Abstract
Plasmacytoid dendritic cells (pDCs) are a unique subset of dendritic cells specialised in secreting high levels of type I interferons. pDCs play a crucial role in antiviral immunity and have been implicated in the initiation and development of many autoimmune and inflammatory diseases. This review summarises the latest advances in recent years in several aspects of pDC biology, with special focus on pDC heterogeneity, pDC development via the lymphoid pathway, and newly identified proteins/pathways involved in pDC trafficking, nucleic acid sensing and interferon production. Finally, we also highlight the current understanding of pDC involvement in autoimmunity and alloreactivity, and opportunities for pDC‐targeting therapies in these diseases. These new insights have contributed to answers to several fundamental questions remaining in pDC biology and may pave the way to successful pDC‐targeting therapy in the future.
Keywords: alloreactivity, autoimmunity, cell development, immunotherapy, plasmacytoid dendritic cells
This review summarises the latest advances in several aspects of plasmacytoid dendritic cell (pDC) biology, with a special focus on pDC heterogeneity, pDC development via the lymphoid pathway, and newly identified proteins/pathways involved in pDC trafficking, nucleic acid sensing and interferon production. We also highlight the current understanding of pDC involvement in autoimmunity and alloreactivity, and opportunities for pDC‐targeting therapies in these diseases.
Introduction
Human plasmacytoid dendritic cells (pDCs) were initially described 20 years ago by the Liu and Colonna groups. 1 , 2 pDCs are continuously generated from haematopoietic stem cells in the bone marrow (BM) via both myeloid and lymphoid precursors. Afterwards, proteins such as CXCR4 context‐dependently mediate the trafficking of pDCs from the BM to peripheral blood and subsequent migration to specific target tissues.
Plasmacytoid dendritic cells constitute 0.1–0.5% of human peripheral blood mononuclear cells (PBMCs). 3 , 4 Freshly purified pDCs manifest a plasmacytoid morphology, with rough endoplasmic reticulum and Golgi apparatus. Upon activation, pDCs gain dendritic cell‐like morphology and produce massive amounts of type I interferons (IFN‐I), for example most of the IFN‐I detectable in the blood following viral infection in mice and humans. 1 , 2 The IFN‐I secretion by pDCs is mainly mediated through the activation of the endosomal Toll‐like receptors (TLRs) TLR7 and TLR9, with cytosolic receptor initiating pathways playing an important supplementary role. 5 Apart from IFN‐I, pDCs could also secrete pro‐inflammatory cytokines and chemokines and express co‐stimulatory or co‐inhibitory molecules which facilitate pDCs to cross‐prime CD8+ T cells and present antigens to CD4+ T cells. 2 , 6
Plasmacytoid dendritic cells have been shown to be implicated in many autoimmune diseases such as systemic lupus erythematosus (SLE) and systemic sclerosis (SSc). 7 , 8 Furthermore, in graft‐versus‐host disease (GVHD), a major immunologic complication after allogeneic haematopoietic cell transplantation (allo‐HCT), our group and others have identified an important role of pDCs during disease occurrence and development. 9 , 10 Based on these observations, several pDC‐targeting drugs such as anti‐interferon‐α (anti‐IFN‐α) monoclonal antibody (mAb) and anti‐type I IFN receptor subunit‐1 (anti‐IFNAR1) mAb are being assessed in SLE in clinical trials and have shown promising outcomes. 11 , 12 , 13
This review summarises the recent advances in pDC biology, including pDC heterogeneity, lymphoid pathway of pDC development and novel nucleic acid sensing patterns during IFN‐I production. Furthermore, the newly identified roles of pDC in immune‐mediated diseases and novel pDC‐targeting drugs assessed in this setting are also described.
Definition of pDCs
Human pDCs were traditionally defined as not expressing the lineage‐associated markers (Lin) CD3, CD19, CD14, CD16 and CD11c, but selectively expressing CD303 (BDCA2), CD304 (BDCA4) and immunoglobulin‐like transcript 7 (ILT7). 14 They also express CD4, CD45RA, CD68, ILT3 and CD123 (IL‐3 receptor α‐subunit). Mouse pDCs were identified with CD11c, CD45RA, B220, Ly6C, bone marrow stromal antigen 2 (BST2; also known as tetherin) and sialic acid‐binding immunoglobulin‐like lectin H (Siglec‐H). 15 However, a specific subset of CD2hiCD5+CD81+ human pDCs was later identified, which express the pDC markers CD123, CD303 and CD304, but do not secrete IFN‐I. Meanwhile, upon activation, they secrete IL‐12 and potently prime T‐ and B‐cell responses. 16 , 17 , 18 Recently, this non‐canonical ‘pDC subset’ has been redefined as Axl+ DCs with the development of single‐cell analysis, which has divided human DCs into 6 putative subsets, namely cDC1, cDC2‐A, cDC2‐B, CD16+ DC, pDC and Axl+ DC (reviewed by Rhodes et al. 2019). 19 , 20 , 21 , 22 Accordingly, the traditionally defined pDCs include two distinct subsets, the canonical IFN‐I‐producing pDCs (referred to as ‘canonical pDC’ hereafter) and the Axl+ DCs, which are inefficient at IFN‐I production but can efficiently activate T/B cells. 22 The Axl+ DCs are a distinct myeloid DC population expressing typical markers Axl and Siglec6 and are a continuum of pDC and cDC2 characteristics. There is considerable diversity within Axl+ DCs, ranging from the pDC‐like state expressing typical pDC markers (CD123, BDCA2, BDCA4, CD45RA) to the cDC2‐like state expressing typical cDC2 markers (CD11c, CD33, CX3CR1, CD1c, CD2). 21 , 22 Meanwhile, from the pDC‐like state to the cDC2‐like state, there is a decreased expression of TCF4 and an increased expression of ID2, the signature transcription factors for pDC and cDC2, respectively. 21 , 22 Moreover, the murine counterpart of Axl+ DCs with identical genetic and functional characteristics is also identified. 23 , 24
The canonical pDC population was initially regarded as ‘bona fide’ IFN‐I‐producing cells without antigen‐presenting capacity. 20 Later, studies revealed that upon activation with IL‐3 and CD40L, influenza virus or oligodeoxyribonucleotides with CpG motifs (CpG ODNs), canonical pDCs show enforced T‐cell activation capacity by expressing higher levels of co‐stimulatory molecules/chemokine receptors and lower levels of co‐inhibitory molecules. 22 , 25 Furthermore, heterogeneity of canonical pDC has started to be investigated. Alculumbre et al. 25 observed that canonical pDCs could be divided into three relatively stable subsets depending on their CD80 and PD‐L1 expression. The P1‐pDCs (PD‐L1+CD80–) have a plasmacytoid morphology and are specialised in IFN‐I production. The P3‐pDCs (PD‐L1−CD80+) have a dendritic morphology and are more potent in T‐cell activation. Finally, the P2‐pDCs (PD‐L1+CD80+) display a phenotype and morphology between the P1‐ and P3‐pDCs. 25
Given their recent discovery, the ontogeny and immune functions of Axl+ DCs remain to be elucidated. Therefore, in the following sections of this review, ‘pDC’ means traditionally defined pDCs unless otherwise indicated. Collectively, the identification of Axl+ DCs and the heterogeneity of canonical pDCs have prompted us to re‐evaluate pDC development and several important aspects of pDC biology.
Development of pDCs
The development of pDCs is schematically shown in Figure 1. pDCs are continuously generated from haematopoietic stem cells in the BM via both myeloid and lymphoid pathways. Flt3 and its ligand Flt3L are crucial for pDC development in the mouse and human. 26 , 27 The other important cytokine promoting pDC development is M‐CSF (encoded by csf‐1), which is able to drive pDCs and cDCs from BM precursor cells in vitro and in vivo. 28 The pDC transcription programme seems to initiate from progenitors expressing IRF8. 29 The specific development of pDCs requires the transcription factor TCF4, as shown in murine pDCs. 30 As the master regulator, TCF4 acts with its transcription co‐factors including SPIB, IRF8 and RUNX2, among others, which are involved in the development, homoeostasis and function of pDCs. 30 , 31 , 32
Figure 1.
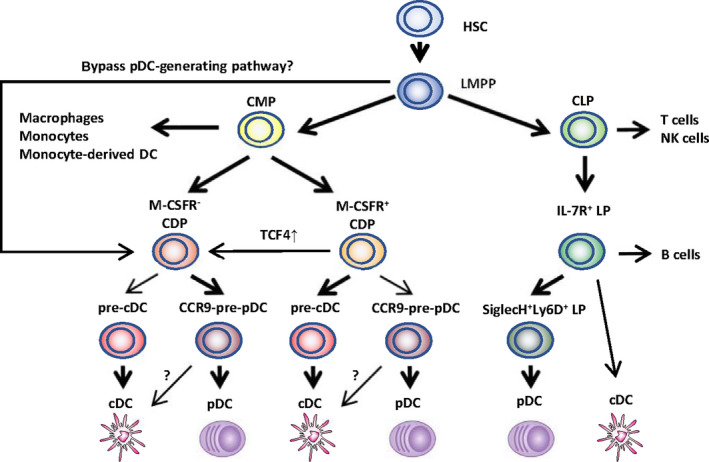
Developmental pathways of pDCs. Major (heavy arrows) and minor (light arrows) haematopoietic pathways found to have the potential to produce plasmacytoid dendritic cells (pDCs) or conventional dendritic cells (cDC) are outlined. The progenitors include the following: HSS, haematopoietic stem cells; LMPP, lymphocyte primed multipotent precursors; CMP, common myeloid precursors; CLP, common lymphoid precursors; CDP, common dendritic precursors; LP, lymphoid precursors; pre‐cDC, precursors of cDC; and pre‐pDC, precursors of pDC. It is not yet clear whether a proportion of M‐CSFR‐ CDP could be derived from LMPP via a more direct ‘bypass pathway’. CCR9− pre‐pDC could differentiate into ‘cDC‐like’ cells context‐dependently, while these cells are not yet identified as real cDCs.
Within the myeloid pathway, the common myeloid progenitors develop firstly into earlier precursors named myeloid precursors, which subsequently differentiate into macrophages and DC precursors (MDPs). Murine MDPs are Lin−CX3CR1+CD11b−c‐KithiFlt3+ and macrophage colony‐stimulating factor receptor (M‐CSFR or CD115) positive. Finally, MDPs give rise to monocytes and common DC progenitors. 33 CDPs express Lin−c‐Kitint/loFlt3+IL‐7R− and comprise M‐CSFR‐positive (M‐CSFR+ CDPs) and M‐CSFR‐negative (M‐CSFR− CDPs) subsets, which preferentially give rise to cDCs and pDCs, respectively. 34 M‐CSFR− CDPs express a high level of TCF4 (E2‐2), while TCF4 expression on M‐CSFR+ CDPs is low.
