Abstract
Spondyloarthropathies are a group of chronic inflammatory disorders that involve the joints of the axial skeleton, peripheral joints and have extra-articular manifestations. Treatment includes inhibitors of tumor necrosis factor α. Currently there are five approved inhibitors: a soluble receptor, Etanercept and four monoclonal.
Etanercept has very low toxicity with pulmonary adverse reactions being very rare. We present the case of a patient who developed respiratory symptoms and pulmonary infiltrates of rapid evolution after the third dose of treatment with Etanercept.
Keywords: Etanercept, Pulmonary granulomatosis, TNF-α, Anti-TNF, Spondyloarthropathy, Case report
1. Background
Spondyloarthropathies are a group of chronic inflammatory disorders that share clinical, pathological, genetic, radiological and epidemiological characteristics as well as response to treatment. This condition affects the joints of the axial skeleton and the peripheral joints having. Moreover, it produces extra-articular manifestations causing limiting symptoms and significantly affecting the quality of life of patients. These disorders have been associated with the major histocompatibility complex class I HLA-B27 and the prototypical disorder in this group is the ankylosing spondylitis. Other spondyloarthropathies include Reiter's syndrome or reactive arthritis, psoriatic arthritis, arthropathy of inflammatory bowel disease, and undifferentiated spondyloarthropathies [1]. It is estimated that the prevalence of spodyloartropathies is of 1–1.9% in the general population worldwide [2].
Disease-modifying antirheumatic drugs (DMARDs), including Sulfasalazine (SSZ), Methotrexate (MTX) and Leflunomide (LFN) may be beneficial for peripheral arthritis, but are not effective in treating diseases of the axial skeleton or enthesitis [3]. The guidelines from the American College of Rheumatology 2015 consistently recommend treatment with inhibitors of tumor necrosis factor α in adult patients with active spondyloarthropathy refractory to treatment with nonsteroidal anti-inflammatory drugs [4]. Currently there are five inhibitors of tumor necrosis factor α approved: Etanercept, Adalimumab, Infliximab, Golimumab and Certolizumab-pegol. These drugs have proven to be effective in reducing symptoms and peripheral manifestations of the disease [5].
The tumor necrosis factor alpha (TNF-??) is a proinflammatory cytosine that exists on a circulating and transmembrane form with important functions in modulation, expansion and maintenance of the inflammatory response. It has important functions in the intracellular response to pathogens and cell death [6]. Etanercept is a dimeric fusion protein that combines 2 binding domains of the TNF-α receptor with an immunoglobulin G1. It binds predominantly to the soluble form of TNF-α and is considered less immunogenic than others TNF-α antagonists. However, an important number of adverse effects with the use of this drug have been reported since it was released [7,8].
Etanercept has a very low toxicity and its main adverse effects are related to inflammation in the injection site and localized cutaneous leukocytoclastic vasculitis, the most frequent adverse events related to anti-TNF-?? therapies are infectious diseases, malignancies, demyelinating diseases, and drug-induced lupus [7,8]. Etanercept has been associated with the development of diverse granulomatous diseases such as sarcoidosis [[9], [10], [11], [12], [13]] granulomatous hepatitis [14], granulomatous thyroiditis [15], granulomatosis with polyangiitis and granulomatous lung disease [13]. Pulmonary reactions are very rare, especially those involving the formation of granulomas. Nevertheless, long-term, since these drugs are used initially to block the emergence and maintenance of granulomas in diseases such as sarcoidosis. sarcoid reactions associated with the use of Etanercept have been described even after 23 months of the start of treatment (1–50 months).
We aim to report present the case of a young patient with a seronegative spondyloarthropathy who developed respiratory symptoms and pulmonary infiltrates of rapid evolution after the third dose of treatment with Etanercept.
2. Case presentation
A 41-year-old female consulted with 3-month history of pain in the sacroiliac region associated with arthralgias and myalgias of the lower limbs that partially improved with nonsteroidal anti-inflammatory drugs (NSAIDs). She denied cutaneous, digestive or ocular symptoms. An MRI of the sacroiliac joints showed subchondral bone marrow edema in both sacroiliac joints suggesting active sacroiliitis. A diagnosis of seronegative spondyloarthropathy with negative HLA-B27 was performed.
