.5.2269-2273.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gail M.H., Pfeiffer R.M., Brown L.M., Zhang L., Ma J.L., Pan K.F., Liu W.D., You W.C. Garlic, vitamin, and antibiotic treatment for Helicobacter pylori: a randomized factorial controlled trial. Helicobacter. 2007;12(5):575–578. doi: 10.1111/j.1523-5378.2007.00528.x. [DOI] [PubMed] [Google Scholar]
- 54.Gu L.K., Zhou P., Zhou J., Wang R.M., Yang W.J., Deng D.J. Effect of selenium-enriched garlic on chronic gastritis of the glandular stomach of Mongolian gerbils induced by H. pylori. Zhonghua Yu Fang Yi Xue Za Zhi. 2007;41:104–107. [PubMed] [Google Scholar]
- 55.Liu S., Sun Y., Li W., Yu H., Li X., Liu Z., Zeng J., Zhou Y., Chen C., Jia J. The antibacterial mode of action of allitridi for its potential use as a therapeutic agent against Helicobacter pylori infection. FEMS Microbiol. Lett. 2010;303(2):183–189. doi: 10.1111/j.1574-6968.2009.01877.x. [DOI] [PubMed] [Google Scholar]
- 56.Cellini L., Di Campli E., Masulli M., Di Bartolomeo S., Allocati N. Inhibition of Helicobacter pylori by garlic extract (Allium sativum) FEMS Immunol. Med. Microbiol. 1996;13(4):273–277. doi: 10.1111/j.1574-695X.1996.tb00251.x. [DOI] [PubMed] [Google Scholar]
- 57.Prabjone R., Thong-Ngam D., Wisedopas N., Chatsuwan T., Patumraj S. Anti-inflammatory effects of Aloe vera on leukocyte–endothelium interaction in the gastric microcirculation of Helicobacter pylori-infected rats. Clin. Hemorheol. Microcirc. 2006;35(3):359–366. [PubMed] [Google Scholar]
- 58.Hsieh S.C., Fang S.H., Rao Y.K., Tzeng Y.M. Inhibition of pro-inflammatory mediators and tumor cell proliferation by Anisomeles indica extracts. J. Ethnopharmacol. 2008;118(1):65–70. doi: 10.1016/j.jep.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 59.Rosas-Acevedo H., Terrazas T., González-Trujano M.E., Guzmán Y., Soto-Hernández M. Anti-ulcer activity of Cyrtocarpa procera analogous to that of Amphipterygium adstringens, both assayed on the experimental gastric injury in rats. J. Ethnopharmacol. 2011;134(1):67–73. doi: 10.1016/j.jep.2010.11.057. [DOI] [PubMed] [Google Scholar]
- 60.Cho C.H., Mei Q.B., Shang P., Lee S.S., So H.L., Guo X., Li Y. Study of the gastrointestinal protective effects of polysaccharides from Angelica sinensis in rats. Planta Med. 2000;66(04):348–351. doi: 10.1055/s-2000-8552. [DOI] [PubMed] [Google Scholar]
- 61.Castillo-Juárez I., González V., Jaime-Aguilar H., Martínez G., Linares E., Bye R., Romero I. Anti-Helicobacter pylori activity of plants used in Mexican traditional medicine for gastrointestinal disorders. J. Ethnopharmacol. 2009;122(2):402–405. doi: 10.1016/j.jep.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 62.Konstantinopoulou M., Karioti A., Skaltsas S., Skaltsa H. Sesquiterpene lactones from Anthemis altissima and their anti-Helicobacter pylori activity. J. Nat. Prod. 2003;66(5):699–702. doi: 10.1021/np020472m. [DOI] [PubMed] [Google Scholar]
- 63.Mafioleti L., da Silva Junior I.F., Colodel E.M., Flach A., de Oliveira Martins D.T. Evaluation of the toxicity and antimicrobial activity of hydroethanolic extract of Arrabidaea chica (Humb. & Bonpl.) B. Verl. J. Ethnopharmacol. 2013;150(2):576–582. doi: 10.1016/j.jep.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 64.Wang K.T., Chen L.G., Wu C.H., Chang C.C., Wang C.C. Gastroprotective activity of atractylenolide III from Atractylodes ovata on ethanol‐induced gastric ulcer in vitro and in vivo. J. Pharm. Pharmacol. 2010;62(3):381–388. doi: 10.1211/jpp.62.03.0014. [DOI] [PubMed] [Google Scholar]
- 65.Cogo L.L., Monteiro C.L.B., Miguel M.D., Miguel O.G., Cunico M.M., Ribeiro M.L., Camargo E.R.D., Kussen G.M.B., Nogueira K.D.S., Costa L.M.D. Anti-Helicobacter pylori activity of plant extracts traditionally used for the treatment of gastrointestinal disorders. Braz. J. Microbiol. 2010;41(2):304–309. doi: 10.1590/S1517-83822010000200007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abdelwahab S.I., Mohan S., Abdulla M.A., Sukari M.A., Abdul A.B., Taha M.M.E., Syam S., Ahmad S., Lee K.H. The methanolic extract of Boesenbergia rotunda (L.) Mansf. and its major compound pinostrobin induces anti-ulcerogenic property in vivo: possible involvement of indirect antioxidant action. J. Ethnopharmacol. 2011;137(2):963–970. doi: 10.1016/j.jep.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 67.Epifano F., Menghini L., Pagiotti R., Angelini P., Genovese S., Curini M. In vitro inhibitory activity of boropinic acid against Helicobacter pylori. Bioorg. Med. Chem. Lett. 2006;16(21):5523–5525. doi: 10.1016/j.bmcl.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 68.Galan M.V., Kishan A.A., Silverman A.L. Oral broccoli sprouts for the treatment of Helicobacter pylori infection: a preliminary report. Dig. Dis. Sci. 2004;49(7–8):1088–1090. doi: 10.1023/B:DDAS.0000037792.04787.8a. [DOI] [PubMed] [Google Scholar]
- 69.Banskota A.H., Tezuka Y., Adnyana I.K., Ishii E., Midorikawa K., Matsushige K., Kadota S. Hepatoprotective and anti-Helicobacter pylori activities of constituents from Brazilian propolis. Phytomedicine. 2001;8(1):16–23. doi: 10.1078/0944-7113-00004. [DOI] [PubMed] [Google Scholar]