A pDC progenitor close to terminal differentiation was identified in mice, which shares most properties with mature pDCs, but does not express CCR9, and expresses low class II major histocompatibility complex (MHC II). 35 CCR9− pDC progenitors account for about 20% of murine BM pDCs. They could migrate into peripheral organs and undergo tissue‐specific differentiation into either terminal CCR9+ pDCs or cDC‐like cells. 36 The plasticity of this CCR9− pDC progenitor indicates that the conversion of pDC to cDC could happen close to terminal differentiation.
The lymphoid origin of pDCs was proposed soon after their identification, with the observation that both common myeloid progenitors and common lymphoid progenitors had the potential to produce pDCs after transfer into irradiated mice. 37 Moreover, murine pDCs of lymphoid origin showed evidence of past recombination activating gene 1 (RAG1) expression and had D‐J rearrangements in IgH genes, which are gene arrangement processes normally restricted to lymphoid lineage cells. 26 Recently, a pDC progenitor within the IL‐7R+ lymphoid precursors was identified in mice. IL‐7R+ lymphoid precursors could differentiate into both pDCs and B cells, with the specific subset of SiglecH+Ly6D+‐double‐positive subset giving rise exclusively to pDCs when cultured in the presence of Flt3L. 38 Similarly, a common IL‐7R+ progenitor of both pDCs and B cells has also been identified in humans. 39
It was initially considered that the majority of pDCs derive from myeloid progenitors, with the evidence that the majority (~80%) of pDCs became labelled with in vivo lineage tracing using the common DC progenitor (myeloid origin) marker Csf1r. 40 Moreover, progenitors with transcriptomic features of pDCs emerge before lymphoid progenitors 29 and pDCs develop from stem cells in vivo with the same kinetics as myeloid cells including cDCs. 41 This theory is, however, challenged with new findings. Rodrigues et al. observed that murine mature BM and splenic pDCs differentiate in vitro and in vivo predominantly from IL‐7R+ lymphoid progenitors. Further single‐cell analysis revealed that mature pDC subsets derived from both myeloid and lymphoid origins are able to secrete IFN‐I, but only myeloid‐derived pDCs share with cDCs the ability to process and present antigen. 38 Given that Axl+ DCs were not excluded in this study, these ‘myeloid‐derived pDCs’ may represent the Axl+ DCs and/or the P3‐pDCs (PD‐L1−CD80+).
Importantly, a series of studies have warranted ‘revisiting’ the DC progenitors previously defined solely by phenotype. Sathe et al. 26 observed that murine pDCs derived from the Lin−c‐Kit−sca‐1− MDPs showed ‘lymphoid’ characteristics of past RAG1 expression and had D‐J IgH gene rearrangements, indicating a possible pDC lineage imprinting in earlier progenitors. In addition, murine MDPs were found to contain predominantly precursors of macrophages/monocytes but few precursors of resident pDCs, thus challenging MDPs as the major source of myeloid pDCs. 42 Indeed, recent studies have observed that several progenitors, such as the lymphocyte primed multipotent progenitor, are heterogeneous at the clonal level and include progenitors of many different functional potentials. 43 In summary, pDCs derive from both myeloid and lymphoid pathways, and the exact programme for pDC lineage imprinting remains to be elucidated, which may occur in earlier haematopoietic progenitors. 44 Further comprehensive studies on the transcriptomic programme are crucial to better trace the fate of pDCs.
Trafficking of pDCs
Plasmacytoid dendritic cells are constantly produced in the BM and migrate to the primary and secondary lymphoid organs via peripheral blood during homoeostasis. Human and murine pDCs constitutively express CXCR4, and the CXCR4–CXCL12 signalling is crucial for the early development of pDCs within the BM stromal cell niches, and their migration towards splenic white pulp. 3 , 4 Circulating pDCs migrate from the blood compartment into lymph nodes mainly through high endothelial venules in both humans and mice. 2 , 15 pDCs constitutively express high levels of L‐selectin, 15 CXCR4 4 and ChemR23, 45 whose ligands are expressed by high endothelial venules. Therefore, these proteins are responsible for pDC trafficking to lymph nodes during homoeostasis. In addition, chemokine receptors including CCR2, CCR5, CCR6, CCR7, CCR9 and CCR10 are expressed on pDCs and facilitate the homing to peripheral blood during homoeostasis or inflammation (reviewed by Swiecki and Colonna 2015). 46 Moreover, during inflammation, additional molecules are involved in pDCs homing to lymph nodes, such as PSGL‐1, the ligand for E‐selectin, β1 and β2 integrins and CXCR3. 46 Other proteins involved in pDC migration and organ localisation include MAdCAM‐1 47 and IFN‐β. 48
In addition to receptors expressed on the surface, several intracellular signalling molecules have been identified as playing a decisive role in pDC migration. CD2‐associated protein, which is specifically expressed in human and murine pDCs, is correlated with pDCs’ lymph node migration under conditions of inflammation in mice. 49 Moreover, dedicator of cytokinesis protein 2 (DOCK2) is found to be indispensable for migration of murine pDCs. 50
Nucleic acid sensing and IFN secretion by pDCs
Plasmacytoid dendritic cells were initially identified as a unique cell subset that respond to viruses with rapid and massive production of IFN‐I, and play a central role in the antiviral immune response. 2 Moreover, pDCs could also respond to certain non‐viral pathogens such as bacteria (e.g. Chlamydia pneumoniae) 51 and apicomplexan parasites (e.g. Plasmodium). 52 , 53 The recent advances in pDCs in anti‐infectious immunity are not within the scope of this review, but have been very well summarised by Reizis. 54 Recognition of either pathogen‐derived nucleic acids or synthetic TLR ligands such as CpG ODNs initiates IFN‐I secretion by pDCs, which is mainly (albeit not exclusively) mediated through the activation of the endosomal TLR7 and TLR9, and the subsequent myeloid differentiation primary response protein 88 (MYD88)–interferon regulatory factor 7 (IRF7) pathway. 55 In addition, the MYD88–NF‐κB pathway is also activated, leading to the secretion of pro‐inflammatory cytokines and chemokines, and the expression of co‐stimulatory molecules. TLR7 senses RNA viruses and endogenous RNA, whereas TLR9 detects prokaryotes containing unmethylated CpG‐rich DNA sequences and endogenous DNA. Both TLR7 and TLR9 sense synthetic CpG ODNs, and different classes of CpG ODNs have been developed to perform different immune functions. CpG‐A is a strong inducer of type I IFNs, whereas CpG‐B is a potent stimulator of maturation and the production of cytokines and chemokines. CpG‐C exhibits properties of both CpG‐A and CpG‐B. 56
Most cell types other than pDCs constitutively express IRF3, but not IRF7 or only at a very low level. Upon viral infection, IFN‐β can be directly induced by IRF3 and promotes both their own secretion and that of IFN‐α in an autocrine manner mediated by type I IFN receptor (IFNAR). 57 This IFNAR‐based feedback signalling is crucial for the massive production of IFN‐I during viral infection. Notably, pDCs constitutively express higher levels of IRF7 than do other cell types, 58 and are able to secrete IFN‐I rapidly and independently of the IFN‐I receptor IFNAR‐based feedback signalling. 59 Consistently, studies have shown that IFNAR is dispensable for pDCs during certain virus infections in vivo including vesicular stomatitis virus (VSV) 59 and mouse cytomegalovirus (MCMV). 60 However, the ultimate IFN‐I responses by pDCs to TLR ligands in vivo 61 or to certain viruses in vitro 62 require IFNAR signalling, iing the necessity for intact IFNAR‐based feedback for optimal pDC function.
Not long after its identification, the TLR‐mediated sensing of pDCs was found to be not exclusive with the observation that pDCs could generate IFN‐α in response to the DNA virus herpes simplex virus type 1 (HSV‐1) independent of TLR9 signalling. 63 Gradually, alternative sensing systems initiated by cytosolic receptors were revealed. Human pDCs could sense cytosolic DNA via the cGAS (cyclic GMP‐AMP (cGAMP) synthase)–STING (stimulator of interferon genes) pathway, which thereby triggers an IRF3‐mediated IFN‐I production independent of TLR9. 5 Moreover, both human and murine pDCs express the cytosolic RNA sensor retinoic acid‐inducible gene I (RIG‐I), which senses replicate viral RNA, recruits the mitochondrial antiviral signalling protein adaptor protein and finally leads to IFN‐I production. 62 Other cytosolic sensors include (DExD/H)‐box helicases DHX36 and DHX9 expressed on human pDCs, with the former selectively binding to CpG‐A and activating the IRF7 pathway and the latter selectively binding to CpG‐B, leading to subsequent activation of the Nf‐ĸB pathway. 64 Collectively, these cytosolic receptor initiating pathways may play an important supplementary role in pDC immunity. Routes of pDC sensing are summarised in Figure 2.
Figure 2.
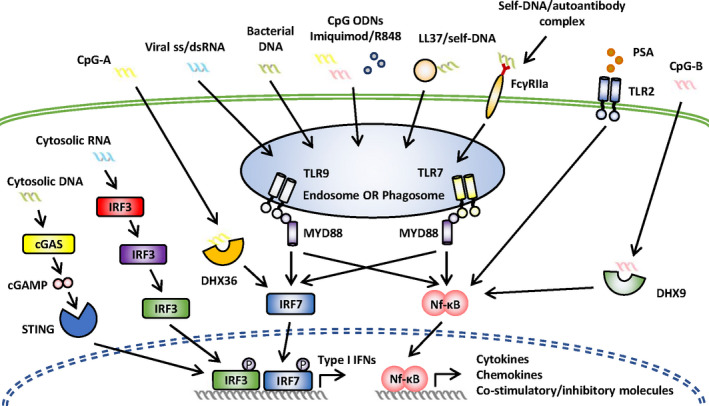
Routes of pDC sensing. Endosomal pathways: TLR7 senses RNA viruses and endogenous RNA, whereas TLR9 detects prokaryotes containing unmethylated CpG‐rich DNA sequences and endogenous DNA. Both TLR7 and TLR9 sense synthetic TLR ligands (CpG ODNs/imiquimod/R848) and immune complexes (self‐DNA/autoantibody and LL37/self‐DNA complexes mediated by FcγIIa). Non‐endosomal pathways: The cGAS (cyclic GMP‐AMP (cGAMP) synthase)–STING (stimulator of interferon genes) pathway senses cytosolic DNA and triggers an IRF3‐mediated IFN‐I production. Retinoic acid‐inducible gene I (RIG‐I) senses replicate viral RNA, recruits the mitochondrial antiviral signalling protein adaptor protein and leads to IFN‐I production. (DExD/H)‐box helicases DHX36 and DHX9 sense CpG ODNs, with the former selectively binding to CpG‐A and activating the IRF7 pathway and the latter selectively binding CpG‐B and activating the Nf‐ĸB pathway. pDCs sense polysaccharide A (PSA) via cytosolic TLR2 and activate the Nf‐ĸB pathway.