Two months later, she started Etanercept 50 mg administered subcutaneously once weekly, showing significant clinical improvement. After the third dose of the drug, she presented dry cough without expectoration and moderate effort dyspnea. She denied orthopnea, paroxysmal nocturnal dyspnea or upper respiratory symptoms. She had no chest pain, syncope or hemoptysis and did not refer digestive, genitourinary, cutaneous or neurological symptoms.
Her past medical history included two cesarean sections and a septorinoplasty. She denied previous exposure to tobacco or allergic diseases. In addition to Etanercept, she received vitamin D 2000 IU and Etoricoxib 90 mg daily. She had not traveled recently, did not have contact with animals, dust, particulate material or ill people.
The respiratory symptoms progressed rapidly. Thus, the patient consulted to the emergency department (ER). On admission to the ER, she was in acceptable general conditions, without signs of respiratory distress or requirement of supplementary oxygen at rest. Her blood pressure was 118/84 mmHg, pulse 96/bpm, respiratory rate 18/bpm, and weight 64.85Kg. She had no neck masses or jugular engorgement, the heart sounds were rhythmic without murmurs, the vesicular murmur was preserved in both lung fields. On pulmonary examination basal scanty pulmonary rales “Velcro”-like were auscultated. The abdomen had no masses, the extremities had neither edema nor inflammatory signs. The Tinel's sign was negative, Phalen's sign negative. Sacroiliac pain provocation tests were positive.
A high-resolution thoracic CT scan showed incipient ground-glass interstitial infiltrates of centrifugal distribution, especially involving the upper lobes. The EKG revealed a normal sinus rhythm. The transthoracic echocardiogram was normal without evidence of pulmonary hypertension. We decided to perform bronchoscopy with bronchoalveolar lavage (BAL), which showed a negative GeneXpert for Mycobacterium tuberculosis and a negative methenamine silver stain. Laboratory tests taken during admission revealed Hb 13 gr/dL, Hct 42%, leukocytes 4100/mm, neutrophils 2000/mm, lymphocytes 1400/mm, eosinophils 50/mm, platelets 325,000/mm, AST 29, ALT 23, total bilirubin 0.3, direct bilirubin 0.1, creatinine 0.6 and BUN 16.
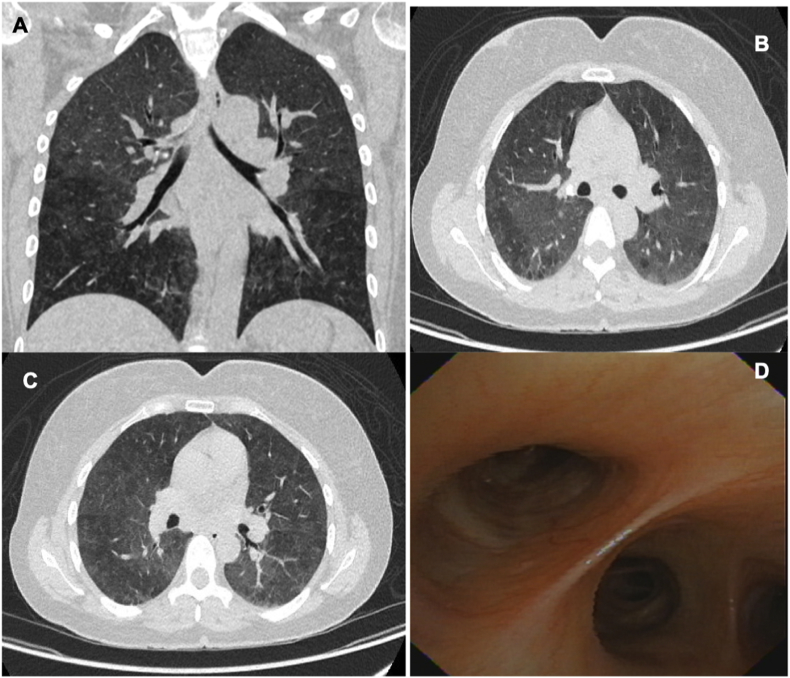
A new high-resolution thoracic CT scan was performed. This scan showed a mosaic pattern of disseminated attenuation in both lungs with foci of ground glass infiltrates (Fig. 1 A, B, C). To dismiss the possibility of an infection a new bronchoscopy with a transbronchial biopsy was also performed. In the bronchoscopy, the aspect of the airway was normal, without endobronchial lesions or bleeding (Fig. 1 D), washing cytology showed a predominance of lymphocytes in 46% without the presence of malignant cells. The methenamine silver stain was negative. The pathology report showed the presence of rounded granulomas, composed of macrophages, epithelioid cells, multinucleated giant cells, and scarce lymphoplasmacytic infiltrate. No central necrosis or microorganisms were identified.