- 70.Okeleye B.I., Bessong P.O., Ndip R.N. Preliminary phytochemical screening and in vitro anti-Helicobacter pylori activity of extracts of the stem bark of Bridelia micrantha (Hochst., Baill., Euphorbiaceae) Molecules. 2011;16(8):6193–6205. doi: 10.3390/molecules16086193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Adefuye A.O., Ndip R.N. Phytochemical analysis and antibacterial evaluation of the ethyl acetate extract of the stem bark of Bridelia micrantha. Pharmacogn. Mag. 2013;9(33):45. doi: 10.4103/0973-1296.108139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bonacorsi C., Da Fonseca L.M., Raddi M.S.G., Kitagawa R.R., Vilegas W. Comparison of Brazilian plants used to treat gastritis on the oxidative burst of Helicobacter pylori-stimulated neutrophil. Evid. Based Complement. Altern. Med. 2013;2013 doi: 10.4103/0973-1296.108139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lima Z.P., dos Santos R.D.C., Torres T.U., Sannomiya M., Rodrigues C.M., dos Santos L.C., Pellizzon C.H., Rocha L.R.M., Vilegas W., Brito A.R.M.S., Cardoso C.R.P. Byrsonima fagifolia: an integrative study to validate the gastroprotective, healing, antidiarrheal, antimicrobial and mutagenic action. J. Ethnopharmacol. 2008;120(2):149–160. doi: 10.1016/j.jep.2008.07.047. [DOI] [PubMed] [Google Scholar]
- 74.Santos R.C., Kushima H., Rodrigues C.M., Sannomiya M., Rocha L.R.M., Bauab T.M., Tamashiro J., Vilegas W., Hiruma-Lima C.A. Byrsonima intermedia A. Juss.: gastric and duodenal anti-ulcer, antimicrobial and antidiarrheal effects in experimental rodent models. J. Ethnopharmacol. 2012;140(2):203–212. doi: 10.1016/j.jep.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 75.Lemos L.M.S., Martins T.B., Tanajura G.H., Gazoni V.F., Bonaldo J., Strada C.L., da Silva M.G., Dall’Oglio E.L., de Sousa Júnior P.T., de Oliveira Martins D.T. Evaluation of antiulcer activity of chromanone fraction from Calophyllum brasiliesnse Camb. J. Ethnopharmacol. 2012;141(1):432–439. doi: 10.1016/j.jep.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 76.Takabayashi F., Harada N., Yamada M., Murohisa B., Oguni I. Inhibitory effect of green tea catechins in combination with sucralfate on Helicobacter pylori infection in Mongolian gerbils. J. Gastroenterol. 2004;39(1):61–63. doi: 10.1007/s00535-003-1246-0. [DOI] [PubMed] [Google Scholar]
- 77.Ruggiero P., Rossi G., Tombola F., Pancotto L., Lauretti L., Del Giudice G., Zoratti M. Red wine and green tea reduce H pylori-or VacA-induced gastritis in a mouse model. World J. Gastroenterol.: WJG. 2007;13(3):349. doi: 10.3748/wjg.v13.i3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nariman F., Eftekhar F., Habibi Z., Massarrat S., Malekzadeh R. Antibacterial activity of twenty Iranian plant extracts against clinical isolates of Helicobacter pylori. Iran. J. Basic Med. Sci. 2009;12(2):105–111. [Google Scholar]
- 79.Spósito L., Oda F.B., Vieira J.H., Carvalho F.A., dos Santos Ramos M.A., de Castro R.C., Crevelin E.J., Crotti A.E.M., Santos A.G., da Silva P.B., Chorilli M. In vitro and in vivo anti-Helicobacter pylori activity of Casearia sylvestris leaf derivatives. J. Ethnopharmacol. 2019;233:1–12. doi: 10.1016/j.jep.2018.12.032. [DOI] [PubMed] [Google Scholar]
- 80.Shikov A.N., Pozharitskaya O.N., Makarov V.G., Kvetnaya A.S. Antibacterial activity of Chamomilla recutita oil extract against Helicobacter pylori. Phytother. Res. 2008;22(2):252–253. doi: 10.1002/ptr.2243. [DOI] [PubMed] [Google Scholar]
- 81.Stamatis G., Kyriazopoulos P., Golegou S., Basayiannis A., Skaltsas S., Skaltsa H. In vitro anti-Helicobacter pylori activity of Greek herbal medicines. J. Ethnopharmacol. 2003;88(2–3):175–179. doi: 10.1016/S0378-8741(03)00217-4. [DOI] [PubMed] [Google Scholar]
- 82.Mabe K., Yamada M., Oguni I., Takahashi T. In vitro and in vivo activities of tea catechins against Helicobacter pylori. Antimicrob. Agents Chemother. 1999;43(7):1788–1791. doi: 10.1128/AAC.43.7.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hosseininejad Z., Moghadam S.D., Ebrahimi F., Abdollahi M., Zahedi M.J., Nazari M., Hayatbakhsh M., Adeli S., Sharififar F. In vitro screening of selected Iranian medicinal plants against Helicobacter pylori. Int. J. Green Pharm. (IJGP) 2011;5(4) doi: 10.22377/ijgp.v5i4.214. [DOI] [Google Scholar]
- 84.Ali S.M., Khan A.A., Ahmed I., Musaddiq M., Ahmed K.S., Polasa H., Rao L.V., Habibullah C.M., Sechi L.A., Ahmed N. Antimicrobial activities of Eugenol and Cinnamaldehyde against the human gastric pathogen Helicobacter pylori. Ann. Clin. Microbiol. Antimicrob. 2005;4(1):20. doi: 10.1186/1476-0711-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tabak M., Armon R., Neeman I. Cinnamon extracts’ inhibitory effect on Helicobacter pylori. J. Ethnopharmacol. 1999;67(3):269–277. doi: 10.1016/S0378-8741(99)00054-9. [DOI] [PubMed] [Google Scholar]
- 86.Nabati F., Mojab F., Habibi-Rezaei M., Bagherzadeh K., Amanlou M., Yousefi B. Large scale screening of commonly used Iranian traditional medicinal plants against urease activity. Daru J. Pharm. Sci. 2012;20(1):72. doi: 10.1186/2008-2231-20-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yeşilada E., Gürbüz I., Shibata H. Screening of Turkish anti-ulcerogenic folk remedies for anti-Helicobacter pylori activity. J. Ethnopharmacol. 1999;66(3):289–293. doi: 10.1016/S0378-8741(98)00219-0. [DOI] [PubMed] [Google Scholar]