Despite their low frequency, pDCs produce most of the IFN‐I detectable in the blood following viral infection. Meanwhile, upon in vivo CpG ODN activation in mice, the IFN‐I response is mediated exclusively by pDCs. 65 Given that TLR7 and TLR9 are also expressed on B cells and several myeloid cell types, an important question is raised: Why and how pDCs, but not other cell types, activate this signalling pathway for IFN‐I induction? So far, it seems that a combination of cellular processes contributes to the answer to this question. Firstly, CpG‐A is retained for long periods in the early endosome of pDCs, together with the MYD88–IRF7 complex, whereas in cDCs, CpG‐A is quickly transferred to lysosomal vesicles. 66 , 67 Moreover, protein kinase C and casein kinase substrate in neurons 1 (PACSIN1) is specifically expressed on human and mouse pDCs and is involved in the type I IFN, but not the pro‐inflammatory cytokine secretion in response to the TLR9 ligand. 68
Given that both the IRF7 and NF‐κB pathways depend on MYD88 and UNC93B, why and how pDCs ‘select’ the IRF7 pathway to secrete IFN‐I has been intensively investigated. The compartment in which TLRs encounter their ligands seems to be the decisive factor. 67 Another important factor mediating the preferential secretion of IFN‐I is the adapter protein‐3 (AP3). 69 The AP3 adaptor complex and the AP‐3‐interacting cation transporter Slc15a4 are responsible for the trafficking of TLR9 from the early endosome to a specialised lysosome‐related organelle (IRF7 endosome), where TLR9 activates the MYD88 signalling this IFN‐I secretion. 70 In addition, a non‐canonical recognition process called microtubule‐associated protein 1A/1B‐light chain 3 (LC3)‐associated phagocytosis (LAP) was identified when pDCs were exposed to large DNA containing immune complexes. 71 It was recently found that LAP is also involved in CpG ODN‐induced TLR9 sensing. 72
Plasmacytoid dendritic cells produce high levels of IFN‐I during MCMV infection in vivo through the TLR9–MYD88–IRF7 signalling pathway. Surprisingly, this process is dependent on neither AP3‐driven endosomal routing nor the autophagy‐related 5 (Atg5)‐dependent LAP, indicating a potentially unknown mechanism involved in TLR sensing. 60
Apart from the cell‐intrinsic mechanism for type I interferon production, recent studies have indicated the involvement of a cooperative mechanism. It was previously observed that in vivo pDC activation by TLR ligands induced their tight clustering. 61 In vitro, CpG ODN‐activated human pDCs produce higher IFN‐I when cultured with high cell density, which was proved in a single‐cell activation assay. 73 This phenomenon is partly explained by an autocrine/paracrine mechanism. Moreover, recent studies reveal that cell–cell contacts may also contribute to the enhanced IFN‐I secretion within clustered pDCs. 60 , 74 , 75 Lymphocyte function‐associated antigen 1 (LFA‐1) is found to be responsible for cell–cell contact of pDCs in humans 74 and mice 75 in vitro. Saitoh et al. 75 observed that murine pDCs lacking LFA‐1 have decreased IFN‐I production in response to TLR ligands and to the influenza virus, which is due to impaired intracellular TLR7 trafficking to the cell–cell contacts and subsequent IFN‐I secretion in the vicinity. Moreover, the optimal in vivo activation of IFN‐I production by murine pDCs during MCMV infection or TLR9 ligand activation also requires LFA‐1 expression. 60
The cooperative mechanism also plays a crucial role in virus sensing. pDCs could respond efficiently to viruses (e.g. influenza virus) without being infected, through internalised virions which initiate the IFN‐I response. 2 However, some viruses (e.g. VSV) only drive the IFN‐I response when replicate‐active. In these cases, cooperative virus sensing would happen between uninfected pDCs and infected pDCs, or between uninfected pDCs and infected cells other than pDCs. The homotypic interaction was supported by studies showing that during certain virus infections (including VSV), the viruses replicated in a certain subset of pDCs while substantial IFN‐I was produced by another subset of pDCs where virus replication does not occur. 76 , 77 Besides the homotypic mechanism, the broader heterotypic interactions may play a more important role in antiviral immunity. It was observed that hepatitis C virus (HCV)‐infected cells trigger a robust IFN response in pDCs via a mechanism that requires active viral replication, direct cell–cell contact and TLR7 signalling. 78 Moreover, the cooperative sensing between pDCs and multiple other infected cells such as cDCs and macrophages has been described. 53 , 77 , 79 Finally, dependent on the pathogen, the close‐range interactions between pDCs and infected cells are mediated through multiple routes including exosomes, 78 enveloped virions, 79 LFA‐1‐mediated adhesion 60 and the integrin‐mediated ‘interferogenic synapse’ 80 (reviewed by Reizis 54 ).
Plasmacytoid dendritic cells also produce another class of potent innate antiviral interferons, the IFN‐λs or type III IFNs (IFN‐III) in response to viruses or synthetic TLR ligands. 81 The IFN‐λs mainly serve as a first line of defence at the mucosal barrier, given that the IFN‐λ receptor (IFN‐λR), the specific receptor for IFN‐λ, is restrictively expressed on cells of epithelial lineage and on certain human leucocytes including pDCs and B cells. 82 Importantly, IFN‐λs are observed to provide non‐redundant antiviral protection at mucosal sites including the respiratory and gastrointestinal tract. 83 , 84 Recently, IFN‐λs have also found to be involved in autoimmunity and antitumor immunity. 85 , 86 Besides, IFN‐λs could positively regulate pDC functions, including interferon‐dependent gene transcription, 87 production of cytokines (including IFN‐I), 88 maturation 81 and survival. 88 Therefore, during virus infection, the local defence by IFN‐λs at mucosal sites may enhance the subsequent systematic IFN‐I responses.
pDCs and T‐/B‐cell responses
Antigen presentation by pDCs could context‐dependently lead to CD4+ T‐cell activation or tolerance induction. Upon in vitro activation by the influenza virus, human pDCs drive a potent Th1 polarisation. 2 Meanwhile, CD40L‐activated human pDCs induce a strong Th2 response. 89 Nevertheless, upon TLR7 activation or TGF‐β exposure, both human and mouse pDCs selectively promote a Th17 response. 90 , 91 When pDCs are either unstimulated or alternatively activated, they express the context‐dependent expression of indoleamine 2,3‐dioxygenase (IDO), inducible costimulator ligand, OX40 ligand (CD252), PD‐L1 and Granzyme B and induce regulatory T‐cell responses during viral infection, tumor and autoimmune disorders (reviewed by Swiecki and Colonna 2015). 46
To identify the antigen‐presenting role of specific surface molecules expressed on pDCs, monoclonal antibodies (mAb) were used. By using a mouse model expressing human CD303 specifically in pDCs together with an anti‐CD303 mAb, it was confirmed that antigen delivery to pDCs through CD303 decreased effector CD4+ T cells and preserved Foxp3+ Tregs. 92 Using similar methods, it was found that Siglec‐H‐mediated antigen delivery induced a hyporesponsive state of T cells via reducing expansion of CD4+ T cells and inhibiting Th1/Th17‐cell polarisation but not conversion to Foxp3+ Tregs. 93 Moreover, antigen delivered to murine pDCs via BST2 in combination with TLR agonists as adjuvants is specifically presented by pDCs in vivo and elicits strong cellular and humoral immune responses. 94
Besides antigen presentation to CD4+ T cells, it was also observed that both human and murine pDCs could cross‐present exogenous antigens to prime CD8+ T cells. 95 Notably, murine pDCs acquire cross‐presentation capacity only when activated by TLR ligands, and mitochondrial reactive oxygen species is involved in the regulation of this process. 96 , 97 The recycling endosomes within pDCs facilitate CD8+ T cross‐priming by offering sites for loading peptide onto MHC class I, and subsequent cross‐presentation to CD8+ T cells. 95 Moreover, it was recently observed that upon viral infection, pDCs would migrate to the CD8+ T‐cell priming sites in the lymph nodes in a strictly CCR5‐dependent manner, indicating a crosstalk between pDCs and CD8+ T cells which is yet to be investigated. 98
A pioneering study showed that in response to influenza virus, human pDCs secrete IFN‐α and IL‐6, which mediate the differentiation of B cells into plasmablasts and the subsequent development into immunoglobulin (Ig)‐secreting plasma cells, respectively. 99 Later, it was also observed that CpG‐stimulated human pDCs could induce plasma cell differentiation in naive and memory B cells in the absence of T‐cell help. 100 Additionally, cell‐to‐cell contact also contributes to B‐cell proliferation and differentiation promoted by CpG‐activated human pDCs. 101 Indeed, during virus infections such as human cytomegalovirus (HCMV) 102 and rotavirus 103 , the activated pDCs play an important role in triggering B‐cell responses and enhance humoral immunity. Meanwhile, in many autoimmune diseases, the pDCs are abnormally activated and drive B‐cell responses involved in disease pathophysiology (introduced in the next section). 104
It is noteworthy that some of the previously regarded capacities for pDCs to induce T‐/B‐cell responses (e.g. IL‐12 secretion and antigen presentation in part) may be attributed to the Axl+ DCs. 16 , 17 , 18 However, since the canonical IFN‐I‐producing pDCs retain antigen‐presenting capacity upon activation, their relationships with T/B cells require re‐evaluation. 22 , 25 Collectively, the correlations between pDCs and T/B cells play either beneficial or deleterious roles during infections and immune‐mediated diseases and warrant further investigation.
pDCs in autoimmunity
The roles of pDCs in immune‐mediated diseases are summarised in Table 1. pDCs play an important pathogenetic role in SLE. Raised serum levels of IFN‐α and constitutive upregulation of IFN‐α‐inducible genes have been observed in SLE patients and are correlated with both disease activity and severity. 7 , 105 Importantly, during SLE and other autoimmune diseases, human pDCs sense the immune complexes formed by autoantibodies and nucleic acids mediated by FcγIIa (CD32A) or FcεRI expressed at the plasma membrane. 106 , 107 The immune complexes are then internalised through phagocytosis and delivered into phagosomal compartments, where TLR7 and/or TLR9 signalling initiates and finally leads to IFN‐α production. 71 , 108 In addition, pDCs are decreased in peripheral blood, activated and accumulated in the tissue lesions of SLE patients. 109 Moreover, in SLE patients, pDCs promote plasmablast differentiation but fail to induce regulatory B cells. 104 Consistent with the predominance of females among SLE patients, pDCs from females produce more IFN‐α upon TLR7 stimulation than those from males, probably due to both the effects of female sex hormone estrogens and the intrinsic X chromosome complement. 110
Table 1.