Fig. 1.
A, B, C. Thoracic CT scan showing few ground glass infiltrates predominantly in the upper lobes, without evidence of lymph node enlargement, nodules, pulmonary masses or pleural involvement. D. Aspect of the airway during bronchoscopy, which is of normal characteristics.
Based on the results obtained, we diagnosed acute toxicity by Etanercept presenting as granulomatous pneumonitis. Consequently, after a multidisciplinary evaluation, we decided to initiate pulses of methylprednisolone 500 mg for three days, continuing with an oral dose of prednisone 50 mg daily for twelve weeks. The dose was reduced progressively until reaching 12.5 mg daily with adequate tolerance and decrease of symptoms of cough and dyspnea.
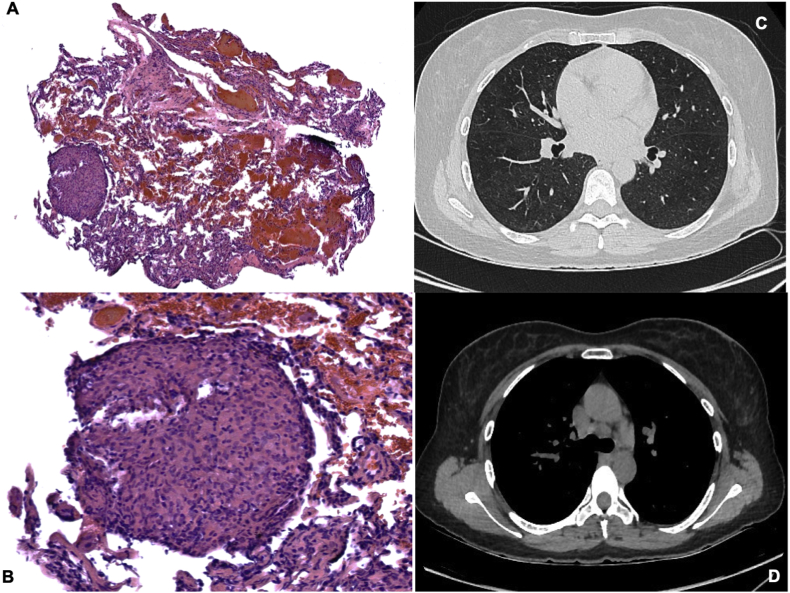
Four weeks after starting the treatment with steroids, the patient was in an excellent general condition, without respiratory symptoms. A new high-resolution thoracic CT scan showed a notable decrease in the mosaic attenuation (Fig. 2 C, D). Two months later, she was in good clinical condition and had returned to work without new relapses nor respiratory symptoms.
Fig. 2.
A, B. H & E stain shows a pulmonary parenchyma with conserved architecture and the presence of rounded granulomas composed of epithelioid macrophages, giant multinucleated cells and scarce lymphoplasmacytic infiltrate. No central necrosis is identified, and no microorganisms are observed with BK, PAS, Mucicarmin and GMS special stains. C, D. Thoracic CT scan with a decrease in the infiltrates and the mosaic attenuation, presence of mediastinal lymph node enlargement in the low precarinal area.
3. Discussion
Tumor necrosis factor-alpha (TNF-α) participates in a constellation of immune responses, which include processes of activation, maintenance, and regulation of inflammation at the cellular level. Moreover, it promotes the secretion of extracellular matrix proteins and the proliferation and migration of fibroblasts. It is relevant in the control of infections by intracellular microorganisms, including fungi and mycobacteria. It is well known that TNF- α overproduction plays a paramount role in rheumatoid arthritis, inflammatory bowel disease, and psoriasis vulgaris; all which has made TNF inhibitors an attractive alternative to treat these diseases.
Etanercept is a dimeric fusion protein that combines two binding domains of the TNF-α receptor with an immunoglobulin G1. It binds predominantly to the soluble form of TNF-α and has a half-life of 4.3 days. This drug has a wide variety of clinical applications, especially in the context of autoimmune diseases. The clinical use of this drug has expanded rapidly in recent years, and thus, the description of possible adverse events is critical to refine the safety of this drug, which in turn drives its clinical use.