- 88.Ustün O., Ozçelik B., Akyön Y., Abbasoglu U., Yesilada E. Flavonoids with anti-Helicobacter pylori activity from Cistus laurifolius leaves. J. Ethnopharmacol. 2006;108(3):457–461. doi: 10.1016/j.jep.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 89.Bonamin F., Moraes T.M., Dos Santos R.C., Kushima H., Faria F.M., Silva M.A., Junior I.V., Nogueira L., Bauab T.M., Brito A.R.S., da Rocha L.R. The effect of a minor constituent of essential oil from Citrus aurantium: the role of β-myrcene in preventing peptic ulcer disease. Chem. Biol. Interact. 2014;212:11–19. doi: 10.1016/j.cbi.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 90.Rozza A.L., de Mello Moraes T., Kushima H., Tanimoto A., Marques M.O.M., Bauab T.M., Hiruma-Lima C.A., Pellizzon C.H. Gastroprotective mechanisms of Citrus lemon (Rutaceae) essential oil and its majority compounds limonene and β-pinene: involvement of heat-shock protein-70, vasoactive intestinal peptide, glutathione, sulfhydryl compounds, nitric oxide and prostaglandin E2. Chem. Biol. Interact. 2011;189(1–2):82–89. doi: 10.1016/j.cbi.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 91.Poovendran P., Kalaigandhi V., Poongunran E. Antimicrobial activity of the leaves of Cocculus hirsutus against gastric ulcer producing Helicobacter pylori. J. Pharm. Res. 2011;4:4294–4295. doi: 10.1186/s12941-014-0054-0. [DOI] [Google Scholar]
- 92.Njume C., Jide A.A., Ndip R.N. Aqueous and organic solvent-extracts of selected South African medicinal plants possess antimicrobial activity against drug-resistant strains of Helicobacter pylori: inhibitory and bactericidal potential. Int. J. Mol. Sci. 2011;12(9):5652–5665. doi: 10.3390/ijms12095652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Suleiman M.M., Tauheed M., Babandi J.S., Umar R., Sulaiman M.H., Shittu M., Isa H.I. An in vivo experimental trial to determine the efficacy of stem-bark extract of Khaya senegalensis A. Juss (Meliaceae) for treating gastric ulcer in rat. Int. J. Med. Aromat. Plants. 2013;3(3):352–361. [Google Scholar]
- 94.Reyes‐Trejo B., Sánchez‐Mendoza M.E., Becerra‐García A.A., Cedillo‐Portugal E., Castillo‐Henkel C., Arrieta J. Bioassay‐guided isolation of an anti‐ulcer diterpenoid from Croton reflexifolius: role of nitric oxide, prostaglandins and sulfhydryls. J. Pharm. Pharmacol. 2008;60(7):931–936. doi: 10.1211/jpp.60.7.0016. [DOI] [PubMed] [Google Scholar]
- 95.Koga T., Kawada H., Utsui Y., Domon H., Ishii C., Yasuda H. In-vitro and in-vivo antibacterial activity of plaunotol, a cytoprotective antiulcer agent, against Helicobacter pylori. J. Antimicrob. Chemother. 1996;37(5):919–929. doi: 10.1093/jac/37.5.919. [DOI] [PubMed] [Google Scholar]
- 96.Nostro A., Cellini L., Bartolomeo S.D., Campli E.D., Grande R., Cannatelli M.A., Marzio L., Alonzo V. Antibacterial effect of plant extracts against Helicobacter pylori. Phytother. Res. 2005;19(3):198–202. doi: 10.1002/ptr.1640. [DOI] [PubMed] [Google Scholar]
- 97.Zaidi S.F.H., Yamada K., Kadowaki M., Usmanghani K., Sugiyama T. Bactericidal activity of medicinal plants, employed for the treatment of gastrointestinal ailments, against Helicobacter pylori. J. Ethnopharmacol. 2009;121(2):286–291. doi: 10.1016/j.jep.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 98.Ohno T., Kita M., Yamaoka Y., Imamura S., Yamamoto T., Mitsufuji S., Kodama T., Kashima K., Imanishi J. Antimicrobial activity of essential oils against Helicobacter pylori. Helicobacter. 2003;8(3):207–215. doi: 10.1046/j.1523-5378.2003.00146.x. [DOI] [PubMed] [Google Scholar]
- 99.Jaguezeski A.M., Perin G., Crecencio R.B., Baldissera M.D., Stefanil L.M., da Silva A.S. Addition of curcumin in dairy sheep diet in the control of subclinical mastitis. Acta Sci. Vet. 2018;46:7. [Google Scholar]
- 100.Escobedo-Hinojosa W.I., del Carpio J.D., Palacios-Espinosa J.F., Romero I. Contribution to the ethnopharmacological and anti-Helicobacter pylori knowledge of Cyrtocarpa procera Kunth (Anacardiaceae) J. Ethnopharmacol. 2012;143(1):363–371. doi: 10.1016/j.jep.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 101.Kushima H., Nishijima C.M., Rodrigues C.M., Rinaldo D., Sassá M.F., Bauab T.M., Di Stasi L.C., Carlos I.Z., Brito A.R.M.S., Vilegas W., Hiruma-Lima C.A. Davilla elliptica and Davilla nitida: gastroprotective, anti-inflammatory immunomodulatory and anti-Helicobacter pylori action. J. Ethnopharmacol. 2009;123(3):430–438. doi: 10.1016/j.jep.2009.03.031. [DOI] [PubMed] [Google Scholar]
- 102.Bergonzelli G.E., Donnicola D., Porta N., Corthesy-Theulaz I.E. Essential oils as components of a diet-based approach to management of Helicobacter infection. Antimicrob. Agents Chemother. 2003;47(10):3240–3246. doi: 10.1128/AAC.47.10.3240-3246.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Uyub A.M., Nwachukwu I.N., Azlan A.A., Fariza S.S. 2010. In-Vitro Antibacterial Activity and Cytotoxicity of Selected Medicinal Plant Extracts from Penang Island Malaysia on Metronidazole-Resistant-Helicobacter Pylori and Some Pathogenic Bacteria.http://hdl.handle.net/10125/21002 [Google Scholar]
- 104.Ramadan M.A., Safwat N.A. Antihelicobacter activity of a flavonoid compound isolated from Desmostachya bipinnata. Aust. J. Basic Appl. Sci. 2009;3(3):2270–2277. https://www.researchgate.net/publication/281526897 [Google Scholar]
- 105.Ibrahim N.H., Awaad A.S., Alnafisah R.A., Alqasoumi S.I., El-Meligy R.M., Mahmoud A.Z. In–vitro activity of Desmostachya bipinnata (L.) Stapf successive extracts against Helicobacter pylori clinical isolates. Saudi Pharm. J. 2018;26(4):535–540. doi: 10.1016/j.jsps.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Miguel G., Faleiro L., Cavaleiro C., Salgueiro L., Casanova J. Susceptibility of Helicobacter pylori to essential oil of Dittrichia viscosa subsp. revoluta. Phytother. Res. 2008;22(2):259–263. doi: 10.1002/ptr.2284. [DOI] [PubMed] [Google Scholar]