Role of pDCs in immune‐mediated diseases
| Investigated disease | Role of pDC | Human/Mouse model & pDC depletion/modulation method | Possible mechanism | References |
|---|---|---|---|---|
| pDC in autoimmunity | ||||
| Systemic lupus erythematosus (SLE) | Disease initiation/promotion |
SLE patients
Mouse models
|
|
7, 105, 115, 116, 117 |
| Systemic sclerosis (SSc) | Disease initiation/promotion |
SSc patients
Mouse models
|
|
8, 121 |
| Type I diabetes | Disease initiation/promotion |
Type I diabetes patients
Mouse models
|
|
122, 123 |
| Psoriasis | Disease initiation/promotion |
Psoriasis patients
Mouse models
|
|
45, 124, 125 |
| Rheumatoid arthritis (RA) | Disease prevention |
RA patients
Mouse models
|
|
126 |
| Inflammatory bowel disease (IBD) |
Controversy Disease promotion |
IBD patients
Mouse models
|
|
127 |
| IBD |
Controversy Dispensable |
Mouse models
|
N/A | 128 |
| Atherosclerosis |
Controversy Disease promotion |
Atherosclerosis patients
Mouse models
|
|
129, 130 |
| Atherosclerosis |
Controversy Disease prevention |
Mouse models
|
|
131 |
| pDC in alloreactivity | ||||
| Graft‐versus‐host disease (GVHD) | Sufficient but not necessary in inducing GVHD |
Mouse models
|
|
133, 134 |
| GVHD | Disease prevention |
GVHD patients: intestinal mucosa & skin
Mouse models
|
|
9, 10, 136, 139, 141, 143 |
DTR, diphtheria toxin receptor; Flt3L, Flt3 ligand; IDO, indoleamine 2,3‐dioxygenase; KO, knockout; mAb, monoclonal antibody.
A positive feedback loop between pDCs and neutrophils is abnormally upregulated during the SLE disease process. The circulating neutrophils in SLE patients may be primed in vivo by type I IFN excessively produced by pDCs and release more neutrophil extracellular traps (NETs) rich in antimicrobial peptides, self‐DNA, HMGB1 and oxidised mitochondrial DNA and will trigger pDC activation and excessive type I IFN secretion via the TLR9 pathway. 111 , 112 , 113 , 114
Genetic models have helped to understand better the pathogenic role of pDCs in SLE. Diphtheria toxin receptor (DTR)‐based transient depletion of pDC in lupus‐prone mice before disease onset resulted in amelioration of disease. Surprisingly, these effects were maintained even though pDCs later recovered, revealing the crucial role of pDC in disease initiation. 115 , 116 In addition, constitutive impairment of pDCs by monoallelic deletion of Tcf4 strongly reduced autoantibody production and all disease manifestations in two different spontaneous models of SLE. 117 However, there remain caveats in genetic ablation or antibody‐mediated pDC depletion in these mouse models due to lower specificity and potency. For instance, besides pDCs, TCF4 is also an important regulator for germinal centre B‐cell and plasma cell development. 118 Meanwhile, BST2 is also expressed on plasmacytes in steady state and on most cell types upon stimulation with IFN‐Is and IFN‐γ. 119 In addition, the antibody‐mediated depletion could only exert transient effects and that certain genetic ablation methods such as the monoallelic deletion of Tcf4 could only induce partial reduction of pDCs. 117 Techniques for in vivo depletion and functional modulation of pDCs, as well as their advantages and caveats, are well summarised by Reizis. 54
Apart from SLE, pDCs were found to be implicated in several other autoimmune diseases. pDCs are responsible for most of the IFN‐α secretion in SSc patients and play a critical role during the process of fibrosis. 120 Abnormally activated pDCs are infiltrated in the target organs such as skin and lung and found in bronchoalveolar lavage, and secrete IFN‐α and CXCL4 (both hallmarks of SSc), in both patients and mouse models. 8 Moreover, in a SSc mouse model with bleomycin‐induced fibrosis, depletion of pDCs not only prevented disease initiation, but also ameliorated the established fibrosis. 8 , 121 In type I diabetes, pDCs are proportionally expanded in patients at disease onset. 122 Indeed, pDCs are recruited and activated in the pancreas of non‐obese diabetic (NOD) mice, and TCF4 knockout in NOD mice has ameliorated insulitis and reduced diabetes incidence. 123
In psoriasis, pDCs were recruited to the skin of patients via the chemerin/ChemR23 axis, became activated and produced IFN‐α early during disease formation. 45 , 124 Moreover, functional inhibition or early depletion of pDCs in a xenograft and a genetic model of psoriasis caused disease amelioration, respectively. 124 , 125
Plasmacytoid dendritic cells are not always disease‐promoting. In certain diseases, the role of pDCs may be protective. Enhanced pDC recruitment and activation to arthritic joints by topical application of the TLR7 agonist imiquimod ameliorated arthritis in a genetic mouse model. 126 In addition, pDCs were found to infiltrate the intestinal mucosa of inflammatory bowel disease (IBD) patients; however, controversy remains over their exact role. Moreover, Arimura et al. 127 reported that pDC depletion using Siglec‐H‐DTR mice attenuated disease development in a chemically induced acute colitis model, while Sawai et al. 128 showed that monoallelic deletion of Tcf4 in two genetic models of IBD had no effect on disease development. pDCs have been shown to decrease in circulation and are detected in plaques during atherosclerosis in patients. However, conflicting results exist in in vivo experiments, as constitutive or transient depletion of pDCs prevented 129 , 130 or aggravated 131 atherosclerosis in genetic mouse models. Therefore, the role of pDCs in certain autoimmune diseases may be spatially and temporally dependent.
pDCs in alloreactivity
Alloreactivity is identified when immunocompetent T cells in the donated tissue (the graft) recognise the recipient (the host) as foreign and migrate to and attack the target organs in the immune‐compromised host. 132 In clinical conditions, alloreactivity happens during GVHD, a major immunologic complication for patients who undergo allogeneic haematopoietic cell transplantation (allo‐HCT). A pioneering study showed that MHC‐expressing host pDCs alone were sufficient to prime alloreactive T cells and cause GVHD in a GVHD‐resistant mouse model, and pDC maturation was mediated by the inflammatory environment created by irradiation. 133 However, in vivo depletion of host pDC, alone or together with cDC depletion, did not ameliorate murine GVHD. 134 This is consistent with studies revealing that in allo‐HCT, many other cells, including donor antigen‐presenting cells (APCs) and recipient non‐haematopoietic APCs, are, with enough potency, sufficient to induce GVHD. 135
The effects of pDC on the major target organ of aGVHD, the gastrointestinal tract (GI), have been under intensive investigation. Hadeiba and colleagues showed that CCR9+ pDCs were recruited to the intestines and attenuated aGVHD in a mouse model induced by allogeneic CD4+ T cells, probably via induction of Tregs. 136 In addition, the pro‐inflammatory Th17 cells, together with pDCs, were upregulated in the intestinal mucosa of patients with aGVHD, as compared with patients without aGVHD. 9 Moreover, this co‐upregulation of pDC and Th17 was also shown in the skin of aGVHD patients, as compared with healthy individuals. 10
The content of pDCs within a graft, or the graft type, may affect GVHD severity. Unrelated BM allograft with a higher content of pDCs led to improved survival in GVHD patients as compared with grafts with lower pDCs. 137 Relatively, in a MHC‐mismatched murine transplant model, recipients of Flt3L‐treated BM (containing a higher proportion of inactivated pDCs) had increased survival and decreased GVHD scores with fewer Th1 and Th17 polarised T cells post‐transplant as compared with recipients of unmanipulated BM. 138 Interestingly, in a murine model of MHC‐mismatched transplantation, the 120G8 mAb‐mediated pDC depletion from BM grafts resulted in an acceleration of GVHD mortality while the pDC depletion from G‐CSF‐mobilised splenic grafts had no effect. 139 This observation indicated the intrinsic difference between pDCs in BM and G‐CSF‐mobilised graft. Indeed, a subset of haematopoietic stem cells, the CD8+TCR− ‘facilitating cells (FCs)’, has long been identified in murine BM but not in G‐CSF‐mobilised graft. FCs could enhance engraftment and promote transplantation tolerance in vivo. 140 Further studies revealed that FCs contain a specific subset of pDC precursors which could attenuate GVHD in mouse models. These cells express Lin−CD11c+B220+PDCA1+ and predominantly develop into mature pDCs upon Flt3L activation. 141 , 142 , 143 The GVHD prevention by these pDC precursors is probably mediated by IFN‐γ produced by donor T cells, which induce IDO synthesis by donor precursor pDCs and subsequent Treg generation in recipient mice. 143 However, it is noteworthy that in vivo expansion by Flt3L is not pDC‐specific, as it would also induce development and proliferation of other cells (e.g. the CD3+ subset) within the FC population and exert anti‐GVHD effects. 144
Post‐transplantation reconstitution of pDCs is predictive for subsequent GVHD risk. Patients developing aGVHD after myeloablative allo‐HCT were shown to have significantly lower numbers of both circulating cDCs and pDCs than non‐GVHD patients, and low DC counts were associated with severe aGVHD. 145 Similar to myeloablative allo‐HCT, low pDC counts in patients receiving reduced‐intensity conditioning allo‐HCT were also correlated with severe grade II–IV aGVHD. 146 Moreover, steroid treatment rapidly decreased pDC counts at all time points after transplantation. 147 Nevertheless, recent studies in mouse models show that not only the quantity, but also the quality of DCs is altered during GVHD. On the one hand, GVHD impairs the murine pDC ability to prime the virus‐specific T cells. 148 On the other hand, antigen presentation through MHC II is also impaired during aGVHD, leading to Treg deficiency and consequent chronic GVHD (cGVHD) in a pre‐clinical mouse model. 149
Targeting pDC functions in autoimmunity and alloreactivity
Given the pathogenetic role of pDCs in autoimmunity and alloreactivity, several molecules targeting pDCs have been assessed in clinical trials (summarised in Table 2). Recently, anifrolumab, the anti‐IFNAR1 mAb, has shown efficacy in moderate‐to‐severe SLE in a Phase III clinical trial, 11 in which a BILAG‐based composite lupus assessment (BICLA) response occurred in 86 of 180 (47.8%) patients who received anifrolumab at week 52, compared with 57 of 182 (31.5%) of those who received placebo. This is the first Phase III trial confirming the efficacy of pDC‐targeting drugs in SLE. Sifalimumab 12 and rontalizumab, 150 the two humanised anti‐IFN‐α mAbs, have also shown efficacy in two Phase II clinical trials in moderate‐to‐severe SLE. Moreover, BIIB059, a humanised anti‐BDCA2 mAb, was shown in a Phase I trial to ameliorate skin lesions in SLE, 13 and a Phase II trial is ongoing. Notably, since it was observed that pDCs depend more on the anti‐apoptotic protein BCL‐2 for survival as compared with cDCs, the BCL‐2 antagonists (e.g. the commercially available drug venetoclax) have been proven to selectively deplete pDCs, but not cDCs, in vitro and in vivo. 151 , 152 A Phase I clinical trial has confirmed the safety of venetoclax for SLE in female patients. 153
Table 2.