Symptoms such as dyspnea, cough, and fever can occur in a timeframe that goes from nine months to five years after initiation of anti-TNF-α therapy. It is known that the development of another type of pulmonary toxicity, such as pulmonary interstitial disease (PID) predominates in older patients with underlying lung disease. The radiological findings consist of ground-glass infiltrates, reticule-nodular opacities, and mediastinal lymphadenopathies.
The utility of bronchoalveolar lavage relies on its capacity to rule-out microorganisms in immunosuppressed patients. Nevertheless, in patients in whom drug toxicity is suspected, the cell differential count has great importance.
Granuloma formation occurs as a result of a Th1-type immune response and is characterized by the infiltration of activated macrophages and CD4+ T lymphocytes. Interferon-γ, TNF-α, interleukin (IL)-12, and IL-18 play an essential role in these granulomatous reactions. A CD4 + or CD8 + predominant lymphocytic alveolitis pattern can be found, and the presence of non-caseating granulomas can be observed in the biopsy. Granulomas are composed for organized aggregates of activated macrophages surrounded by lymphocytes and fibroblasts, the latter forming a fibrous capsule that isolates the content of the granuloma. Differential diagnoses concerning these findings include mycobacterial infections, histoplasmosis, cryptococcosis of hypersensitivity pneumonitis, chronic interstitial pneumonia, granulomatosis with polyangiitis, and drug reactions (14,15). Cases of histopathology consistent with PID have been described; in these cases, the typical findings include a pattern of usual interstitial pneumonia or nonspecific interstitial pneumonia.
Increased levels of TNF have been related to idiopathic pulmonary fibrosis (IPF) and studies with animal models have revealed that TNF antagonists may be a promising agent for their treatment. Based on the physiological aspects, the antiTNF-α therapy may be indicated in patients with refractory sarcoidosis. Favorable response has been reported with infliximab. In contrast, few cases have been described in relation to the development of granulomatous inflammation after the beginning of therapy with Etanercept. (9–13). Other granulomatous reactions also have been reported (14, 15), occurring in 0.04% of the individuals who use the medicament. These granulomatous reactions occur equally in men and women and are independent of the underlying rheumatic disease. it is suggested that lung injury can be a direct effect through a hypersensitivity reaction or indirect effect inducing a condition similar to sarcoidosis.
The differences in the mode of neutralizing TNF-α action between TNF-α antagonists could be implicated in the genesis of granulomatous reaction. For example, Infliximab is an anti-TNF-α monoclonal antibody and Etanercept is a recombinant TNF receptor/IgG fusion protein. Infliximab binds both soluble and transmembrane TNF, whereas Etanercept has a 4-fold lower binding affinity for transmembrane TNF. Infliximab bind TNF in a fast and irreversible mode in vitro. In contrast, Etanercept has both high on-and high off-binding kinetics, shedding about 50% of soluble TNF and 90% of transmembrane TNF only 10 minutes after binding. Infliximab, after binding to membrane-anchored TNF receptors on monocytes or lymphocytes, activate complement and cause antibody-dependent cellular cytotoxicity via their Fc tail. These differences could explain why Etanercept could generate rapid-onset adverse effects, such as the case presented with an unusually early pulmonary and radiographic manifestations with only three doses (one every week).
The treatment of pulmonary lesions induced by drugs consists in suspending the implicated drug coupled with the use of systemic steroids.
4. Conclusions
Granulomatous pneumonitis is a real and important adverse effect of the use of drugs that inhibit tumor necrosis factor-α, particularly Etanercept. Pulmonologist dealing with these patients should have a high level of clinical awareness regarding drug toxicity if patients develop granulomas similar to sarcoidosis during the treatment with these agents. The diagnostic flowchart should always start with the study of the infectious causes of granulomas before considering drug toxicity/adverse reaction, which is always a diagnosis of exclusion.
Patient consent
Written informed consent was obtained from the participant for the publication of this case report and any potentially identifying images/information. We have approval letter of Ethics Committee in biomedical research IRB/EC No. 203–2019 of the Fundación Valle del Lili to publish this manuscript. All data and material are available for sharing if needed.
Funding
We have financial support from the Clinical Research Center of Fundación Valle del Lili.