- 107.Adeniyi C.B.A., Lawal T.O., Mahady G.B. In vitro susceptibility of Helicobacter pylori to extracts of Eucalyptus camaldulensis and Eucalyptus torelliana. Pharm. Biol. 2009;47(1):99–102. doi: 10.1080/13880200802448708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li Y., Xu C., Zhang Q., Liu J.Y., Tan R.X. In vitro anti-Helicobacter pylori action of 30 Chinese herbal medicines used to treat ulcer diseases. J. Ethnopharmacol. 2005;98(3):329–333. doi: 10.1016/j.jep.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 109.Sánchez-Mendoza M.E., Reyes-Trejo B., Sánchez-Gómez P., Rodríguez-Silverio J., Castillo-Henkel C., Cervantes-Cuevas H., Arrieta J. Bioassay-guided isolation of an anti-ulcer chromene from Eupatorium aschenbornianum: role of nitric oxide, prostaglandins and sulfydryls. Fitoterapia. 2010;81(1):66–71. doi: 10.1016/j.fitote.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 110.Hamasaki N., Ishii E., Tominaga K., Tezuka Y., Nagaoka T., Kadota S., Kuroki T., Yano I. Highly selective antibacterial activity of novel alkyl quinolone alkaloids from a Chinese herbal medicine, Gosyuyu (Wu-Chu-Yu), against Helicobacter pylori in vitro. Microbiol. Immunol. 2000;44(1):9–15. doi: 10.1111/j.1348-0421.2000.tb01240.x. https://www.jstage.jst.go.jp/article/mandi1977/44/1/44_1_9/_article/-char/ja/ [DOI] [PubMed] [Google Scholar]
- 111.Basile A., Conte B., Rigano D., Senatore F., Sorbo S. Antibacterial and antifungal properties of acetonic extract of Feijoa sellowiana fruits and its effect on Helicobacter pylori growth. J. Med. Food. 2010;13(1):189–195. doi: 10.1089/jmf.2008.0301. [DOI] [PubMed] [Google Scholar]
- 112.Basile A., Sorbo S., Spadaro V., Bruno M., Maggio A., Faraone N., Rosselli S. Antimicrobial and antioxidant activities of coumarins from the roots of Ferulago campestris (Apiaceae) Molecules. 2009;14(3):939–952. doi: 10.3390/molecules14030939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rosselli S., Maggio A.M., Faraone N., Spadaro V., Morris-Natschke S.L., Bastow K.F., Lee K.H., Bruno M. The cytotoxic properties of natural coumarins isolated from roots of Ferulago campestris (Apiaceae) and of synthetic ester derivatives of aegelinol. Nat. Prod. Commun. 2009;4(12) doi: 10.1177/1934578X0900401219. p.1934578X0900401219. [DOI] [PubMed] [Google Scholar]
- 114.Jadhav S.G., Meshram R.J., Gond D.S., Gacche R.N. Inhibition of growth of Helicobacter pylori and its urease by coumarin derivatives: molecular docking analysis. J. Pharm. Res. 2013;7(8):705–711. doi: 10.1016/j.jopr.2013.09.002. [DOI] [Google Scholar]
- 115.Kawase M., Tanaka T., Sohara Y., Tani S., Sakagami H., Hauer H., Chatterjee S.S. Structural requirements of hydroxylated coumarins for in vitro anti-Helicobacter pylori activity. In Vivo. 2003;17(5):509–512. [PubMed] [Google Scholar]
- 116.Niero R., Dal Molin M.M., Silva S., Damian N.S., Maia L.O., Delle Monache F., Cechinel Filho V., de Andrade S.F. Gastroprotective effects of extracts and guttiferone A isolated from Garcinia achachairu Rusby (Clusiaceae) against experimentally induced gastric lesions in mice. Naunyn Schmiedebergs Arch. Pharmacol. 2012;385(11):1103–1109. doi: 10.1007/s00210-012-0788-1. [DOI] [PubMed] [Google Scholar]
- 117.Zhang X.Q., Gu H.M., Li X.Z., Xu Z.N., Chen Y.S., Li Y. Anti-Helicobacter pylori compounds from the ethanol extracts of Geranium wilfordii. J. Ethnopharmacol. 2013;147(1):204–207. doi: 10.1016/j.jep.2013.02.032. [DOI] [PubMed] [Google Scholar]
- 118.Shahani S., Monsef-Esfahani H.R., Saeidnia S., Saniee P., Siavoshi F., Foroumadi A., Samadi N., Gohari A.R. Anti-Helicobacter pylori activity of the methanolic extract of Geum iranicum and its main compounds. Z. Naturforschung C. 2012;67(3–4):172–180. doi: 10.1515/znc-2012-3-409. [DOI] [PubMed] [Google Scholar]
- 119.Fukai T., Marumo A., Kaitou K., Kanda T., Terada S., Nomura T. Anti-Helicobacter pylori flavonoids from licorice extract. Life Sci. 2002;71(12):1449–1463. doi: 10.1016/s0024-3205(02)01864-7. [DOI] [PubMed] [Google Scholar]
- 120.Aly A.M., Al-Alousi L., Salem H.A. Licorice: a possible anti-inflammatory and anti-ulcer drug. AAPS PharmSciTech. 2005;6(1):E74–E82. doi: 10.1016/S0024-3205(02)01864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.de Mello Moraes T., Rodrigues C.M., Kushima H., Bauab T.M., Villegas W., Pellizzon C.H., Brito A.R.M.S., Hiruma-Lima C.A. Hancornia speciosa: indications of gastroprotective, healing and anti-Helicobacter pylori actions. J. Ethnopharmacol. 2008;120(2):161–168. doi: 10.1016/j.jep.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 122.Shang X., Tan Q., Liu R., Yu K., Li P., Zhao G.P. In vitro anti-Helicobacter pylori effects of medicinal mushroom extracts, with special emphasis on the Lion’s Mane mushroom, Hericium erinaceus (higher Basidiomycetes) Int. J. Med. Mushrooms. 2013;15(2):165–174. doi: 10.1615/IntJMedMushr.v15.i2.50. [DOI] [PubMed] [Google Scholar]
- 123.Mahady G.B., Pendland S.L., Stoia A., Chadwick L.R. In vitro susceptibility of Helicobacter pylori to isoquinoline alkaloids from Sanguinaria canadensis and Hydrastis canadensis. Phytother. Res. 2003;17(3):217–221. doi: 10.1002/ptr.1108. [DOI] [PubMed] [Google Scholar]
- 124.Mohtar M., Johari S.A., Li A.R., Isa M.M., Mustafa S., Ali A.M., Basri D.F. Inhibitory and resistance-modifying potential of plant-based alkaloids against methicillin-resistant Staphylococcus aureus (MRSA) Curr. Microbiol. 2009;59(2):181–186. doi: 10.1007/s00284-009-9416-9. [DOI] [PubMed] [Google Scholar]
- 125.Markham P.N., Westhaus E., Klyachko K., Johnson M.E., Neyfakh A.A. Multiple novel inhibitors of the NorA multidrug transporter of Staphylococcus aureus. Antimicrob. Agents Chemother. 1999;43(10):2404–2408. doi: 10.1128/AAC.43.10.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rao K.N., Venkatachalam S.R. Inhibition of dihydrofolate reductase and cell growth activity by the phenanthroindolizidine alkaloids pergularinine and tylophorinidine: the in vitro cytotoxicity of these plant alkaloids and their potential as antimicrobial and anticancer agents. Toxicol. Vitro. 2000;14(1):53–59. doi: 10.1016/S0887-2333(99)00092-2. [DOI] [PubMed] [Google Scholar]