Clinical trials targeting pDCs in immune‐mediated diseases
| Drug | Antigen | Format | Status | Disease | Results | References |
|---|---|---|---|---|---|---|
| Anifrolumab | IFNAR1 | Blocking antibody | Phase III | SLE | Phase III: response at week 52: anifrolumab (47.8%) vs placebo (37.5%) | Phase III: 11; NCT02446912; NCT02794285 |
| Anifrolumab | IFNAR1 | Blocking antibody | Phase II | Lupus nephritis | Phase II ongoing | NCT02547922 |
| Anifrolumab | IFNAR1 | Blocking antibody | Phase II | Rheumatoid arthritis | Phase II ongoing | NCT03435601 |
| Sifalimumab | IFN‐α | Blocking antibody | Phase II | SLE | Phase II met primary endpoint | 12; NCT01031836; NCT00979654 |
| Rontalizumab | IFN‐α | Blocking antibody | Phase II | SLE | Primary endpoint not met in Phase II, but disease improved in patients with low ISM scores | 150 |
| IFN‐α kinoid | IFN‐α | Vaccine | Phase II | SLE | IFN‐α kinoid was well tolerated in Phase I; Phase II ongoing | 154 |
| IFN‐α kinoid | IFN‐α | Vaccine | Phase II | DM | Ongoing | NCT02980198 |
| BIIB059 | BDCA2 | Functional antagonist | Phase II | SLE | BIIB059 ameliorated skin lesion in Phase I; Phase II ongoing |
Phase I: 13 Phase II: NCT02847589 |
| DV1179 | TLR7/9 | Oligonucleotide inhibitor | Phase IIa | SLE | Primary pharmacodynamic endpoints not met in Phase IIa | – |
| PF‐06650833 | IRAK4 | Small‐molecule inhibitor | Phase II | Rheumatoid arthritis | Phase II completed and results submitted | NCT02996500 |
| Venetoclax | BCL‐2 | Small‐molecule inhibitor | Phase I | SLE | Venetoclax was well tolerated in Phase I; | 153 |
| CPG 52364 | TLR7/8/9 | Oligonucleotide inhibitor | Phase I | SLE | Phase I completed, no results posted | NCT00547014 |
| VIB7734/MEDI7734 | ILT7 | Functional antagonist | Phase I | SLE, CLE, SSc, DM, PM, Sjogren's | Phase I completed, no results posted |
BCL‐2, B‐cell lymphoma 2; CLE, cutaneous lupus erythematosus; DM, dermatomyositis; IFNAR1, type I IFN receptor subunit‐1; ISM, interferon signature metric; PM, polymyositis; SLE, systemic lupus erythematosus; SSc, systemic sclerosis.
Several new molecules are also progressing in the pipeline with the focus on depleting or inhibiting pDC. These molecules bind to surface receptors (such as BDCA2 or ILT7) or block endosomal TLRs, or TLR’s downstream signalling. Moreover, these molecules may not only inhibit the IFN‐I pathway, but also affect other pDC functions such as the production of TNF‐α, IL‐6 and chemokines and antigen presentation. 154 Some of them have been assessed in pioneering clinical trials (Table 2).
Arsenic trioxide (As2O3), a well‐established drug for acute promyelocytic leukaemia, was observed to have therapeutic potential in pre‐clinical mouse models of SLE, 155 SSc 156 and sclerodermatous GVHD, 157 with unknown mechanism. 158 Our group has recently offered a potential explanation by demonstrating that clinically relevant concentrations of As2O3 preferentially block IFN‐α secretion from pDCs through IRF7 inhibition and also impair the capacity of pDCs to induce T‐/B‐cell responses. 159 We are currently running a prospective multicentre clinical trial testing As2O3 in the setting of cGVHD (ClinicalTrials.gov identifier: NCT02966301).
Concluding remarks
The heterogeneity of pDCs has been revealed recently, especially in the last five years. With the development of single‐cell analysis, the previously identified pDC population has been separated into Axl+ DCs and canonical IFN‐I‐producing DCs. Therefore, although Axl+ DCs constitute only a small proportion (10–15%) of the traditionally defined pDCs, 20 , 21 , 22 this putative DC subset must be independently investigated in future studies of pDCs. Moreover, recent evidence has indicated that both the Axl+ DCs and canonical DCs are indeed heterogeneous at both phenotypic and genetic levels, prompting us to study pDCs with more precise and comprehensive techniques in the future. 21 , 22 , 25
Plasmacytoid dendritic cells could be derived from both lymphoid and myeloid origins. However, recent studies have provided strong evidence that the lineage imprinting of pDCs happens early before the emergence of the myeloid/lymphoid progenitors, and probably at the level of haematopoietic stem cells. 26 , 29 These observations have challenged the current theory of leucocyte development and indicated that the previously regarded ‘homogenous’ progenitors are indeed heterogeneous. Moreover, the capacity of freshly isolated pDCs to differentiate into cDC‐like cells discovered in both humans and mice reveals an intrinsic plasticity of differentiated pDCs. 35 , 160 , 161 To answer these questions requires a better characterisation of pDC fate and poses important challenges for future studies.
The exact roles of pDC in most autoimmune diseases are still far from elucidation. Positive results for the anti‐IFNAR1 mAb anifrolumab in a Phase III SLE trial have provided encouraging evidence for the use of pDC‐targeting drugs in SLE. In addition, in alloreactivity, pDCs may play either a protective 136 , 143 or deleterious role, 9 , 10 making the effects of pDC depletion unpredictable. Collectively, more studies must be done to understand more fully the biology of pDCs in the initiation/development of autoimmunity and alloreactivity, and novel pDC‐targeting modulation drugs are to be expected.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
The authors acknowledge the Association for Training, Education and Research in Hematology, Immunology and Transplantation for the generous and continuous support to the research work. YY thanks the China Scholarship Council for financial support (CSC No. 201606320257).
References
- 1. Siegal FP, Kadowaki N, Shodell M, Fitzgerald‐bocarsly PA, Shah K, Ho S. The nature of the principal type 1 interferon – producing cells in human blood. Science 1999; 284: 1835–1838. [DOI] [PubMed] [Google Scholar]
- 2. Cella M, Facchetti F, Lanzavecchia A, Colonna M. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat Immunol 2000; 1: 305–310. [DOI] [PubMed] [Google Scholar]
- 3. Kohara H, Omatsu Y, Sugiyama T, Noda M, Fujii N, Nagasawa T. Development of plasmacytoid dendritic cells in bone marrow stromal cell niches requires CXCL12‐CXCR4 chemokine signaling. Blood 2007; 110: 4153–4160. [DOI] [PubMed] [Google Scholar]
- 4. Umemoto E, Otani K, Ikeno T et al Constitutive plasmacytoid dendritic cell migration to the splenic white pulp is cooperatively regulated by CCR7‐ and CXCR4‐mediated signaling. J Immunol 2012; 189: 191–199. [DOI] [PubMed] [Google Scholar]
- 5. Bode C, Fox M, Tewary P et al Human plasmacytoid dendritic cells elicit a Type I Interferon response by sensing DNA via the cGAS‐STING signaling pathway. Eur J Immunol 2016; 46: 1615–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hoeffel G, Ripoche A, Matheoud D et al Antigen crosspresentation by human plasmacytoid dendritic cells. Immunity 2007; 27: 481–492. [DOI] [PubMed] [Google Scholar]
- 7. Baechler E, Batliwalla F, Karypis G et al Interferon‐inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci USA 2003; 100: 2610–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ah Kioon MD, Tripodo C, Fernandez D et al Plasmacytoid dendritic cells promote systemic sclerosis with a key role for TLR8. Sci Transl Med 2018; 10: eaam8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bossard C, Malard F, Arbez J et al Plasmacytoid dendritic cells and Th17 immune response contribution in gastrointestinal acute graft‐versus‐host disease. Leukemia 2012; 26: 1471–1474. [DOI] [PubMed] [Google Scholar]
- 10. Malard F, Bossard C, Brissot E et al Increased plasmacytoid dendritic cells and RORγt‐expressing immune effectors in cutaneous acute graft‐versus‐host disease. J Leukoc Biol 2013; 94: 1337–1343. [DOI] [PubMed] [Google Scholar]
- 11. Morand EF, Furie R, Tanaka Y et al Trial of anifrolumab in active systemic lupus erythematosus. N Engl J Med 2020; 382: 211–221. [DOI] [PubMed] [Google Scholar]
- 12. Khamashta M, Merrill JT, Werth VP et al Sifalimumab, an anti‐interferon‐α monoclonal antibody, in moderate to severe systemic lupus erythematosus: A randomised, double‐blind, placebo‐controlled study. Ann Rheum Dis 2016; 75: 1909–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Furie R, Werth VP, Merola JF et al Monoclonal antibody targeting BDCA2 ameliorates skin lesions in systemic lupus erythematosus. J Clin Invest 2019; 129: 1359–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cao W, Rosen DB, Ito T et al Plasmacytoid dendritic cell‐specific receptor ILT7‐FcεRIγ inhibits Toll‐like receptor‐induced interferon production. J Exp Med 2006; 203: 1399–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nakano H, Yanagita M, Gunn MD. CD11c+B220+Gr‐1+ cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J Exp Med 2001; 194: 1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bryant C, Fromm PD, Kupresanin F et al A CD2 high‐expressing stress‐resistant human plasmacytoid dendritic‐cell subset. Immunol Cell Biol 2016; 94: 447–457. [DOI] [PubMed] [Google Scholar]
- 17. Matsui T, Connolly JE, Michnevitz M et al CD2 distinguishes two subsets of human plasmacytoid dendritic cells with distinct phenotype and functions. J Immunol 2009; 182: 6815–6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang H, Gregorio JD, Iwahori T et al A distinct subset of plasmacytoid dendritic cells induces activation and differentiation of B and T lymphocytes. Proc Natl Acad Sci USA 2017; 114: 1988–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rhodes JW, Tong O, Harman AN, Turville SG. Human dendritic cell subsets, ontogeny, and impact on HIV infection. Front Immunol 2019; 10: 1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. See P, Dutertre CA, Chen J et al Mapping the human DC lineage through the integration of high‐dimensional techniques. Science 2017; 356: eaag3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Villani AC, Satija R, Reynolds G et al Single‐cell RNA‐seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science 2017; 356: eaah4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alcántara‐Hernández M, Leylek R, Wagar LE et al High‐dimensional phenotypic mapping of human dendritic cells reveals interindividual variation and tissue specialization. Immunity 2017; 47: 1037–1050.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dekker JD, Rhee C, Hu Z et al Lymphoid origin of a lineage of intrinsically activated plasmacytoid dendritic cell in mice and humans. bioRxiv 2018; 310680.