Declaration of competing interest
We have no conflicts of interest to disclose. This manuscript has not been published and is not under consideration for publication elsewhere. Additionally, all of the authors have approved the contents of this paper and have agreed to the journal's submission policies.
Acknowledgments
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmcr.2020.101079.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zochling J., Smith E.U. Seronegative spondyloarthritis. Best Pract. Res. Clin. Rheumatol. 2010;24:747–756. doi: 10.1016/j.berh.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Reveille J.D., Witter J.P., WeismanMH Prevalence of axial spondylarthritis in the United States: estimates from a cross-sectional survey. Arthritis Care Res. 2012;64:905–910. doi: 10.1002/acr.21621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duba Ayyappa S., Stephanie D., Mathew D.O. The seronegative spondyloarthropathies. Prim. Care Clin. Off. Pract. 2018;45(2):271–287. doi: 10.1016/j.pop.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Ward M.M., Deodhar A., Akl E.A., Lui A., Emann J., Gensler L.S. American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network 2015 recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheum. 2016;68:282–298. doi: 10.1002/art.39298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strand V., Singh J.A. Patient burden of axial spondyloarthritis. J. Clin. Rheumatol. 2017;23(7):383–391. doi: 10.1097/RHU.0000000000000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Díez Piña Juan M., Vázquez Gómez Óscar, Mayoralas Alises Sagrario, García Jiménez José D., Álvaro Álvarezay Dolores, Paz Rodríguez Bolado M. Fibrosis pulmonar mortal, con Etanercept como posible desencadenante. Arch. Bronconeumol. 2008;44(7):393–395. doi: 10.1016/s1579-2129(08)60067-1. [DOI] [PubMed] [Google Scholar]
- 7.Hochberg M.C., Lebwohl M.G., Plevy S.E., Hobbs F., Yocum D.E. The benefit/risk profile of TNF-blocking agents: findings of a consensus panel. Semin. Arthritis Rheum. 2005;34:819–836. doi: 10.1016/j.semarthrit.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Diaz J.C., Vallejo S., Cañas C.A. Drug-induced lupus in anti-TNF-alpha therapy and its treatment with rituximab. Rheumatol. Int. 2012;32:3315–3317. doi: 10.1007/s00296-011-2137-y. [DOI] [PubMed] [Google Scholar]
- 9.Ishiguro T., Takayanagi N., Kurashima K., Matsushita A., Harasawa K. Development of sarcoidosis during etanercept therapy. Intern. Med. 2008;47:1021–1025. doi: 10.2169/internalmedicine.47.0602. [DOI] [PubMed] [Google Scholar]
- 10.Hostettler K.E., Studler U., Tamm M., Brutsche M.H. Long-term treatment with infliximab in patients with sarcoidosis. Respiration. 2012;83:218–224. doi: 10.1159/000328738. [DOI] [PubMed] [Google Scholar]
- 11.Kudrin A., Chilvers E.R., Ginawi A., Hazleman B.L., Griffiths M.H. Sarcoid-like granulomatous disease following etanercept treatment for rheumatoid arthritis. J. Rheumatol. 2007;34:648–649. [PubMed] [Google Scholar]
- 12.Gifre L., Ruiz-Esquide V., Xaubet A., Gomez-Puerta J.A., Hernandez M.V., Sanmarti R. Sarcoidosis pulmonar inducida por antagonistas del factor de necrosis tumoral en la artritis reumatoide: presentación de un caso y revisión de la literatura. Arch. Bronconeumol. 2011;47:208–212. doi: 10.1016/j.arbres.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 13.Petrovici A., Kaiser M.J., Louis R., Nguyen Dang D. Sarcoid-like granulomatosis in patients treated with anti-TNFα. Rev. Med. Liege. 2016;71:124–128. [PubMed] [Google Scholar]
- 14.Farah M., Al Rashidi A., Owen D.A., Yoshida E.M., Reid G.D. Granulomatous hepatitis associated with etanercept therapy. J. Rheumatol. 2008;35:349–351. [PubMed] [Google Scholar]
- 15.Cañas C.A., Tobón G.J., Arango L.G., Guarín N. Developing of granulomatous thyroiditis during etanercept therapy. Clin. Rheumatol. 2009;28:S17–S19. doi: 10.1007/s10067-008-1046-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.