- 127.Vera-Arzave C., Antonio L.C., Arrieta J., Cruz-Hernández G., Velázquez-Méndez A.M., Reyes-Ramírez A., Sánchez-Mendoza M.E. Gastroprotection of suaveolol, isolated from Hyptis suaveolens, against ethanol-induced gastric lesions in Wistar rats: role of prostaglandins, nitric oxide and sulfhydryls. Molecules. 2012;17(8):8917–8927. doi: 10.3390/molecules17088917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lu M.C., Chiu H.F., Lin C.P., Shen Y.C., Venkatakrishnan K., Wang C.K. Anti-Helicobacter pylori effect of various extracts of ixeris chinensis on inflammatory markers in human gastric epithelial AGS cells. J. Herb. Med. 2018;11:60–70. doi: 10.1016/j.hermed.2017.08.002. [DOI] [Google Scholar]
- 129.Pertino M., Schmeda-Hirschmann G., Rodríguez J.A., Theoduloz C. Gastroprotective effect and cytotoxicity of terpenes from the Paraguayan crude drug “yagua rova” (Jatropha isabelli) J. Ethnopharmacol. 2007;111(3):553–559. doi: 10.1016/j.jep.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 130.Hajimahmoodi M., Shams-Ardakani M., Saniee P., Siavoshi F., Mehrabani M., Hosseinzadeh H., Foroumadi P., Safavi M., Khanavi M., Akbarzadeh T., Shafiee A. In vitro antibacterial activity of some Iranian medicinal plant extracts against Helicobacter pylori. Nat. Prod. Res. 2011;25(11):1059–1066. doi: 10.1080/14786419.2010.501763. [DOI] [PubMed] [Google Scholar]
- 131.Stege P.W., Davicino R.C., Vega A.E., Casali Y.A., Correa S., Micalizzi B. Antimicrobial activity of aqueous extracts of Larrea divaricata Cav (jarilla) against Helicobacter pylori. Phytomedicine. 2006;13(9–10):724–727. doi: 10.1016/j.phymed.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 132.Bae E.A., Han M.J., Kim N.J., Kim D.H. Anti-Helicobacter pylori activity of hearbal medicines. Biol. Pharm. Bull. 1998;21(9):990–992. doi: 10.1248/bpb.21.990. [DOI] [PubMed] [Google Scholar]
- 133.Neelima N., Sudhakar M., Patil M.B., Lakshmi B.V.S. Anti-ulcer activity and HPTLC analysis of Mangifera indica L. leaves. Int. J. Pharm. Phytopharm. Res. 2012;1(4):146–155. [Google Scholar]
- 134.Carvalho A.C.S., Guedes M.M., de Souza A.L., Trevisan M.T., Lima A.F., Santos F.A., Rao V.S. Gastroprotective effect of mangiferin, a xanthonoid from Mangifera indica, against gastric injury induced by ethanol and indomethacin in rodents. Planta Med. 2007;73(13):1372–1376. doi: 10.1055/s-2007-990231. [DOI] [PubMed] [Google Scholar]
- 135.Abdulla M.A., Ali H.M., Ahmed K.A.A., Noor S.M., Ismail S. 2009. Evaluation of the Anti-Ulcer Activities of Morus alba Extracts in Experimen-Tally-Induced Gastric Ulcer in Rats. [Google Scholar]
- 136.Ahmad A., Gupta G., Afzal M., Kazmi I., Anwar F. Antiulcer and antioxidant activities of a new steroid from Morus alba. Life Sci. 2013;92(3):202–210. doi: 10.1016/j.lfs.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 137.Sidahmed H.M.A., Azizan A.H.S., Mohan S., Abdulla M.A., Abdelwahab S.I., Taha M.M.E., Hadi A.H.A., Ketuly K.A., Hashim N.M., Loke M.F., Vadivelu J. Gastroprotective effect of desmosdumotin C isolated from Mitrella kentii against ethanol-induced gastric mucosal hemorrhage in rats: possible involvement of glutathione, heat-shock protein-70, sulfhydryl compounds, nitric oxide, and anti-Helicobacter pylori activity. BMC Complement. Altern. Med. 2013;13(1):183. doi: 10.1186/1472-6882-13-183. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 138.Lewis D.A., Shaw G.P. A natural flavonoid and synthetic analogues protect the gastric mucosa from aspirin-induced erosions. J. Nutr. Biochem. 2001;12(2):95–100. doi: 10.1016/S0955-2863(00)00133-9. [DOI] [PubMed] [Google Scholar]
- 139.Jain D.L., Baheti A.M., Parakh S.R., Ingale S.P., Ingale P.L. PHCOG MAG.: research article study of antacid and diuretic activity of ash and extracts of Musa sapientum L. fruit peel. Phcog. Mag. 2007;3(10):116. [Google Scholar]
- 140.Bhamarapravati S., Pendland S.L., Mahady G.B. Extracts of spice and food plants from Thai traditional medicine inhibit the growth of the human carcinogen Helicobacter pylori. In Vivo (Athens, Greece) 2003;17(6):541–544. https://europepmc.org/article/med/14758718 [PubMed] [Google Scholar]
- 141.Ohsaki A., Takashima J., Chiba N., Kawamura M. Microanalysis of a selective potent anti-Helicobacter pylori compound in a Brazilian medicinal plant, Myroxylon peruiferum and the activity of analogues. Bioorg. Med. Chem. Lett. 1999;9(8):1109–1112. doi: 10.1016/S0960-894X(99)00141-9. [DOI] [PubMed] [Google Scholar]
- 142.Deriu A., Branca G., Molicotti P., Pintore G., Chessa M., Tirillini B., Paglietti B., Mura A., Sechi L.A., Fadda G., Zanetti S. In vitro activity of essential oil of Myrtus communis L. against Helicobacter pylori. Int. J. Antimicrob. Agents. 2007;30(6):562. doi: 10.1016/j.ijantimicag.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 143.Sudjana A.N., D’Orazio C., Ryan V., Rasool N., Ng J., Islam N., Riley T.V., Hammer K.A. Antimicrobial activity of commercial Olea europaea (olive) leaf extract. Int. J. Antimicrob. Agents. 2009;33(5):461–463. doi: 10.1016/j.ijantimicag.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 144.Singh S., Majumdar D.K. Evaluation of the gastric antiulcer activity of fixed oil of Ocimum sanctum (Holy Basil) J. Ethnopharmacol. 1999;65(1):13–19. doi: 10.1016/S0378-8741(98)00142-1. [DOI] [PubMed] [Google Scholar]