- 24. Leylek R, Alcántara‐Hernández M, Lanzar Z et al Integrated cross‐species analysis identifies a conserved transitional dendritic cell population. Cell Rep 2019; 29: 3736–3750.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alculumbre SG, Saint‐André V, Di Domizio J et al Diversification of human plasmacytoid predendritic cells in response to a single stimulus article. Nat Immunol 2018; 19: 63–75. [DOI] [PubMed] [Google Scholar]
- 26. Sathe P, Vremec D, Wu L, Corcoran L, Shortman K. Convergent differentiation: myeloid and lymphoid pathways to murine plasmacytoid dendritic cells. Blood 2013; 121: 11–19. [DOI] [PubMed] [Google Scholar]
- 27. Chen W, Antonenko S, Sederstrom JM et al Thrombopoietin cooperates with FLT3‐ligand in the generation of plasmacytoid dendritic cell precursors from human hematopoietic progenitors. Blood 2004; 103: 2547–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fancke B, Suter M, Hochrein H, O’Keeffe M. M‐CSF: a novel plasmacytoid and conventional dendritic cell poietin. Blood 2008; 111: 150–159. [DOI] [PubMed] [Google Scholar]
- 29. Upadhaya S, Sawai C, Papalexi E et al Kinetics of adult hematopoietic stem cell differentiation in vivo . J Exp Med 2018; 215: 2815–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cisse B, Caton ML, Lehner M et al Transcription factor E2–2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell 2008; 135: 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sasaki I, Hoshino K, Sugiyama T et al Spi‐B is critical for plasmacytoid dendritic cell function and development. Blood 2012; 120: 4733–4743. [DOI] [PubMed] [Google Scholar]
- 32. Sawai CM, Sisirak V, Ghosh HS et al Transcription factor Runx2 controls the development and migration of plasmacytoid dendritic cells. J Exp Med 2013; 210: 2151–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Auffray C, Fogg DK, Narni‐Mancinelli E et al CX3CR1+ CD115+ CD135+ common macrophage/DC precursors and the role of CX3CR1 in their response to inflammation. J Exp Med 2009; 206: 595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Onai N, Kurabayashi K, Hosoi‐Amaike M et al A clonogenic progenitor with prominent plasmacytoid dendritic cell developmental potential. Immunity 2013; 38: 943–957. [DOI] [PubMed] [Google Scholar]
- 35. Schlitzer A, Loschko J, Mair K et al Identification of CCR9‐ murine plasmacytoid DC precursors with plasticity to differentiate into conventional DCs. Blood 2011; 117: 6562–6570. [DOI] [PubMed] [Google Scholar]
- 36. Schlitzer A, Heiseke AF, Einwachter H et al Tissue‐specific differentiation of a circulating CCR9‐ pDC‐like common dendritic cell precursor. Blood 2012; 119: 6063–6071. [DOI] [PubMed] [Google Scholar]
- 37. D’Amico A, Wu L. The early progenitors of mouse dendritic cells and plasmacytoid predendritic cells are within the bone marrow hemopoietic precursors expressing Flt3. J Exp Med 2003; 198: 293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rodrigues PF, Alberti‐Servera L, Eremin A, Grajales‐Reyes GE, Ivanek R, Tussiwand R. Distinct progenitor lineages contribute to the heterogeneity of plasmacytoid dendritic cells. Nat Immunol 2018; 19: 711–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Herman JS, Grün S, Grün D. FateID infers cell fate bias in multipotent progenitors from single‐cell RNA‐seq data. Nat Methods 2018; 15: 379–386. [DOI] [PubMed] [Google Scholar]
- 40. Loschko J, Rieke GJ, Schreiber HA et al Inducible targeting of cDCs and their subsets in vivo . J Immunol Methods 2016; 434: 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sawai CM, Babovic S, Upadhaya S et al Hematopoietic stem cells are the major source of multilineage hematopoiesis in adult animals. Immunity 2016; 45: 597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sathe P, Metcalf D, Vremec D et al Lymphoid tissue and plasmacytoid dendritic cells and macrophages do not share a common macrophage‐dendritic cell‐restricted progenitor. Immunity 2014; 41: 104–115. [DOI] [PubMed] [Google Scholar]
- 43. Karamitros D, Stoilova B, Aboukhalil Z et al Single‐cell analysis reveals the continuum of human lympho‐myeloid progenitor cells. Nat Immunol 2018; 19: 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Helft J, Anjos‐Afonso F, van der Veen AG, Chakravarty P, Bonnet D, Reis e Sousa C. Dendritic cell lineage potential in human early hematopoietic progenitors. Cell Rep 2017; 20: 529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Albanesi C, Scarponi C, Pallotta S et al Chemerin expression marks early psoriatic skin lesions and correlates with plasmacytoid dendritic cell recruitment. J Exp Med 2009; 206: 249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Swiecki M, Colonna M. The multifaceted biology of plasmacytoid dendritic cells. Nat Rev Immunol 2015; 15: 471–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Clahsen T, Pabst O, Tenbrock K, Schippers A, Wagner N. Localization of dendritic cells in the gut epithelium requires MAdCAM‐1. Clin Immunol 2015; 156: 74–84. [DOI] [PubMed] [Google Scholar]
- 48. Gao Y, Majchrzak‐kita B, Fish EN, Gommerman JL. Dynamic accumulation of plasmacytoid dendritic cells in lymph nodes is regulated by IFN‐β. Blood 2009; 114: 2623–2632. [DOI] [PubMed] [Google Scholar]
- 49. Srivatsan S, Swiecki M, Otero K, Cella M, Shaw AS. CD2‐associated protein regulates plasmacytoid dendritic cell migration, but is dispensable for their development and cytokine production. J Immunol 2013; 191: 5933–5940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gotoh K, Tanaka Y, Nishikimi A et al Differential requirement for DOCK2 in migration of plasmacytoid dendritic cells versus myeloid dendritic cells. Blood 2008; 111: 2973–2976. [DOI] [PubMed] [Google Scholar]
- 51. Crother TR, Ma J, Jupelli M et al Plasmacytoid dendritic cells play a role for effective innate immune responses during chlamydia pneumoniae infection in mice. PLoS One 2012; 7: e48655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yu X, Cai B, Wang M et al Cross‐regulation of two type i interferon signaling pathways in plasmacytoid dendritic cells controls anti‐malaria immunity and host mortality. Immunity 2016; 45: 1093–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Spaulding E, Fooksman D, Moore JM et al STING‐licensed macrophages prime type I IFN production by plasmacytoid dendritic cells in the bone marrow during severe Plasmodium yoelii malaria. PLoS Pathog 2016; 12: 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Reizis B. Plasmacytoid dendritic cells: development, regulation, and function. Immunity 2019; 50: 37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Honda K, Yanai H, Negishi H et al IRF‐7 is the master regulator of type‐I interferon‐dependent immune responses. Nature 2005; 434: 772–777. [DOI] [PubMed] [Google Scholar]
- 56. Vollmer J, Weeratna R, Payette P et al Characterization of three CpG oligodeoxynucleotide classes with distinct immunostimulatory activities. Eur J Immunol 2004; 34: 251–262. [DOI] [PubMed] [Google Scholar]
- 57. Green NM, Laws A, Kiefer K et al Murine B cell response to TLR7 ligands depends on an IFN‐β feedback loop. J Immunol 2009; 183: 1569–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Izaguirre A, Barnes B, Amrute S et al Comparative analysis of IRF and IFN‐alpha expression in human plasmacytoid and monocyte‐derived dendritic cells. J Leukoc Biol 2003; 74: 1125–1138. [DOI] [PubMed] [Google Scholar]
- 59. Barchet W, Cella M, Odermatt B, Asselin‐Paturel C, Colonna M, Kalinke U. Virus‐induced interferon α production by a dendritic cell subset in the absence of feedback signaling in vivo . J Exp Med 2002; 195: 507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tomasello E, Naciri K, Chelbi R et al Molecular dissection of plasmacytoid dendritic cell activation in vivo during a viral infection. EMBO J 2018; 37: e98836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Asselin‐Paturel C, Brizard G, Chemin K et al Type I interferon dependence of plasmacytoid dendritic cell activation and migration. J Exp Med 2005; 201: 1157–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kumagai Y, Kumar H, Koyama S, Kawai T, Takeuchi O, Akira S. Cutting edge: TLR‐dependent viral recognition along with type I IFN positive feedback signaling masks the requirement of viral replication for IFN‐ production in plasmacytoid dendritic cells. J Immunol 2009; 182: 3960–3964. [DOI] [PubMed] [Google Scholar]
- 63. Hochrein H, Schlatter B, O’Keeffe M et al Herpes simplex virus type‐1 induces IFN‐α production via toll‐like receptor 9‐dependent and ‐independent pathways. Proc Natl Acad Sci USA 2004; 101: 11416–11421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kim T, Pazhoor S, Bao M et al Aspartate‐glutamate‐alanine‐histidine box motif (DEAH)/RNA helicase A helicases sense microbial DNA in human plasmacytoid dendritic cells. Proc Natl Acad Sci USA 2010; 107: 15181–15186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Blasius A, Vermi W, Krug A, Facchetti F, Cella M, Colonna M. A cell‐surface molecule selectively expressed on murine natural interferon‐producing cells that blocks secretion of interferon‐alpha. Blood 2004; 103: 4201–4206. [DOI] [PubMed] [Google Scholar]
- 66. Honda K, Ohba Y, Yanai H et al Spatiotemporal regulation of MyD88–IRF‐7 signalling for robust type‐I interferon induction. Nature 2005; 434: 1035–1040b. [DOI] [PubMed] [Google Scholar]
- 67. Guiducci C, Ott G, Chan J et al Properties regulating the nature of the plasmacytoid dendritic cell response to Toll‐like receptor 9 activation. J Exp Med 2006; 203: 1999–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Esashi E, Bao M, Wang YH, Cao W, Liu YJ. PACSIN1 regulates the TLR7/9‐mediated type I interferon response in plasmacytoid dendritic cells. Eur J Immunol 2012; 42: 573–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sasai M, Linehan M, Iwasaki A. Bifurcation of Toll‐like receptor 9 signaling by adaptor protein 3. Science 2010; 329: 1530–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Blasius A, Arnold C, Georgel P et al Slc15a4, AP‐3, and Hermansky‐Pudlak syndrome proteins are required for Toll‐like receptor signaling in plasmacytoid dendritic cells. Proc Natl Acad Sci USA 2010; 107: 19973–19978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Henault J, Martinez J, Riggs JM et al Noncanonical autophagy is required for type i interferon secretion in response to DNA‐immune complexes. Immunity 2012; 37: 986–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hayashi K, Taura M, Iwasaki A. The interaction between IKKα and LC3 promotes type I interferon production through the TLR9‐containing LAPosome. Sci Signal 2018; 11: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wimmers F, Subedi N, van Buuringen N et al Single‐cell analysis reveals that stochasticity and paracrine signaling control interferon‐alpha production by plasmacytoid dendritic cells. Nat Commun 2018; 9: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hagberg N, Berggren O, Leonard D et al IFN‐α production by plasmacytoid dendritic cells stimulated with RNA‐containing immune complexes is promoted by NK cells via MIP‐1 and LFA‐1. J Immunol 2011; 186: 5085–5094. [DOI] [PubMed] [Google Scholar]
- 75. Saitoh SI, Abe F, Kanno A et al TLR7 mediated viral recognition results in focal type I interferon secretion by dendritic cells. Nat Commun 2017; 8: 1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Deal EM, Jaimes MC, Crawford SE, Estes MK, Greenberg HB. Rotavirus structural proteins and dsRNA are required for the human primary plasmacytoid dendritic cell IFNα response. PLoS Pathog 2010; 6: e1000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Frenz T, Graalmann L, Detje CN et al Independent of plasmacytoid dendritic cell (pDC) infection, pDC triggered by virus‐infected cells mount enhanced type I IFN responses of different composition as opposed to pDC stimulated with free virus. J Immunol 2014; 193: 2496–2503. [DOI] [PubMed] [Google Scholar]
- 78. Takahashi K, Asabe S, Wieland S et al Plasmacytoid dendritic cells sense hepatitis C virus‐infected cells, produce interferon, and inhibit infection. Proc Natl Acad Sci USA 2010; 107: 7431–7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Décembre E, Assil S, Hillaire MLB et al Sensing of immature particles produced by dengue virus infected cells induces an antiviral response by plasmacytoid dendritic cells. PLoS Pathog 2014; 10: e1004434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Assil S, Coléon S, Dong C et al Plasmacytoid dendritic cells and infected cells form an interferogenic synapse required for antiviral responses. Cell Host Microbe 2019; 25: 730–745.e6. [DOI] [PubMed] [Google Scholar]
- 81. Megjugorac NJ, Gallagher GE, Gallagher G. Modulation of human plasmacytoid DC function by IFN‐λ1 (IL‐29). J Leukoc Biol 2009; 86: 1359–1363. [DOI] [PubMed] [Google Scholar]
- 82. Sommereyns C, Paul S, Staeheli P, Michiels T. IFN‐lambda (IFN‐λ) is expressed in a tissue‐dependent fashion and primarily acts on epithelial cells in vivo . PLoS Pathog 2008; 4: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Galani IE, Triantafyllia V, Eleminiadou EE et al Interferon‐λ mediates non‐redundant front‐line antiviral protection against influenza virus infection without compromising host fitness. Immunity 2017; 46: 875–890.e6. [DOI] [PubMed] [Google Scholar]
- 84. Nice TJ, Baldridge MT, McCune BT et al Interferon‐λ cures persistent murine norovirus infection in the absence of adaptive immunity. Science 2015; 347: 269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wolk K, Witte K, Witte E et al IL‐29 is produced by TH17 cells and mediates the cutaneous antiviral competence in psoriasis. Sci Transl Med. 2013; 5: 204ra129. [DOI] [PubMed] [Google Scholar]
- 86. Numasaki M, Tagawa M, Iwata F et al IL‐28 elicits antitumor responses against murine fibrosarcoma. J Immunol 2007; 178: 5086–5098. [DOI] [PubMed] [Google Scholar]
- 87. Kelly A, Robinson MW, Roche G, Biron CA, O’Farrelly C, Ryan EJ. Immune cell profiling of IFN‐λ response shows pDCs express highest level of IFN‐λR1 and are directly responsive via the JAK‐STAT pathway. J Interf Cytokine Res 2016; 36: 671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Finotti G, Tamassia N, Calzetti F, Fattovich G, Cassatella MA. Endogenously produced TNF‐α contributes to the expression of CXCL10/IP‐10 in IFN‐λ3‐activated plasmacytoid dendritic cells. J Leukoc Biol 2016; 99: 107–119. [DOI] [PubMed] [Google Scholar]
- 89. Rissoan MC, Soumelis V, Kadowaki N et al Reciprocal control of T helper cell and dendritic cell differentiation. Science 1999; 283: 1183–1186. [DOI] [PubMed] [Google Scholar]
- 90. Yu C‐F, Peng W‐M, Oldenburg J et al Human plasmacytoid dendritic cells support Th17 cell effector function in response to TLR7 ligation. J Immunol 2010; 184: 1159–1167. [DOI] [PubMed] [Google Scholar]
- 91. Bonnefoy F, Couturier M, Clauzon A et al TGF‐β‐exposed plasmacytoid dendritic cells participate in Th17 commitment. J Immunol 2011; 186: 6157–6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chappell C, Giltiay N, Draves K et al Targeting antigens through blood dendritic cell antigen 2 on plasmacytoid dendritic cells promotes immunologic tolerance. J Immunol 2014; 192: 5789–5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Loschko J, Heink S, Krug AB et al Antigen targeting to plasmacytoid dendritic cells via siglec‐H inhibits Th cell‐dependent autoimmunity. J Immunol 2011; 187: 6346–6356a. [DOI] [PubMed] [Google Scholar]
- 94. Loschko J, Schlitzer A, Dudziak D et al Antigen delivery to plasmacytoid dendritic cells via BST2 induces protective T cell‐mediated immunity. J Immunol 2011; 186: 6718–6725b. [DOI] [PubMed] [Google Scholar]
- 95. Di Pucchio T, Chatterjee B, Smed‐Sörensen A et al Direct proteasome‐independent cross‐presentation of viral antigen by plasmacytoid dendritic cells on major histocompatibility complex class I. Nat Immunol 2008; 9: 551–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kool M, GeurtsVanKessel C, Muskens F et al Facilitated antigen uptake and timed exposure to TLR ligands dictate the antigen‐presenting potential of plasmacytoid DCs. J Leukoc Biol 2011; 90: 1177–1190. [DOI] [PubMed] [Google Scholar]
- 97. Oberkampf M, Guillerey C, Mouriès J et al Mitochondrial reactive oxygen species regulate the induction of CD8+ T cells by plasmacytoid dendritic cells. Nat Commun 2018; 9: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Brewitz A, Eickhoff S, Dähling S et al CD8+ T cells orchestrate pDC‐XCR1+ dendritic cell spatial and functional cooperativity to optimize priming. Immunity 2017; 46: 205–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity 2003; 19: 225–234. [DOI] [PubMed] [Google Scholar]
- 100. Poeck H, Wagner M, Battiany J et al Plasmacytoid dendritic cells, antigen, and CpG‐C license human B cells for plasma cell differentiation and immunoglobulin production in the absence of T‐cell help. Blood 2004; 103: 3058–3064. [DOI] [PubMed] [Google Scholar]
- 101. Shaw J, Wang YH, Ito T, Arima K, Liu YJ. Plasmacytoid dendritic cells regulate B‐cell growth and differentiation via CD70. Blood 2010; 115: 3051–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Varani S, Cederarv M, Feld S et al Human cytomegalovirus differentially controls B cell and T cell responses through effects on plasmacytoid dendritic cells. J Immunol 2007; 179: 7767–7776. [DOI] [PubMed] [Google Scholar]
- 103. Deal EM, Lahl K, Narváez CF, Butcher EC, Greenberg HB. Plasmacytoid dendritic cells promote rotavirus‐induced human and murine B cell responses. J Clin Invest 2013; 123: 2464–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Menon M, Blair PA, Isenberg DA, Mauri C. A regulatory feedback between plasmacytoid dendritic cells and regulatory B cells is aberrant in systemic lupus erythematosus. Immunity 2016; 44: 683–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Bengtsson A, Sturfelt G, Truedsson L et al Activation of type I interferon system in systemic lupus erythematosus correlates with disease activity but not with antiretroviral antibodies. Lupus 2000; 9: 664–671. [DOI] [PubMed] [Google Scholar]
- 106. Lövgren T, Eloranta ML, Båve U, Alm GV, Rönnblom L. Induction of interferon‐α production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum 2004; 50: 1861–1872. [DOI] [PubMed] [Google Scholar]
- 107. Henault J, Riggs JM, Karnell JL et al Self‐reactive IgE exacerbates interferon responses associated with autoimmunity. Nat Immunol 2016; 17: 196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Means TK, Latz E, Hayashi F, Murali MR, Golenbock DT, Luster AD. Human lupus autoantibody‐DNA complexes activate DCs through cooperation of CD32 and TLR9. J Clin Invest 2005; 115: 407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Blomberg S, Eloranta M, Cederblad B, Nordlin K, Alm G, Rönnblom L. Presence of cutaneous interferon‐a producing cells in patients with systemic lupus erythematosus. Lupus 2001; 10: 484–490. [DOI] [PubMed] [Google Scholar]
- 110. Laffont S, Rouquié N, Azar P et al X‐chromosome complement and estrogen receptor signaling independently contribute to the enhanced TLR7‐mediated IFN‐α production of plasmacytoid dendritic cells from women. J Immunol 2014; 193: 5444–5452. [DOI] [PubMed] [Google Scholar]
- 111. Lande R, Ganguly D, Facchinetti V et al Neutrophils activate plasmacytoid dendritic cells by releasing self‐DNA‐peptide complexes in systemic lupus erythematosus. Sci Transl Med 2011; 3: 73ra19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Garcia‐Romo G, Caielli S, Vega B et al Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med 2011; 3: 73ra20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lood C, Blanco L, Purmalek M et al Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus‐like disease. Nat Med 2016; 22: 146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Caielli S, Athale S, Domic B et al Oxidized mitochondrial nucleoids released by neutrophils drive type I interferon production in human lupus. J Exp Med 2016; 213: 697–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Rowland S, Riggs J, Gilfillan S et al Early, transient depletion of plasmacytoid dendritic cells ameliorates autoimmunity in a lupus model. J Exp Med 2014; 211: 1977–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Davison LM, Jørgensen TN. Sialic acid‐binding immunoglobulin‐type lectin H‐positive plasmacytoid dendritic cells drive spontaneous lupus‐like disease development in B6.Nba2 mice. Arthritis Rheumatol 2015; 67: 1012–1022. [DOI] [PubMed] [Google Scholar]
- 117. Sisirak V, Ganguly D, Lewis K et al Genetic evidence for the role of plasmacytoid dendritic cells in systemic lupus erythematosus. J Exp Med 2014; 211: 1969–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Wöhner M, Tagoh H, Bilic I et al Molecular functions of the transcription factors E2A and E2–2 in controlling germinal center B cell and plasma cell development. J Exp Med 2016; 213: 1201–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Blasius AL, Giurisato E, Cella M, Schreiber RD, Shaw AS, Colonna M. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN‐producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J Immunol 2006; 177: 3260–3265. [DOI] [PubMed] [Google Scholar]