- 145.Sumbul S., Ahmad M.A., Mohd A., Mohd A. Role of phenolic compounds in peptic ulcer: an overview. J. Pharm. Bioallied Sci. 2011;3(3):361. doi: 10.4103/0975-7406.84437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ares J.J., Outt P.E., Randall J.L., Johnston J.N., Murray P.D., O’Brien L.M., Weisshaar P.S., Ems B.L. Synthesis and biological evaluation of flavonoids and related compounds as gastroprotective agents. Bioorg. Med. Chem. Lett. 1996;6(8):995–998. doi: 10.1016/0960-894X(96)00134-5. [DOI] [Google Scholar]
- 147.Afroz S., Yagi A., Fujikawa K., Rahman M.M., Morito K., Fukuta T., Watanabe S., Kiyokage E., Toida K., Shimizu T., Ishida T. Lysophosphatidic acid in medicinal herbs enhances prostaglandin E2 and protects against indomethacin-induced gastric cell damage in vivo and in vitro. Prostaglandins Other Lipid Mediat. 2018;135:36–44. doi: 10.1016/j.prostaglandins.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 148.Sun X.B., Matsumolo T., Yamada H. Purification of an anti-ulcer polysaccharide from the leaves of Panax ginseng. Planta Med. 1992;58(05):445–448. doi: 10.1055/s-2006-961510. [DOI] [PubMed] [Google Scholar]
- 149.Yamada H. Pectic polysaccharides from Chinese herbs: structure and biological activity. Carbohydr. Polym. 1994;25(4):269–276. doi: 10.1016/0144-8617(94)90052-3. [DOI] [Google Scholar]
- 150.Cowan M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999;12(4):564–582. doi: 10.1128/CMR.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rojas-Martínez R., Arrieta J., Cruz-Antonio L., Arrieta-Baez D., Velázquez-Méndez A., Sánchez-Mendoza M. Dillapiole, isolated from Peperomia pellucida, shows gastroprotector activity against ethanol-induced gastric lesions in Wistar rats. Molecules. 2013;18(9):11327–11337. doi: 10.3390/molecules180911327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ranilla L.G., Apostolidis E., Shetty K. Antimicrobial activity of an Amazon medicinal plant (Chancapiedra) (Phyllanthus niruri L.) against Helicobacter pylori and lactic acid bacteria. Phytother. Res. 2012;26(6):791–799. doi: 10.1002/ptr.3646. [DOI] [PubMed] [Google Scholar]
- 153.Wang Y., Wang S.L., Zhang J.Y., Song X.N., Zhang Z.Y., Li J.F., Li S. Anti-ulcer and anti-Helicobacter pylori potentials of the ethyl acetate fraction of Physalis alkekengi L. var. franchetii (Solanaceae) in rodent. J. Ethnopharmacol. 2018;211:197–206. doi: 10.1016/j.jep.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 154.Quílez A., Berenguer B., Gilardoni G., Souccar C., De Mendonça S., Oliveira L.F.S., Martín-Calero M.J., Vidari G. Anti-secretory, anti-inflammatory and anti-Helicobacter pylori activities of several fractions isolated from Piper carpunya Ruiz & Pav. J. Ethnopharmacol. 2010;128(3):583–589. doi: 10.1016/j.jep.2010.01.060. [DOI] [PubMed] [Google Scholar]
- 155.Rüegg T., Calderón A.I., Queiroz E.F., Solis P.N., Marston A., Rivas F., Ortega-Barría E., Hostettmann K., Gupta M.P. 3-Farnesyl-2-hydroxybenzoic acid is a new anti-Helicobacter pylori compound from Piper multiplinervium. J. Ethnopharmacol. 2006;103(3):461–467. doi: 10.1016/j.jep.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 156.Al-Said M.S., Ageel A.M., Parmar N.S., Tariq M. Evaluation of mastic, a crude drug obtained from Pistacia lentiscus for gastric and duodenal anti-ulcer activity. J. Ethnopharmacol. 1986;15(3):271–278. doi: 10.1016/0378-8741(86)90165-0. [DOI] [PubMed] [Google Scholar]
- 157.Marone P., Bono L., Leone E., Bona S., Carretto E., Perversi L. Bactericidal activity of Pistacia lentiscus mastic gum against Helicobacter pylori. J. Chemother. 2001;13(6):611–614. doi: 10.1179/joc.2001.13.6.611. [DOI] [PubMed] [Google Scholar]
- 158.Dabos K.J., Sfika E., Vlatta L.J., Giannikopoulos G. The effect of mastic gum on Helicobacter pylori: a randomized pilot study. Phytomedicine. 2010;17(3–4):296–299. doi: 10.1016/j.phymed.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 159.Paraschos S., Magiatis P., Mitakou S., Petraki K., Kalliaropoulos A., Maragkoudakis P., Mentis A., Sgouras D., Skaltsounis A.L. In vitro and in vivo activities of Chios mastic gum extracts and constituents against Helicobacter pylori. Antimicrob. Agents Chemother. 2007;51(2):551–559. doi: 10.1128/AAC.00642-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.de Araújo Rodrigues P., de Morais S.M., de Souza C.M., Silva A.R.A., de Andrade G.M., Silva M.G.V., Albuquerque R.L., Rao V.S., Santos F.A. Gastroprotective effect of barbatusin and 3-beta-hydroxy-3-deoxibarbatusin, quinonoid diterpenes isolated from Plectranthus grandis, in ethanol-induced gastric lesions in mice. J. Ethnopharmacol. 2010;127(3):725–730. doi: 10.1016/j.jep.2009.11.031. [DOI] [PubMed] [Google Scholar]
- 161.Wang Y.C., Huang T.L. High-performance liquid chromatography for quantification of plumbagin, an anti-Helicobacter pylori compound of Plumbago zeylanica L. J. Chromatogr. A. 2005;1094(1–2):99–104. doi: 10.1016/j.chroma.2005.07.092. [DOI] [PubMed] [Google Scholar]
- 162.Klein L.C., Gandolfi R.B., Santin J.R., Lemos M., Cechinel Filho V., de Andrade S.F. Antiulcerogenic activity of extract, fractions, and some compounds obtained from Polygala cyparissias St. Hillaire & Moquin (Polygalaceae) Naunyn Schmiedeberg’s Arch. Pharmacol. 2010;381(2):121–126. doi: 10.1007/s00210-009-0485-x. [DOI] [PubMed] [Google Scholar]
- 163.Hashimoto T., Aga H., Chaen H., Fukuda S., Kurimoto M. Isolation and identification of anti-Helicobacter pylori compounds from Polygonum tinctorium Lour. Nat. Med.= 生薬學雜誌. 1999;53(1):27–31. [Google Scholar]