- 120. Kim D, Peck A, Santer D et al Induction of interferon‐α by scleroderma sera containing autoantibodies to topoisomerase I: association of higher interferon‐α activity with lung fibrosis. Arthritis Rheum 2008; 58: 2163–2173. [DOI] [PubMed] [Google Scholar]
- 121. Kafaja S, Valera I, Divekar AA et al pDCs in lung and skin fibrosis in a bleomycin‐induced model and patients with systemic sclerosis. JCI Insight 2018; 3: e98380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Allen JS, Pang K, Skowera A et al Plasmacytoid dendritic cells are proportionally expanded at diagnosis of type 1 diabetes and enhance islet autoantigen presentation to T‐cells through immune complex capture. Diabetes 2009; 58: 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Hansen L, Schmidt‐Christensen A, Gupta S et al E2–2 dependent plasmacytoid dendritic cells control autoimmune diabetes. PLoS One 2015; 10: e0144090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Nestle FO, Conrad C, Tun‐Kyi A et al Plasmacytoid predendritic cells initiate psoriasis through interferon‐α production. J Exp Med 2005; 202: 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Glitzner E, Korosec A, Brunner P et al Specific roles for dendritic cell subsets during initiation and progression of psoriasis. EMBO Mol Med 2014; 6: 1312–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Nehmar R, Alsaleh G, Voisin B et al Therapeutic modulation of plasmacytoid dendritic cells in experimental arthritis. Arthritis Rheumatol 2017; 69: 2124–2135. [DOI] [PubMed] [Google Scholar]
- 127. Arimura K, Takagi H, Uto T et al Crucial role of plasmacytoid dendritic cells in the development of acute colitis through the regulation of intestinal inflammation. Mucosal Immunol 2017; 10: 957–970. [DOI] [PubMed] [Google Scholar]
- 128. Sawai C, Serpas L, Neto A et al Plasmacytoid dendritic cells are largely dispensable for the pathogenesis of experimental inflammatory bowel disease. Front Immunol 2018; 9: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. MacRitchie N, Grassia G, Sabir SR et al Plasmacytoid dendritic cells play a key role in promoting atherosclerosis in apolipoprotein e‐deficient mice. Arterioscler Thromb Vasc Biol 2012; 32: 2569–2579. [DOI] [PubMed] [Google Scholar]
- 130. Sage AP, Murphy D, Maffia P et al MHC Class II‐restricted antigen presentation by plasmacytoid dendritic cells drives proatherogenic T cell immunity. Circulation 2014; 130: 1363–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Yun TJ, Lee JS, Machmach K et al Indoleamine 2,3‐dioxygenase‐expressing aortic plasmacytoid dendritic cells protect against atherosclerosis by induction of regulatory T cells. Cell Metab 2016; 23: 852–866. [DOI] [PubMed] [Google Scholar]
- 132. Sackstein R. A revision of Billingham’s tenets: the central role of lymphocyte migration in acute graft‐versus‐host disease. Biol Blood Marrow Transplant 2006; 12: 2–8. [DOI] [PubMed] [Google Scholar]
- 133. Koyama M, Hashimoto D, Aoyama K et al Plasmacytoid dendritic cells prime alloreactive T cells to mediate graft‐versus‐host disease as antigen‐presenting cells. Blood 2009; 113: 2088–2095. [DOI] [PubMed] [Google Scholar]
- 134. Li H, Demetris A, McNiff J et al Profound depletion of host conventional dendritic cells, plasmacytoid dendritic cells, and B cells does not prevent graft‐versus‐host disease induction. J Immunol 2012; 188: 3804–3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Koyama M, Kuns R, Olver S et al Recipient nonhematopoietic antigen‐presenting cells are sufficient to induce lethal acute graft‐versus‐host disease. Nat Med 2011; 18: 135–142. [DOI] [PubMed] [Google Scholar]
- 136. Hadeiba H, Sato T, Habtezion A, Oderup C, Pan J, Butcher EC. CCR9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft‐versus‐host disease. Nat Immunol 2008; 9: 1253–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Waller E, Logan B, Harris W et al Improved survival after transplantation of more donor plasmacytoid dendritic or naïve T cells from unrelated‐donor marrow grafts: results from BMTCTN 0201. J Clin Oncol 2014; 32: 2365–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Hassan M, Ulezko Antonova A, Li JM et al Flt3L treatment of bone marrow donors increases graft plasmacytoid dendritic cell content and improves allogeneic transplantation outcomes. Biol Blood Marrow Transplant 2019; 25: 1075–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Banovic T, Markey KA, Kuns RD et al Graft‐versus‐host disease prevents the maturation of plasmacytoid dendritic cells. J Immunol 2009; 182: 912–920. [DOI] [PubMed] [Google Scholar]
- 140. Kaufman CL, Colson YL, Wren SM, Watkins S, Simmons RL, Ildstad ST. Phenotypic characterization of a novel bone marrow‐derived cell that facilitates engraftment of allogeneic bone marrow stem cells. Blood 1994; 84: 2436–2446. [PubMed] [Google Scholar]
- 141. Huang Y, Xu H, Miller T, Wen Y, Ildstad ST. Fms‐like tyrosine kinase 3‐ligand contributes to the development and function of the subpopulation of CD8α+ plasmacytoid precursor dendritic cells in CD8+/TCR− facilitating cells. Stem Cells 2018; 36: 1567–1577. [DOI] [PubMed] [Google Scholar]
- 142. Li J, Southerland LT, Lu Y et al Activation, immune polarization, and graft‐versus‐leukemia activity of donor T cells are regulated by specific subsets of donor bone marrow antigen‐presenting cells in allogeneic hemopoietic stem cell transplantation. J Immunol 2009; 183: 7799–7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Lu Y, Giver C, Sharma A et al IFN‐ and indoleamine 2,3‐dioxygenase signaling between donor dendritic cells and T cells regulates graft versus host and graft versus leukemia activity. Blood 2011; 119: 1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Fugier‐Vivier IJ, Rezzoug F, Huang Y et al Plasmacytoid precursor dendritic cells facilitate allogeneic hematopoietic stem cell engraftment. J Exp Med 2005; 201: 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Vakkila J, Thomson AW, Hovi L, Vettenranta K, Saarinen‐Pihkala UM. Circulating dendritic cell subset levels after allogeneic stem cell transplantation in children correlate with time post transplant and severity of acute graft‐versus‐host disease. Bone Marrow Transplant 2005; 35: 501–507. [DOI] [PubMed] [Google Scholar]
- 146. Mohty M, Gastaut J‐A, Viens P et al Impact of plasmacytoid dendritic cells on outcome after reduced‐intensity conditioning allogeneic stem cell transplantation. Leukemia 2005; 19: 1–6. [DOI] [PubMed] [Google Scholar]
- 147. Arpinati M, Chirumbolo G, Urbini B et al Acute graft‐versus‐host disease and steroid treatment impair CD11c+ and CD123+ dendritic cell reconstitution after allogeneic peripheral blood stem cell transplantation. Biol Blood Marrow Transplant 2004; 10: 106–115. [DOI] [PubMed] [Google Scholar]
- 148. Wikstrom ME, Fleming P, Kuns RD et al Acute GVHD results in a severe DC defect that prevents T‐cell priming and leads to fulminant cytomegalovirus disease in mice. Blood 2015; 126: 1503–1514. [DOI] [PubMed] [Google Scholar]
- 149. Leveque‐El Mouttie L, Koyama M, Le Texier L et al Corruption of dendritic cell antigen presentation during acute GVHD leads to regulatory T‐cell failure and chronic GVHD. Blood 2016; 128: 794–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Kalunian KC, Merrill JT, Maciuca R et al A phase II study of the efficacy and safety of rontalizumab (rhuMAb interferon‐α) in patients with systemic lupus erythematosus (ROSE). Ann Rheum Dis 2015; 75: 196–202. [DOI] [PubMed] [Google Scholar]
- 151. Carrington EM, Zhang J‐G, Sutherland RM et al Prosurvival Bcl‐2 family members reveal a distinct apoptotic identity between conventional and plasmacytoid dendritic cells. Proc Natl Acad Sci USA 2015; 112: 4044–4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Zhan Y, Carrington EM, Ko HJ et al Bcl‐2 antagonists kill plasmacytoid dendritic cells from lupus‐prone mice and dampen interferon‐α production. Arthritis Rheumatol 2015; 67: 797–808. [DOI] [PubMed] [Google Scholar]
- 153. Lu P, Fleischmann R, Curtis C et al Safety and pharmacodynamics of venetoclax (ABT‐199) in a randomized single and multiple ascending dose study in women with systemic lupus erythematosus. Lupus 2018; 27: 290–302. [DOI] [PubMed] [Google Scholar]
- 154. Lauwerys BR, Hachulla E, Spertini F et al Down‐regulation of interferon signature in systemic lupus erythematosus patients by active immunization with interferon α‐kinoid. Arthritis Rheum 2013; 65: 447–456. [DOI] [PubMed] [Google Scholar]
- 155. Bobe P, Bonardelle D, Benihoud K, Opolon P, Chelbi‐Alix MK. Arsenic trioxide: A promising novel therapeutic agent for lymphoproliferative and autoimmune syndromes in MRL/lpr mice. Blood 2006; 108: 3967–3975. [DOI] [PubMed] [Google Scholar]
- 156. Kavian N, Marut W, Servettaz A et al Reactive oxygen species‐mediated killing of activated fibroblasts by arsenic trioxide ameliorates fibrosis in a murine model of systemic sclerosis. Arthritis Rheum 2012; 64: 3430–3440a. [DOI] [PubMed] [Google Scholar]
- 157. Kavian N, Marut W, Servettaz A et al Arsenic trioxide prevents murine sclerodermatous graft‐versus‐host disease. J Immunol 2012; 188: 5142–5149b. [DOI] [PubMed] [Google Scholar]
- 158. Ye Y, Gaugler B, Mohty M, Malard F. Old dog, new trick: trivalent arsenic as an immunomodulatory drug. Br J Pharmacol 2020; 177: 2199–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Ye Y, Ricard L, Siblany L et al Arsenic trioxide induces regulatory functions of plasmacytoid dendritic cells through interferon‐α inhibition. Acta Pharm Sin B 2020. 10.1016/j.apsb.2020.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. O’Keeffe M, Hochrein H, Vremec D et al Mouse plasmacytoid cells: Long‐lived cells, heterogeneous in surface phenotype and function, that differentiate into CD8‐ dendritic cells only after microbial stimulus. J Exp Med 2002; 196: 1307–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Grouard G, Rissoan MC, Filgueira L, Durand I, Banchereau J, Liu YJ. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)‐3 and CD40‐ligand. J Exp Med 1997; 185: 1101–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]