- 164.Tomczyk M., Leszczyńska K., Jakoniuk P. Antimicrobial activity of Potentilla species. Fitoterapia. 2008;79(7–8):592–594. doi: 10.1016/j.fitote.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 165.Bisignano C., Filocamo A., La Camera E., Zummo S., Fera M.T., Mandalari G. Antibacterial activities of almond skins on cagA-positive and-negative clinical isolates of Helicobacter pylori. BMC Microbiol. 2013;13(1):103. doi: 10.1186/1471-2180-13-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yin S., Fan C.Q., Dong L., Yue J.M. Psoracorylifols A–E, five novel compounds with activity against Helicobacter pylori from seeds of Psoralea corylifolia. Tetrahedron. 2006;62(11):2569–2575. doi: 10.1016/j.tet.2005.12.041. [DOI] [Google Scholar]
- 167.De Leo M., De Tommasi N., Sanogo R., D’Angelo V., Germanò M.P., Bisignano G., Braca A. Triterpenoid saponins from Pteleopsis suberosa stem bark. Phytochemistry. 2006;67(24):2623–2629. doi: 10.1016/j.phytochem.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 168.Voravuthikunchai S.P., Limsuwan S., Mitchell H. Effects of Punica granatum pericarps and Quercus infectoria nutgalls on cell surface hydrophobicity and cell survival of Helicobacter pylori. J. Health Sci. 2006;52(2):154–159. doi: 10.1248/jhs.52.154. [DOI] [Google Scholar]
- 169.Moghaddam M.N. In vitro inhibition of Helicobacter pylori by some spices and medicinal plants used in Iran. Glob. J. Pharmacol. 2011;5(3):176–180. [Google Scholar]
- 170.Mazzolin L.P., Nasser A.L.M., Moraes T.M., Santos R.C., Nishijima C.M., Santos F.V., Varanda E.A., Bauab T.M., da Rocha L.R.M., Di Stasi L.C., Vilegas W. Qualea parviflora Mart.: an integrative study to validate the gastroprotective, antidiarrheal, antihemorragic and mutagenic action. J. Ethnopharmacol. 2010;127(2):508–514. doi: 10.1016/j.jep.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 171.Kadota S., Basnet P., Ishii E., Tamura T., Namba T. Antibacterial activity of trichorabdal A from Rabdosia trichocarpa against Helicobacter pylori. Zent. Bakteriol. 1997;286(1):63–67. doi: 10.1016/S0934-8840(97)80076-X. [DOI] [PubMed] [Google Scholar]
- 172.Chung J.G., Tsou M.F., Wang H.H., Lo H.H., Hsieh S.E., Yen Y.S., Wu L.T., Chang S.H., Ho C.C., Hung C.F. Rhein affects arylamine N‐acetyltransferase activity in Helicobacter pylori from peptic ulcer patients. J. Appl.Toxicol. 1998;18(March (2)):117–123. doi: 10.1002/(SICI)1099-1263(199805/06)18:3<179::AID-JAT494>3.0.CO;2-W. Chichester: John Wiley & Sons, Ltd. [DOI] [PubMed] [Google Scholar]
- 173.Berté P.E., da Silva Lopes J., Comandulli N.G., Rangel D.W., Delle Monache F., Cechinel Filho V., Niero R., de Andrade S.F. Evaluation of the gastroprotective activity of the extracts, fractions, and pure compounds obtained from aerial parts of Rubus imperialis in different experimental models. Naunyn Schmiedebergs Arch. Pharmacol. 2014;387(4):313–319. doi: 10.1007/s00210-013-0954-0. [DOI] [PubMed] [Google Scholar]
- 174.Atapour M., Zahedi M.J., Mehrabani M., Safavi M., Keyvanfard V., Foroughi A., Siavoshi F., Foroumadi A. In vitro susceptibility of the Gram-negative bacterium Helicobacter pylori to extracts of Iranian medicinal plants. Pharm. Biol. 2009;47(1):77–80. doi: 10.1080/13880200802434401. [DOI] [Google Scholar]
- 175.Gharashi A. vol. 295. Tehran University of Medical Sciences; 2008. (Al-mogez-dar-tebb. Tehran: Translated by Tafghad Khabbaz R). [Google Scholar]
- 176.Ochi T., Shibata H., Higuti T., Kodama K.H., Kusumi T., Takaishi Y. Anti-Helicobacter pylori compounds from Santalum album. J. Nat. Prod. 2005;68(6):819–824. doi: 10.1021/np040188q. [DOI] [PubMed] [Google Scholar]
- 177.La Casa C., Villegas I., De La Lastra C.A., Motilva V., Calero M.M. Evidence for protective and antioxidant properties of rutin, a natural flavone, against ethanol induced gastric lesions. J. Ethnopharmacol. 2000;71(1–2):45–53. doi: 10.1016/S0378-8741(99)00174-9. [DOI] [PubMed] [Google Scholar]
- 178.Njume C., Afolayan A.J., Green E., Ndip R.N. Volatile compounds in the stem bark of Sclerocarya birrea (Anacardiaceae) possess antimicrobial activity against drug-resistant strains of Helicobacter pylori. Int. J. Antimicrob. Agents. 2011;38(4):319–324. doi: 10.1016/j.ijantimicag.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 179.Júnior G.M.V., da Rocha C.Q., de Souza Rodrigues T., Hiruma-Lima C.A., Vilegas W. New steroidal saponins and antiulcer activity from Solanum paniculatum L. Food Chem. 2015;186:160–167. doi: 10.1016/j.foodchem.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 180.Areche C., Schmeda‐Hirschmann G., Theoduloz C., Rodríguez J.A. Gastroprotective effect and cytotoxicity of abietane diterpenes from the Chilean Lamiaceae sphacele chamaedryoides (Balbis) Briq. J. Pharm. Pharmacol. 2009;61(12):1689–1697. doi: 10.1211/jpp.61.12.0015. [DOI] [PubMed] [Google Scholar]
- 181.Khanavi M., Safavi M., Siavoi F., Fallah Tafti A., Hajimahmoodi M., Hadjiakhoondi A., Rezazadeh S., Foroumadi A. Evaluation of anti-Helicobacter pylori activity of methanol extracts of some species of Stachys and Melia. J. Med. Plants. 2008;4(28):74–80. [Google Scholar]
- 182.Bonamin F., Moraes T.M., Kushima H., Silva M.A., Rozza A.L., Pellizzon C.H., Bauab T.M., Rocha L.R.M., Vilegas W., Hiruma-Lima C.A. Can a Strychnos species be used as antiulcer agent? Ulcer healing action from alkaloid fraction of Strychnos pseudoquina St. Hil.(Loganiaceae) J. Ethnopharmacol. 2011;138(1):47–52. doi: 10.1016/j.jep.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 183.Magaji R.A., Okasha M.A.M., Abubakar M.S., Fatihu M.Y. Anti-ulcerogenic and anti-secretory activity of the n-butanol portion of Syzygiumaromaticum in rat. Nig. J. Pharm. Sci. 2007;6:119–126. [Google Scholar]
- 184.Babu T.H., Manjulatha K., Kumar G.S., Hymavathi A., Tiwari A.K., Purohit M., Rao J.M., Babu K.S. Gastroprotective flavonoid constituents from Oroxylum indicum Vent. Bioorg. Med. Chem. Lett. 2010;20(1):117–120. doi: 10.1016/j.bmcl.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 185.Park B.S., Lee H.K., Lee S.E., Piao X.L., Takeoka G.R., Wong R.Y., Ahn Y.J., Kim J.H. Antibacterial activity of Tabebuia impetiginosa Martius ex DC (Taheebo) against Helicobacter pylori. J. Ethnopharmacol. 2006;105(1–2):255–262. doi: 10.1016/j.jep.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 186.Chatterjee A., Khatua S., Chatterjee S., Mukherjee S., Mukherjee A., Paloi S., Acharya K., Bandyopadhyay S.K. Polysaccharide-rich fraction of Termitomyces eurhizus accelerate healing of indomethacin induced gastric ulcer in mice. Glycoconj. J. 2013;30(8):759–768. doi: 10.1007/s10719-013-9479-5. [DOI] [PubMed] [Google Scholar]
- 187.Fabry W., Okemo P., Ansorg R. Activity of East African medicinal plants against Helicobacter pylori. Chemotherapy. 1996;42(5):315–317. doi: 10.1159/000239460. [DOI] [PubMed] [Google Scholar]
- 188.Malekzadeh F., Ehsanifar H., Shahamat M., Levin M., Colwell R.R. Antibacterial activity of black myrobalan (Terminalia chebula Retz) against Helicobacter pylori. Int. J. Antimicrob. Agents. 2001;18(1):85–88. doi: 10.1016/S0924-8579(01)00352-1. [DOI] [PubMed] [Google Scholar]
- 189.Mishra V., Agrawal M., Onasanwo S.A., Madhur G., Rastogi P., Pandey H.P., Palit G., Narender T. Anti-secretory and cyto-protective effects of chebulinic acid isolated from the fruits of Terminalia chebula on gastric ulcers. Phytomedicine. 2013;20(6):506–511. doi: 10.1016/j.phymed.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 190.Bag A., Bhattacharyya S.K., Chattopadhyay R.R. The development of Terminalia chebula Retz. (Combretaceae) in clinical research. Asian Pac. J. Trop. Biomed. 2013;3(3):244–252. doi: 10.1016/S2221-1691(13)60059-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Esmaeili D., Mobarez A.M., Tohidpour A. Anti-Helicobacter pylori activities of shoya powder and essential oils of thymus vulgaris and eucalyptus globulus. Open Microbiol. J. 2012;6:65. doi: 10.2174/1874285801206010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sánchez-Mendoza M.E., Reyes-Ramírez A., Cruz Antonio L., Martínez Jiménez L., Rodríguez-Silverio J., Arrieta J. Bioassay-guided isolation of an anti-ulcer compound, tagitinin C, from Tithonia diversifolia: role of nitric oxide, prostaglandins and sulfhydryls. Molecules. 2011;16(1):665–674. doi: 10.3390/molecules16010665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Nariman F., Eftekhar F., Habibi Z., Falsafi T. Anti-Helicobacter pylori activities of six Iranian plants. Helicobacter. 2004;9(2):146–151. doi: 10.1111/j.1083-4389.2004.00211.x. [DOI] [PubMed] [Google Scholar]
- 194.Burger O., Ofek I., Tabak M., Weiss E.I., Sharon N., Neeman I. A high molecular mass constituent of cranberry juice inhibits Helicobacter pylori adhesion to human gastric mucus. FEMS Immunol. Med. Microbiol. 2000;29(4):295–301. doi: 10.1111/j.1574-695X.2000.tb01537.x. [DOI] [PubMed] [Google Scholar]
- 195.Shmuely H., Burger O., Neeman I., Yahav J., Samra Z., Niv Y., Sharon N., Weiss E., Athamna A., Tabak M., Ofek I. Susceptibility of Helicobacter pylori isolates to the antiadhesion activity of a high-molecular-weight constituent of cranberry. Diagn. Microbiol. Infect. Dis. 2004;50(4):231–235. doi: 10.1016/j.diagmicrobio.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 196.Ghannadi A., Sajjadi S.E., Abedi D., Yousefi J., Daraei-Ardekani R. The in vitro activity of seven Iranian plants of the Lamiaceae family against Helicobacter pylori. Niger. J. Nat. Prod. Med. 2004;8(1):40–42. doi: 10.4314/njnpm.v8i1.11812. [DOI] [Google Scholar]
- 197.Backon J. Ginger: inhibition of thromboxane synthetase and stimulation of prostacyclin: relevance for medicine and psychiatry. Med. Hypotheses. 1986;20(3):271–278. doi: 10.1016/0306-9877(86)90043-5. [DOI] [PubMed] [Google Scholar]
- 198.Johji Y., Michihiko M., Rong H.Q., Hisashi M., Hajime F. The anti-ulcer effect in rats of ginger constituents. J. Ethnopharmacol. 1988;23(2–3):299–304. doi: 10.1016/0378-8741(88)90009-8. [DOI] [PubMed] [Google Scholar]
- 199.Al-Yahya M.A., Rafatullah S., Mossa J.S., Ageel A.M., Parmar N.S., Tariq M. Gastroprotective activity of ginger zingiber officinale rosc., in albino rats. Am. J. Chin. Med. 1989;17(01n02):51–56. doi: 10.1142/S0192415X89000097. [DOI] [PubMed] [Google Scholar]
- 200.Yoshikawa M., Hatakeyama S., Taniguchi K., Matuda H., Yamahara J. 6-gingesulfonic acid, a new anti-ulcer principle, and gingerglycolipids A, B and C, three new monoacyldigalactosylglycerols, from Zingiberis rhizoma originating in Taiwan. Chem. Pharm. Bull. 1992;40(8):2239–2241. doi: 10.1248/cpb.40.2239. [DOI] [PubMed] [Google Scholar]
- 201.Yoshikawa M., Yamaguchi S., Kunimi K., Matsuda H., Okuno Y., Yamahara J., Murakami N. Stomachic principles in ginger. III. An anti-ulcer principle, 6-gingesulfonic acid, and three monoacyldigalactosylglycerols, gingerglycolipids A, B, and C, from Zingiberis Rhizoma originating in Taiwan. Chem. Pharm. Bull. 1994;42(6):1226–1230. doi: 10.1248/cpb.42.1226. [DOI] [PubMed] [Google Scholar]
- 202.Banerjee S., Mullick H.I., Banerjee J., Ghosh A. Zingiber officinale: ‘a natural gold’. Int. J. Pharm. Biol.-Sci. 2011;2:283–294. [Google Scholar]